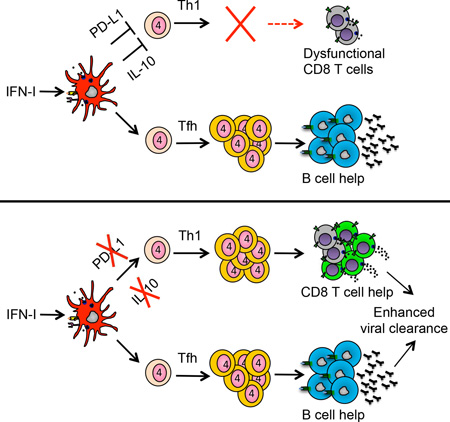
SUMMARY
Viral persistence specifically inhibits CD4 Th1 responses and promotes Tfh immunity, yet the mechanisms that suppress Th1 cells and the disease consequences of their loss are unclear. We demonstrate that the loss of CD4 Th1 cells specifically leads to the progressive CD8 T cell decline and dysfunction during viral persistence. Therapeutically reconstituting CD4 Th1 cells restored CD4 T cell polyfunctionality, enhanced antiviral CD8 T cells numbers and function and enabled viral control. Mechanistically, combined interaction of PD-L1 and IL-10 by suppressive dendritic cell subsets inhibited new CD4 Th1 cells in both acute and persistent virus infection, demonstrating an unrecognized suppressive function for PD-L1 in virus infection. Thus, the loss of CD4 Th1 cells is a key event leading to the progressive CD8 T cell demise during viral persistence with important implications for restoring antiviral CD8 T cell immunity to control persistent viral infection.
Graphical Abstract
INTRODUCTION
In response to many persistent virus infections, virus-specific T cells are either physically deleted or persist in an attenuated (exhausted) state characterized by a distinct transcriptional program, alterations in antiviral and immune-stimulatory cytokines and a decreased ability to proliferate or lyse virally infected cells (Ng et al., 2013; Wherry and Kurachi, 2015). Although CD4 T cells are the central orchestrators of the immune response, to date the majority of work analyzing T cell dynamics and exhaustion during persistent viral infection has focused on CD8 T cells. In fact, effectively directed and sustained CD4 T cell responses are the best correlate of control and clearance of multiple persistent virus infections (Ng et al., 2013). Thus, understanding how CD4 T cells mediate control of persistent infection will be critical for designing therapies to fight viral infections.
Upon activation, naïve CD4 T cells differentiate into specific subsets (Th1, Tfh, Th17, Treg, etc.), based on signals from the antigenic environment and interactions with antigen presenting cells (APCs) (Zhu et al., 2010). The CD4 Th response generated is tailored to control individual types of pathogens, with misdirection often leading to ineffective immunity (Hegazy et al., 2010; MacDonald et al., 2002). In response to viral infections, CD4 T cells predominantly differentiate into Th1 cells that sustain CD8 T cell responses to kill virus infected cells (Battegay et al., 1994; Elsaesser et al., 2009; Frohlich et al., 2009; Matloubian et al., 1994; Yi et al., 2009) or develop into T follicular helper (Tfh) cells that mediate B cell differentiation and antibody production (Crotty, 2014; Fahey et al., 2011). Although CD4 help at the onset of persistent virus infection is initially required to promote the CD8 T cell and antibody responses required for long-term control of infection (Bergthaler et al., 2009; Fahey et al., 2011), the subsets best suited to maintain long-term immunity and control an established persistent virus infection are unclear.
During viral persistence, continuous T cell receptor (TcR) stimulation progressively drives transformation of virus-specific CD4 Th1 cells into Tfh cells (Fahey et al., 2011), and ultimately, the CD4 Th1 cells generated at the onset of persistent infection are lost, leaving primarily B cell helping CD4 Tfh cells. Furthering the skew towards Tfh, we recently identified a severe defect in the ability to generate new virus specific CD4 Th1 cells in the midst of an established persistent virus infection (Osokine et al., 2014). In the established persistent virus infection, only new CD4 Tfh cells were generated (Osokine et al., 2014), leaving a hole in the CD4 Th subset repertoire and skewing the type of help available as persistent infection progressed.
A similar decrease in CD4 Th1 cells and accumulation of Tfh cells is observed in HIV, SIV and HCV infections (Feng et al., 2012; Lindqvist et al., 2012; Petrovas et al., 2012), suggesting a conserved loss of Th1 responses in persistent virus infection. Interestingly, prolonged CD4 Th1 responses in HIV and HCV infections correlate with enhanced viral control (Gerlach et al., 1999; Rosenberg et al., 1997; Thimme et al., 2001). However, it remains unclear whether sustained Th1 responses actually facilitate viral control or whether decreased viral loads simply enable Th1 priming and maintenance. Differentiating between these scenarios will be critical to determine the role of Th1 cells in persistent infection and further the understanding of which CD4 T cell subsets are optimal to promote therapeutically for viral control.
Herein, we demonstrate the fundamental disease consequences of the loss and inability to generate new CD4 Th1 cells during viral persistence. We demonstrate that the loss of CD4 Th1 cells underlies the progressive CD8 T cell decline and dysfunction that prevents control of persistent infection and that therapeutically restoring CD4 Th1 cells enhances virus-specific CD8 T cell numbers and function facilitating control of the persistent infection. Mechanistically, expression of PD-L1 and IL-10, two immunosuppressive molecules integrally linked to the failure to control persistent virus infections (Barber et al., 2006; Brooks et al., 2006; Day et al., 2006; Ejrnaes et al., 2006) by suppressive dendritic cells (DCs) simultaneously inhibits CD4 Th1 immunity. Further, these same mechanisms also impede CD4 Th1 differentiation at the onset of acute and persistent viral infections, identifying a continuous mechanism limiting Th1 differentiation and preventing control of persistent infection.
RESULTS
Combined PD-L1 and IL-10 suppression inhibits de novo CD4 Th1 generation
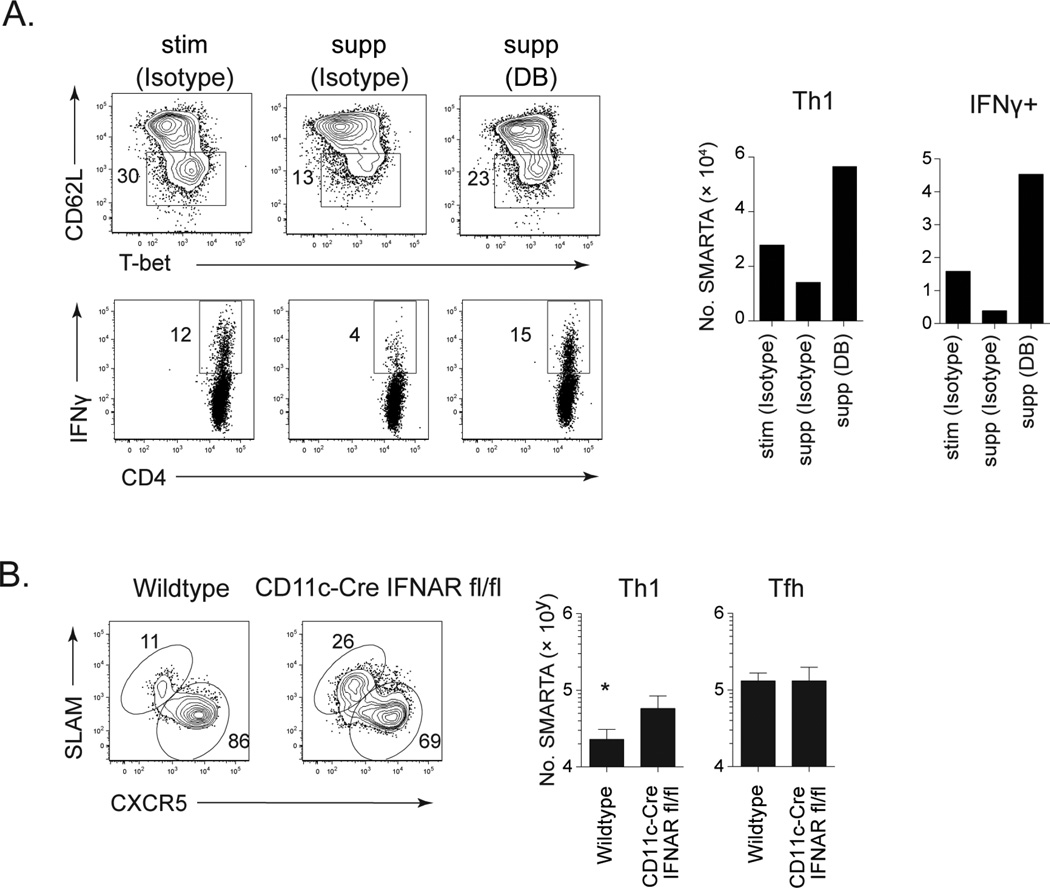
To explore CD4 T cell dynamics and dysfunction in persistent viral infection, we use the lymphocytic choriomeningitis virus (LCMV) model. Infection with the LCMV variant Clone 13 (Cl13) establishes a persistent infection due to enhanced viral replication and receptor affinity that outcompetes the developing immune response, thereby inducing immunosuppression and T cell exhaustion (Ahmed et al., 1984; Ng et al., 2013). To identify the mechanisms that inhibit CD4 Th1 cell generation in the midst of persistent infection, we isolated naïve LCMV-specific CD4 TCR transgenic (SMARTA) cells and adoptively transferred them into mice one day prior to LCMV-Cl13 infection, or 21 days after LCMV-Cl3 infection (i.e., in an established persistent infection) (Osokine et al., 2014). Importantly, virus-specific CD4 SMARTA T cells mount analogous responses to their endogenous polyclonal LCMV-specific I-Ab restricted GP61–80 CD4 T cell counterparts. On day 8 post-transfer the virus-specific CD4 SMARTA T cells primed at the onset of LCMV-Cl13 infection (termed early primed) differentiated into both Th1 cells (SLAMhi CXCR5lo) and Tfh cells (SLAMlo CXCR5hi), whereas virus-specific CD4 SMARTA T cells primed in the midst of persistent viral infection (termed late primed) almost exclusively generated Tfh (Figure 1A and (Osokine et al., 2014)). The suppression of late Th1 priming was overcome by blocking IFN-I signaling prior to priming (Figure 1A), but interestingly the effect was indirect on the T cells (Osokine et al., 2014). IFNβ is associated with the suppressive qualities of the IFN-I system (Ng et al., 2015) however, similar to untreated WT mice, no enhancement of Th1 priming was observed in IFNβ−/− mice (Figure S1A). Thus, our data suggest that IFNβ alone does not mediate the Th1 suppressive capacity in the midst of persistent viral infection.
