Abstract
Objective
The precision and accuracy of a quantitative magnetic resonance (EchoMRI-Infants™) system in newborn was determined. Methods: Canola oil and drinking water phantoms (increments of 10g to 1.9kg) were scanned four times. Instrument reproducibility was assessed from 3 scans (within 10-minutes) in 42 healthy term newborns (12–70 hours post-birth). Instrument precision was determined from the coefficient of variation (CV) of repeated scans for total water, lean, and fat measures for newborns and the mean difference between weight and measurement for phantoms. In newborns, the system accuracy for total body water (TBW) was tested against deuterium dilution (D2O).
Results
In phantoms, the repeatability and accuracy of fat and water measurements increased as the weight of oil and water increased. TBW was overestimated in amounts >200g. In newborns weighing 3.14kg, fat, lean and TBW were 0.52kg (16.48%), 2.28kg and 2.40kg, respectively. EchoMRI’s reproducibility (CV) was 3.27%, 1.83% and 1.34% for total body fat, lean, and TBW, respectively. EchoMRI-TBW values did not differ from D2O; mean difference − 1.95±6.76%, p=0.387; mean bias (limits of agreement) 0.046 kg (−0.30 to 0.39 kg).
Conclusions
EchoMRI infant system’s precision and accuracy for total body fat and lean are better than established techniques and equivalent to D2O for TBW in phantoms and newborns.
Keywords: Body composition, water, measurement, infants pediatric
INTRODUCTION
Obesity rates have increased among obstetric populations (1, 2). Increased maternal weight at conception and delivery is associated with increased risk of macrosomia, specifically higher body fat of newborns (3, 4). This increase in body fat may be a significant risk factor for obesity in early childhood and in later life. The extent to which fatness/adiposity, fat distribution, and the composition of fat-free mass are determinants of diseases like obesity and comorbidities later in life remain to be elucidated. There is a need to understand what constitutes a healthy body fat at birth and with growth during the first years of life.
The measurement of body composition in infants continues to be ‘work in progress’ due largely to the lack of validated measurement techniques in this age group (5). Available measurement methods are limited by issues relating to accuracy, practicality, invasiveness, and safety. There is no single technique that allows for the measurement of body composition from birth through adulthood that is radiation free and with good precision. The EchoMRI Infant technology (EchoMRI-Infant; Echo Medical Systems, Houston, TX) provides an opportunity to measure fat mass, lean mass, and total body water (TBW) in infants beginning at birth and longitudinally for body weights up to 12 kg.
Quantitative magnetic resonance (QMR) is a non-imaging technique that uses an electromagnetic field to detect the hydrogen atoms in three groups: fat, lean tissue, and free water. Once excited by radiofrequency pulses, these hydrogen protons have different relaxation times per their environment or the tissue in which they are embedded (protein, fat, or unbound, as water in the bladder or stomach). The processed signal is obtained from the whole body at once as a linear combination of fat, lean, and free water. The lean signal originates mainly from water bound within the lean tissues (6). Regression formulas optimized by an algorithm based on previous validation studies separate the different components based on the pulse sequences obtained (7). To assess the total water, a second independent physical measure is obtained for all the hydrogen present in the body. All hydrogen measured is the sum of all water (TBW + free water) and fat; thus, lean and fat are estimated independent of each other. Total body water minus free water (unbound water is that found in the bladder and stomach) is the water contained in a bound state in tissues.
This technique does not pose any health or safety concerns (no use of ionizing radiation) and can be repeated many times within or across days, allowing for the assessment of short-term changes in infant body composition. The latter theoretically would allow for greater sensitivity and smaller sample sizes in clinical research studies. The advantages of the QMR device for studies involving humans include rapid data collection, no special participation requirements on the part of the subject, no sedation, no ionizing radiation, and the highest precision (in adult and animal studies) to date for available measurement methods.
The QMR measurement approach has been validated in adults (6, 8) and small animals (9, 10, 11, 12) where it detected small changes in body fat with high precision, making it a highly promising tool for use in longitudinal studies involving infants. Two studies in piglets, a well-accepted research model for infant body composition, reported high precision for the measurement of fat mass, with a mean CV of 1.8% compared to 3.1% for dual-energy x-ray absorptiometry (DXA) in piglets weighing 2 to 12 kg (12) and CV of 1.5% for QMR versus CV of 3.5% for DXA in pigs weighing 3 to 50 kg (11). A smaller version of the adult QMR (EchoMRI-AH Small) also performed with high precision for infants, children, and adolescents weighing 3 to 50 kg, and its accuracy was improved after mathematical adjustment (13). The EchoMRI-Infants™ system has not previously been validated for accuracy and precision in infants.
