Abstract
Transfection of rat skeletal muscle in vivo is a widely used research model. However, gene electrotransfer protocols have been developed for mice and yield variable results in rats. We investigated whether changes in hyaluronidase pre-treatment and plasmid DNA delivery can improve transfection efficiency in rat skeletal muscle. We found that pre-treating the muscle with a hyaluronidase dose suitable for rats (0.56 U/g b.w.) prior to plasmid DNA injection increased transfection efficiency by >200% whereas timing of the pre-treatment did not affect efficiency. Uniformly distributing plasmid DNA delivery across the muscle by increasing the number of plasmid DNA injections further enhanced transfection efficiency whereas increasing plasmid dose from 0.2 to 1.6 µg/g b.w. or vehicle volume had no effect. The optimized protocol resulted in ~80% (CI95%: 79–84%) transfected muscle fibers with a homogenous distribution. We also show that transfection was stable over five weeks of regular exercise or inactivity. Our findings show that species-specific plasmid DNA delivery and hyaluronidase pre-treatment greatly improves transfection efficiency in rat skeletal muscle.
Abbreviations: TA, Tibialis anterior; GFP, Green fluorescent protein; CMV, Cytomegalovirus
Keywords: Skeletal muscle, Gene electrotransfer, Rat, Hyaluronidase, Exercise
Highlights
-
•
Parameters for effective in vivo skeletal muscle transfection are species specific.
-
•
Pre-treatment with a rat-specific hyaluronidase dose greatly improves transfection efficiency.
-
•
Delivering plasmid DNA more uniformly enhances transfection efficiency in rat skeletal muscle.
-
•
Transfection efficiency is not improved by increasing plasmid DNA dose.
-
•
Exercise training does not affect transfection stability.
1. Introduction
Direct plasmid DNA injection into skeletal muscle is a simple method to perform gene transfer in vivo. However, a major limitation is the very low transfection efficacy. Manipulating with a number of factors, such as plasmid dose, plasmid construct design, injection volume, injection vehicle, number of injections and injection technique have been tested with some success [1], [2], but the advent of electroporation has resulted in the most dramatic improvements in transfection efficiency [3], [4]. DNA electrotransfer has developed into an effective means to carry out gene transfer in vivo [3], [4]. DNA electrotransfer is widely used as a research tool for studying the biological effects of proteins in skeletal muscle [5], [6], [7], [8], [9], [10], [11] and it has been successfully introduced in the clinic to facilitate intramuscular plasmid delivery in cancer patients [12]. In order to acquire reliable data or an effective treatment the transfection efficiency needs to be high. Different electroporation protocols have been developed and a combination of one short high-voltage pulse followed by one or more microsecond-long low-voltage pulses have been shown to result in efficient transfection in mice skeletal muscle [13], [14], [15].
Rats are commonly used in electrotransfer studies for investigating the effect of different proteins on a wide variety of areas such as: angiogenesis [16], muscle hypertrophy [17] and atrophy [18], glucose uptake [11] and lipid metabolism [7], [10]. However, high transfection efficiencies with low variability have only been achieved in mice [19]. In larger animals such as rats [11], dogs [20] and non-human primates [21], [22], the available transfection protocols lead to moderate transfection success and great variability in the percentage of transfected muscle fibers. Similar electroporation parameters seem to be effective in large [20], [23] and small animals [13], suggesting that other factors such as intramuscular plasmid delivery and distribution might influence transfection efficiency.
Hyaluronidase is thought to enhance the distribution of plasmid DNA in the muscle by catalyzing the hydrolysis of hyaluronan, a constituent of the extracellular matrix. Hyaluronidase has been shown to increase protein expression in dose-dependent manner in mice and rabbits [24]. However, the hyaluronidase dose resulting in the highest transfection efficiency is much lower in rabbits compared with mice, suggesting that the optimal hyaluronidase dose might be species-specific [24]. When pre-treating the muscle with hyaluronidase [24], the concentration [25] and dose of plasmid DNA administered [21], [26], [27] as well as the injection technique [2] all affect transfection efficiency in mice skeletal muscle, but these parameters have never systematically been tested in rats.
When other interventions, such as a period of exercise training, are added in conjunction with plasmid transfection, stability over time is important to ensure that changes in transgene expression are maintained throughout the intervention period. Although skeletal muscle cells are terminally differentiated and individual fibers are long-lived there is a small degree of muscle fiber turn-over [28], which can increase slightly with exercise training [29]. Whether stability of transfection is affected by a period of exercise training has not previously been tested.
In this study, we investigated whether changes in hyaluronidase pre-treatment and plasmid DNA delivery can improve transfection efficiency in rat skeletal muscle. Specifically, we manipulated with dose and timing of hyaluronidase pre-treatment, plasmid dilution volume, number of plasmid injections and dose of plasmid DNA injected. Using the optimized protocol we have also tested whether exercise training affects transfection stability.
2. Materials and methods
2.1. Animals
All procedures were approved by the Danish Animal Experimental Inspectorate (License number: 2012-15-2934-00406) and were performed in accordance with the European Convention for the Protection of Vertebrate Animals Used for Experiments and Other Scientific Purposes. Male Sprague Dawley rats (200–250 g; Taconic Europe A/S, Lille Skensved, Denmark) were housed at a constant temperature (22–23 °C) and 35–55% relative humidity on a 12/12 h light/dark cycle with free access to food (Altromin #1324, Brogaarden, Denmark) and water. The animals were acclimatized for one week before being allocated into their respective groups and interventions. Animals were anaesthetized with a 2 ml/kg b.w. injection (s.c.) of an anesthetic cocktail (Hypnorm, 5 mg/ml, Vetapharma, UK and Midazolam, 2.5 mg/ml, Hameln pharmaceuticals, Germany) prior to harvesting of Tibialis anterior muscle (TA) and euthanasia by cervical dislocation of the neck.
2.2. Plasmid
A plasmid encoding green fluorescent protein (GFP) under the control of a cytomegalovirus (CMV) promoter (PS100048, Origene Technologies Inc., USA) was used to assess transfection efficiency in skeletal muscle. The plasmid was amplified in Escherichia coli and purified using EndoFree® Giga Kits (QIAGEN®, Life technologies, Thermo Fisher Scientific, USA) according to the manufacturer's instructions. The only deviation from this protocol was the use of sterile de-mineralized water (pH 7.1) for the plasmid suspension. The concentration and purity of plasmid DNA suspension was determined spectrophotometrically (NanoDrop 1000, Thermo Scientific, USA). The absorbance ratio at 260 and 280 nm was used to assess the purity of suspended plasmid DNA. All suspensions had a ratio of >1.8.
Prior to injection additional sterile demineralized water and sterile saline was added to create the desired plasmid concentration (see below) dissolved in a 75 mM NaCl solution as has previously been recommended [30]. A saline solution containing 75 mM NaCl was used as a vehicle in all experiments.
2.3. Injection and electroporation procedure
In all experiments, the animals were anesthetized with gaseous Isoflurane (Baxter A/S, Allerød, Denmark) mixed with 100% oxygen (Air Liquide, A/S, Denmark) in a 5.5 l induction-chamber (Midmark Animal Health, Stockholm, Sweden). The Isoflurane was administered with an Isoflurane-vaporizer (E-Vet, Manchester, United Kingdom). When anesthetized, the rat was placed on a heating plate (Peco Services Ltd., Brough, United Kingdom) in the supine position. The hind legs were shaved and swabbed with 0.5% Chlorohexidine (Ceduren, Mediq, Denmark).
