Abstract
Post-mastectomy radiotherapy (PMRT) has been shown to improve disease-free survival and overall survival for locally advanced breast cancer. However, long term survivors may develop life threatening acute and chronic treatment-related toxicities after radiotherapy, like cardiac toxicity and second cancers. The more advanced techniques like volumetric arc therapy (VMAT), and proton therapy have the potential to improve treatment outcome by constraining doses to radiosensitive organs, but evidence from outcome study will not be available until years or decades later. Furthermore, the literature is largely incomplete regarding systematic comparison of potential benefits of advanced technologies for PMRT. The purpose of this study was to compare proton therapy, both passively scattered (PSPT) and intensity modulated (IMPT), to VMAT and develop an evidence-based rationale for selecting a treatment modality for left sided post-mastectomy radiotherapy (PMRT) patients. Eight left-sided PMRT patients previously treated with VMAT were included in this study. Planning target volumes (PTV) included the chest wall and regional lymph nodes. PSPT and IMPT plans were created using a commercial proton treatment planning system. The resulting plans were compared to the corresponding VMAT on the basis of dosimetric and radiobiological endpoints. The uncertainties in risk from proton range, set-up errors, and dose-response models were also evaluated. All modalities produced clinically acceptable treatment plans with nearly 100% tumor control probability. Both proton techniques provided significantly lower normal tissue complication probability values for the heart (p < 0.02) and lung (p < 0.001). Patient-averaged second cancer risk for the contralateral breast and lungs were also significantly lower (p < 0.001) with protons compared to VMAT. The findings of this study were upheld by the uncertainty analysis. All three techniques provided acceptable PMRT treatment plans. Proton therapy showed significant advantages in terms of predicted normal tissue sparing compared to VMAT, taking into account possible uncertainties.
Keywords: Breast Cancer, Post-Mastectomy, Volumetric Modulated Arc Therapy, Proton Therapy
1. Introduction
Post-mastectomy radiotherapy (PMRT) has been shown to improve disease-free survival and overall survival for locally advanced breast cancer.1 However, radiation to the chest wall and regional lymphatics carries risks of cardiopulmonary and other toxicities.2–5 While modern PMRT techniques such as volumetric modulated arc therapy (VMAT) minimize high dose radiation to surrounding normal tissues, there is an increase in low dose to normal tissues due to the complex geometry of the treatment field, which can lead to radiation induced complications in organs such as the heart and lungs.6 Comparative treatment planning studies have consistently predicted proton beam therapy can substantially decrease dose to OARs for the treatment of early or locally-advanced breast cancers compared to conventional and IMRT photon techniques.7–11 However, none of these studies considered uncertainties associated with radiation therapies, even though advanced technology like proton therapy is known to contain considerable range and other uncertainties.12 No previous study has compared VMAT and proton therapy for PMRT or predicted risks of radiogenic side effects after these modalities.
The purpose of this study was to compare VMAT, passively scattered proton therapy (PSPT), and intensity modulated proton therapy (IMPT) plans for a clinically representative cohort of PMRT patients. PSPT and IMPT plans were created for eight left-sided PMRT patients who were previously treated clinically with VMAT. The resulting plans were compared based on dosimetric quality and radiobiological calculations, along with a sensitivity analysis of the results.
2. Materials and Methods
2.1 Patient selection
Eight patients that received a left-sided modified radical mastectomy and VMAT radiation treatments at Mary Bird Perkins Cancer Center were consecutively sampled. All patients were planned with both PSPT and IMPT techniques using a commercial treatment planning system (TPS) (Eclipse, version 11, Varian Medical Systems, Palo Alto, CA, USA). The average age was 54 and the average planning target volume (PTV) was 954 cm3. All plans had the same prescription dose, 50.4 Gy (RBE) in 28 fractions, as were the original VMAT plans. Patients were treated in the supine position and the free breathing CT scans were obtained from the top of the head to the lower abdomen. PTVs were drawn by a board certified radiation oncologist for all original VMAT plans and subsequently transferred to the proton TPS. The VMAT PTV also included a 1 cm tissue-equivalent bolus, which was placed on the whole chest wall prior to the patient’s initial CT scan and used during the course of treatment. The planning target volume for PSPT and IMPT was the physician drawn VMAT PTV excluding any portion that extended into the bolus. Recording the dose within the bolus was not necessary for this study, and therefore the original PTV was altered to exclude the bolus for plan evaluation purposes and was renamed the evaluation target volume (TVeval).
