Abstract
Variation in extent of the brain’s collateral circulation is an important determinant of variation in the severity of stroke and efficacy of revascularization therapies. However, the number and diameter of pial collateral “arterioles” decrease with aging in associated with reduced eNOS and increased oxidative stress. We tested whether exercise reduces this aging-induced rarefaction. Twelve-month-old mice were randomized to sedentary or voluntary wheel-running. At 26 months’ age, permanent MCA occlusion was followed 72 h later by determination of infarct volume and vascular casting after maximal dilation. The decline in collateral number and diameter and 2.4-fold increase in infarct volume evident in 26-versus 3-month-old sedentary mice were prevented by exercise-training. In contrast, number and diameter of the posterior communicating collateral “arteries” were unaffected by aging or exercise. Interestingly, diameter of the primary intracranial arteries increased with aging. Mechanistically, genetic overexpression of eNOS inhibited age-induced collateral rarefaction, and exercise increased eNOS and SOD2 and decreased the inflammatory marker NFkB assessed in hindlimb arteries. In conclusion, exercise prevented age-induced rarefaction of pial collaterals and reduced infarct volume. Aging also promoted outward remodeling of intracranial arteries. These effects were associated with increased eNOS and reduced markers of inflammation and aging in the vascular wall.
Keywords: Collateral circulation, aging brain, ischemic stroke, aerobic exercise, cerebral arteries, posterior communicating artery
Introduction
Increased age is among the strongest predictors of infarct size and poor functional outcome following acute ischemic stroke.1,2 While moderate-to-high levels of physical activity reduce the risk of stroke in humans,3–5 little is known regarding whether an extended history of regular aerobic exercise (RAEx) affects infarct volume and functional outcome. Studies in rodents reported that several weeks of daily voluntary wheel-running reduced subsequent infarct volume and neurological sensory-motor deficit post-stroke, findings supported by clinical studies.6–8 However, no experimental studies have investigated the impact on stroke severity of a prior history of RAEx extending from the middle to senior years of life when the incidence stroke becomes most prominent. Moreover, the effects of exercise-training are complex, thus mechanisms responsible for the above benefits remain unclear.
The major determinants of infarct volume after large-vessel occlusion are the location and duration of occlusion and the amount of collateral-dependent blood flow.9 In the absence of spontaneous or therapeutic revascularization, collateral flow becomes paramount. Collaterals are anastomoses that interconnect arterial trees supplying adjacent vascular territories. Wide variation in their number and lumen diameter (i.e. “extent” determined at maximal dilation) within the pial (leptomeningeal) circulation occurs among different mouse strains and is a major determinant of their similarly wide variation in infarct volume after permanent middle cerebral artery occlusion (pMCAO).10 Large variation in collateral extent is also seen in other tissues of these strains that mirrors that seen in their pial circulation. Collateral score measured in patients with acute ischemic stroke also varies widely and correlates indirectly with infarct volume, directly with efficacy of revascularization therapies, and inversely with risk of hemorrhagic transformation.11,12 Besides variation in collateral extent, which depends strongly on genetic factors at least in mice,13 environmental factors cause rarefaction of pial collaterals, i.e. loss of number and/or a decline in the diameter of those that remain, and more severe strokes. For example, collaterals “age” faster than other vessels in brain and lower extremities of mice, resulting in rarefaction from middle-age onward which is not seen in nearby similarly-sized arterioles and that is associated with decreased endothelial nitric oxide synthase activity and bioavailability of nitric oxide (eNOS-NO) and increased oxidative stress in endothelial and smooth muscle cells of the collateral wall.14–16 In fact, deficiency of eNOS in mice causes collateral rarefaction by even three months’ age that worsens by six months’ age and results in a smaller penumbra and larger infarct volume,17–19 findings indicating that eNOS protects against age-induced collateral rarefaction. Hypertension, diabetes, obesity and other cardiovascular/ stroke risk factors also cause rarefaction of collaterals, in association with decreased eNOS and increased oxidative stress.17
It is not known whether rarefaction of collaterals can be slowed or prevented. Regular physical exercise is well recognized to decrease the age-associated decline in eNOS-NO and endothelial dysfunction in peripheral arteries,20 and to reduce the increases in oxidative stress, inflammation and DNA damage in vascular wall cells that accompany aging.5,20–22 Moreover, RAEx lessens the reduction in eNOS-NO and endothelial dysfunction caused by hypertension, obesity and other risk factors/co-morbidities.5 In addition, bradycardia and lower arterial pressure seen at rest in the exercise-trained individual decrease mechanical stress on the vascular wall.22
The present study tested the hypothesis that RAEx reduces rarefaction of the collateral circulation that occurs with aging. We examined the effect of RAEx in mice, beginning at 12 and continuing through 26 months’ age (∼40-to-70 human-year equivalents23), on the extent of pial collaterals and severity of stroke. Since collateral arterioles of the pial circulation and collateral arteries of the circle of Willis differ both developmentally and anatomically,24 and since their extent is affected differently by genetic and environmental factors,25 we also examined the posterior communicating collateral arteries (PComs).
Little is known concerning whether changes in diameter of the primary intracranial arteries in humans occur with aging or RAEx, and findings in rodents are inconsistent.16,22,26–29 Lumen diameter of peripheral conduit arteries increases with aging in humans.21 It is well-established that exercise-training induces outward remodeling (increase in anatomic lumen diameter) of arteries supplying the muscles recruited during the exercise.30 Interestingly, recent studies have found that exercise-training also promotes, to a lesser extent, outward remodeling of peripheral arteries to muscles not engaged in the exercise.30,31 Shear-stressed induced increase in eNOS activity is an important proximal signal in the trophic pathway mediating outward remodeling.18,20–22,30–33 Moreover, RAEx increases eNOS-NO and endothelial function of the basilar artery (BA), internal carotid artery (ICA) and posterior cerebral artery (PCA) in rodents.22,32,33 Since remodeling of intracranial arteries would favor increased vascular conductance to the aging brain and thus increased collateral-dependent blood flow following stroke, we also examined the effect of aging and exercise-training on lumen diameter of the BA, ICA, PCA, MCA and anterior cerebral artery (ACA).
