Abstract
Upon pathogen encounter, naïve CD8+ T cells are primed and undergo massive clonal expansion. A fraction of effector CD8+ T cells remains during the contraction phase and differentiate into memory T cells critical for mounting robust recall responses in response to secondary infection. Low frequency of memory T cells in vivo is a major obstacle to investigate their functional aspects including migration capacity and genetic regulation. Here, we describe detailed protocol for memory T cell differentiation developed by von Andrian’s group to generate large number of CD44hiCD62Lhi antigen-specific memory T cells in vitro.
Materials and Reagents
Recombinant mouse IL-15 (rmIL15) (BioLegend, catalog number: 566302)
RPMI-1640 medium (Life Technologies, Gibco®, catalog number: 11875-119)
Fetal bovine serum (Atlanta Biologicals, catalog number: S11055H)
Penicillin/streptomycin (Gemini Bio-Products, catalog number: F52M00E)
L-Glutamine (Life Technologies, Gibco®, catalog number: 25030-081)
100x 1 M Hepes (Life Technologies, Gibco®, catalog number: 15630-080)
100x MEM Non-essential amino acids (Life Technologies, Gibco®, catalog number: 11140-050)
100x sodium pyruvate (100 mM) (Life Technologies, Gibco®, catalog number: 11360-070)
100x 2-mercaptoethanol (Life Technologies, Gibco®, catalog number: 21985-023)
OVA257-264 synthetic peptide (Sigma-Aldrich, catalog number: S7951)
Ficoll-Paque™ Premium 1.084 (GE Healthcare, catalog number: 17-5446-02)
-
Antibodies:
Anti-CD44 PerCpCy5.5 (clone: IM7) (eBioscience, catalog number: 45-0441)
Anti-CD62L APC (clone: MEL-14) (eBioscience, catalog number: 17-0621)
RBC lysis buffer (eBioscience, catalog number: 00-4333-57)
Bovine serum albumin (Thermo Fisher Scientific, catalog number: BP1605-100)
NaN3 (Sigma-Aldrich, catalog number: S8032)
T cell media (see Recipes)
Staining buffer (in PBS) (see Recipes)
Equipment
Centrifuge (Thermo Fischer Scientific, Sorvall™ Legend RT)
70 μm cell strainer (BD Biosciences, Falcon®, catalog number: 352350)
15 ml and 50ml Falcon tubes
24 well plates (BD Biosciences, Falcon®, catalog number: 353226)
T75 culture flask (Corning, catalog number: 430641)
37 °C 5% CO2 Cell Culture incubator
Procedure
A. CD44hiCD62Llo Memory T cell differentiation proceeds under sterile tissue culture conditions
-
1
Euthanize a OT-1 CD8 TCR transgenic mouse and take spleen, and (optional) lymph nodes.
-
2
Splenocytes are RBC lysed followed by washing with PBS twice.
B. OT-1 TCR stimulation with cognate peptide antigen
-
3
Resuspend cells in 1 ml of T cell media and add OVA257-264 synthetic peptide to 1 μM.
-
4
Incubate in the 5% CO2 at 37 °C for 1 h.
-
5
Spin down cells at 1,500 rpm for 3 min at 4 °C and wash once with T cell media.
-
6
Resuspend cells in 12 ml of T cell media and plate 1ml/well of a 24 well plate.
-
7
Incubate in the 5% CO2 at 37 °C for 2 days.
-
8
Harvest the cells by pipetting up and down, and pellet cells.
-
9
Resuspend cells in 5 ml of T cell media, and load on to 2.5 ml of Ficoll.
-
10
Spin down at 400 x g for 15 min at 4 °C.
-
11
Transfer live cells on the interphase to a new 15 ml tube and fill up the tube with T cell media.
-
12
Spin down cells at 1,500 rpm for 3 min at 4 °C.
C. Memory T cell culture in the presence of IL-15
-
13
Resuspend cells in 24 ml of T cell media containing rmIL15 (20 ng/ml). Culture cells in T75 flask for four days.
-
14
Harvest and pellet cells for Ficoll gradient (repeat steps 9–12).
-
15
Resuspend cells in 40 ml of T cell media containing rmIL15 (20 ng/ml). Culture in T75 flask for two days.
-
16
Staining cells with anti-CD44 and CD62L antibodies in staining buffer for 15 min on ice.
-
17
Wash with staining buffer twice, then proceeds flow cytometry analysis.
Recipes
-
T cell media
RPMI-1640
10% fetal bovine serum
1% penicillin/streptomycin
1% L-Glutamine
1x 1 M Hepes
1x MEM non-essential amino acids
1x sodium pyruvate 100 mM
1x 2-mercaptoethanol
-
Staining buffer (in PBS)
1% BSA
0.02% NaN3
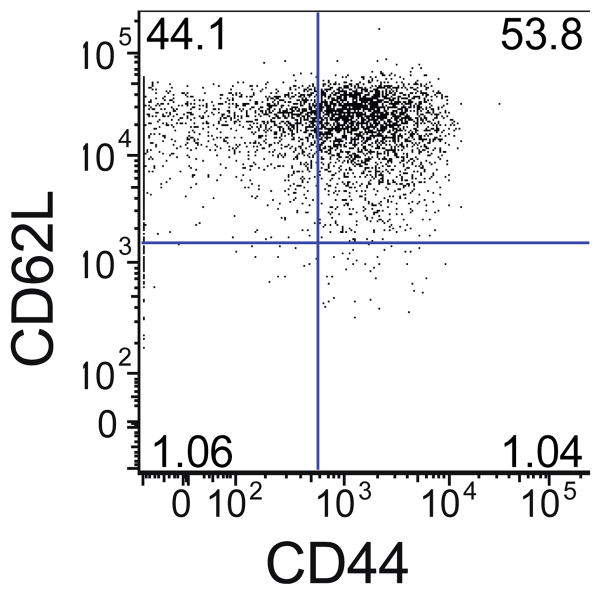
Figure 1.
CD44 and CD62L expression of differentiated memory T cells
Acknowledgments
The protocol was adapted from a previously described study (Manjunath et al., 2001). This work was supported by the Starr Cancer Consortium (13-A123 to M.O.L. and M.Q.Z.), the Rita Allen Foundation (M.O.L.), the NBRPC (2012CB316503 to M.Q.Z), and the NIH (HG001696 to M.Q.Z.).
References
- 1.Kim MV, Ouyang W, Liao W, Zhang MQ, Li MO. The transcription factor Foxo1 controls central-memory CD8+ T cell responses to infection. Immunity. 2013;39(2):286–297. doi: 10.1016/j.immuni.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manjunath N, Shankar P, Wan J, Weninger W, Crowley M, Hieshima K, Springer T, Fan X, Shen H, Lieberman J. Effector differentiation is not prerequisite for generation of memory cytotoxic T lymphocytes. J Clin Invest. 2001;108(6):871–878. doi: 10.1172/JCI13296. [DOI] [PMC free article] [PubMed] [Google Scholar]