Abstract
Introduction
Hypoglycemia occurs commonly in insulin requiring individuals with either Type 1 or Type 2 Diabetes.
Areas Covered
This article will review recent information on the pro-inflammatory and pro-atherothrombotic effects of hypoglycemia. Additionally, effects of hypoglycemia on arrhythmogenic potential and arterial endothelial dysfunction will be discussed. Effects of hypoglycemia on cardiovascular morbidity and mortality from large clinical studies in Type 1 and Type 2 DM will also be reviewed.
Expert Commentary
The relative and absolute risk of severe hypoglycemia leading to death and serious adverse events in both cardiovascular and other organ systems has been highlighted following the publication of recent large clinical trials focused on glucose control and outcomes. It would be helpful if future studies could develop broader end points to include minor and moderate hypoglycemia as well as more robust methods for capturing hypoglycemia contemporaneously with adverse events. In addition, perhaps consideration of including hypoglycemia as a primary outcome, may help identify the possible cause and effect of hypoglycemia on cardiovascular morbidity and mortality.
Keywords: Hypoglycemia, Inflammation, Atherothrombosis, Fibrinolytic Balance, Dysrhythmia, Diabetes
1. Introduction
Cardiovascular (CV) disease is the leading cause of death for patients with either Type 1 (T1DM) or Type 2 Diabetes Mellitus (T2DM).1 A substantial body of work indicates that insulin resistance, hypertension, obesity, dyslipidemia which are all associated with T1DM and/or T2DM play a role in the increased cardiovascular disease occurring in Diabetes.2,3 Recently, evidence suggests that hypoglycemia also contributes to the pathophysiology of CV disease in both T1DM and T2DM.4
Hypoglycemia is a common complication of patients with diabetes who receive insulin or insulin secretagogues. A prospective study reveals that over 85% of T1DM patients suffer at least one confirmed hypoglycemic episodes over 30 days.5 Multiple studies demonstrate that T1DM patients experience an average of 0.5 to 5 severe hypoglycemic events every year.6,7,8,9 Hypoglycemia is less frequent for T2DM patients, but T2DM patients treated intensively with insulin can also experience hypoglycemic episodes with a prevalence of ≥80%.10 The incidence of severe hypoglycemia in intensively treated Type 2 DM is less than T1DM but is still relatively common, approaching parity with T1DM individuals with disease duration less than 5 years and 50% with disease duration over 15 years.11,12 Severe hypoglycemia can occur in Type 2 DM following insulin secretagogue therapy.8 The risk is exacerbated in the elderly and may result in severe cardiovascular, neurologic sequelae and even death. Of note, recent observational trials suggest that HbA1c is not predictive of hypoglycemia. Emphasizing that severe hypoglycemia can and does occur at HBA1c levels representing moderate to frankly sub-optimal glucose control. 5,9 In addition to the association of severe cardiac events with hypoglycemia per se there is also evidence that certain sulfonylureas may increase the risk of cardiac disease by inhibiting ischemic preconditioning. (Please see reference for review of this topic.)13 Overall, due to the greater number of individuals with T2DM, the burden of hypoglycemia is actually greater in T2DM as compared to T1DM.14
Additionally, inpatient, ambulatory care and epidemiologic studies have reported the association of hypoglycemia with increased mortality.15,16 It should be noted that there is no evidential cause and effect that hypoglycemia was responsible for all the deaths reported in these studies. It is possible that hypoglycemia occurred because of accompanying severe underlying co-morbid condition(s) (e.g. organ failure) and was therefore a secondary phenomenon and not directly responsible for death but a marker of serious illness in a vulnerable population. Given the high incidence of CV mortality in patients with diabetes mellitus and the clinical frequency of hypoglycemia, this article aims to first review the pathophysiological changes caused by hypoglycemia that may lead to CV disease and then discuss the findings of multicenter trials that have investigated the effects of improved glucose control (with attendant hypoglycemia) on cardiovascular outcomes in DM patients.
2. Pathophysiology
Hypoglycemia causes a sympathetic nervous system response, altered t-wave morphology, an increased pro-coagulant state, inflammation, pro-atherothrombotic responses as well as endothelial dysfunction. All of which may explain the associative link between hypoglycemia and cardiovascular disease.
2.1 Sympathetic Nervous System Response
Hypoglycemia induces several homeostatic responses aimed at preventing and defending against a falling plasma glucose. Paramount in the acute defense against a falling glucose is the release of glucagon and catecholamines.17 In Type 1 DM individuals with duration longer than five years and severely insulin deficient Type 2 DM, the glucagon response to hypoglycemia is either lost or significantly reduced. Thus, the acute defense against a falling glucose occurs only with an intact epinephrine (sympathoadrenal) response. Of note, although both the duration of diabetes and improved metabolic control may reduce epinephrine responses to hypoglycemia, work by Amiel et al has demonstrated that rate of fall of plasma glucose does not affect catecholamine release in response to hypoglycemia.18 The release of catecholamines (both sympathoadrenal and sympathetic neural combined with direct sympathetic activation), in response to hypoglycemia, has profound hemodynamic changes such as increased myocardial contractility, stroke volume, and cardiac output. Collectively, these effects including the release of epinephrine and norepinephrine into the circulation increase cardiac workload and may worsen an already compromised heart in patients with coronary vessel disease, or those with existing impaired endothelial function and atherosclerotic disease.19,20 The sympathoadrenal response, which acts in a gluco-protective role to increase circulating glucose levels (by increasing glucose production by the liver, inhibiting glucose uptake by muscle and elevating lipolysis to raise NEFA levels which also restrict peripheral glucose utilization), can be paradoxically detrimental for diabetic patients who already deal with significant cardiovascular stress. Epinephrine can also prompt hypokalemia, which can induce fatal cardiac arrhythmias.21,22 There exists widespread evidence for a hypokalemic effect of catecholamines mediated through β receptors. Brown et al. infused epinephrine at substantial rates into normal subjects to reach concentrations of around 9 nmol/l, equivalent to those reached during acute myocardial infarction. These epinephrine levels lowered plasma potassium by around 0.8 mmol/l.23 In order to test the hypothesis that the sympathoadrenal response increases risk of fatal cardiac arrhythmias during severe hypoglycemia, Reno et al. infused adrenergic receptor blockers into rats during a hyperinsulinemic/severe hypoglycemic clamp. Severe hypoglycemia–induced mortality was 33% in control rats, while combined α/β-blocker infusion and β-blocker infusion alone completely prevented death.24 It was therefore concluded that the hypoglycemia-induced sympathoadrenal response, mediated primarily through β-adrenergic receptors, acts in a proarrythmic fashion. However, it should be noted that studies performed in animals using a profoundly deep model of hypoglycemia often below 1 mmol/L are not readily applicable to clinical situations.