The aim of this study was to assess the accuracy and precision of the infant QMR system (EchoMRI Infant) for infant body composition, particularly fat and TBW. A secondary aim was to assess the level of agreement between body composition measures by QMR and PEAPOD.
METHODS
Four linked studies were conducted to test the QMR system, one with phantoms and three with human subjects. Canola oil and water phantoms were separately scanned in increments of 10 g through 100 g and increments of 100 g through 1.9 kg at 37°C, with two consecutive scans in the morning and two scans in the afternoon performed on the same day. Three consecutive scans were acquired in a convenience sample of 42 healthy term newborns born at Mount Sinai-Roosevelt Hospital (MS-R), between 12–70 hours post birth. Instrument reproducibility was assessed from 3 scans with repositioning between scans (within 10-min period) in newborns. Instrument precision was determined from CV of repeated scans for TBW, lean, and fat measures for newborns and the mean difference between known weight and measurement for phantoms. The infant studies were conducted in three parts: 1. Infant scanned alone; 2. A subset of infants scanned with phantoms; 3. A subset of infants who completed deuterium dilution assessment and QMR scanning. Thirteen infants were measured with 10g of oil, 21 infants were measured with 30 g and 50 g of oil, and 20 infants were measured with 100 g of oil. Fifteen infants were measured with 10 g of water, 23 infants were measured with 30 and 50 g of water, and 22 infants measured with 100 g of water. The accuracy of the QMR for TBW measurement was compared to deuterium dilution in this subsample of 10 infants.
After screening for inclusion (women 18–40 years with singleton newborns that were healthy term (>37 weeks) or preterms that were stable and did not require intubation or mechanical ventilation) and exclusion criteria (mothers with poorly controlled gestational diabetes (fasting capillary blood glucose level higher than 105 mg per dL and postprandial capillary blood glucose level higher than 140 mg per dL at one hour and higher than 120 mg per dL at two hours), preclampsia, HIV or other infectious diseases were excluded. Infants with known birth defects, congenital abnormality, inability to urinate, or an admission to the NICU were excluded. Informed consent was obtained and while still inpatient, the infants were tested. Delivery and newborn data (gestational age, mode of delivery, obstetric complications, birth weight, APGAR scores, and immediate neonatal complications) were retrieved from the medical chart. The study was approved by the IRBs of MSSM (IRB#12-159) and Columbia University (IRB-AAAP4521).
Infant QMR
The infant was placed supine on a flat tray with an infant pad that slides into an enclosed chamber that can be visually monitored through a screen door. The QMR chamber was at room temperature, and the infant was wrapped in a blanket for comfort. The body composition of the infant was measured. The infant QMR system has a subject capacity of up to 12 kg. The system’s external dimensions are 120 × 60 × 180 cm3 (L x W x H). The resistive magnet generates a static magnetic field of about 0.0145 Tesla in a bore size of 120 x 30 x 30 cm3 (L x W x H). The field of view is a 25 cm diameter, 60-cm long cylinder in the center of the bore and the system is self-shielded. The operating system is based on Windows XP Professional Edition. Measuring time is <4 min and there is a recommended daily system calibration test. The system output includes fat mass, lean tissue mass, free water, and TBW in units of grams.
Deuterium dilution (D2O) method
After obtaining maternal consent, a subsample of 10 infants was given an oral dose of D2O, calculated at 100 mg per kg body weight that was administered using a syringe with a volume of ~1 mL/kg body weight of a 10% deuterium oxide stock solution within at least 24 hours after birth but prior to discharge from the hospital (14). At this dose, the D2O enrichment of body fluids at equilibrium is <0.03% (15, 16). Saliva samples (0.5 – 0.7 mL) were collected at baseline prior to dosing and 3 to 5 hours after dosing using a 1.0-mL syringe with a small tube to suction the saliva from the sides of the mouth (14). Exact timing of dosing and sampling were recorded. The saliva samples were stored at -20°C in cryovials until analysis. Deuterium abundance in the saliva samples was measured by gas-isotope-ratio mass spectrometry (17) in the Gas-Isotope-Ratio Mass Spectrometry Laboratory at Baylor College of Medicine.
Infant Anthropometry
Infant length was measured to the nearest 0.5 cm with an infant Length Board. Weight was measured using the PEA POD electronic scale to the nearest 0.001 kg.
PEA POD
Following the daily PEA POD calibration (18), the infant was weighed and placed on a tray that slid into the transparent plastic chamber. The infant was undressed during the PEA POD measurement, except for a nylon hat, umbilical clamp, and identification bands. The PEA POD chamber was about 88°F. Infant body volume was measured using the infant’s weight and length to estimate body surface area and surface area artifact (18). Body density was obtained from body volume and body weight, from which fat free mass was derived, using Fomon et al.’s age and sex-specific equations (19). From these measures, fat mass was calculated. In a previous study of healthy newborns, same day repeated tests on 29 infants gave CV’s of 6.6% for %fat, 6.5% for fat, and 1.1% for FFM.