Methods
Detailed methods are in the online Supplement. We randomized 30 male and 30 female, 12-month-old C57BL/6J (B6) mice (∼40 human-year equivalents23) to sedentary (SED) or voluntary wheel-running (see Supplement for average distance and time run per day). Both sexes were included as required by the funding agency (NIH). The experimental design, allocation of subjects, and reasons for loss of animals or data points are in Supplemental Figure I. At 26 months’ age (∼70 human-year equivalents23), pMCAO was performed distal to the lenticulostriate branches, followed three days later by determination of infarct volume (2,3,5-triphenyltetrazolium chloride staining) and vascular casting (after maximal dilation and fixation) for subsequent measurement of number and lumen diameter of the pial collaterals (between the MCA and ACA trees), PComs and primary intracranial arteries.10,13,14 Controls for aging were approximately equal numbers of three-month-old SED male and female B6 mice (∼14 human-year equivalents23). eNOS, phospho-eNOS and markers of anti-oxidative stress (SOD2, HO-1), aging (8OHdG, p16INK4) and inflammation (NFkB) were assessed by immunohistochemistry in a non-cerebral vessel, i.e. the proximal caudal femoral artery. This was done because the number of computer-monitored running cages (1 mouse per cage) available for 14 months of uninterrupted use was limited to 30, and because the methods used to obtain infarct volume and vascular morphometry precluded simultaneous use of the brains for immunohistochemistry of cerebral vessels (that requires immediate fixation, sucrose protection and freezing). However, changes in the above proteins that occur with aging and exercise-training in arteries supplying muscles recruited by the exercise also occur in arteries supplying other tissues including brain.14,15,17,20–22,26,27 Our experimental design also specified examining this artery and its surrounding adductor muscle to allow analysis of changes in lumen diameter and wall thickness of an artery supplying a muscle involved in the exercise (Supplemental Figure II), as well as fiber type (ATPase isoform) switching within the latter (Supplemental Figure III). These endpoints are routinely assessed in exercise studies to document the level of training effect achieved by the exercise regimen.17 Two additional routine endpoints, body weight and heart weight,17 were also obtained (Supplemental Figure IV). eNOS transgenic mice (eNOSTG, B6 background;34 see supplement for details) were maintained SED, and morphometry of their cerebral vessels was obtained at 3 and 26 months’ age. Morphometric and immunohistochemical measurements were quantified with ImageJ (NIH). No significant sex-dependent differences were identified. However, dichotomizing for sex yielded n-sizes that lacked the power required to detect any potential changes in the endpoints,35 due to the above-mentioned limitations in the number of mice that could undergo exercise-training.
Experiments were performed in accordance with the University of North Carolina’s Institutional Animal Care and Use Committee, the NIH Guide for the Care and Use of Laboratory Animals, the ARRIVE guidelines, and the following suggested STAIR criteria: n-sizes were specified based on our previous studies demonstrating sufficient power to test the endpoints measured herein; approximately equal number of males and females were included; mice were randomized to treatment groups; controls and treatment groups were singly housed in the same conditions with the exception of the presence of running wheels; investigators were blinded during data analysis; pMCAO was used to permanently recruit pial collaterals; no data points were determined to be outlier by statistical criterion and excluded; negative results were reported; and the literature was reviewed in support and in disagreement with the results.
Statistics
Data are mean ± SEM for the n-sizes given in the figures (number of animals), with significance defined as p < 0.05 determined by ANOVA, Bonferroni protected t-, Student’s t- and X2 tests. For all figures unless stated otherwise in the legend: data were subjected to the above tests with significance set at two-tailed p values; each three-bar group (3-month-old Sed, 26-month-old Sed, 26-month-old RAEx or eNOSTG) was tested, if significant by ANOVA, for three post hoc comparisons using Bonferroni-protected two-tailed t-tests, where *,**,*** denotes p < 0.05, < 0.01, < 0.001 versus 3-month-old Sed and where #, ##, ### denotes p < 0.05, < 0.01, < 0.001 versus 26-month-old Sed mice. If a comparison was not p < 0.05 by the above test, the two-tailed Bonferroni test was constrained to comparison of two groups/bars among the following groups: 3-month-old Sed, 26-month-old Sed, 26-month-old RAEx or 26-month-old eNOSTG, where †, ††, ††† denotes p < 0.025, < 0.005, < 0.0005 versus 3-month-old Sed and where ‡, ‡‡, ‡‡‡ denotes p < 0.025, < 0.005, < 0.0005 versus 26-month-old Sed. Constraining was justified since the above two-bar comparisons were pre-specified in the experimental design based on previous results;14–18,20–22,30–33 nevertheless, the p values were halved, as above, to maintain protection against a type-II error in the two-group comparison. See figure legends and Supplement for additional details.
Results
RAEx prevents rarefaction of pial collaterals and increase in infarct volume that occur with aging
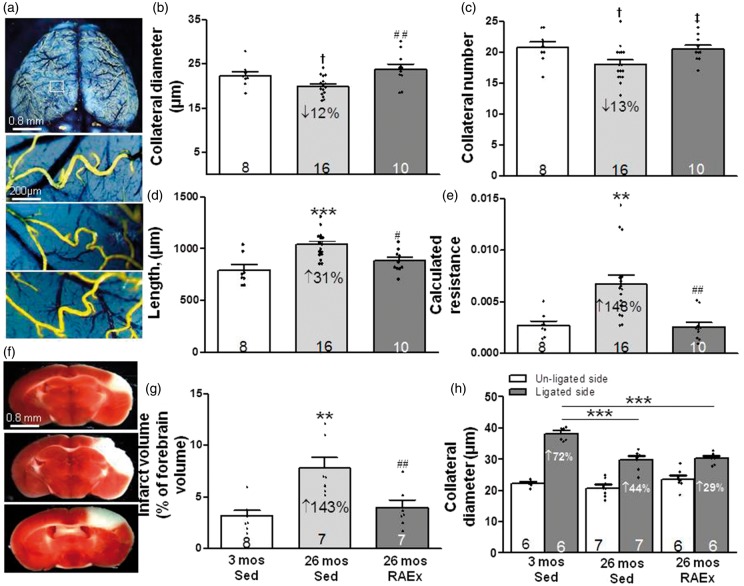
Aging caused a decline in number, diameter and increased length of pial collaterals, resulting in an increase in calculated relative resistance of the collateral network (Figure 1), findings which confirmed previous reports.14,16 The age-induced collateral rarefaction and increased resistance were prevented by RAEx. Moreover, exercise prevented the accompanying 2.5-fold increase in infarct volume after pMCAO seen in 26-month-old SED mice.
Figure 1.
