Abstract
Large vessel ischemic stroke represents the most disabling subtype. While t-PA and endovascular thrombectomy can recanalize the occluded vessel, good clinical outcomes are not uniformly achieved. We propose that supplementing endovascular thrombectomy with superselective intra-arterial (IA) verapamil immediately following recanalization could be safe and effective. Verapamil, a calcium channel blocker, has been shown to be an effective IA adjunct in a pre-clinical mouse focal ischemia model. To demonstrate translational efficacy, mechanism, feasibility, and safety, we conducted a group of translational experiments. We performed in vivo IA dose–response evaluation in our animal stroke model with C57/Bl6 mice. We evaluated neuroprotective mechanism through in vitro primary cortical neuron (PCN) cultures. Finally, we performed a Phase I trial, SAVER-I, to evaluate feasibility and safety of administration in the human condition. IA verapamil has a likely plateau or inverted-U dose–response with a defined toxicity level in mice (LD50 16–17.5 mg/kg). Verapamil significantly prevented PCN death and deleterious ischemic effects. Finally, the SAVER-I clinical trial showed no evidence that IA verapamil increased the risk of intracranial hemorrhage or other adverse effect/procedural complication in human subjects. We conclude that superselective IA verapamil administration immediately following thrombectomy is safe and feasible, and has direct, dose–response-related benefits in ischemia.
Keywords: Ischemic stroke, emergent large vessel occlusion, thrombectomy, verapamil, neuroprotection
Introduction
Stroke is the fifth leading cause of death, but the leading cause of long-term disability in the USA, with over 800,000 affected annually.1–4 While stroke encompasses both ischemic and hemorrhagic pathologies, 87% are ischemic.5,6 Currently, intravenous tissue-plasminogen activator (IV t-PA) is the only FDA approved pharmacotherapy for ischemic stroke.7,8 With a number of exclusion criteria, IV t-PA is only administered to 3–37% of acute ischemic stroke (AIS) patients leaving the majority of ischemic stroke patients with few options.9,10
Endovascular thrombectomy (ET), or the mechanical removal of a clot, has been shown to be an effective therapeutic approach for emergent large vessel occlusion (ELVO) stroke.11 Multiple randomized trials, including MR CLEAN, EXTEND IA and SWIFT PRIME, have established the significant clinical benefit of thrombectomy for ELVO.12–14 However, clinical outcomes vary significantly, with 90-day independence ranging from 32.6% to 71%.1,8,12,13 Furthermore, these trials used varying forms of pre-thrombectomy imaging criteria and/or time limitations to exclude patients with significant infarcted core. Thus, while clinical outcomes continue to lag the high rates of technical success, there may also be opportunity to use adjuvant neuroprotectants to extend the window for intervention, or reverse damage that is currently viewed as unsalvageable based on radiographic imaging alone, thereby increasing the number of patients eligible for thrombectomy.
To address this, we propose coupling ET with a potential neuroprotective pharmacotherapy by selectively administering a compound directly to the site of ischemia immediately following thrombectomy/reperfusion. Representing the first clinical study to purposely administer an IA neuroprotective agent as an adjunct to thrombectomy, the overall aims were to demonstrate the feasibility of such an approach and evaluate the safety of a neuroprotective compound administered in this setting.
In selecting a potentially neuroprotective agent, we chose to evaluate verapamil, an L-type calcium channel blocker. Verapamil is already routinely administered intra-arterially in the cerebral circulation to treat cerebral vasospasm secondary to subarachnoid hemorrhage. Preclinical studies evaluating calcium channel blockers for stroke neuroprotection have shown some promise.15–17 Recently, our laboratory has published in vivo studies demonstrating the neuroprotective effects of coupling recanalization with IA verapamil in a mouse model of focal cortical ischemia (transient middle cerebral artery occlusion).18 We demonstrated that, when administered IA following recanalization, verapamil was physiologically safe (heart rate and blood pressure), and it significantly reduced infarct volume and significantly improved functional outcome.19 Furthermore, IA verapamil did not significantly alter post-reperfusion flow or cerebral perfusion, suggesting a therapeutic mechanism separate from its vasodilatory effects, possibly related to reduction of excitotoxic damage through its calcium channel activity.19 We hypothesize that verapamil is a safe, feasible, and efficacious neuroprotectant for direct administration after thrombectomy. With these data as a foundation, we performed a translational set of experiments including in vivo dose–response studies, in vitro experiments to determine verapamil’s potential for direct neuroprotection separate from vasodilation, and finally a Phase I clinical trial, Superselective Administration of VErapamil During Recanalization in Acute Ischemic Stroke (SAVER-I) to further demonstrate evidence for therapeutic potential.
Materials and methods
In vivo dose–response evaluation
In order to further elucidate the therapeutic window of IA verapamil, we conducted toxicity and dose–response studies based on the initial dose used in our prior in vivo studies (weight-based dose equivalent to 10 mg in 70 kg human).18,19 We used an identical animal surgery protocol as previously published for the tandem transient common carotid CCA-MCA middle cerebral artery occlusion model (MCAo) with IA internal carotid injection immediately following recanalization. Animal experiments were conducted in accordance with and following approval by the University of Kentucky Institutional Animal Care and Use Committee (IACUC), and results were reported in accordance with ARRIVE Guidelines.19,20 Sixteen-week-old male C57/Bl6 mice (25–30 g) from Jackson Laboratories were housed with free access to food and water. In order to estimate the possible dosing scale, we evaluated the body surface area (BSA) of the subjects enrolled in SAVER-I (see below), and calculated a mean BSA of 2.091 ± 0.076 (data not shown). Based on previous studies of translational dosing strategies, we used a mouse BSA of 0.007, with a Km value of 37 for humans and 3 for mice.21 In order to maintain similar injection volumes and pressures (to minimize any potential volume effect on neuroprotection or complications), we dosed verapamil as a concentration (mg/mL) during the experiments, though weight based in our calculations, maintaining a stable total injection volume of isotonic solution (10 µL). Each dose group had an N of 5 animals. Based on the known toxicity of verapamil in mice (intravenous LD50 of 5.8 mg/kg), we began our toxicity studies at 50 mg/mL (500 µg), higher than the peripheral IV LD50, with decreasing doses to demonstrate the point of IA lethal toxicity. Once this was identified, we began a randomized, blinded, dose escalation experiment from a log-10 dose higher than our previously published effective dose, followed by two dose intervals between that dose (10 mg/kg) and the discovered lowest lethal dose (17.5 mg/kg as noted in the Results section) in order to identify the dose–response curve between our previously published dose and the lowest toxicity. For surviving animals, euthanasia occurred via cervical dislocation at seven days, whereupon the whole brain was removed, and either cut and stained with TTC, or flash frozen, sectioned on the cryostat (20 µm), and stained with Cresyl Violet for infarct volume using methods previously detailed.18,19 NeuN immunohistochemistry was performed to evaluate mature neuron survival (1:1000 antibody dilution, Abcam) in the peri-infarct region. This corresponds to the cortical region that was the epicenter of the stroke morphologically identified based on cryostat sectioning to include the greatest affected area. Stains were visualized with a Nikon Eclipse Ti microscope at 20× magnification and images were collected via a CCD camera attached to a computer. Nikon NIS Element BR Analysis imaging software was used to analyze brain sections for positive pixel density. Analysis of results for comparison between groups was performed using Student’s t-test, with significance defined as p ≤ 0.05.