2.2 T Wave Morphology and Hypoglycemia
A hypoglycemic state is known to affect cardiac electrical properties. QT interval is a commonly used measure of cardiac repolarization and predictor of arrhythmia risk and sudden death. Several studies have shown that hypoglycemia is associated with a significant lengthening of the corrected QT interval (QTc) in subjects with and without diabetes.25,26,27 QT prolongation can occur as a result of compromised cardiac autonomic regulation, increased catecholamine release during hypoglycemia, and lower serum potassium levels. Robinson et al. measured cardiac repolarization, specifically QTc, during experimental hypoglycemia with and without β-blockade and potassium infusion to uncover possible mechanisms.28 Their data confirmed that acute experimental hypoglycemia leads to abnormal cardiac repolarization with an increase of QTc from baseline of 75 ms; such an increase is able to cause severe cardiac arrhythmias in certain illnesses.29,30,31,32 This pronounced increase in QTc was reduced only by β-blockade rather than potassium replacement. Researchers thus concluded that sympathoadrenal activation is largely responsible for abnormal cardiac repolarization during hypoglycemia through a direct effect on the myocardium. While it has been shown that the episodes of severe hypoglycemia seen most notably in patients with T1D are independently associated with a lengthened QTc interval,33 it should be noted, however, that it was also suggested that hypoglycemia has only slight effects on QTc and that repolarization analyses have produced somewhat ambiguous results.34 It is also important to note that in clinical practice insulin levels and changes in potassium and catecholamines are much less than the above reported experimental models.35
2.3 Inflammation and endothelial dysfunction during hypoglycemia
The effects of acute hypoglycemia on the upregulation and release of inflammatory cytokines and vasoactive substances has been studied to elucidate possible mechanisms of hypoglycemia-induced vascular injury. There is increasing evidence that acute hypoglycemia induces a pro-inflammatory environment that may contribute to cardiovascular complications. During episodes of hypoglycemia, T1DM individuals had increases of both CD40 expression (a soluble marker of inflammation) on monocytes and plasma sCD40L concentrations.36 In addition pro-atheromatous biomarkers such as ICAM, VCAM, E-selectin and VEGF37,38 and pro-inflammatory cytokines, such as C-reactive protein, interleukin (IL)-6, IL-8 and tumor necrosis factor (TNF)-α have been shown to be elevated during acute hypoglycemia. The increase in inflammatory markers, resultant on acute hypoglycemia, may cause endothelial dysfunction and ultimately lead to vascular dysfunction due to pro-atherogenic actions of the cytokines.39,40 Recent studies suggest that endothelial function is impaired during hypoglycemia, and worsened by repeated episodes of hypoglycemia. Joy et al. demonstrated in healthy individuals that hyperinsulinemic-hypoglycemia, compared with hyperinsulinemic-euglycemia, reduced endogenous nitric oxide (NO)-mediated endothelial vasodilation, stimulated pro-inflammatory responses, diminished fibrinolytic balance, and increased pro-atherothrombotic mechanisms. Repeated episodes of hypoglycemia on two successive study days resulted in greater endothelial dysfunction by reducing both endogenous and exogenous NO-mediated vasodilation compared with a single episode of hypoglycemia.41 Additionally, Gimenéz M et al. demonstrated in T1DM patients that repeated hypoglycemic episodes are associated with flow-mediated endothelial dysfunction, increased intima-media thickness in the carotid and femoral arteries and increases of von Willebrand factor, fibrinogen, leukocyetes and ICAM-1.42 Furthermore, Ceriello et al. have reported that rebound hyperglycemia following hypoglycemia (which often occurs in clinical practice) produces even greater endothelial dysfunction and thrombosis activation in Type 1 DM and healthy individuals.43
These results demonstrate that both of the protective endothelial nitric oxide vasodilator mechanisms are reduced by hypoglycemia. Arterial vasodilation is an important in vivo homeostatic adaptive mechanism and lack of responsiveness is a risk factor for future arterial and cardiovascular disease. Arterial vasodilation can occur by activating arterial wall smooth muscle (endogenous nitric oxide pathways) or by exogenous nitric oxide (NO) donors activating vasodilator mechanisms. Hypoglycemia appears to be the only physiologic stress that can impair both NO mediated protective mechanisms. Additionally troubling is the finding that acute, moderate hypoglycemia of only 2.9 mmol/L can produce a pro-coagulant state in Type 1 DM, Type 2 DM and non-diabetic individuals. Acute hypoglycemia can produce increased platelet activation (as measured by increased p-selectin), diminished fibrinolytic balance (increased PAI-1) and elevated hemostatic mechanisms (increased thrombin-anti-thrombin complexes, von Willebrand factor.)31
Therefore, as can be determined from the above, hypoglycemia can activate multiple putative pathways that can result in adverse cardiovascular events. Hypoglycemia’s ability to increase pro-inflammatory, pro-atherothrombotic, pro-coagulant responses while reducing in-vivo endothelial function and increasing dysrhythmic potential makes it a potent and unique pathophysiologic cardiovascular stress.
3. “Dead in Bed” Syndrome
“Dead in bed” is a tragic consequence occurring in T1DM Patients in which death occurs overnight despite seeming well prior to sleeping. “Dead in Bed” is believed to account for up to 27% of all unexplained deaths in T1DM patients.”44 The exact mechanism for “Dead in Bed” syndrome has not been elucidated however it has been speculated that severe, nocturnal hypoglycemia leads to fatal cardiac arrhythmias in these patients. Supporting this, Bougneres et al performed hypoglycemic clamps in 16 adolescent patients with T1DM and demonstrated an average increase in QTc of 146ms correlated with increased heart rate (P=0.02) and urinary metanephrine levels (P=0.003) all of which have pro-arrhythmic potential.45 Reno et al explored the pathophysiology of this hypothesis in rats and found that mortality due to severe hypoglycemia was mediated by central neuroglycopenia which activated a vigorous sympathoadrenal response leading to cardiac arrhythmias, resultant cardiac failure and eventually respiratory arrest.20 Interestingly, the study also revealed that although diabetes per se worsened severe hypoglycemia-induced mortality, paradoxically recurrent antecedent hypoglycemia had a protective effect to mitigate the damaging effects of subsequent severe hypoglycemia.
Recently, post-mortem studies have deepened our understanding of the genetic factors underpinning “Dead in Bed” syndrome. A review of 22 cases in Australia identified 3 silent polymorphisms in the SNC5A gene- A29A, E1061E and D1819D and a protein-changing variant H558R as possible pathogenic mechanisms.46 Another post-mortem case from New Zealand presents an individual with T1DM who died suddenly at night but with a glucose level of 7 mmol/L- excluding hypoglycemia as a direct cause of the sudden death. The individual had a missense mutation c.370A>G in the GPD1L gene which is associated with the SCN5A ion channel. Mutations in this gene are associated with Brugada syndrome as well as sudden infant death syndrome.47
4. Multicenter Trials
Recently, a number of large multicenter trials have studied the effect of glucose control on cardiovascular outcomes. Rates of severe hypoglycemia were measured in these studies which allowed the risk association of hypoglycemia and the occurrence of severe cardiovascular events and death to be estimated.
4.1 DCCT/EDIC
The Diabetes Control and Complications Trial (DCCT) ran from 1983 to 1993 and sought to examine the effect of glycemic control on microvascular complications (retinopathy, nephropathy and neuropathy) in 1441 T1DM patients. Important secondary outcomes included CVD and quality of life. The study found significant beneficial effects of intensive treatment on reducing microvascular complications (retinopathy, nephropathy, and neuropathy) as well as a non-significant trend of cardiovascular disease reduction (P = 0.059). Additionally, the study found an increased prevalence of severe hypoglycemia and increased weight in the intensive treatment group.48 The subsequent EDIC follow-up study determined that after at least seven years with equivalent standard of care and identical glycemic control in the two groups, the overall risk of CVD decreased by 42 percent (P = 0.02) and the risk of nonfatal myocardial infarction, stroke or death from CVD decreased by 57% (P = 0.02.)49 At year 27 follow up, severe hypoglycemia was associated with increase mortality within the overall EDIC group. The previously intensively treated group did suffer increased severe hypoglycemia and thus may have been predicted to have an increased mortality risk compared to the conventionally treated group. Indeed, severe hypoglycemia did increase the risk of mortality in the intensive group but not enough to prevent an overall reduction in all-cause mortality (Hazard ratio = 0.67 95% CI, 0.46–0.99; P = .045) as well as fewer cardiovascular deaths when compared to the conventional group (9 vs 15).50 Taken together, these results reveal that a period of intensive glycemic control has beneficial long term effects on microvascular, macrovascular, and mortality outcomes in middle-aged T1DM patients that can outweigh the potential serious adverse events associated with severe hypoglycemia.
The DCCT/EDIC investigators studied the progression of Coronary Artery Calcification (CAC) as a marker of atherosclerotic coronary artery disease. Overall, there was less increase in CAC in the intensive group compared to the conventionally treated group.51 This was despite a significant increase of severe hypoglycemia occurring in the intensive group. Thus, this may appear to suggest that hypoglycemia does not result in increased coronary artery atherosclerosis. However, it should be noted that the number of patients with potentially clinically relevant CAC scores were relatively low, thus reducing the predictive power of the observation. Secondly, a more recent study by Fahrmann at al have determined that the role of severe hypoglycemia in intensively treated DCCT participants with an HBA1C of less than 7.5% was significantly associated with increased CAC score after adjusting for baseline albuminuria, smoking status, body mass index, blood pressure, LDC-cholesterol, diabetes duration, and DCCT-HBA1C.52 Thus, as discussed above, it would appear that severe hypoglycemia per se did increase the risk of cardiovascular disease in the DCCT/EDIC but was less of a risk as compared to the more traditional macrovascular cardiovascular risk factors.
4.2 UKPDS
The UK Prospective Diabetes study (UKPDS) was a multi-center study examining the effect of glycemic control on complications, morbidity and mortality in 5102 newly-diagnosed T2DM patients running from 1977 to 1997.53 The study found that intensive blood glucose control with an average HbA1c of 7.0% compared to a conventionally treated group (HbA1c 7.9%) resulted in primarily a decrease of microvascular complications (25% decrease in microvascular risk, 95% CI 7–40, p-0.0099). There was a very strong trend but no significant difference overall between the groups for macrovascular risk. Of note patients in the intensive group had significantly more hypoglycemic episodes (p<0.0001) as compared to the conventionally treated group.54 What role, if any, severe hypoglycemia could have played in reducing the benefits of intensive control on macrovascular disease is unknown and speculative.