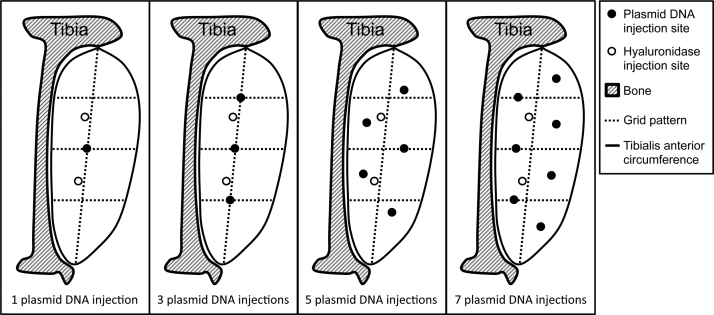
The muscle was pre-treated with hyaluronidase (Type IV-S, Sigma-Aldrich, Denmark) diluted in vehicle (0.8 µl/g b.w.) or vehicle alone (0.8 µl/g b.w.) delivered by 2 injections in all experiments as depicted in Fig. 1. Following the pre-treatment, the plasmid was injected into the TA as described below (Fig. 1). For pre-treatment and plasmid injections, we used a disposable sterile plastic syringe with a 27-gauge needle (0.5 ml, Myjector U-100 Insulin, Terumo, Leuven, Belgium). The syringe needle was fitted with a 9 mm collar cut from plastic tubing (Tygon S-54-HL Microbore Tubing, i.d.: 0.41 mm, Norton Performance Plastics, Ohio, USA) effectively reducing the injection depth to 3 mm. The depth of needle penetration is important for the transfection efficacy [31]. In preliminary experiments, we had found that 3 mm is the optimal injection depth resulting in the most homogenous distribution of Brilliant Blue in the TA muscle (data not shown). All injections were performed at a right angle to the muscle.
Fig. 1.
The figure depicts the pattern that was drawn on the skin to guide the injections. Tibialis anterior muscle was injected at a right angle to the muscle with a depth of 3 mm. Plasmid DNA was injected at 1, 3, 5 or 7 sites indicated by a filled circle. Hyaluronidase or vehicle, marked with an open circle, was injected at 2 sites in all experiments.
Immediately following the injections, a pair of 30×8 mm2 plate electrodes (P-30-8B, Cliniporation, IGEA Clinical Biophysics, Italy) was applied covering the ventral and dorsal sides of the distal hind limb. A conductive gel (Combiscan Ultrasound & Tens Gel, Lina Medical Aps., Denmark) was applied to the skin to improve contact with the electrodes and reduce electrical impedance. The electrical pulse was generated with an electroporation pulse generator (EPS01, Clinivet, IGEA Clinical Biophysics, Italy) and comprised a single pulse of 800 V/cm amplitude lasting 100 μs followed by a time lag of 1 s and a series of four 80 V/cm pulses lasting 100 ms interspersed by pauses of 900 ms as has previously been shown to be very effective [14] and result in minimal disturbance in muscle function [23].
2.4. Tissue harvesting
Following the treatment period, the animals were anaesthetized with a 2 ml/kg b.w. injection (s.c.) of an anesthetic cocktail (Hypnorm, 5 mg/ml, Vetapharma, UK and Midazolam, 2.5 mg/ml, Hameln pharmaceuticals, Germany) and had both of their TA muscles excised. The TA muscles were immediately frozen in liquid nitrogen and stored at −80 °C until further analysis. Subsequently, all animals were euthanized with cervical dislocation.
2.5. Interventions
2.5.1. Hyaluronidase dose
To test for an optimal hyaluronidase pre-treatment dose, TA muscles of 23 Sprague Dawley were transfected with 0.8 µg/g b.w. plasmid solution diluted in vehicle (1.5 µl/g b.w.) delivered with seven injections 1 min following 0.06 (n=10), 0.28 (n=10), 0.56 (n=10), 1.12 (n=10) U/g b.w. hyaluronidase or vehicle (n=6) pre-treatment. The muscles were electroporated immediately after plasmid injection and harvested 7 days later as described above.
2.5.2. Pre-treatment time
To investigate the effect of timing of pre-treatment, both TA muscles of 14 rats were pre-treated with either hyaluronidase (0.56 U/g b.w.) or vehicle. In 8 rats, we injected hyaluronidase (0.56 U/g b.w.) and vehicle 10 min prior to plasmid injection and 6 rats were injected with hyaluronidase (0.56 U/g b.w.) and vehicle 1 min prior to plasmid injection. We injected a plasmid solution 0.8 µg/g b.w. diluted in a low volume (1.5 µl/g b.w.) into the muscles with 7 injections (Fig. 1) and electroporated. Muscles were harvested after 7 days as described above.
2.5.3. Vehicle volume
We investigated the effect of plasmid vehicle volume by transfecting both TA muscles of 14 rats. The muscles were pre-treated with 0.56 U/g b.w. hyaluronidase 10 min prior to plasmid injection. We injected a plasmid DNA solution 0.8 µg / g b.w. diluted in a large volume (3 µl/g b.w.) of vehicle into the TA of one leg and in a low volume (1.5 µl/g b.w.) into the contralateral leg. The large and low volume injections were equally distributed between the right and left legs. In half of the rats we used 3 injections and in the other half we used 5 injections (Fig. 1). The injections were immediately followed by electroporation. Muscles were harvested after 7 days as described above.
2.5.4. Number of injections
To investigate the effect of the number of injections, both TA muscles of 10 rats were injected with plasmid (0.8 µg/g b.w.) diluted in vehicle (1.5 µl/g b.w.) distributed evenly over 1, 3, 5, or 7 sites (Fig. 1) 1 min following hyaluronidase pre-treatment (0.56 U/g b.w.). The muscles were electroporated immediately after plasmid injection and harvested 7 days later as described above.
2.5.5. Plasmid DNA dose
We investigated the relationship between plasmid dose and transfection in 16 rats. Both TA muscles were injected with either 0.2 (n=8), 0.4 (n=8), 0.8 (n=8) or 1.6 (n=8) µg/g b.w. plasmid in vehicle (1.5 µl/g b.w.) delivered with seven injections (Fig. 1) 1 min following hyaluronidase pre-treatment (0.56 U/g b.w.). The muscles were electroporated immediately after plasmid injection and harvested 7 days later as described above.
2.5.6. Distribution of transfection
To investigate the distribution of transfection, both TA muscles of 2 rats were pre-treated with hyaluronidase (0.56 U/g b.w.) 1 min prior to plasmid (0.8 µg/g b.w.) injection in vehicle (1.5 µl/g b.w.) delivered by 7 injections (Fig. 1) and electroporated immediately after. The muscles were removed one week later and a proximal, mid-belly, and distal portion of the muscle were cut and immediately embedded in mounting medium, frozen in pre-cooled isopentane and stored at −80 °C until further analysis.
2.5.7. Effect of exercise training on transfection stability
We investigated whether transfection stability is affected by exercise training by transfecting the right TA muscle of 22 rats and comparing the percentage of transfected muscle fibers against the mid-belly portion of the 4 TA muscles used to investigate distribution of transfection. All rats were pre-treated with hyaluronidase (0.56 U/g b.w.) 1 min prior to plasmid (0.8 µg/g b.w.) injection in vehicle (1.5 µl/g b.w.) delivered by 7 injections (Fig. 1) and electroporated immediately after. Seven days later, 11 rats were allocated to exercise training, while 11 rats remained sedentary. The rats trained on a motorized treadmill at a speed of 25 m min−1 and an incline of 8% 5 times per week for 5 weeks. During the first 5 days of training we increased the duration from 20 min to 60 min in 10 min increments. During the remaining 4 weeks the rats ran for 60 min during each training session. The training intensity corresponded to approximately 75% of VO2 max [32] and all training was supervised. The muscles were harvested 48 h after the last training session under pentobarbital anesthesia (55 mg/kg b.w., i.v.). Since we had found that the transfection distribution was homogenous in the muscle (Fig. 5), only a mid-belly portion of the muscle was cut and immediately embedded in mounting medium, frozen in pre-cooled isopentane and stored at −80 °C until further analysis. Subsequently, the animals were euthanized with an overdose of pentobarbital (110 mg/ kg b.w., i.v.).
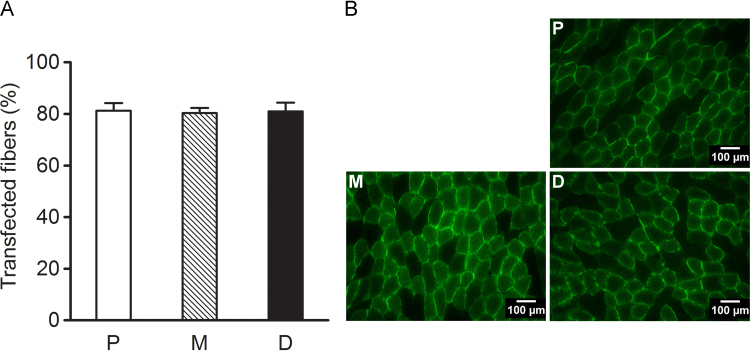
Fig. 5.
