Abstract
Objective
The understanding of complex heritable psychiatric disorders such as schizophrenia could be clarified by examining endophenotypes within genetically isolated populations, such as the one found in the Central Valley of Costa Rica. The reduction of familial variability within a sample could allow the relationship between the cognitive and symptomatic manifestations of the illness and the genetic underpinnings to become more observable. This study investigates the neuropsychological test performances of 41 family members from four extended multiplex families within the Spanish origin population of the Central Valley of Costa Rica as potential endophenotypes for genetic studies.
Methods
Individuals with a diagnosis of schizophrenia or schizoaffective disorder were compared with unaffected relatives and 15 unrelated controls with no family history of schizophrenia.
Results
Although the sample size is small, the results confirm previous reports in the literature of deficits in working memory, executive function, processing speed, and verbal fluency in individuals with schizophrenia compared with controls and intermediate performance in nonpsychotic family members compared with controls. We also found several suggestive quantitative cognitive trait loci with log of the odds greater than 1.75.
Conclusion
These findings suggest that the cognitive deficits in schizophrenia are consistent aspects of the illness, although their usefulness as endophenotypes for genetic studies remains unclear.
Keywords: endophenotype, neuropsychological, schizophrenia
Introduction
Several studies designed to investigate the genetic etiology of complex hereditary illnesses such as schizophrenia have examined homogeneous populations with high prevalence of these diagnoses (Egeland and Hostetter, 1983; Myles-Worsley et al., 1999; DeLisi et al., 2001; Escamilla, 2001; DeLisi et al., 2002; Wijsman, et al., 2003; Peltonen, et al., 2006). One such relatively isolated population has been identified in the Central Valley of Costa Rica surrounding the city of San Jose. Costa Rica is a Central American nation initially occupied by a small group of Spanish settlers who immigrated during the 1500–1800s (Melendez, 1982; Escamilla et al., 1996). These early settlers were geographically isolated by large impassible mountain ranges surrounding the region, and it has only been in the last decade that travel to and from the area has been frequent and tourism has escalated. The historical combination of high consanguinity and low outbreeding within this region has produced a relatively isolated population that has been shown to be useful for the study of a number of genetic disorders, including schizophrenia. Recently, some chromosomal regions of interest have been identified in this population for both schizophrenia (DeLisi et al., 2002; Cooper-Casey et al., 2005; Walss-Bass et al., 2006) and bipolar disorder (Freimer et al., 1996).
In addition to the clinical features of schizophrenia, neuropsychological dysfunction is known to be a characteristic of the disorder and neuropsychological traits have been used as endophenotypes in genetic studies of schizophrenia (Green, 2006). Patients with schizophrenia have deficits in various neuropsychological domains such as attention, memory, language skills, executive function, verbal fluency, and processing speed (Bowie and Harvey, 2005; Nemoto et al., 2005; Morrens et al., 2006). These findings have been replicated even in developing countries such as Brazil (Ayres et al., 2006). Interestingly, studies comparing patterns of cognitive deficiencies between patients with schizophrenia, their unaffected relatives, and healthy controls have suggested that unaffected siblings tend to perform intermediate to the other groups on various measures of cognition such as immediate and delayed memory tasks and on tests of executive function (Cannon et al., 1994; Shedlack et al., 1997; Byrne et al., 1999; Staal et al., 2000; Hoff et al., 2005; Hughes et al., 2005; Delawalla et al., 2006; Szoke et al., 2005, 2006). Findings from other population isolates such as in the Pacific Island Republic of Palau have also shown that unaffected relatives at risk for schizophrenia have impairments in verbal memory, attention, and motor skills (Myles-Worsley et al., 2007). This study was designed to further evaluate the cognitive traits previously shown to be altered in schizophrenia and to see whether they could also be valuable as phenotypes in gene linkage studies.