2.2 Treatment planning
VMAT treatment plans were created in Pinnacle3 v9.2 by certified radiation dosimetrists following the current standard of care at our institution. Patients were treated on a linear accelerator (Elekta Ltd., Infinity, Crawley, UK), with two 220° 6 MV photon arcs to a prescribed dose of 50.4 Gy in 28 fractions. The beam geometry consisted of a 0 degree couch angle and a 45 degree collimator angle. The SmartArc optimization algorithm was used for VMAT treatment planning.
For PSPT plans, one left-anterior oblique (LAO) field was used for six of the eight patients with beams angles ranging from 25° to 45°. Angles were chosen to be as enface as possible to the chest wall while allowing adequate lateral margins. Due to a suboptimal dose distribution with a single LAO field, patient CW-2 was planned with an anterior-posterior (AP) and LAO field combination with different beam weighting with each field completely covering the PTV. Patient CW-4 was unable to have a PSPT plan due to the extent of the target size and snout size limitation of 25×25 cm2. To be consistent with other patients in this study, we did not try beam abutment for patient CW-4. Beam specific proximal margin (PM), distal margin (DM) and lateral margin (LM) were calculated using a methodology similar to that used in previous proton planning studies11, and following the methods originally outlined by Moyers and Miller13 and Moyers et al.14. Distal margins ranged from 0.6 to 0.7 cm for all patients and a proximal margin of 0.3 cm was used for each patient. Lateral margins varied from 0.8 to 1.2 cm. A smearing radius of 3 mm was used to account for any uncertainty in the alignment of the range compensator to the patient and possible motion of the patient during the treatment. A border smoothing radius of 1 cm (default) was used for all range compensators.
For IMPT plans, two fields were used for all patients: an anterior-posterior (AP) field (0°) and a LAO field with gantry angles ranging from 30° to 45°. Distal, proximal, and lateral margins were added to the plans in the same fashion as the PSPT proton plans. The optimization objectives were similar to the objectives set by the VMAT plans and were consistent with the standard protocol for IMRT chest wall plans. All plans were then reviewed and approved by a board certified radiation oncologist (MS).
2.3 Plan comparison metrics
The photon and proton PMRT treatment plans were compared on dosimetric endpoints. Specifically, target coverage (D95%), dose homogeneity index15, conformity index16, and select dose-volume metrics for the organs at risk (OARs). Dose homogeneity index (DHI) was computed for the TVeval such that
| (1) |
where, Dmax represents the dose to 2% of the TVeval, Dmin denotes the dose to 98% of the TVeval, and DRX is the prescribed dose. A value of zero represents the ideal homogeneity index. The target volume was also evaluated on the conformity index (Cl) given by
| (2) |
where TV is the target volume (TVeval), RI is the reference isodose, and TVRI denotes the volume of the target that is covered by the reference isodose (47.8 Gy RBE). This CI takes into account irradiation of the target volume and irradiation of healthy tissues. The CI ranges from zero to one, where one represents the ideal target conformity.
