Abstract
Purpose
Determine the antitumor activity of the epidermal growth factor receptor (EGFR) inhibitor gefitinib in patients with recurrent/metastatic salivary gland cancer.
Methods
Phase II study in adenoid cystic (ACC) and non-adenoid cystic (non-ACC) carcinomas. Gefitinib was administered 250mg orally daily. Primary endpoint was tumor response. Secondary endpoints included progression-free survival (PFS), overall survival (OS) and disease control rates (DCR). EGFR and HER2 expression were evaluated and correlated with outcomes.
Results
Thirty-seven patients were enrolled, and 36 were evaluable (18 with ACC and 18 with non-ACC). No responses were observed. Median PFS was 4.3 months and 2.1 months, and median OS was 25.9 months and 16 months for patients with ACC and non-ACC respectively. DCR at 8 weeks was higher in ACC patients. No unexpected toxicities occurred. EGFR and HER2 overexpression did not correlate with outcomes.
Conclusions
We did not observe significant clinical activity of gefitinib in advanced salivary gland cancer. NCT00509002.
Keywords: salivary gland cancer, adenoid cystic carcinoma, non-adenoid cystic carcinoma, gefitinib, response to therapy
INTRODUCTION
Salivary malignancies represent 5% of all head and neck cancers and are diverse with respect to origin and pathology. The parotid gland is the most common major gland site of origin and the oral cavity the most common site for minor salivary gland primaries. The most frequent pathologic diagnoses are adenoid cystic carcinoma (ACC), adenocarcinoma, and mucoepidermoid carcinoma (MEC) (1,2). Primary treatment most often is surgical resection, followed by postoperative radiotherapy in patients at high risk for local recurrence (3). However, treatment strategies for patients with locally advanced disease not amenable to curative therapy or widespread metastases remain investigational.
The epidermal growth factor receptor (EGFR) is expressed in normal salivary glands and is likely involved in salivary tissue homeostasis (4). EGFR activation of downstream signaling leads to increased cell survival and proliferation. Preclinical work on ACC models has shown that EGFR inhibition can control tumor growth (5). Further, molecular studies suggest the EGFR may be a vulnerable tumor target in salivary gland cancer. Immunohistochemical studies have shown increased expression of EGFR in 85% of primary salivary ACC (4) and 100 % in primary MEC (6). Activating mutations in EGFR have been reported in 9 % of primary salivary duct carcinomas (7), and high EGF levels have been reported in 23 % and 70 % of primary ACC and MEC lesions, respectively (8). Collectively, these studies suggest that targeting EGFR family proteins in salivary gland carcinomas may be an effective approach for patients with local recurrent and metastatic disease. Herein, we report the results of a non-randomized, open label, single institutional, phase II study of the oral EGFR tyrosine kinase inhibitor gefitinib, in patients with unresectable local recurrent and metastatic salivary cancer.
PATIENTS AND METHODS
Patients
Eligible patients were 18 years or older, with pathologically confirmed recurrent and/or metastatic salivary gland cancer who were not candidates for curative surgery or radiation therapy, had an Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0 to 2, measurable disease by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0 (9), and adequate organ bone marrow, kidney, and liver function. Documented progression of disease by radiographs was not an eligibility criterion for study entry. Prior treatment with EGFR-targeted agents was not permitted, though patients may have had unlimited number of prior systemic therapies. Patient were excluded if pregnant or breastfeeding, had clinically evident interstitial lung disease, or another uncontrolled systemic disease. In addition, the concurrent use CYP3A4 inducers, was not permitted among patients enrolled in the trial.
Pre-study evaluations included history and physical examination, assessment of PS, complete blood count, and chemical profile within 2 weeks prior to study entry. Patients had to have computed tomography (CT) or magnetic resonance imaging (MRI) within 4 weeks prior to study entry.