In vitro verification of neuroprotection
Primary neuronal culture
Primary cortical neuronal (PCN) cultures were prepared from embryonic day-18 C57/Bl6 mice by using methods described previously.22 Briefly, cortices were isolated in Hanks’ Balanced Salt Solution (Corning, Manassas, VA; calcium, magnesium and phenol red free) and the hippocampi removed. Tissues were trypsinized and mechanically dissociated. Cortical neurons were plated on Poly-d-Lysine (PDL, Sigma St. Louis, MO, USA) coated glass disk (15 mm) in a 12-well plate containing NBactive4 (BrainBits) supplemented with antimicrobial/antibiotic solution (10,000 units/mL of penicillin, 10,000 µg/mL of streptomycin, and 25 µg/mL of Fungizone® Antimycotic, Gibco, Grand Island, NY, USA) at a density of 100,000 cells/well. Cortical neurons were used at seven days in vitro for cell survival and neurite outgrowth following oxygen glucose deprivation (OGD).
Oxygen glucose deprivation
OGD was performed in hypoxic chambers (Billups-Rothenberg Inc, Del Mar, CA, USA) as previously described.23 Briefly, at 37℃ and 5% CO2 PCNs were exposed to control (NBactive4) or OGD (Neurobasal-A, no glucose, within a sealed hypoxic chamber flushed with 100 % N2) conditions for 30 min. Thirty minutes was chosen after a preliminary experiment evaluating cell survival showed a mean cell survival of more than 50% of cells for 30 min versus a mean cell survival of less than 20% for 60 min of OGD (Supplemental Figure 1); 30 min was selected over 60 min to ensure some PCN survival at baseline control conditions for comparison. Following a media change with vehicle (saline) or verapamil (250, 300, 500 ng/mL), neurons were allowed to reperfuse for 24 h at 37℃.
Cell survival
Nuclei were imaged using the fluorescent DNA binding dye Hoechst 33342 (Sigma) as previously described.24 Briefly, at 23.5 h following reperfusion, Hoechst 33342 (Sigma) was added to the media for 30 min at 37℃ and 5% CO2. PCNs were fixed with ice-cold 4% PFA for 20 min, washed with PBS, and mounted with Vectorshield (H 1400). Nuclei from at least 200 neurons per experimental condition were counted on a Nikon Eclipse Ti microscope using a 40× oil immersion objective. Nuclei with condensed or fragmented chromatin were considered apoptotic.
Neurite outgrowth
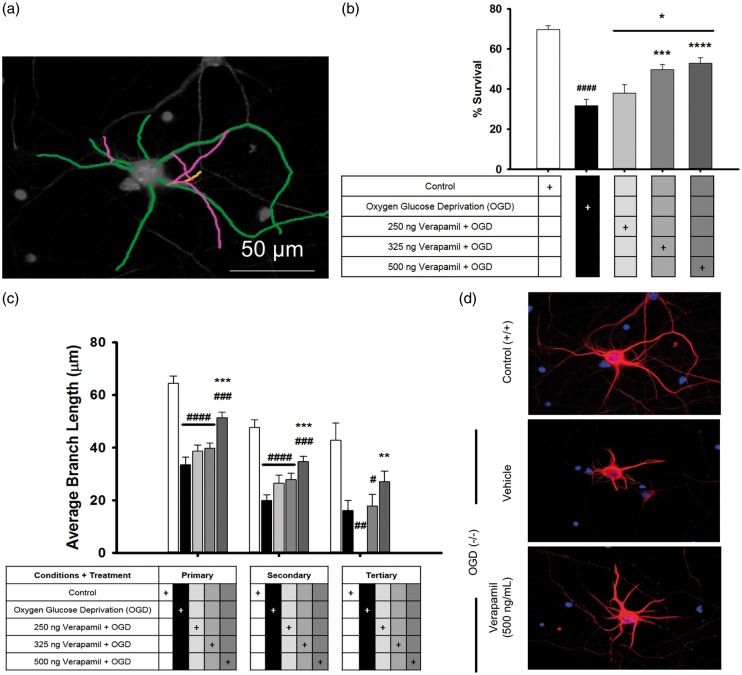
Neurons were fixed with ice-cold 4% PFA for 20 min, rinsed twice with TBS, and blocked for 1 h with TBS containing Trition X-100 (0.1%; Sigma), and BSA (5%). Cells were incubated overnight at 4℃ with mouse anti-MAP2 (1:500 Sigma), in TBS containing Triton X (0.1% Trition X-100) and a reduced amount of BSA (2.5%). Cells were washed three times with TBS containing Trition-X and incubated for 2 h at room temperature with Alexa Flour 594 goat anti-mouse (Life Technologies, Carlsbad, CA, USA). Cells were washed and mounted with Vectorshield. Images were captured using a Nikon Eclipse Ti microscope with a 40× oil immersion objective and analyzed using the NeuronJ plugin for the Image J software.25 Neurites were counted, measured, and labeled as primary, secondary, or tertiary (Figure 2(a)). Conditions were performed in triplicate and at least five images were analyzed per well, N = 15.
Figure 2.
Verapamil promotes cell survival and preservation of neurons after administration immediately following exposure to 30 min of OGD. An example shows the process of labeling each neuron to count the dendritic branches (a). OGD significantly reduced neuron survival, as well as dendritic branching in all levels (b). Addition of verapamil, in an increasing dose–response manner, resulted in rescuing of some neurons from the effects of OGD (c). Examples of the damaging effect of OGD and neuroprotective effect of verapamil can be seen (d).