The photon and proton PMRT treatment plans were also compared on these radiobiological endpoints: tumor control probability (TCP) for the TVeval, secondary cancer complication probability (SCCP) and normal tissue complication probability (NTCP) for select OARs. TCP values were computed for the TVeval using the standard Poisson dose-response model17 assuming homogeneous tumor cell distribution. SCCP was chosen as an endpoint because radiation induced secondary cancers in contralateral breast and lung have been reported for breast cancer patients who received radiotherapy.18, 19 Baseline SCCP values were calculated using the product of the linear organ equivalent dose (OED) and the organ specific absolute cancer incidence rate in percent per gray (Inorg).20 The linear OED is simply the average organ absorbed dose given by,
| (3) |
where vi is the volume receiving dose Di and the summation runs over all voxels of organ T with volume VT. SCCP was calculated for the lungs (Inorg = 1.68% Gy−1) and the contralateral breast (Inorg = 0.78% Gy−1) and these values represent lifetime risk, and assume a residual life expectancy of 50 years.21,22 The Lyman-Kutcher-Burman (LKB) probit model23–26 was used to calculate the NTCP for the lungs with radiation pneumonitis grade two or higher as an endpoint using the following values: D50 = 30.8 Gy, n = 0.99, and m = 0.3726. NTCP for radiation-induced cardiac mortality was computed for the heart using the relative seriality model27 with the following values: D50 = 52.3 Gy, s = 1.0, and γ = 1.2828.
2.4 Uncertainty analysis
We investigated the impact of isocenter shifts (both 5 mm and 10 mm) and proton range uncertainty (by simulating a 3.5% and 10% error in CT number to relative linear proton stopping power conversion) for the representative patient. Since there is uncertainty in the form of the radiation dose-response curve for absorbed dose above 2 Gy, we performed a sensitivity analysis of the risk models for SCCP by including linear-exponential and linearplateau models22 in addition to the linear (baseline) model. These other dose-response models take into account cell killing and sterilization, which may become more important at higher doses. A sensitivity analysis of NTCP was accomplished by varying NTCP model parameters to their respective 95% confidence interval based on literature26,28 for all the patients. Statistical significance was determined for each comparison (VMAT vs PSPT and VMAT vs IMPT) using a paired, two-tailed Student’s t-test and Wilcoxon signed-rank test with a significance level of p = 0.05.
3. Results
Figure 1 plots isodose distributions for a representative patient from the VMAT, PSPT, and IMPT plans. All treatment plans were normalized to have the same mean dose in the TVeval, shown as the red contour. All three techniques had comparable coverage of the TVeval while achieving the desired OARs dose limitations. However, VMAT had noticeably larger volumes of low dose extending beyond the target area and well into the contralateral side; whereas there was minimal dose in those regions for the proton plans. The corresponding dose-volume histogram (DVH) comparisons are shown in Figure 2. Proton therapy, especially IMPT, achieved superior coverage with a noticeably more homogeneous plan and decreased maximum dose or “hot spot”. Substantial normal tissue sparing was observed with both proton techniques compared to VMAT.
Figure 1.
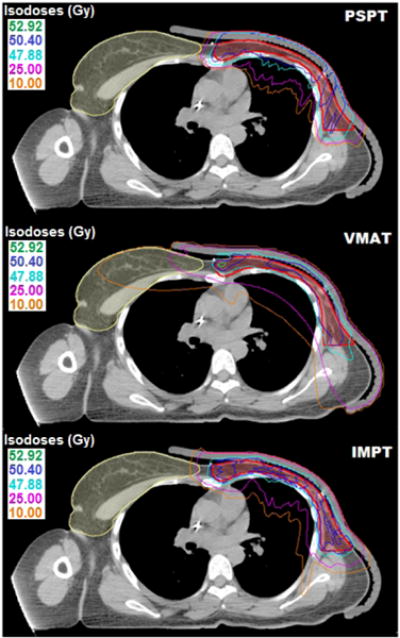
Comparison plans for a representative patient for PSPT, VMAT, and IMPT at the level of VMAT isocenter.
Figure 2.

DVH comparing IMPT (dashed line), VMAT (solid line), and PSPT (dotted line) for the same representative patient seen in Figure 1.