Study design
This was an open label non-randomized phase II study to evaluate gefitinib monotherapy in patients with unresectable local recurrent or metastatic salivary cancer. Two separate cohorts were included: ACC and non-ACC. The primary endpoint was tumor response (RR) defined as complete response (CR) or partial response (PR). Secondary endpoints included progression-free survival (PFS) and overall survival (OS) disease control rate (DCR) at 8 weeks, duration of stable disease (SD) and safety. Exploratory objectives included evaluation of the EGFR and HER2 expression in tumor tissues and their correlation with probability of response. Enrolled patients were treated with gefitinib 250mg orally once daily in 4-week cycles. Treatment continued until objective evidence of disease progression or intolerable drug related toxicity. Radiographic evaluations for response or stabilization by RECIST were performed every eight weeks by CT or MRI. In patients with demonstrated disease progression by clinical or radiographic assessment, gefitinib was discontinued and the patients were removed from study.
The clinical trial was reviewed yearly and approved by the University of Texas, MD Anderson Cancer Center Institutional Review Board. All patients were required to sign written informed consent before study entry.
Study therapy and dose modifications
Study treatment consisted of gefitinib 250mg once daily in the morning without food. Gefitinib was supplied by Astra Zeneca (Wilmington, DE). Cycle length was 4 weeks and treatment was continued until disease progression, unacceptable toxicity, patient refusal, or physician’s decision to withdraw the patient. Toxicity was graded according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 2.0. If toxicity occurred, the appropriate treatment was used to ameliorate signs and symptoms. If grade 3 or 4 toxicity occurred, drug was held until the toxicity was grade 1 or less. If grade 3 or 4 toxicity recurred, continuation of treatment was discussed between the trial physician and the patient’s physician with a decision made after consideration of the relative risks and benefits to the patient.
EGFR and HER2 assessment
EGFR and HER2 expressions were measured by immunohistochemistry (IHC) on archieved tumor tissues. IHC analysis was done using the automated BOND MAX immunohistochemistry stainer by Vision BioSystems (Norwell, MA) on 4-μm paraffin sections of the tumor tissue. In brief, following dewaxing, washing, and rehydration of the slides through xylene and graded alcohols, Tris-EDTA buffer was used for antigen retrieval. Slides were subsequently treated with 3% hydrogen peroxide. Following incubation with the primary antibodies, HER2 (clone e2-4001, mouse, 1:300), (LabVision, Kalamazoo, MI) and EGFR (clone 31G7, mouse, 1:50), (Zymed/Invitrogen, Carlsbad, CA), secondary conjugate antibody was applied. Each specimen-containing slide was developed using the chromogen 3,3′-diaminobenzidine and counterstained with hematoxylin. Scoring of expression was performed blindly and independent of the clinical outcomes. The intensity of the membranous expression was recorded as: 3+, strong in more than 50%; 2+, intermediate, interrupted and incomplete membranes and heterogenous cytoplasmic staining in >10%; 1+, weak cytoplasmic staining of tumor cells in >10%; 0, negative staining.
Statistical Analysis
Patients were placed in one of two groups based on histological type: ACC or non-ACC. The aim was to detect a response rate of 15 % or greater in each histological group. The Gehan’s design model was used to determine whether to continue or terminate accrual after first stage (10). This design required accrual of 19 patients first to each group (ACC and non-ACC), and if no objective responses (partial and complete) were observed in the first 19 patients, accrual was discontinued and the treatment deemed ineffective. If one or more responses were observed, 21 more patients in each subgroup would be enrolled with a goal of 40 patients per subgroup. Progression free survival (PFS) time was calculated from treatment initiation date to progression date or the death date. Overall survival (OS) time was calculated from treatment initiation date to death date or the last follow-up date (censored). Median PFS time and OS time were estimated using the Kaplan-Meier method and compared between patients’ characteristics groups using log-rank test. Disease control rate (DCR) was defined as the proportion of patients who did not meet RECIST criteria for disease progression at or before the first follow-up imaging at 8 weeks. A toxicity frequency table was constructed to summarize the adverse events. Response was evaluated in all patients who received at least one cycle of gefitinib. Patients who received at least one dose of gefitinib were included in the safety analysis. EGFR and HER2 expression was completed. Tumors with 2+ or 3+ expression were considered positive.