The graph depicts the percentage (%) of transfected muscle fibers (A) and representative cross-sections (B) in the proximal (P, n=4), mid-belly (M, n=4) or distal (D, n=4) portions of the Tibialis anterior muscle of Sprague-Dawley rats (n=2) 1 week after transfection by electroporation. TA muscles were injected with 0.8 µg/g b.w. plasmid in vehicle (1.5 µl/g b.w.) delivered with 7 evenly spaced injections 1 min following hyaluronidase pre-treatment (0.56 U/g b.w.). The data are presented as mean±SE.
2.6. Western blotting
GFP expression was determined by western blotting in TA on whole muscle lysates. Frozen muscle samples were pulverized and a sample of approximately 95 mg muscle was collected and homogenized (Qiagen Tissuelyzer II, Retsch GmbH, Haan, Germany) in a fresh batch of buffer containing (in mM): 10% glycerol, 20 mM Na-pyrophosphate, 150 mM NaCl, 50 mM HEPES (pH 7.5), 1% NP-40, 20 mM β-glycerophosphate, 2 mM Na3VO4, 10 mM NaF, 2 mM PMSF, 1 mM EDTA (pH 8), 1 mM EGTA (pH 8), 10 µg/ml Aprotinin, 10 µg/ml Leupeptin and 3 mM Benzamidine. Samples were rotated end over end for 1 h at 4 °C and centrifuged at 18,320 g for 20 min at 4 °C to exclude non-dissolved structures, and the supernatant (lysate) was used for further analysis. Total protein concentration in each sample was determined by a BSA standard kit (Pierce), and samples were mixed with 6 x Laemmli buffer (7 ml 0.5 M Tris-base, 3 ml glycerol, 0.93 g DTT, 1 g SDS and 1.2 mg bromophenol blue) and ddH2O to reach equal protein concentration before protein expression were determined by western blotting.
Equal amount of total protein were loaded in each well of pre-casted 10% gels (Bio-Rad Laboratories, USA). All samples from each experiment were loaded on the same gel. Proteins were separated by SDS page gel electrophoresis and semi-dry transferred to a PVDF membrane (BioRad, Denmark). The membranes were blocked in 2% skimmed milk in Tris-buffered saline including 0.1% Tween-20 (TBST) before an overnight incubation in primary antibody (AB121, Evrogen JSC, Russia) at 4 °C and a subsequent 1 hour incubation in horseradish-peroxidase conjugated secondary goat anti-rabbit antibody (4010-05, Southern Biotech, USA or P-0448, DAKO, Denmark) at room temperature. The bands were visualized with ECL (Millipore) and recorded with a digital camera (ChemiDoc MP Imaging System, Bio-Rad Laboratories, USA). Densitometry quantification of the western blot band intensity was done using Image Lab version 4.0 (Bio-Rad Laboratories, USA) and determined as the total band intensity adjusted for background intensity.
2.7. Transfection efficiency quantification
Transverse sections (8 µm) of the proximal, mid-belly, and distal muscle regions were cut using a microtome (Mikron HM 500 M, Zeiss, Denmark). Transfection efficiency was determined from a series of four images per sample consisting of 86±3 muscle fibers per image and totaling 345±14 muscle fibers per sample acquired using a High-Resolution Interline CCD Camera (CoolSNAP cf. Photometrics, Tucson, USA) through a light microscope (Axioplan 2 Imaging, Zeiss, Denmark) and bandpass filters limiting excitation and emission wavelength ranges to 450–490 and 505–530 nm, respectively. The excitation and emission maximum of mGFP are 483 and 506 nm, respectively. No autoflourescence was detected in sections of non-transfected muscle samples (data not shown). The images were analyzed by counting the number of fluorescent structures using the computer program ImageJ (National Institute of Health, Maryland, USA). Transfection efficiency was represented as percentage of transfected fibers, which was calculated by dividing the number of transfected fibers by the total number of counted fibers and multiplying by 100.
2.8. Statistics
Distribution of data was evaluated using probability plots and Kolmogorov-Smirnov tests. Data are presented as means±SEM or 95% confidence interval. The effect of plasmid dilution volume was tested using a paired t-test (PROC TTEST). The effect of pre-treatment time was analyzed using a 2-way mixed model analysis (PROC MIXED) and to investigate the effect of hyaluronidase dose, plasmid dose, number of plasmid injections, transfection distribution or training a 1-way mixed model analysis (PROC ANOVA) was used (SAS version 9.2, SAS Institute, Cary, NC). Post hoc analysis was performed using Tukey-adjusted t-tests as appropriate. Distribution and variance homogeneity of the residuals derived from the variance analysis were evaluated using probability plots and scatter plots. Significance for all tests was set at P<0.05.
3. Results
3.1. Hyaluronidase dose affects transfection efficiency
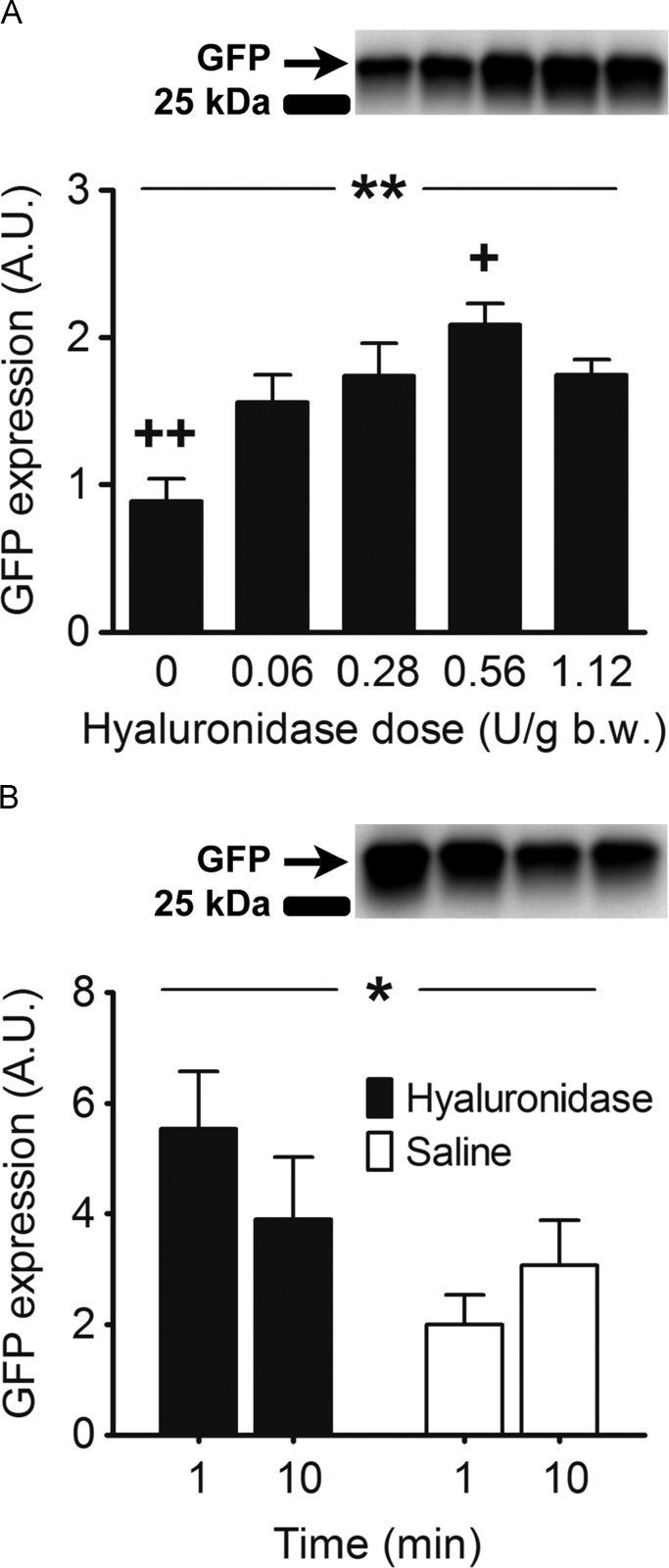
All hyaluronidase doses increased transfection efficiency compared with vehicle pre-treatment (p<0.01, Fig. 2A), and the effect with 0.56 U/g b.w. was greater than 0.06 U/g b.w. (p<0.05, Fig. 2A). The dose-dependent increase that was observed from low (0.06 U/g b.w.) to high (0.56 U/g b.w.) doses of hyaluronidase disappeared when the dose was increased to 1.12 U/g b.w. The highest hyaluronidase dose (1.12 U/g b.w.) tended to result in lower transfection efficiency than the second highest dose (0.56 U/g b.w.) (p=0.08, Fig. 2).