Phase I clinical trial: superselective administration of verapamil during recanalization in acute ischemic stroke – SAVER-I
Consecutive subjects presenting to our institution with a large vessel occlusive stroke deemed eligible for thrombectomy as standard of care were considered for enrollment. This open-label unblinded Phase I safety trial was conducted with University of Kentucky IRB approval, was reported in accordance with the Harms extension of the CONSORT checklist, and was listed on www.clinicaltrials.gov (NCT02235558). Inclusion criteria were: (1) 21–85-year-old male or female; (2) suspected acute ischemic stroke based on clinical and radiographic evidence as determined by the Stroke Neurology team; (3) candidate for thrombectomy as determined by a Neurointerventionalist with an acute thromboembolus within an intracranial artery (internal carotid, anterior cerebral, middle cerebral, posterior cerebral, basilar, vertebral); (4) undergo endovascular thrombectomy with a TICI 2A or better recanalization. TICI (Thrombolysis in Cerebral Infarction) score indicates how much of the cerebral vasculature is reopened during any thrombectomy procedure.26,27 TICI 2A would indicate flow beyond the occlusion, but could include perfusion of less than half the downstream branches (TICI 2B would indicate greater than half of the downstream branches are open, while TICI 3 would indicate complete revascularization).27 Subjects with impaired capacity were included if their legally authorized representative signed consent. Consent was performed in-person prior to thrombectomy by a member of the study group (care was taken to ensure no time was lost in the treatment of the patient as a result of the consent process). During consent, it was explained that successful enrollment would be contingent upon successful thrombectomy as defined by TICI 2a (above). Exclusion criteria were: (1) pregnant women; (2) those undergoing thrombectomy with only a TICI 0-1 revascularization; (3) tandem occlusion of the cervical common or internal carotid artery; and (4) subjects on therapeutic anticoagulation, as it is a relative contraindication to thrombectomy, and could be a confounding variable predisposing to intracranial hemorrhage.
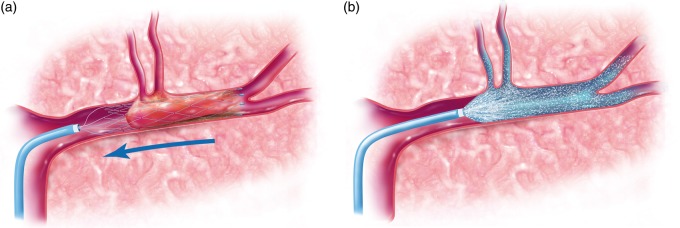
Subjects underwent standard of care thrombectomy using both stent-retrievers and reperfusion catheters as indicated. Subjects were not excluded if they received IV t-PA as standard of care. Once the thrombectomy was performed (Figure 4(a)), the microcatheter was used initially as part of the thrombectomy was re-navigated over a microwire using roadmap technique to the most proximal location of the previous thrombus. The study procedure was as follows: 10 mg of verapamil in 20 cc of normal saline was administered over 20 min (1 cc/minute) through the microcatheter and into the vessel previously obstructed by the clot (Figure 4(b)). This dose was selected for several reasons. First, the dose is within the range safely used routinely for intra-arterial injection in the cerebral circulation to treat vasospasm.28,29 As such, there is a familiarity with this dose in human patients. Second, this dose corresponds closely to the human equivalent dose of that used by our lab previously in our IA mouse model which showed a neuroprotective effect.19 At the conclusion of infusion, the microcatheter was removed. Angiography through the guide catheter was performed to verify no thromboembolic complications secondary to IA infusion. Following that, the procedure was completed and the groin access removed using standard of care procedures. Subjects underwent a follow-up MRI (or CT if MR contraindicated) within 24 h after thrombectomy.
Figure 4.
Illustrations demonstrating the study protocol. This includes performing the thrombectomy (the illustration shows a stent-triever device) (a) followed by microcatheter navigation to the site of the thrombus and infusion of the study agent (b).
The predetermined primary endpoint was the presence or absence of significant intracranial hemorrhage (sICH) within 24 h after treatment. Hemorrhage was considered significant if it resulted in a 4-point or more increase in NIH Stroke Scale, or if it required surgical intervention; these criteria were consistent with prior stroke trials.1,30,31 Secondary objectives included presence/absence of systemic side effects of verapamil at the time of administration, technical feasibility and safety of IA drug delivery with a microcatheter immediately following thrombectomy, and follow-up modified Rankin scale for clinical outcome within 90 days. We also recorded pre-thrombectomy ASPECTS and CTA collateral score.32–34 Post-thrombectomy cerebral infarct volume was measured on diffusion-weighted images or on CT by manual segmentation using ITK-SNAP software (www.itksnap.org).35 Radiographic gradings and outcomes were assessed by an independent neuroradiologist (DL).
Results
In vivo dose–response evaluation
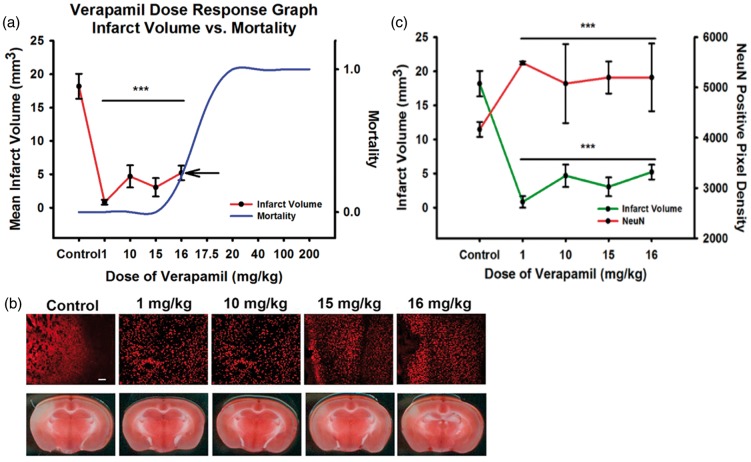
An N of 40 mice was used to perform toxicity analysis, and results were compared to data from our previously published in vivo experiments with IA verapamil. The initial high starting dose was 200 mg/kg, resulting in immediate 100% lethality from cardiopulmonary instability. This dose was decreased in a step-wise fashion as shown in Figure 1(a). Lethality dropped off at a dose of 17.5 mg/kg where all mice in that group (N of 5) died in a delayed fashion within 30 min after administration. With the next decrease of dose (16 mg/kg), all mice survived (as did all at lower doses) demonstrating a likely LD50 estimated between 16 and 17.5 mg/kg dose. Thereafter, blinded experiments of N of 5 mice each were performed to evaluate stroke volume and immunohistochemistry at therapeutic doses between that used in our previous study (0.9 mg/kg) and the lethal dose (17.5 mg/kg). Figure 1(a) again demonstrates the calculated mean stroke volume at each dose plotted with the lethal dose curve. Of note, TTC and cresyl violet staining demonstrated similar infarct patterns (Figure 1(b), cresyl violet not shown). Furthermore, stroke volume plotted against neuronal NeuN preservation demonstrated an expected inverse relationship (Figure 1(c)). While there may be a trend toward maximal verapamil effect on reducing infarct volume at a dose around 1 mg/kg, there was no statistically significant difference among the therapeutic doses for either infarct volume or NeuN preservation. Based on this, verapamil has a likely plateau or inverted U-shaped efficacy effect up to the lethal dose.
