Abstract
Despite the fact that prefrontal cortex (PFC) function declines with age, aged individuals generally show an enhanced ability to delay gratification, as evident by less discounting of delayed rewards in intertemporal choice tasks. The current study was designed to evaluate relationships between two aspects of PFC-dependent cognition (working memory and cognitive flexibility) and intertemporal choice in young (6 mo.) and aged (24 mo.) Fischer 344 x Brown-Norway F1 hybrid rats. Rats were also evaluated for motivation to earn rewards using a progressive ratio task. As previously reported, aged rats showed attenuated discounting of delayed rewards, impaired working memory, and impaired cognitive flexibility compared to young. Among aged rats, greater choice of delayed reward was associated with preserved working memory, impaired cognitive flexibility, and less motivation to work for food. These relationships suggest that age-related changes in PFC and incentive motivation contribute to variance in intertemporal choice within the aged population. Cognitive impairments mediated by PFC are unlikely, however, to fully account for the enhanced ability to delay gratification that accompanies aging.
Keywords: aging, intertemporal choice, delay discounting, working memory, cognitive flexibility, incentive motivation
1.0 INTRODUCTION
Intertemporal choice refers to decision making between options at one time point that dictate outcomes arriving at a later time point. Most individuals strongly prefer large over small rewards in the absence of delays, but reliably “discount” the subjective value of the large reward the longer they have to wait for its delivery (Fobbs and Mizumori, 2017; Green et al., 1994; Odum, 2011; Smits et al., 2013; Vanderveldt et al., 2016). During adolescence and early adulthood, the ability to wait for reward, or delay gratification, is generally considered adaptive and is associated with better overall life outcomes (Banich et al., 2013; Steinbeis et al., 2016). Indeed, children and adolescents, as well as individuals with psychiatric conditions such as attention deficit hyperactive disorder and substance use disorders, reliably display elevated preference for immediate over delayed rewards (Martinelli et al., 2016; Perry and Carroll, 2008; Scheres et al., 2013, 2008). These disorders are also associated with, and have been in part attributed to, prefrontal cortical-mediated cognitive deficits. For example, working memory, which depends on the dorsolateratal prefrontal cortex (dlPFC) in primates (Levy and Goldman-Rakic, 2000), is the ability to temporarily hold information in mind and to use such internal representations to guide current and future action. As delaying gratification requires the ability to maintain an internal representation of a prospective reward until its delivery, it is perhaps not surprising that poor working memory is generally associated with greater preference for small, immediate over large, delayed rewards on intertemporal choice tasks (Gunn and Finn, 2013; Huckans et al., 2011; Shamosh et al., 2008). Consistent with these associations, damage to dlPFC in humans reduces the ability to forgo immediate gratification in favor of delayed rewards (Bechara and Van Der Linden, 2005; Figner et al., 2010), and cognitive training to improve working memory abilities can increase choice of delayed rewards on an intertemporal choice task (Bickel et al., 2011).
Deficits in working memory and other cognitive functions supported by the dlPFC are widely reported in older adults (Alexander et al., 2012; Daigneault and Braun, 1993; Langley and Madden, 2000; MacPherson et al., 2002; Noda et al., 2017). In stark contrast to the associations between working memory impairment and intertemporal choice described above, however, advanced age is accompanied by an increased ability to forgo immediate gratification in favor of delayed rewards (Green et al., 1999, 1996; Jimura et al., 2013; Samanez-Larkin et al., 2011; Simon et al., 2010). In humans, this preference for delayed rewards has largely been ascribed to experiential factors (i.e., learning over the lifetime that forgoing immediate over delayed rewards can be beneficial (reviewed in Beas et al., 2015)). Importantly, however, our laboratory has shown that enhanced preference for delayed rewards in aging is also evident in rats, in which experiential differences across age groups are minimal. Specifically, Simon et al. (2010) assessed intertemporal choice in young and aged Fischer 344 (F344) rats on a task that presented discrete-trial choices between small, immediate and large, delayed food rewards. Consistent with data from human subjects, aged rats displayed greater preference for large, delayed rewards compared to young (Simon et al., 2010). Other work from our laboratory with F344 rats has identified age-related deficits in tests of several forms of PFC-mediated executive function, including a delayed response task that assesses working memory and an attentional set-shifting task that assesses cognitive flexibility (Bañuelos et al., 2014; Beas et al., 2017, 2013). Cognitive flexibility is not as well characterized in the context of intertemporal choice; however, both an exaggerated ability to delay gratification and behavioral inflexibility are associated with the eating disorder anorexia nervosa (Steinglass et al., 2012; Tchanturia et al., 2004), suggesting the possibility that such relationships may exist. In addition, tasks used to study intertemporal choice in rodents often incorporate a block design, which places demands on cognitive flexibility as response contingencies shift over the course of test sessions (Hamilton et al., 2015). As our laboratory has reported both an enhanced ability to delay gratification (Simon et al., 2010) and impaired cognitive flexibility (Beas et al., 2017, 2013) in aged rats, it is reasonable to hypothesize that cognitive inflexibility in aged subjects may in part mediate age-associated differences in intertemporal choice using the block task design (Breton et al., 2015).
The current experiments were designed to test relationships between age-associated impairments in PFC-dependent cognitive functions and intertemporal choice. In addition, although much of the previous work assessing decision making in aged rat models has employed rats of the F344 strain, the National Institute on Aging also supports Fischer 344 × Brown Norway F1 hybrid (FBN) rats. Compared to F344 rats, FBN rats have better visual acuity and a more robust aging phenotype, including both increased physical vigor and an extended lifespan (36 mo. compared to 27 mo. for F344 rats; Spangler et al., 1994). These characteristics render FBN rats ideal for aging research involving longitudinal experimental designs or extended testing periods. As such, a secondary goal of the current experiments was to characterize both PFC-mediated cognition and intertemporal choice in the FBN strain, in order to provide a behavioral context for future research on neural mechanisms of age-related cognitive decline.
2.0 METHODS
2.1 Subjects
Young (6 months old; n=12) and aged (24 months old, n=35) male FBN rats were obtained from the National Institute on Aging colony (Charles River Laboratories) and housed in the Association for Assessment and Accreditation of Laboratory Animal Care International-accredited vivarium facility in the McKnight Brain Institute Building at the University of Florida in accordance with the rules and regulations of the University of Florida Institutional Animal Care and Use Committee and National Institutes of Health guidelines. The facility was maintained at a consistent temperature of 25° with a 12-hour ligh t/dark cycle (lights on at 0600) with free access to food and water except as otherwise noted. Rats were acclimated in this facility for at least one week prior to the onset of behavior testing.
An initial cohort of rats (total of n=3 young and n=9 aged) was tested on the delayed response, set shifting, and progressive ratio tasks, performed in that order. From this initial cohort, n=1 aged died prior to completing the progressive ratio task. A second cohort of rats (total of n=8 young and n=20 aged) was tested on the intertemporal choice, delayed response, set shifting, and progressive ratio tasks, performed in that order. From this second cohort, n=6 aged rats died before completing the working memory task, and an additional n=3 aged rats died before completing the set shifting task. Set-shifting data were lost for an additional n=2 young and n=1 aged rats due to technical problems.
Across both cohorts, final group sizes for each task were: n=11 young and n=23 aged for working memory, n=9 young and n=19 aged for set shifting, n= 8 young and n= 20 aged for intertemporal choice, and n=11 young and n=20 aged for progressive ratio. A total of n=8 young and n=14 aged rats completed both intertemporal choice and working memory, n=6 young and n=10 aged rats completed both intertemporal choice and set shifting, and n=7 young and n=11 aged rats completed both intertemporal choice and progressive ratio.
2.2 General Behavioral Testing Procedures
2.2.1 Apparatus
Testing was conducted in 8 identical standard rat behavioral test chambers (Coulbourn Instruments) with metal front and back walls, transparent Plexiglas side walls, and a floor composed of steel rods (0.4 cm in diameter) spaced 1.1 cm apart. Each test chamber was housed in a sound-attenuating cubicle, and was equipped with a recessed food pellet delivery trough located 2 cm above the floor in the center of the front wall. The trough was fitted with a photobeam to detect head entries and a 1.12 W lamp for illumination. Food rewards consisted of 45-mg grain-based food pellets (PJAI; Test Diet, Richmond, IN, USA). Two retractable levers were positioned to the left and right of the food trough (11 cm above the floor), and a 1.12 W cue lamp was located 3.8 cm above each lever. An additional 1.12 W house light was mounted near the top of the rear wall of the sound-attenuating cubicle. A computer interfaced with the behavioral test chambers and equipped with Graphic State 3.01 software (Coulbourn Instruments) was used to control experiments and collect data.
2.2.2 Shaping
Behavioral testing commenced at least one week after rats arrived in the vivarium. Before the start of testing, rats were food-restricted to 85% of their free-feeding weights over the course of 5 d and maintained at this weight for the duration of testing. Rats progressed through three stages of shaping designed to train them to reliably press each of the two response levers prior to starting task-specific procedures. New shaping stages began on the day immediately following completion of the previous stage.
On the day before Shaping Stage 1, each rat was given five 45 mg food pellets in its home cage to reduce neophobia to the food reward used in the task. Shaping Stage 1 consisted of a 64 min session of magazine training, involving 38 deliveries of a single food pellet with an intertrial interval of 100 ± 40 s. Shaping Stage 2 consisted of lever press training, in which a single lever (left or right, counterbalanced across age groups) was extended and a press resulted in delivery of a single food pellet. After reaching a criterion of 50 lever presses in 30 min, rats were then trained on the opposite lever using the same procedures. During Shaping Stage 3, a nosepoke into the food trough resulted in extension of the left or right lever (counterbalanced across trials in this Stage of testing), and a lever press resulted in a single food pellet delivery. Rats were trained in Shaping Stage 3 until achieving 80 lever presses in a 30 min session.
2.3 Procedures for delayed response task used to assess working memory
The design of this task was based on Sloan et al., 2006, and has been used by our lab previously to demonstrate age-related working memory impairments in F344 rats (e.g., Bañuelos et al., 2014; Beas et al., 2013; McQuail et al., 2016).