Methods
Participants
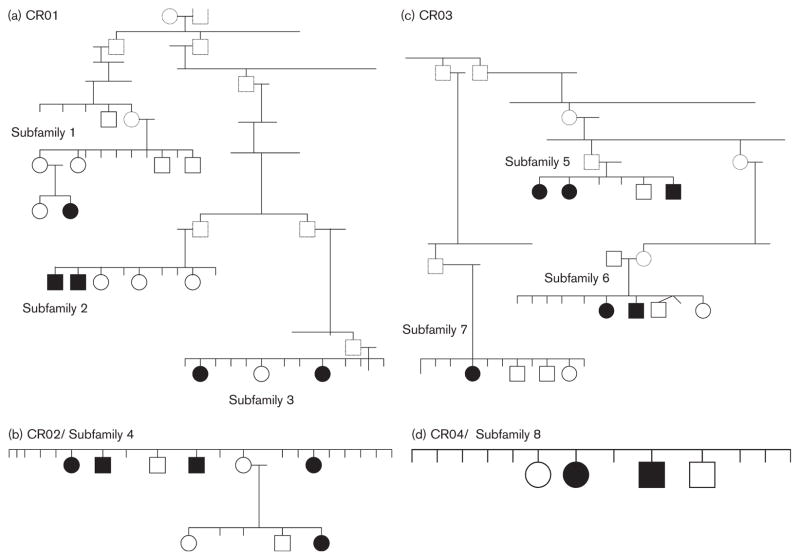
A large registry of families with multiple members diagnosed with schizophrenia or schizoaffective disorder was established through screening patients admitted to the National Psychiatric Hospital of Costa Rica as previously described (DeLisi et al., 2001, 2002). Patients admitted to this hospital were systematically screened, family informants were interviewed, and hospital records were reviewed to identify and evaluate this cohort. Only families in which all four grandparents of a proband were of Spanish descent were included. This project was approved by the Ministry of Health of Costa Rica and the ethics committee for the Hospital Nacional Psiquiatrico. In addition, USA OPRR Single Project Assurance was obtained, and the project is currently approved by the Institutional Review Boards at New York University School of Medicine and the University of California at Irvine. All participants gave written informed consent for participation. Three of the four families (CR01, CR02, CR03) used for this study were each traced back multiple generations to unique pairs of founders. The fourth family (CR04) originally was considered an extension of CR01 but later found only to be related through marriage and thus separated out in this study as an unrelated set of siblings. The four extended families were composed of eight distally related familial subsets (Fig. 1a–d). Although these subbranches shared a common ancestry within each family, they may also evidence comparative genetic variability between them because of the familial and generational distances between them. Identification of family relationships was made with the assistance of a genealogist who researched archival family records to relate previously identified multiplex nuclear families with one another (DeLisi et al., 2001, 2002).
Fig. 1.
(a–d) The four families and their relationships for all individuals who were tested. Circles represent females, squares represent males. Data were collected only on those individuals with solid outlines. Dark circles or squares indicate a diagnosis of schizophrenia or schizoaffective disorder. All other individuals are unaffected.
In addition to the patients with schizophrenia and their unaffected relatives within the families described above, 15 healthy controls from unique families participated as comparison participants. These individuals were unrelated to any other individual in this study and there was no genetic overlap between them.
All participants were interviewed using the Diagnostic Interview for Genetic Studies (Nurnberger et al., 1994) and given an extensive neuropsychological assessment battery as described below. Diagnoses were made by consensus of three independent trained research psychiatrists using Diagnostic and statistical manual of mental disorders, 4th Edition (American Psychiatric Association, 1994) criteria. A total of 56 individuals completed the evaluations. These included 14 with a diagnosis of schizophrenia or schizoaffective disorder, four with other psychoses, 23 family members with no psychotic illness, and 15 unrelated controls without significant psychopathology. Of the individuals diagnosed with a psychotic disorder, all but one was medicated with a conventional antipsychotic and one had an unknown medication status. As described above, ill and well family members from the four large extended families were also subdivided into eight smaller distantly related branches. The pedigrees and demographics for the family members are shown in Fig. 1a–d and in Table 1.
Table 1.
Demographics of final sample
| Diagnosis | Sex | Age | Education | Family/subfamily | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| CR01 | CR02 | CR03 | CR04 | |||||||||||
|
| ||||||||||||||
| M | F | M | SD | M | SD | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Schizophrenia/schizoaffective/psychosis (N=18) | 7 | 11 | 50.22 | 10.40 | 6.17 | 2.81 | 1 | 2 | 2 | 5 | 3 | 2 | 1 | 2 |
| Discordant family member (N=23) | 11 | 12 | 52.61 | 11.58 | 6.00 | 3.97 | 6 | 3 | 1 | 4 | 1 | 3 | 3 | 2 |
| Control (N=15) | 7 | 8 | 53.33 | 7.57 | 7.33 | 3.85 | – | – | – | – | – | – | – | – |
Neuropsychological measures
Before the commencement of testing, a panel of Costa Rican neuropsychologists determined the translated tests described below to be linguistically and culturally comparable with Costa Rican language and culture. The only exception was in items related to currency, where the Costa Rican currency (the colon) was substituted.
Wechsler Adult Intelligence Scale-Third Revision
This widely used test is composed of 14 subtests that can be combined to measure a Full Scale Intelligence Quotient, which can be subdivided into either a Verbal IQ and Performance IQ, or four index scores: the Verbal Comprehension Index (VCI), the Perceptual Organization Index, the Working Memory Index (WMI), and the Processing Speed Index (PSI) (Wechsler, 1997). For the purposes of this study, the Spanish translation of the Wechsler Adult Intelligence Scale-Third Revision (WAIS-III) was used (translation by Silvina Vizzini, 2002, authorized by The Psychological Corporation, USA. Edited by PAIDOS, Buenos Aires, Barcelona y México). It is important to note that although this Spanish translation of the WAIS-III is the version most frequently used with Hispanic populations, the test has no norms specific to Costa Rica. Scores included in the final analyses were therefore the total raw scores of the indices described above.
Boston Naming Test
The Boston Naming Test (Kaplan et al., 1983) is a 60-item test designed to measure word retrieval and expressive speech. The total number of correct responses was used as the final score.