RESULTS
Patients and Treatment
Between June 2004 and May 2007, 19 patients with ACC and 18 patients with non-ACC were enrolled at MD Anderson. Of the 37 patients enrolled onto the study, one ACC patient never received study drug therefore was excluded from the analysis and two patients (one ACC and one non-ACC) received therapy but had rapid disease progression. Therefore, 34 were eligible for response assessment, and 36 patients were eligible for OS, PFS and toxicity assessment. One non- ACC patient had no measurable disease. Patient characteristics are listed in Table 1. Median age was 50 years for the ACC patients and 59 years for the non-ACC patients. Most patients with ACC were male (72%), and all patients but one had an ECOG PS of 0 or 1. Non-ACC included multiple histologies including acinic cell carcinoma, mucoepidermoid carcinoma, myoepithelial carcinoma. Tumors were located mostly in the parotid glands (72%). There was one patient that presented with an unknown primary metastatic to the lymph nodes. All patients received prior treatment. Most patients underwent surgical resection and or radiation therapy in both histological groups. More than half of the patients receive prior chemotherapy. The median number of prior chemotherapy regimens were 3 (range 1–5) and 2 (range 1–6) for the patients with ACC and non-ACC respectively.
TABLE 1.
Patient characteristics for ACC and non-ACC cohorts
| Characteristic | ACC | Non-ACC |
|---|---|---|
| No(%) | No(%) | |
| 18 (100%) | 18 (100%) | |
|
| ||
| Age | ||
| Median | 50 | 59 |
| Range | (26–80) | (29–76) |
|
| ||
| Gender | ||
| Male | 13 (72%) | 10 (56%) |
| Female | 5 (28%) | 8 (44%) |
|
| ||
| Performance status | ||
| ECOG 0–1 | 17 (94%) | 18 (100%) |
| ECOG2 | 1 (6%) | 0 (0%) |
|
| ||
| Histology | ||
| Adenoid cystic carcinoma | 18 (100%) | - |
| Acinic cell carcinoma | - | 2 (11%) |
| High grade adenocarcinoma | - | 6 (33%) |
| Mucoepidermoid | - | 2 (11%) |
| Myoepithelial carcinoma | - | 1 (6%) |
| Salivary duct carcinoma | - | 3 (17%) |
| Salivary adenocarcinoma | - | 3 (17%) |
|
| ||
| Primary site | ||
| Parotid | 2 (11%) | 11 (61%) |
| Sublingual | 2 (11%) | 1 (6%) |
| Submandibular | 1 (6%) | 2 (11%) |
| Buccal mucosa | - | 1 (6%) |
| Tongue | - | 2 (11%) |
| Gingiva | 1 (6%) | - |
| Floor of the mouth | 1 (6%) | - |
| Sinuses | 2 (11%) | - |
| Trachea | 2 (11%) | - |
| Lung | 2 (11%) | - |
| Palate | 4 (22%) | - |
| Skin of ear | 1 (6%) | - |
| Unknown Primary Site | - | 1 (6%) |
|
| ||
| Previous Systemic Therapies, | ||
| Yes | 6 (33%) | 10 (56%) |
| No | 12 (67%) | 8 (44%) |
| Median (range) | 3 (1–5) | 2 (1–6) |
|
| ||
| Prior Surgery | ||
| Yes | 17 (94%) | 15 (83%) |
| No | 1 (6%) | 3 (17%) |
|
| ||
| Prior Radiation Therapy | ||
| Yes | 15 (83%) | 13 (72%) |
| No | 3 (17%) | 5 (28%) |
|
| ||
| EGFR Status* | ||
| Positive (2+ or 3+) | 6 (38%) | 4 (24%) |
| Negative (0 or 1+) | 10 (63%) | 13 (76%) |
|
| ||
| HER2 Status* | ||
| Positive (2+ or 3+) | 0 (0%) | 9 (50%) |
| Negative (0 or 1+) | 16 (100%) | 9 (50%) |
Tissue available for EGFR in 16 ACC and 17 non-ACC, and for HER2 in 16 ACC and 18 non-ACC
Tissue was available for EGFR staining in 16 ACC and 17 non-ACC, and for HER2 staining in 16 ACC and 18 non-ACC. EGFR staining was positive in 38% of ACC and of 24% non-ACC. As expected, none of the ACC were HER2-positive; however 50% of non-ACC stained positive for HER2.