Regular aerobic exercise prevents age-induced rarefaction of pial collaterals and associated increase in infarct volume. (a, f) Representative images of pial collaterals (after maximal dilation, formaldehyde fixation and filling with MicrofilR) and infarctions (2,3,5-triphenyltetrazolium chloride staining measured 72 h after permanent MCA occlusion) in 3-month-old sedentary (Sed, top), 26-month-old Sed (middle) and 26-month-old regular aerobic exercised mice (RAEx, bottom). (b–g) Exercise prevented age-induced collateral rarefaction (decline in number and diameter of MCA-to-ACA collaterals), increase in collateral length, increase in calculated resistance of the MCA-to-ACA collateral network [collateral length/(collateral number x diameter4)], and increase in infarct volume. (h) Exercise did not prevent the decrease in collateral remodeling with aging (measured three days after MCA occlusion). For all figures unless stated otherwise in the legend: Data are mean ± SEM for the n-sizes given in the figures (number of animals), with significance defined as p < 0.05 determined by ANOVA, Bonferroni protected t-, Student’s t- and X2 tests; each three-bar group (3-month-old sedentary (Sed), 26-month-old Sed, 26-month-old RAEx or eNOSTG) was tested, if significant by ANOVA, for three post hoc comparisons using Bonferroni-protected two-tailed t-tests, where *,**,*** denotes p < 0.05, < 0.01, < 0.001 versus 3-month-old Sed and where #, ##, ### denotes p < 0.05, < 0.01, < 0.001 versus 26 month-old Sed mice. If a comparison was not p < 0.05 by the above test, the two-tailed Bonferroni test was constrained to comparison of two groups/bars among the following groups: 3-month-old Sed, 26-month-old Sed, 26-month-old RAEx or 26-month-old eNOSTG, where †, ††, ††† denotes p < 0.025, < 0.005, < 0.0005 versus 3-month-old Sed and where ‡, ‡‡, ‡‡‡ denotes p < 0.025, < 0.005, < 0.0005 versus 26-month-old Sed. Constraining was justified since the above two-bar comparisons were pre-specified in the experimental design based on previous results; 14–18,20–22,30–33 nevertheless, the p values were halved, as above, to maintain protection against a type-II error in the 2-group comparison. ANOVAs for panels B-D,E,G,H are p < 0.05– < 0.001.
Permanent occlusion of the MCA causes flow-dependent outward remodeling of pial collaterals on the ipsilesional side that requires three days to reach completion in three month-old B6 mice.10 Although this study was not designed to explore this process, we measured pial collateral remodeling because it is reduced in aged animals14,15 and no studies have examined whether this inhibition can be mitigated by exercise-training. We confirmed that aging reduces collateral remodeling measured three days after pMCAO (Figure 1(h)). However, RAEx did not affect the inhibition.
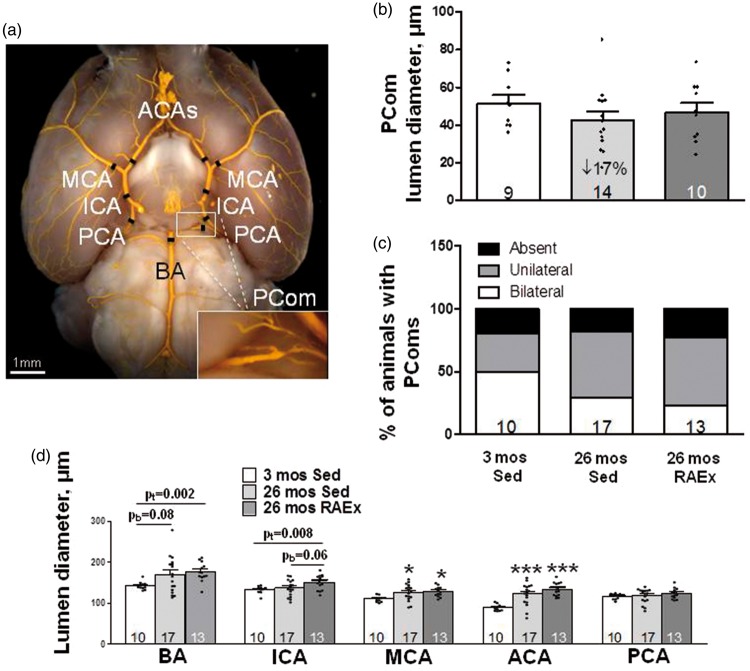
Unilateral pMCAO augments flow across the ipsi- and contralesional PCom collaterals, which presumably aids perfusion of the ipsilesional PCA tree and its downstream pial collaterals.25 Thus, if PCom collateral arteries also undergo rarefaction with aging, like pial collateral arterioles, this could contribute to the increased lesion size that accompanies aging (Figure 1(g)), and RAEx may inhibit the rarefaction. We therefore examined PCom diameter and number, i.e. whether PComs are present bilaterally, unilaterally or absent. In contrast to pial collaterals, PCom diameter and number were not affected by aging or RAEx, although PCom diameter evidenced similar trends as seen for pial collaterals (Figure 2; the source of the large intrinsic variability in PCom number is addressed in Discussion).
Figure 2.
Lumen diameters of primary intracranial arteries increase with aging and exercise-training further augments this. (a) Black hashes identify site of measurement (250 µm from bifurcations; at midpoint for ICA, PCA and PComs). Statistics symbols are defined in the legend for Figure 1; the values above the bracketed two-group comparisons for BA and ICA are double the Bonferroni value, computed for the comparisons defined in Figure 1 legend, to protect against a type-II error in the two-group comparison. (b, c) Diameter and number of PComs (% of animals with PComs present bilaterally, unilaterally or absent) did not change with aging or exercise-training (ANOVA for diameter p = 0.20, two-tailed X2 test for percentage of animals with absent + unilateral p = 0.25). Panel D, ANOVAs for BA, ICA and MCA are p < 0.05, ACA p < 0.001. pb = p-value for Bonferroni constrained to two comparisons (26 mos Sed or 26 mos RAEx vs. 3 mos Sed). pt = p-value for one-tailed t-test.
Intracranial arteries undergo outward remodeling with aging
As noted in the Introduction, little is known concerning whether diameter of the primary intracranial arteries is affected by aging or RAEx. Such changes could contribute to the effects of aging and RAEx on infarct volume shown in Figure 1(g). We therefore examined diameter of the intracranial arteries. Aging significantly increased diameter of the MCA (15%) and ACA (37%), with the BA exhibiting the same trend (18%, p = 0.08) (Figure 2(d)). Diameters did not differ significantly between RAEx and age-matched SED animals, although ICA tended to be larger in RAEx mice (p = 0.06). ICA and PCA diameters were not increased by aging alone; however, lumen diameter of both vessels was increased in age-matched exercise-trained mice. When the diameters of the five vessel types were averaged for each animal and compared among the three groups, “intracranial artery diameter” increased by 12% in 26-month-old SED mice and by 18% in the exercise-trained age-matched mice (ANOVA p = 0.02; p < 0.01 and p < 0.05, respectively, for these groups compared to three-month-old SED mice by two-tailed Bonferroni t-tests for three comparisons), and RAEx tended to further increase it over aging alone (Bonferroni: two-tail p = 0.097, one-tail p < 0.05).