2.3.1 Delayed response task description
Each 40 min session began with illumination of the house light, which remained illuminated throughout the entire session except during timeout periods (see below). Rats received a single test session each day. Each trial in the task began with extension of a single “sample” lever into the chamber (Fig 1A). The sample lever (left or right) was randomly selected within each pair of trials to ensure equal representation of both levers across the test session. A press on the sample lever caused it to retract and initiated the delay interval. During the delay interval, rats were required to nosepoke into the food trough to minimize their use of mediating strategies (e.g., positioning themselves in front of the sample lever during the delay). The first nosepoke executed after the delay interval expired initiated the “choice” phase, in which both levers were extended. During the choice phase, a response on the same lever pressed during the sample phase was “correct” and resulted in retraction of both levers and delivery of a food pellet into the food trough. A nosepoke into the food trough to retrieve the food initiated a 5 s intertrial interval, after which the next trial began. A response on the opposite lever from that chosen during the sample phase was “incorrect” and resulted in retraction of both levers and initiation of a 5 s “timeout” period during which the house light was extinguished. Immediately following this timeout, the house light was re-illuminated, signaling the start of the next trial.
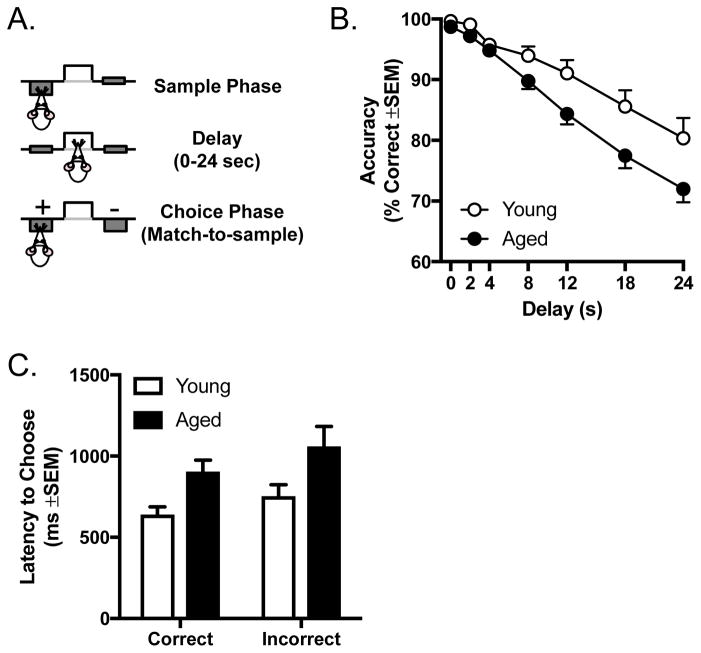
Figure 1.
A) Schematic of the delayed response task, illustrating the three phases of each trial. B) Performance (% accuracy) in the delayed response task of young and aged rats. Aged rats were disproportionately impaired relative to young at long delays. C) Latency to lever press during the choice phase of trials on the delayed response task (averaged across delays). Rats were slower on incorrect trials than correct trials and aged rats were slower than young, but there was no interaction between the two variables.
During initial sessions in this task, there were no delays between the sample and choice phases, and a correction procedure was used such that the sample lever was repeated on the same side following an incorrect response to reduce development of side biases. Once rats reached a criterion of 80% correct choices across a test session for two consecutive sessions, this correction procedure was discontinued and a set of seven delays was introduced. The presentation of delay durations was randomized within each block of seven trials, such that each delay was presented once within a block. Upon establishing >80% correct responses across two consecutive sessions in a “delay set”, rats were progressed to the next set, which contained increasingly longer delays (delay set 1: 0, 1, 2, 3, 4, 5, 6 s; delay set 2: 0, 2, 4, 8, 12, 16 s; delay set 3: 0, 2, 4, 8, 12, 18, and 24 s).
2.3.2 Delayed response statistical analyses
Raw data files were exported from Graphic State software and compiled using a custom macro written for Microsoft Excel (Dr. Jonathan Lifshitz, University of Kentucky). Statistical analyses were conducted in SPSS 24.0, using an alpha of p ≤ 0.05 for this and all other analyses. Huynh-Feldt corrections were used when sphericity was violated.
Accuracy (percent correct at each delay) was the primary measure of delayed response performance (Bañuelos et al., 2014; Beas et al., 2013). Upon reaching delay set 3, performance accuracy (% correct) was evaluated daily across a sliding 5 session window. For each subject, stable performance was defined as a 5-session window in which there was less than 10% variability in accuracy across all delays. Performance at each delay was averaged across these 5 sessions, and age comparisons were conducted using a two factor ANOVA, with age as a between-subjects factor and delay (7 levels) as a within-subjects factor. Latency to respond during the choice phase was also assessed in the same 5 sessions. Latency to respond during the choice phase was calculated separately for correct and incorrect trials (averaged across all delays). Latency data were analyzed using a multi-factor ANOVA, with age as a between-subjects factor, and choice type (2 levels, correct vs. incorrect) as a within-subjects factor. Finally, the number of trials completed per session was monitored and compared between ages using an independent-samples Student’s t-test.
2.4 Procedures for the set shifting task used to assess cognitive flexibility
This task was originally developed by (Floresco et al., 2008a) and was used previously to demonstrate impaired cognitive flexibility in aged Fischer 344 rats (e.g., Beas et al., 2017, 2013).
2.4.1 Side bias assessment
Rats were first assessed on a protocol designed to determine their individual “side bias” or inherent preference for the left or right lever. This protocol was composed of 45 trials, each of which consisted of two phases. In the first phase of each trial, the house light was illuminated and both levers extended into the test chamber. A response on either lever resulted in retraction of both levers and delivery of a single food pellet. In the second phase of each trial, both levers were extended into the chamber, but only a response on the lever opposite to the choice made in the first phase was rewarded. If the rat made an “incorrect” response (i.e., chose the same lever as in the first phase), the levers were retracted and the house light was extinguished. The second phase of the trial was then repeated until the rat made a correct response, and then a new trial was initiated. An individual rat’s “side bias” reflected the lever position on which it made the greatest number of responses across the entire test session.
2.4.2 Initial (visual cue) discrimination
The day after side bias determination, rats began discrimination training on the initial (visual cue) discrimination learning rule. Each 20 s trial began by illuminating one of the cue lights positioned over the left or right lever for 3 s (the position was randomly selected within each pair of trials). Both levers were then inserted into the chamber for 4 s, during which the cue light remained illuminated (the house light was also illuminated during this phase). If the rat made a correct response (pressed the lever beneath the cue light), both levers were retracted, the cue light was extinguished, and a single food pellet was delivered. If the rat made an incorrect response (pressed the lever opposite from the cue light), the levers were retracted and the house light extinguished, but no food was delivered. As in our previous work (Beas et al., 2016, 2013), rats were trained on the visual cue discrimination for a minimum of 30 trials and until reaching criterion performance of 8 consecutive correct choices. The visual discrimination task included a maximum of 120 trials/session. If a rat failed to reach criterion performance in the course of a single session, the task was repeated on subsequent days. Upon reaching criterion performance, the session was ended. To reinforce the formation of an attentional “set”, on the day after reaching criterion performance, rats received one additional session of 120 trials of visual discrimination training.
2.4.3 Set shift (left/right) discrimination
The day after completing visual cue discrimination training, rats received a “set shift” in which the contingencies for making a correct (reinforced) choice were changed. The presentation of the trials during the set shift was identical to that in the visual cue discrimination phase of the task; however, to receive a food reward, rats were now required to ignore the cue light and instead respond to a particular lever position (either left or right, whichever was not their “biased” side as determined during the side bias assessment) to receive a food reward. Rats were trained on the set shift until achieving criterion performance of 10 consecutive correct trials (Beas et al., 2017, 2013). As in the initial discrimination phase, each session included a maximum of 120 trials.
2.4.4 Set shifting statistical analyses
The total numbers of trials and errors required to achieve criterion performance on the initial discrimination and set shift were the primary measures of performance. In addition, errors during the set shift were subdivided into those that were previously-reinforced (i.e., errors attributable to perseveration on the previously learned rule) and those that were never-reinforced (i.e., errors that were inconsistent with both the initial rule and the rule following the set shift). Independent samples Student’s t-tests were used for age comparisons on all of these measures.
2.5 Procedures for the Intertemporal Choice Task
This task was based on Cardinal et al., 2001 and Evenden and Ryan, 1996, and was used previously to demonstrate age-related alterations in decision making in Fischer 344 rats (Simon et al., 2010). Before commencing the intertemporal choice task, rats were trained in a shaping protocol to reinforce each lever with a reward outcome. Briefly, rats nose poked into the food trough to initiate the extension of one lever (either left or right, randomized within every two trials), and a lever press resulted in a single food pellet. After two consecutive days of 45 presses on each lever, rats were advanced to the final delay discounting task.
2.5.1 Intertemporal choice task description
Each 80 min session consisted of 5 blocks of 12 trials each. Each 80 s trial began with a 10 s illumination of the food trough and house lights. A nosepoke into the food trough during this time extinguished the food trough light and triggered extension of either a single lever (forced choice trials) or of both levers simultaneously (free choice trials). Each block consisted of 2 forced choice trials (one for each lever) followed by 10 free choice trials. Trials on which rats failed to nosepoke during this 10 s window were scored as omissions. The forced choice trials were designed to remind rats of the delay contingencies in effect for that block. A press on one lever (either left or right, counterbalanced across age groups) resulted in one food pellet (the small reward) delivered immediately. A press on the other lever resulted in 4 food pellets (the large reward) delivered after a variable delay. The identities of the levers remained consistent throughout testing. Failure to press either lever within 10 s of their extension resulted in the levers being retracted and lights extinguished, and the trial was scored as an omission. Once either lever was pressed, both levers were retracted for the remainder of the trial (i.e., the inter-trial interval). The duration of the delay preceding large reward delivery decreased between each block of trials (60, 40, 20, 10, 0 s), but remained constant within each block. The delays were presented in descending order to exclude the possibility that aged rats’ elevated preference for the large, delayed reward observed in our previous work (Simon et al., 2010) was due to an impaired ability to adjust choice behavior as delay contingencies changed across blocks of trials (i.e., impaired cognitive flexibility).
2.5.2 Intertemporal choice statistical analyses
Percent choice of the large, delayed reward in each block of trials was used as the primary measure of performance. Rats were trained for 15 sessions, after which performance was compared daily across a sliding 5 session window using a two factor ANOVA (delay × session) as in our previous work (Simon et al., 2010, 2007). Stability was defined as the absence of both a main effect of session and a session × delay interaction. Data from the first stable 5 session block were averaged to calculate a mean percent choice of the large reward at each delay (60, 40, 20, 10, 0 s). Age comparisons were conducted using a two factor ANOVA, with age as a between-subjects factor and delay (5 levels) as a within-subjects factor. Latency to lever press during the forced choice trials was also evaluated, with mean latency calculated separately for large and small reward choices at each delay (Shimp et al., 2015). Age comparisons of latency data were conducted using a multi-factor ANOVA, with age as a between-subjects factor, and both choice type (2 levels, small and large) and delay (5 levels) as within-subjects factors.
