Abstract
In this protocol, we use a CyTOF™ mass cytometry to collect single-cell data on a large number of cytokines/chemokines as well as cell-surface proteins that characterize T cells and other immune cells. The current selected mass window in AW 103–203 includes the lanthanides used for most antibody labeling, along with iridium and rhodium for DNA intercalators. The output data are in the format as .txt and .fcs files, which is compatible with many analysis programs. This protocol could be adapted to include tetramers into the staining panel, but we have not optimized for that purpose.
The principal steps of intracellular cytokine staining are as follows: First, cells are activated for a few hours using either a specific peptide or a non-specific activation cocktail. An inhibitor of protein transport (e.g. Brefeldin A) is added to retain the cytokines within the cell. Next, EDTA is added to remove adherent cells from the activation vessel. After washing, antibodies to cell surface markers are added to the cells. The cells are then fixed in paraformaldehyde and permeabilized. We use a gentle detergent, saponin, as the permealization buffer because it is less destructive to surface and intracellular epitopes compared to harsh detergents or methanol. After permeabilization, the metal-conjugated anti-cytokine antibodies are added into the cell suspension. The stained cells are then sequentially introduced into the mass cytometry for signal intensity analysis.
Materials and Reagents
PBMC (fresh or thawed frozen)
RPMI-1640 (Hyclone, catalog number: SH30027.01)
FBS (Atlanta Biologicals, catalog number: S11150)
Pen-strep-Glutamin 100X (Hyclone, catalog number: SV30082.01)
Benzonase (2.5 × 105 U/ml) (Pierce, catalog number: 88701)
Brefeldin A (Sigma-Aldrich, catalog number: B7651)
Monensin (Sigma-Aldrich, catalog number: M5273)
0.5 M EDTA (Hoefer, catalog number: GR-123-100)
Sodium azide (10% w/v solution) (Teknova, catalog number: S0209)
16% para-formaldehyde (PFA) (Alfa Aesar, catalog number: 43368))
10× PBS (ROCKLAND, catalog number: MB-008)
BSA (Sigma-Aldrich, catalog number: A7284)
Maleimide-DOTA (Macrocyclics, catalog number: B-272)
Lanthanum (III) chloride heptahydrate (Sigma-Aldrich, catalog number: 203521)
Indium (III) chloride (Sigma-Aldrich, catalog number: 203440)
-
MilliQ water
Note: Beakers or bottles used here are not washed with soap due to barium content of most commercial soaps.
Phenotyping antibodies (filtered with 0.1 µm spin filters) (Millipore, catalog number: UFC30VV00)
-
Ir-intercalator stock solution (Fluidigm, catalog number: 201192)
Note: Rh103-intercalator can be used.
10× saponin-based permeabilization buffer (eBioscience, catalog number: 00 8333-56)
Phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich, catalog number: P8139)
Ionomycine (Sigma-Aldrich, catalog number: I0634)
Phytohemagglutinin (PHA) (Sigma-Aldrich, catalog number: 61764)
SEB (Sigma-Aldrich, catalog number: S0812)
Anti-CD3/CD28 (various vendors)
Peptide mixes (JPT)
Complete RPMI (see Recipes)
CyPBS (see Recipes)
CyFACS buffer (see Recipes)
Live-dead stain (see Recipes)
Equipment
96- well round-bottom plates
37 °C water bath
Biosafety cabinet
Centrifuge
CO2 incubator at 37 °C
Calibrated pipettes
Procedure
- Thaw PBMC
- Warm complete RPMI media to 37 °C in water bath. Each sample will require 22 ml of media with benzonase. Calculate the amount needed to thaw all samples, and prepare a separate aliquot of warm media with 1:10,000 benzonase (final concentration 25 U/ml). Benzonase is added into the media to prevent dead cell aggregation. Thaw no more than 3 samples at a time. Run one control PBMC with each batch of samples.
- Remove samples from liquid nitrogen and transport to lab on dry ice.
- Place 10 ml of warmed benzonase media into a 15 ml tube, making a separate tube for each sample.
- Thaw frozen vials in 37 °C water bath.
- When cells are nearly completely thawed, carry to hood.
- Add 1 ml of warm benzonase media from appropriately labeled centrifuge tube slowly to the cells, then transfer the cells to the centrifuge tube. Rinse vial with more media from centrifuge tube to retrieve all cells.