Age-induced increase in resistance of the pial collateral network is prevented in eNOS transgenic mice, and eNOS overexpression increases diameter of the ICA, MCA and PCom collateral arteries
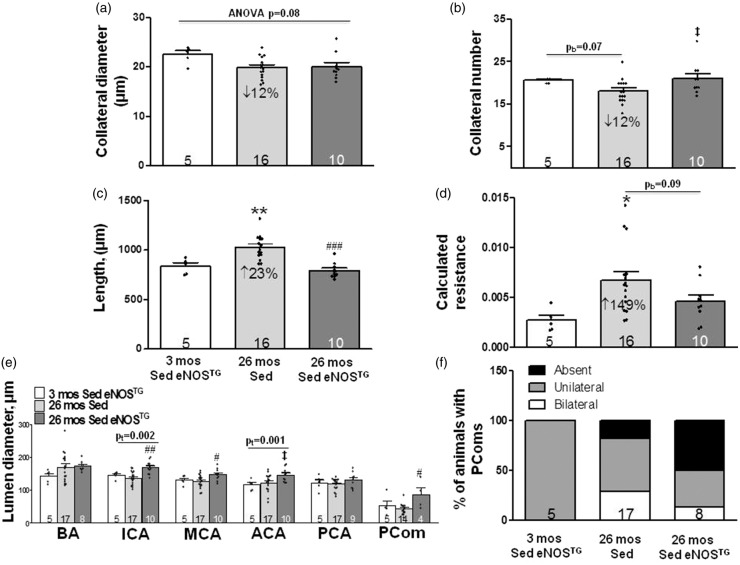
Aging is accompanied by decreased eNOS-NO bioavailability,14,15,17,18,20,21,34 and RAEx prevents or lessens this.20 Furthermore, age-induced rarefaction of pial collaterals is strongly accelerated in mice deficient in eNOS.17,18 We therefore examined eNOS transgenic mice (eNOSTG, 2-fold increase in endothelial cell-specific NO production34) to determine if increased eNOS-NO, alone, inhibits rarefaction. Figure 3 shows data for pial collaterals, PComs and intracranial arteries of SED 3 and 26-month-old eNOSTG mice compared to the SED 26-month-old B6 (wildtype) mice shown in Figure 1. The declines in pial collateral diameter and number in the aged mice were no longer significant (p = 0.07 by two-tailed Bonferroni constrained to two comparisons), possibly due to the smaller n-size for the three-month-old eNOSTG mice. However, collateral length and calculated resistance evidenced increases (23% and 149%) similar to those in Figure 1 (31% and 148%). The trend toward fewer collaterals and significant increases in length and resistance in 26-month-old B6 mice were not observed in age-matched eNOSTG mice. The above four parameters did not differ between three-month-old B6 and eNOSTG mice (Supplemental Figure V).
Figure 3.
Age-induced increase in resistance of the pial collateral network is prevented in eNOSTG mice, and eNOS overexpression increases diameter of the ICA, MCA and PCom collateral arteries. (a–f) Data for pial collaterals, PComs and intracranial arteries of 26-month-old sedentary B6 (wildtype) mice are from Figure 1. Statistics symbols are defined in the legend for Figure 1; the value above the bracketed two-group comparison in panel A is double the Bonferroni value, computed for the comparisons defined in Figure 1 legend, to protect against a type-II error in the two-group comparison. (e) Diameter for ICA was larger (BA and ACA trended similarly) in sedentary 26 month versus 3-month-old eNOSTG mice. Diameters for ICA, MCA and PComs were larger in sedentary 26-month-old eNOSTG versus age-matched wildtype mice. (f) PCom number did not significantly differ (two-tailed X2 test). ANOVAs for ICA, MCA, ACA and PCom were p < 0.01, <0.05, 0.06, <0.05. Supplemental Figure V gives comparison of values of the parameters in Figure 3 for three-month-old eNOSTG mice versus the sedentary three-month-old wildtype mice shown in Figures 1 and 2; the following are not significantly different: pial collateral number, diameter, length, calculated resistance, diameters of BA, ICA, PCA, PCom and PCom number; MCA and ACA diameters are 18% (p < 0.01) and 33% (p < 0.001) larger, respectively, in eNOSTG mice. pb = p-value for Bonferroni constrained to two comparisons (26 mos Sed or 26 mos RAEx vs. 3 mos Sed). pt = p-value for one-tailed t-test.
PCom diameter of 26-month-old eNOSTG mice was larger than age-matched wildtype mice (Figure 3(e)). No significant differences were observed for PCom number (Figure 3(f)), potentially due to the same large intrinsic variation seen in Figure 2. PCom diameter and number did not differ significantly for three-month-old B6 and eNOSTG mice (Supplemental Figure V(e) and (f)). Similar to the increase in PCom diameter, ICA and MCA diameters were significantly larger and ACA trended similarly (p = 0.017 by Bonferroni two-tailed t test constrained to two comparisons) in 26-month-old eNOSTG mice compared to age-matched B6 mice (Figure 3(e)). Diameters of the MCA and ACA were greater (p < 0.01), and ICA trended similarly (p = 0.05) in the three-month-old eNOSTG compared to age-matched B6 mice (Supplemental Figure V). Taken together, the above data indicate that genetic increase in eNOS-NO promotes larger diameters of the PCom and anterior (but interestingly not posterior) intracranial arteries—the latter which become evident at a young age and are seen to have progressed with advanced aging.
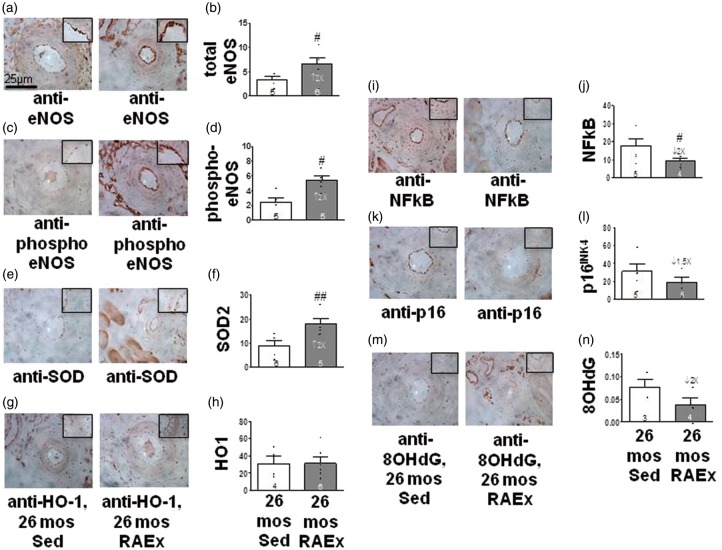
Exercise induces protective changes in eNOS, inflammation and oxidative stress in the vascular wall of aged mice
To provide additional insight regarding how RAEx may inhibit pial collateral rarefaction, we performed immunohistochemistry for eNOS and markers of oxidative stress, inflammation and aging in the hindlimb’s proximal caudal femoral artery (Figure 4). Compared to 26-month-old SED mice, RAEx increased eNOS, phospho-eNOS and superoxide dismutase-2 (SOD2), and decreased NFkB, p16INK4a and 8-hydroxy-2′-deoxyguanosine (8OHdG), although p16INK4a and 8OHdG were not significant; heme-oxygenase-1 was not increased, confirming a previous study.36 These findings demonstrate that the RAEx regimen increased eNOS and reduced oxidative stress and inflammation in the arterial wall and suggest.