Statistics
Instrument precision was determined from the CV of the repeated scans for TBW, lean mass, and fat mass for newborns and phantoms, and the mean difference between weight and measurement for phantoms. In newborns, the accuracy of the system was tested against D2O dilution for TBW. Bland-Altman pair-wise comparison plots were generated to examine agreement between methods. Data were analyzed using SPSS for Windows v15 (SPSS, Chicago, Il). Statistical significance was set at p < 0.05.
RESULTS
Phantoms
Precision
For each day, the CV for oil and the CV for water of the four consecutive scans were calculated. Small amounts (10–100 g) of oil and water resulted in moderate CV values (−33.2 ± 27.1% for fat and 14.3 ± 7.2% for water measures). For 500 g oil the CV was 2.4%. For 1000 g oil, the CV was 1.5%. For TBW, the CV was 4.3% for 500 g and 4.6% for 1000 g of water.
Accuracy
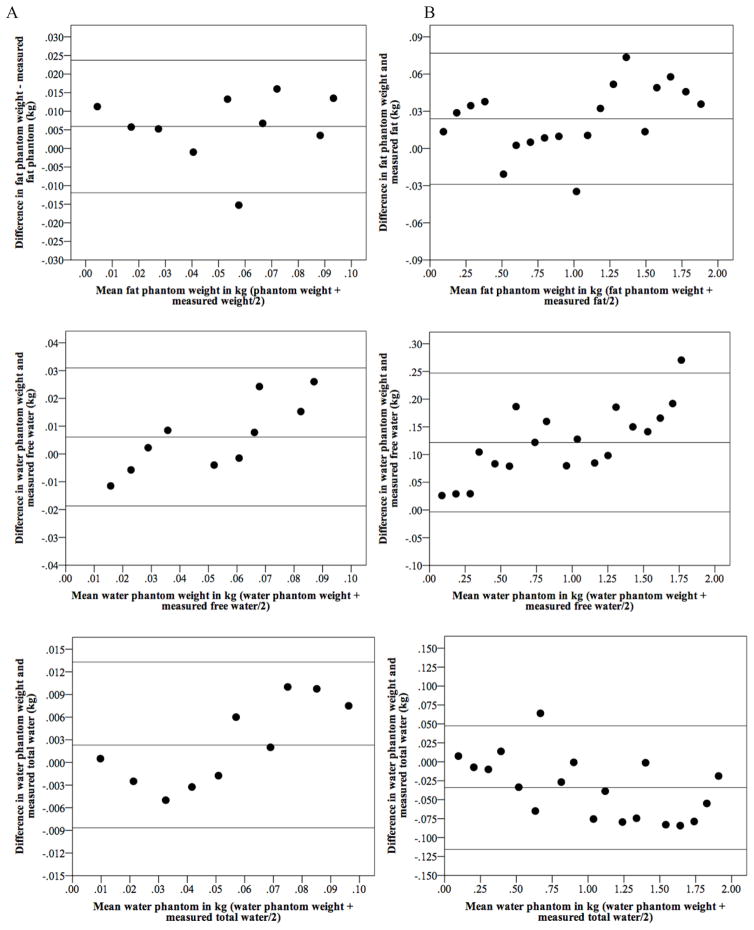
We investigated the effect of adding incremental quantities of oil to the QMR fat measurement. It was hypothesized that the mean difference between known phantom weight and QMR measured weight would be zero (i.e., the addition of 100 g of oil would increase the QMR fat measurement by 100 g). Figure 1 presents Bland-Altman plots showing the limits of agreement (bias ± 2 SD) between known oil and water weight and EchoMRI-Infants measurements of fat, free water, and total water (TBW). Column A reflects weights of 10–100 g and column B 100–1900g. The QMR underestimated fat by 5.9 g and 23.9 g (Figure 1A-B top) and the variability was consistent throughout the mean weight. The QMR underestimated free water by 6.1 g and 121.8 g (Figure 1A–B center), and there was a tendency for the difference between known phantom weight and estimated weight to increase as mean weight increased. For TBW, the QMR underestimated phantom weights between 10–100 by 2.3 g (Figure 1A bottom) with a tendency for the difference to increase as mean increased; and the QMR overestimated TBW by 34.1 g for phantoms 100–1900 g (Figure 1B bottom) with a tendency for the difference to decrease as mean weight increased.
Figure 1.