Spanish Auditory and Verbal Learning and Memory Test
The Test de Memoria de Perri (Perri et al., 1995) is a version of the California Verbal Learning Test developed for use with a Hispanic population. Immediate and delayed recall scores were used in the comparisons for this project.
Trail Making Test
The Trail Making Test (Reitan and Wolfson, 1985), a part of the Halstead–Reitan Neuropsychological Test Battery, is designed to measure visual scanning, conceptual flexibility, and motor speed. Final scores are measured as the time taken to complete each part of the task.
Stroop Test
The Stroop Test (Golden, 1978) is composed of three sections, the first two in which the participant is timed as he/she reads columns of colors and words, respectively, and the final one in which he/she is asked to inhibit his/her response to the text of the stimuli while responding only to the color. An interference score is then calculated to reflect the differences between the scores in the neutral and interference sections.
Controlled Oral Word Association Test
A timed verbal fluency task. Total scores are calculated (Spreen and Strauss, 1998).
Purdue Pegboard Test
The Purdue Pegboard Test (Tiffen, 1968) is a measure of functional asymmetry where participants are timed as they insert pegs into a pegboard. Final scores are the average of three trials across each hand and the comparisons between them. From these averages, a laterality index was calculated for each participant.
Wisconsin Card Sorting Test
The Wisconsin Card Sorting Test (Heaton and Goldin, 2003) is a measure of executive function and abstract thinking in which participants are required to shift mental set as they match 128 stimulus cards on the basis of either color, shape, or number with minimal instruction or feedback from the examiner. Statistical analyses were carried out using the number of categories completed and total number of errors.
Statistical analyses of the cognitive data
Owing to the very low education levels of the individuals who participated in this study (6.41 ± 3.58 years of education) and the limited normative data available specifically for the population in Costa Rica, raw scores were used in the analyses. Statistical tests were conducted using analyses of covariance (ANCOVAs), with age, sex, and level of education completed as covariates. Family membership and diagnosis were the independent variables and cognitive tests the dependent variables. Bonferroni correction was used to adjust for multiple pairwise comparisons within each dependent variable that produced a significant omnibus finding.
Families were analyzed in two ways, the first as four independent extended families and second, given that the four extended families had eight branches that were distally related, the data were also analyzed considering the cohort as eight discrete familial subsets (Fig. 1).
Various statistical ways are available to examine complex family relationships combined with additional independent variables in quantitative studies and this method was selected as it is clear, uncomplicated, and provides the necessary findings about both pathologic diagnosis and familial segregation in a straightforward manner. Comparable methods were previously used in published studies with similar multiplex family analyses (DeLisi et al., 1986; Shedlack et al., 1997; Hoff et al., 2005). Although alternative methods are described elsewhere (i.e. Gelman and Hill, 2007) and the authors recognize that this method is not the only manner in which these data can be analyzed, it has been acknowledged in the literature as a valid means by which to study the hypotheses described above.
Linkage analyses
As an exploratory effort, we attempted to identify quantitative trait loci (QTL) for performance on all neuropsychological tests with significant family or diagnosis effects. A total of 81 participants were genotyped by deCODE Genetics using an 8-cM whole-genome scan (522 microsatellite markers). Blood samples were collected in Costa Rica and sent to the laboratory at University of California, Irvine. DNA was isolated from the blood through phenol-chloroform extraction, followed by a sodium acetate precipitation. All DNA samples had an OD260/OD280 ratio of at least 1.8. A total of 10 μg genomic DNA was used for genotyping.
We had both cognitive data and genotypes available for 39 participants from six of the eight family subsets (from kindreds CR01 and CR03). There were an additional related 42 genotyped participants without cognitive data who were included in the analyses to estimate allele frequencies.
Variance components linkage analyses were run using Merlin statistical software (available with source code online at http://bioinformatics.well.ox.ac.uk/Merlin and http://www.sph.umich.edu/csg/abecasis/Merlin) (Abecasis et al., 2002) using a total of 522 microsatellite markers for 39 participants. The false discovery rate using a logarithm of the odds (LOD) cutoff of 1.5 and 3.0 were calculated by 1000 permutations on each of the five traits using random marker data generated through genotype dropping simulations. Permutations were run on chromosome 22 and extended to the whole genome.
Results
Cognitive data
No significant differences were found between age (F=0.44, d.f.=2, 53, P=0.65), education (F=0.68, d.f.=2, 53, P=0.51), or sex [χ2(2)=0.36, P=0.84] among affected or nonaffected family members and controls. Table 2 depicts the means and standard deviations of test scores for the diagnostic groups, the comparisons between them by diagnosis and family membership (with values for the four extended families presented in the table) and the interactions. The second analysis is similar to the first, but the significance values differ because of the change in the definition of ‘family’ (i.e. eight smaller subsets).
Table 2.