Positional uncertainty or patient set-up error was simulated by a 5 mm and 10 mm isocenter shift in all directions for the representative patient. An isocenter shift of 5 mm in any direction did not cause V95% to fall below the minimal acceptable criteria of 95% for the TVeval for any modality except for a 5 mm shift to the patient’s right for IMPT (94%). However, an isocenter shift of 10 mm caused a larger degradation in TVeval dose coverage, specifically V95%, with a range of 58% to 89%, 79% to 93%, and 94% to 98% for VMAT, IMPT, and PSPT, respectively. Table 1 compares dosimetric and radiobiological characteristics of proton and photon treatment plans. The values represent the means for the eight patients, with PSPT values having a sample size of seven. In general, both protons plans showed a statistically significant advantage in dose coverage of the TVeval and sparing of the OARs. All techniques met the required TVeval coverage, with 95% of the TVeval receiving more than 95% of the prescribed dose (47.9 Gy or Gy RBE), and the volume receiving 107% or more of the prescription dose (53.9 Gy or Gy RBE) was less than 2% of the target volume. All three techniques showed a high degree of conformity, with statistically better conformity in the proton plans. IMPT showed a statistical advantage over PSPT for several of PTV metrics, specifically DV95%, DHI, and CI. IMPT also showed an advantage over PSPT for Dmax to the skin (5 mm shell contour), V5Gy for total lung and contralateral breast. For the other dosimetric and radiobiological metrics no noticeable statistical difference was observed between IMPT and PSPT.
Table 1.
Summary comparison of VMAT and proton plans. Values shown are averages of the eight patients (± 1σ) with the exception of PSPT (n=7). Specific p-values refer to PSPT vs VMAT or IMPT vs VMAT, or IMPT vs PSPT, separately.
| Structure/Item | Metric | VMAT | PSPT | IMPT |
p-value PSPT |
p-value IMPT |
p-value Proton |
|---|---|---|---|---|---|---|---|
| TVeval | DV95% (Gy RBE) | 47.8 ± 0.6 | 48.6 ± 0.4 | 49.4 ± 0.4 | 0.043 | <0.001 | 0.001 |
| VD107% (%) | 0.2 ± 0.3 | 0.1 ± 0.2 | 0.003 ± 0.005 | 0.628 | 0.134 | 0.174 | |
| DHI | 0.13 ± 0.02 | 0.085 ± 0.018 | 0.058 ± 0.014 | 0.013 | <0.001 | 0.005 | |
| CI | 0.72 ± 0.08 | 0.81 ± 0.03 | 0.86 ± 0.02 | 0.016 | 0.001 | <0.001 | |
| TCP (%) | 98.8 ±2.6 | 99.9 ±0.2 | 100.0 ±0.1 | 0.220 | 0.220 | 0.349 | |
|
| |||||||
| Skin | Mean dose (Gy RBE) | 50.9 ±0.3 | 50.6 ±0.1 | 50.5 ±0.0 | 0.02* | 0.006 | 0.119 |
| Dmax (Gy RBE) | 52.9 ± 0.4 | 52.4 ± 0.3 | 51.7 ± 0.3 | 0.086 | <0.001 | 0.004 | |
| Dmin (Gy RBE) | 47.5 ±1.8 | 47.5 ±0.9 | 49.0 ±0.5 | 0.724 | 0.046* | 0.063 | |
|
| |||||||
| Total lung | V5Gy (%) | 53.2 ± 10.6 | 13.1 ±4.6 | 19.0 ±3.9 | <0.001 | <0.001 | <0.001 |
| V20Gy (%) | 14.7 ± 1.6 | 8.73 ± 4.13 | 8.72 ± 3.04 | 0.002 | <0.001 | 0.411 | |
| NTCP (%) | 2.1 ± 0.2 | 0.91 ± 0.39 | 0.83 ± 0.22 | <0.001 | <0.001 | 0.632 | |
| SCCP (%) | 12.50 ±0.88 | 5.92 ±2.91 | 5.61 ±1.81 | <0.001 | <0.001 | 0.925 | |
|
| |||||||
| Heart | V5Gy (%) | 88.0 ±16.0 | 2.02 ± 1.21 | 3.05 ±2.25 | <0.001 | <0.001 | 0.140 |
| V22.5Gy (%) | 12 ± 3 | 0.49 ± 0.52 | 0.56 ± 0.76 | <0.001 | <0.001 | 0.647 | |
| NTCP(%) | 0.82 ± 0.67 | 0.036± 0.061 | 0.024 ± 0.051 | 0.017 | 0.014 | 0.761 | |
| NTCPmyo (%) | 0.8 ±0.8 | 0.002 ±0.005 | 0.005 ±0.014 | 0.019 | 0.020 | 0.605 | |
|
| |||||||
| Contralateral breast | Mean dose (Gy RBE) | 7.3 ±2.5 | 0.17 ± 0.11 | 0.34 ± 0.16 | <0.001 | <0.001 | 0.085 |
| V5Gy (%) | 57 ± 23 | 0.99 ± 0.64 | 2.1 ± 0.9 | 0.001 | <0.001 | 0.048 | |
| SCCP (%) | 3.9 ± 1.4 | 0.12 ± 0.06 | 0.20 ± 0.09 | <0.001 | <0.001 | 0.130 | |
Abbreviations: TVeval: Evaluation target volume; DHI: Dose homogeneity index; CI: Conformity index; TCP: Tumor control probability; NTCP: Normal tissue complication probability; NTCPmyo: Normal tissue complication probability associated with the myocardium; SCCP: Secondary cancer complication probability;
: Statistical significance only detected using Student’s t-test.