Figure 1. Dual PD-L1 and IL-10 expression suppress de novo CD4 Th1 generation during an established persistent virus infection.
Mice infected for late prime experiments were CD8 depleted prior to LCMV-Cl13 infection to prevent control of infection by blocking antibodies. Virus-specific CD4 SMARTA T cells were transferred into mice 1 hour prior to LCMV-Cl13 infection (Early priming) or into mice infected 21 days earlier with LCMV-Cl13 (Late priming). One day prior to late priming, mice were treated with either isotype control, anti-IFNAR, anti-IL-10R, anti-PD-L1, or anti-PD-L1 and IL-10R (dual block - DB) blocking antibodies. Antibody treatment continued on day 1, day 4 and day 7 post priming. Analysis was performed on spleen at day 8 after priming.
A. Flow plots depict the frequency and bar graphs the number of CD4 Th1 (SLAMhi CXCR5lo) and Tfh (SLAMlo CXCR5hi) cells or IFNγ and TNFα producing SMARTA CD4 T cells
B. Flow plots and bar graphs depict the frequency of IFNγ, IFNγ and TNFα, and granzyme B producing SLAM+ Th1 (red) and SLAM− Tfh (black) SMARTA cells following ex vivo peptide stimulation. Also depicted in a bar graph is the geometric mean fluourescent intensity (GMFI) of IFNγ on Th1 and Tfh SMARTA cells.
* p < 0.05. Data are representative of 3–4 independent experiments with 3 – 6 mice per group. See also Figure S1.
IFN-I drives IL-10 expression and IL-10 is well recognized to suppress Th1 differentiation (Cunningham et al., 2016; Moore et al., 2001). To test the role of IL-10 in preventing new CD4 Th1 cell generation, an anti-IL-10R antibody was used. All mice were depleted of CD8 T cells prior to infection to prevent the decrease in virus titers by anti-IL-10R (Brooks et al., 2006; Ejrnaes et al., 2006) or other blocking antibodies (Barber et al., 2006) in persistent LCMV infection. Unexpectedly, IL-10R blockade had minimal restorative effect for late primed CD4 Th1 cell differentiation compared to isotype antibody treated mice (Figure 1A). IFN-I signaling also directly induces PD-L1 expression during viral persistence (Cunningham et al., 2016), and although PD-L1 is not specifically identified to inhibit CD4 Th1 differentiation, its CD8 T cell suppressive capacity is well established (Barber et al., 2006). PD-L1 blockade alone induced a moderate increase in CD4 Th1 cell priming during persistent infection, however the simultaneous blockade of both PD-L1 and IL-10R completely restored CD4 Th1 differentiation, in fact exceeding the recovery generated by blocking IFNAR (Figure 1A). The Th1 cells that formed after the dual IL-10R and PD-L1 blockade produced increased IFNγ, TNFα and granzyme B (Figure 1A and 1B). Consistent with previous data, a proportion of Tfh cells also produced IFNγ after dual blockade (Fahey et al., 2011), but at a substantially decreased percentage of the population and dramatically lower per cell levels than Th1 cells (Figure 1B). Inhibition of late Th1 priming and its restoration upon PD-L1 and IL-10R blockade was also observed using CD4 TCR transgenic cells specific for the LCMV-nucleoprotein311–325 (NIP TCR-transgenic mice) (Nance et al., 2015) (Figure S1B), indicating the effect was a general biological mechanism and not due to a specific TCR or a particular LCMV epitope.
IFN-I signaling upregulates PD-L1 expression by DCs (activation inducible PD-L1), but does not control the basal level of PD-L1 expression on DCs in the absence of inflammatory signals (Wilson et al., 2013). Consistent with regulating activation induced expression, PD-L1 expression on DCs was decreased by anti-IFNAR treatment, whereas it was completely blocked by anti-PD-L1 (Figure S1C), suggesting that the remaining basal levels of PD-L1 following anti-IFNAR blockade further diminish Th1 priming. Changes in virus titers in the CD8 T cell depleted mice are not responsible for the enhanced Th1 priming as increased Th1 differentiation was observed in IL-10R/PD-L1 dual blocked mice where virus loads were slightly decreased, as well as in anti-IFNAR treated mice where titers were increased due to the loss of IFN-I antiviral activity (Figure S1D). Thus, IFN-I mediated induction of the suppressive factors IL-10 and PD-L1 combinatorially suppress de novo CD4 Th1 priming and the production of polyfunctional cytokine producing CD4 T cells during an established persistent virus infection.
Dendritic cells (DCs) are required for priming CD4 T cells in the midst of persistent LCMV infection (Osokine et al., 2014). We recently identified two populations of DCs that emerge during persistent infection, stimulatory (stim) DCs that robustly stimulate CD4 T cells and suppressive (supp) DCs that are induced by IFN-I to express multiple inhibitory molecules, including PD-L1 and IL-10 (Cunningham et al., 2016). Of note, suppDCs have similar or elevated expression of MHC I and II and of costimulatory molecules CD80 and CD86 when compared to stimDCs (Cunningham et al., 2016; Wilson et al., 2012). To determine how these different DC subsets affect Th1 priming, we isolated suppDCs and stimDCs from LCMV-Cl13 infected mice and cultured them with naïve LCMV-specific CD4 T cells. SuppDCs suppressed CD4 Th1 priming, generating a lower frequency and particularly number of T-bet expressing virus-specific CD4 T cells, as well as fewer IFNγ producing cells than the stimDCs (Figure 2A). PD-L1 and IL-10R blockade reversed suppDC mediated inhibition and enabled robust CD4 Th1 activation and IFNγ production even greater than observed by the stimDC (Figure 2A), indicating that interaction of naïve CD4 T cells with IL-10/PD-L1 producing suppDCs from persistent virus infection potently inhibits de novo CD4 Th1 cell priming. Additionally, virus-specific CD4 SMARTA T cells primed in persistently infected CD11c-Cre IFNARfl/fl mice exhibited increased Th1 generation compared to late priming in wildtype mice (Figure 2B), confirming DCs as the target of IFN-I suppression of de novo CD4 Th1 differentiation in vivo in the midst of persistent viral infection.