Similar to the DCCT, a 10 year follow-up of the UKPDS indicated that the differences of HbA1c between the intensive and conventionally treated groups shrank as glycemic control stabilized after the first post study year. However, reductions of risks from diabetes related tissue complications persisted for 10 years in the intensively treated group; microvascular disease was reduced 24% (p=0.001), risk of myocardial infarction was reduced 15% (p=0.01), and there was a 13% decrease in overall death from any cause (p=0.007).55 Notably, the prevalence of severe hypoglycemia in the UKPDS follow-up study was reported to be very low, even in patients receiving sulfonylureas and insulin. Thus, it is possible that the low rates of hypoglycemia allowed the benefits of improved glycemic control on cardiovascular end points to be more fully demonstrated.
4.3 ACCORD
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) study examined the effect of intensive and standard therapy on a composite of severe cardiovascular events and death from any cause in 10,251 T2DM patients. These patients were 62.2±6.8 years old, had longer prior durations of DM (10 years) and more prior cardiovascular events (~35%) than patients in UKPDS and DCCT. The study was conducted from 2001 to 2008, when it was terminated prematurely due to a significantly increased risk of death in the intensively treated group (hazard ratio, 1.22; 95% CI, 1.01 to 1.46; p=0.04). Stable HbA1c’s of the intensively and conventionally treated groups obtained during the first year of the study were 6.4% and 7.5%, respectively. Severe hypoglycemic events were reported more frequently in the intensively vs. conventionally treated groups (830 vs 261 p<0.001) respectively.56
A retrospective epidemiological analysis of the ACCORD data examined the effect of severe hypoglycemia on mortality. The analysis reported that patients who experienced severe hypoglycemia had a greater risk of death than patients who did not experience severe hypoglycemia events, with unadjusted annual mortality rates of 2.8% for the intensively treated group who had one or more severe hypoglycemia events vs. 1.2% for patients with no severe hypoglycemia (HR 1.41; 95% CI 1.03 to 1.93). In the standard treated group, annual mortality was increased for patients who experienced severe hypoglycemia 3.7% vs 1.0% for patients without a severe hypoglycemia (HR 2.30, 95% CI 1.46 to 3.65). There was a relatively higher risk associated with adverse cardiac events and severe hypoglycemia in the standard controlled group compared to the intensive group.57 Also, patients with a higher A1c at baseline as well as patients in the intensive arm who were unable to achieve A1c < 7% were both at higher risk for severe hypoglycemia.58
Much work by the ACCORD study group has been performed to determine if severe hypoglycemia was the cause of the increased mortality in the intensively treated group. The group have now determined that severe hypoglycemia was not the cause for the increased mortality and thus early ending of the glucose control arm of ACCORD. However, the group have clearly demonstrated that severe hypoglycemia was increased with an accompanying elevated risk of death within both study arms. The risk of death following severe hypoglycemia was in fact higher in the standard treated group. Additionally, another important clinical point was that patients with poorer glycemic control had a greater risk of severe hypoglycemia as compared to metabolically well controlled individuals. In other words severe hypoglycemia does not only occur in intensively treated Type 2 DM patients or only in individuals with good glycemic control. Secondly, this point also identifies that severe hypoglycemia occurring in an individual with poorer glycemic control can be more devastating leading to an increased risk of serious cardiac events and death, as compared to an intensively treated type 2 DM individual. As an explanation, it is also worth noting that the magnitude of autonomic nervous system and neuroendocrine counterregulatory responses are dependent upon prevailing glycemic control. Thus, the magnitude of the counterregulatory response and the glucose level at which the powerful anti-insulin hormones are released (glucose thresholds) are much higher in individuals with poor glycemic control and much lower in individuals with good glycemic control. Furthermore, glucose thresholds can be dynamic, shifting to lower glycemic values after only 1–2 recent hypoglycemia events.9 Thus, the individuals in the standard group that suffered severe hypoglycemia most likely had a much greater counterregulatory response as compared to individuals in the intensive group. Although, the counterregulatory response may be primarily responsible for defending against a falling glucose, as outlined in the above pathophysiologic section, certain elements of the counter-regulatory response can also activate pro-atherothrombotic and potentially dysrhythmic mechanisms.
4.4 ADVANCE
The Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) study examined 11,140 T2DM patients that underwent standard or intensive glucose control using gliclazide plus other drugs. After a median of 5 years follow-up, the HbA1c was 6.5% for the intensively treated group versus 7.3% for group that received standard treatment. Compared to the UKPDS and DCCT, the ADVANCE patients were more similar to the ACCORD patients: older (66±6), longer duration of diabetes (8±6.3 years) and increased macrovascular (32.2%) and microvascular (10.4%) events. ADVANCE reported that the intensively treated group had a significantly decreased risk of major microvascular events (HR, 0.86; 95% CI, 0.77 to 0.97; p=0.01). However, there was not a significant reduction in risk for major macrovascular events (HR, 0.94; 95% CI, 0.84 to 1.06; p=0.32). Severe hypoglycemia was uncommon but significantly increased in the intensively treated group (HR, 1.86; 95% CI, 1.42 to 2.40; P<0.001).59
A post-hoc analysis of ADVANCE examined the effect of both severe and non-severe hypoglycemia on various clinical outcomes. The study reported that severe hypoglycemia resulted in increased risk of major cardiovascular events (hazard ratio 2.88), major microvascular events (hazard ratio 1.81), death from cardiovascular cause (hazard ratio 2.68), and death from any cause (hazard ratio 2.69) p<0.001 for all of the above. The study also found that severe hypoglycemia was also associated (p<0.01) with an increased risk of skin, digestive, and respiratory conditions. The study also specifically pointed out the long temporal relationship between an episode of severe hypoglycemia and the subsequent serious adverse event. The median time from severe hypoglycemia to first major macrovascular or microvascular effect was 1.56 years and median time from severe hypoglycemia to death was 1.05 years.11 In addition, the authors stated that although severe hypoglycemia may well be directly involved in causing serious adverse events, it may also be a clinical marker in a vulnerable individual in which other serious co-morbid conditions are present. The analysis reported that minor hypoglycemia resulted in a decreased risk of macrovascular events (OR 0.7 p<0.0001) and an increase in microvascular events (OR 1.29 p=0.0002). The study did not find a dose-response of repeated hypoglycemic events on vascular outcomes (although the authors noted that few patients had repeated events).
4.5 VADT
The Veterans Affairs Diabetes Trial (VADT) investigated the effects of intensive glucose control on cardiovascular events in 1791 long-standing, poorly-controlled T2DM patients. The patients, similar to ACCORD and ADVANCE, were older (60.4±9 years), had long DM durations (11.5±7.5 years) and 40% had previous cardiovascular events. The HbA1c’s of the standard-therapy and intensive-therapy were 8.4% and 6.9%, respectively. The primary outcome (a composite of CV events) occurred in 235 of the intensively treated group versus 264 in the group receiving standard treatment (hazard ratio in the intensive-therapy group, 0.88; 95% confidence interval [CI], 0.74 to 1.05; p=0.14). The study reported no significant difference between the groups for any component of the primary outcome, rate of death from any cause, nor microvascular complications. The study also reported significantly more episodes of hypoglycemia in the intensively treated group (p=0.001) and identified a greater risk of cardiovascular events following hypoglycemia.60 A subgroup analysis indicated that severe hypoglycemia was associated with a greater rate of coronary artery calcium progression in the group receiving standard treatment as compared to group given intensive treatment.61 In the 10 year VADT follow up study, similar to the EDIC and UKPDS, the intensive control group was found to have 8.6 fewer major CV events per 1000 person-years as compared to standard therapy, however there was no difference in the rates of survival.62