PSPT was less susceptible to set-up errors largely in part due to compensator smearing. It should be noted that in current treatment practice, a patient set-up error of 1 cm is an extreme case yet still plausible. The impact of both 5 mm and 10 mm patient-setup errors on NTCP were small (<1%) for the heart and lungs for all modalities. SCCP for the lungs did not deviate more than 2% and 4% from the nominal risk prediction for any of the modalities when a 5 mm and 10 mm isocenter shift was simulated for the representative patient, respectively. Additionally, absolute SCCP values for the lung resulting from the 10 mm isocenter shift were 4.4% to 9.9% (PSPT), and 5.2% to 9.1% (IMPT) and were still lower than the nominal risk associated with VMAT (12%).
PSPT and IMPT plans were robust for up to a 10% error in relative linear proton stopping power conversion. The TVeval maintained adequate dose coverage, and the predicted risk of late effects had small changes (<1.2%) from the nominal dose distribution for both PS and IMPT.
The dose-response relationship is not well known for doses typically above 5 Gy. It has been postulated that the relationship in this dose range could be linear, bell shaped (exponential) due to sterilization, or plateau shaped due to a balance between cell killing and repopulation. In the present study both proton techniques statistically significantly reduced the patient-averaged SCCP for all dose-risk models (linear, linear-exponential, and linearplateau) compared to VMAT. Patient-averaged SCCP values for the lung using the linear model were 13%, 5.9%, and 5.6% for VMAT, PSPT, and IMPT, respectively. Patient-averaged SCCP values using the linear-exponential and linear-plateau model were 4.1% and 5.3% (VMAT), 0.70% and 1.4% (PSPT), and 1.3% and 1.9% (IMPT), respectively. Similarly, patient-averaged SCCP values for the contralateral breast ranged from 2.0% to 3.9% for VMAT using the three different dose-risk models, while values for PSPT and IMPT were less than 0.50%.
Despite fluctuations in the patient-averaged NTCP values due to uncertainties in dose-risk model parameter values, VMAT plans produced significantly higher predicted risks relative to both proton modalities. Patient-averaged NTCP values depending on which parameter value used for each heart structure ranged from 0.10% to 3.1% for VMAT, and from <0.01% to 0.33% for both PSPT and IMPT. Similar results were observed for the lungs with values fluctuating between 0.40% to 8.8% for VMAT, and 0.10% to 5.8% for both PSPT and IMPT techniques depending on model parameter value used.
4. Discussion
The present study compared PSPT and IMPT to VMAT for a clinically representative sample of left-sided breast cancer patients treated with PMRT. DVHs, NTCP, and SCCP metrics were used to assess the dose to the chest wall and the risks of radiation pneumonitis, cardiac mortality, and second cancers. PSPT and IMPT plans showed statistically significantly better PTV conformity and dose homogeneity compared to VMAT, and all treatment techniques had nearly 100% TCP. PSPT and IMPT also showed significantly lower average predicted risks of side effects for the sample population compared to VMAT.