Response and Survival Outcomes
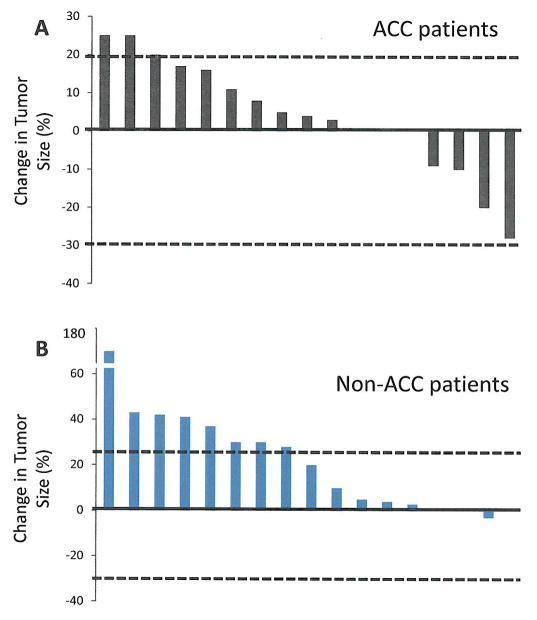
Although there were no objective responses, a group of patients had reduction on their measurable disease. Figure 1 summarizes the maximum percentage reduction of target lesions in patients by histological type.
FIGURE 1.
Maximal percentage of tumor reduction for target lesions by Response Evaluation Criteria in Solid Tumors Committee (RECIST). Percentages are calculated using the summed unidimensional measurements of target lesions per RECIST.
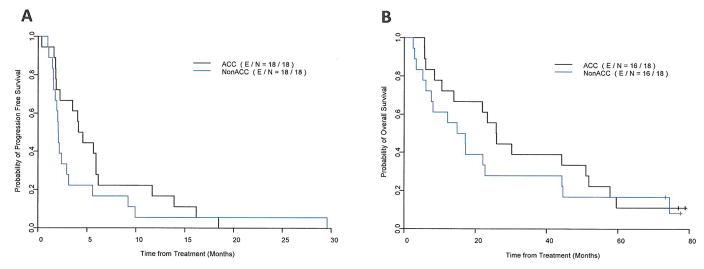
Thirty-six patients are included in PFS and OS analysis. At a median follow up of 77.3 months (range 73 – 79 months), 32 patients have died, 16 in the ACC group, and 16 in the non-ACC group; 33 patients had disease progression, 16 in the ACC group, and 17 in the non-ACC group; and 3 patients died without progression;. Median PFS time was 4.3 months (95%CI: 2.2–11.7 months) for the patients with ACC, and 2.1 months (95%CI: 1.7–5.6 months) for the patients with non-ACC. Median OS time was 25.9 months (95%CI: 13.9–57.8 months) for the patients with ACC, and 16.0 months (95%CI: 7.6–44.6 months) for the patients with non-ACC. Figure 2 shows the Kaplan-Meier survival curves for PFS and OS by histology. Disease control rates (DCR) at 8 weeks, were higher for the patients with ACC (82%) compared to patients with non-ACC (35%). In each histologic group there were patients with prolonged disease stabilization of more than nine months, seven in the ACC group, and 4 in the non-ACC group. There were two patients with stable disease for over 5 years, a patient with adenocarcinoma of the parotid (63 months), and a patient with a lung ACC (87 months).
FIGURE 2.
Progression-free survival (A) and overall survival (B) for patients with adenoid cystic carcinoma (ACC) and non-ACC malignant salivary gland tumors treated with gefitinib.
Table 2 summarizes the PFS and OS estimates, and DCR by histology and EGFR and HER2 expression. There was no clear association between PFS, OS or DCR at 8 weeks and EGFR or HER2 expression. There was no correlation between EGFR or HER2 overexpression and outcomes even in the patients with long stabilization of their disease.