4.6 ORIGIN
The Outcome Reduction with Initial Glargine Intervention (ORIGIN) Trial was a prospective study of 12,537 people with cardiovascular risk factors with either T2DM or impaired glucose tolerance to measure the effects of insulin glargine therapy or standard care with or without n-3 omega fatty acids on cardiovascular outcomes as well as incident diabetes, hypoglycemia, weight and caner. The patients were ~63±8 years old, ~60% had prior cardiovascular events and ~88% had diabetes with a duration of 5.5±6.1 years. After an average of 6.2 years, insulin glargine had a neutral effect on cardiovascular outcomes and cancers, and the insulin glargine group had a higher incidence of first episode of severe hypoglycemia: 1.00/100 person years compared to 0.31/100 person years in the standard group (p<0.001). The incidence of confirmed, first episode of non-severe hypoglycemia was higher in the insulin glargine group compared to the standard group, 9.83/100 person years vs. 2.68/100 person years, respectively (p<0.001).63
A post-hoc analysis of the ORIGIN trial examined the relationship between hypoglycemia and cardiovascular outcomes: 1) composite of CV death, non-fatal MI or stroke 2) mortality 3) CV mortality and 4) arrhythmic death. The study found that after 6.2 years, non-severe hypoglycemia (blood sugar ≤54 mg/dL) was not associated with an increase of any outcome, but severe hypoglycemia (requirement for assistance or glucose ≤ 36 mg/dL) was associated with greater risk of 1) the composite outcome (CV death, non-fatal MI or stroke (HR: 1.58, p=0.001)) 2) mortality (HR: 1.74, p=0.001) 3) CV mortality (HR: 1.71, p= 0.001) and 4) arrhythmic death (HR: 1.77, p=0.007). The study also reported that severe nocturnal hypoglycemia increased the risk of the composite outcome and mortality. Again, the relative risk of adverse cardiovascular outcomes following hypoglycemia was higher in the standard treatment group as compared to insulin glargine.64
4.7 NICE-SUGAR
The Normoglycemia in Intensive Care Evaluation - Survival Using Glucose Algorithm Regulation (NICE-SUGAR) trial examined the associations between moderate (41–70 mg/dl blood glucose) and severe hypoglycemia (≤40 mg/dl blood glucose) on death in 6026 critically-ill inpatients in Intensive Care Units who were randomized to intensive (target blood sugar of 81 to 108 mg/dl) or conventional glucose control (target of 180mg/dl or less). The study reported that the acute risk of death for patients who experienced moderate hypoglycemia was increased compared to those without hypoglycemia at HR: 1.41 (p<0.001). The risk of death was further increased for severe hypoglycemia compared to no hypoglycemia at HR: 2.10 (p<0.001). Patients who experienced moderate hypoglycemia on more than one day had increased mortality compared to patients who had moderate hypoglycemia on 1 day (p=0.01). Consistent with the ORIGIN, ADVANCE, ACCORD and VADT trials, severe hypoglycemia in the standard group increased the risk of mortality - HR: 3.84 (p<0.001).65
4.8 Edinburgh Type 2 Diabetes Study (ET2DS)
ET2DS was a prospective study of a cohort of 1066 patients with T2DM in order to assess whether a history of severe hypoglycemia increased the risk of macrovascular events through an inflammatory mechanism (C-reactive protein, fibrinogen, IL-6, and TNF-α). The patients were ~68 years old with ~8.1 years of prior T2DM and an HbA1c of 7.4%. At 4 years the study reported that hypoglycemia was associated with increased odds of macrovascular events (OR: 2.11 (p=0.035)) and increased odds of coronary heart events (OR: 2.44 (p=0.023)) primarily due to an increased risk of myocardial infarction (OR: 4.02 (p=0.004)). While hypoglycemia was associated with increased levels of inflammatory markers, the significant association between hypoglycemia and macrovascular events remained after statistical adjustment for inflammatory markers.66
5. Conclusion
Hypoglycemia acutely elicits a multitude of cardiovascular changes within the body. Evidence suggests that hypoglycemia places an increased demand on the heart via hemodynamic changes, which could be detrimental to those with underlying cardiovascular disease. Further, studies have shown QTc prolongation and epinephrine-induced hypokalemia during hypoglycemia, indicating an increased risk of fatal arrhythmias. Such arrhythmias may also be responsible for the syndrome known as “dead in bed.” Clinical mechanistic studies have revealed increases in inflammatory and pro-atherothrombotic markers, as well as reductions in endothelial function. Several large, multi-center clinical trials have examined the relationship between glycemic control and cardiovascular disease and death. Results of such studies have been mixed, with some long-term follow-up studies (EDIC, UKPDS, VADT) finding a reduction in events following intensive glycemic control. Others however have found no improvement in cardiovascular outcomes (ADVANCE, ORIGIN) with improved glycemic control, or even an increase in mortality with such treatment (ACCORD, NICE-SUGAR). In fact, severe hypoglycemia was uniformly associated with an increased risk of death (ACCORD, ORIGIN, NICE-SUGAR, VADT) and cardiovascular events (ADVANCE, VADT, ORIGIN) in all of the recent large Type 2 DM glucose control studies. However, within these studies, it remains uncertain whether hypoglycemia could present as a confounding factor (i.e. as a late sign of an underlying severe illness in a vulnerable patient). A recent large meta-analysis by Goto et al. however, suggests that comorbid illness doesn’t fully explain the correlation between severe hypoglycemia and cardiovascular disease risk thus suggesting that severe hypoglycemia, per se, is associated with a higher risk of cardiovascular disease.67 Several pieces of evidence suggest that hypoglycemia in those with poorer control may be associated with worse outcomes. Additional research is required to determine if the hypoglycemia-induced cardiovascular changes observed in pre-clinical and mechanistic studies have the long-term capacity to cause the increased risk of cardiovascular events and death observed in the large clinical trials.
6. Expert Commentary
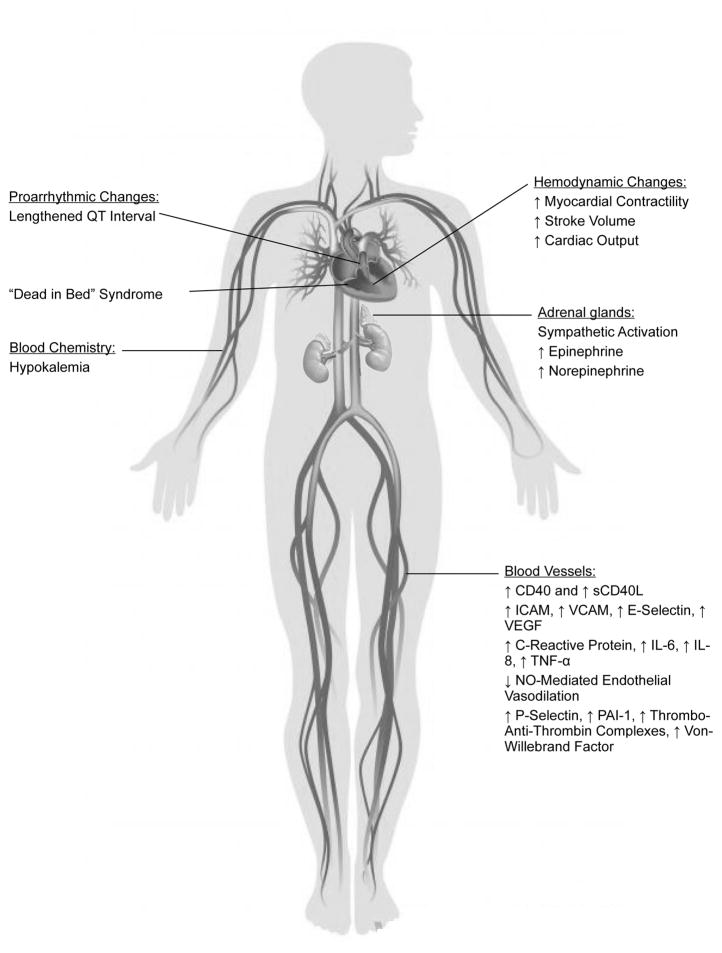
In several large glycemic control trials, severe hypoglycemia has been associated with increased cardiovascular events, cardiovascular death, and all-cause mortality. A recent large retrospective cohort study has also demonstrated that severe hypoglycemia is associated with increased serious cardiovascular events and all cause mortality in insulin requiring individuals with Type 1 or Type 2 Diabetes. Furthermore, this increased risk persists for months or even years after the episode of severe hypoglycemia.4 Basic and mechanistic research indicates that hypoglycemia could contribute to cardiovascular risk via a number of mechanisms, including induction of dysrhythmias, up-regulation of inflammation, thrombosis, and coagulation processes, and reduction of endothelial function. (Figure 2) It not yet possible to extrapolate from results of these small acute studies to the findings of large clinical trials, but it is plausible and certainly arguable that hypoglycemia-induced cardiovascular changes contributed to the relationship between hypoglycemia and risk of cardiovascular events and death.
Figure 2.
Integrated pathophysiologic pathways depicting possible mechanisms of cardiovascular disease following acute hypoglycemia.
In an attempt to link acute changes that occur during hypoglycemia to cardiovascular outcomes, the ET2DS looked at the relationship between hypoglycemic events, inflammation, and macrovascular events. Although the ET2DS found an elevated inflammatory state associated with severe hypoglycemia, inflammation was also considered not to be the mediator between severe hypoglycemia and increased macrovascular events. It is important to point out though that only four inflammatory markers were measured and measurements were only conducted at baseline and at four-years follow-up. Thus, measurements did not capture changes in inflammation throughout out the study, nor following hypoglycemic events. Further, it is likely that a number of factors contribute to the enhanced cardiovascular risk of hypoglycemia, rather than inflammation alone. Further studies asking a similar question as the ET2DS, that incorporate a larger array of biochemical measurements and an increased number of follow-up visits could certainly be helpful in enhancing our understanding.