- Continue with the rest of the samples as quickly as possible.
- Centrifuge cells at 1,550 rpm (RCF = 473) for 8 min at room temperature.
- Remove supernatant from the cells and resuspend the pellet by tapping the tube.
- Gently resuspend the pellet in 1 ml warmed benzonase media. Filter cells through a 70 micron cell strainer if needed. Add 9 ml more warmed benzonase media to the tube.
- Centrifuge cells at 1,550 rpm (RCF = 473) for 8 min at room temperature. Remove supernatant from the cells and resuspend the pellet by tapping the tube.
- Resuspend cells in 1 ml warm media.
- Count cells with Vicell (or hemocytometer if necessary). To count, take 20 µl cells and dilute with 480 µl PBS in Vicell counting chamber. Load onto Vicell as PBMC with a 1:25 dilution factor.
- Adjust the cell concentration to 5–10 × 106 cells/ml with warm media (no more benzonase at this point).
- Using a multichannel pipette, add 200 µl cells (for at least 1 × 106 cells) into each well of a 96-well deep well plate. Split each sample equally into two or more wells keeping one as an unstimulated control and the others for different types of stimulation.
- Rest overnight (6–18 h) at 37 °C in CO2 incubator.
- Stimulate cells
-
After overnight rest at 37 °C, add the activation reagents and secretion inhibitor (brefeldin A/monensin) to the well for stimulation. Add only the secretion inhibitor to the unstimulated control well. If doing CD107a staining, add CD107a antibody during the stimulation.Notes:
- It is important to avoid solvent toxicity. Final DMSO+ethanol concentration from all sources (peptides, brefeldin A, monensin) should not exceed 0.5%.
- For most cytokines: Use brefeldin A at 10 µg/ml final concentration (see stock preparation table). For CD107 and CD154: Use monensin at 10 µg/ml final concentration (see stock preparation table). For assays combining cytokines and CD107 or CD154: Use brefeldin A and monensin at 5 µg/ml final concentration each.
- Metal-labeled CD107 and CD154 can be added into the culture during the stimulation at a concentration of 2 µg/ml. This allows for staining of target molecules that are re-internalized by cells during the activation process.
- Addition of costimulatory antibodies is optional. Add 1 µg/ml final concentration of CD28 and/or CD49d (labeled antibody can be used if analysis of the marker is desired).
-
Incubate the cells for 4 h (PMA + ionomycin stimulation, PHA + ionomycin stimulation) or 6–8 h (SEB, anti-CD3/CD28 stimulation, peptide stimulation) at 37 °C, in a CO2 incubator.Note: For most cytokines 6–12 h incubation at 37 °C is sufficient; For IL-10 optimal incubatation time is 12–24 h, but it is possible to detect in 6 h.
- At the end of stimulation, add EDTA to a final concentration of 2 mM and incubate for 15 min at room temperature.
-
- Staining
- Wash 2× with 250 µl CyFACS buffer per well 1,550 rpm (RCF = 473), 10 min at room temperature. The same volume and centrifuge conditions are used in additional wash steps before fixation with PFA (step C6).
- Make surface Ab cocktail in CyFACS buffer (filter with 0.1 µm spin filter) 100 µl per reaction. Incubate on ice for 45 min. Use vendor’s recommended concentration (or optimal titer as determined for self-made conjugates) for each antibody.
- Wash 2× in CyFACS buffer.
- Resuspend cells in 100 µl of 1:3,000 diluted 5 mg/ml 115-DOTA maleimide (titrated if new stock) in CyPBS, Incubate 30 min on ice.
- Wash 3× in CyFACS buffer.
- Resuspend in 100 µl of 2% PFA in CyPBS, Incubate 4 °C overnight.
- Wash 2× in 1× eBioscience perm buffer (1× in milliQ water), 2,000 rpm (RCF = 787), 10 min at 4 °C. The same volume and centrifuge conditions are used in the following wash steps.
- Make intracellular staining cocktail in 1× perm buffer and filter with 0.1 µm spin filter, 100 µl per reaction. Incubate on ice for 45 min.
- Wash 3× in CyFACS buffer.
- Resuspend in 100 µl 1:2,000 Ir-Interchelator diluted in 2% PFA (in CyPBS).
- Incubate 20 min at room temp.
- Wash 2× in CyFACS buffer.
- Wash 3× in MilliQ water.