A sensitivity analysis revealed that the average NTCP and SCCP values for PSPT and IMPT were consistently lower than that of VMAT for different risk model parameter values and different OED models. Results from the uncertainty analysis for a single patient suggest PSPT and IMPT plans are robust for up to ±5% HU to proton linear stopping power conversion error. An isocenter setup shift of 1 cm could degrade PTV dose coverage, and PSPT is the least susceptible to patient-setup errors. The impact of patient-setup errors on NTCP and SCCP was small. Our findings were different from a pioneer study29, which investigated the setup and dosimetric errors in conventional photon beam therapy for breast cancer. They concluded that 1 cm isocenter shift and 4 degrees error in gantry angle did not produce significant deviations from the planned dose distribution. The possible reason for this discrepancy is that different radiotherapy modalities were used the two studies. While the uncertainty analysis provides some insights, additional uncertainty analyses for a larger population are needed to confirm these findings.
The predicted risks investigated within this study generally agree with the literature. The average NTCP for the lung with radiation pneumonitis Grade 2 or higher was 2.05%±0.23%, 0.91%±0.39%, and 0.83%±0.22% for VMAT, PSPT, and IMPT, respectively. These values fall within previously reported clinical incidence rates of 1 to 5% following breast cancer radiotherapy.30 Average SCCP values for the lungs using the linear-exponential model in a PMRT study by Nichols et al.6 predicted a value of 5.3%±0.1% for VMAT. We had a similar result at 4.1%±0.6% for VMAT using the linear-exponential model as well. Average NTCP values for the whole heart were 0.82%±0.67%, 0.04%±0.06%, and 0.02%±0.05% for VMAT, PS, and IMPT, respectively. In a previous study, Johansson et al.7 estimated the risk of cardiac mortality following a left-sided breast cancer radiotherapy treatment using passively scattered proton therapy for 11 patients. They reported a value of 0.5%±0.5% calculated using the relative-seriality model for a single LAO proton field. The PSPT results from our study agreed within uncertainties.
According to Surveillance, Epidemiology, and End Results (SEER) data, one in eight women will develop invasive breast cancer in their lifetime, and there are about 2.8 million breast cancer survivors in the US (seer.cancer.gov). Approximately 1% excess deaths resulting mainly from cardiovascular causes, to a lesser extent secondary malignancies were observed in patients having received radiotherapy.31 These data translate to around 30,000 patients who will suffer radiogenic side effects and indicate the potential clinical importance of dose sparing to OARs in the treatment of locoregional breast cancer. This paper shows that the advanced technology like proton therapy can significantly reduce unnecessary doses to the organs at risk and reduce predicted risk of side effects, and can meet the urgent need for research on effective approaches to reduce breast-cancer-related medical problems.
Our study had some limitations. Only eight patients were used for this work and, although that provided satisfactory statistical power, a larger sample would provide more reliable estimate of the descriptive statistics of the doses and risks. A follow-up study with a larger sample size is in progress. Only one representative patient was used to investigate the impact of isocenter shifts and proton range uncertainty. Uncertainty analysis based on all eight patients will provide more robust information and will be included in the follow-up study. Our predicted risks were calculated using only the therapeutic doses. Including the stray dose may have an impact on the predicted outcomes, and studies are underway in our laboratory to quantify this. Finally, this study did not investigate the effects of respiratory and cardiac motion on the nominal dose distribution. However, we don’t consider this as a serious limitation because Ares et al.9 looked at this issue and concluded that the effects were small for en face proton beams because the beam’s axis is parallel to the direction of organ motion.
5. Conclusion
The current study revealed advantages of PSPT and IMPT over VMAT for representative patients with left sided breast cancer requiring PMRT. Specifically, PSPT and IMPT significantly reduced dose to the cardiopulmonary structures and the contralateral breast, and significantly reduced the predicted risk of radiation pneumonitis, cardiac toxicity, and radiogenic second cancers compared to VMAT, taking into account the possible treatment plan, delivery and calculation uncertainties. These findings strongly suggest proton therapy can potentially improve quality of life for PMRT patients.