From the DCCT and the follow-up EDIC trials, intensified glycemic control appeared to be greatly effective in reducing cardiovascular risks in type 1 diabetes patients. The UKPDS too, showed a protective benefit of intensified glycemic control on cardiovascular outcomes in type 2 diabetes, primarily at the 10-year follow-up. Since then, the large glycemic control trials involving type 2 diabetes patients have shown none or only a small association between intensive glucose control and improvements in cardiovascular outcomes. Given the metabolic complexity of type 2 diabetes, and the long time course of atherosclerotic arterial disease it is not necessarily surprising that researchers have not been able to generate significant reductions in macrovascular events. Positive results from studies using multifactorial intervention strategies (i.e., targeting improvements in glycemia, cholesterol, triglycerides, blood pressure, microalbuminuria, etc.) indicate that prevention of cardiovascular events in people with diabetes requires more than the one-dimensional approach of glycemic control.68,69 However, hypoglycemic events, which occurred frequently in the glucose control trials, especially in the intensive control cohorts, may have complicated researchers’ ability to fully explore a relationship between glycemic control and cardiovascular risk.
In the ACCORD and NICE-SUGAR trials, excess mortality occurred in the intensive control groups. In the latter study, hypoglycemia occurred much more frequently in the intensive control group, but the study was not designed to determine if the increased hypoglycemia was the cause of the increased mortality. In ACCORD, hypoglycemic events were higher in the intensive control group, but a post hoc analysis did not find an association between hypoglycemia and the excess mortality. Thus, it is curious that if hypoglycemia is associated with increased events and death, and hypoglycemia is occurring more frequently with intensive control, why was intensive control not linked to increased mortality in this study? For one, it may be that analyses are limited by the way hypoglycemia data is collected.
The methods by which hypoglycemia is defined and recorded in large clinical trials may be preventing a more nuanced analysis of effects of hypoglycemia in these studies. Among other difficulties, hypoglycemia is inconsistently defined and assessed, and the duration of hypoglycemic episodes goes unmeasured. Quantification relies upon patients to detect symptoms and/or measure blood glucose and document events for future reporting. Thus many events could go undetected and/or unreported. In the ACCORD study, for example, hypoglycemia was defined as severe hypoglycemia requiring assistance and either a blood glucose measurement of ≤2.8 mmol/l or symptoms of hypoglycemia that resolved with corrective action.70 Under these parameters, therefore, hypoglycemia would go unrecorded for patients with hypoglycemia unawareness that did not sense a low blood glucose level. Further, recording of episodes relied upon patients to contact study investigators to inform them of hypoglycemic events. Finger sticks <3.9 mmol/l were documented at follow-up visits but only for the 7 days preceding the visit,38 so that mild hypoglycemia was not documented for a majority of the study period.
Omission of data involving mild to moderate episodes of hypoglycemia leaves the relevance of these episodes to cardiovascular and mortality risk in question. Blood glucose levels of ~3.6–3.8 mmol/l are sufficient to initiate the sympathoadrenal counterregulatory response to a falling blood glucose.71 Given this and the potential impact of sympathetic output on acute changes in cardiovascular function and biomarkers during hypoglycemia, it seems plausible that such mild episodes could also be implicated in cardiovascular damage over time. Unfortunately, symptoms are not generated until a lower glucose level, ~3.2 mmol/l (and can be much lower in some patients with hypoglycemic unawareness), making detection and quantification of mild hypoglycemia much more challenging. Furthermore, detection of hypoglycemic symptoms can change acutely following antecedent hypoglycemia. Thus, repeated hypoglycemia will lower the glucose level (threshold) for sensing hypoglycemia while poor metabolic control will increase the threshold for hypoglycemic symptom awareness.5 It is possible then, that many episodes of mild hypoglycemia would have been overlooked, especially in the intensive control groups, in which hypoglycemia was more prevalent. Interestingly, in the ACCORD trial, those with a history of hypoglycemia requiring medical assistance actually had a lower rate of death with increasing incidence of finger sticks <3.9 mmol/l.38 In ADVANCE too, increased episodes of mild hypoglycemia were associated with lower rates of macrovascular and microvascular events, all-cause death, cardiovascular death, and non-cardiovascular death.40 It is plausible that mild, prior hypoglycemia could be protective against future damage from repeated hypoglycemia similar to preconditioning seen in the setting of hypoglycemia in the brain.72 Further, while hypoglycemia has been observed to increase nearly universally with tight glycemic control, in several studies, including ACCORD, ADVANCE, ORIGIN, and NICE-SUGAR, hypoglycemia has been associated with an increased risk of cardiovascular events and/or mortality in those with poorer glycemic control – either those randomized to the standard glycemic control groups or those who did not respond to intensified glycemic control. These findings raise the question of why those with poorer control are more susceptible to negative outcomes with hypoglycemia.
One explanation is that individuals with poorer metabolic control experience a relative reduction in the frequency of hypoglycemia events. This would tend to preserve autonomic nervous system and neuroendocrine counterregulatory responses. This if it is the magnitude of the physiologic response to hypoglycemia that initiates the pathway to serious adverse cardiovascular events, then the preserved counterregulatory responses in standard treated patients (with poorer metabolic control) may effectively defend against the falling plasma glucose as compared to intensively treated individuals but may also be increasing the risk of serious adverse events.
Experts have suggested that the treatment regimen and corresponding level of glycemic control is not the driver of differences between treatment groups in relationships between hypoglycemia and cardiovascular events.38,73 Rather, it is hypothesized, there is a subset of patients – as yet not identified – that are more susceptible (vulnerable) to negative cardiovascular effects of hypoglycemia. While this is certainly possible, it is curious that multiple studies have now found that hypoglycemia is associated with more negative cardiovascular outcomes in standard control groups.
VADT researchers recently conducted a post-hoc analysis of serious hypoglycemia and progression of coronary artery calcification from baseline to ~4.6 years follow-up in a subset of VADT participants. Despite a higher incidence of serious hypoglycemia in the intensive glucose control group, serious hypoglycemia was associated with a greater progression of coronary artery calcification in the standard treatment group only.43 The reason for this is only speculative at this point. One possible explanation offered by the study authors is that those with a higher HbA1c (and therefore a higher fasting blood glucose) that experience an episode of hypoglycemia undergo a larger change in blood glucose from euglycemia to hypoglycemia.43 Greater glucose variability may have more detrimental effects on the vasculature.74,75,76 It has also been shown that over-correction for a low blood glucose that results in hyperglycemia is associated with prolonged derangements in thrombosis and oxidative stress, as compared to appropriate corrective actions that result in euglycemia.28 Study authors have also suggested that because those in the intensive control groups experienced hypoglycemia and were in contact with study investigators more frequently, these participants were better prepared to respond to hypoglycemic events more quickly and appropriately than those in the standard control groups, leaving the standard control participants susceptible to lower and longer episodes of hypoglycemia.38 Newer technologies such as Continuous Glucose Monitoring Systems and methods for measuring glucose variability may capture “minor and moderate” episodes of hypoglycemia that may go unrecognized and thus may be able to more accurately assign cause and effect of hypoglycemia and subsequent adverse events.77,43 It is also worth noting that the large Type 2 DM glucose control and complications studies (ADVANCE, ACCORD, VADT) have all reported a delayed association between hypoglycemia and subsequent severe adverse events or death. One possible explanation is that severe hypoglycemia sets up a cascade of pathophysiologic mechanisms in a vulnerable patient that can be amplified by subsequent “minor or moderate hypoglycemic events” that in of themselves are not considered to have serious clinical relevance.
With respect to when some of the newer anti-diabetes agents (i.e., long-and rapid-acting insulins, DPP4 inhibitors, GLP-1 receptor agonists, and SGLT2 inhibitors) came to market, the treatment algorithms used by the large glucose control trials are relatively outdated, relying more heavily on the previously available sulfonylureas and insulin, which are associated with greater risk of hypoglycemic events. In treatment of type 1 diabetes, glucose monitoring and insulin replacement have improved, so that while risk of hypoglycemia is still substantial, more physiologic insulin replacement does mitigate risk. The newer drug classes approved for type 2 diabetes have much lower rates of hypoglycemia. Therefore, targeting glycemic control with these agents could drastically diminish the untoward effects of hypoglycemia in those who have not progressed to dependence on insulin. For the most part, treatment with the newer medications has not led to reductions in cardiovascular risk in clinical trials. However, such trials have mainly been designed to determine non-inferiority rather than superiority to comparator groups for cardiovascular risk, and studies may have to be much longer in order to observe any improvements in cardiovascular outcomes. Additionally, as noted above, development of new multi-intervention trials that incorporate these new medications into a regimen that also addresses body weight, blood pressure, lipids, etc. may be necessary to realize reductions in cardiovascular risk. Importantly, long-standing metformin has been shown to improve cardiovascular outcomes.35 More recently, empagliflozin (an SGLT-2 inhibitor with a low risk for hypoglycemia) was shown to significantly reduce the composite endpoint of cardiovascular death, myocardial infarction, and stroke,78 as well as the composite endpoint of heart failure hospitalization and cardiovascular death in a population at high cardiovascular risk.79 Additionally the GLP-1 receptor agonist, liraglutide has also been demonstrated to reduce cardiovascular mortality and morbidity in Type 2 DM.80 While we have yet to see how these findings play out in clinical practice among different populations of patients, it is promising that clinicians are slowly gaining tools to simultaneously improve glycemia and reduce cardiovascular events without introducing a greater risk for hypoglycemia.