Figure 1.
In vivo dose–response experiments to evaluate IA verapamil. (a) Infarct volume was mapped against the dose-dependent mortality. All animals died at 17.5 mg/kg, with an estimated LD50 between 16 and 17.5 mg/kg. Infarct volume reduction demonstrated a likely plateau or inverted-U curve in dose-dependent efficacy up to the mortality curve. Examples of NeuN immunohistochemistry and TTC infarct volumes are shown (NeuN green fluorescence; TTC white is ischemia) (b) and plotted (c), demonstrating an expected inverse relationship between reduction of infarct volume with verapamil and improvement in NeuN staining that matches.
In vitro verification of neuroprotection
Primary cortical neurons were used to determine if verapamil is directly neuroprotective following OGD; such a result would demonstrate an independent therapeutic mechanism from its well-known vasodilatory role, while remaining consistent with its known pharmacology as an l-type calcium channel-blocker. Overall neuronal health was assessed by cell death and dendritic damage 24 h following OGD. OGD conditions had been optimized to 30 min in a prior experiment (Supplemental Figure 1). Dendritic damage was assessed by tracing the dendrites and measuring the length of the primary, secondary, and tertiary branches (Figure 2(a)). Significant loss of cell viability (70.0 ± 1.9% to 31.64 ± 3.3% with OGD, p ≤ 0.001) was seen following 30 min of OGD, while verapamil increased cell survival in a dose-dependent manner (325 ng/mL, 49.7 ± 2.5%, p ≤ 0.001 and 500 ng/mL, 52.9 ± 2.8%, p ≤ 0.001; Figure 2(b)). Of note, the overall number of cells did not significantly differ across groups, and could not account for the difference (Supplemental Figure 2). OGD caused significant dendritic damage by reducing the branch length of primary dendrites by 48% (64.4 ± 2.7 µm to 33.5 ± 2.9 µm, p ≤ 0.001) and secondary dendrites by 58% (47.7 ± 2.9 µm to 19.9 ± 2.1 µm, p ≤ 0.001) compared to control (Figure 2(c)). Verapamil (500 ng/mL) significantly reduced the dendritic damage by decreasing the primary dendritic loss to only 19.5% (51.9 ± 2.0 µm, p = 0.01) and secondary to only 25% (35.7 ± 2.0 µm, p ≤ 0.001; Figure 2(c)). Representative images are shown in Figure 2(d).
Phase I clinical trial: SAVER-I
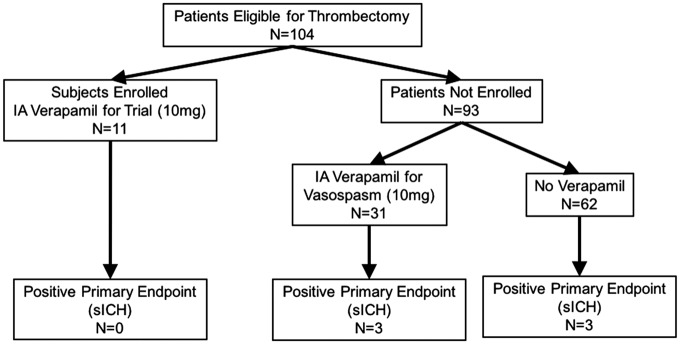
From 2013 to 2015, of 104 consecutive patients undergoing thrombectomy at our institution, 11 were successfully enrolled (Figure 3). Ninety-three were excluded, of whom one was therapeutically anticoagulated at the time of presentation, two were initially consented but underwent thrombectomy which did not achieve TICI 2 A or better, one was consented but angiogram showed no thrombus so no thrombectomy was performed, and 89 presented with no family available for in-person consent prior to time of thrombectomy. Table 1 shows the subject demographics and presenting radiographic information. Table 2 displays the treatment technique and outcome variables. All subjects successfully underwent the procedure, during which thrombectomy was performed (Figure 4(a)), followed immediately by superselective intra-arterial administration of 10 mg of verapamil (Figure 4(b)). Of the 11 subjects enrolled and successfully treated, none met the primary safety endpoint (significant intracranial hemorrhage). However, as the same dose of verapamil (10 mg) is often administered non-selectively through the guide catheter in the cervical internal carotid artery for treatment of radiographic vasospasm encountered during the thrombectomy procedure, we also reported the incidence of the primary safety endpoint among patients excluded from the study. After obtaining IRB approval, we tracked the patients for the incidence of significant ICH, which is reported as a standard of care measure at our stroke center. Of those patients, 93 patients underwent thrombectomy outside the study, of which 31 (33%) received nonselective IA verapamil as standard of care. There were three cases of sICH in the outside-of-study verapamil group (9.6%) versus three in the group that did not receive verapamil (4.8%); the difference was not significant. No subject in the study or patient in the non-enrolled group died during or immediately following the procedure. Of note, one of the patients who received verapamil outside the study had a contraindication to study enrollment; they were on therapeutic anticoagulation prior to presentation. The patient underwent thrombectomy and verapamil treatment, and did develop a significant intracerebral hemorrhage. There were two periprocedural adverse events recorded during the thrombectomy procedure. One subject had a self-limited desaturation with premature ventricular contractions approximately 5 min after thrombectomy and IA verapamil administration were complete. The patient was already on oxygen, was given additional oxygen via facemask, and no further intervention after it resolved quickly; post-procedure workup did not reveal any source or complication (such as flash pulmonary edema) requiring treatment. A second subject had a seizure 5 min after thrombectomy and verapamil administration, but may have had a seizure prior to admission, and had a history of alcohol abuse. A total of four subjects died during the follow-up study period. Two subjects died within 30 days. One subject had massive sepsis and organ failure after dislodgement of a PEG tube required for feeding. A second subject died of respiratory failure after his family elected to extubate him when he did not recover within two days of admission. Two subjects died after 30 days but before 90 days. One subject with a history of kidney transplant did well initially with an mRS of 1 and was discharged. She re-presented with chest pain and neurological deterioration, and was found to have a de novo ruptured mycotic aneurysm contralateral to the prior stroke. A second subject was discharged but died after 30 days with a likely myocardial infarction. In all of these cases, given the late timing and the nature of these complications as physically and chronologically distant to site of IA injection, it was unlikely that single-dose verapamil was a significant causative factor. All but one subject received intravenous tPA as standard of care. Successful drug delivery was achieved in all cases without thromboembolic complication. Median infarct volume was 24.0 cc with a range of 1.7–163.3 cc. Of the subjects who survived initial hospitalization, the rate of independence (reported as percentage of subjects with mRS of 0–2 at 90 days) was 44.4%. An example case is shown in Figure 5.