Figure 4.
Exercise induces protective changes in eNOS and markers of inflammation and oxidative stress in the aged vascular wall. (a, c, e, g, i ,k, m) Representative images of immuno-stained proximal caudal femoral artery (630×). (b, d) Endothelial staining normalized to lumen circumference.(f) Endothelia + media staining normalized to media thickness. (h, j, l, n) Media staining normalized to media thickness. #, ## p < 0.05, < 0.01 by one-tailed t-tests (see Supplement for justification).
The RAEx regime used herein had the expected effects of long-term aerobic exercise training on endpoints routinely obtained in such studies: RAEx reduced the increase in body weight that occurred in SED mice with aging (Supplemental Figure IV). The tendency of the wall thickness-to-lumen diameter ratio of the proximal caudal femoral artery to increase with aging (Supplemental Figure II) was no longer evident. RAEx also induced physiologic/exercise cardiac hypertrophy (Supplemental Figure IV) and an increase in the percentage of slow-twitch fibers in the adductor muscles of the hindlimb (Supplemental Figure III).
Discussion
Patients with a history of physical activity have better outcome post-stroke;7 however, the responsible mechanisms are unknown. Findings in the present study demonstrate that RAEx is protective against the effect of aging on the brain’s collateral circulation. RAEx beginning in middle age prevented: (1) the loss of pial collaterals and decline in their diameter, (2) increase in calculated resistance of the pial collateral network, and (3) more than doubling of infarct volume following acute ischemic stroke, that occurred in age-matched SED mice. Interestingly, in addition to the above collateral rarefaction, aging was also accompanied by an increase in lumen diameter of the primary intracranial arteries (12% increase overall, p < 0.01), and exercise tended to increase it further. Genetic overexpression of eNOS had similar effects as exercise, preventing the increase in collateral resistance seen with aging, and increasing diameter of the ICA, MCA and PComs over that seen in age-matched SED mice. The effects of exercise were associated with increased vascular wall eNOS and the antioxidant enzyme SOD and reduced NFkB, a marker of inflammation. Thus, RAEx protected the collateral circulation from rarefaction and prevented the increase in stroke severity seen with aging. The finding that rarefaction with aging was strongly accelerated when eNOS is deficient14,17,18 and that the effects of RAEx were mimicked by overexpression of eNOS, suggest that the well-known effect of exercise-training to inhibit the decline in eNOS-NO that occurs with aging5,20–22,33 contributes significantly to the protective effects we observed with exercise-training.
The decrease in collateral diameter in 26-month-old SED B6 mice (12%, Figure 1) is smaller than we previously reported14 for 24-month-old B6 mice (23%; the decreases were relative to three-month-old mice in both studies). Decrease in collateral number was comparable in both studies (13% in Figure 1 and 11%14). The different results for diameter likely reflect that both sexes were examined herein, whereas only males in the previous study, and that a smaller decline in diameter (and smaller infarct volume) occurs in females with aging35 (n-sizes in the present study were insufficient to confirm the latter). Hecht et al.16 also found reduced collateral number in 18-versus 3-month-old male B6 mice (27% fewer; diameter was not measured). While the above loss of collateral number and diameter with aging is small, when combined with the accompanying increase in collateral length, the result is a 2.5- (Figures 1,3) to-5 fold14 increase in calculated resistance across the ACA-MCA collateral network and a 2.4- (Figure 2) to-3 fold14 increase in infarct volume after pMCAO.
In contrast to pial collateral arterioles, diameter and number of PCom collateral arteries were not affected by aging or exercise. Hecht et al.16 also found no difference in PCom number and diameter in one versus 18-month-old mice. The dissimilar effects of aging and exercise-training on pial versus PCom collaterals may reflect their differences in size and anatomic location, and thus differences in hemodynamic environment and sensitivity of their endothelial and smooth muscle cells to aging and exercise. Pial and PCom collaterals also exhibit significant differences in time of formation during embryogenesis,24 sensitivity to cardiovascular risk factor presence, and effect of genetic background and stochastic factors on their extent in the adult.25 With regard to the latter, PCom number displayed unusually large variation in all groups (Figures 2(c), 3(f) and V(f)). We recently examined the cause of this large variation, which is not shared by pial collateral number, and found that stochastic factors are a major contributor, along with genetic and environmental factors including age.25 For example, n-sizes of 22 B6 and 27 BALB/cBy four-month-old mice were required to detect a significant strain-dependent difference in their number of PComs. This large variation limits our conclusions regarding the sensitivity of PCom number to aging, RAEx and eNOS overexpression. While previous studies have not examined these factors for collaterals, exercise-training inhibited eNOS-dependent endothelial dysfunction in diabetic rats (but had no effect on diameter of pial arterioles)37 and age-associated decline in capillary density.22,38
Similar to RAEx, overexpression of eNOS prevented the increase in pial collateral resistance seen with aging, and increased ICA, MCA and PCom diameters. The tendency of overexpression to prevent loss of pial collateral number but not diameter (Figure 3) is consistent with our previous findings that eNOS deficiency caused loss of pial collateral number but not diameter.17,18 No experimental or human studies have examined whether overexpression of eNOS or chronic treatment with a NO donor affects collateral extent or increases diameter of intracranial arteries. However, Yamashita et al.39 reported that lumen diameters of the posterior tibial artery of four to five-month-old eNOSTG mice were larger than wildtype (219 vs. 205 µm, respectively).39 Potential mechanisms for the above effects include that eNOS protects pial collaterals against rarefaction caused by aging and other risk factors17,18 and that chronic administration of vasodilators (NO is such) induces outward remodeling of arteries.40
We have proposed that the sensitivity of collaterals within the microcirculation to rarefaction with aging and other risk factors arises from the unusual hemodynamic environment in which they reside: 14,17,18,41 in the absence of obstruction, collaterals experience little or no net flow and thus are presumably exposed to increased circumferential wall stress and lower PO2. These conditions, when prolonged, favor reduced eNOS-NO and pro-oxidative/-inflammatory changes that promote, in arteries, arteriosclerosis and atherosclerosis. In contrast, collaterals (but not nearby similarly sized pial arterioles) evidence increased cell proliferation, length and tortuosity with aging (e.g. Figure 1(a)), accompanied by accelerated senescence, apoptosis and loss of collateral number and diameter.14,17 It is well known that with aging, endothelial cells undergo decreased NO and SOD and increased oxidative stress, inflammatory changes, DNA damage and apoptosis.14,20–22 Age-induced reduction in eNOS-NO increases susceptibility to apoptosis and impairs recruitment of progenitor cells.15,42 Our observation that RAEx increased eNOS and SOD and tended to lower 8OHdG may reflect the effect of NO to reduced free radical-mediated DNA damage.43 As well, the reduction we saw for NFkB with exercise-training is consistent with the effect of exercise to inhibit increased oxidative stress and inflammation in arteries of aged individuals.5,20–22,26,27,44,45 Vascular dysfunction in senescence is associated with cell cycle arrest,21 a finding supported by increased p16INK4 seen in aging humans and rodents.46 The trend toward lower p16INK4 in our exercise-trained mice is consistent with reduced expression of p16INK4 in leukocytes of aged individuals that correlates inversely with their physical activity.46