Hypoglycemia is a prevalent, major complication of diabetes treatment and can acutely induce an array of cardiovascular derangements. Results from large glycemic control trials have shown an association between hypoglycemia and cardiovascular events and death, especially in those with poorer glucose control. The physiologic mechanisms underlying this increase in adverse outcomes remain to be established. Further, analyses of hypoglycemia and outcomes in the large glycemic control trials have been post hoc, resulting in less robust findings. As hypoglycemia has been the subject of much scrutiny and debate following the publication of the large clinical trials, future studies should develop more specific definitions and monitoring for hypoglycemia, include hypoglycemia as a primary outcome, and utilize newer anti-hyperglycemic medications. Additionally, incorporating fear of hypoglycemia surveys and recent studies identifying predictors of hypoglycemia would also be clinically useful and relevant.81,82 This will help to provide clinicians with an updated and more precise portrait of the long-term effects of hypoglycemia and contemporary intensive glycemic control.
7. Five Year View
Accumulating data, primarily in individuals with Type 2 DM, clearly points to an associative risk between severe hypoglycemia and serious adverse cardiovascular events and death. Despite repetitive finding there remains doubt about whether hypoglycemia in fact caused adverse cardiac outcomes/death or whether hypoglycemia was merely a consequence or marker of a serious underlying fatal condition. This uncertainty about the role played by hypoglycemia per se in causing fatal outcomes needs to be addressed by “on line” glucose monitoring at the time of a serious adverse event. Similarly, research needs to be performed to understand why patients with poorer glucose control are at a greater risk of dying following an episode of severe hypoglycemia as compared to individuals with good metabolic control. Could the counterregulatory response be maladaptive and in addition to defending against a falling glucose might be causing self-harm? Finally, are any episode of hypoglycemia really minor? Could an episode of hypoglycemia further stimulate pathophysiologic mechanisms that in a vulnerable patient could have devastating consequences? All of the above questions are answerable and will give invaluable insight as to how to manage individuals with diabetes that require insulin replacement therapy.
8. Key Issues
Type 1 Diabetes and longer duration Type 2 Diabetes require insulin replacement. Since the discovery of insulin, hypoglycemia has been an unwelcome adverse side effect of this life saving molecule. More recently focus has shifted from the almost exclusive understanding of hypoglycemia in Type 1 DM to the potentially catastrophic effects of hypoglycemia in Type 2 DM. Hypoglycemia in Type 1 and Type 2 DM are equally important and research needs to focus on management models that can prevent hypoglycemia while maintaining appropriate and individualized glycemic control. While much has been learned about causes of insufficient counterregulatory responses to hypoglycemia in diabetic individuals, perhaps the time has now arrived for research to determine the non-metabolic effects of hypoglycemia and its attendant pathophysiologic vascular consequences.
Figure 1.
Pathophysiologic changes in the vasculature following hypoglycemia.
Table 1.
Serious cardiovascular adverse events and mortality associated with severe hypoglycemia in randomized controlled trials evaluating the effects of intensive or standard therapy in individuals with type 2 diabetes.
| Study | Population | HbA1c (intensive: standard/conventional group) | Incidence of Severe Hypoglycemia (intensive:standard/conventional group) | Overall hypoglycemiaglycemia Findings | hypoglycemiaglycemia-Related Intensive Group Findings | hypoglycemiaglycemia-Related Standard/Conventional Group Findings | ||
|---|---|---|---|---|---|---|---|---|
| DCCT/EDIC | 1441 T1DM patients | 7% | 9% | 65% | 35% | Increased hypoglycemia events. | ||
| UKPDS | 5102 T2DM patients | 7% | 7.9% | 1.1% | 0.7% | Low number of severe hypoglycemia events reported. | ||
| ACCORD | 10,251 T2DM patients- 62 y.o | 6.4% | 7.5% | 16.2% | 5.1% | Increased severe hypoglycemia events. Increased mortality with increased severe hypoglycemia. | Relatively increased mortality with severe hypoglycemia. | |
| ADVANCE | 11,140 T2DM patients- 66 y.o, | 6.5% | 7.3% | 2.7% | 1.5% | Increased cardiovascular adverse events with severe hypoglycemia. Decreased macrovascular and increased microvascular events with minor hypoglycemia. No dose response or temporal relation. | Increased hypoglycemia events. | Relatively increased mortality associated with severe hypoglycemia. |
| VADT | 1791 T2DM- 60 y.o, | 6.9% | 8.4% | 8.5% | 3.1% | Increased severe hypoglycemia events. Severe adverse cardiac events and mortality associated with severe hypoglycemia. | Relatively increased risk of mortality in standard/conventional group following severe hypoglycemia. | |
| Origin | 12,537 T2DM and pre-T2DM- 63 y.o, | 6.2% | 6.5% | 1.0% | 0.3% | Severe hypoglycemia-increased cardiovascular and total mortality | Increased severe and non-severe hypoglycemia events. | Increased relative risk of composite CV outcome, mortality, CV mortality and arrhythmic mortality |
| NICE-SUGAR | 6026 critically ill, ICU inpatients | 6.9% | 0.5% | Increased mortality with moderate hypoglycemia. Further increased mortality with severe hypoglycemia. | Increased severe hypoglycemia in standard group | |||
| ET2DS | 1066 T2DM patients- 68 y.o | 7.4% | Hypoglycemia associated with increased macrovascular, myocardial infarction and all adverse coronary heart events. | |||||
Acknowledgments
Funding
This work was supported by the following NIH grants: PO1 HLO56693 NIH/NHLBI, T35DK095737.
Footnotes
Declaration of interest
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Contributor Information
Ian Charles Davis, University of Maryland School of Medicine, Baltimore, Maryland 21201-1544, United States.
Ida Ahmadizadeh, University of Maryland School of Medicine, Baltimore, Maryland 21201-1544, United States.
Jacqueline Randell, Johns Hopkins University, Baltimore, Maryland 21205, United States.
Lisa Younk, University of Maryland School of Medicine, Baltimore, Maryland 21201-1544, United States.
Stephen N Davis, University of Maryland School of Medicine – Medicine, 22 S. Greene Street, Baltimore, Maryland 21201, United States.