Figure 3.
Enrollment schedule for the SAVER-I Study. Eleven subjects were successfully enrolled of 104 eligible patients. Of the 93 patients not enrolled, 31 also received 10 mg of IA verapamil outside the study protocol, while 62 did not; there was no significant difference in the rate of significant intracranial hemorrhage between those groups.
Table 1.
Demographics and baseline information for enrolled subjects.
| Patient ID | Gender | Age | Vessel Occlusion | Initial NIHSS | ASPECTS Score | CTA Score |
|---|---|---|---|---|---|---|
| 1 | F | 70 | Right MCA | 18 | 8 | 1 |
| 2 | M | 44 | Left ICA, ACA, MCA | 21 | 8 | 1 |
| 3 | M | 40 | Right ICA | 12 | 7 | 4 |
| 4 | M | 32 | Left ICA/MCA | 18 | 7 | 1 |
| 5 | F | 63 | Basilar Artery | 21 | N/A | N/A |
| 6 | F | 79 | Right MCA | 15 | 8 | 3 |
| 7 | M | 73 | Right MCA | 17 | 10 | 1 |
| 8 | M | 53 | Right MCA | 16 | 9 | 4 |
| 9 | F | 75 | Left MCA | 19 | 10 | 2 |
| 10 | M | 84 | Left MCA | 22 | 6 | 2 |
| 11 | F | 49 | Right MCA | 12 | 10 | 2 |
Table 2.
Procedural and outcome data for enrolled subjects.
| Patient ID | Interval from last known normal to recanalization (min) | IV tPA | Thrombectomy technique | TICI Score | Successful delivery without complication | Significant ICH | Infarct volume (cm3) | Periprocedural event | 30-day mRS | 90-day mRS |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 264 | Yes | Stent-Triever | 3 | Yes | No | 24.0 | Self-limited desaturation | 4 | 4 |
| 2 | 206 | Yes | Stent-Triever | 2B | Yes | No | 24.0 | None | 5 | 4 |
| 3 | 350 | Yes | ADAPT | 3 | Yes | No | 83.8 | None | 4 | 4 |
| 4 | 210 | Yes | ADAPT | 3 | Yes | No | 34.2 | None | 2 | 1 |
| 5 | 437 | No | SOLUMBRA | 3 | Yes | No | 51.7 | None | 6 | 6 |
| 6 | 285 | Yes | ADAPT | 3 | Yes | No | 2.1 | None | 0 | 0 |
| 7 | 181 | Yes | ADAPT | 2B | Yes | No | 163.3 | None | 6 | 6 |
| 8 | 203 | Yes | ADAPT | 2B | Yes | No | 16.8 | Single seizure | 1 | 2 |
| 9 | 146 | Yes | ADAPT | 2B | Yes | No | 2.3 | None | 5 | 6 |
| 10 | 194 | Yes | SOLUMBRA | 3 | Yes | No | 1.7 | None | 4 | 6 |
| 11 | 455 | Yes | SOLUMBRA | 3 | Yes | No | 53.2 | None | 3 | 1 |
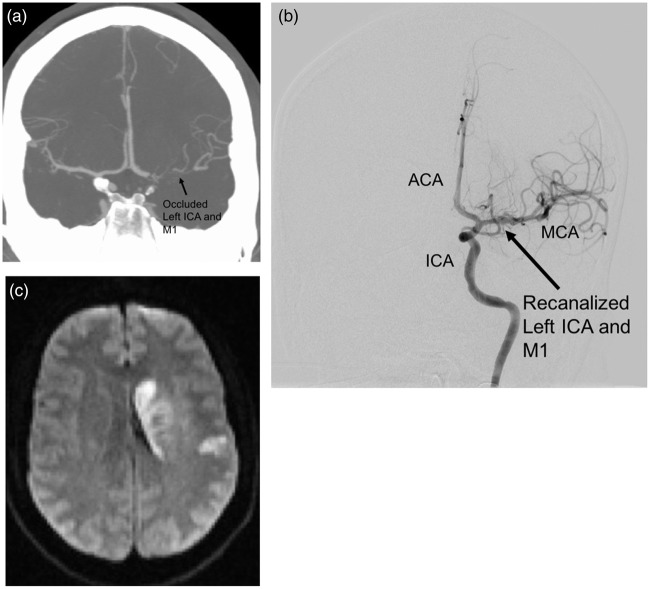
Figure 5.
Case illustration of a study subject. A 32-year-old male smoker with migraine, diabetes, and hyperlipidemia presented with last known normal time of 0830 with an NIH Stroke Scale of 18 (aphasia, right hemisensory loss, and right hemiparesis). The subject received intravenous t-PA. CTA (a) and angiography demonstrated a left ICA distal occlusion extending to the MCA, with a CTA collateral score of 1. He underwent TICI 3 recanalization via thrombectomy approximately 3.5 h after last known normal (b). IA verapamil was administered per study protocol. Postoperative MRI demonstrated a small stroke (34.2 cc) limited to the basal ganglia (c). The subject was discharged to acute rehabilitation, and had an mRS score of 1 at 90 days.
Discussion
During ischemia, ion-pump failure and ATP depletion result in a cytotoxic increase in cytosolic calcium.36 Verapamil is an l-type calcium-channel blocker (CCB) currently used clinically for intra-arterial administration in the treatment of cerebral vasospasm with minimal adverse effects.37–39 Previous studies also support a possible therapeutic role for CCBs in stroke neuroprotection.15,16 Flunarizine, another calcium channel blocker, showed promise in early animal data and clinical application, but failed in the double-blind Flunarizine in Stroke Trial (FIST) of 331 subjects to show clinical benefit; however, mean time interval to treatment was 13.5 h, and subjects did not undergo thrombolysis as part of the protocol.40 While these data are discouraging, they suffer from a disconnect between the bench (pretreated rodent models via intraperitoneal injection) and bedside (human subjects given intravenous/oral treatment approximately 12–14 h after insult). Morikawa et al. explored the post-ischemic ‘therapeutic window’ for (S)-emopamil; with repeated doses i.p., they showed that significant reduction of infarct volume was found with 1 h of post-occlusion administration (48% reduction, p < 0.05), with a time-dependent decline in effect.17 The addition of transvenous perfusion of verapamil through an artery to vein graft to the brain after MCAO in rodent models resulted in significant improvement in cerebral blood flow, with significant reduction in total cerebral infarct.41 Finally, verapamil is a first-pass metabolic agent, with 90% binding to plasma proteins. As such, it is ideally suited for local delivery to maximize focal effects and minimize systemic effects, while representing one of the best known and most widely used calcium channel blockers in cerebrovascular disease. Thus, combining the use of verapamil with direct intra-arterial administration in a rapidly recanalized cerebral artery may offer an opportunity to translate bench-side neuroprotective effects into bed-side success. Finally, verapamil is known to have overall enhanced passage across the blood–brain barrier after stroke via paracellular pathways that are facilitated by stroke-induced blood–brain barrier breakdown.42 This pathological condition, thus, provides an optimal environment to enhance the therapeutic potential of verapamil directly on neuronal tissues.