Figure 2. IFN-I induced PD-L1 and IL-10 expression on suppressive DCs inhibit the priming of Th1 cells.
A. Stimulatory (stim) DCs and suppressive (sup) DCs from LCMV-Cl13 infected mice were co-cultured for 5 days with naïve CD4 SMARTA T cells in the presence of isotype or anti-PD-L1 + anti-IL-10R blocking antibodies (DB). Flow plots show the frequency and bar graphs the number of activated Th1+ (CD62Llo T-bet+) and IFNγ producing CD4 SMARTA T cells. No exogenous peptide was added to the cultures for stimulation. Data are shown from 1 of 2 experiments using DC pooled from 20 mice to obtain adequate numbers of each population.
B. Virus-specific CD4 SMARTA T cells were transferred into wildtype or CD11c-Cre IFNARfl/fl mice infected 21 days earlier with LCMV-Cl13. Flow plots depict the frequency and bar graphs the number of CD4 Th1 and Tfh SMARTA cells in the spleen 8 days after priming.
* p < 0.05. Data are representative of 2 independent experiments with 5 mice per group.
IL-10 and PD-L1 inhibit Th1 differentiation at the onset of acute and persistent virus infection
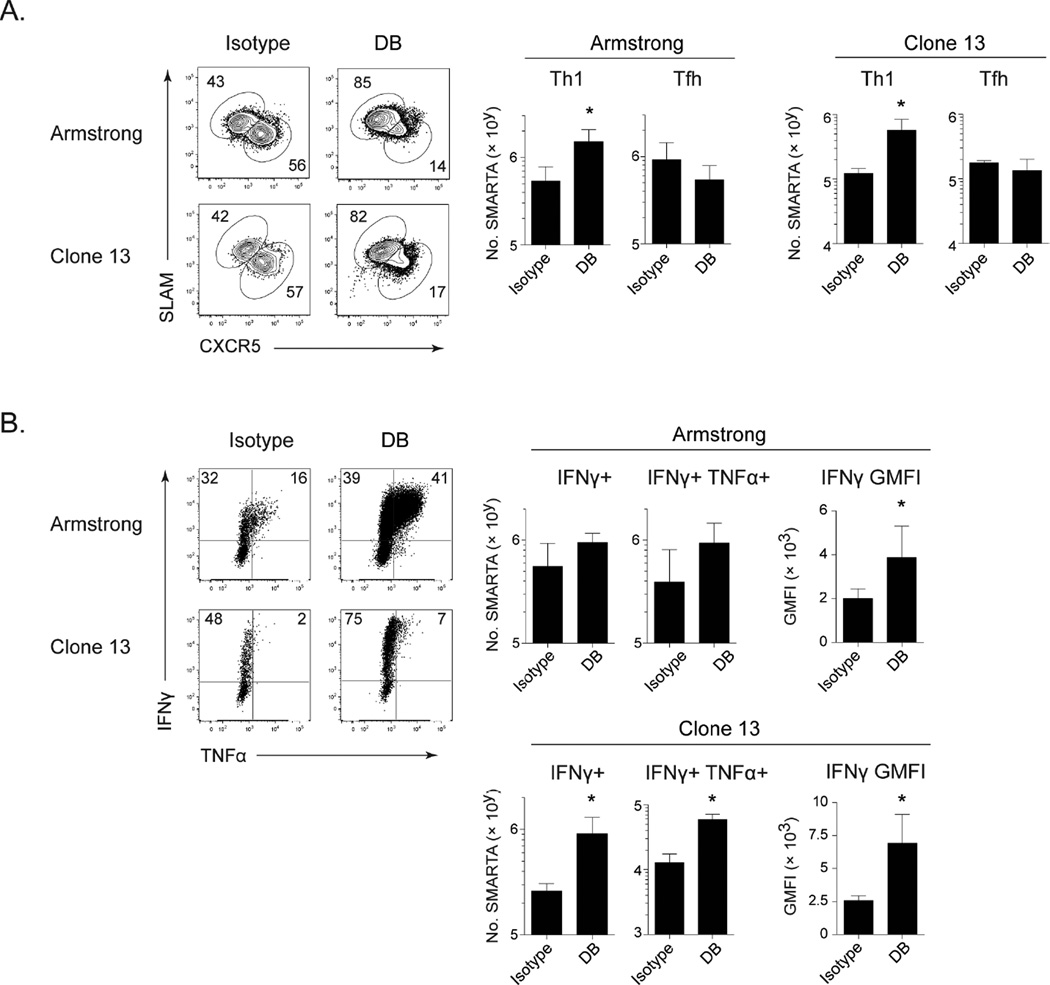
IL-10 and PD-L1 are also expressed early in viral infection (Barber et al., 2006; Brooks et al., 2010). To investigate their role in suppressing CD4 Th1 differentiation at the onset of infection, PD-L1 and IL-10R were blocked prior to acute LCMV-Armstrong (Arm) infection or persistent LCMV-Cl13 infection. To avoid the mortality associated with anti-PD-L1 treatment at the onset of LCMV-Cl13 infection (Barber et al., 2006) mice were CD8 depleted prior to infection. Similar to their effects during persistent infection, IL-10R plus PD-L1 blockade at the onset of acute and persistent LCMV did not affect overall CD4 Tfh responses, but dramatically increased the number of SLAM+/Tbet+ virus-specific CD4 Th1 cells, almost entirely reorienting the CD4 T cell response toward Th1 (Figure 3A and S2). PD-L1 and IL-10R blockade increased the number of IFNγ and of dual IFNγ/TNFα producing SMARTA cells in persistent LCMV infection, as well as the level of IFNγ produced per cell in the acute and persistent infections (Figure 3B). Thus, IL-10 and PD-L1 suppress CD4 Th1 differentiation at the onset of acute and persistent viral infection.
Figure 3. PD-L1 and IL-10 suppress Th1 responses at the onset of acute and persistent viral infection.
Virus-specific CD4 SMARTA T cells were transferred into mice 1 day prior to LCMV-Arm or LCMV-Cl13 infection. LCMV-Cl13 infected mice were CD8 depleted prior to infection. Mice were treated with anti-PD-L1 and anti-IL-10R or isotype control on day 0, 2, 5 and 8 after infection. Flow plots depict the frequency and bar graphs the number of (A) CD4 Th1 (SLAMhi CXCR5lo) and Tfh (SLAMlo CXCR5hi) SMARTA cells and (B) IFNγ and TNFα production and IFNγ GMFI by splenic CD4 SMARTA T cells 9 days after infection. Data are representative of 2 independent experiments with 4 mice per group. * p < 0.05. See also Figure S2.