Footnotes
Conflict of Interest
The author declares he has no conflicts of interest. The author alone is responsible for the content and writing of the paper.
References
- 1.McGale P, Taylor C, Correa C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383:2127–35. doi: 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rutqvist LE, Rose C, Cavallin-ståhl E. A systematic overview of radiation therapy effects in breast cancer. Acta Oncologica. 2003;42:532–45. doi: 10.1080/02841860310014444. [DOI] [PubMed] [Google Scholar]
- 3.Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–98. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 4.Grantzau T, Mellemkjaer L, Overgaard J. Second primary cancers after adjuvant radiotherapy in early breast cancer patients: a national population based study under the Danish Breast Cancer Cooperative Group (DBCG) Radiother Oncol. 2013;106:42–9. doi: 10.1016/j.radonc.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Lind PA, Marks LB, Hardenbergh PH, et al. Technical factors associated with radiation pneumonitis after local +/− regional radiation therapy for breast cancer. Int J Radiat Oncol Biol Phys. 2002;52:137–43. doi: 10.1016/s0360-3016(01)01715-1. [DOI] [PubMed] [Google Scholar]
- 6.Nichols GP, Fontenot JD, Gibbons JP, et al. Evaluation of volumetric modulated arc therapy for postmastectomy treatment. Radiat Oncol. 2014;9:66. doi: 10.1186/1748-717X-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johansson J, Isacsson U, Lindman H, et al. Nodepositive left-sided breast cancer patients after breast-conserving surgery: potential outcomes of radiotherapy modalities and techniques. Radiother Oncol. 2002;65:89–98. doi: 10.1016/s0167-8140(02)00266-9. [DOI] [PubMed] [Google Scholar]
- 8.Weber DC, Ares C, Lomax AJ, et al. Radiation therapy planning with photons and protons for early and advanced breast cancer: an overview. Radiat Oncol. 2006;1:22. doi: 10.1186/1748-717X-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ares C, Khan S, Macartain AM, et al. Postoperative proton radiotherapy for localized and locoregional breast cancer: potential for clinically relevant improvements? Int J Radiat Oncol Biol Phys. 2010;76:685–97. doi: 10.1016/j.ijrobp.2009.02.062. [DOI] [PubMed] [Google Scholar]
- 10.Jimenez RB, Goma C, Nyamwanda J, et al. Intensity modulated proton therapy for postmastectomy radiation of bilateral implant reconstructed breasts: a treatment planning study. Radiother Oncol. 2013;107:213–7. doi: 10.1016/j.radonc.2013.03.028. [DOI] [PubMed] [Google Scholar]
- 11.Macdonald SM, Jimenez R, Paetzold P, et al. Proton radiotherapy for chest wall and regional lymphatic radiation; dose comparisons and treatment delivery. Radiat Oncol. 2013;8:71. doi: 10.1186/1748-717X-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Zhang X, Li X, et al. Accelerated partial-breast irradiation using intensity-modulated proton radiotherapy: do uncertainties outweigh potential benefits? Br J Radiol. 2013;86:20130176. doi: 10.1259/bjr.20130176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moyers MF, Miller DW. Range, range modulation, and field radius requirements for proton therapy of prostate cancer. Technol Cancer Res Treat. 2003;2:445–7. doi: 10.1177/153303460300200509. [DOI] [PubMed] [Google Scholar]
- 14.Moyers MF, Miller DW, Bush DA, et al. Methodologies and tools for proton beam design for lung tumors. Int J Radiat Oncol Biol Phys. 2001;49:1429–38. doi: 10.1016/s0360-3016(00)01555-8. [DOI] [PubMed] [Google Scholar]