Fig. 2.
The figure depicts western blot band intensity, which represents GFP protein content in arbitrary units (A.U.) in Tibialis anterior (TA) muscles of Sprague-Dawley rats 1 week after transfection. A) Dose-response relationship between hyaluronidase pre-treatment dose and GFP expression. Both TA muscles of 23 rats were pre-treated with either 0.06 (n=10), 0.28 (n=10), 0.56 (n=10), 1.12 (n=10) U/g b.w. Hyaluronidase or vehicle (0 U/g b.w., n=6). B) Effect of timing of hyaluronidase pre-treatment on GFP expression. TA muscles of 14 rats were pretreated 1 (n=6) or 10 min (n=8) prior to plasmid injection with hyaluronidase (0.56 U/g b.w.) in one TA muscle and vehicle in the other. The data are presented as mean±SE. *: effect of hyaluronidase pre-treatment, P<0.05, **: effect of hyaluronidase dose, P<0.01, +: different from 0.06 U/g b.w. Hyaluronidase, P<0.05, ++: different from all doses of hyaluronidase, P<0.05.
3.2. Timing of hyaluronidase pre-treatment does not affect transfection efficiency
Pre-treating the muscle with hyaluronidase significantly increased transfection efficiency compared to vehicle pre-treatment (p<0.05, Fig. 2B). However, transfection efficiency was not affected by whether the pre-treatment was administered 1 or 10 min prior to plasmid injection (p=0.78, Fig. 3). There was no interaction between pre-treatment and time (p=0.15). Due to these findings we decided to administer hyaluronidase 1 min prior to plasmid injection in all subsequent experiments.
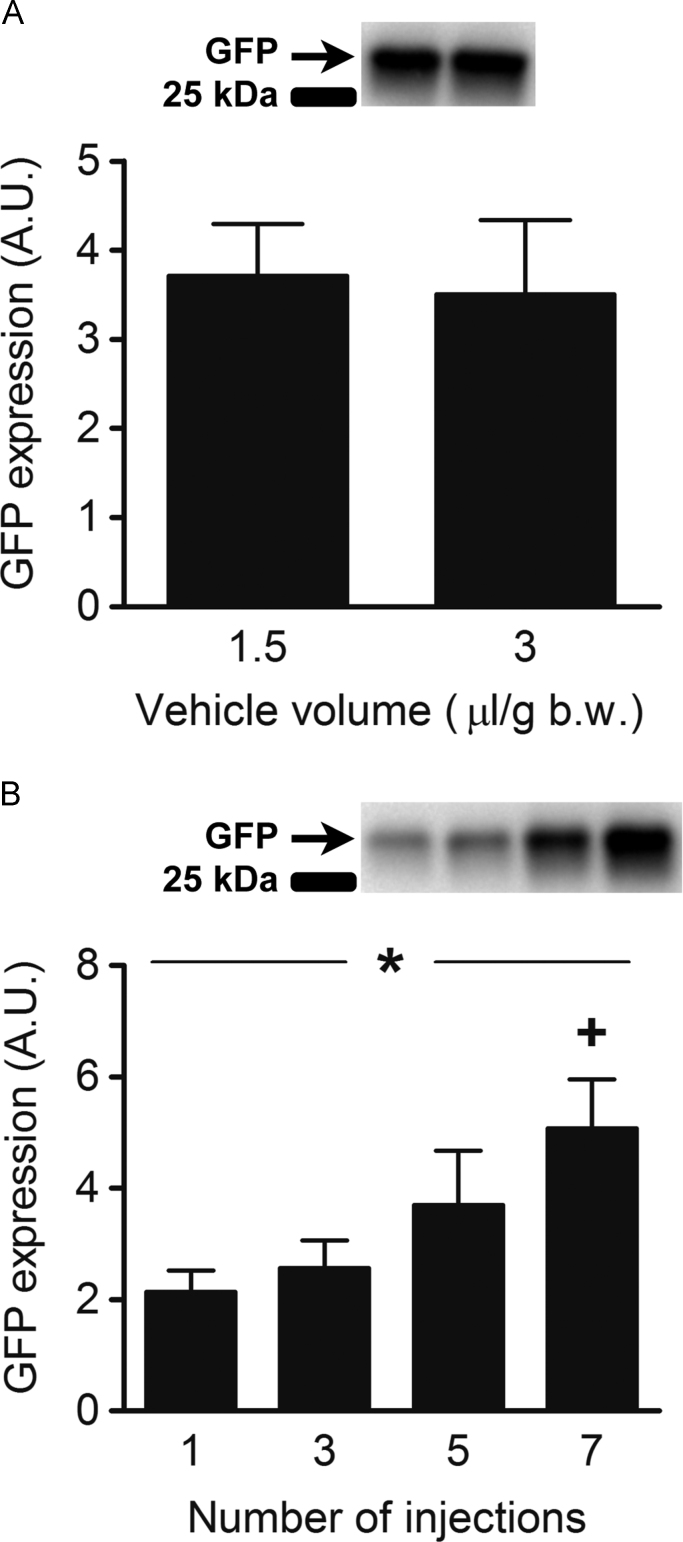
Fig. 3.
The figure depicts western blot band intensity, which represents protein content of GFP in arbitrary units (A.U.) in Tibialis anterior (TA) muscles of Sprague-Dawley rats 1 week after transfection. (A) Effect of plasmid dilution volume and GFP expression. The muscles of 12 rats were pre-treated with 0.56 U/g b.w. Hyaluronidase 10 min prior to plasmid injection. The plasmid (0.8 µg/g b.w.) was injected in one TA muscle diluted in a small (1.5 µl/g b.w., n=12) and in the other in a large (3 µl/g b.w., n=12) vehicle volume. (B) Effect of the number of plasmid injection sites and GFP expression. Both TA muscles of 10 rats were injected with DNA plasmid (0.8 µg/g b.w.) diluted in vehicle (1.5 µl/g b.w.) at either 1 (n=5), 3 (n=5), 5 (n=5), or 7 (n=5) evenly spaced sites 1 min following hyaluronidase pre-treatment (0.56 U/g b.w.). The data are presented as mean±SE. +: different from 1 and 3 injections, P<0.05 *: effect of number of injections, P<0.05.
3.3. No effect of plasmid dilution volume on transfection efficiency
There was no difference in muscle GFP expression when plasmid was delivered in a small (1.5 µl/g b.w.) or large (3 µl/g b.w.) volume of vehicle using 3 or 5 injection sites (p=0.37, Fig. 3A).
3.4. Increasing the number of plasmid injection sites increases transfection efficiency
Increasing the number of plasmid solution injections, thereby lowering the plasmid solution amount of every injection site and increasing the total tissue coverage, increased transfection efficiency in a dose-dependent manner (p<0.05, Fig. 3B). Delivering the plasmid using 7 evenly spaced injection sites was superior to 1 or 3 injections (p<0.05), but not significantly different from 5 (p=0.20).