In vitro Th1 polarized virus-specific CD4 T cells maintain Th1 responses
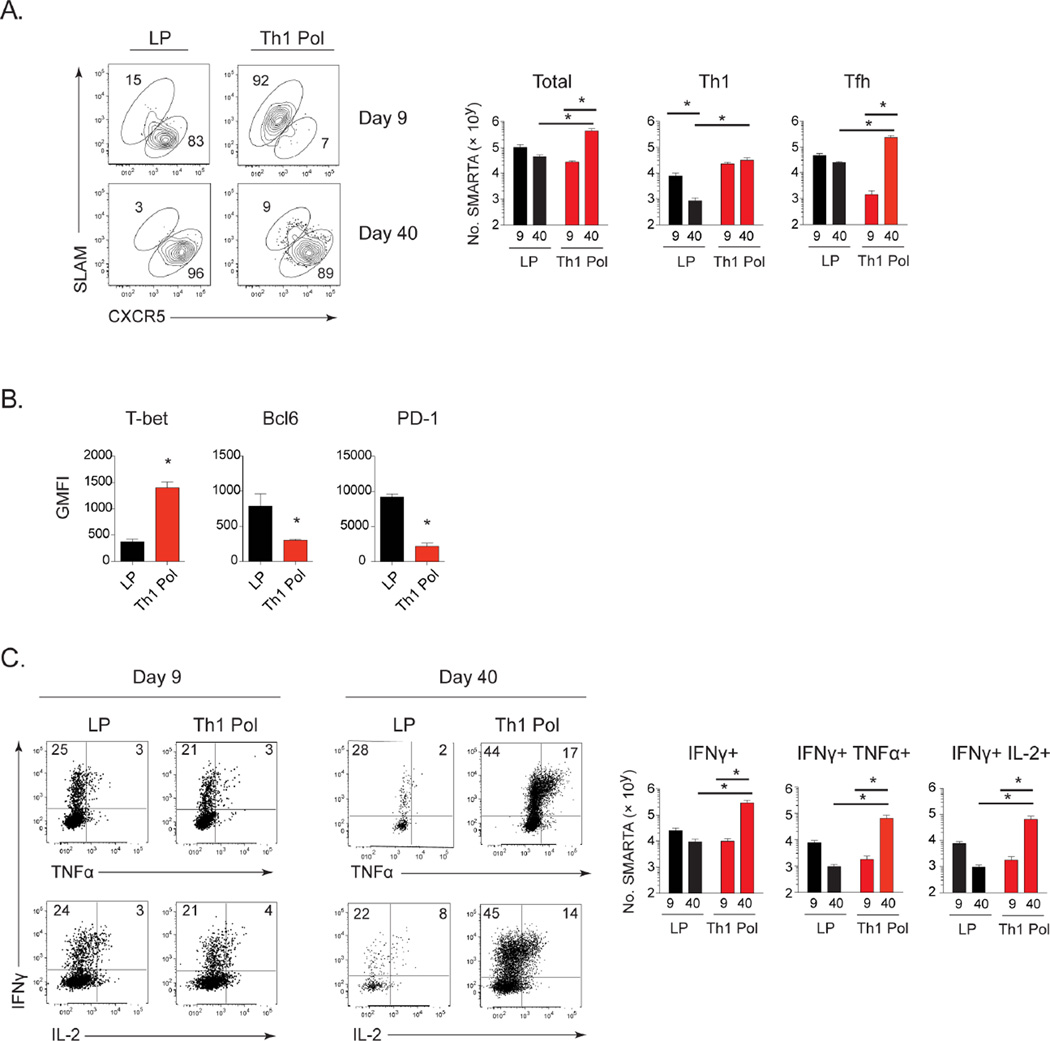
Viral persistence converts CD4 Th1 cells to Tfh and inhibits new Th1 cell generation (Fahey et al., 2011; Osokine et al., 2014). Thus, whether generation of new Th1 cells can be maintained to provide help for CD8 T cells in persistent infection is unclear. To investigate the sustainability of CD4 Th1 cells during viral persistence without the multiple direct and indirect effects induced by PD-L1 and IL-10R blockade, virus-specific CD4 SMARTA T cells were Th1 polarized in vitro for 5 days and then transferred into persistently infected mice 26 days after infection. Further, mice were CD4 depleted prior to LCMV infection to generate a life-long viremic infection and enable long-term analysis of CD4 Th1 cells in the continued presence of high levels of virus replication (Matloubian et al., 1994). Depletion of CD4 T cells prior to infection does not alter late primed CD4 T cell differentiation or function (Osokine et al., 2014). In parallel, naïve CD4 SMARTA T cells were transferred into a cohort of mice on day 21 after LCMV-Cl13 infection for in vivo late priming to enable direct comparison with cells primed at the same time that become Tfh after priming in vivo (late primed). The number of Th1 polarized cells for transfer was chosen to achieve roughly equal amounts to the in vivo late primed CD4 T cells on day 9 after priming, thus enabling direct comparison (Figure 4A). Prior to transfer, in vitro polarized Th1 cells produced IFNγ and TNFα upon restimulation, expressed the Th1 transcription factor T-bet, high levels of SLAM and little to no expression of CXCR5, confirming Th1 polarization and demonstrating minimal contamination of Tfh (Figure S3A). Four days after transfer into persistent infection (9 days after priming), Th1 polarized virus-specific CD4 T cells maintained cellular and molecular markers of Th1 differentiation, including high expression of SLAM, T-bet, and P-selectin glycoprotein 1 (PSGL1), which is down-regulated in Tfh (Poholek et al., 2010) (Figure 4A, 4B, S3B and S3C). Further, Th1 polarized cells exhibited minimal expression of CXCR5 and the Tfh transcription factor Bcl6, in contrast to the late primed cells that were predominantly Tfh (Figure 4A, 4B, and S3C). PD-1 levels were lower on Th1 polarized cells as compared to late primed CD4 T cells (Figure 4B and S3C), in accordance with T-bet mediated repression of PD-1, and increased PD-1 expression by Tfh (Haynes et al., 2007; Kao et al., 2011). However, upon ex vivo restimulation, the amount of IFNγ producing Th1 polarized virus-specific CD4 T cells was markedly decreased compared to their pre-transfer levels (Figure 4C and S3A) and was now similar to the production by late primed virus-specific CD4 T cells (Figure 4C), suggesting maintenance of CD4 Th1 cells, but exhaustion of Th1 cytokine production upon entry into the persistent infection.
Figure 4. In vitro Th1 polarized virus-specific CD4 T cells sustain long term Th1 responses.
Mice were CD4 depleted prior to LCMV-Cl13 infection to generate a life-long viral infection. Naïve virus-specific CD4 SMARTA T cells were transferred 21 days after LCMV-Cl13 infection (in vivo late prime; LP), or primed in vitro under Th1 polarizing conditions for 5 days (Th1 Pol) and injected into mice at day 26 after LCMV-Cl13 infection.
A. Flow plots demonstrate the frequency and bar graphs the number of total, Th1 (SLAMhi CXCR5lo) and Tfh (SLAMlo CXCR5hi) CD4 SMARTA T cells in the late primed (black) and Th1 polarized (red) SMARTA populations in the spleen at day 9 and day 40 after priming.
B. Bar graphs show GMFI of T-bet, Bcl6 and PD-1 in the in vivo late primed (black) or Th1 polarized (red) SMARTA CD4 T cells at day 9 after priming.
C. Flow plots depict frequency and bar graphs the number of IFNγ, TNFα, and IL-2 producing late primed (black) or Th1 polarized (red) SMARTA CD4 T cells following ex vivo peptide stimulation of splenocytes at day 9 and day 40 after priming.
Data are representative of 3 independent experiments with 3 – 5 mice per group. * p < 0.05. See also Figure S3.
We next determined whether the CD4 Th1 cells could be stably maintained long-term in the presence of an ongoing persistent infection. Whereas total numbers of in vivo late primed virus-specific CD4 T cells stayed relatively similar between day 9 and 40 after priming, the total number of polarized CD4 T cells increased during this same timeframe (Figure 4A). Of the polarized cells initially transferred, the total number of Th1 cells remained the same between day 9 and 40 after priming despite their decrease in proportion (Figure 4A). At day 40 after transfer, the Th1 cells continued to express increased T-bet, decreased PD-1 and lacked Bcl6 (Figure S3D, S3E), confirming the maintenance of Th1 differentiation. Despite initially being a homogeneous Th1 population, a high frequency of the Th1 polarized virus-specific CD4 T cells now expressed CXCR5 and had down-regulated SLAM (Figure 4A), indicating generation of a sustained, polyfunctional CD4 Th1 response that could additionally give rise to Tfh cells during persistent virus infection. Further, the initial functional exhaustion observed at day 9 after transfer of the Th1 polarized cells was no longer evident, and at day 40 a large population of the polarized CD4 Th1 cells expressed IFNγ, TNFα, IL-2, and in particular, there were increased amounts of double and triple cytokine producing cells (Figure 4C). Due to the initial CD4 depletion of mice prior to infection virus titers remained high in all mice (Figure S4A), indicating that the ability to sustain polyfunctional CD4 Th1 cells occurred despite the presence of ongoing viral persistence.