- Resuspend in MilliQ water (1 to 1.5 ml) for running on CyTOF.
Representative data
Recipes
-
Complete RPMI
RPMI1640
10% FBS
1× pen-strep -Glutamine
-
CyPBS
1× PBS without heavy metal contaminants, such as 10× PBS
Made in MilliQ water
-
CyFACS buffer
1× CyPBS with 0.1% BSA
2 mM EDTA and 0.05% sodium azide
Made in MilliQ water
Note: Do not use FBS!
-
Live-dead stain
5 mg/ml maleimide-DOTA loaded with 139-Lanthanum* or 115-Indium*
(*Natural-abundance metal chloride salt used; >95% specified isotope; trace-metal pure 99.99%)
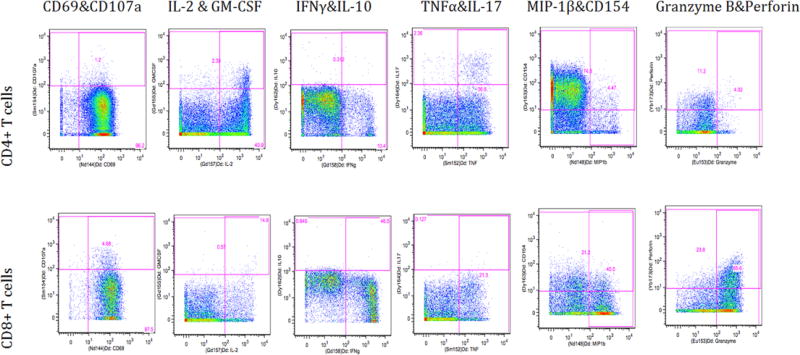
Figure 1.
Flowjo gating of intracelluar markers upon PMA/Ionomycin stimulation
Table 1.
Protein secretion inhibitors
| Reagent | Stock concentration | Intermediate dilution | Final concentration |
|---|---|---|---|
| Brefeldin A | 5 mg/ml in DMSO (stored in aliquots at −20 °C) | 1:10 in PBS | 10 µg/ml (1:50) or 5 µg/ml (1:100) with monensin |
| Monensin | 5 mg/ml in ethanol (stored at −20 °C) | 1:10 in PBS | 10 µg/ml (1:50) or 5 µg/ml (1:100) with brefeldinA |
Table 2.
Activators
| Reagent | Stock concentration | Intermediate dilution |
Final concentration |
|---|---|---|---|
| Phorbol 12-myristate 13-acetate (PMA) | 1 mg/ml in DMSO (stored in aliquots at −20 °C) | 1:1,000 in PBS | 10 ng/ml |
| Ionomycine | 1 mg/ml in DMSO (stored in aliquots at −20 °C) | 1:10 in PBS | 1 µg/ml |
| Phytohemagglutinin (PHA) | 1 mg/ml in DMSO (stored at 4 °C) | 1:10 in PBS | 1 µg/ml |
| SEB | 50 µg/ml in PBS | None | 1 µg/ml (1:50) |
| Anti-CD3/CD28 | Follow manufacturer instruction | - | - |
| Peptide mixes | 0.5–1 mg/ml/pep in DMSO (stored in aliquots at −20 °C) | 1:10 in PBS | 1 µg/ml/peptide (1:50 – 1:100) |
Acknowledgments
This work was supported by grants # S10RR027582 and 5U19AI057229 from the National Institutes of Health. We thank Evan Newell and Mark Davis for help in the initial protocol development.
References
- 1.Bendall SC, Simonds EF, Qiu P, Amir el AD, Krutzik PO, Finck R, Bruggner RV, Melamed R, Trejo A, Ornatsky OI, Balderas RS, Plevritis SK, Sachs K, Pe'er D, Tanner SD, Nolan GP. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science. 2011;332(6030):687–696. doi: 10.1126/science.1198704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maecker HT. Multiparameter flow cytometry monitoring of T cell responses. Methods Mol Biol. 2009;485:375–391. doi: 10.1007/978-1-59745-170-3_25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newell EW, Sigal N, Bendall SC, Nolan GP, Davis MM. Cytometry by time-of-flight shows combinatorial cytokine expression and virus-specific cell niches within a continuum of CD8+ T cell phenotypes. Immunity. 2012;36(1):142–152. doi: 10.1016/j.immuni.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]