Bland-Altman plot showing the limits of agreement (bias ± 2 SD) between known oil (fat) and water weight and EchoMRI Infants measurements of fat, free water, and total water. Column A reflects weights of 10–100 g and column B 100–1900g
Table 2 shows mean fat and water differences between actual weights and QMR measured weights. Total water differences were 6.1 g (10–100 g) and -34.1 g (100–1900 g). While free water was underestimated, total water was overestimated for amounts above 100 g.
Table 2.
QMR accuracy for oil and water phantom measurements*
| 10–100 g | 100–1900 g | |||
|---|---|---|---|---|
|
| ||||
| Mean (g) | SD | Mean (g) | SD | |
| Fat | 5.9 | 9.1 | 23.9 | 27.0 |
| Total Water | 6.1 | 12.7 | −34.1 | 35.6 |
| Free Water | 2.3 | 5.6 | 121.8 | 61.4 |
Mean is the mean difference between known phantom weight and QMR estimated weight in 4 scans, where 2 consecutive scans were completed in the morning and 2 scans in the afternoon of the same day for each increment of oil or water phantom.
Newborns
Newborn characteristics are shown in Table 3. Mean weight was 3.14 ± 0.38 kg, length 49.1 ± 1.61 cm, fat mass 521 g (16.59%), lean mass 2.27 kg, and TBW 2.40 kg, and fat mass was a function of body weight (Supplementary Figure 1).
Table 3.
Newborn descriptive characteristics (n=42)
| N | Mean | SD | |
|---|---|---|---|
| Weight (kg) | 38 | 3.14 | 0.38 |
| Length (cm) | 39 | 49.1 | 1.61 |
| Gestational Age (weeks) | 37 | 39.7 | 1.00 |
| Fat (kg) | 42 | 0.52 | 0.14 |
| Fat (%) | 42 | 16.53 | 3.24 |
| Lean (kg) | 42 | 2.27 | 0.25 |
| Total body water (kg) | 42 | 2.40 | 0.25 |
Mean and SD values represent the mean of 3 consecutive scans with repositioning
Precision
The CV for fat mass was 3.27% (Table 4), which translates to a precision of 17.04 g of fat in newborns. A CV of 1.83% for lean equates to a precision of 41.54 g and a CV of 1.34% for TBW translates to a precision of 32.16 g in newborns.
Table 4.
Reproducibility (with repositioning) of 3 repeated measures in newborn [CV%= (SD/mean)*100] (N=42)
| Fat | Lean | Total body water | Free Water | |
|---|---|---|---|---|
| Mean CV % | 3.27 | 1.83 | 1.34 | 14.43 |
| SD (CV) % | 2.30 | 1.86 | 0.89 | 14.16 |
| Range (min) % | 0.67 | 0.18 | 0.29 | 0.59 |
| Range (max) % | 10.72 | 8.81 | 3.94 | 67.87 |
Accuracy
Newborns with phantoms
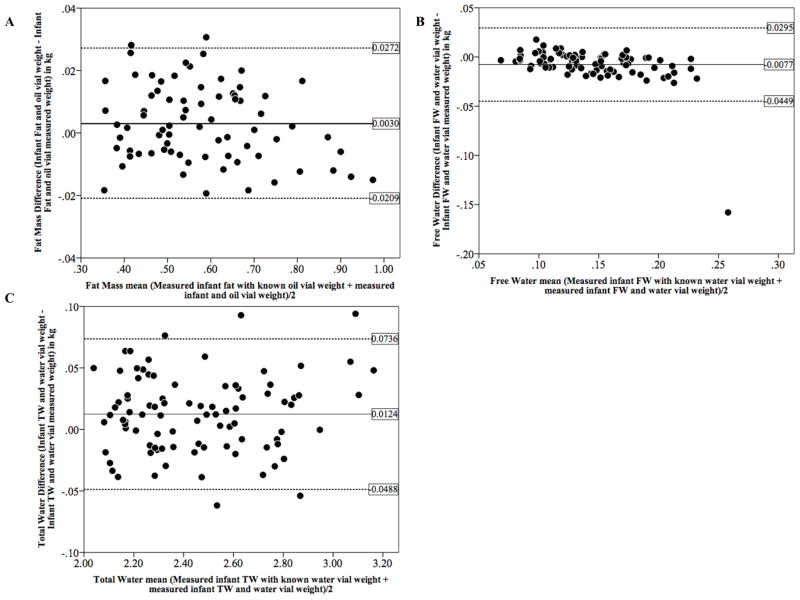
The effect of adding increments of oil or water with a newborn in the QMR is shown by Bland-Altman plots in Figure 2. Adding 10, 30, 50, and 100 g of oil or water resulted in QMR measured overall differences of 3 g for fat, −8 g for FW; and 12 g for TBW. Specifically, we found differences (SD) of 0.7 g (10 g) for 10 g, 9 g (11 g) for 30 g, −2 g (10 g) for 50 g, and 5 g (13 g) for 100 g of fat; −2 g (7 g) for 10 g, −4 g (8 g) for 30 g, −6 g (9 g) for 50 g, −17 g (33g) for 100 g of FW; and 12 g (31 g) for 10 g, 12 g (29 g) for 30 g, 12 g (32 g) for 50 g, 16 g (38 g) for 100 g of TBW.