Th1 polarized virus-specific CD4 T cells enhance LCMV-specific CD8 T cells and accelerate viral control
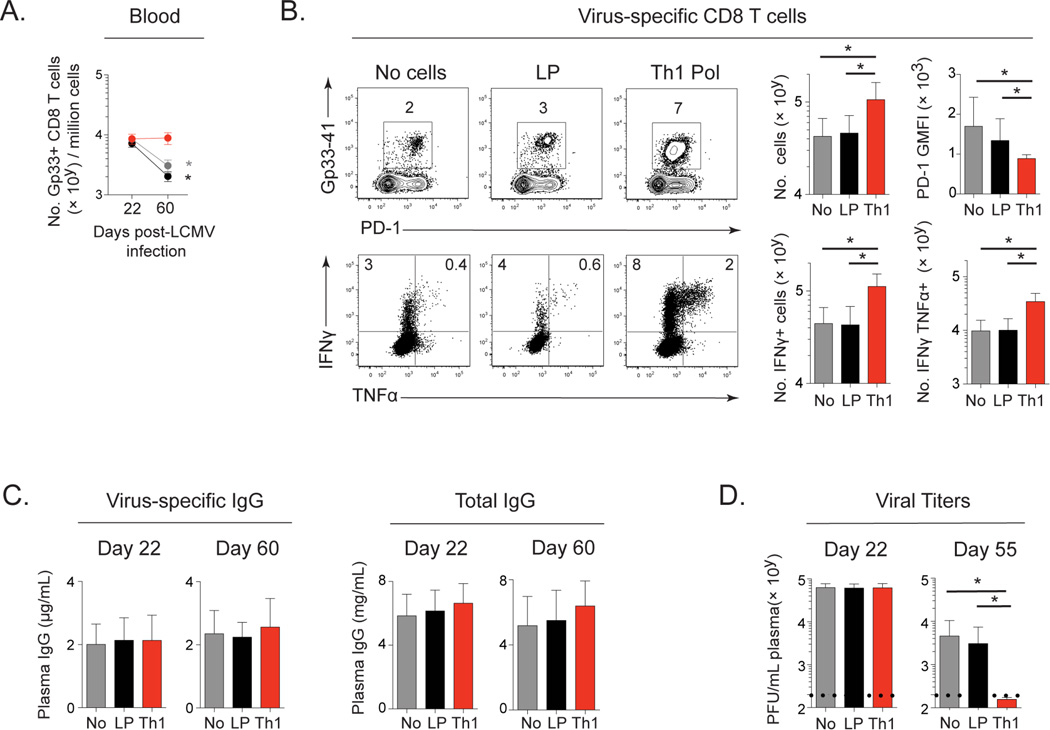
CD8 T cells become progressively exhausted in persistent infection (Wherry et al., 2003), corresponding to the loss of CD4 Th1 cells (Fahey et al., 2011). We next determined how the loss of CD4 Th1 cells as persistent infection progressed contributed to the demise of antiviral immunity. CD4 Th1 polarized cells were transferred into persistently infected mice at a time point when the initial virus-specific CD4 Th1 cells are lost and primarily only CD4 Tfh cells remain (day 27 after infection). In parallel, no SMARTA cells or naïve SMARTA cells were transferred at day 22 after persistent infection to generate Tfh cells. The number of naïve SMARTA and Th1 polarized SMARTA were chosen so that similar numbers of each were observed at day 9 after priming and a relatively small number of CD4 T cells were transferred compared to other studies (Aubert et al., 2011). Interestingly, unlike the progressive decline in LCMV-specific CD8 T cells in persistently infected mice that received naïve (late primed) SMARTA cells or that received no CD4 T cells, in mice that received Th1 polarized cells the total number of LCMV-specific CD8 T cells in the blood was maintained throughout persistent infection (Figure 5A). Further, mice that received Th1 polarized CD4 T cells had increased numbers of LCMV-specific CD8 T cells in the spleen 60 days after infection, as well as increased numbers of cytokine producing CD8 T cells and decreased PD-1 expression compared to those that received no cells or late primed Tfh (Figure 5B). Despite the development of Tfh in the Th1 polarized transfer mice, neither the addition of Th1 polarized nor in vivo activated Tfh cells significantly enhanced LCMV-specific IgG or total IgG over levels generated by endogenous Tfh responses (Figure 5C). Thus, maintaining CD4 Th1 cells sustained and enhanced virus-specific CD8 T cell numbers and function, but did not affect virus-specific IgG levels.
Figure 5. Th1 polarized virus-specific CD4 T cells enhance LCMV-specific CD8 T cells and accelerate viral control.
Virus-specific CD4 SMARTA T cells were primed in vitro under Th1 polarizing conditions for 5 days (Th1) (red) and transferred into mice at day 27 after infection. Additional cohorts of mice received naïve virus-specific CD4 SMARTA T cells (late primed; LP; black) on day 22 after LCMV-Cl13 infection or no CD4 T cells (gray).
A. Kinetics of LCMV Db/GP33–41 tetramer positive CD8 T cell numbers in the blood.
B. LCMV Db/GP33–41 tetramer positive CD8 T cell numbers, PD-1 expression and IFNγ and TNFα producing CD8 T cells following ex vivo peptide restimulation with Gp33 peptide in the spleen at day 60 after infection.
C. LCMV-specific and total plasma IgG.
D. Plasma virus titers.
Data are representative of 3 independent experiments with 5–9 mice per group. * p < 0.05. See also Figure S4.
The effects of sustaining CD4 Th1 help were not due to changes in virus titers since Th1 polarized CD4 T cells similarly enhanced virus-specific CD8 T cell numbers and function when transferred into mice that had been CD4 depleted prior to LCMV-Cl13 infection and that maintain life-long viremic infection (Figure S4A and S4B). In the CD4 depleted system where B cells had not received CD4 help at the onset of infection, the transferred Th1 polarized CD4 T cells stimulated IgG production similar to late primed CD4 Tfh cells, consistent with gradual Tfh accumulation in the Th1 polarized subset (Figure S4C). This is distinctly different from the situation wherein initial CD4 help had been provided but then Th1 cells waned while Tfh were maintained and no further help to antibody production was evident (compare Figure 5C and S4C). Thus, CD4 Th1 transfer enabled help to CD8 T cells and B cells independent of changes in viral titers, when endogenous CD4 T cell help is absent.
The ability of Th1 polarized cells to sustain virus-specific CD8 T cell responses in the absence of enhanced antibody production led us to question whether the reconstitution of CD4 Th1 immunity would enhance viral control. Viral titers were initially similar between the groups at the time of cell transfer (Figure 5D). However, by day 55 after infection, plasma virus titers were below detection in mice that received CD4 Th1 cells, whereas viremia remained elevated in mice that received no cells or late primed Tfh cells (Figure 5D). Thus, the loss of CD4 Th1 cells impedes viral clearance and their maintenance enhances antiviral immunity to accelerate control of an established persistent virus infection.
DISCUSSION
Viral persistence severely restricts the breadth of CD4 T cell help, favoring B cell help over Th1 mediated CD8 T cell help. Yet the mechanisms that inhibit CD4 Th1 differentiation, the stability of Th1 cells if they could be generated, the overall impact of Th1 loss and the disease consequences if these cells were reconstituted in persistent viral infection has remained unclear. Our data demonstrate that PD-L1 and IL-10 expression by a specific population of suppressive DCs inhibits de novo Th1 responses in the midst of persistent viral infection. We demonstrate that the loss of CD4 Th1 cells is a critical immune event driving progressive CD8 T cell decline and dysfunction, thereby impeding control of persistent infection. Strikingly, therapeutically restoring CD4 Th1 cells after they would have otherwise been lost stopped the progressive decline in antiviral CD8 T cell responses, diminished CD8 T cell exhaustion and enabled viral control. Thus, our data indicate that the loss of CD4 Th1 cells is a critical factor leading to the progressive CD8 T cell demise during viral persistence and undermining the ability to control persistent infection.