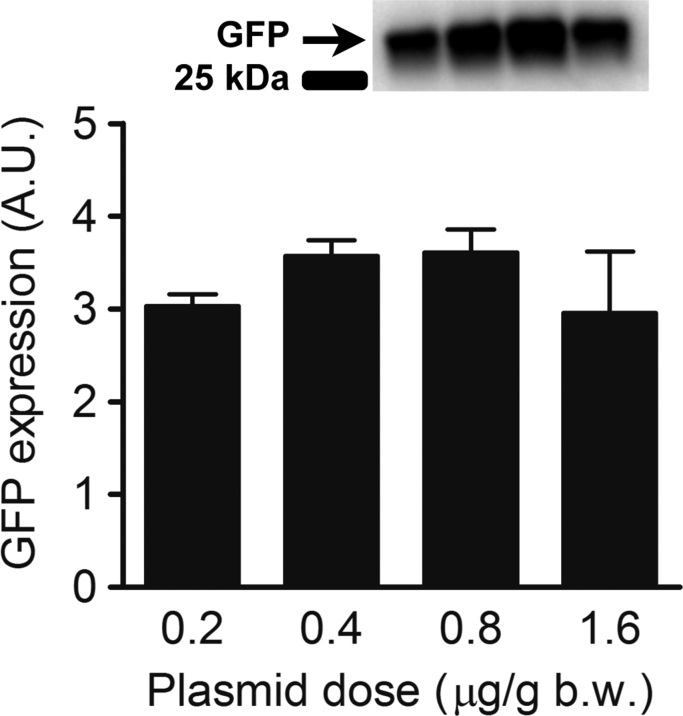
3.5. Plasmid dose does not affect transfection efficiency
The injected plasmid dose did not affect transfection efficiency significantly (p=0.47, Fig. 4). There was no difference in GFP expression of muscle that was injected with either 0.2, 0.4, 0.8 or 1.6 µg/g b.w. plasmid.
Fig. 4.
Effect of plasmid dose and GFP expression in Tibialis anterior (TA) muscles of Sprague-Dawley rats (n=16). Bar graph depicts western blot band intensity, which represents GFP expression 1 week after transfection by electroporation in arbitrary units (A.U.). Both TA muscles were injected with either 0.2 (n=8), 0.4 (n=8), 0.8 (n=8) or 1.6 (n=8) µg/g b.w. plasmid in vehicle (1.5 µl/g b.w.) delivered with 7 evenly spaced injections 1 min following hyaluronidase pre-treatment (0.56 U/g b.w.). The data are presented as mean±SE.
3.6. The percentage of transfected fibers was equally distributed across the whole length of the muscle
The optimized transfection protocol resulted in a high percentage of transfected muscle fibers in all three evaluated parts, i.e. the proximal, mid-belly and distal portions of the muscle and in all areas the percentage of transfected fibers was ~80% with no difference between the different regions of the muscle (p=0.97, Fig. 5).
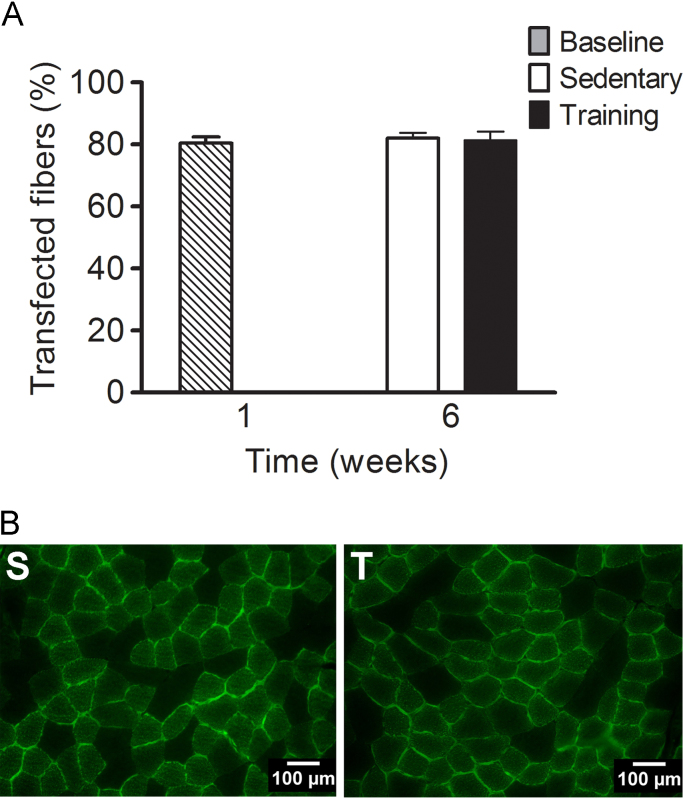
3.7. Exercise does not affect transfection stability
After 5 weeks of exercise training or sedentary life, the percentage of transfected muscle fibers were still ~80% and was not different from 1 week after transfection with the optimized protocol (p=0.94, Fig. 6). The within group variability was low, indicated by a range of 77–83, 70–90 and 76–90% transfected fibers in the baseline, sedentary and training groups, respectively. In addition, the 95% confidence intervals of percentage of transfected fibers were low both at baseline (CI95%: 79–84%, n=4) and after 5 weeks of training (CI95%: 76–87%, n=11) or sedentary life (CI95%: 78–86%, n=11). Importantly, 5 weeks of exercise training had no effect on transfection stability, suggesting that exercise training lasting up to 5 weeks can be combined with DNA electrotransfer for functional studies without concerns of changes in transgene expression.
Fig. 6.
The graph depicts the percentage (%) of transfected muscle fibers (A) and representative cross-sections (B) in the mid-belly portion of the Tibialis anterior muscle of Sprague-Dawley rats (n=24). All rats were pre-treated with hyaluronidase (0.56 U/g b.w.) 1 min prior to plasmid (0.8 µg/g b.w.) injection in vehicle (1.5 µl/g b.w.) delivered by 7 evenly spaced injections and electroporated immediately after. Muscles were harvested 1 week after transfection (B, n=4) or after an additional 5 weeks of sedentary life (S, n=11) or exercise training on a motorized treadmill (T, n=11). The data are presented as mean±SE.
4. Discussion
The present study investigated whether improvements in plasmid DNA delivery and hyaluronidase pre-treatment can improve transfection efficiency in rat skeletal muscle. We found that a hyaluronidase dose suitable for rats (0.56 U/g b.w.) injected into the muscle immediately prior to plasmid DNA injection increased transfection efficiency by >200%. Increasing the number of plasmid DNA injections further enhanced transfection efficiency whereas increasing plasmid dose from 0.2 to 1.6 µg/g b.w. or vehicle volume from 1.5 to 3.0 µl/g b.w. had no effect. The optimized protocol resulted in ~80% of muscle fibers transfected with a low animal to animal variability. The transfection was homogenously distributed throughout the muscle and stable over five weeks of regular exercise or inactivity. Our findings show that hyaluronidase pre-treatment and plasmid DNA delivery adapted to rat skeletal muscle greatly improves transfection efficiency.
The abundant connective tissue surrounding the muscle in the extracellular matrix is a barrier for adequate DNA plasmid distribution and transfer to the muscle [33]. Hyaluronidase degrades the extracellular matrix, which is thought to enhance distribution of fluids and soluble molecules in the tissue. This notion is supported by the dose-dependent increase in transgenic protein expression observed with hyaluronidase pre-treatment in rats (Fig. 2A), mice and rabbits [24]. However, in the present study the effect leveled off at the highest dose (1.12 U/g b.w.), which tended to be less effective than a dose of 0.56 U/g b.w. (p=0.08). It has been postulated that the extracellular matrix is a major limitation to plasmid gene transfer especially in larger animals [33]. In mice, the dose-dependency continuum stretches to very high doses of hyaluronidase [24], but this is not the case in rats (Fig. 2A) or rabbits [24]. When comparing the results of the present study (Fig. 2A) to previous results in mice [24], the most effective dose is ~3 times larger in mice than rats when size has been corrected for. Doing the same calculation using data from rabbit TA muscle [24], the most effective hyaluronidase dose is only one tenth and one quarter of the most effective dose in mice and rats, respectively. When a dose equivalent to the most effective dose in the present study (Fig. 2A) was used in rabbits the positive effect of hyaluronidase on transfection efficacy disappeared [24]. This suggests that there is a species difference in the optimal hyaluronidase dose and that larger hyaluronidase doses might have detrimental effects on transfection efficiency in larger animals when pre-treatment with hyaluronidase is combined with electroporation. In mice, the effect of hyaluronidase pre-treatment on transfection efficacy is also dependent on strain and age [34], indicating that it might be necessary to determine an optimal hyaluronidase dose for each experimental set-up depending on the species and characteristics of the animal used.