2.6 Procedures for the progressive ratio task
This task evaluated rats’ motivation to press a lever to obtain food reward, and was based on a design used previously by our lab and others (Barr and Phillips, 1999; Cetin et al., 2004; Kheramin et al., 2005; Mendez et al., 2009).
2.6.1 Progressive ratio task description
Instrumental responding for food reward was assessed using a progressive ratio schedule of reinforcement, on which the number of lever presses required to earn a reward increased with each successive reward earned (1, 4, 10, 20, 35, …). Rats were tested in the progressive ratio task for seven consecutive sessions (one session per day). These sessions varied in length, ending only after 30 min elapsed with no reward delivery.
2.6.2 Progressive ratio statistical analyses
The number of lever presses per session on the progressive ratio task was used as the primary measure of performance. This value was averaged across the final three test sessions to provide a mean value for each rat, which was compared between young and aged rats via an independent-samples Student’s t-test.
2.7 Relationships between intertemporal choice and cognitive/motivational measures
Two approaches were used to assess relationships among cognitive and motivational variables and intertemporal choice. First, bivariate correlations were conducted between performance on the intertemporal choice task (mean % choice of the large reward across 60–10 s delay blocks) and primary measures of performance on the delayed response (mean % accuracy at 18–24 s delays; Bañuelos et al., 2014), set shifting (number of trials to reach criterion performance on the set shift; Beas et al., 2017), and progressive ratio (mean number of lever presses across the final three test sessions) tasks. These correlations were performed separately for young and aged rats. Second, because attrition resulted in a relatively small number of rats cross-characterized on some tasks, aged rats were divided (based on a median split) to create subgroups corresponding to the upper and lower halves of the distribution on each behavioral measure. Performance on the intertemporal choice task was then compared between these subgroups and young rats using a two-factor ANOVA (subgroup × delay) as in previous work from our laboratories and others (e.g., Beas et al., 2013; Bizon et al., 2009; Leal et al., 2017; Lee et al., 2005; Yoder et al., 2017).
3.0 RESULTS
3.1 Influence of age on working memory (delayed response performance)
A total of n=11 young and n=23 aged rats were tested in the delayed response task to assess working memory (Figure 1A). Both young and aged rats acquired the working memory task at a similar rate, with a similar number of sessions required to progress through the shaping stages and reach the final (0–24 s) set of delays (mean (SEM) number of shaping sessions: young = 20.9 (1.34); aged = 21.6 (1.45); t(32) = 0.305, p=0.763). As described above, trials in the delayed response task were self-paced across the 40 min session. Although aged rats completed fewer trials than young rats (t(32) = 2.189, p = 0.036), the magnitude of this difference was small (mean (SEM) total trials: young: 140.6 (1.4); aged: 127.1 (4.2), with both young and aged rats completing a mean of more than 18 trials at each delay per session.
Figure 1B shows mean accuracy of young and aged rats in the delayed response task. A two-factor ANOVA (age × delay) was used to compare the effects of age on working memory accuracy across delays. A main effect of delay (F(6, 192) = 79.889, p < 0.0001) indicated that all rats decreased accuracy as the delay interval over which they had to remember the sample lever position increased. Across delays, however, aged rats performed significantly worse than young (main effect of age: F(1, 32) = 6.642, p = 0.015) and were disproportionally less accurate than young as delays increased (delay × age interaction: F(6, 192) = 2.886, p = 0.041).
The design of the delayed response task required rats to nosepoke into the food trough during the delay phase of the trials in order to initiate the choice phase (lever extension); hence, the actual delay durations could depend on how rapidly rats nosepoked following the end of the delay interval. To determine whether age differences in actual delay durations might account for differences in choice accuracy, a two-factor ANOVA (age × delay) compared actual durations between young and aged rats. Consistent with our previous findings in F344 rats (Beas et al., 2013), the actual delay durations were slightly longer than the programmed durations (see Table 1); but there was neither a main effect of age (F(1, 32) = 3.637, p = 0.066) nor an age × delay interaction (F(6, 192) = 1.961, p = 0.157), indicating that age differences in experienced delays likely did not account for impaired choice accuracy in aging.
Table 1.
Actual delay durations (in seconds) in the delayed response working memory task.
| Programmed Delays | 0 | 2 | 4 | 8 | 12 | 18 | 24 |
|---|---|---|---|---|---|---|---|
| Actual Delays: Young | 0.70 | 2.32 | 4.48 | 8.70 | 12.84 | 18.93 | 25.05 |
| Actual Delays: Aged | 1.11 | 2.52 | 4.69 | 9.25 | 13.49 | 19.82 | 26.14 |
Previous studies using operant procedures have shown that latency to respond on a choice trial tends to reflect the certainty of the choice, with subjects taking more time to respond on incorrect compared to correct trials (Abraham et al., 2012, 2004; Higgins et al., 2007; Yoder et al., 2015), and choice latency has been shown to increase with choice uncertainty (or decreased confidence) in humans (Rutishauser et al., 2015). In addition, given that both slower reaction times and reduced speed of processing have been reported in aged subjects (Grottick and Higgins, 2002; Muir et al., 1999), analysis of response latencies could help identify the extent to which accuracy differences in aged rats are influenced by reaction speed or other nonmnemonic factors. Figure 1C shows the latency to respond in the “choice” phase of the delayed response trials. A two factor ANOVA (age × trial type (correct vs incorrect, averaged across all delays) revealed a main effect of age (F(1, 32) = 4.207, p = 0.049), with aged rats having longer choice latencies compared to young rats, irrespective of trial type. A main effect of trial type on latency to respond was also evident (F(1, 32) = 5.736, p = 0.023) such that both young and aged rats took longer to respond prior to making incorrect compared to correct choices. No interaction was present, however, between choice accuracy and age (F(1, 32) = 0.137, p = 0.714).
3.2 Influence of age on cognitive flexibility (set shifting performance)
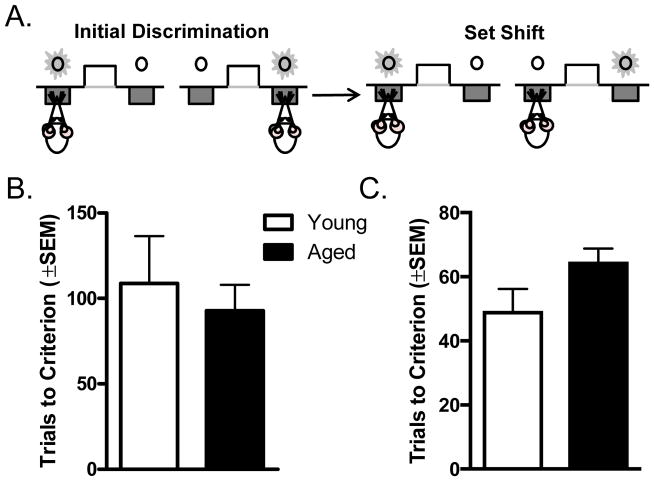
A total of n=9 young and n=19 aged rats were tested in the set shifting task used to assess cognitive flexibility (Figure 2A). There was no age difference in the magnitude of lever bias in the side bias determination session conducted prior to acquisition of the initial (visual) discrimination (ratio of the number of presses on the preferred vs. non-preferred side: young = 1.928 ± 0.397; aged = 1.625 ± 0.106; t(26) = 0.979, p = 0.337). On the visual discrimination, young and aged rats took similar numbers of trials to reach criterion performance (Figure 2B; t(26) = 0.547, p = 0.589). In addition, there was no difference in performance (percent accuracy) in the session following acquisition of the visual discrimination that was used to reinforce the attentional “set” (t(26) = 0.423, p = 0.676). In contrast, aged rats took more trials to reach criterion performance after the reinforcement contingencies were shifted (i.e., ignore the light cue and only respond to position of the lever; Figure 2C; t(26) = 2.047, p = 0.05). Aged rats also made numerically more errors than young during acquisition of the set shift discrimination, although this difference did not reach statistical reliability (total errors: t(26) = 1.475, p = 0.152; previously-reinforced errors: t(26) = 1.259, p = 0.219; never-reinforced errors: t(26) = 1.458, p = 0.157). Finally, there was no correlation between performance on the delayed response and set shifting tasks among either aged (r = 0.011, p = 0.963) or young (r = 0.391, p = 0.299) rats.
Figure 2.
A) Schematic of the set shifting task, illustrating both initial (visual) discrimination and set shift (left/right) discrimination trials. B) Performance (trials to criterion) on the initial (visual) discrimination. Young and aged rats required similar numbers of trials to reach criterion performance. C) Performance on the set shift (left/right) discrimination. Aged rats took more trials to reach criterion performance compared to young rats.
3.3 Influence of age on motivation to press a lever for food (progressive ratio performance)
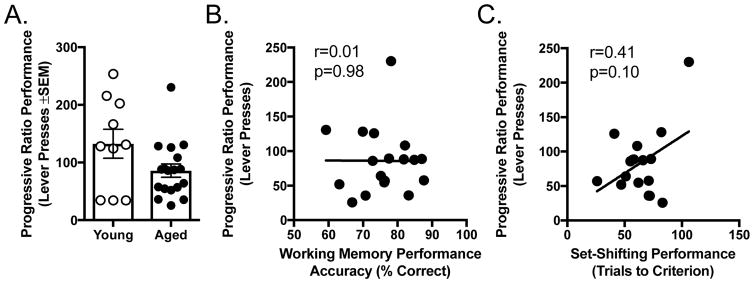
A total of n=11 young and n=20 aged rats were tested on a progressive ratio schedule of reinforcement to provide a measure of incentive motivation for food. Three rats (n=1 young and n=2 aged rats were statistical outliers on this task, (with numbers of lever presses greater than two times the standard deviation of their respective age group means) and were excluded from further analysis. After four sessions of training on this task, rats received three sessions of testing, which were averaged and compared between young and aged rats. A t-test comparing mean lever presses between young and aged rats trended toward an effect of age (t(26) = 1.942, p = 0.063), such that aged rats reached their breakpoints with fewer lever presses than young. As shown in Figure 3A, which depicts both mean and individual rat performance on this task, it is notable that the age differences on progressive ratio were not large in magnitude, with the vast majority of aged rats falling within the range of young. As shown in Figures 3B and C, amongst aged rats there were no significant correlations between either working memory and progressive ratio performance (r = −0.008, p = 0.975) or set-shifting and progressive ratio performance (r = 0.408, p = 0.104). There were also no relationships between these measures amongst young rats (working memory and progressive ratio, r = −0.111, p = 0.761; set-shifting and progressive ratio, r = −0.513, p = 0.157).