TABLE 2.
Progression-free survival, overall survival, and disease control rates by histology and EGFR and HER2 expression
| ACC (N=18) | Non-ACC (N=18) | |
|---|---|---|
|
| ||
| Median progression-free survival (95% CI) | ||
| All | 4.4 mo (2.2–11.7 mo) | 2.1 mo (1.7–5.6 mo) |
| EGFR-positive | 6.0 mo (3.5-NR mo) | 2.1 mo (1.1-NR mo) |
| EGFR-negative | 4.3 mo (1.8-NR mo) | 2.1 mo (1.7-NR mo) |
| HER2-positive | 2.0 mo (1.6-NR mo) | |
| HER2-negative | 5.1 mo (2.2–13.9 mo) | 2.1 mo (1.9-NR mo) |
|
| ||
| Median overall survival (95% CI) | ||
| All | 25.9 (13.9, 57.8) (n=18) | 16.0 mo (7.6–44.6 mo) |
| EGFR-positive | 18.6 (10.5, NR) (n=6) | 30.8 mo (3.4-NR mo) |
| EGFR-negative | 37.2 (25.9, NR) (n=10) | 14.9 mo (7.6-NR mo) |
| HER2-positive | 17.2 mo (5.2-NR mo) | |
| HER2-negative | 28.1 (22.0, 59.5) (n=16) | 12.2 mo (7.6-NR mo) |
|
| ||
| Disease control rates * | ||
| All | 14/17(82% ) | 6/17(35%) |
| EGFR-positive | 5/5(100%) | 1/3(33%) |
| EGFR-negative | 8/10 (80%) | 5/13(38%) |
| HER2-positive | 3/9(33%) | |
| HER2-negative | 13/15(87%) | 3/8(38%) |
Estimated from patients evaluable for response.
ACC: adenoid cystic carcinoma; non ACC: non-adenoid cystic carcinoma; NR: Not reached.
Drug delivery and safety
Patients with ACC received a median of 4.9 cycles (range: 0.7 to 17.5) of treatment, and patients with non-ACC patients received a median of 2.3 cycles (range: 0.5 to 16.4). Two ACC patients did not complete one cycle (4 weeks) of gefitinib, precluding assessment for response to the study medication. Two patients discontinued gefitinib secondary to toxicities attributed to the study drug. One patient experienced grade 3 emesis, which prompted hospitalization and the initiation of parenteral nutrition and discontinuation of the study medication. Another patient discontinued the study drug because of unacceptable folliculitis (grade 2) after ten cycles. A third patient self-discontinued gefitinib for unknown reasons after completing 12 months of treatment and did not have documented progression until 19 months after initiation of therapy.
Gefitinib was tolerated well by most patients (Table 3) with the majority of adverse events being grade 1 or 2. The most common reported events included diarrhea (78%), rash (78%), fatigue (41%), anorexia (24%), and nausea (11%). All serious adverse events were considered unrelated to gefitinib therapy. They included a fatal mycocardial infarction, and the development of second malignancies in two patients, one mycosis fungoides and one ovarian cancer. In these two patients gefitinib was discontinued prior to diagnosis of their new cancer. Two patients required hospitalization while on study drug for aspiration pneumonia and hemoptysis due to rapid disease progression.
TABLE 3.