Figure 2.
Bland-Altman plot showing the limits of agreement (bias ± 2 SD) between known oil (fat) and water weight added to newborns in increments of 10g, 30g, 50g, and 100g and EchoMRI Infants measurements of fat, free water, and total water.
A: n=13 infants measured with 10g of oil, n=21 measured with 30 g and 50 g of oil, and n=20 measured with 100 g of oil.
B and C: n=15 infants measured with 10 g of water, n=23 measured with 30 and 50 g of water, and n=22 measured with 100 g of water.
Deuterium dilution validation
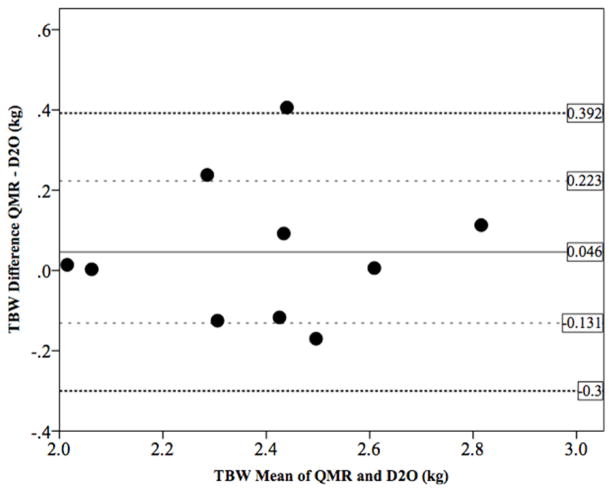
We examined the accuracy of QMR estimates of TBW in newborns using deuterium dilution (D2O) as the criterion method. TBW measurements did not differ between methods with QMR estimating 2.41 kg and D2O 2.37 kg (mean difference −0.046 ± 0.18 kg, p=0.43). The QMR overestimated TBW by 46 g, as evident from the Bland-Altman plot (Figure 3) but the variability was consistent across the range and 70% of subjects fell within one standard deviation of the bias. The limits of agreement were clinically acceptable (1 SD: −0.131 − 0.223; 2 SD: −0.300 – 0.392).
Figure 3.
Bland-Altman plot for total body water from EchoMRI-Infants (QMR) vs. Deuterium Dilution in newborns (n=10)
QMR and PEA POD comparison
Compared to PEA POD, the QMR measured higher FM with a bias of −191 g (p >0.001). The difference in both directions (scatter) between methods increased at a mean FM of 500 g (Supplementary Figure 2). The QMR measured lower lean mass than PEAPOD FFM, with a bias of 502 g (p >0.001) (Supplementary Figure 3).
DISCUSSION
We conducted a series of phantom and human experiments to assess the accuracy and precision of a new infant body composition instrument. Known phantom weights over 100g were measured with small differences between measured weight and QMR estimated fat, free water, and total water. Small, simulated changes in body composition, as created by the addition of phantoms were detected accurately. Furthermore, newborn QMR-TBW did not differ from the reference deuterium dilution estimate of TBW. In infants, this technique has significant potential to measure small changes in body composition with comparable precision and accuracy to any other available technique but with no health risks and without the need for sedation.
Studies investigating infant body composition and longitudinal changes are limited by the size capacity of available methods and the unestablished validity of some measurement techniques in this young age group. Air displacement plethysmography is the most commonly used body composition technique in infants through ~6 months (PEA POD with an upper weight limit of 8 kg). The adult sized BODPOD can be used in children (>5 years or whose body volume >50 L) who can remain motionless during the required testing periods. The BODPOD with the pediatric option (child sized seat) can be used in children greater than 2 years (as per the manufacturer) but success in the 2–5 year age group is low (20) due to inability to remain motionless. No option is available for the age group 6 month to 2 years. A series of QMR devices exist for a wide range of weights that are capable of high accuracy and precision. These allow for the assessment of longitudinal changes in fat, lean, and body water from infancy to adulthood, eliminating error arising from the use of different body composition approaches.