Figure 3.
A) Performance (number of lever presses) on the progressive ratio task. Bars represent group means and circles represent individual rats’ values. Aged rats emitted fewer lever presses than young but this difference did not reach statistical reliability. B) Scatterplot relationship between progressive ratio and delayed response task performance among aged rats. C) Scatterplot relationship between progressive ratio and set shifting task performance among aged rats. See text for statistical details.
3.4 Influence of age on intertemporal choice
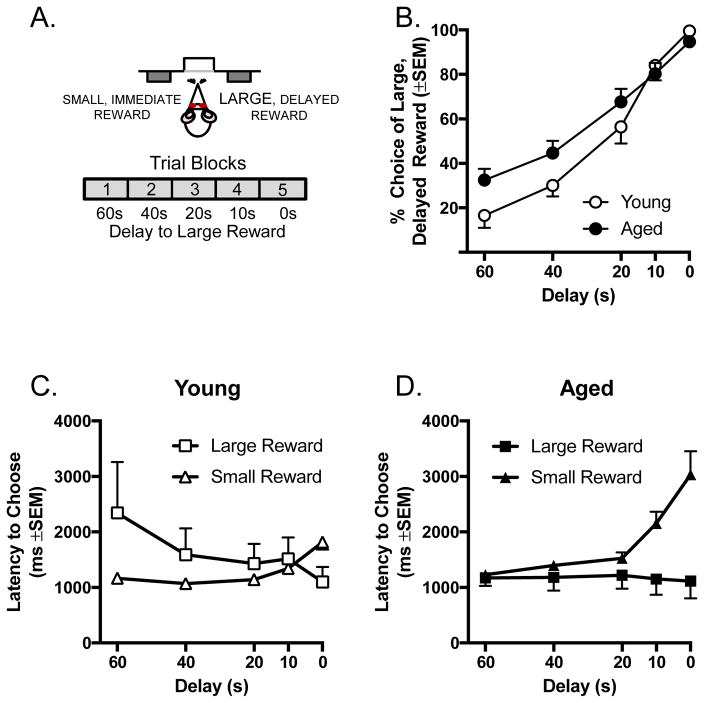
A total of n=8 young and n=20 aged rats were tested in the intertemporal choice task (Figure 4A). Unlike the self-paced delayed response task, the intertemporal choice task used a block design with fixed numbers of trials per block. Young and aged rats did not differ in their percentage of omitted trials (mean percentage (SEM) omitted trials: young: 0.307% (±0.308); aged: 1.11% (±0.382); t(26) = 1.257, p = 0.220). On the primary measure of intertemporal choice task performance, a two-factor ANOVA (delay × age) confirmed that young and aged rats’ preference for the large, delayed reward increased as delays to the large reward decreased (i.e., shorter delays increased the value of the large reward relative to the small reward; Figure 4B; F(4, 104) = 126.603, p < 0.0001). A delay × age interaction, however, indicated that aged rats showed a disproportionately greater preference for the large reward at long delays (F(4, 104) = 3.553, p = 0.017).
Figure 4.
A) Schematic of the intertemporal choice task, illustrating the choices and trial blocks across which the duration of the delay to the large reward decreased. B) Performance (% choice of the large reward plotted as a function of the delay to its delivery) on the intertemporal choice task. Aged rats showed a disproportionately greater choice of the large reward at longer delays. C & D) Latency to choose the large and small rewards in young (C) and aged (D) rats plotted as a function of the delay to large reward delivery. See text for statistical details.
As described above, latency measures can provide information beyond that provided by binary choice behavior. In this task design, rats are required to choose between two levers, both of which are associated with rewards. Once the lever contingencies are learned, the latency to respond likely reflects the current incentive value of the associated reward (Bohn et al., 2003; Calaminus and Hauber, 2007; Holland and Straub, 1979; Sage and Knowlton, 2000; Schoenbaum et al., 2004). Forced choice trials, on which only one lever was presented, preceded each block of choice trials in order to signal the contingencies in effect for that trial block. These trials provided an opportunity to analyze latency to respond for each reward type, at least partially independent of the value of the other reward (Shimp et al., 2015). Response latencies (time from lever insertion to lever press) on these forced choice trials are shown for young and aged rats across delay blocks in Figures 4C and 4D, respectively. A three-factor ANOVA (reward type (large or small) × delay block (60 – 0 s) × age (young or aged)) confirmed a main effect of delay block (F(4, 104) = 3.184, p = 0.048), indicating that there were reliable differences in latency across delay blocks in both young and aged rats. While there was no main effect of reward type (F(1, 26) = 1.090, p = 0.306), there were significant interactions between age and delay block (F(4, 104) = 4.198, p = 0.003), and between age and reward type (F(1, 26) = 6.317, p = 0.018). These interactions reflected the fact that, consistent with the influence of age on choice trials, the patterns of latency differences across delay blocks were distinct between young and aged rats. While young rats took longer to respond for the large, delayed reward when the delay interval was long (e.g., 60 s), aged rats took longer to respond for the small reward when delay intervals to reward delivery were absent or very short (0–2 s). Importantly, there was no main effect of age on response latencies (F(1, 26) = 0.045, p = 0.833), suggesting an absence of obvious age differences in motor or motivational abilities necessary for task performance.
3.5 Relationships between age-associated cognitive and motivational factors and performance on the intertemporal choice task
The overarching motivation for these experiments was to determine relationships between age-associated changes in cognitive and motivational factors and intertemporal choice. These relationships were initially assessed by conducting bivariate correlations between performance on the intertemporal choice task, and on the delayed response, set shifting, and progressive ratio tasks. In addition, given that attrition resulted in relatively small group sizes on some tasks, relationships were also assessed by subgrouping aged rats (via a median split) on the basis of their performance in the delayed response, set shifting, or progressive ratio tasks. Intertemporal choice was then evaluated in these subgroups (e.g., intertemporal choice was compared among young rats, aged rats with “best” working memory and aged rats with the “worst” working memory).
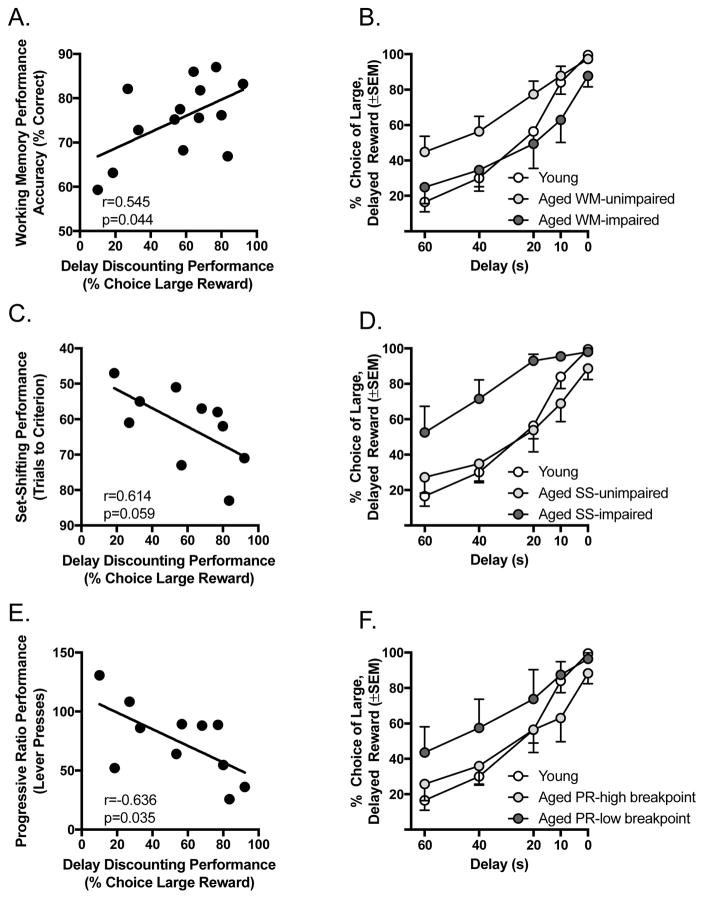
On the delayed response task, better working memory among aged rats was associated with a greater ability to delay gratification and to wait longer for larger rewards (Fig. 5A & B). This was reflected in both a significant correlation between delayed response and intertemporal choice performance (r = 0.545, p = 0.044) and a significant group × delay interaction in the two-factor ANOVA (main effect of group, F(2, 19) = 2.892, p = 0.080; group × delay interaction, F(8, 76) = 2.930, p = 0.011). Follow-up two-factor ANOVAs (pairwise comparisons between individual subgroups) further revealed that the aged working memory-unimpaired subgroup had significantly greater choice of the large, delayed reward on the intertemporal choice task compared to young rats (F(1, 14) = 4.782, p = 0.046) and trended toward different from aged working memory-impaired rats (F(1, 12) = 3.897, p = 0.072), whereas aged working memory-impaired and young rats did not differ (F(1, 12) = 0.322, p = 0.581).
Figure 5.
Relationships between performance on the delayed response, set shifting, and progressive ratio tasks with performance on the intertemporal choice task among aged rats. A) Mean performance across 18 and 24 s delays on the delayed response task plotted against performance in the intertemporal choice task (mean % choice of the large reward from 60–10 s) among aged rats. Aged rats with better working memory showed greater preference for delayed gratification. B) Performance on the intertemporal choice task in young rats as well as aged rats median split on the basis of their delayed response task performance into “working memory (WM) impaired” and “working memory (WM) unimpaired” subgroups. Aged rats with better working memory showed enhanced preference for delayed gratification compared to both young and aged working memory impaired rats. C) Mean set shifting performance (trials to criterion) plotted against performance in the intertemporal choice task (mean % choice of the large reward from 60–10 s) among aged rats. D) Performance on the intertemporal choice task in young rats as well as aged rats median split on the basis of their set shifting task performance into “set shifting (SS) impaired” and “set shifting (SS) unimpaired” subgroups. Aged rats with worse cognitive flexibility showed enhanced preference for delayed gratification compared to both young and aged set shifting unimpaired rats. E) Mean progressive ratio performance (number of lever presses) plotted against performance in the intertemporal choice task (mean % choice of the large reward from 60–10 s) among aged rats. Aged rats that were less motivated to earn food rewards showed greater preference for delayed gratification. F) Performance on the intertemporal choice task in young rats as well as aged rats’ median split on the basis of their progressive ratio task performance into “progressive (PR) ratio high breakpoint” (more lever presses) and “progressive ratio (PR) low breakpoint” (fewer lever presses) subgroups. Aged rats with lower breakpoints showed enhanced preference for delayed gratification compared to both young and aged high breakpoint rats.