Comparisons of neuropsychological tests across diagnostic groups and families (age, sex, and education covariates)
| Test/index | Control (0) (N= 15) | Family member (1) (N= 23) | Scz (2) (N= 18) | Main effect Diagnosis | Main effect Family | Interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| M | SD | M | SD | M | SD | F | Sig | F | Sig | F | Sig | |
| Raw VIQ | 40.87 | 9.43 | 34.52 | 9.08 | 31.06 | 8.68 | 1.46 | 0.24 | 0.62 | 0.84 | 0.59 | 0.63 |
| Raw PIQ | 28.73 | 4.98 | 23.39 | 6.53 | 21.78 | 6.46 | 1.93 | 0.18 | 0.66 | 0.81 | 2.18 | 0.11 |
| Raw FSIQ | 69.60 | 13.61 | 57.91 | 13.86 | 53.39 | 14.48 | 1.60 | 0.22 | 0.51 | 0.93 | 1.47 | 0.24 |
| Raw VCI | 20.80 | 6.16 | 16.87 | 4.53 | 16.28 | 5.00 | 0.32 | 0.58 | 1.25 | 0.29 | 0.30 | 0.83 |
| Raw POI | 19.27 | 2.99 | 14.48 | 4.63 | 13.61 | 4.42 | 0.92 | 0.35 | 0.62 | 0.85 | 2.52 | 0.08 |
| Raw WMI | 19.80 | 4.21 | 16.91 | 4.62 | 13.94 | 4.78 | 4.16 | 0.05b | 0.56 | 0.90 | 1.04 | 0.39 |
| Raw PSI | 10.67 | 3.39 | 8.43 | 3.92 | 6.89 | 3.55 | 2.55 | 0.12 | 0.60 | 0.87 | 0.20 | 0.90 |
| BNT total | 44.13 | 6.42 | 36.26 | 7.49 | 34.56 | 8.40 | 1.32 | 0.26 | 0.60 | 0.87 | 0.28 | 0.84 |
| Perri Short | 99.36 | 24.48 | 80.21 | 24.36 | 84.62 | 30.22 | 0.34 | 0.56 | 0.82 | 0.66 | 0.28 | 0.84 |
| Perri Long | 105.60 | 36.84 | 93.00 | 18.76 | 91.26 | 33.65 | 0.15 | 0.71 | 2.42 | 0.02* | 1.39 | 0.26 |
| Trails A | 76.20 | 23.33 | 116.52 | 88.17 | 127.22 | 66.16 | 0.57 | 0.45 | 0.27 | 1.00 | 0.27 | 0.85 |
| Trails B | 246.80 | 113.12 | 287.83 | 162.31 | 332.78 | 194.64 | 4.14 | 0.05b | 0.67 | 0.80 | 0.65 | 0.59 |
| Stroop Int | −1.66 | 9.68 | 0.35 | 8.71 | 0.92 | 7.49 | 0.23 | 0.64 | 1.87 | 0.06 | 0.14 | 0.93 |
| COWA PAM | 29.60 | 9.00 | 29.04 | 9.81 | 26.28 | 11.75 | 1.33 | 0.26 | 0.66 | 0.82 | 0.12 | 0.95 |
| COWA Anim | 16.33 | 3.31 | 15.13 | 4.20 | 13.00 | 3.61 | 3.06 | 0.09 | 1.25 | 0.29 | 0.87 | 0.47 |
| Laterality | 59.42 | 72.35 | 35.44 | 70.69 | 9.25 | 68.52 | 1.55 | 0.22 | 0.90 | 0.58 | 0.52 | 0.67 |
| WCST Errors | 61.80 | 23.47 | 68.00 | 20.30 | 59.00 | 19.62 | 0.87 | 0.36 | 1.55 | 0.14 | 0.82 | 0.49 |
| Categories | 2.60 | 2.13 | 1.78 | 1.88 | 2.33 | 1.64 | 0.53 | 0.47 | 1.37 | 0.22 | 0.86 | 0.47 |
Sig, at P < 0.05 after Bonferroni correction: a=0,1 b=0,2 c =1,2.
BNT, Boston naming test total correct responses; COWA anim, controlled oral word association total number of animal names; COWA PAM, Controlled Oral Word Association total correct responses beginning with P, A and M; Laterality index, laterality index from Purdue Pegboard Test; Perri Long, test de Memoria de perri long delayed recall; Perri Short, test de memoria de perri immediate recall; Raw FSIQ, WAIS-III full scale IQ total raw score; Raw PIQ, WAIS-III performance IQ total raw score; Raw POI, WAIS-III perceptual organization index total raw score; Raw VCI, WAIS-III verbal comprehension index total raw score; Raw VIQ, Wechsler Adult Intelligence Scale, 3rd Revision (WAIS-III) verbal IQ total raw score; Raw WMI,WAIS-III working memory index total raw score; Raw PSI, WAIS-III processing speed index total raw score; Stroop int, stroop interference score; Trails A, trail making test part A completion time; Trails B, trail making test part B completion time; WCST categories, Wisconsin Card Sort test total categories completed; WCST errors, total number errors on Wisconsin Card Sort Test.