References
- 1.Morrish NJ, Wang SL, Stevents LK, et al. Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetes. 2001;2:S14–21. doi: 10.1007/pl00002934. [DOI] [PubMed] [Google Scholar]
- 2.Laakso M. Hyperglycemia and cardiovascular disease in type 2 diabetes. Diabetes. 1999;48(5):937–42. doi: 10.2337/diabetes.48.5.937. [DOI] [PubMed] [Google Scholar]
- 3.Schnell O, Cappuccio F, Genovese S, et al. Type 1 diabetes and cardiovascular disease. Cardiovasc Diabetol. 2013;12:156. doi: 10.1186/1475-2840-12-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khunti K, Davies M, Majeed A. Hypoglycemia and risk of cardiovascular disease and all-cause mortality in insulin-treated people with type 1 and type 2 diabetes: a cohort study. Diabetes Care. 2015;38(2):316–22. doi: 10.2337/dc14-0920. [DOI] [PubMed] [Google Scholar]
- 5.Cariou B, Fontaine P, Eschwege E. Frequency and predictors of confirmed hypoglycemia in type 1 and insulin-treated type 2 dabetes mellitus patients in a real-life setting: results from the DIALOG study. Diabetes metabolism. 2015;41(2):116–25. doi: 10.1016/j.diabet.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 6.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 7.Reichard P, Berglund B, Britz A. Intensified conventional insulin treatment retards the microvascular complications of insulin-dependent diabetes mellitus (IDDM): the Stockholm Diabetes Intervention Study (SDIS) after 5 years. J Intern Med. 1991;230:101–108. doi: 10.1111/j.1365-2796.1991.tb00415.x. [DOI] [PubMed] [Google Scholar]
- 8.MacLeod KM, Hepburn DA, Frier BM. Frequency and morbidity of severe hypoglycaemia in insulin-treated diabetic patients. Diabet Med. 1993;10:238–245. doi: 10.1111/j.1464-5491.1993.tb00051.x. [DOI] [PubMed] [Google Scholar]
- 9.Khunti K, Alsifri S, Aronson R. Rates and predictors of hypoglycaemia in 27585 people from 24 countries with insulin-treated type 1 and type 2 diabetes: the global HAT study. Diabetes Obes Metabolism. 2016;18(9):907–15. doi: 10.1111/dom.12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herman WH, Ilag LL, Johnson SL, et al. A clinical trial of continuous subcutaneous insulin infusion versus multiple daily injections in older adults with Type 2 Diabetes. Diabetes Care. 2005;28:1568–1573. doi: 10.2337/diacare.28.7.1568. [DOI] [PubMed] [Google Scholar]
- 11.UK hypoglycemia Study Group. Rise of hypoglycemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia. 2007;50:1140–1147. doi: 10.1007/s00125-007-0599-y. [DOI] [PubMed] [Google Scholar]
- 12**.Cryer PE, Davis SN, Shamoom H. Hypoglycemia in diabetes. Diabetes Care. 2003;26(6):1902–12. doi: 10.2337/diacare.26.6.1902. A comprehensive review of the incidence and pathophysiology of hypoglycemia in diabetes. [DOI] [PubMed] [Google Scholar]
- 13.Brady PA, Terzic AJ. The sulfonylurea controversy: more questions from the heart. Am Coll Cardiol. 1998 Apr;31(5):950–6. doi: 10.1016/s0735-1097(98)00038-2. [DOI] [PubMed] [Google Scholar]
- 14.Williams SA, Shi L, Brenneman SK. The burden of hypoglycemia on healthcare utilization, costs, and quality of life amongtype 2 diabetes mellitus patients. 2012;26(5):399–406. doi: 10.1016/j.jdiacomp.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Zoungas S, Patel A, Chalmers J. Severe hypoglycemia and risks of vascular events and death. N Engl J Med. 2010;363(15):1410–8. doi: 10.1056/NEJMoa1003795. [DOI] [PubMed] [Google Scholar]
- 16.McCoy RG, Van Houten HK, Ziegenfuss JY. Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care. 2012 Sep;35(9):1897–901. doi: 10.2337/dc11-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benson JW, Jr, Johnson DG, Palmer JP. Glucagon and catecholamine secretion during hypoglycemia in normal and diabetic man. J Clin Endocrinol Metab. 1977 Mar;44(3):459–64. doi: 10.1210/jcem-44-3-459. [DOI] [PubMed] [Google Scholar]
- 18.Amiel Stephanie A, Simonson Donald C, William V. Tamborlane Rate of Glucose Fall Does Not Affect Counterregulatory Hormone Responses to Hypoglycemia in Normal and Diabetic Humans. Diabetes. 1987 Apr;36(4):518–522. doi: 10.2337/diab.36.4.518. [DOI] [PubMed] [Google Scholar]
- 19.Keen H. The Diabetes Control and Complications Trial (DCCT) Health Trends. 1994;26:41–43. [PubMed] [Google Scholar]
- 20.Wright RJ, Frier BM. Vascular disease and diabetes: is hypoglycaemia an aggravating factor? Diabetes Metab Res Rev. 2008;24(5):353–363. doi: 10.1002/dmrr.865. [DOI] [PubMed] [Google Scholar]
- 21.Petersen KG, Schluter KJ, Kerp L. Regulation of serum potassium during insulin-induced hypoglycemia. Diabetes. 1982;31(7):615–617. doi: 10.2337/diab.31.7.615. [DOI] [PubMed] [Google Scholar]
- 22.Heller SR, Robinson RT. Hypoglycaemia and associated hypokalaemia in diabetes: mechanisms, clinical implications and prevention. Diabetes Obes Metab. 2000;2(2):75–82. doi: 10.1046/j.1463-1326.2000.00050.x. [DOI] [PubMed] [Google Scholar]
- 23.Brown MJ, Brown DC, Murphy MB. Hypokalemia from beta2 receptor stimulation by circulating adrenaline. N Engl J Med. 1983;309:1414–1419. doi: 10.1056/NEJM198312083092303. [DOI] [PubMed] [Google Scholar]
- 24*.Reno CM, Daphna-Iken D, Chen YS, et al. Severe hypoglycemia-induced lethal cardiac arrhythmias are mediated by sympathoadrenal activation. Diabetes. 2013;62:3570–3581. doi: 10.2337/db13-0216. An experiment in animals that explores the pathophysiology of fatal cardiac changes caused by hypoglycemia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laitinen T, Lyyra-Laitinen T, Huopio H, et al. Electrocardiographic alterations during hyperinsulinemic hypoglycemia in healthy subjects. Ann Noninvasive Electrocardiol. 2008;13:97–105. doi: 10.1111/j.1542-474X.2008.00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gill GV, Woodward A, Casson IF, et al. Cardiac arrhythmia and nocturnal hypoglycaemia in type 1 diabetes: the ‘dead in bed’ syndrome revisited. Diabetologia. 2009;52:42–45. doi: 10.1007/s00125-008-1177-7. [DOI] [PubMed] [Google Scholar]
- 27.Robinson RT, Harris ND, Ireland RH, et al. Mechanisms of abnormal cardiac repolarization during insulin-induced hypoglycemia. Diabetes. 2003;52:1469–1474. doi: 10.2337/diabetes.52.6.1469. [DOI] [PubMed] [Google Scholar]
- 28.Schouten EG, Dekker JM, Meppelink P, et al. QT interval prolongation predicts cardiovascular mortality in an apparently healthy population. Circulation. 1991;84:1516–1523. doi: 10.1161/01.cir.84.4.1516. [DOI] [PubMed] [Google Scholar]
- 29.Barr CS, Naas A, Freeman M, et al. QT dispersion and sudden unexpected death in chronic heart failure. Lancet. 1994;343:327–329. doi: 10.1016/s0140-6736(94)91164-9. [DOI] [PubMed] [Google Scholar]
- 30.Hii JTY, Wyse DG, Gillis AM, et al. Precordial QT interval dispersion as a marker of torsades de pointes: disparate effects of class Ia antiarrhythmic drugs and amiodarone. Circulation. 1986;86:1376–1382. doi: 10.1161/01.cir.86.5.1376. [DOI] [PubMed] [Google Scholar]
- 31.Buja J, Miorelli M, Turrini P, et al. Comparison of QT dispersion in hypertrophic cardiomyopathy between patients with and without ventricular arrhythmias and sudden death. Am J Cardiol. 1993;72:973–976. doi: 10.1016/0002-9149(93)91118-2. [DOI] [PubMed] [Google Scholar]
- 32.Higham J, Furniss SS, Campbell RWF. QT dispersion and components of the QT interval in ischaemia and infarction. Br Heart J. 1995;73:32–36. doi: 10.1136/hrt.73.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gruden G, Giunti S, Barutta F, et al. QTc interval prolongation is independently associated with severe hypoglycemic attacks in type 1 diabetes from the EURODIAB IDDM complications study. Diabetes Care. 2012;35(1):125–127. doi: 10.2337/dc11-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christensen TF, Tarnow L, Randlov J, et al. QT interval prolongation during spontaneous episodes of hypoglycaemia in type 1 diabetes: the impact of heart rate correction. Diabetologia. 2010;53(9):2036–2041. doi: 10.1007/s00125-010-1802-0. [DOI] [PubMed] [Google Scholar]
- 35.Davis SN, Shavers C, Collins L. Effects of physiological hyperinsulinemia on counterregulatory response to prolonged hypoglycemia in normal humans. Am J Physiol. 1994 Sep;267(3 Pt 1):E402–10. doi: 10.1152/ajpendo.1994.267.3.E402. [DOI] [PubMed] [Google Scholar]
- 36.Wright RJ, Newby DE, Stirling D, et al. Effects of acute insulin-induced hypoglycemia on indices of inflammation: putative mechanism for aggravating vascular disease in diabetes. Diabetes Care. 2010;33(7):1591–1597. doi: 10.2337/dc10-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gogitidze Joy N, Hedrington MS, et al. Effects of acute hypoglycemia on inflammatory and pro-atherothrombotic biomarkers in individuals with type 1 diabetes and healthy individuals. Diabetes Care. 2010;33(7):1529–1535. doi: 10.2337/dc09-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ceriello A, Novials A, Ortega E, et al. Evidence that hyperglycemia after recovery from hypoglycemia worsens endothelial function and increases oxidative stress and inflammation in healthy control subjects and subjects with type 1 diabetes. Diabetes. 2012;61(11):2993–2997. doi: 10.2337/db12-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galloway PJ, Thomson GA, Fisher BM, et al. Insulin-induced hypoglycemia induces a rise in C-reactive protein. Diabetes Care. 2000;23:861–862. doi: 10.2337/diacare.23.6.861. [DOI] [PubMed] [Google Scholar]