On the set shifting task, better cognitive flexibility among aged rats was associated with reduced choice of delayed gratification and greater preference for the small, immediate reward (Fig. 5C & D). Worse set shifting was generally associated with a greater preference for large, delayed rewards on the intertemporal choice performance, although this relationship did not reach statistical reliability (r = 0.614, p = 0.059). The two-factor ANOVA comparing subgroup performance on the intertemporal choice task, however, revealed both a main effect of group (F(1, 13) = 4.022 p = 0.044) and a group × delay interaction (F(8, 52) = 3.280, p = 0.006). Follow-up pairwise comparisons showed that the aged set shifting-impaired subgroup had significantly greater choice of the large, delayed reward compared to both aged set shifting-unimpaired (F(1, 8) = 5.404, p = 0.049) and young rats (F(1, 8) = 9.913, p = .014), whereas aged set shifting-unimpaired and young rats did not differ (F(1, 10) = 0.007, p = 0.935).
On the progressive ratio task, more lever presses (greater reward motivation) were associated with reduced choice of delayed gratification and greater preference for the small, immediate reward (Fig. 5E & F). This was reflected in both a significant correlation between progressive ratio and intertemporal choice performance (r = −0.636, p = 0.035) and a significant group × delay interaction in the two-factor ANOVA (main effect of group, F(1, 15) = 1.320, p = 0.297; group × delay interaction, F(8, 60) = 2.568, p = 0.026). Follow-up pairwise comparisons between individual subgroups further revealed that aged rats with lower breakpoints had significantly greater choice of the large reward at longer delays compared to both aged rats with higher break points (significant group × delay interaction: F(4, 44) = 3.586, p = 0.024) and young rats (significant group × delay interaction: F(4, 40) = 4.147, p = 0.010), but there were no differences between aged rats with higher breakpoints and young rats (main effect of group: F(1, 9)= 1.552, p = 0.244; group × delay interaction: F(4, 36) = 0.432, p = 0.757).
Finally, no relationships were observed in young rats between performance on the intertemporal choice task and performance on the delayed response (r = −0.409, p = 0.315); set shifting (r = 0.457, p = 0.363) or progressive ratio (r = −0.149, p = 0.751) tasks.
4.0 DISCUSSION
4.1 Aging is associated with greater preference for delayed gratification
Intertemporal choice is a form of prospective decision making that involves deliberation between options that dictate outcomes (e.g., rewards) occurring at some point in the future. While almost all subjects will choose large over small rewards when both rewards are delivered immediately, preference for large rewards tends to decrease the longer a subject is required to wait for their delivery (i.e., the delay to reward delivery “discounts” the value of the large reward). In comparison to young adult rats, aged FBN rats in the current study showed attenuated discounting of delayed rewards, with aged rats maintaining greater preference for the large reward compared to young, even at the longest delay interval tested (60 s). These findings from aged FBN rats are consistent with previous findings from other rat strains and across species (Eppinger et al., 2013, 2012, Green et al., 1999, 1996, 1994; Samanez-Larkin et al., 2011; Simon et al., 2010). Notably, in the current study, the age-associated change in preference for delayed rewards was reflected not only in actual choices, but also in the rats’ latency to respond on the levers associated with the small vs. large rewards (Fig. 4C & D). Overall, response latencies on the intertemporal choice task (averaged across all choices) did not differ between young and aged rats, demonstrating that aged rats maintain the physical vigor to complete procedural aspects of this task. Interactions among age, delay, and reward type were observed, however, such that young rats generally showed shorter latencies to choose the small, immediate reward whereas aged rats showed shorter latencies to choose the large, delayed reward. Response latencies have been suggested to reflect the incentive properties of rewards, and to represent a more sensitive measure of preference compared to evaluation of discrete choices (Abraham et al., 2012, 2004; Higgins et al., 2007; Schoenbaum et al., 2004; Yoder et al., 2015). The fact that aged rats’ large reward latencies were unchanged by delay (as opposed to young rats, for which large reward latencies were greater at long than short delays) suggests that the incentive properties of the large reward remained high even when delay intervals were long and the large reward was less frequently chosen than the small reward.
4.2 Working Memory Impairments and Intertemporal Choice in Aged Rats
Performance on the delayed response working memory task was significantly impaired in aged compared to young adult rats, a result similar to those observed previously in aged F344 rats in the same task (Beas et al., 2013; Dunnett et al., 1988; McQuail et al., 2016) as well as in aged monkeys and humans (Lamar and Resnick, 2004; Oscar-Berman and Bonner, 1985; Rapp and Amaral, 1989; Shamy et al., 2011). Importantly, aged rats performed comparably to young adults at short delays, demonstrating intact abilities to conduct the procedural aspects of the task and suggesting that the deficit in aged rats was specific to maintenance of the position of the sample lever over longer delays. While aged rats completed fewer trials per session than young adults, the aged rats still completed an average of more than 18 trials at each delay per session. This high number of trials completed indicates that aged rats are readily able to complete the procedural demands of the delayed response task. Aged rats did demonstrate somewhat longer latencies than young adults to choose a lever during the choice phase of the trials (potentially accounting for the modest reduction in the number of trials completed). In isolation, such longer response latencies might be indicative of motor deficits in aged rats; however, the fact that aged rats’ response latencies were comparable to young in the intertemporal choice task argues against this interpretation. Instead, longer response latencies in aged rats could reflect “cognitive slowing”, or a decline in the speed at which the neural computations required for delayed response performance are conducted (Salthouse, 2000). Consistent with the idea that choice latencies in this task reflect (at least in part) cognitive processing speed, both young and aged rats had longer latencies preceding incorrect compared to correct choices, potentially reflecting uncertainty in their choices (Rutishauser et al., 2015). While the current data suggest possible processing speed deficits in aged rats, such decline cannot fully account for aged rats’ decreased choice accuracy as aged rats showed longer latencies than young on both correct and incorrect trials (i.e. – there was no interaction between age and choice accuracy (Salthouse, 1994, 1992)). Future experiments that tax processing speed with time-limited trials or manipulations that increase cognitive load may be useful for better dissociating the degree to which cognitive slowing contributes to working memory deficits in rat models.
A primary goal of these experiments was to determine how impairments in executive function contribute to age-related alterations in intertemporal choice. Among young adult subjects (both rats and humans), better working memory is associated with greater choice of large, delayed over small, immediate rewards, possibly due to a greater ability to maintain representations of future events (Bobova et al., 2009; Shamosh et al., 2008; Shimp et al., 2015). In the current study, the age group differences in working memory do not easily account for the age group difference in intertemporal choice, as overall, aged rats demonstrated worse working memory than young but greater choice of the large delayed reward (i.e., aged rats showed a greater ability to delay gratification). This mismatch between the effects of age on the two tasks suggests that factors beyond working memory account for age differences in intertemporal choice. It is notable, however, that when examining individual performance among aged population, rats with better working memory did show greater preference for the large, delayed rewards. Together, these patterns of performance suggest that, within the aged population, declining working memory could drive subjects toward greater preference for immediate gratification, but that other factors, such as age-related changes in cognitive flexibility or motivation (see sections 4.3 and 4.4 below), must be involved in driving the increased preference for delayed rewards in aged compared to young rats.
4.3 Set shifting impairments and intertemporal choice in aged rats
Similar to the findings in the delayed response task, aged FBN rats were impaired relative to young adults on the set shifting task, which required them to shift from a previously-learned response rule (visual cue discrimination) to a new rule (left/right discrimination) after it stopped producing the desired outcome. This age difference could not be accounted for by general discrimination learning deficits, as aged rats performed comparably to young on the initial discrimination rule. These results are in agreement with previously reported results using a multidimensional set-shifting paradigm in aged FBN rats (Nieves-Martinez et al., 2012) and are comparable with studies shown previously in aged rats of other strains, as well as aged monkeys and humans (Alexander et al., 2012; Barense et al., 2002; Beas et al., 2017, 2013; Berg, 1948; Moore et al., 2003). Together, these findings suggest that deficits in cognitive flexibility are a common feature of aging.
In contrast to the results described above which show a positive relationship between choice of the large, delayed reward and working memory, there was an inverse relationship between choice of the large, delayed reward and cognitive flexibility (greater choice of the large, delayed reward associated with worse set shifting performance). As aged rats were impaired relative to young on the set shifting task, this finding suggests that deficits in cognitive flexibility could account for aged rats’ preference for the large, delayed reward. Indeed, it has been suggested that such an interpretation could account for results of our previous work in F344 rats, in which aged rats maintained their preference for the large reward across blocks of trials in which the delay to the large reward increased (Breton et al., 2015; Simon et al., 2010). In other words, it could be the case that the maintained preference for the large reward observed in that study reflected not the aged rats’ preference for that choice, but rather an inability to flexibly shift to the small reward lever across blocks of trials as the delays to the large reward were increased. In the current study, however, the sequence of the reward/delay contingencies was intentionally reversed, such that the first block required choices between a small, immediate reward and a large reward delivered after 60 seconds. Aged rats showed enhanced choice of the large, delayed reward in the first block of trials (before any shifts in large reward delays), suggesting that failures to adapt choice strategies in response to changes in reward delays cannot easily account for aged rats’ performance compared to young.
Previous work from our labs showed an inverse relationship between working memory and cognitive flexibility among aged F344 rats, such that aged rats that performed accurately on the delayed response task at long delays performed poorly on the set shifting task, and vice versa. This relationship was not evident among aged FBN rats in the present study, likely in part because of reduced parametric space in which to detect such relationships, given the more restricted range of delayed response performance in aged rats (75–90% accuracy at the 18–24 s delays in FBN rats compared to 55–85% in our previous work in F344 rats, Beas et al., 2013). Notably, however, there were opposite relationships with intertemporal choice between cognitive flexibility and working memory among aged rats (i.e., greater choice of the large, delayed reward was associated with better working memory but worse cognitive flexibility). These opposite relationships suggest that, as in F344 rats, the two forms of executive function are inversely related, although future studies with a broader range of working memory performance will be needed to more fully evaluate this hypothesis.