Precision
The results of this study extend Mitchell’s findings, which validated the EchoMRI-Infants in 2–12 kg piglets (12). Precision of the EchoMRI-Infant was evaluated in repeat phantom and newborn measures. In phantoms, precision increased as the weight of the phantom increased and was the highest for free water. Phantom water simulates free water in the QMR, as it is unbound in the body (6). It is unknown why TBW did not reflect the same precision, although it could be related to a difference in the algorithms used by the system to determine the different body composition components. Based on the study by Mitchel (12), we hypothesized that in infants the mean within subject difference (re-test minus test) would be at most 1.8 % and the within subject standard deviation across repeated measures would be at most 1.86 % for fat mass measured by infant QMR. The precision in the current study was good, but lower than hypothesized. Precision in newborns was 3.3%, 1.8% and 1.3% for fat mass, lean mass, and TBW, respectively. Using the same device, Mitchel (12) reported better precision of 1.8%, 0.6%, and 0.85% for fat mass, lean mass, and TBW in piglets. Anesthetized piglet measurements had a higher precision compared to awake piglets (12). In the current study, newborns were not sedated and were repositioned between scans, which could explain the difference in precision. In comparison, using DXA, Mitchell (12) reported 3.09% CV while others reported 9.1% CV for FM in piglets in piglets (21). A recent study (22) reported a CV of 0.6% for fat mass in newborns using DXA. The PEA POD’s precision is lower in piglets and newborns compared to DXA and the QMR, with a CV for fat mass of 17% (23) and 7.9% (24), respectively. In phantoms, precision improved as weight increased (Table 1). In newborns, fat mass was a function of body weight (Supplementary Figure 1).
Table 1.
QMR precision for oil and water phantom measurements*
| 10–100 g | 100–1900 g | |||
|---|---|---|---|---|
|
| ||||
| Mean CV | SD (CV) | Mean CV | SD (CV) | |
| % | % | % | % | |
| Fat | 33.2** | 27.1 | 3.9 | 3.8 |
| Free Water | 14.3 | 7.2 | 3.0 | 3.0 |
| Total Water | 31.9 | 15.2 | 5.6 | 8.0 |
Average CV of 4 scans in one day, where 2 consecutive scans were completed in the morning and 2 scans in the afternoon for each increment of oil or water phantom.
Showing mean CV% for 20–100 g. CV% for 10 g was an extreme outlier at -1765%.
Accuracy
A previous study reported high accuracy (within 2% versus carcass analysis) for body composition estimates in piglets using a larger version of the QMR (EchoMRI-AH) with a capacity up to 50 kg (11). In children ≥ 6 years, the EchoMRI-AH overestimated fat mass by 10% compared to a four-compartment model, which the authors speculated could be due to possible differences in data processing and acquisition that are species-dependent (13). The EchoMRI-Infants was less accurate in piglets up to 12 kg (12) when compared to the larger EchoMRI-AH, with an average difference from carcass analysis of 4% for fat mass, 2.1% for lean mass, and −3.1% for TBW. In comparison, the PEA POD has shown accuracy of 0.6% for percent fat in infants (24), whereas DXA in children overestimated FM by 1.7 kg (25), compared to a 4-compartment model. We found high accuracy for phantoms in amounts greater than 100 g and in newborns when small phantom amounts were added. The QMR was unable to measure accurately and precisely small phantoms (≤100 g) placed alone (without a newborn). However, small changes in fat mass and TBW in newborns, simulated using small phantoms, were detected accurately, where, for example, the addition of 10 g, 30 g, 50, and 100 of oil, resulted in a mean increase of 9.3 g, 21g, 48 g, and 95 of fat, respectively (overall mean difference 3 g), and mean differences in TBW measurements were 12 g overall. TBW was overestimated in phantoms and underestimated in newborns, in agreement with findings by Mitchell in piglets, where TBW was also underestimated (12).
In vivo QMR TBW accuracy is very high. The Bland-Altman plot (Figure 3) demonstrates that QMR overestimates TBW by 46 g or 1.95% compared to deuterium dilution. There is a trend towards an increase in the difference between methods as the average increases (regression slope = 0.06), with overestimation of QMR TBW as the mean TBW increases. However, the scatter around the bias line does not increase as the average increased.
QMR and PEAPOD comparison
FM and FFM comparisons to the PEA POD showed significant differences between the methods where the QMR measured higher FM with a bias of -191 g (36.7%) and lower FFM, where the bias was 502 g (22.1%). These differences could be attributed to dissimilarities in the measurement approaches to assess fat, fat-free mass and lean mass. Specifically, the PEA POD employs a 2-compartment model and estimates body fat from body weight and volume using assumed values for the density of fat and fat-free mass. The density of body fat is generally considered to be constant at 0.9007 g/mL, while the density of fat-free mass varies with age and maturation. The PEA POD uses fixed age and sex-specific fat-free mass density constants to estimate fat-free mass from birth to 10 years. Newborn or birth constants were derived from infants of different ages (days) after birth but placed in a single category (19), which may not accurately represent body composition of 3 day old infants, due to rapid changes in hydration following birth (20). Importantly, the water content of fat-free mass, an important determinant of fat-free mass density, decreases during fetal life. Fomon reported TBW at birth 68.6% in girls and 69.6% in boys (19). In the current study, we found that TBW was 76.43%, suggesting that the constants established by Fomon at birth were based on older infants. QMR estimates TBW by subtracting the signal of hydrogen bound in fat from the total hydrogen signal, and is acquiring a measure of water in real time. Therefore, the PEA POD and QMR each measure different components using different approaches and the QMR may give a more accurate estimate of each body component due to measuring TBW.