Toxicities experienced by patients on study attributable to gefitinib
| Toxicity | Grade 1 | Grade 2 | Grade 3 | All Grades |
|---|---|---|---|---|
|
| ||||
| Abdominal cramps | 2 (5%) | 2 (5%) | ||
| Anorexia | 9 (24%) | 9 (24%) | ||
| Diarrhea | 28 (76%) | 1 (3%) | 29 (78%) | |
| Dizziness | 2 (5%) | 2 (5%) | ||
| Dry Eyes | 1 (3%) | 1 (3%) | ||
| Dyspepsia | 3 (8%) | 3 (8%) | ||
| Dyspnea | 1 (3%) | 1 (3%) | ||
| Emesis | 1 (3%) | 1 (3%) | 2 (5%) | |
| Fatigue | 12 (32%) | 3 (8%) | 15(41%) | |
| Nausea | 4 (11%) | 4 (11%) | ||
| Nail changes | 2 (5%) | 2 (5%) | ||
| Rash/Folliculitis | 22 (59%) | 7 (19%) | 29 (78%) | |
DISCUSSION
This phase II single agent study did not meet its primary endpoint as there were no objective responses with single agent gefitinib in patients with advanced salivary gland cancer in either histologic group. As expected, the median PFS, OS, and DCR were better in the ACC group compared with the non-ACC group. Although not a protocol specific eligibility criterion, all patients had radiographic evidence of tumor progression prior to enrollment. Interestingly, in each histologic group there were patients with prolonged disease stabilization of more than nine months, seven in the ACC group, and four in the non-ACC group. The median PFS and OS were 4.6 and 26 months for the ACC group and 2.1 and 17.1 months for the non-ACC group. These results are consistent with those reported in a number of phase II trials with different molecularly directed agents in patients with ACC or a mixture of ACC and non-ACC. Salivary cancer is a heterogeneous disease with a number of different potential therapeutic targets. Both EGFR and HER2 are overexpressed in carcinomas of the salivary glands. A phase II trial of single agent cetuximab in patients with ACC (n=23) or non-ACC (n=7) reported no responses, but stable disease rates of 87% (n=20) and 57% (n=4) with a combined time to tumor progression (TTP) of approximately 6 months (11). In a biomarker based clinical trial, single agent lapatinib therapy was evaluated in patients with salivary cancers. Patients with EGFR or HER2 expressing ACC and non-ACC received single agent lapatinib with no responses reported. For all patients the PFS was 15.8 months with a median OS of 13.8 months, 6-month OS rate of 69.3%, and median PFS of 2.1 months for the non-ACC group. The ACC group achieved a median PFS of 3.5 months with 6-month OS of 90% although median OS was not reached (12). Trastuzumab has also been evaluated in patients with salivary tumors overexpressing HER2. In a phase II trial of 14 treated patients, the median TTP was 4.2 months and there was one response in a patient with mucoepidermoid cancer. This trial closed after screening demonstrated that HER2 overexpression was relatively uncommon (17%) (13,14). Most cases of ACC express the c-kit protein, therefore, imatinib has been evaluated in a number of single arm phase II clinical trials as a single agent or in combination with chemotherapy (15–17) including a single arm trial evaluating the efficacy of imatinib in patients with c-kit expressing ACC. Despite biomarker-based patient selection there were no responses to single agent imatinib. Median survival was 30 weeks and median PFS was 10 weeks (15). In addition to c-Kit, there is high expression of vascular endothelial growth factor (VEGF) in ACC which is associated with biologically aggressive behavior (18). A multicenter phase II trial of sunitinb in patients with recurrent and/or metastatic ACC of the salivary glands enrolled 14 patients. There were no responses reported and the TTP was 7.2 months with a 6-month TTP of 57% (19).
Single agent gefitinib proved to be well tolerated and no unexpected safety signals were observed in this patient population. The type and rates of adverse events were similar to that reported with gefitinib in other solid tumors including lung cancer (20).