When the cohort was analyzed as four large families, significant differences between members with schizophrenia and controls were observed for the raw WMI and the Trail Making Test, part B (F=4.16, d.f.=1, 30, P=0.05; and F=4.14, d.f.=1, 30, P=0.05, respectively). A main effect of family membership emerged on the delayed memory trial of the Perri Verbal Memory Test (F=2.42, d.f.=17, 30, P=0.02).
The analysis comparing the eight separate family subbranches appeared even more sensitive to group differences. Differences between siblings with schizophrenia and controls were still found for the raw WMI, but also for the raw PSI, and on a number of animals named on the verbal fluency task (F=5.65, d.f.=1, 22, P=0.03; F=4.51, d.f.=1, 22, P=0.05 and F=4.91, d.f.=1, 22, P=0.04, respectively) in the expected directions. There was also a main effect of family on the raw VCI (F=2.14, d.f.=21, 22, P=0.04) and on delayed memory performance of the Perri Verbal Memory Test (F=2.09, d.f.=21, 22, P=0.05). Diagnosis by family interactions on the raw WMI (F=3.59, d.f.=7, 22, P=0.01) and the raw PSI (F=2.88, d.f.=7, 22, P=0.03) were carried out.
To include the control individuals in these large ANCOVAs, it was required that each be assigned a unique family code number. This subsequently inflated the degrees of freedom reported above to include the control ‘families’. To verify the findings from the initial ANCOVAs exclusively within the schizophrenia families of interest, confirmatory post-hoc analyses on significant findings were conducted to study the main effect of family membership without control participants. Results were similar to the original findings, with significance in the Perri Delayed Memory (F=2.97, d.f.=3,31, P=0.05) across the four large families, significance in the raw VCI (F=4.91, d.f.=7, 27, P=0.01) and marginal significance in the Perri Delayed Memory (F=2.30, d.f.=7, 27, P=0.056) in the eight familial subsets.
Quantitative trait loci mapping
Variance components linkage analyses were run to identify putative QTL for the neuropsychological performance of five traits with significant diagnosis or family effects: VCI scores, WMI scores, PSI scores, the number of animals named on the verbal fluency task and the Trail Making Test B performance. For these five traits, all LOD scores were less than 3.0 (false discovery rate=0.2%). However, suggestive QTLs with LOD scores greater than 1.75 were found for the VCI and the Controlled Oral Word Association (COWA) Test. One suggestive QTL for COWA (total number of animals named) was identified at D13S1315 (LOD=2.08, P=0.001), with one family, CR03-02, making the primary contribution to this LOD score (LOD=1.78). A second suggestive QTL for COWA was seen near marker D3S1308 (LOD=1.76, P=0.002). A suggestive QTL was also identified for the VCI score with a peak at marker D10S1661 (LOD=1.95, P=0.0014) (Table 3).
Table 3.
Suggestive QTLs for performance on cognitive tests
| Neuropsychological test | P value | Peak marker | Proximal marker | Distal marker | Peak LOD score |
|---|---|---|---|---|---|
| COWA animals | 0.0010 | D13S1315 | D13S1809 | D13S293 | 2.08 |
| Verbal comprehension index | 0.0014 | D10S1661 | D10S600 | D10S570 | 1.95 |
| Processing speed index | – | – | – | – | – |
| Trail making test part B | – | – | – | – | – |
| Working memory index | – | – | – | – | – |
‘–’ Not reported, all LOD scores were < 1.75.
COWA, Controlled Oral Word Association; LOD, log of the odds; QTL, quantitative trait loci.
Discussion
Despite the small number of participants, the findings of this study are consistent with the earlier literature showing a clear pattern of impairment in working memory, executive function, verbal fluency, and processing speed in patients with schizophrenia compared with controls. In addition, there were nonsignificant trends for the well family members to score between the controls and their ill relatives on many of the tasks. Well family members were, however, generally not significantly different from controls or their ill relatives in neuropsychological function. No cognitive variables that discriminated between affected individuals and controls were found, and at the same time showed consistent heritability, and segregated with schizophrenia within families (e.g. family by diagnosis interactions). Therefore, no variables could be defined as valid endophenotypes in this small sample. However, some suggestive linkages between cognitive factors and genetic loci were found.
We observed a significant effect of family on the raw VCI score and variance components linkage analyses revealed a suggestive QTL on chromosome 10 for this trait. The raw VCI scores, however, did not distinguish patients with schizophrenia from controls. On the COWA Test, we identified a suggestive QTL at 13q34 but there was not a significant family effect on performance. Closer examination showed that linkage of COWA performance to these loci was mainly restricted to one family. Notably, we observed a significant effect of diagnosis on performance of the COWA Test locus (13q34) that was previously identified as a potential region for a susceptibility locus for schizophrenia (Chumakov et al., 2002). These results raise the possibility that deficits in verbal fluency and risk for schizophrenia in this family may be related pleiotropic effects of a single gene within this susceptibility locus. The findings of this study, however, need further investigation in a larger sample.