Study Limitations
In vivo accuracy was solely reported for TBW using deuterium dilution, but FM accuracy was not assessed (with a 4-compartment model). The 4-compartment model implies a small radiation exposure from DXA and is therefore of limited use in infants and not appropriate for such a study (20). Furthermore, although it is the gold standard technique, any error introduced in one of the components will influence the overall result.
CONCLUSIONS
In conclusion, the EchoMRI infant system shows high precision and accuracy for measures of fat, lean, and water in phantoms and newborns and presents as a method that will provide reliable longitudinal measures in body composition beginning after birth.
Supplementary Material
What is already known about this subject
Available body composition measurement methods during infancy are limited by issues relating to accuracy, practicality, and safety.
There is no single technique that allows for the measurement of body composition from birth through adulthood that is radiation free and with good precision.
What this study adds
The EchoMRI Infant system shows comparable precision and accuracy for the measurement of fat and lean mass in infants compared to available and commonly used infant techniques including the PEA POD and DXA.
The EchoMRI Infant system shows precision and accuracy for total body water measurements not different from the reference deuterium dilution technique.
Acknowledgments
We thank all study participants. Thaisa Lemos, Ph.D. for editorial assistance.
Funding: Supported by National Institutes of Health Grants P30-DK-26687; UL1 TR000040. T. Toro-Ramos was supported by a diversity supplement UO1 DK094463 and T32-DK007559-25.
Footnotes
Disclosure: Drs. Gallagher and Pi-Sunyer report grants from National Institutes of Health during the conduct of the study. Dr. Thornton was affiliated with the New York Obesity Nutrition Research Center at the time of study design. He has since retired and continues to serve as a biostatistician for data analysis and manuscript writing as an independent contractor.
Author contributions: TT-R and DG designed the study. TT-R and CP contributed to recruitment, study procedures, and database management. WY contributing to setting up D2O procedures, preparation of D2O samples for administration to infants, and processing and shipping of collected specimens. WW contributed to deuterium procedures and performed analysis of specimens. TT-R and JT conducted the statistical analyses. TT-R, JT, XP, and DG wrote the paper. All authors were involved in writing the paper and had final approval of the submitted and published versions.
References
- 1.Ehrenberg HM, Dierker L, Milluzzi C, Mercer BM. Prevalence of maternal obesity in an urban center. American journal of obstetrics and gynecology. 2002;187:1189–1193. doi: 10.1067/mob.2002.127125. [DOI] [PubMed] [Google Scholar]
- 2.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. Jama. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 3.Brown JE, Murtaugh MA, Jacobs DR, Jr, Margellos HC. Variation in newborn size according to pregnancy weight change by trimester. Am J Clin Nutr. 2002;76:205–209. doi: 10.1093/ajcn/76.1.205. [DOI] [PubMed] [Google Scholar]
- 4.Lepercq J, Hauguel-De Mouzon S, Timsit J, Catalano PM. Fetal macrosomia and maternal weight gain during pregnancy. Diabetes & metabolism. 2002;28:323–328. [PubMed] [Google Scholar]
- 5.Brodie DA, Stewart AD. Body composition measurement: a hierarchy of methods. J Pediatr Endocrinol Metab. 1999;12:801–816. doi: 10.1515/jpem.1999.12.6.801. [DOI] [PubMed] [Google Scholar]
- 6.Gallagher D, Thornton JC, He Q, Wang J, Yu W, Bradstreet TE, et al. Quantitative magnetic resonance fat measurements in humans correlate with established methods but are biased. Obesity (Silver Spring) 2010;18:2047–2054. doi: 10.1038/oby.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kovner I, Taicher GZ, Mitchell AD. Calibration and validation of EchoMRI whole body composition analysis based on chemical analysis of piglets, in comparison with the same for DXA. International journal of body composition research. 2010;8:17–29. [PMC free article] [PubMed] [Google Scholar]