There is overexpression of EGFR and HER2 in carcinomas of the salivary glands. In our cohorts, EGFR staining was positive in 38% of ACC and of 24% non-ACC. As expected, none of the ACC were HER2-positive; however 50% of non-ACC stained positive for HER2. However EGFR and HER2 overexpression did not correlate with outcomes. Fluorescence in situ hybridization (FISH) analysis for both EGFR and HER2 gene amplification was performed in the archival tumor specimens of 20 patients with ACC and 17 patients with non-ACC, treated with lapatinib in a clinical trial (12). There were no EGFR or HER2 amplifications detected in ACC. For non-ACC, no EGFR gene amplifications were detected, but 3 tumors had HER2 amplified tumors and all had a 3+ staining for both EGFR and HER2 by IHC. Two of these patients had time-to-progression of 8.3 months and 18.4 months, the longest clinical benefit. However, as an interesting finding, patients with a low or a high HER2/chromosome-specific centromeric enumeration probe (CEP) 17 ratio had a longer time to progression compared with those with moderate ratios (21). Activating EGFR mutations are well described in lung cancer and have been reported in cancers of the salivary glands. Tissue samples taken from 25 patients with salivary gland cancers were evaluated for the presence of exon 19 and exon 21 L858R point mutations. No mutations in exon 21 were found though two exon 19 deletions were found; one in a patient with ACC and the 2nd in a patient with mucoepidermoid cancer. This observation had not been reported at the time our protocol was written and unfortunately we did not have enough DNA to perform the mutational testing after completing the planned biomarker evaluations (22). Improving outcomes for patients with salivary gland cancers will require novel approaches in terms of therapeutics and clinical trial design. Continued work toward the development of predictive biomarkers/signatures to better select treatments for patients is needed to improve outcomes in this disease. With the access of new technologies including next generation sequencing, comprehensive molecular assessments of these tumors will be essential to identify effective therapies and drive pathway specific clinical trial design.
CONCLUSION
In this study we aimed to determine the antitumor activity of the EGFR inhibitor gefitinib in patients with recurrent/metastatic salivary gland cancer. Patients with ACC and non-ACC were treated. Although the drug was well tolerated, there were no responses, however, there were prolonged SD in some patients. EGFR and HER2 overexpression did not correlate with outcomes.
Acknowledgments
This study was supported by Astra-Zeneca. Institutional grant, IRUSIRE0198.
Footnotes
Presented in part at the American Society for Clinical Oncology, Orlando, Florida, May 13–17, 2005
References
- 1.Adelstein DJ, Rodriguez CP. What is new in the management of salivary gland cancers? Curr Opin Oncol. 2011;23:249–53. doi: 10.1097/CCO.0b013e328344f59c. [DOI] [PubMed] [Google Scholar]
- 2.Speight PM, Barrett AW. Salivary gland tumours. Oral Dis. 2002;8(5):229–40. doi: 10.1034/j.1601-0825.2002.02870.x. [DOI] [PubMed] [Google Scholar]
- 3.NCCN. National Comprehensive Cancer Network v2.2012 ed. 2012. [Accessed 11/6/212]. Head and Neck Cancers: Salivary Gland Cancers. [Google Scholar]
- 4.Vered M, Braunstein E, Buchner A. Immunohistochemical study of epidermal growth factor receptor in adenoid cystic carcinoma of salivary gland origin. Head Neck. 2002;24:632–6. doi: 10.1002/hed.10104. [DOI] [PubMed] [Google Scholar]
- 5.Choi S, Sano D, Cheung M, Zhao M, Jasser SA, Ryan AJ, Mao L, Chen WT, El-Naggar AK, Myers JN. Vandetanib inhibits growth of adenoid cystic carcinoma in an orthotopic nude mouse model. Clin Cancer Res. 2008;14:5081–9. doi: 10.1158/1078-0432.CCR-08-0245. [DOI] [PubMed] [Google Scholar]