A schizophrenia candidate gene, G72, is in the region of 13q34 and this gene has been previously implicated in cognitive abnormalities in schizophrenia (Goldberg et al., 2006). A meta-analysis shows modest association of schizophrenia and G72 (Shi et al., 2007). No previously implicated schizophrenia or cognition candidates in the other two regions (10p13 and 3q25), although evidence for linkage of the 3p25 region and the 10p13 region to bipolar disorder has been demonstrated (Badenhop et al., 2002; Maziade et al., 2005).
This study has an advantage over previous work in that we assessed a culturally homogeneous set of families, thus controlling for nongenetic factors that may contribute to clustering of cognitive variables within families. Nevertheless, it is likely that we failed to observe inherited effects because of the small number of participants or because of the possibility that the tests we used were not the ones that were most sensitive to detect heritable cognitive endophenotypes in this population. Power issues may have been moderated, however, by reducing within-group variability (and therefore the size of the error term in the F ratios) by categorizing participants by family in addition to diagnostic group. Although a pattern of cognitive deficits across diagnostic groups similar to earlier reports seemed to emerge in this sample, it is also important to consider that none of the tests administered were standardized for the specific Spanish-speaking population of Costa Rica. As stated above, however, before the beginning of this study, a panel of Costa Rican psychologists with expertise in cognitive assessment determined these tests to be culturally and linguistically comparable to Costa Rican culture and language. In addition, despite the fact that education level was used as a covariate in the statistical analyses, the very low educational levels in this sample may have further reduced the sensitivity of the tests, many of which are very educationally based. Although this bias certainly limits the clinical utility of the tests, however, the fact that educational level did not differ across diagnostic groups in this sample may have made the raw scores still useful for our research comparisons. Given the absence of neuropsychological instruments standardized for the specific population of Costa Rica, the battery used within this study was determined to be the best possible alternative for measuring cognitive functioning within this sample. It is always possible that a study of a larger number of families and individuals using more specific and culturally sensitive tests may lead to differences that we failed to observe.
Despite the limitations of this particular study, the consistency between the pattern of cognitive deficits within this small sample in Costa Rica and the cognitive deficits seen in more heterogeneous populations in industrialized countries along with the limited suggestions of heritability is encouraging and leaves open the possibility for the use of cognitive variables as endophenotypes for schizophrenia. Specifically, verbal comprehension or fluency tests may be useful in future studies for examining genetic linkages or associations, and would be worth pursuit in a larger cohort study.
Acknowledgments
This study was made possible in part by funding from a NARSAD junior investigator award to A. Mesen (LE DeLisi as mentor). Some of the contributors to this project are members of the Pritzker Neuropsychiatric Disorders Research Consortium, which is supported by the Pritzker Neuropsychiatric Disorders Research Fund L.L.C. In addition to the affiliated authors, other authors would like to acknowledge the contributions of Huda Akil, Jack Barchas, Edward Jones, Rick Myers, Alan Schatzberg, and Stanley Watson from the Pritzker Consortium. The authors also thank statistics consultant, Dr Carl Goodrich, for his support and guidance on this project. A shared intellectual property agreement exists between this philanthropic fund and the University of Michigan, Stanford University, the Weill Medical College of Cornell University, the Universities of California at Davis, and at UC Irvine, to encourage the development of appropriate findings for research and clinical applications.
References
- Abecasis GR, Cherny SS, Cookson WO, Cardon LR. Merlin-rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet. 2002;30:97–101. doi: 10.1038/ng786. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Ayres AM, Busatto GF, Menezes PR, Schaufelberger MS, Coutinho L, Murray RM, et al. Cognitive deficits in first-episode psychosis: a population-based study in Sao Paulo, Brazil. Schizophr Res. 2007;90:338–343. doi: 10.1016/j.schres.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Badenhop RD, Moses MJ, Scimone A, Mitchell PB, Ewen-White KR, Rosso A, et al. A genome screen of 13 bipolar affective disorder pedigrees provides evidence for susceptibility loci on chromosome 3 as well as chromosomes 8, 13 and 19. Mol Psychiatry. 2002;7:851–859. doi: 10.1038/sj.mp.4001114. [DOI] [PubMed] [Google Scholar]