Our previous studies established a preclinical model for intra-arterial (IA) delivery of pharmacologics after reperfusion in large vessel occlusion.18 Furthermore, a blinded, randomized pre-clinical study demonstrated a direct neuroprotective effect of verapamil when administered IA after stroke reperfusion.19 This initial animal study demonstrated benefits in overall infarct volume, functional outcome, and immunohistochemical markers of neuron preservation, reduction of astrogliosis, and reduction of apoptosis.19 These data opened several questions about IA verapamil as a neuroprotective adjunct to thrombectomy.
The first question we asked was: with IA administration to the brain, what is the toxicity of verapamil relative to the expected dose–response? In the material safety data-sheet printed by Hospira, the LD50 listed for intravenous verapamil hydrochloride is 16 mg/kg for rats, and 5.8 mg/kg for mice. With these dosages in mind, we initiated a toxicity study for IA administration to mirror the methods used in the angiography suite.18 We estimated the LD50 for IA administration to be between 16 and 17.5 mg/kg for mice. As expected, selective IA administration allows for higher dose-toxicity than intravenous administration using the same formulation (16 mg/kg vs. 5.8 mg/kg). Thus, selective IA infusion may allow for administration of higher doses of neuroprotective therapies that would be unfeasible or unsafe if administered systemically. However, in mapping the dose–response, we found beneficial effects of verapamil across therapeutic doses up to the level of toxicity, demonstrated by both stroke volume assessment and immunohistochemical analysis. Furthermore, based on body surface area, the dose used in SAVER-I, a mean dose of 0.11 mg/kg, would translate to 1.35 mg/kg in the mouse.21 This is well within the expected efficacy curve in Figure 2(a). As such, we have shown further translational support for dosing IA verapamil in further clinical studies in the current range. While we did not evaluate doses lower than 1 mg/kg in the mouse, it would be reasonable to do so for future studies. However, given that we have shown good therapeutic efficacy in stroke reduction in this study and in our prior publication at doses similar in mice to those currently used in humans, we did not track lower doses at this time.
The second question we asked was: is there a direct neuroprotective mechanism on neurons? As noted, several previous studies have suggested a role for verapamil in ischemia.41,43 In particular, El-Zammar et al. suggested a role for vasodilators in improving perfusion during intra-arterial treatment of stroke.43 While vasodilation may play a role in the treatment of ischemia, we believe that verapamil may act through an additional mechanism as a neuroprotectant. Given that it crosses the blood–brain barrier during ischemia, we studied its effect on primary cortical neurons. In both cell survival and dendritic sprouting, verapamil administered after OGD (30 min) prevented significant ischemia-induced injury in a graded dose–response manner. This demonstrates a clear direct effect, which would be expected given its known role as an l-type calcium channel blocker. As verapamil is known to cross the blood–brain barrier, and increasingly so in stroke, and as calcium channels and their activity are known to play a key role in the excitotoxic pathway of ischemia, we would expect a direct neuroprotective potential for verapamil. Indeed, these results are consistent with the known important and early role that calcium-mediated apoptosis plays in the excitotoxic pathway of ischemia.
The third question we asked was: Is IA verapamil safe and feasible as an adjunctive injection procedure in the setting of clinical thrombectomy in the human condition? To date, there have been no published studies specifically evaluating superselective intra-arterial infusion of pharmacotherapy immediately following reperfusion after thrombectomy. The SAVER-I trial demonstrated a feasible workflow for adding IA pharmacotherapy to the thrombectomy procedure without any thromboembolic complications, despite the additional time (20 min) added to the endovascular procedure. Furthermore, given its recognized vasodilatory properties, we needed to demonstrate safety of IA infusion after thrombectomy. None of our enrolled subjects who met inclusion criteria incurred the primary safety endpoint; none had a significant intracranial hemorrhage. Additionally, while IA verapamil at the same dose is often infused from the guide catheter to treat vasospasm encountered during the thrombectomy procedure, we separately tracked patients not enrolled in the study (Figure 2). In this uncontrolled but practice-based comparison, there was no significant difference between the rate of significant intracranial hemorrhage in patients who did or did not receive IA verapamil. Thus, while our data were not powered to show efficacy of IA verapamil in reducing infarct or improving outcomes, they demonstrate the safety of IA adjuncts during thrombectomy in general, and of IA verapamil specifically. Based on these data, larger randomized controlled trials evaluating IA verapamil as a therapeutic adjunct to thrombectomy for large vessel occlusive stroke are justified.