- 40.Razavi Nematollahi L, Kitabchi AE, Kitabchi AE, et al. Proinflammatory cytokines in response to insulin-induced hypoglycemic stress in healthy subjects. Metabolism. 2009;58:443–448. doi: 10.1016/j.metabol.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 41*.Joy NG, Tate DB, Younk LM, et al. Effects of acute and antecedent hypoglycemia on endothelial function and markers of atherothrombotic balance in healthy humans. Diabetes. 2015;64:2571–2580. doi: 10.2337/db14-1729. A trial in humans that relates hypoglycemia to endothelial dysfunction and pro-atherothrombotic changes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gimenéz M, et al. Repeated episodes of hypoglycemia as a potential aggravating factor for preclinical atherosclerosis in subjects with type 1 diabetes. Diabetes Care. 2011;34:198–203. doi: 10.2337/dc10-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ceriello A, Novials A, Ortega E. Hyperglycemia following recovery from hypoglycemia worsens endothelial damage and thrombosis activation in type 1 diabetes and in healthy controls. Nutr Metab Cardiovasc Dis. 2014 Feb;24(2):116–23. doi: 10.1016/j.numecd.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 44.O’Reilly M, O’Sullivan EP, Davenport C, et al. “Dead in bed”: a tragic complication of type 1 diabetes mellitus. Ir J Med Sci. 2010;179(4):585–7. doi: 10.1007/s11845-010-0519-x. [DOI] [PubMed] [Google Scholar]
- 45.Effects of a controlled hypoglycaemia test on QTc in adolescents with Type 1 diabetes. Diabet Med. 2008;25(12):1483–5. doi: 10.1111/j.1464-5491.2008.02599.x. [DOI] [PubMed] [Google Scholar]
- 46.Emily Tu, Richard Bagnall, Johan Duflou, et al. Post-mortem pathologic and genetic studies in “dead in bed syndrome” cases in type 1 diabetes mellitus. Human Pathology. 2010;41(3):392–400. doi: 10.1016/j.humpath.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 47.Skinner JR, Marquis-Nicholson R, Luangpraseuth A. Diabetic Dead-in-Bed Syndrome: A Possible Link to a Cardiac Ion Channelopathy. Case Rep Med. 2014;2014:647252. doi: 10.1155/2014/647252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nathan David M, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study at 30 Years: Advances and Contributions. Diabetes. 2013;62(12):3976–3986. doi: 10.2337/db13-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive Diabetes Treatment and Cardiovascular Disease in Patients with Type 1 Diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Writing Group for the DCCT/EDIC Research Group. Association Between 7 Years of Intensive Treatment of Type 1 Diabetes and Long-term Mortality. JAMA. 2015;313(1):45–53. doi: 10.1001/jama.2014.16107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cleary PA, Orchard TJ, Genuth S, et al. The effect of intensive glycemic treatment on Coronary Artery Calcification on Type 1 Diabetic participants of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes. 2006;55 doi: 10.2337/db06-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Farhmann ER, Adkins L, Loader CJ, et al. Severe hypoglycemia and coronary artery calcification during the diabetes control and complcations trial/epidemiology of diabetes interventions and complications (DCCT/EDIC) study. Diabetes Res Clin Pract. 2015;107(2):280–9. doi: 10.1016/j.diabres.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.UK Prospective Diabetes Study (UKPDS) VIII. Study design, progress and performance. Diabetologia. 1991;34(12):877–90. [PubMed] [Google Scholar]
- 54.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) The Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 55.Holman RR1, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–89. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 56.The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of Intensive Glucose Lowering in Type 2 Diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ. 2010;340:b4909. doi: 10.1136/bmj.b4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Riddle MC. Effects of intensive glucose lowering in the management of patients with type 2 diabetes mellitus in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Circulation. 2010;122(8):844–6. doi: 10.1161/CIRCULATIONAHA.110.960138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patel A, MacMahon S, Chalmers J, et al. ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–72. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 60.Duckworth W, Abraira C, Moritz T VADT Investigators et al. Glucose Control and Vascular Complications in Veterans with Type 2 Diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 61.Saremi A, Bahn GD, Reaven PD. A Link Between Hypoglycemia and Progression of Atherosclerosis in the Veterans Affairs Diabetes Trial (VADT) Diabetes Care. 2016 Jan 19; doi: 10.2337/dc15-2107. pii: dc152107 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hayward RA, Reaven PD, Emanuele NV. Follow-up of Glycemic Control and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2015;373(10):978. doi: 10.1056/NEJMc1508386. [DOI] [PubMed] [Google Scholar]
- 63.Gerstein HC, Bosch J, Dagenais GR, et al. ORIGIN Trial Investigators. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367(4):319–28. doi: 10.1056/NEJMoa1203858. [DOI] [PubMed] [Google Scholar]
- 64.Mellbin LG, Rydén L, Riddle MC, et al. ORIGIN Trial Investigators. Does hypoglycaemia increase the risk of cardiovascular events? A report from the ORIGIN trial. Eur Heart J. 2013;34(40):3137–44. doi: 10.1093/eurheartj/eht332. [DOI] [PubMed] [Google Scholar]
- 65.Finfer S, Liu B, Chittock DR, et al. NICE-SUGAR Study Investigators. Hypoglycemia and risk of death in critically ill patients. N Engl J Med. 2012;367(12):1108–1. doi: 10.1056/NEJMoa1204942. [DOI] [PubMed] [Google Scholar]
- 66.Bedenis R, Price AH, Robertson CM, et al. Association between severe hypoglycemia, adverse macrovascular events, and inflammation in the Edinburgh Type 2 Diabetes Study. *A large clinical trial with some markers of inflammation that may serve as a starting-point for future trials. Diabetes Care. 2014;37(12):3301–8. doi: 10.2337/dc14-0908. [DOI] [PubMed] [Google Scholar]
- 67.Gota A, Arah OA, Goto M. Severe hypoglycaemia and cardiovascular disease: systematic review and meta-analysis with bias analysis. BMJ. 2013 Jul 29;347:f4533. doi: 10.1136/bmj.f4533. [DOI] [PubMed] [Google Scholar]
- 68.Gaede P, Lund-Andersen H, Parving HH. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008 Feb 7;358(6):580–91. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 69.Rubin RR, Gaussoin SA, Peyrot M. Cardiovascular disease risk factors, depression symptoms and antidepressant medicine use in the Look AHEAD (Action for Health in Diabetes) clinical trial of weight loss in diabetes. Diabetologia. 2010 Aug;53(8):1581–9. doi: 10.1007/s00125-010-1765-1. Epub 2010 Apr 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70*.Miller ME, Bonds DE, Gerstein HC. The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010 Jan 8;340:b5444. doi: 10.1136/bmj.b5444. Important analysis of the results from the ACCORD study pertaining to hypoglycemia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mitrakou A, Ryan C, Veneman T. Hierarchy of glycemic thresholds for counterregulatory hormone secretion, symptoms, and cerebral dysfunction. Am J Physiol. 1991 Jan;260(1 Pt 1):E67–74. doi: 10.1152/ajpendo.1991.260.1.E67. [DOI] [PubMed] [Google Scholar]
- 72.Litvin M, Clark AL, Fisher SJ. Recurrent hypoglycemia: boosting the brain’s metabolic flexibility. J Clin Invest. 2013;123(5):1922–4. doi: 10.1172/JCI69796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Desouza CV, Bolli GB, Fonseca V. Hypoglycemia, diabetes, and cardiovascular events. Diabetes Care. 2010 Jun;33(6):1389–94. doi: 10.2337/dc09-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Monnier L, Mas E, Ginet C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006 Apr 12;295(14):1681–7. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 75.Torimoto K, Okada Y, Mori H. Cardiovasc Diabetol. Relationship between fluctuations in glucose levels measured by continuous glucose monitoring and vascular endothelial dysfunction in type 2 diabetes mellitus. 2013 Jan 2;12(1) doi: 10.1186/1475-2840-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saisho Y. Glycemic variability and oxidative stress: a link between diabetes and cardiovascular disease? Int J Mol Sci. 2014 Oct 13;15(10):18381–406. doi: 10.3390/ijms151018381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.DeVries JH. Glucose Variability: Where It Is Important and How to Measure It Diabetes. 2013 May;62(5):1405–1408. doi: 10.2337/db12-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zinman B, Wanner C, Lachin JM. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015 Nov 26;373(22):2117–28. doi: 10.1056/NEJMoa1504720. Epub 2015 Sep 17. [DOI] [PubMed] [Google Scholar]
- 79.Fitchett D, Zinman B, Wanner C. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME® trial. Eur Heart J. 2016 Jan 26; doi: 10.1093/eurheartj/ehv728. pii: ehv728. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marso S, Daniels G, Brown-Frandsen K. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016 Jul 28;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grabman J, Vajda Bailey K, Schmidt K. An empirically derived short form of the Hypoglycaemia Fear Survey II. Diabet Med. 2016 Jun 9; doi: 10.1111/dme.13162. [DOI] [PubMed] [Google Scholar]
- 82.Bordier L, Buysschaert M, Bauduceau B. Predicting factors of hypoglycaemia in elderly type 2 diabetes patients: Contributions of the GERODIAB study. Diabetes Metab. 2015;41(4):301–3. doi: 10.1016/j.diabet.2015.03.001. [DOI] [PubMed] [Google Scholar]