- Bowie CR, Harvey PD. Cognition in schizophrenia: impairments, determinants, and functional importance. Psychiatr Clin North Am. 2005;28:613–633. 626. doi: 10.1016/j.psc.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Byrne M, Hodges A, Grant E, Owens C, Johnstone EC. Neuropsychological assessment of young people at high genetic risk for developing schizophrenia compared with controls: preliminary findings of the Edinburgh high risk study. Psychol Med. 1999;29:1161–1173. doi: 10.1017/s0033291799001002. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Zorrilla LE, Shtasel D, Gur RE, Gur RC, Marco EJ, et al. Neuropsychological functioning in siblings discordant for schizophrenia and healthy volunteers. Arch Gen Psychiatry. 1994;51:651–661. doi: 10.1001/archpsyc.1994.03950080063009. [DOI] [PubMed] [Google Scholar]
- Chumakov I, Blumenfeld M, Guerassimenko O, Cavarec L, Palicio M, Abderrahim H, et al. Genetic and physiological data implicating the new human gene G72 and the gene for D-amino acid oxidase in schizophrenia. Proc Natl Acad Sci. 2002;99:13675–13680. doi: 10.1073/pnas.182412499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper-Casey K, Mesen-Fainardi A, Galke-Rollins B, Llach M, Laprade B, Rodriguez C, et al. Suggestive linkage of schizophrenia to 5p13 in Costa Rica. Mol Psychiatry. 2005;10:651–656. doi: 10.1038/sj.mp.4001640. [DOI] [PubMed] [Google Scholar]
- Delawalla Z, Barch DM, Fisher-Eastep JL, Thomason ES, Hanewinkel MJ, Thompson PA, et al. Factors mediating cognitive deficits and psychopathology among siblings of individuals with schizophrenia. Schizophr Bull. 2006;32:525–537. doi: 10.1093/schbul/sbj082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisi LE, Goldin LR, Hamovit JR, Maxwell ME, Kurtz D, Gershon ES. A family study of the association of increased ventricular size with schizophrenia. Arch Gen Psychiatry. 1986;43:148–153. doi: 10.1001/archpsyc.1986.01800020058007. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Mesen A, Rodriguez C, Bertheau A, LaPrade B, Llach M, et al. Clinical characteristics of schizophrenia in multiply affected Spanish origin families from Costa Rica. Psychiatr Genet. 2001;11:145–152. doi: 10.1097/00041444-200109000-00006. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Mesen A, Rodriguez C, Bertheau A, LaPrade B, Llach M, et al. Genome-wide scan for linkage to schizophrenia in a Spanish-origin cohort from Costa Rica. Am J Med Genet B Neuropsychiatr Genet. 2002;114:497–508. doi: 10.1002/ajmg.10538. [DOI] [PubMed] [Google Scholar]
- Egeland JA, Hostetter AM. Amish study, I: affective disorders among the Amish, 1976–1980. Am J Psychiatry. 1983;140:56–61. doi: 10.1176/ajp.140.1.56. [DOI] [PubMed] [Google Scholar]
- Escamilla MA. Population isolates: their special value for locating genes for bipolar disorder. Bipolar Disord. 2001;3:299–317. doi: 10.1034/j.1399-5618.2001.30605.x. [DOI] [PubMed] [Google Scholar]
- Escamilla MA, Spesny M, Reus VI, Gallegos A, Meza L, Molina J, et al. Use of linkage disequilibrium approaches to map genes for bipolar disorder in the Costa Rican populations. Am J Med Genet. 1996;67:244–253. doi: 10.1002/(SICI)1096-8628(19960531)67:3<244::AID-AJMG2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Freimer NB, Reus VI, Escamilla MA, McInnes LA, Spesny M, Leon P, et al. Genetic mapping using haplotype, association and linkage methods suggests a locus for severe bipolar disorder (BPI) at 18q22–q23. Nat Genet. 1996;12:436–441. doi: 10.1038/ng0496-436. [DOI] [PubMed] [Google Scholar]
- Gelman A, Hill J. Data analysis using regression and multilevel/hierarchical models. New York: Cambridge University Press; 2007. [Google Scholar]
- Golden CJ. Stroop color and word test. Chicago, Illinois: Stoelting Co; 1978. [Google Scholar]
- Goldberg TE, Straub RE, Callicott JH, Hariri A, Mattay VS, Bigelow L, et al. The G72/G30 gene complex and cognitive abnormalities in achizophrenia. Neuropsychopharmacology. 2006;31:2022–2032. doi: 10.1038/sj.npp.1301049. [DOI] [PubMed] [Google Scholar]
- Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry. 2006;67:e12. [PubMed] [Google Scholar]
- Heaton RK, Goldin JN. Wisconsin card sorting test computer version 4, research edition. Lutz, FL: Psychological Assessment Resources, Inc; 2003. [Google Scholar]
- Hoff AL, Svetina C, Maruizio AM, Crow TJ, Spokes K, DeLisi LE. Familial cognitive deficits in schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2005;133:43–49. doi: 10.1002/ajmg.b.30120. [DOI] [PubMed] [Google Scholar]
- Hoff AL, Svetina C, Shields G, Stewart J, DeLisi LE. Ten year longitudinal stud of neuropsychological functioning subsequent to a first episode of schizophrenia. Schizophr Res 1. 2005;78:27–34. doi: 10.1016/j.schres.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Hughes C, Kumari V, Das M, Zachariah E, Ettinger U, Sumich A, et al. Cognitive functioning in siblings discordant for schizophrenia. Acta Psychiatr Scand. 2005;111:185–192. doi: 10.1111/j.1600-0447.2004.00392.x. [DOI] [PubMed] [Google Scholar]