Mechanisms underlying inhibition of collateral rarefaction by exercise are undoubtedly complex. It is possible that collaterals experience increases in wall stresses during RAEx that maintain collateral “health” by “exercising” their endothelial and smooth muscle cells, which has been proposed for the general circulation. Exercise increases cerebral blood flow (CBF).22,38,47,48 Arteriolar dilation upstream and/or downstream of collaterals, together with their characteristic convergence of blood flow41 predict that collaterals experience a larger fraction of the increased pulse pressure that accompanies exercise, compared to similarly sized pial arterioles. As well, fluctuating levels of exercise-associated hyperemia in the MCA, ACA and PCA territories will induce phasic flow/shear stress across collaterals. The above forces exerted on collateral vessels during prolonged exercise training may inhibit collateral rarefaction by opposing the endothelial dysfunction, oxidative stress and inflammation that occur with aging. In support, exercise training restores impaired eNOS activity in the BA and ICA of aged as well as diabetic rodents and humans.20,32,33,37 and promotes an NO-dependent increase in circulating endothelial progenitor cells that exhibit prolonged lifespan and reduced apoptosis.42 Also, reduced tissue oxygen during exercise favors increased hypoxia-inducible factor 1, VEGF-A and stromal cell-derived factor-1 that promote increased circulating endothelial progenitor cells.42 Indeed in young adult mice, several weeks of daily aerobic exercise reduced infarct volume after MCA occlusion, in association with increased VEGF-A (which activates eNOS), endothelial progenitor cells and microvascular density in the ischemic region.7 Increased VEGF-A and eNOS-NO (which both protect against collateral rarefaction18,49) and endothelial progenitor cell homing to the aging collateral wall could contribute to exercise-induced inhibition of collateral rarefaction with aging.
Aging was accompanied by an increase in lumen diameter of the primary intracranial arteries, and exercise tended to increase it further. Outward remodeling with aging also occurs in peripheral arteries including the carotid.21,50 However, conflicting results have been reported for intracranial arteries,16,22,26–29,30 possibly due to presence of hypertension and other risk factors which can instead promote intima-medial thickening and lumen loss. For example, PCA diameter was larger in 23 month-versus 4-month-old male SED B6 mice,27 while in our mixed-sex cohort, aged mice had larger diameter ICA and MCA (and ACA trended) but not PCA. Exercise-training favored additional increase in age-associated remodeling of intracranial arteries (Figure 2), possibly from eNOS-NO dependent flow-mediated remodeling caused by the increased CBF during exercise.22,38 With endurance training, elevated blood flow/shear stress are accompanied by increased lumen diameter and decreased intima-media thickness of arteries supplying the exercising muscles,26 with the latter also occurring to a lesser extent in arteries supplying tissues not involved in the exercise including the carotid.30 This may extend from the regular bouts of increased circumferential and fluid shear stresses31 during exercise, which increase angiogenic factors that induce proliferation of vascular wall cells,48 or from changes in pulse pressure-dependent wall distension caused by resting bradycardia.22 Voluntary wheel-running increased endothelial-dependent function in the ICA of 29–32-month-old B6 mice,33 in the BA of type 1 diabetic rats,32 and in the PCA of mice exposed to a western diet.22
The increase in lumen diameter of intracranial arteries that occurred with aging and RAEx may have functional significance. Unlike peripheral conduit arteries, the primary intracranial arteries account for a significant fraction of cerebral vascular resistance (∼40 percent) and contribute to regulation of blood flow during exercise and other conditions.22,51 Lower capillary density, and impaired reactivity of resistance vessels that occur with aging—changes that are diminished or prevented by voluntary wheel-running15,22,38,48,50,52—may cause a compensatory autoregulatory decrease in the smooth muscle tone of intracranial arteries that could, like the effect of prolonged administration of vasodilators,40 contribute to the outward remodeling we observed. This could mitigate the decline in CBF with aging that is favored not only by the above microvascular changes, but also by the medial hypertrophy and lumen loss that occur in resistance vessels with aging.27–29 Thus, remodeling of intracranial arteries could aid preservation of CBF in the aging brain and, in addition, increase collateral blood flow following stroke.
This study has several limitations. Additional investigations are needed to determine the effect of reducing the intensity or duration of the exercise regimen or beginning it at an earlier age. In a previous study, collateral rarefaction became evident in B6 mice at ∼ 55 human-years equivalent and increased progressively at each age examined thereafter, the last being at 90 human-years equivalent.10 Thus, beginning an exercise regimen at an earlier age and continuing it for a shorter duration may delay or slow collateral rarefaction and provide protection well-after the exercise-training has diminished or stopped. Our ex-vivo measurements of maximally dilated vessels do not address the dynamics of the cerebral circulation in vivo where significant basal tone is present. N-sizes and statistical power were reduced in several groups because vascular casting and measurement of infarct volume required separate animals, and because the number of computer-monitored wheel-running cages was restricted to 30. These exigencies limited the number of mice that could be studied and precluded use of an animal for multiple measurements, including requiring immunostaining of a peripheral artery, the proximal caudal femoral artery, which may not predict intracranial arteries. However, the changes we observed have also been reported with aging and exercise-training in other arteries supplying muscles recruited during exercise as well as other tissues including brain.14,15,17,20–22 Outward remodeling of arteries, including pial collaterals after pMCAO, is mediated by an increase in fluid shear stress that induces eNOS-NO and downstream signaling.18,26,31 Collateral remodeling is inhibited by aging, in part from impaired eNOS-NO.14,15 We therefore examined whether RAEx in aged mice restores remodeling measured three days after pMCAO, at which time it has reached maximum in three-month-old B6 mice.14 It is possible that our observation that exercise did not reverse the age-induced inhibition of remodeling (Figure 1(h)) may reflect that it occurs more slowly in aged mice.14 Thus, examining the amount of remodeling at a later time point may have given a different result.