- 6.Gibbons MD, Manne U, Carroll WR, Peters GE, Weiss HL, Grizzle WE. Molecular differences in mucoepidermoid carcinoma and adenoid cystic carcinoma of the major salivary glands. Laryngoscope. 2001;111:1373–8. doi: 10.1097/00005537-200108000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Williams MD, Roberts DB, Kies MS, Mao L, Weber RS, El-Naggar AK. Genetic and expression analysis of HER-2 and EGFR genes in salivary duct carcinoma: empirical and therapeutic significance. Clin Cancer Res. 2010;16:2266–74. doi: 10.1158/1078-0432.CCR-09-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ito FA, Ito K, Coletta RD, Graner E, de Almeida OP, Lopes MA. Salivary gland tumors: immunohistochemical study of EGF, EGFR, ErbB-2, FAS and Ki-67. Anal Quant Cytol Histol. 2009;31:280–7. [PubMed] [Google Scholar]
- 9.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2:205–216. doi: 10.1093/jnci/92.3.205. 200. [DOI] [PubMed] [Google Scholar]
- 10.Kramar A, Potvin D, Hill C. Multistage designs for phase II clinical trials: statistical issues in cancer research. Br J Cancer. 1996;74:1317–20. doi: 10.1038/bjc.1996.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Locati LD, Bossi P, Perrone F, et al. Cetuximab in recurrent and/or metastatic salivary gland carcinomas: A phase II study. Oral Oncol. 2009;45:574–8. doi: 10.1016/j.oraloncology.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Agulnik M, Cohen EW, Cohen RB, et al. Phase II study of lapatinib in recurrent or metastatic epidermal growth factor receptor and/or erbB2 expressing adenoid cystic carcinoma and non adenoid cystic carcinoma malignant tumors of the salivary glands. J Clin Oncol. 2007;25:3978–84. doi: 10.1200/JCO.2007.11.8612. [DOI] [PubMed] [Google Scholar]
- 13.Haddad R, Colevas AD, Krane JF, et al. Herceptin in patients with advanced or metastatic salivary gland carcinomas. A phase II study. Oral Oncol. 2003;39:724–7. doi: 10.1016/s1368-8375(03)00097-6. [DOI] [PubMed] [Google Scholar]
- 14.Glisson B, Colevas AD, Haddad R, et al. HER2 Expression in Salivary Gland Carcinomas: Dependence on Histological Subtype. Clin Cancer Res. 2004;10:944–946. doi: 10.1158/1078-0432.ccr-03-0253. [DOI] [PubMed] [Google Scholar]
- 15.Hotte SJ, Winquist EW, Lamont E, et al. Imatinib mesylate in patients with adenoid cystic cancers of the salivary glands expressing c-kit: a Princess Margaret Hospital phase II consortium study. J Clin Oncol. 2005;23:585–90. doi: 10.1200/JCO.2005.06.125. [DOI] [PubMed] [Google Scholar]
- 16.Pfeffer MA, Talmi Y, Catane R, et al. A phase II study of imatinib for advanced adenoid cystic carcinoma of head and neck salivary glands. Oral Oncol. 2007;43:33–6. doi: 10.1016/j.oraloncology.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 17.Ghosal N, Mais K, Shenjere P, et al. Phase II study of cisplatin and imatinib in advanced salivary adenoid cystic carcinoma. British J Maxillofacial Surg. 2011;49:510–5. doi: 10.1016/j.bjoms.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, Peng B, Chen X, et al. Expressions on nuclear factor kappaB, induceble nitic oxide synthase and vascular endothelial growth factor in adenoid cystic carcinoma of salivary glands: correlations with the angiogenesis and clincal outcome. Clin Cancer Res. 2005;11:7334–43. doi: 10.1158/1078-0432.CCR-05-0241. [DOI] [PubMed] [Google Scholar]
- 19.Chau NG, Hotte SJ, Chen EX, et al. A phase II study of sunitinib in recurrent and/or metastatic adenoid cystic carcinoma (ACC) of the salivary glands: current progress and challenges in evaluating moleculrly targeted agents in ACC. Ann Oncol. 2012;23:1562–70. doi: 10.1093/annonc/mdr522. [DOI] [PubMed] [Google Scholar]
- 20.Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet. 2008;372:1809–18. doi: 10.1016/S0140-6736(08)61758-4. [DOI] [PubMed] [Google Scholar]
- 21.Vidal L, Tsao MS, Pond GR, et al. Fluorescence in situ hybridization gene amplification analysis of EGFR and HER2 in patients with malignant salivary gland tumors treated with lapatinib. Head Neck. 2009:1006–12. doi: 10.1002/hed.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dahse R, Kosmehl H. Detection of drug-sensitizing EGFR exon 19 deletion mutations in salivary gland carcinoma. Br J Cancer. 2008;99:90–2. doi: 10.1038/sj.bjc.6604430. [DOI] [PMC free article] [PubMed] [Google Scholar]