4.4 Reward motivation and intertemporal choice in aged rats
On the progressive ratio task, aged rats on average made numerically fewer lever presses than young prior to reaching breakpoint, although this difference was not statistically reliable. This finding of relatively minimal age differences in motivation to work for reward is consistent with our previous findings in aged F344 rats (Simon et al., 2010), as well as with other data in this rat strain (e.g., Frutos et al., 2012). Reward motivation also did not appear to account for either working memory or cognitive flexibility deficits in aged rats, as there were no relationships between progressive ratio and delayed response or set shifting performance. Considered together, these data suggest that age differences in executive functions cannot easily be attributed to impaired reward motivation. In contrast, there was a significant relationship between performance on the progressive ratio and intertemporal choice tasks, such that aged rats that were less motivated to obtain reward on the progressive ratio task (fewer lever presses) displayed greater preference for the large, delayed rewards. This relationship could be viewed as counterintuitive, as greater reward (incentive) motivation might be expected to drive preference for the large reward despite the additional costs of the delay. Greater reward motivation might also be expected to bias choices toward the more immediate reward, however, as its immediacy could endow it with greater incentive salience despite its smaller magnitude (Berridge, 2007). The fact that response latencies were shorter for the small reward than for the large reward at long delays in young rats (Figure 4C) is consistent with the idea that reward immediacy can confer greater incentive salience than reward magnitude (although note that incentive motivation is only one of several factors that can bias choice behavior, and that sufficiently long delays or large reward magnitudes could overshadow any motivational influences). Several prior studies in young rats that have compared intertemporal choice with reward motivation using progressive ratio schedules of reinforcement have yielded conflicting results, however, finding either greater food motivation associated with less preference for immediate reward, or no relationship between the two variables (Marshall et al., 2014; Narayanaswami et al., 2013). Irrespective of the underlying mechanisms, the relationship between the progressive ratio and intertemporal choice tasks observed in the present study does suggest that age-associated reductions in incentive motivation may be an important contributor to the greater preference for large, delayed rewards observed in aged subjects. Future work directed at manipulating underlying neurobiology related to both cognitive and motivational circuits in aged rats should more fully elucidate these relationships.
4.5 Neural mechanisms supporting altered intertemporal choice and executive functions in aging
Aging is accompanied by a range of neural alterations in brain systems that mediate intertemporal choice, executive functions, and reward motivation (Bailey et al., 2016; Bickel et al., 2011; Sasse et al., 2017; Shamosh et al., 2008). For example, biochemical, electrophysiological and pharmacological evidence indicates that the normal balance of excitatory and inhibitory signaling within the mPFC is markedly altered with advanced age (Bañuelos et al., 2014; Beas et al., 2017; Carpenter et al., 2016; McQuail et al., 2016). Such alterations have been linked to impaired working memory and cognitive inflexibility, and could directly account for some proportion of the variance in intertemporal choice inasmuch as working memory and/or cognitive flexibility contribute to the increased ability of aged rats to delay gratification. In addition to these cognitive capacities supported directly by the mPFC, this brain region is highly interconnected with other brain regions implicated in intertemporal choice, cognitive flexibility, and incentive motivation, including the orbitofrontal cortex, ventral striatum, and basolateral amygdala (Bailey et al., 2016; Churchwell et al., 2009; Floresco et al., 2008b; Ghods-Sharifi et al., 2009; Hosking et al., 2014; Ishikawa et al., 2008; Jimura et al., 2013; McClure et al., 2004; Samanez-Larkin et al., 2011; Smith et al., 2016; Stalnaker et al., 2009; Tye and Janak, 2007; Wassum and Izquierdo, 2015; Winstanley et al., 2004). Age-associated shifts in excitatory/inhibitory dynamics within PFC may also influence intertemporal choice by altering interactions with these brain regions that integrate cognitive and motivational variables. The current findings provide a framework for future studies in which to probe both the circuit and molecular drivers of age-associated alterations in intertemporal choices.
4.6 Conclusion
The results of this study show that, consistent with previous work in both humans and other rat strains, aging is accompanied by increased preference for large, delayed over small, immediate rewards (i.e., greater ability to delay gratification). Comparisons with performance on other behavioral tasks suggest that executive functions (working memory and cognitive flexibility) and reward motivation could at least partially account for both group differences (between young and aged) and individual differences among aged rats in intertemporal choice. In addition, the results of these experiments provide the most comprehensive cognitive characterization to date of aged FBN rats. Rats of this strain have considerable utility in studies of cognitive aging due to their long lifespan and relatively robust health, and the current results will provide a useful framework for future studies of neural mechanisms of cognitive aging. There is growing appreciation that age-related changes in decision making can cause declines in health and finances (James et al., 2012; Weierich et al., 2011), and hence a better understanding of such changes has the potential to enhance quality of life for older adults. Finally, it should be noted that alterations in intertemporal choice are thought to play a major role in several psychiatric disorders, particularly in substance use and attention deficit-hyperactivity disorders, which are characterized by elevated preference for small, immediate rewards, or “impulsive choice.” Investigation of intertemporal choice in aging, in which preference for small, immediate rewards declines, has the potential to yield novel insights about how behavioral and neural mechanisms can support such behavior, which could ultimately lead to new approaches for reducing impulsivity.
HIGHLIGHTS.
Aged rats show enhanced preference for large, delayed vs. small, immediate rewards
Aged rats show impaired working memory and cognitive flexibility compared to young
Preference for delayed reward associates with better working memory, worse flexibility
Preference for delayed reward associates with less reward motivation
Altered intertemporal choice with age is only partially mediated by prefrontal cortex
Acknowledgments
We thank Vicky S. Kelly, Shannon C. Wall, and Matthew Bruner for technical assistance. Supported by R01AG029421 and the McKnight Brain Research Foundation (JLB), a McKnight Predoctoral Fellowship and the Pat Tillman Foundation (CMH), a Thomas H. Maren Fellowship (CAO), F32AG051371 (JAM), and a University of Florida University Scholars Program Award (LMV).
ABBREVIATIONS
- PFC
prefrontal cortex
- mPFC
medial prefrontal cortex
- dlPFC
dorsolateral prefrontal cortex
- F344
Fischer 344
- FBN
Fischer 344 x Brown Norway hybrid
Footnotes
DISCLOSURES.
The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham NM, Guerin D, Bhaukaurally K, Carleton A. Similar odor discrimination behavior in head-restrained and freely moving mice. PloS One. 2012;7:e51789. doi: 10.1371/journal.pone.0051789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham NM, Spors H, Carleton A, Margrie TW, Kuner T, Schaefer AT. Maintaining accuracy at the expense of speed: stimulus similarity defines odor discrimination time in mice. Neuron. 2004;44:865–876. doi: 10.1016/j.neuron.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Ryan L, Bowers D, Foster TC, Bizon JL, Geldmacher DS, Glisky EL. Characterizing cognitive aging in humans with links to animal models. Front Aging Neurosci. 2012;4:21. doi: 10.3389/fnagi.2012.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey MR, Simpson EH, Balsam PD. Neural substrates underlying effort, time, and risk-based decision making in motivated behavior. Neurobiol Learn Mem. 2016;133:233–256. doi: 10.1016/j.nlm.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banich MT, De La Vega A, Andrews-Hanna JR, Mackiewicz Seghete K, Du Y, Claus ED. Developmental trends and individual differences in brain systems involved in intertemporal choice during adolescence. Psychol Addict Behav J Soc Psychol Addict Behav. 2013;27:416–430. doi: 10.1037/a0031991. [DOI] [PubMed] [Google Scholar]
- Bañuelos C, Beas BS, McQuail JA, Gilbert RJ, Frazier CJ, Setlow B, Bizon JL. Prefrontal cortical GABAergic dysfunction contributes to age-related working memory impairment. J Neurosci Off J Soc Neurosci. 2014;34:3457–3466. doi: 10.1523/JNEUROSCI.5192-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barense MD, Fox MT, Baxter MG. Aged rats are impaired on an attentional set-shifting task sensitive to medial frontal cortex damage in young rats. Learn Mem Cold Spring Harb N. 2002;9:191–201. doi: 10.1101/lm.48602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr AM, Phillips AG. Withdrawal following repeated exposure to d-amphetamine decreases responding for a sucrose solution as measured by a progressive ratio schedule of reinforcement. Psychopharmacology (Berl) 1999;141:99–106. doi: 10.1007/s002130050812. [DOI] [PubMed] [Google Scholar]
- Beas BS, McQuail JA, Bañuelos C, Setlow B, Bizon JL. Prefrontal cortical GABAergic signaling and impaired behavioral flexibility in aged F344 rats. Neuroscience, Cognitive Flexibility: Development, Disease, and Treatment. 2017;345:274–286. doi: 10.1016/j.neuroscience.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beas BS, Setlow B, Bizon JL. Effects of acute administration of the GABA(B) receptor agonist baclofen on behavioral flexibility in rats. Psychopharmacology (Berl) 2016;233:2787–2797. doi: 10.1007/s00213-016-4321-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beas BS, Setlow B, Bizon JL. Distinct manifestations of executive dysfunction in aged rats. Neurobiol Aging. 2013;34:2164–2174. doi: 10.1016/j.neurobiolaging.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beas BS, Setlow B, Gregory R, Samanez-Larkin Bizon JL. Aging and Decision Making: Empirical and Applied Perspectives. Elsevier; 2015. Modeling Cost-Benefit Decision Making in Aged Rodents; pp. 16–40. [Google Scholar]