Pre-treatment with hyaluronidase is typically performed 1–4 h prior to injection of the plasmid in both mice [24], [31], [34], [35], [36] and rats [7], [8], [11]. The rationale is that the enzyme needs time to act on the extracellular matrix in order to have the desired effect. This requires anesthetizing the animal twice, which can be stressful for the animal, as well as being impractical and time-consuming for the operator. However, pre-treating the muscle only 10 min prior to plasmid injection elicits comparable effects [24], suggesting that the positive effect of hyaluronidase is achieved rapidly. In line with this, we show in the present study that a pre-injection of hyaluronidase only 1 min prior to plasmid injection is just as effective as injecting hyaluronidase 10 min prior to plasmid delivery (Fig. 2B). Using a shorter pre-injection time will save undue stress to the animals and time for the operator.
The exact mechanism of DNA electrotransfer is not fully elucidated, but it is clear from experiments both in vitro [37] and in vivo [21] that DNA has to be present for electroporation to enhance transfection efficiency. Increasing the proximity of plasmid DNA to the cell membrane [38] and the surface of interaction with the cell membrane leads to higher gene expression [39], but plasmid DNA only distributes a few hundred µm from the site of injection in muscle [40]. A better distribution of plasmid within the muscle using guided injections enhances transfection in combination with electroporation [2]. In an attempt to achieve better distribution of the plasmid prior to electroporation, we manipulated the volume of vehicle the plasmid was diluted in and the number of injection sites. Injecting plasmid diluted in a larger volume of vehicle had no effect on expression of GFP within the muscle (Fig. 3A), which is in line with previous observations in mice [1]. On the other hand, the number of injection sites had a clear effect on reporter expression (Fig. 3B). Others have not been able to find the same association in mice [1], but in that study electroporation was not used to enhance transfection efficiency. However, the delivery of electric pulses does not affect intramuscular plasmid DNA distribution [40] and is therefore an unlikely explanation for the observed difference. Thus the difference in size between the rat and mice TA muscle seems a more likely explanation. Rats are in the range of 5–9 times larger than mice and the size of the TA muscle is proportional to body size [41], [42]. With 7 evenly spaced injections of plasmid DNA, which we find to be most effective (Fig. 3B), the number of injections per mg of muscle weight is approximately the same as 1 injection in a mouse and the distribution of plasmid DNA can therefore be expected to be comparable to that in mice. Increasing the number of plasmid DNA injection sites does not seem to be warranted in mice [1], [43], but we show that increasing the number of plasmid DNA injection sites in rats increases total expression of reporter protein several fold (Fig. 3B).
Dose of plasmid DNA is another parameter that has previously been shown to have a large impact on transfection efficacy. Many studies show a clear dose-response between the amount of plasmid injected and the transfection efficacy [21], [26], [27]. In our study, the dose of plasmid DNA had no effect on transfection efficiency (Fig. 4). However, the dose response relationship between plasmid dose and gene expression has not previously been investigated after other transfection parameter have been optimized. We used an effective electroporation protocol [14] and plasmid vehicle solution [30] in addition to the most effective number of injections (Fig. 3B) and hyaluronidase dose (Fig. 2A) arrived at in the present study. The result is a very high percentage of transfected fibers in all parts of the muscle (Fig. 5). Thus, plasmid dose might be of lesser important for the transfection efficacy when other parameters of the protocol are optimal. For effective gene electrotransfer, it is important that an adequate amount of DNA plasmid is in close proximity to the cell [38], which increases the probability of a high number of transfected muscle fibers. It is possible that the amount of DNA plasmid in close proximity to most muscle fibers was adequate for successful transfection at all doses in the present study. In that case, further increasing the plasmid dose might have a negative impact on the transfection efficacy. Some studies have shown that transfection with excessive doses of plasmid can have deleterious effects on protein expression [27] and induce muscle damage [44].
Injection of plasmid without electroporation causes a limited [44], [45] inflammatory response, but does not result in muscle damage or loss of function. However, the combination of plasmid injection and electroporation has frequently been reported to induce small [23], [46], [47] to moderate [36], [44] inflammatory responses, muscle damage and a transient loss of function. In the present study, muscle damage was not assessed, but we used a gene electrotransfer protocol consisting of a single high voltage pulse followed by low voltage pulses which has been shown to only cause a small and brief inflammatory response [23], [26], [47]. In addition, any detectable muscle damage, reduction in force generation or functional limitation following the procedure subsides within 6–7 days [23], [26], [47]. Pre-treating the muscle with hyaluronidase has been reported to cause an increased inflammatory response [35], but it does not increase muscle damage [31] and using a 50% vol/vol saline solution as vehicle for the plasmid DNA [30] reduces muscle damage. Although muscle damage was not assessed it seems unlikely due to the nature of the gene electrotransfer protocol that there would be remnants of inflammation or muscle damage at the 1 and 5 week time-points when muscles were harvested [23], [26], [46], [47].
Our transfection protocol resulted in ~80% transfection in the proximal, mid-belly and distal portions of the TA muscle (Fig. 5), indicating that the number of transfected muscle cells was high and homogenously distributed throughout the muscle. There is a small degree of cell turn-over in skeletal muscle [28] and the turn-over can increase slightly with exercise training [29], which we thought might affect transfection stability. However, we found no evidence of a reduction in the percentage of transfected fibers, which was maintained at ~80% after 5 weeks, whether the rats exercised or not (Fig. 6).
There appears to be an additive effect of hyaluronidase pre-treatment and increasing plasmid doses on transfection longevity [24]. We used a hyaluronidase dose suitable for rats (Fig. 2A) and a comparable plasmid dose to the one eliciting the greatest response over time in mice [24]. A combination of these factors might explain why the high percentage of transfected fibers was maintained.
Transfection stability is also affected by which protein is encoded by the plasmid [48] and it is therefore possible that the high percentage of transfected muscle fibers observed in the present study would not be maintained if the plasmid encoded for a therapeutic protein. Previous studies have reported stable expression of reporter genes in skeletal muscle for up to a year [3], [21], [26], but electrotransfer of plasmids encoding a human secreted protein with therapeutic potential such as erythropoietin [27], [49], interleukin-10 [50] or factor IX [51] lead to a transient concentration peak of the encoded protein in the blood a few days after transfection followed by a return to low or undetectable levels within a few weeks or months. This reduction in protein expression is typically due to a humoral immune response targeting the xenogeneic protein [51] which is not seen when a syngeneic protein is expressed [48]. Expression of the encoded protein regardless of whether it is a xenogeneic or syngeneic protein is considerably more stable in muscle than in blood [48], [49], [50], [51] and in most cases stable expression can be detected in skeletal muscle for at least two months [50], [51]. It therefore seems unlikely that the number of transfected muscle fibers observed in this study five weeks after transfection would be lower if the transfected plasmid coded for a therapeutic gene rather than GFP.
In tissues such as skin using a plasmid with a tissue-specific promoter region seems to stabilize the expression of the therapeutic gene [52] whereas the muscle-specific creatine kinase promoter does not seem to have that effect in muscle tissue when compared to the ubiquitous CMV promoter [53]. Rather, transfection stability in skeletal muscle is affected largely by the electroporation protocol [26] and pre-treatment with hyaluronidase [24], [34]. In line with this, Hojman and coworkers showed that using a gene electrotransfer protocol similar to the one employed in the present study consisting of a single high voltage pulse followed by low voltage pulses resulted in stable expression of erythropoietin for 8 weeks [27].
5. Conclusions
There are a host of factors that affect the efficacy and stability of transgene expression in skeletal muscle. These factors seem to vary depending on the species and phenotype of the animal used in the experimental set-up. In the present study, we show that the main parameter for ensuring high transfection efficiency in rat skeletal muscle is a uniform distribution of the plasmid DNA across the whole muscle, demonstrated by the efficacy of increasing the number of injection sites and pre-treating the muscle with an effective dose of hyaluronidase prior to plasmid injection. Our optimized protocol results in a high percentage of transfected muscle fibers, which is stable over time and not affected by exercise training.