In conclusion, our results show that RAEx beginning in middle age prevents age-induced rarefaction of pial collaterals and reduces infarct volume. Such findings, if confirmed in humans, would add these benefits to the list of advantages provided by regular physical exercise.
Supplementary Material
Acknowledgments
We thank Kirk McNaughton, Ashley Ezzell and Kara Clissold of the Research Histology Core for tissue sectioning and Massons staining. Metabolic phenotypes in sedentary versus exercised mice (see Supplement) were collected using the Animal Metabolism Phenotyping (AMP) core facility within UNC’s Nutrition Obesity Research Center (NORC) funded by National Institutes of Health Grant DK056350.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health, National Institutes of Neurological Diseases and Stroke grant NS083633 (JEF)
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
WR and HZ performed the experiments; BB oversaw the exercise training with assistance from KH; DP directed the UNC Exercise Core; JF designed the study; WR and JF wrote the paper.
Supplementary material
Supplementary material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Weimar C, Konig IR, Kraywinkel K, et al. Age and the National Institutes of Health Stroke Scale within 6h after onset are accurate predictors of outcome after cerebral ischemia: development and external validation of prognostic models. Stroke 2004; 35: 158–162. [DOI] [PubMed] [Google Scholar]
- 2.Vogt G, Laage R, Shuaib A, et al. Initial lesion volume is an independent predictor of clinical stroke outcome at day 90: an analysis of the Virtual International Stroke Trials Archive (VISTA) database. Stroke 2012; 43: 1266–1272. [DOI] [PubMed] [Google Scholar]
- 3.Shinton R, Sagar G. Lifelong exercise and stroke. BMJ 1993; 307: 231–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wendel-Vos GC, Schuit AJ, Feskens EJ, et al. Physical activity and stroke. A meta-analysis of observational data. Int J Epidemiol 2004; 33: 787. [DOI] [PubMed] [Google Scholar]
- 5.Howard VJ, McDonnell MN. Physical activity in primary stroke prevention: just do it!. Stroke 2015; 46: 1735–1739. [DOI] [PubMed] [Google Scholar]
- 6.Gertz K, Priller J, Kronenberg G, et al. Physical activity improves long-term stroke outcome via endothelial nitric oxide synthase-dependent augmentation of neovascularization and cerebral blood flow. Circ Res 2006; 99: 1132–1140. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt W, Endres M, Dimeo F, et al. Train the vessel, gain the brain: physical activity and vessel function and the impact on stroke prevention and outcome in cerebrovascular disease. Cerebrovasc Dis 2013; 35: 303–312. [DOI] [PubMed] [Google Scholar]
- 8.Egan KJ, Janssen H, Sena ES, et al. Exercise reduces infarct volume and facilitates neurobehavioral recovery: results from a systematic review and meta-analysis of exercise in experimental models of focal ischemia. Neurorehabil Neural Repair 2014; 28: 800–812. [DOI] [PubMed] [Google Scholar]
- 9.Ginsberg M. Expanding the concept of neuroprotection for acute ischemic stroke: the pivotal roles of reperfusion and the collateral circulation. Prog Neurobiol 2016. 145–146: 46–77. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H, Prabhakar P, Sealock R, et al. Wide genetic variation in the native pial collateral circulation is a major determinant of variation in severity of stroke. J Cereb Blood Flow Metab 2010; 30: 923–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leng X, Fang H, Leung TW, et al. Impact of collaterals on the efficacy and safety of endovascular treatment in acute ischaemic stroke: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2016; 87: 537–544. [DOI] [PubMed] [Google Scholar]
- 12.Mokin M, Rojas H, Levy EI, et al. 2016 Randomized trials of endovascular therapy for stroke—impact on stroke care. Nat Rev Neurol 2016; 12: 86–94. [DOI] [PubMed] [Google Scholar]
- 13.Lucitti JL, Sealock R, Buckley B, et al. Variants of Rab GTPase-effector binding protein-2 cause variation in the collateral circulation and severity of stroke. Stroke 2016; 47: 3022–3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faber JE, Zhang H, Lassance-Soares RM, et al. Aging causes collateral rarefaction and increased severity of ischemic injury in multiple tissues. Arterioscler Thromb Vasc Biol 2011; 31: 1748–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Peng X, Lassance-Soares RM, et al. Aging-induced collateral dysfunction: impaired responsiveness of collaterals and susceptibility to apoptosis via dysfunctional eNOS signaling. J Cardiovasc Trans Res 2011; 4: 779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hecht N, He J, Kremenetskaia I, et al. Cerebral hemodynamic reserve and vascular remodeling in C57/BL6 mice are influenced by age. Stroke 2012; 43: 3052–3062. [DOI] [PubMed] [Google Scholar]
- 17.Moore SM, Zhang H, Maeda N, et al. Cardiovascular risk factors cause premature rarefaction of the collateral circulation and greater ischemic tissue injury. Angiogenesis 2015; 18: 265–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dai X, Faber JE. Endothelial nitric oxide synthase deficiency causes collateral vessel rarefaction and impairs activation of a cell cycle gene network during arteriogenesis. Circ Res 2010; 106: 1870–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atochin DN, Huang PL. Endothelial nitric oxide synthase transgenic models of endothelial dysfunction. Pflugers Arch 2010; 460: 965–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seals DR, Edward F. Adolph distinguished lecture: the remarkable anti-aging effects of aerobic exercise on systemic arteries. J Appl Physiol 2014; 117: 425–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thijssen DH, Carter SE, Green DJ, et al. Arterial structure and function in vascular ageing: are you as old as your arteries? J Physiol 2016; 594: 2275–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bolduc V, Thorin-Trescases N, Thorin E, et al. Endothelium-dependent control of cerebrovascular functions through age: exercise for healthy cerebrovascular aging. Am J Physiol Heart Circ Physiol 2013; 305: H620–H633. [DOI] [PubMed] [Google Scholar]
- 23.Flurkey K, Currer JM, Harrison DE. Mouse models in aging research. In: Fox JG, et al. (ed). The mouse in biomedical research 20072nd ed Elsevier, pp. 637–672. [Google Scholar]
- 24.Faber JE, Chilian WM, Deindl E, et al. A brief etymology of the collateral circulation. Arterioscler Thromb Vasc Biol 2014; 34: 1854–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faber JE, Rzechorzek W, Dai KZ. Genetic, environmental and stochastic factors contribute to variation in the posterior communicating collaterals of the circle of Willis. Stroke 2017; 48: ATMP120. International Stroke Conference: 22-24 2017 (abstract).