- Bechara A, Van Der Linden M. Decision-making and impulse control after frontal lobe injuries. Curr Opin Neurol. 2005;18:734–739. doi: 10.1097/01.wco.0000194141.56429.3c. [DOI] [PubMed] [Google Scholar]
- Berg EA. A simple objective technique for measuring flexibility in thinking. J Gen Psychol. 1948;39:15–22. doi: 10.1080/00221309.1948.9918159. [DOI] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Yi R, Landes RD, Hill PF, Baxter C. Remember the future: working memory training decreases delay discounting among stimulant addicts. Biol Psychiatry. 2011;69:260–265. doi: 10.1016/j.biopsych.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizon JL, LaSarge CL, Montgomery KS, McDermott AN, Setlow B, Griffith WH. Spatial reference and working memory across the lifespan of male Fischer 344 rats. Neurobiol Aging. 2009;30:646–655. doi: 10.1016/j.neurobiolaging.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobova L, Finn PR, Rickert ME, Lucas J. Disinhibitory psychopathology and delay discounting in alcohol dependence: personality and cognitive correlates. Exp Clin Psychopharmacol. 2009;17:51–61. doi: 10.1037/a0014503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn I, Giertler C, Hauber W. NMDA receptors in the rat orbital prefrontal cortex are involved in guidance of instrumental behaviour under reversal conditions. Cereb Cortex N Y N 1991. 2003;13:968–976. doi: 10.1093/cercor/13.9.968. [DOI] [PubMed] [Google Scholar]
- Breton YA, Seeland KD, Redish AD. Aging impairs deliberation and behavioral flexibility in inter-temporal choice. Front Aging Neurosci. 2015;7:41. doi: 10.3389/fnagi.2015.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calaminus C, Hauber W. Intact discrimination reversal learning but slowed responding to reward-predictive cues after dopamine D1 and D2 receptor blockade in the nucleus accumbens of rats. Psychopharmacology (Berl) 2007;191:551–566. doi: 10.1007/s00213-006-0532-y. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 2001;292:2499–2501. doi: 10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- Carpenter HE, Kelly KB, Bizon JL, Frazier CJ. Age-related changes in tonic activation of presynaptic versus extrasynaptic γ-amniobutyric acid type B receptors in rat medial prefrontal cortex. Neurobiol Aging. 2016;45:88–97. doi: 10.1016/j.neurobiolaging.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetin T, Freudenberg F, Füchtemeier M, Koch M. Dopamine in the orbitofrontal cortex regulates operant responding under a progressive ratio of reinforcement in rats. Neurosci Lett. 2004;370:114–117. doi: 10.1016/j.neulet.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Churchwell JC, Morris AM, Heurtelou NM, Kesner RP. Interactions between the prefrontal cortex and amygdala during delay discounting and reversal. Behav Neurosci. 2009;123:1185–1196. doi: 10.1037/a0017734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daigneault S, Braun CM. Working memory and the Self-Ordered Pointing Task: further evidence of early prefrontal decline in normal aging. J Clin Exp Neuropsychol. 1993;15:881–895. doi: 10.1080/01688639308402605. [DOI] [PubMed] [Google Scholar]
- Dunnett SB, Evenden JL, Iversen SD. Delay-dependent short-term memory deficits in aged rats. Psychopharmacology (Berl) 1988;96:174–180. doi: 10.1007/BF00177557. [DOI] [PubMed] [Google Scholar]
- Eppinger B, Nystrom LE, Cohen JD. Reduced sensitivity to immediate reward during decision-making in older than younger adults. PloS One. 2012;7:e36953. doi: 10.1371/journal.pone.0036953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppinger B, Schuck NW, Nystrom LE, Cohen JD. Reduced striatal responses to reward prediction errors in older compared with younger adults. J Neurosci Off J Soc Neurosci. 2013;33:9905–9912. doi: 10.1523/JNEUROSCI.2942-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology (Berl) 1996;128:161–170. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- Figner B, Knoch D, Johnson EJ, Krosch AR, Lisanby SH, Fehr E, Weber EU. Lateral prefrontal cortex and self-control in intertemporal choice. Nat Neurosci. 2010;13:538–539. doi: 10.1038/nn.2516. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Block AE, Tse MTL. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav Brain Res. 2008a;190:85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Floresco SB, St Onge JR, Ghods-Sharifi S, Winstanley CA. Cortico-limbic-striatal circuits subserving different forms of cost-benefit decision making. Cogn Affect Behav Neurosci. 2008b;8:375–389. doi: 10.3758/CABN.8.4.375. [DOI] [PubMed] [Google Scholar]
- Fobbs WC, Mizumori SJY. A framework for understanding and advancing intertemporal choice research using rodent models. Neurobiol Learn Mem. 2017;139:89–97. doi: 10.1016/j.nlm.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frutos MGS, Pistell PJ, Ingram DK, Berthoud HR. Feed efficiency, food choice, and food reward behaviors in young and old Fischer rats. Neurobiol Aging. 2012;33:206.e41–53. doi: 10.1016/j.neurobiolaging.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghods-Sharifi S, St Onge JR, Floresco SB. Fundamental contribution by the basolateral amygdala to different forms of decision making. J Neurosci Off J Soc Neurosci. 2009;29:5251–5259. doi: 10.1523/JNEUROSCI.0315-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianotti LRR, Figner B, Ebstein RP, Knoch D. Why Some People Discount More than Others: Baseline Activation in the Dorsal PFC Mediates the Link between COMT Genotype and Impatient Choice. Front Neurosci. 2012;6:54. doi: 10.3389/fnins.2012.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Fry AF, Myerson J. Discounting of Delayed Rewards: A Life-Span Comparison. Psychol Sci. 1994;5:33–36. doi: 10.1111/j.1467-9280.1994.tb00610.x. [DOI] [Google Scholar]
- Green L, Myerson J, Lichtman D, Rosen S, Fry A. Temporal discounting in choice between delayed rewards: the role of age and income. Psychol Aging. 1996;11:79–84. doi: 10.1037//0882-7974.11.1.79. [DOI] [PubMed] [Google Scholar]
- Green L, Myerson J, Ostaszewski P. Discounting of delayed rewards across the life span: age differences in individual discounting functions. Behav Processes. 1999;46:89–96. doi: 10.1016/S0376-6357(99)00021-2. [DOI] [PubMed] [Google Scholar]
- Grottick AJ, Higgins GA. Assessing a vigilance decrement in aged rats: effects of pre-feeding, task manipulation, and psychostimulants. Psychopharmacology (Berl) 2002;164:33–41. doi: 10.1007/s00213-002-1174-3. [DOI] [PubMed] [Google Scholar]
- Gunn RL, Finn PR. Impulsivity partially mediates the association between reduced working memory capacity and alcohol problems. Alcohol Fayettev N. 2013;47:3–8. doi: 10.1016/j.alcohol.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton KR, Mitchell MR, Wing VC, Balodis IM, Bickel WK, Fillmore M, Lane SD, Lejuez CW, Littlefield AK, Luijten M, Mathias CW, Mitchell SH, Napier TC, Reynolds B, Schütz CG, Setlow B, Sher KJ, Swann AC, Tedford SE, White MJ, Winstanley CA, Yi R, Potenza MN, Moeller FG. Choice Impulsivity: Definitions, Measurement Issues, and Clinical Implications. Personal Disord. 2015;6:182–198. doi: 10.1037/per0000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins GA, Grzelak ME, Pond AJ, Cohen-Williams ME, Hodgson RA, Varty GB. The effect of caffeine to increase reaction time in the rat during a test of attention is mediated through antagonism of adenosine A2A receptors. Behav Brain Res. 2007;185:32–42. doi: 10.1016/j.bbr.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Holland PC, Straub JJ. Differential effects of two ways of devaluing the unconditioned stimulus after Pavlovian appetitive conditioning. J Exp Psychol Anim Behav Process. 1979;5:65–78. doi: 10.1037//0097-7403.5.1.65. [DOI] [PubMed] [Google Scholar]
- Hosking JG, Cocker PJ, Winstanley CA. Dissociable contributions of anterior cingulate cortex and basolateral amygdala on a rodent cost/benefit decision-making task of cognitive effort. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2014;39:1558–1567. doi: 10.1038/npp.2014.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckans M, Seelye A, Woodhouse J, Parcel T, Mull L, Schwartz D, Mitchell A, Lahna D, Johnson A, Loftis J, Woods SP, Mitchell SH, Hoffman W. Discounting of delayed rewards and executive dysfunction in individuals infected with hepatitis C. J Clin Exp Neuropsychol. 2011;33:176–186. doi: 10.1080/13803395.2010.499355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa A, Ambroggi F, Nicola SM, Fields HL. Contributions of the amygdala and medial prefrontal cortex to incentive cue responding. Neuroscience. 2008;155:573–584. doi: 10.1016/j.neuroscience.2008.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James BD, Boyle PA, Bennett JS, Bennett DA. The Impact of Health and Financial Literacy on Decision Making in Community-Based Older Adults. Gerontology. 2012;58:531–539. doi: 10.1159/000339094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimura K, Chushak MS, Braver TS. Impulsivity and self-control during intertemporal decision making linked to the neural dynamics of reward value representation. J Neurosci Off J Soc Neurosci. 2013;33:344–357. doi: 10.1523/JNEUROSCI.0919-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheramin S, Body S, Herrera FM, Bradshaw CM, Szabadi E, Deakin JFW, Anderson IM. The effect of orbital prefrontal cortex lesions on performance on a progressive ratio schedule: implications for models of inter-temporal choice. Behav Brain Res. 2005;156:145–152. doi: 10.1016/j.bbr.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Lamar M, Resnick SM. Aging and prefrontal functions: dissociating orbitofrontal and dorsolateral abilities. Neurobiol Aging. 2004;25:553–558. doi: 10.1016/j.neurobiolaging.2003.06.005. [DOI] [PubMed] [Google Scholar]
- Langley LK, Madden DJ. Functional neuroimaging of memory: implications for cognitive aging. Microsc Res Tech. 2000;51:75–84. doi: 10.1002/1097-0029(20001001)51:1<75::AID-JEMT8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Latzman RD, Taglialatela JP, Hopkins WD. Delay of gratification is associated with white matter connectivity in the dorsal prefrontal cortex: a diffusion tensor imaging study in chimpanzees (Pan troglodytes) Proc Biol Sci. 2015;282:20150764. doi: 10.1098/rspb.2015.0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal SL, Noche JA, Murray EA, Yassa MA. Age-related individual variability in memory performance is associated with amygdala-hippocampal circuit function and emotional pattern separation. Neurobiol Aging. 2017;49:9–19. doi: 10.1016/j.neurobiolaging.2016.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Min SS, Gallagher M, Kirkwood A. NMDA receptor-independent long-term depression correlates with successful aging in rats. Nat Neurosci. 2005;8:1657–1659. doi: 10.1038/nn1586. [DOI] [PubMed] [Google Scholar]
- Levy R, Goldman-Rakic PS. Segregation of working memory functions within the dorsolateral prefrontal cortex. Exp Brain Res. 2000;133:23–32. doi: 10.1007/s002210000397. [DOI] [PubMed] [Google Scholar]