Conflicts of interest statements
We have no potential conflicts of interest relevant to this article to report.
Acknowledgment
The authors thank Martin Thomassen for his expert technical assistance. Thorbjorn Akerstrom was supported by a grant from The Danish Council for Independent Research (Grant # 09-073821) and the work was supported by a grant from the Novo Nordisk Research Foundation (Grant #R168a14300).
References
- 1.Manthorpe M., Cornefert-Jensen F., Hartikka J., Felgner J., Rundell A., Margalith M., Dwarki V. Gene therapy by intramuscular injection of plasmid DNA: studies on firefly luciferase gene expression in mice. Hum. Gene Ther. 1993;4:419–431. doi: 10.1089/hum.1993.4.4-419. [DOI] [PubMed] [Google Scholar]
- 2.Levy M.Y., Barron L.G., Meyer K.B., Szoka F.C., Jr Characterization of plasmid DNA transfer into mouse skeletal muscle: evaluation of uptake mechanism, expression and secretion of gene products into blood. Gene Ther. 1996;3:201–211. [PubMed] [Google Scholar]
- 3.Mir L.M., Bureau M.F., Rangara R., Schwartz B., Scherman D. Long-term, high level in vivo gene expression after electric pulse-mediated gene transfer into skeletal muscle. C. R. Acad. Sci. III. 1998;321:893–899. doi: 10.1016/s0764-4469(99)80003-1. [DOI] [PubMed] [Google Scholar]
- 4.Andre F.M., Gehl J., Sersa G., Preat V., Hojman P., Eriksen J., Golzio M., Cemazar M., Pavselj N., Rols M.P., Miklavcic D., Neumann E., Teissie J., Mir L.M. Efficiency of high- and low-voltage pulse combinations for gene electrotransfer in muscle, liver, tumor, and skin. Hum. Gene Ther. 2008;19:1261–1271. doi: 10.1089/hum.2008.060. [DOI] [PubMed] [Google Scholar]
- 5.Peng B., Zhao Y., Lu H., Pang W., Xu Y. In vivo plasmid DNA electroporation resulted in transfection of satellite cells and lasting transgene expression in regenerated muscle fibers. Biochem. Biophys. Res. Commun. 2005;338:1490–1498. doi: 10.1016/j.bbrc.2005.10.111. [DOI] [PubMed] [Google Scholar]
- 6.Wells K.E., Fletcher S., Mann C.J., Wilton S.D., Wells D.J. Enhanced in vivo delivery of antisense oligonucleotides to restore dystrophin expression in adult mdx mouse muscle. FEBS Lett. 2003;552:145–149. doi: 10.1016/s0014-5793(03)00904-9. [DOI] [PubMed] [Google Scholar]
- 7.Bruce C.R., Brolin C., Turner N., Cleasby M.E., van der Leij F.R., Cooney G.J., Kraegen E.W. Overexpression of carnitine palmitoyltransferase I in skeletal muscle in vivo increases fatty acid oxidation and reduces triacylglycerol esterification. Am. J. Physiol. Endocrinol. Metab. 2007;292:E1231–E1237. doi: 10.1152/ajpendo.00561.2006. [DOI] [PubMed] [Google Scholar]
- 8.Bruce C.R., Hoy A.J., Turner N., Watt M.J., Allen T.L., Carpenter K., Cooney G.J., Febbraio M.A., Kraegen E.W. Overexpression of carnitine palmitoyltransferase-1 in skeletal muscle is sufficient to enhance fatty acid oxidation and improve high-fat diet-induced insulin resistance. Diabetes. 2009;58:550–558. doi: 10.2337/db08-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright L.E., Brandon A.E., Hoy A.J., Forsberg G.B., Lelliott C.J., Reznick J., Lofgren L., Oscarsson J., Stromstedt M., Cooney G.J., Turner N. Amelioration of lipid-induced insulin resistance in rat skeletal muscle by overexpression of Pgc-1beta involves reductions in long-chain acyl-CoA levels and oxidative stress. Diabetologia. 2011;54:1417–1426. doi: 10.1007/s00125-011-2068-x. [DOI] [PubMed] [Google Scholar]
- 10.Holloway G.P., Lally J., Nickerson J.G., Alkhateeb H., Snook L.A., Heigenhauser G.J., Calles-Escandon J., Glatz J.F., Luiken J.J., Spriet L.L., Bonen A. Fatty acid binding protein facilitates sarcolemmal fatty acid transport but not mitochondrial oxidation in rat and human skeletal muscle. J. Physiol. 2007;582:393–405. doi: 10.1113/jphysiol.2007.135301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cleasby M.E., Davey J.R., Reinten T.A., Graham M.W., James D.E., Kraegen E.W., Cooney G.J. Acute bidirectional manipulation of muscle glucose uptake by in vivo electrotransfer of constructs targeting glucose transporter genes. Diabetes. 2005;54:2702–2711. doi: 10.2337/diabetes.54.9.2702. [DOI] [PubMed] [Google Scholar]
- 12.Spanggaard I., Snoj M., Cavalcanti A., Bouquet C., Sersa G., Robert C., Cemazar M., Dam E., Vasseur B., Attali P., Mir L.M., Gehl J. Gene electrotransfer of plasmid antiangiogenic metargidin peptide (AMEP) in disseminated melanoma: safety and efficacy results of a phase I first-in-man study. Hum. Gene Ther. Clin. Dev. 2013;24:99–107. doi: 10.1089/humc.2012.240. [DOI] [PubMed] [Google Scholar]
- 13.Bureau M.F., Gehl J., Deleuze V., Mir L.M., Scherman D. Importance of association between permeabilization and electrophoretic forces for intramuscular DNA electrotransfer. Biochim. Biophys. Acta. 2000;1474:353–359. doi: 10.1016/s0304-4165(00)00028-3. [DOI] [PubMed] [Google Scholar]
- 14.Satkauskas S., Bureau M.F., Puc M., Mahfoudi A., Scherman D., Miklavcic D., Mir L.M. Mechanisms of in vivo DNA electrotransfer: respective contributions of cell electropermeabilization and DNA electrophoresis. Mol. Ther. 2002;5:133–140. doi: 10.1006/mthe.2002.0526. [DOI] [PubMed] [Google Scholar]
- 15.Satkauskas S., Andre F., Bureau M.F., Scherman D., Miklavcic D., Mir L.M. Electrophoretic component of electric pulses determines the efficacy of in vivo DNA electrotransfer. Hum. Gene Ther. 2005;16:1194–1201. doi: 10.1089/hum.2005.16.1194. [DOI] [PubMed] [Google Scholar]
- 16.Jiang J., Jiangl N., Gao W., Zhu J., Guo Y., Shen D., Chen G., Tang J. Augmentation of revascularization and prevention of plasma leakage by angiopoietin-1 and vascular endothelial growth factor co-transfection in rats with experimental limb ischaemia. Acta Cardiol. 2006;61:145–153. doi: 10.2143/AC.61.2.2014327. [DOI] [PubMed] [Google Scholar]
- 17.Magee T.R., Artaza J.N., Ferrini M.G., Vernet D., Zuniga F.I., Cantini L., Reisz-Porszasz S., Rajfer J., Gonzalez-Cadavid N.F. Myostatin short interfering hairpin RNA gene transfer increases skeletal muscle mass. J. Gene Med. 2006;8:1171–1181. doi: 10.1002/jgm.946. [DOI] [PubMed] [Google Scholar]
- 18.Schakman O., Gilson H., de C., V, Lause P., Verniers J., Havaux X., Ketelslegers J.M., Thissen J.P. Insulin-like growth factor-I gene transfer by electroporation prevents skeletal muscle atrophy in glucocorticoid-treated rats. Endocrinology. 2005;146:1789–1797. doi: 10.1210/en.2004-1594. [DOI] [PubMed] [Google Scholar]
- 19.Dona M., Sandri M., Rossini K., Dell’Aica I., Podhorska-Okolow M., Carraro U. Functional in vivo gene transfer into the myofibers of adult skeletal muscle. Biochem. Biophys. Res. Commun. 2003;312:1132–1138. doi: 10.1016/j.bbrc.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 20.Pavlin D., Tozon N., Sersa G., Pogacnik A., Cemazar M. Efficient electrotransfection into canine muscle. Technol. Cancer Res. Treat. 2008;7:45–54. doi: 10.1177/153303460800700106. [DOI] [PubMed] [Google Scholar]