Prolonged CD4 Th1 responses are associated with enhanced control of persistent virus infections, including HIV and HCV, and the loss of Th1 cells correlates with decreased viral control (Gerlach et al., 1999; Rosenberg et al., 1997; Thimme et al., 2001). Yet it has been difficult to differentiate whether sustained Th1 responses are the cause of virus control or a consequence of the lower virus titers. Our data now answer this question and demonstrate that the maintenance of CD4 Th1 cells prevents the progressive decline in antiviral CD8 T cell numbers and function to accelerate viral control. Unlike help to CD8 T cells when Th1 are lost, the ongoing Tfh response is sufficient to sustain antibody production during viral persistence and no further enhancement in LCMV-specific IgG was afforded by transfer of relatively small amounts of either Th1 polarized or naïve CD4 T cells that became Tfh upon priming. On the other hand, in situations where B cells had never received CD4 T cell help, antibody production was enhanced by transfer of Th1 polarized cells, indicating that even after prolonged periods of time in the persistent infection, B cells retain the ability to produce IgG if help is provided. Interestingly, the initial Th1 polarized cells did not contain Tfh, indicating that the rise of CD4 Tfh cells was derived from Th1 that became Tfh. This is similar to Th1 cells primed at the onset of infection that convert into Tfh, but without the loss of the initial Th1 cells that was observed in these studies (Fahey et al., 2011), suggesting that established CD4 Th1 can give rise to Tfh offspring while maintaining their initial identity. Thus, the loss of CD4 Th1 cells as persistent infection progresses has an important impact on the decay of antiviral CD8 T cell responses and importantly, preventing this loss leads to enhanced CD8 T cell numbers and function and control of persistent infection.
It is important to note that a balanced CD4 Th response consisting of both Th1 and Tfh cells is likely optimal to control virus infection. However, in the situation of viral persistence this balance is disrupted and skewed toward Tfh. Our data now indicate that restoring the Th1 side of the Th1/Tfh balance can complement the already ongoing B cell help promoted by the accumulating Tfh response to simultaneously prevent the decay and sustain the antiviral CD8 T cell responses required to optimally fight persistent viral infection.
There is considerable interest in understanding the mechanisms through which immune check point inhibition restores immune function to control disease. PD-L1 blockade has been shown to augment antigen-specific CD4 T cell responses (Aubert et al., 2011; Barber et al., 2011; Day et al., 2006), however, a specific modulation of Th subsets by PD-L1 as opposed to a general suppression of all CD4 T cells has not been demonstrated. Our data now add an additional mechanism of suppression by PD-L1 during persistent viral infection: the prevention of new CD4 Th1 cells into the antiviral repertoire. Functionally, almost all of the restored CD4 Th1 cells from the dual blockade produced IFNγ in conjunction with additional cytokines/antiviral effector molecules, and these functional Th1 cells produced significantly more of these molecules on a per cell basis. Surprisingly, the effect of PD-L1 and IL-10 was specific to CD4 Th1 cells and had little impact on Tfh cells, demonstrating a bifurcation in the factors and priming cell types that lead to Th1 vs. Tfh fate decisions. In contrast to the midst of persistent infection where Th1 cell priming is significantly impaired, Th1 do form at the onset of infection, but the generation of CD4 Th1 cells can still be further enhanced by blocking IL-10 and PD-L1 signaling. Thus, at the onset of infection PD-L1 and IL-10 most likely serve to dampen Th1 differentiation and diversify the CD4 helper response, as exacerbated Th1 responses are associated with pathology and lethality (Barber et al., 2011; Gazzinelli et al., 1996; Sun et al., 2009). Thus, we now establish an additional mechanism of PD-L1 induced immunosuppression during viral persistence and demonstrate that the joint action of PD-L1 and IL-10 serve as a continuous CD4 Th1 suppressing mechanism throughout persistent infection.
PD-L1 and IL-10 signaling are important suppressive factors in many persistent viral infections, including HIV, SIV and HCV, which also show gradual erosion of CD4 Th1 cells, accumulation of Tfh, and progressively diminished CD8 T cell responses (Feng et al., 2012; Lindqvist et al., 2012; Petrovas et al., 2012). Our data now suggest that the decline in antiviral CD8 T cells in these infections may also be due in part to an inability to maintain and invoke new CD4 Th1 cells. Consistent with the mechanism we observe in persistent LCMV infection, de novo induction of HIV-specific CD4 Th1 cells only occurred in patients with suppressed virus loads (Lichterfeld et al., 2004), suggesting that active viral replication also limits the induction of de novo Th1 responses in multiple persistent infections. Thus, the ability to block PD-L1 and IL-10 and restore Th1 priming may be therapeutically beneficial to generate the CD4 Th1 help needed to sustain and enhance antiviral CD8 T cell responses. Although PD-L1 therapy is most often recognized for its direct effects on antiviral CD8 T cells, it likely functions through multiple mechanisms (Nguyen and Ohashi, 2015) and our data suggest that an additional aspect of PD-L1 blockade therapy may be to enhance CTL responses indirectly by allowing for the generation of new CD4 Th1 cells and the cytokines they produce.
With the success of adoptive immunotherapy to fight persistent virus infection and cancer, the ability to reconstitute and re-establish a patient’s T cells to fight disease is becoming a reality. Our results have important implications for CD4 T cell immunotherapy and demonstrate that therapeutic reconstitution of CD4 Th1 cells can accelerate control of persistent infection. Thus, de novo induction and/or adoptive transfer of antigen-specific CD4 Th1 cells might provide enhanced and prolonged help to sustain CTL responses and facilitate viral control. Similarly this approach has been hypothesized for cancer immunotherapy (Dobrzanski, 2013). Indeed, in various cancer models, polarized CD4 Th1 cells and the cytokines they produce can mediate tumor rejection, typically through a CD8 T cell dependent manner (Moeller et al., 2007; Nishimura et al., 1999), indicating that the ability to maintain strong Th1 effector functions over time in the tumor setting may also be pivotal for prolonged CD8 T cell help. Since CD4 T cells are central to sustain the diverse facets of the immune response in situations of chronic antigen stimulation, future immunotherapeutic strategies should consider the reconstitution of CD4 Th1 cells in their regiments, but should keep mindful of how the antigenic environment can prevent new and skew established CD4 Th1 cell responses.
EXPERIMENTAL PROCEDURES
Mice and Virus Infection
C57BL/6 mice were purchased from Jackson Laboratories, or the rodent breeding colony at the University of California, Los Angeles or at the Princess Margaret Cancer Center. LCMV-GP61–80 specific transgenic (SMARTA) mice have been characterized previously and were bred on a Ly5.1 background. LCMV-NP311-25 specific transgenic (NIP) mice (Nance et al., 2015) and IFNβ−/− mice were generously provided by Dr. Shane Crotty (La Jolla Institute for Allergy and Immunology) and Dr. Eleanor Fish (University of Toronto) respectively. CD11c-Cre IFNARfl/fl mice were generated by crossing CD11c-Cre mice (Jackson Laboratories) by IFNARfl/fl mice (Detje et al., 2009) generously provided by Dr. Ulrich Kalinke (Hannover Medical School). All mice were housed under specific-pathogen free conditions. Animal studies were approved by the UCLA Chancelor’s Animal Research Committee (ARC) or the OCI Animal Care Committee at the Princess Margaret Cancer Center/University Health Network. Mice were infected intravenously in the retro-orbital sinus with 2×106 PFU of LCMV-Armstrong or Clone 13. Viral stocks were prepared and titered as previously described (Brooks et al., 2005).
In vivo cell depletion and blocking antibody treatments
Mice were administered 125 µg of anti-CD4 antibody (GK1.5) or anti-CD8α (2.43) 4 days and then 1 day prior to infection. CD4 or CD8 depletion was confirmed with flow cytometry using a non-blocking CD4 clone (RM4.4) or an antibody to CD8β. For in vivo blocking experiments either 500 µg anti-IFNAR (MAR1), 250 µg anti-PD-L1 (10F.9G2), 250 µg anti-IL-10R (1B1.3A), 250 µg anti-PD-L1 plus 250 µg IL-10R, or appropriate isotype controls (HPRN, LTF-2 or mouse IgG1) were administered one day prior to CD4 T cell transfer and then at day 1 post priming and every 3 days following for 2 treatments. Anti-IFNAR dose was decreased to 250 µg for the last 2 injections. In blocking experiments during acute and persistent LCMV infection, mice were administered 250 µg of anti-PD-L1 and anti-IL-10R or isotype controls at days 0, 2, 5 and 8 of infection. In vivo blocking and depletion antibodies were obtained from BioXcell, except anti-IFNAR which was obtained from Leinco Technologies.