- MacPherson SE, Phillips LH, Della Sala S. Age, executive function, and social decision making: a dorsolateral prefrontal theory of cognitive aging. Psychol Aging. 2002;17:598–609. [PubMed] [Google Scholar]
- Marshall AT, Smith AP, Kirkpatrick K. Mechanisms of impulsive choice: I. Individual differences in interval timing and reward processing. J Exp Anal Behav. 2014;102:86–101. doi: 10.1002/jeab.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli MK, Mostofsky SH, Rosch KS. Investigating the Impact of Cognitive Load and Motivation on Response Control in Relation to Delay Discounting in Children with ADHD. J Abnorm Child Psychol. 2016 doi: 10.1007/s10802-016-0237-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- McQuail JA, Beas BS, Kelly KB, Simpson KL, Frazier CJ, Setlow B, Bizon JL. NR2A-Containing NMDARs in the Prefrontal Cortex Are Required for Working Memory and Associated with Age-Related Cognitive Decline. J Neurosci Off J Soc Neurosci. 2016;36:12537–12548. doi: 10.1523/JNEUROSCI.2332-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez IA, Williams MT, Bhavsar A, Lu AP, Bizon JL, Setlow B. Long-lasting sensitization of reward-directed behavior by amphetamine. Behav Brain Res. 2009;201:74–79. doi: 10.1016/j.bbr.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TL, Killiany RJ, Herndon JG, Rosene DL, Moss MB. Impairment in abstraction and set shifting in aged rhesus monkeys. Neurobiol Aging. 2003;24:125–134. doi: 10.1016/s0197-4580(02)00054-4. [DOI] [PubMed] [Google Scholar]
- Muir JL, Fischer W, Björklund A. Decline in visual attention and spatial memory in aged rats. Neurobiol Aging. 1999;20:605–615. doi: 10.1016/s0197-4580(99)00098-6. [DOI] [PubMed] [Google Scholar]
- Narayanaswami V, Thompson AC, Cassis LA, Bardo MT, Dwoskin LP. Diet-induced obesity: dopamine transporter function, impulsivity and motivation. Int J Obes 2005. 2013;37:1095–1103. doi: 10.1038/ijo.2012.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieves-Martinez E, Haynes K, Childers SR, Sonntag WE, Nicolle MM. Muscarinic receptor/G-protein coupling is reduced in the dorsomedial striatum of cognitively impaired aged rats. Behav Brain Res. 2012;227:258–264. doi: 10.1016/j.bbr.2011.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda Y, Zomorrodi R, Backhouse F, Cash RFH, Barr MS, Rajji TK, Chen R, Daskalakis ZJ, Blumberger DM. Reduced Prefrontal Short-Latency Afferent Inhibition in Older Adults and Its Relation to Executive Function: A TMS-EEG Study. Front Aging Neurosci. 2017;9:119. doi: 10.3389/fnagi.2017.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odum AL. Delay discounting: I’m a k, you’re a k. J Exp Anal Behav. 2011;96:427–439. doi: 10.1901/jeab.2011.96-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Bonner RT. Matching- and delayed matching-to-sample performance as measures of visual processing, selective attention, and memory in aging and alcoholic individuals. Neuropsychologia. 1985;23:639–651. doi: 10.1016/0028-3932(85)90065-x. [DOI] [PubMed] [Google Scholar]
- Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology (Berl) 2008;200:1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- Peters J, Büchel C. Episodic future thinking reduces reward delay discounting through an enhancement of prefrontal-mediotemporal interactions. Neuron. 2010;66:138–148. doi: 10.1016/j.neuron.2010.03.026. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Amaral DG. Evidence for task-dependent memory dysfunction in the aged monkey. J Neurosci Off J Soc Neurosci. 1989;9:3568–3576. doi: 10.1523/JNEUROSCI.09-10-03568.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U, Ye S, Koroma M, Tudusciuc O, Ross IB, Chung JM, Mamelak AN. Representation of retrieval confidence by single neurons in the human medial temporal lobe. Nat Neurosci. 2015;18:1041–1050. doi: 10.1038/nn.4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage JR, Knowlton BJ. Effects of US devaluation on win-stay and win-shift radial maze performance in rats. Behav Neurosci. 2000;114:295–306. doi: 10.1037//0735-7044.114.2.295. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Aging and measures of processing speed. Biol Psychol. 2000;54:35–54. doi: 10.1016/s0301-0511(00)00052-1. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The aging of working memory. Neuropsychology. 1994;8:535–543. doi: 10.1037/0894-4105.8.4.535. [DOI] [Google Scholar]
- Salthouse TA. Influence of processing speed on adult age differences in working memory. Acta Psychol (Amst) 1992;79:155–170. doi: 10.1016/0001-6918(92)90030-h. [DOI] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Mata R, Radu PT, Ballard IC, Carstensen LL, McClure SM. Age Differences in Striatal Delay Sensitivity during Intertemporal Choice in Healthy Adults. Front Neurosci. 2011;5:126. doi: 10.3389/fnins.2011.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasse LK, Peters J, Brassen S. Cognitive Control Modulates Effects of Episodic Simulation on Delay Discounting in Aging. Front Aging Neurosci. 2017;9:58. doi: 10.3389/fnagi.2017.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres A, Lee A, Sumiya M. Temporal reward discounting and ADHD: task and symptom specific effects. J Neural Transm Vienna Austria 1996. 2008;115:221–226. doi: 10.1007/s00702-007-0813-6. [DOI] [PubMed] [Google Scholar]
- Scheres A, Tontsch C, Thoeny AL. Steep temporal reward discounting in ADHD-Combined type: acting upon feelings. Psychiatry Res. 2013;209:207–213. doi: 10.1016/j.psychres.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Saddoris MP, Ramus SJ, Shaham Y, Setlow B. Cocaine-experienced rats exhibit learning deficits in a task sensitive to orbitofrontal cortex lesions. Eur J Neurosci. 2004;19:1997–2002. doi: 10.1111/j.1460-9568.2004.03274.x. [DOI] [PubMed] [Google Scholar]
- Shamosh NA, Deyoung CG, Green AE, Reis DL, Johnson MR, Conway ARA, Engle RW, Braver TS, Gray JR. Individual differences in delay discounting: relation to intelligence, working memory, and anterior prefrontal cortex. Psychol Sci. 2008;19:904–911. doi: 10.1111/j.1467-9280.2008.02175.x. [DOI] [PubMed] [Google Scholar]
- Shamy JL, Habeck C, Hof PR, Amaral DG, Fong SG, Buonocore MH, Stern Y, Barnes CA, Rapp PR. Volumetric correlates of spatiotemporal working and recognition memory impairment in aged rhesus monkeys. Cereb Cortex N Y N 1991. 2011;21:1559–1573. doi: 10.1093/cercor/bhq210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimp KG, Mitchell MR, Beas BS, Bizon JL, Setlow B. Affective and cognitive mechanisms of risky decision making. Neurobiol Learn Mem. 2015;117:60–70. doi: 10.1016/j.nlm.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, LaSarge CL, Montgomery KS, Williams MT, Mendez IA, Setlow B, Bizon JL. Good things come to those who wait: attenuated discounting of delayed rewards in aged Fischer 344 rats. Neurobiol Aging. 2010;31:853–862. doi: 10.1016/j.neurobiolaging.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Mendez IA, Setlow B. Cocaine exposure causes long-term increases in impulsive choice. Behav Neurosci. 2007;121:543–549. doi: 10.1037/0735-7044.121.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan HL, Good M, Dunnett SB. Double dissociation between hippocampal and prefrontal lesions on an operant delayed matching task and a water maze reference memory task. Behav Brain Res. 2006;171:116–126. doi: 10.1016/j.bbr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Smith CT, Wallace DL, Dang LC, Aarts E, Jagust WJ, D’Esposito M, Boettiger CA. Modulation of impulsivity and reward sensitivity in intertemporal choice by striatal and midbrain dopamine synthesis in healthy adults. J Neurophysiol. 2016;115:1146–1156. doi: 10.1152/jn.00261.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits RR, Stein JS, Johnson PS, Odum AL, Madden GJ. Test-retest reliability and construct validity of the Experiential Discounting Task. Exp Clin Psychopharmacol. 2013;21:155–163. doi: 10.1037/a0031725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler EL, Waggie KS, Hengemihle J, Roberts D, Hess B, Ingram DK. Behavioral assessment of aging in male Fischer 344 and brown Norway rat strains and their F1 hybrid. Neurobiol Aging. 1994;15:319–328. doi: 10.1016/0197-4580(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Stalnaker TA, Takahashi Y, Roesch MR, Schoenbaum G. Neural substrates of cognitive inflexibility after chronic cocaine exposure. Neuropharmacology. 2009;56(Suppl 1):63–72. doi: 10.1016/j.neuropharm.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbeis N, Haushofer J, Fehr E, Singer T. Development of Behavioral Control and Associated vmPFC-DLPFC Connectivity Explains Children’s Increased Resistance to Temptation in Intertemporal Choice. Cereb Cortex N Y N 1991. 2016;26:32–42. doi: 10.1093/cercor/bhu167. [DOI] [PubMed] [Google Scholar]
- Steinglass JE, Figner B, Berkowitz S, Simpson HB, Weber EU, Walsh BT. Increased capacity to delay reward in anorexia nervosa. J Int Neuropsychol Soc JINS. 2012;18:773–780. doi: 10.1017/S1355617712000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchanturia K, Anderluh MB, Morris RG, Rabe-Hesketh S, Collier DA, Sanchez P, Treasure JL. Cognitive flexibility in anorexia nervosa and bulimia nervosa. J Int Neuropsychol Soc JINS. 2004;10:513–520. doi: 10.1017/S1355617704104086. [DOI] [PubMed] [Google Scholar]
- Tye KM, Janak PH. Amygdala neurons differentially encode motivation and reinforcement. J Neurosci Off J Soc Neurosci. 2007;27:3937–3945. doi: 10.1523/JNEUROSCI.5281-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderveldt A, Oliveira L, Green L. Delay discounting: Pigeon, rat, human--does it matter? J Exp Psychol Anim Learn Cogn. 2016;42:141–162. doi: 10.1037/xan0000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, Izquierdo A. The basolateral amygdala in reward learning and addiction. Neurosci Biobehav Rev. 2015;57:271–283. doi: 10.1016/j.neubiorev.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weierich MR, Kensinger EA, Munnell AH, Sass SA, Dickerson BC, Wright CI, Barrett LF. Older and wiser? An affective science perspective on age-related challenges in financial decision making. Soc Cogn Affect Neurosci. 2011;6:195–206. doi: 10.1093/scan/nsq056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DEH, Cardinal RN, Robbins TW. Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. J Neurosci Off J Soc Neurosci. 2004;24:4718–4722. doi: 10.1523/JNEUROSCI.5606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder WM, Gaynor L, Windham E, Lyman M, Munizza O, Setlow B, Bizon JL, Smith DW. Characterizing olfactory binary mixture interactions in Fischer 344 rats using behavioral reaction times. Chem Senses. 2015;40:325–334. doi: 10.1093/chemse/bjv014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder WM, Gaynor LS, Burke SN, Setlow B, Smith DW, Bizon JL. Interaction between age and perceptual similarity in olfactory discrimination learning in F344 rats: relationships with spatial learning. Neurobiol Aging. 2017;53:122–137. doi: 10.1016/j.neurobiolaging.2017.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]