- 26.Dinenno FA, Tanaka H, Monahan KD, et al. Regular endurance exercise induces expansive arterial remodeling in the trained limbs of healthy men. J Physiol 2001; 534: 287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diaz-Otero JM, Garver H, Fink GD, et al. Aging is associated with changes to the biomechanical properties of the posterior cerebral artery and parenchymal arterioles. Am J Physiol Heart Circ Physiol 2016; 310: H365–H375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fonck E, Feigl GG, Fasel J, et al. Effect of aging on elastin functionality in human cerebral arteries. Stroke 2009; 40: 2552–2556. [DOI] [PubMed] [Google Scholar]
- 29.Mandala M, Pedatella AL, Morales Palomares S, et al. Maturation is associated with changes in rat cerebral artery structure, biomechanical properties and tone. Acta Physiol 2012; 205: 363–371. [DOI] [PubMed] [Google Scholar]
- 30.Rowley NJ, Dawson EA, Hopman MT, et al. Conduit diameter and wall remodeling in elite athletes and spinal cord injury. Med Sci Sports Exerc 2012; 44: 844–849. [DOI] [PubMed] [Google Scholar]
- 31.Vita JA, Mitchell GF. Effects of shear stress and flow pulsatility on endothelial function: insights gleaned from external counterpulsation therapy. J Am Coll Cardiol 2003; 42: 2096–2098. [DOI] [PubMed] [Google Scholar]
- 32.Mayhan WG, Sun H, Mayhan JF, et al. Influence of exercise on dilatation of the basilar artery during diabetes mellitus. J Appl Physiol 2004; 96: 1730−1737. [DOI] [PubMed] [Google Scholar]
- 33.Durrant JR, Seals DR, Connell ML, et al. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol 2009; 587: 3271–3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haperen VR, Cheng C, Mees BME, et al. Functional expression of endothelial nitric oxide synthase fused to green fluorescent protein in transgenic mice. Am J Pathol 2003; 163: 1677–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Faber JE, Zhang H, Lucitti JL, et al. Sex differences in the murine cerebral collateral circulation. Transl Stroke Res 2016; 14: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niess AM, Passek F, Lorenz I, et al. Expression of the antioxidant stress protein heme oxygenase-I (HO-I) in human leukocytes. Acute and adaptational response to endurance exercise. Free Rad Biol Med 1999; 26: 184–192. [DOI] [PubMed] [Google Scholar]
- 37.Mayhan WG, Arrick DM, Patel KP, et al. Exercise training normalizes impaired NOS-dependent response of cerebral arterioles in type 1 diabetic rats. Am J Physiol Heart Circ Physiol 2011; 300: H1013–H1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas AG, Dennis A, Bandettini PA, et al. The effects of aerobic activity on brain structure. Front Psychology 2012; 3: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamashita T, Kawashima S, Ozaki M, et al. Role of endogenous nitric oxide generation in the regulation of vascular tone and reactivity in small vessels as investigated in transgenic mice using synchrotron radiation microangiography. Nitric Oxide 2001; 5: 494–503. [DOI] [PubMed] [Google Scholar]
- 40.Skalak TC, Price RJ. The role of mechanical stresses in microvascular remodeling. Microcirculation 1996; 3: 143–165. [DOI] [PubMed] [Google Scholar]
- 41.Chalothorn D, Faber JE. Formation and maturation of the native cerebral collateral circulation. J Mol Cell Cardiol 2010; 49: 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koutroumpi M, Dimopulos S, Psarra K. Circulating endothelial and progenitor cells: evidence from acute and long term exercise effects. World J Cardiol 2012; 4: 12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mizutani K, Ikeda K, Nishikata T, et al. Phytoestrogens attenuate oxidative DNA damage in vascular smooth muscle cells from stroke-prone spontaneously hypertensive rats. J Hypertens 2000; 18: 1833–1840. [DOI] [PubMed] [Google Scholar]
- 44.Ungvari Z, Koller A, Toth P, et al. Systems biology of free radicals and antioxidants. In: Ismail Laher (ed). Vascular aging and free radicals, 3rd ed Berlin Heidelberg: Springer, 2014, pp. 1365–1382. [Google Scholar]
- 45.Zou Y, Yoon S, Jung KJ, et al. Upregulation of aortic adhesion molecules during aging. J Gerontol A Biol Sci Med Sci 2006; 61: 232–244. [DOI] [PubMed] [Google Scholar]
- 46.Liu Y, Sanoff HK, Cho H, et al. Expression of p16(INK4a) in peripheral blood T-cells is a biomarker of human aging. Aging Cell 2009; 8: 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ogoh S, Ainslie PN. Cerebral blood flow during exercise: mechanisms of regulation. J Appl Physiol 2009; 107: 1370–1380. [DOI] [PubMed] [Google Scholar]
- 48.Olver TD, Ferguson BS, Laughlin MH. Molecular mechanisms for exercise training-induced changes in vascular structure and function: skeletal muscle, cardiac muscle, and the brain. Prog Mol Biol Transl Sci 2015; 135: 227–257. [DOI] [PubMed] [Google Scholar]
- 49.Clayton JA, Chalothorn D, Faber JE. Vascular endothelial growth factor-A specifies formation of native collaterals and regulates collateral growth in ischemia. Circ Res 2008; 103: 1027–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nagata K, Yamazaki T, Takano D, et al. Cerebral circulation in aging. Ageing Res Rev 2016; 30: 49–60. [DOI] [PubMed] [Google Scholar]
- 51.Warnert EA, Hart EC, Hall JE, et al. The major cerebral arteries proximal to the circle of Willis contribute to cerebrovascular resistance in humans. J Cereb Blood Flow Metab 2016; 36: 1384–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tyndall AV, Davenport MH, Wilson BJ, et al. The brain-in-motion study: effect of a 6-month aerobic exercise intervention on cerebrovascular regulation and cognitive function in older adults. BMC Geriatr 2013; 13: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.