- 8.Napolitano A, Miller SR, Murgatroyd PR, Coward WA, Wright A, Finer N, et al. Validation of a quantitative magnetic resonance method for measuring human body composition. Obesity. 2008;16:191–198. doi: 10.1038/oby.2007.29. [DOI] [PubMed] [Google Scholar]
- 9.Jones AS, Johnson MS, Nagy TR. Validation of quantitative magnetic resonance for the determination of body composition of mice. International journal of body composition research. 2009;7:67–72. [PMC free article] [PubMed] [Google Scholar]
- 10.Nixon JP, Zhang M, Wang C, Kuskowski MA, Novak CM, Levine JA, et al. Evaluation of a quantitative magnetic resonance imaging system for whole body composition analysis in rodents. Obesity (Silver Spring) 2010;18:1652–1659. doi: 10.1038/oby.2009.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andres A, Mitchell A, Badger T. QMR: validation of an infant and children body composition instrument using piglets against chemical analysis. Int J Obes (Lond) 2010;34:775–780. doi: 10.1038/ijo.2009.284. Epub 2010 Jan 2012. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell AD. Validation of quantitative magnetic resonance body composition analysis for infants using piglet model. Pediatr Res. 2011;69:330–335. doi: 10.1203/PDR.0b013e31820a5b9c. [DOI] [PubMed] [Google Scholar]
- 13.Andres A, Gomez-Acevedo H, Badger TM. Quantitative nuclear magnetic resonance to measure fat mass in infants and children. Obesity (Silver Spring) 2011;19:2089–2095. doi: 10.1038/oby.2011.215. [DOI] [PubMed] [Google Scholar]
- 14.Traver LA, Martinez FE, Ferriolli E, Marchini JS, Monteiro JP, Pfrimer K, et al. Deuterium equilibrium time in saliva of newborn infants. J Pediatr Gastroenterol Nutr. 2009;48:471–474. doi: 10.1097/MPG.0b013e31818bba05. [DOI] [PubMed] [Google Scholar]
- 15.Schoeller DA. Hydrometry. In: Heymsfield S, Lohman T, Wang Z-M, Going S, editors. Human Body Composition. Human Kinetics; 2005. p. 536. [Google Scholar]
- 16.Yu WFO, Gallagher D, Heymsfield SB, Horlick M, Thornton JC, Wang J. An easy and inexpensive phantom for calibrating longitudinal water measurements by tracer dilution. FASEB J. 2005;19:A66. [Google Scholar]
- 17.Wong WW, Lee LS, Klein PD. Deuterium and oxygen-18 measurements on microliter samples of urine, plasma, saliva, and human milk. Am J Clin Nutr. 1987;45:905–913. doi: 10.1093/ajcn/45.5.905. [DOI] [PubMed] [Google Scholar]
- 18.Urlando A, Dempster P, Aitkens S. A new air displacement plethysmograph for the measurement of body composition in infants. Pediatr Res. 2003;53:486–492. doi: 10.1203/01.PDR.0000049669.74793.E3. [DOI] [PubMed] [Google Scholar]
- 19.Fomon SJ, Haschke F, Ziegler EE, Nelson SE. Body composition of reference children from birth to age 10 years. Am J Clin Nutr. 1982;35:1169–1175. doi: 10.1093/ajcn/35.5.1169. [DOI] [PubMed] [Google Scholar]
- 20.Toro-Ramos T, Paley C, Pi-Sunyer FX, Gallagher D. Body composition during fetal development and infancy through the age of 5 years. Eur J Clin Nutr. 2015;69:1279–1289. doi: 10.1038/ejcn.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koo WW, Hammami M, Hockman EM. Validation of bone mass and body composition measurements in small subjects with pencil beam dual energy X-ray absorptiometry. J Am Coll Nutr. 2004;23:79–84. doi: 10.1080/07315724.2004.10719346. [DOI] [PubMed] [Google Scholar]
- 22.de Knegt VE, Carlsen EM, Bech Jensen JE, Lade Rasmussen AM, Pryds O. DXA performance in a pediatric population: precision of body composition measurements in healthy term-born infants using dual-energy X-ray absorptiometry. J Clin Densitom. 2015;18:117–123. doi: 10.1016/j.jocd.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Frondas-Chauty A, Louveau I, Le Huerou-Luron I, Roze JC, Darmaun D. Air-displacement plethysmography for determining body composition in neonates: validation using live piglets. Pediatr Res. 2012;72:26–31. doi: 10.1038/pr.2012.35. [DOI] [PubMed] [Google Scholar]
- 24.Ellis KJ, Yao M, Shypailo RJ, Urlando A, Wong WW, Heird WC. Body-composition assessment in infancy: air-displacement plethysmography compared with a reference 4-compartment model. Am J Clin Nutr. 2007;85:90–95. doi: 10.1093/ajcn/85.1.90. [DOI] [PubMed] [Google Scholar]
- 25.Fields DA, Goran MI. Body composition techniques and the four-compartment model in children. Journal of applied physiology. 2000;89:613–620. doi: 10.1152/jappl.2000.89.2.613. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.