SAVER-I also had some additional findings. First, our study group was a good representation of typical large volume occlusive strokes, with subjects that included middle cerebral artery occlusions, internal carotid occlusions, and also basilar artery occlusion. The high NIH stroke scales upon presentation (ranging from 12 to 21) indicate the severity of the target disease. Of important note, all but one subject received intravenous tPA as standard of care; despite the combination of tPA, thrombectomy, and IA verapamil, no subject had a significant intracranial hemorrhage. Finally, the median radiographic infarct was small (24.0 cc). While there were several deaths due to unrelated causes, and two likely unrelated complications (self-limited desaturation and a single seizure), IA verapamil appears safe and potentially important as an adjunctive therapy. While efficacy of IA verapamil cannot be determined from this study, the case presented in Figure 5 exemplifies a scenario where preoperative imaging would have predicted a potentially large infarct (CTA collateral score of 1). Despite this, with tPA, thrombectomy, and IA verapamil, the subject had a small infarct and good clinical outcome. Therefore, in combination, the in vivo dosing/toxicity experiments, the in vitro neuroprotection studies, and the clinical Phase I SAVER-I study demonstrate the feasibility, safety, and potential neuroprotective efficacy of direct verapamil administration after reperfusion from thrombectomy. This opens questions for further study, including efficacy of verapamil, feasibility of IA therapeutics in general for stroke, and the need for understanding the distribution of IA infusions after stroke. Collaborations using other novel techniques such as real-time MRI and infarct probability maps may yield improvements in targeting of therapeutics.44,45
Despite the cross-model translation of this study, there were some notable limitations. As a Phase I study, SAVER-I did not have a control group, and was not powered to show efficacy. While a limitation, these factors were not particular of the study design and are not necessary for a Phase I study. Furthermore, while we focused on the primary safety endpoint of significant ICH, we did not collect other radiographic endpoints such as petechial hemorrhaging and cerebral edema. Future, well-controlled clinical trials should address these issues. In regard to the in vivo preclinical studies, we only evaluated verapamil in young male mice without comorbidities. As such, further studies should include evaluation of neuroprotection with reference to age and gender-based comparisons as well as efficacy on animals with co-morbid conditions typical of the ischemic stroke population (i.e. hypertension, diabetes). Furthermore, the stroke model we use entails a 1-h occlusion (versus a 3–6 h occlusion typical in patients). This is a limitation of the model, by which occlusion times of 3–6 h followed by recanalization/IA infusion would be unfeasible. As such, our results should be further evaluated in other models and animal species. Finally, the in vitro experiments evaluated only direct neuroprotection on neurons. While this is a logical effect, the mechanism of therapeutic efficacy may be multifaceted with both direct and indirect (effects on other cell types such as endothelial cells, astrocytes, etc.) components. For example, evaluating potential indirect neuroprotective effects via endothelial cells would initially require in vitro transwell experimental design with verapamil administration to endothelial cells grown on an insert separated from primary neurons. Furthermore, it is possible that verapamil also has beneficial effects on the endothelium itself. However, given that verapamil readily crosses the blood–brain barrier in ischemic conditions, our direct neuroprotective results suggest that verapamil could also be directly neuroprotective, apart from its vasodilatory effects, in vivo.
We have shown for the first time that IA administration of a neuroprotective agent immediately following thrombectomy removal of thrombus is safe and feasible in the human condition. We have shown the altered dose–response-toxicity of intra-arterial verapamil, demonstrating efficacy at doses in range of the clinically applicable dose. Finally, we have illustrated that verapamil may have direct neuroprotective effects apart from vasodilation to prevent ischemic damage. Collectively, these experiments demonstrate the potential safety and efficacy for superselective IA administered neuroprotective agents to treat a highly morbid and mortal condition – emergent large vessel occlusion stroke.
Supplementary Material
Acknowledgements
The authors thank Mary Faulkner, the clinical study coordinator, for her support and dedication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This publication was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR001998.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
JFF is the lead author, contributor, and principal investigator for this work. He organized and led the clinical trial, and oversaw the design and completion of the in vivo and in vitro experiments. MM performed the in vivo dose–response experiments, and aided in design of the figures, and construction of the manuscript. AT performed the in vitro primary cortical neuron experiments, and aided in design of the figures, and construction of the manuscript. DL was the core neuroradiologist for interpretation of the pre- and post-procedural radiographic images in the clinical trial. LP was the core clinical assessor, who performed the clinical outcome assessments in the clinical trial. WLS assisted with data acquisition, tabulation, and interpretation in the clinical trial enrollment and outcome. AA performed some of the procedures in the subjects included in the clinical trial. JR assisted with the in vivo experiments. GJB supported the laboratory experiments with resources and intellectual input, served as an independent reviewer of the data and advisor to the clinical trial, and mentor to the principal investigator.
Supplementary material
Supplementary material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. New Engl J Med 2015; 372: 2285–2295. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation 2014; 129: e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bretz MN, Graves A, West A, et al. Steps against recurrent stroke plus: patient transition program. J Neurosci Nurs: J Am Assoc Neurosci Nurse 2014; 46: E3–13. quiz E1-E2. [DOI] [PubMed] [Google Scholar]
- 4.Thacker C. Stroke falls to No. 5 cause of death in U.S. American Heart Association 30 December 2014. http://newsroom.heart.org/news/stroke-falls-to-no-5-cause-of-death-in-u-s. [Google Scholar]
- 5.Summers D, Leonard A, Wentworth D, et al. Comprehensive overview of nursing and interdisciplinary care of the acute ischemic stroke patient: a scientific statement from the American Heart Association. Stroke; a journal of cerebral circulation 2009; 40: 2911–44. [DOI] [PubMed] [Google Scholar]
- 6.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics–2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2009; 119: e21–e181. [DOI] [PubMed] [Google Scholar]
- 7.Parsons MW, Albers GW. MR RESCUE: is the glass half-full or half-empty? Stroke; a journal of cerebral circulation 2013; 44: 2055–2057. [DOI] [PubMed] [Google Scholar]
- 8.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. New Engl J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 9.Chalouhi N, Dressler JA, Kunkel ES, et al. Intravenous tissue plasminogen activator administration in community hospitals facilitated by telestroke service. Neurosurgery 2013; 73: 667–671; discussion 71-72. [DOI] [PubMed] [Google Scholar]