- Kaplan EF, Goodglass H, Weintraub S. Boston Naming Test. 2. San Antonio, Texas: The Psychological Corporation; 1983. [Google Scholar]
- Maziade M, Roy MA, Chagnon YC, Cliché D, Fournier JP, Montgrain N, et al. Shared and specific susceptibility loci for schizophrenia and bipolar disorder: a dense genome scan in Eastern Quebec families. Mol Psychiatry. 2005;10:486–499. doi: 10.1038/sj.mp.4001594. [DOI] [PubMed] [Google Scholar]
- Melendez C. Conquerors and habitants: the historic-social origins of Costa Ricans (Conquistadores y pabladores: origenes historico-sociales de los Costarricenses) Costa Rica: EUNED Press; 1982. pp. 105–159. [Printed in Spanish] [Google Scholar]
- Morrens M, Hulstijn W, Sabbe B. Psychomotor slowing in schizophrenia. Schizophr Bull. 2006;33:1038–1053. doi: 10.1093/schbul/sbl051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myles-Worsley M, Coon H, Tiobech J, Collier J, Dale P, Wender P, et al. Genetic epidemiological study of schizophrenia in Palau, Micronesia: prevalence and familiarity. Am J Med Genet. 1999;88:4–10. doi: 10.1002/(sici)1096-8628(19990205)88:1<4::aid-ajmg2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Myles-Worsley M, Ord LM, Ngiralmau H, Weaver S, Blailes F, Faraone SV. The Palau early psychosis study: neurocognitive functioning in high-risk adolescents. Schizophr Res. 2007;89:299–307. doi: 10.1016/j.schres.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Nemoto T, Mizuno M, Kashima H. Qualitative evaluation of divergent thinking in patients with schizophrenia. Behav Neurol. 2005;16:217–224. doi: 10.1155/2005/386932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Blehar MC, Kaurmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH genetics initiative. Arch Gen Psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. Discussion 863–864. [DOI] [PubMed] [Google Scholar]
- Peltonen L, Perola M, Naukkarinen J, Palotie A. Lessons from studying monogenic disease for common disease. Hum Mol Genet. 2006;15:R67–R74. doi: 10.1093/hmg/ddl060. [DOI] [PubMed] [Google Scholar]
- Perri B, Naplin NA, Carpenter GA. A Spanish auditory verbal learning and memory test. Assessment. 1995;2:245–253. [Google Scholar]
- Reitan RM, Wolfson D. The halstead–reitan neuropsychological test battery: therapy and clinical interpretation. Tucson, Arizona: Neuropsychological Press; 1985. [Google Scholar]
- Shedlack K, Lee G, Sakuma M, Xie SH, Kushner M, Pepple J. Language processing and memory in ill and well siblings from multiplex families affected with schizophrenia. Schizophr Res. 1997;25:43–52. doi: 10.1016/s0920-9964(97)00004-2. [DOI] [PubMed] [Google Scholar]
- Shi J, Badner JA, Gershon ES, Liu C. Allelic association of G72/G30 with schizophrenia and bipolar disorder: a comprehensive meta-analysis. Schizophr Res. 2007 doi: 10.1016/j.schres.2007.10.004. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A compendium of neuropsychological tests, 2nd edition. Administration, norms, and commentary. New York: Oxford University Press; 1998. [Google Scholar]
- Staal WG, Hijman R, Hulshoff Pol HE, Kahn RS. Neuropsychological dysfunctions in siblings discordant for schizophrenia. Psychiatry Res. 2000;95:227–235. doi: 10.1016/s0165-1781(00)00172-4. [DOI] [PubMed] [Google Scholar]
- Szoke A, Schurhoff F, Golmard JL, Alter C, Roy I, Meary A, et al. Familial resemblance for executive functions in families of schizophrenic and bipolar patients. Psychiatry Res. 2006;144:131–138. doi: 10.1016/j.psychres.2005.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szoke A, Schurhoff F, Mathieu F, Meary A, Ionescu S, Leboyer M. Tests of executive functions in first-degree relatives of schizophrenic patients: a meta-analysis. Psychol Med. 2005;35:771–782. doi: 10.1017/s0033291704003460. [DOI] [PubMed] [Google Scholar]
- Tiffen J. Purdue pegboard test. Chicago: Scientific Research Associates; 1968. [Google Scholar]
- Walss-Bass C, Liu W, Lew DF, Villegas R, Montero P, Dassori A, et al. A novel missense mutation in the transmembrane domain of neuregulin 1 is associated with schizophrenia. Biol Psychiatry. 2006;60:548–553. doi: 10.1016/j.biopsych.2006.03.017. [Epub 2006 May 30] [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale and Wechsler Memory Scale Technical Manual. 3. San Antonio, Texas: The Psychological Corporation; 1997. Spanish translation by Silvina Vizzini, 2002, authorized by The Psicologycal Corporation USA. Edited by PAIDOS, Buenos Aires, Barcelona y México. [Google Scholar]
- Wijsman EM, Rosenthal EA, Hall D, Blundell ML, Sobin C, Heath SC. Genome-wide scan in a large complex pedigree with predominantly male schizophrenics from the island of Kosrae: evidence for linkage to chromosome 2q. Mol Psychiatry. 2003;8:695–705. 643. doi: 10.1038/sj.mp.4001356. [DOI] [PubMed] [Google Scholar]