Isolation and polarization of virus specific CD4 T cells
CD4 T cells were isolated from the spleens of naïve SMARTA or NIP transgenic mice using a negative selection mouse CD4 T cell enrichment kit (Stem Cell Technologies). For in vivo priming, 5000 naïve SMARTA or NIP transgenic cells were injected i.v. via the retro-orbital sinus at day 21 or day 22 post LCMV-Cl13 infection. For in vitro polarization, naïve SMARTA cells were activated with plate bound anti-CD3 (10µg/mL) and soluble anti-CD28 (1 µg/mL). Cells were further cultured with IL-12 (10 ng/mL) and anti-IL-4 (10 µg/mL) for Th1 polarization. Antibodies were purchased from BioXcell and cytokines from Peprotech Inc. T cells were polarized for 5 days with a media and cytokine change on day 3 of culture. Five days after in vitro activation, 500,000 Th1 polarized SMARTA cells were transferred into day 26 or day 27 LCMV-Cl13 infected mice. This time point was chosen to temporally match time after priming with the late primed cells. Transfer of this amount of in vitro Th1 polarized cells was chosen to attain equal numbers of in vivo late primed and in vitro primed SMARTA at day 9 after priming. For in vivo priming at the onset of LCMV-Arm or LCMV-Cl13 infection, 1000 and 5000 SMARTA cells were respectively transferred 1 day prior to LCMV infection.
Tissue Isolation, intracellular cytokine restimulation, and flow cytometry
Single cell suspensions were prepared from all organs. Cells isolated from organs were stained ex vivo using antibodies to CD4 (GK1.5), CD8 (53–6.7), CD45.1 (A20), SLAM (TC15-12F12.2), CXCR5, (2G8), PSGL1 (2PH1), Ly6C (HK1.4), PD-1 (29F.1A12), PD-L1 (10F.9G2), IL-10R (1B1.3A). Dendritic cells were identified by staining for CD45 (30-F11), CD11c (N418), CD11b (M1/70), CD95 (15A7), CD39 (24DNS1), CD90.2 (30-H12), NK1.1 (PK136) and Ly6G (1A8). All antibodies were from Biolegend with the exception of CXCR5 (BD Biosciences). Staining for T-bet (4B10) (Biolegend), Bcl6 (K112-91) (BD Biosciences), and Granzyme B (GB11) (Biolegend) was performed as directed using the Foxp3 staining kit (EBiosciences). H-2Db Gp33 tetramer was obtained from the NIH Tetramer core. Samples were run on a FACS Verse (BD Biosciences) and data analyzed using Flow Jo software (Treestar).
For cytokine quantification, splenocytes were restimulated for 5 hours at 37°C with 5 µg/ml of MHC class II-restricted LCMV peptide GP61–80 in the presence of 50 U/ml recombinant murine IL-2 and 1 mg/ml brefeldin A (Sigma). Pre-transfer polarized T cells were restimulated with 50 ng/mL PMA + 1 µM ionomycin for 5 hours to assess cytokine production. Following the 5 hours in vitro restimulation, cells were stained with a fixable viability stain, zombie aqua (Biolgend), extracellularly stained as above with CD4, CD45.1, and fixed, permeabilized and stained with IFNγ (XMG1.2), TNFα (MP6-XT22), and IL-2 (JES6-5H4) (Biolgend).
APC Subset sort and in vitro T cell stimulation
Sorting of suppressive and stimulatory DC was carried out as previously described (Cunningham et al., 2016). Briefly, mouse splenocytes were pooled and enriched for CD11c positive cells using CD11c MicroBeads (Miltenyi) and an autoMACS (Miltenyi). Enriched cells were extracellularly stained for suppressive DCs (CD45+, CD11c hi, CD11b+, CD95hi, CD39hi, CD90.2−, NK1.1−, Ly6G−) and stimulatory DCs (CD45+, CD11c hi, CD11b+, CD95lo, CD39lo, CD90.2−, NK1.1−, Ly6G−) and FACSorted on a Moflo Astrios (Beckman Coulter). Post sort purity was >95%. Antigen specific CD4+ T cells (SMARTA) were isolated from the spleens of naïve mice as above and cultured for 5 days with sorted iregDCs or stimDCs at a ratio of 2:1 T cells to dendritic cell populations (80,000 T cells to 40,000 DCs). Antibodies against PD-L1 (1F.9G2) and IL-10R (1B1.3A) or appropriate isotype controls (HPRN and LTF-2) were added at a concentration of 10µg/mL to the appropriate wells. For IFNγ quantification the cultures were stimulated with 50 ng/mL PMA + 1 µM ionomycin in the presence of 1 mg/ml brefeldin A (Sigma) for the last 5 hours of incubation.
Antibody ELISA
Plasma total IgG or LCMV-specific IgG ELISAs were performed using Maxisorp ELISA plates (Nunc) coated overnight with goat anti-mouse IgG (Southern Biotech) or 5 ×10^6 PFU/well of LCMV-Cl13. Plates were blocked with BSA/PBS/0.05% Tween20. Serial dilutions of mouse plasma were incubated on the plates, washed and incubated with HRP-labeled goat anti-mouse IgG (Life Technologies) followed by the addition of the substrate o-phenylenediamine in 0.05 M phosphate buffer. The reaction was stopped with 2 N H2SO4 and the OD values read at 490 nm. Antibody concentrations were quantified by interpolating plasma dilutions falling within a standard curve (500 – 0.245 ng) generated from serial dilutions of purified mouse IgG (Aviva Systems Biology Corporation).
Statistical Analysis
Student t tests (2 tailed, unpaired) and Mann-Whitney nonparametric tests (two tailed, unpaired) were performed using Graph Pad Prism Software.
Supplementary Material
Acknowledgments
We thank past and present members of the Brooks laboratory for technical help and discussion. We thank Shane Crotty for providing LCMV-NP311-25 specific transgenic (NIP) mice, Eleanor Fish for providing IFNβ−/− mice and Ulrich Kalinke for providing IFNARfl/fl mice. This work was supported by the National Institutes of Health (NIH) grants AI085043, AI109627, the Stein/Oppenheimer Award, the Scotiabank Research Chair to D.G.B, a training grant from the Fonds de la Recherche en Santé du Québec to L.M.S, a Microbial Pathogenesis Training Grant T32-AI07323 to I.O, and a dissertation year fellowship to D.H.Y.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
L.M.S., I.O., D.G.B. designed research. L.M.S., I.O., D.H.Y., J.R.F. H.J.E. performed experiments. L.M.S., I.O., D.H.Y. analyzed data. L.M.S. and D.G.B. wrote the paper.