- 15.Wu Q, Mohan R, Morris M, et al. Simultaneous integrated boost intensity-modulated radiotherapy for locally advanced head-and-neck squamous cell carcinomas. I: dosimetric results. Int J Radiat Oncol Biol Phys. 2003;56:573–85. doi: 10.1016/s0360-3016(02)04617-5. [DOI] [PubMed] [Google Scholar]
- 16.Feuvret L, Noel G, Mazeron JJ, et al. Conformity index: a review. Int J Radiat Oncol Biol Phys. 2006;64:333–42. doi: 10.1016/j.ijrobp.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 17.Wigg DR. Applied radiobiology and bioeffect planning. Med Phys. 2001;489 [Google Scholar]
- 18.Neugut AI, Robinson E, Lee WC, et al. Lung cancer after radiation therapy for breast cancer. Cancer. 1993;71:3054–7. doi: 10.1002/1097-0142(19930515)71:10<3054::aid-cncr2820711027>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 19.Gao X, Fisher SG, Emami B. Risk of second primary cancer in the contralateral breast in women treated for early-stage breast cancer: a population-based study. Int J Radiat Oncol Biol Phys. 2003;56:1038–45. doi: 10.1016/s0360-3016(03)00203-7. [DOI] [PubMed] [Google Scholar]
- 20.Schneider U, Zwahlen D, Ross D, et al. Estimation of radiation-induced cancer from three-dimensional dose distributions: Concept of organ equivalent dose. Int J Radiat Oncol Biol Phys. 2005;61:1510–5. doi: 10.1016/j.ijrobp.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 21.Schneider U, Kaser-Hotz B. A simple dose-response relationship for modeling secondary cancer incidence after radiotherapy. Z Med Phys. 2005;15:31–7. doi: 10.1078/0939-3889-00242. [DOI] [PubMed] [Google Scholar]
- 22.Abo-Madyan Y, Aziz MH, Aly MM, et al. Second cancer risk after 3D-CRT, IMRT and VMAT for breast cancer. Radiother Oncol. 2014;110:471–6. doi: 10.1016/j.radonc.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Lyman JT. Complication probability as assessed from dose-volume histograms. Radiat Res Suppl. 1985;8:S13–9. [PubMed] [Google Scholar]
- 24.Kutcher GJ, Burman C. Calculation of complication probability factors for non-uniform normal tissue irradiation: the effective volume method. Int J Radiat Oncol Biol Phys. 1989;16:1623–30. doi: 10.1016/0360-3016(89)90972-3. [DOI] [PubMed] [Google Scholar]
- 25.Mohan R, Mageras GS, Baldwin B, et al. Clinically relevant optimization of 3-D conformal treatments. Med Phys. 1992;19:933–44. doi: 10.1118/1.596781. [DOI] [PubMed] [Google Scholar]
- 26.Seppenwoolde Y, Lebesque JV, de Jaeger K, et al. Comparing different NTCP models that predict the incidence of radiation pneumonitis. Normal tissue complication probability. Int J Radiat Oncol Biol Phys. 2003;55:724–35. doi: 10.1016/s0360-3016(02)03986-x. [DOI] [PubMed] [Google Scholar]
- 27.Kallman P, Agren A, Brahme A. Tumour and normal tissue responses to fractionated non-uniform dose delivery. Int J Radiat Biol. 1992;62:249–62. doi: 10.1080/09553009214552071. [DOI] [PubMed] [Google Scholar]
- 28.Gagliardi G, Lax I, Rutqvist LE. Partial irradiation of the heart. Semin Radiat Oncol. 2001;11:224–33. doi: 10.1053/srao.2001.23483. [DOI] [PubMed] [Google Scholar]
- 29.Das IJ, Cheng CW, Fosmire H, et al. Tolerances in setup and dosimetric errors in the radiation treatment of breast cancer. Int J Radiat Oncol Biol Phys. 1993;26:883–90. doi: 10.1016/0360-3016(93)90505-p. [DOI] [PubMed] [Google Scholar]
- 30.Marks LB, Bentzen SM, Deasy JO, et al. Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys. 2010;76:S70–6. doi: 10.1016/j.ijrobp.2009.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Group EBCTC. Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomised trials. Lancet. 2000;355:1757–70. [PubMed] [Google Scholar]