- 10.Lichtman JH, Watanabe E, Allen NB, et al. Hospital arrival time and intravenous t-PA use in US Academic Medical Centers, 2001–2004. Stroke; a journal of cerebral circulation. 2009; 40: 3845–3850. [DOI] [PubMed] [Google Scholar]
- 11.Rozeman AD, Wermer MJ, Vos JA, et al. Evolution of intra-arterial therapy for acute ischemic stroke in The Netherlands: MR CLEAN Pretrial Experience. J Stroke Cerebrovasc Dis: The official journal of National Stroke Association 2016; 25: 115–121. [DOI] [PubMed] [Google Scholar]
- 12.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. New Engl J Med 2015; 372: 11–20. [DOI] [PubMed] [Google Scholar]
- 13.Campbell BC, Mitchell PJ, Investigators E-I. Endovascular therapy for ischemic stroke. New Engl J Med 2015; 372: 2365–2366. [DOI] [PubMed] [Google Scholar]
- 14.Saver JL, Jahan R, Levy EI, et al. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet 2012; 380: 1241–1249. [DOI] [PubMed] [Google Scholar]
- 15.Silverstein FS, Buchanan K, Hudson C, et al. Flunarizine limits hypoxia-ischemia induced morphologic injury in immature rat brain. Stroke; a journal of cerebral circulation 1986; 17: 477–482. [DOI] [PubMed] [Google Scholar]
- 16.Limburg M, Hijdra A. Flunarizine in acute ischemic stroke: a pilot study. Eur Neurol 1990; 30: 121–122. [DOI] [PubMed] [Google Scholar]
- 17.Morikawa E, Ginsberg MD, Dietrich WD, et al. Postischemic (S)-emopamil therapy ameliorates focal ischemic brain injury in rats. Stroke; a journal of cerebral circulation 1991; 22: 355–360. [DOI] [PubMed] [Google Scholar]
- 18.Maniskas M, Bix G, Fraser J. Selective intra-arterial drug administration in a model of large vessel ischemia. J Neurosci Meth 2015; 240: 22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maniskas ME, Roberts JM, Aron I, et al. Stroke neuroprotection revisited: intra-arterial verapamil is profoundly neuroprotective in experimental acute ischemic stroke. J Cereb Blood Flow Metab: Official journal of the International Society of Cerebral Blood Flow and Metabolism 2016; 36: 721–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kilkenny C, Browne WJ, Cuthill IC, et al. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 2010; 8: e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J 2008; 22: 659–61. [DOI] [PubMed] [Google Scholar]
- 22.Harris J, Lee H, Tu CT, et al. Preparing e18 cortical rat neurons for compartmentalization in a microfluidic device. J Vis Exp 2007; 8: 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saini MG, Bix GJ. Oxygen-glucose deprivation (OGD) and interleukin-1 (IL-1) differentially modulate cathepsin B/L mediated generation of neuroprotective perlecan LG3 by neurons. Brain Res 2012; 1438: 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haughey NJ, Nath A, Mattson MP, et al. HIV-1 Tat through phosphorylation of NMDA receptors potentiates glutamate excitotoxicity. J Neurochem 2001; 78: 457–467. [DOI] [PubMed] [Google Scholar]
- 25.Meijering E, Jacob M, Sarria JC, et al. Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry A 2004; 58: 167–176. [DOI] [PubMed] [Google Scholar]
- 26.Higashida RT, Furlan AJ, Roberts H, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke; a journal of cerebral circulation 2003; 34: e109–e137. [DOI] [PubMed] [Google Scholar]
- 27.Wintermark M, Albers GW, Broderick JP, et al. Acute Stroke imaging research roadmap II. Stroke; a journal of cerebral circulation 2013; 44: 2628–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sehy JV, Holloway WE, Lin SP, et al. Improvement in angiographic cerebral vasospasm after intra-arterial verapamil administration. AJNR Am J Neuroradiol 2010; 31: 1923–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jun P, Ko NU, English JD, et al. Endovascular treatment of medically refractory cerebral vasospasm following aneurysmal subarachnoid hemorrhage. AJNR Am J Neuroradiol 2010; 31: 1911–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. New Engl J Med 2015; 372: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 31.Wahlgren N, Ahmed N, Davalos A, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet 2007; 369: 275–282. [DOI] [PubMed] [Google Scholar]
- 32.Menon BK, Puetz V, Kochar P, et al. ASPECTS and other neuroimaging scores in the triage and prediction of outcome in acute stroke patients. Neuroimag Clin N Am 2011; 21: 407–423, xii. [DOI] [PubMed] [Google Scholar]
- 33.Souza LC, Yoo AJ, Chaudhry ZA, et al. Malignant CTA collateral profile is highly specific for large admission DWI infarct core and poor outcome in acute stroke. AJNR Am J Neuroradiol 2012; 33: 1331–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pexman JH, Barber PA, Hill MD, et al. Use of the Alberta Stroke Program Early CT Score (ASPECTS) for assessing CT scans in patients with acute stroke. AJNR Am J Neuroradiol 2001; 22: 1534–1542. [PMC free article] [PubMed] [Google Scholar]
- 35.Yushkevich PA, Piven J, Hazlett HC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 2006; 31: 1116–1128. [DOI] [PubMed] [Google Scholar]
- 36.White BC, Sullivan JM, DeGracia DJ, et al. Brain ischemia and reperfusion: molecular mechanisms of neuronal injury. J Neurol Sci 2000; 179: 1–33. [DOI] [PubMed] [Google Scholar]
- 37.Albanese E, Russo A, Quiroga M, et al. Ultrahigh-dose intraarterial infusion of verapamil through an indwelling microcatheter for medically refractory severe vasospasm: initial experience. Clinical article. J Neurosurg 2010; 113: 913–922. [DOI] [PubMed] [Google Scholar]
- 38.Feng L, Fitzsimmons BF, Young WL, et al. Intraarterially administered verapamil as adjunct therapy for cerebral vasospasm: safety and 2-year experience. AJNR Am J Neuroradiol 2002; 23: 1284–1290. [PMC free article] [PubMed] [Google Scholar]
- 39.Keuskamp J, Murali R, Chao KH. High-dose intraarterial verapamil in the treatment of cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J Neurosurg 2008; 108: 458–463. [DOI] [PubMed] [Google Scholar]
- 40.Franke CL, Palm R, Dalby M, et al. Flunarizine in stroke treatment (FIST): a double-blind, placebo-controlled trial in Scandinavia and the Netherlands. Acta Neurol Scand 1996; 93: 56–60. [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto YL, Ueda T, Shimauchi M, et al. Efficacy of bypass between extracerebral artery and cerebral vein with retrograde verapamil infusion into focal cerebral ischemic tissue in rats. Neurosurgery 1991; 29: 719–725. discussion 25-26. [DOI] [PubMed] [Google Scholar]
- 42.Fang W, Lv P, Geng X, et al. Penetration of verapamil across blood brain barrier following cerebral ischemia depending on both paracellular pathway and P-glycoprotein transportation. Neurochem Int 2013; 62: 23–30. [DOI] [PubMed] [Google Scholar]
- 43.El-Zammar ZM, Latorre JG, Wang D, et al. Intra-arterial vasodilator use during endovascular therapy for acute ischemic stroke might improve reperfusion rate. Ann New York Acad Sci 2012; 1268: 134–140. [DOI] [PubMed] [Google Scholar]
- 44.Boers AM, Berkhemer OA, Slump CH, et al. Topographic distribution of cerebral infarct probability in patients with acute ischemic stroke: mapping of intra-arterial treatment effect. J Neurointervention Surg. Epub ahead of print 25 April 2016. DOI: 10.1136/neurintsurg-2016-012387. [DOI] [PubMed] [Google Scholar]
- 45.Walczak P, Wojtkiewicz J, Nowakowski A, et al. Real-time MRI for precise and predictable intra-arterial stem cell delivery to the central nervous system. J Cereb Blood Flow Metab. Epub ahead of print 12 September 2016. DOI: 10.1177/0271678X16665853. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.