REFERENCES
- Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160:521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert RD, Kamphorst AO, Sarkar S, Vezys V, Ha SJ, Barber DL, Ye L, Sharpe AH, Freeman GJ, Ahmed R. Antigen-specific CD4 T-cell help rescues exhausted CD8 T cells during chronic viral infection. Proc Natl Acad Sci U S A. 2011;108:21182–21187. doi: 10.1073/pnas.1118450109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber DL, Mayer-Barber KD, Feng CG, Sharpe AH, Sher A. CD4 T cells promote rather than control tuberculosis in the absence of PD-1-mediated inhibition. J Immunol. 2011;186:1598–1607. doi: 10.4049/jimmunol.1003304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- Battegay M, Moskophidis D, Rahemtulla A, Hengartner H, Mak TW, Zinkernagel RM. Enhanced establishment of a virus carrier state in adult CD4+ T-cell-deficient mice. J Virol. 1994;68:4700–4704. doi: 10.1128/jvi.68.7.4700-4704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergthaler A, Flatz L, Verschoor A, Hegazy AN, Holdener M, Fink K, Eschli B, Merkler D, Sommerstein R, Horvath E, et al. Impaired antibody response causes persistence of prototypic T cell-contained virus. PLoS Biol. 2009;7:e1000080. doi: 10.1371/journal.pbio.1000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DG, Teyton L, Oldstone MB, McGavern DB. Intrinsic functional dysregulation of CD4 T cells occurs rapidly following persistent viral infection. J Virol. 2005;79:10514–10527. doi: 10.1128/JVI.79.16.10514-10527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006;12:1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DG, Walsh KB, Elsaesser H, Oldstone MB. IL-10 directly suppresses CD4 but not CD8 T cell effector and memory responses following acute viral infection. Proc Natl Acad Sci U S A. 2010;107:3018–3023. doi: 10.1073/pnas.0914500107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41:529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CR, Champhekar A, Tullius MV, Dillon BJ, Zhen A, de la Fuente JR, Herskovitz J, Elsaesser H, Snell LM, Wilson EB, et al. Type I and Type II Interferon Coordinately Regulate Suppressive Dendritic Cell Fate and Function during Viral Persistence. PLoS Pathog. 2016;12:e1005356. doi: 10.1371/journal.ppat.1005356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- Detje CN, Meyer T, Schmidt H, Kreuz D, Rose JK, Bechmann I, Prinz M, Kalinke U. Local type I IFN receptor signaling protects against virus spread within the central nervous system. J Immunol. 2009;182:2297–2304. doi: 10.4049/jimmunol.0800596. [DOI] [PubMed] [Google Scholar]
- Dobrzanski MJ. Expanding roles for CD4 T cells and their subpopulations in tumor immunity and therapy. Front Oncol. 2013;3:63. doi: 10.3389/fonc.2013.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejrnaes M, Filippi CM, Martinic MM, Ling EM, Togher LM, Crotty S, von Herrath MG. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J Exp Med. 2006;203:2461–2472. doi: 10.1084/jem.20061462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324:1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey LM, Wilson EB, Elsaesser H, Fistonich CD, McGavern DB, Brooks DG. Viral persistence redirects CD4 T cell differentiation toward T follicular helper cells. J Exp Med. 2011;208:987–999. doi: 10.1084/jem.20101773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Hu X, Guo H, Sun X, Wang J, Xu L, Jiang Z, Xu B, Niu J, Jiang Y. Patients with chronic hepatitis C express a high percentage of CD4(+)CXCR5(+) T follicular helper cells. J Gastroenterol. 2012;47:1048–1056. doi: 10.1007/s00535-012-0568-1. [DOI] [PubMed] [Google Scholar]
- Frohlich A, Kisielow J, Schmitz I, Freigang S, Shamshiev AT, Weber J, Marsland BJ, Oxenius A, Kopf M. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 2009;324:1576–1580. doi: 10.1126/science.1172815. [DOI] [PubMed] [Google Scholar]
- Gazzinelli RT, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kuhn R, Muller W, Trinchieri G, Sher A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- Gerlach JT, Diepolder HM, Jung MC, Gruener NH, Schraut WW, Zachoval R, Hoffmann R, Schirren CA, Santantonio T, Pape GR. Recurrence of hepatitis C virus after loss of virus-specific CD4(+) T-cell response in acute hepatitis C. Gstroenterology. 1999;117:933–941. doi: 10.1016/s0016-5085(99)70353-7. [DOI] [PubMed] [Google Scholar]
- Haynes NM, Allen CD, Lesley R, Ansel KM, Killeen N, Cyster JG. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J Immunol. 2007;179:5099–5108. doi: 10.4049/jimmunol.179.8.5099. [DOI] [PubMed] [Google Scholar]
- Hegazy AN, Peine M, Helmstetter C, Panse I, Frohlich A, Bergthaler A, Flatz L, Pinschewer DD, Radbruch A, Lohning M. Interferons direct Th2 cell reprogramming to generate a stable GATA-3(+)T-bet(+) cell subset with combined Th2 and Th1 cell functions. Immunity. 2010;32:116–128. doi: 10.1016/j.immuni.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Kao C, Oestreich KJ, Paley MA, Crawford A, Angelosanto JM, Ali MA, Intlekofer AM, Boss JM, Reiner SL, Weinmann AS, Wherry EJ. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nat Immunol. 2011;12:663–671. doi: 10.1038/ni.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichterfeld M, Kaufmann DE, Yu XG, Mui SK, Addo MM, Johnston MN, Cohen D, Robbins GK, Pae E, Alter G, et al. Loss of HIV-1-specific CD8+ T cell proliferation after acute HIV-1 infection and restoration by vaccine-induced HIV-1-specific CD4+ T cells. J Exp Med. 2004;200:701–712. doi: 10.1084/jem.20041270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist M, van Lunzen J, Soghoian DZ, Kuhl BD, Ranasinghe S, Kranias G, Flanders MD, Cutler S, Yudanin N, Muller MI, et al. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J Clin Invest. 2012;122:3271–3280. doi: 10.1172/JCI64314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AS, Patton EA, La Flamme AC, Araujo MI, Huxtable CR, Bauman B, Pearce EJ. Impaired Th2 development and increased mortality during Schistosoma mansoni infection in the absence of CD40/CD154 interaction. J Immunol. 2002;168:4643–4649. doi: 10.4049/jimmunol.168.9.4643. [DOI] [PubMed] [Google Scholar]
- Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller M, Kershaw MH, Cameron R, Westwood JA, Trapani JA, Smyth MJ, Darcy PK. Sustained antigen-specific antitumor recall response mediated by gene-modified CD4+ T helper-1 and CD8+ T cells. Cancer Res. 2007;67:11428–11437. doi: 10.1158/0008-5472.CAN-07-1141. [DOI] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Nance JP, Belanger S, Johnston RJ, Takemori T, Crotty S. Cutting Edge: T Follicular Helper Cell Differentiation Is Defective in the Absence of Bcl6 BTB Repressor Domain Function. J Immunol. 2015;194:5599–5603. doi: 10.4049/jimmunol.1500200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng CT, Snell LM, Brooks DG, Oldstone MB. Networking at the level of host immunity: immune cell interactions during persistent viral infections. Cell Host Microbe. 2013;13:652–664. doi: 10.1016/j.chom.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng CT, Sullivan BM, Teijaro JR, Lee AM, Welch M, Rice S, Sheehan KC, Schreiber RD, Oldstone MB. Blockade of interferon Beta, but not interferon alpha, signaling controls persistent viral infection. Cell Host Microbe. 2015;17:653–661. doi: 10.1016/j.chom.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LT, Ohashi PS. Clinical blockade of PD1 and LAG3--potential mechanisms of action. Nat Rev Immunol. 2015;15:45–56. doi: 10.1038/nri3790. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Iwakabe K, Sekimoto M, Ohmi Y, Yahata T, Nakui M, Sato T, Habu S, Tashiro H, Sato M, Ohta A. Distinct role of antigen-specific T helper type 1 (Th1) and Th2 cells in tumor eradication in vivo. J Exp Med. 1999;190:617–627. doi: 10.1084/jem.190.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osokine I, Snell LM, Cunningham CR, Yamada DH, Wilson EB, Elsaesser HJ, de la Torre JC, Brooks D. Type I interferon suppresses de novo virus-specific CD4 Th1 immunity during an established persistent viral infection. Proc Natl Acad Sci U S A. 2014;111:7409–7414. doi: 10.1073/pnas.1401662111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovas C, Yamamoto T, Gerner MY, Boswell KL, Wloka K, Smith EC, Ambrozak DR, Sandler NG, Timmer KJ, Sun X, et al. CD4 T follicular helper cell dynamics during SIV infection. J Clin Invest. 2012;122:3281–3294. doi: 10.1172/JCI63039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poholek AC, Hansen K, Hernandez SG, Eto D, Chandele A, Weinstein JS, Dong X, Odegard JM, Kaech SM, Dent AL, et al. In vivo regulation of Bcl6 and T follicular helper cell development. J Immunol. 2010;185:313–326. doi: 10.4049/jimmunol.0904023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg ES, Billingsley JM, Caliendo AM, Boswell SL, Sax PE, Kalams SA, Walker BD. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- Sun J, Madan R, Karp CL, Braciale TJ. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med. 2009;15:277–284. doi: 10.1038/nm.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimme R, Oldach D, Chang KM, Steiger C, Ray SC, Chisari FV. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med. 2001;194:1395–1406. doi: 10.1084/jem.194.10.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15:486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson EB, Kidani Y, Elsaesser H, Barnard J, Raff L, Karp CL, Bensinger S, Brooks DG. Emergence of distinct multiarmed immunoregulatory antigen-presenting cells during persistent viral infection. Cell Host Microbe. 2012;11:481–491. doi: 10.1016/j.chom.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson EB, Yamada DH, Elsaesser H, Herskovitz J, Deng J, Cheng G, Aronow BJ, Karp CL, Brooks DG. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science. 2013;340:202–207. doi: 10.1126/science.1235208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009;324:1572–1576. doi: 10.1126/science.1175194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.