- 21.Mir L.M., Bureau M.F., Gehl J., Rangara R., Rouy D., Caillaud J.M., Delaere P., Branellec D., Schwartz B., Scherman D. High-efficiency gene transfer into skeletal muscle mediated by electric pulses. Proc. Natl. Acad. Sci. U. S. A. 1999;96:4262–4267. doi: 10.1073/pnas.96.8.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fattori E., Cappelletti M., Zampaglione I., Mennuni C., Calvaruso F., Arcuri M., Rizzuto G., Costa P., Perretta G., Ciliberto G., La M.N. Gene electro-transfer of an improved erythropoietin plasmid in mice and non-human primates. J. Gene Med. 2005;7:228–236. doi: 10.1002/jgm.652. [DOI] [PubMed] [Google Scholar]
- 23.Hojman P., Gissel H., Andre F.M., Cournil-Henrionnet C., Eriksen J., Gehl J., Mir L.M. Physiological effects of high- and low-voltage pulse combinations for gene electrotransfer in muscle. Hum. Gene Ther. 2008;19:1249–1260. doi: 10.1089/hum.2008.059. [DOI] [PubMed] [Google Scholar]
- 24.Mennuni C., Calvaruso F., Zampaglione I., Rizzuto G., Rinaudo D., Dammassa E., Ciliberto G., Fattori E., La M.N. Hyaluronidase increases electrogene transfer efficiency in skeletal muscle. Hum. Gene Ther. 2002;13:355–365. doi: 10.1089/10430340252792495. [DOI] [PubMed] [Google Scholar]
- 25.Wang X.D., Tang J.G., Xie X.L., Yang J.C., Li S., Ji J.G., Gu J. A comprehensive study of optimal conditions for naked plasmid DNA transfer into skeletal muscle by electroporation. J. Gene Med. 2005;7:1235–1245. doi: 10.1002/jgm.765. [DOI] [PubMed] [Google Scholar]
- 26.Tevz G., Pavlin D., Kamensek U., Kranjc S., Mesojednik S., Coer A., Sersa G., Cemazar M. Gene electrotransfer into murine skeletal muscle: a systematic analysis of parameters for long-term gene expression. Technol. Cancer Res. Treat. 2008;7:91–101. doi: 10.1177/153303460800700201. [DOI] [PubMed] [Google Scholar]
- 27.Hojman P., Gissel H., Gehl J. Sensitive and precise regulation of haemoglobin after gene transfer of erythropoietin to muscle tissue using electroporation. Gene Ther. 2007;14:950–959. doi: 10.1038/sj.gt.3302951. [DOI] [PubMed] [Google Scholar]
- 28.Schmalbruch H., Lewis D.M. Dynamics of nuclei of muscle fibers and connective tissue cells in normal and denervated rat muscles. Muscle Nerve. 2000;23:617–626. doi: 10.1002/(sici)1097-4598(200004)23:4<617::aid-mus22>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 29.Shefer G., Rauner G., Stuelsatz P., Benayahu D., Yablonka-Reuveni Z. Moderate-intensity treadmill running promotes expansion of the satellite cell pool in young and old mice. FEBS J. 2013;280:4063–4073. doi: 10.1111/febs.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee M.J., Cho S.S., Jang H.S., Lim Y.S., You J.R., Park J., Suh H., Kim J.A., Park J.S., Kim D.K. Optimal salt concentration of vehicle for plasmid DNA enhances gene transfer mediated by electroporation. Exp. Mol. Med. 2002;34:265–272. doi: 10.1038/emm.2002.37. [DOI] [PubMed] [Google Scholar]
- 31.McMahon J.M., Signori E., Wells K.E., Fazio V.M., Wells D.J. Optimisation of electrotransfer of plasmid into skeletal muscle by pretreatment with hyaluronidase -- increased expression with reduced muscle damage. Gene Ther. 2001;8:1264–1270. doi: 10.1038/sj.gt.3301522. [DOI] [PubMed] [Google Scholar]
- 32.Shepherd R.E., Gollnick P.D. Oxygen uptake of rats at different work intensities. Pflugers Arch. 1976;362:219–222. doi: 10.1007/BF00581173. [DOI] [PubMed] [Google Scholar]
- 33.Jiao S., Williams P., Berg R.K., Hodgeman B.A., Liu L., Repetto G., Wolff J.A. Direct gene transfer into nonhuman primate myofibers in vivo. Hum. Gene Ther. 1992;3:21–33. doi: 10.1089/hum.1992.3.1-21. [DOI] [PubMed] [Google Scholar]
- 34.Molnar M.J., Gilbert R., Lu Y., Liu A.B., Guo A., Larochelle N., Orlopp K., Lochmuller H., Petrof B.J., Nalbantoglu J., Karpati G. Factors influencing the efficacy, longevity, and safety of electroporation-assisted plasmid-based gene transfer into mouse muscles. Mol. Ther. 2004;10:447–455. doi: 10.1016/j.ymthe.2004.06.642. [DOI] [PubMed] [Google Scholar]
- 35.Chiarella P., De S.S., Fazio V.M., Signori E. Hyaluronidase contributes to early inflammatory events induced by electrotransfer in mouse skeletal muscle. Hum. Gene Ther. 2013;24:406–416. doi: 10.1089/hum.2012.215. [DOI] [PubMed] [Google Scholar]
- 36.Schertzer J.D., Plant D.R., Lynch G.S. Optimizing plasmid-based gene transfer for investigating skeletal muscle structure and function. Mol. Ther. 2006;13:795–803. doi: 10.1016/j.ymthe.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 37.Klenchin V.A., Sukharev S.I., Serov S.M., Chernomordik L.V., Chizmadzhev Y. Electrically induced DNA uptake by cells is a fast process involving DNA electrophoresis. Biophys. J. 1991;60:804–811. doi: 10.1016/S0006-3495(91)82115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neumann E., Kakorin S., Tsoneva I., Nikolova B., Tomov T. Calcium-mediated DNA adsorption to yeast cells and kinetics of cell transformation by electroporation. Biophys. J. 1996;71:868–877. doi: 10.1016/S0006-3495(96)79288-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faurie C., Phez E., Golzio M., Vossen C., Lesbordes J.C., Delteil C., Teissie J., Rols M.P. Effect of electric field vectoriality on electrically mediated gene delivery in mammalian cells. Biochim. Biophys. Acta. 2004;1665:92–100. doi: 10.1016/j.bbamem.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 40.Bureau M.F., Naimi S., Torero I.R., Seguin J., Georger C., Arnould E., Maton L., Blanche F., Delaere P., Scherman D. Intramuscular plasmid DNA electrotransfer: biodistribution and degradation. Biochim. Biophys. Acta. 2004;1676:138–148. doi: 10.1016/j.bbaexp.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 41.Armstrong R.B., Phelps R.O. Muscle fiber type composition of the rat hindlimb. Am. J. Anat. 1984;171:259–272. doi: 10.1002/aja.1001710303. [DOI] [PubMed] [Google Scholar]
- 42.Delp M.D., Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J. Appl. Physiol. 1985;80(1996):261–270. doi: 10.1152/jappl.1996.80.1.261. [DOI] [PubMed] [Google Scholar]
- 43.Hojman P. Basic principles and clinical advancements of muscle electrotransfer. Curr. Gene Ther. 2010;10:128–138. doi: 10.2174/156652310791110994. [DOI] [PubMed] [Google Scholar]
- 44.Durieux A.C., Bonnefoy R., Busso T., Freyssenet D. In vivo gene electrotransfer into skeletal muscle: effects of plasmid DNA on the occurrence and extent of muscle damage. J. Gene Med. 2004;6:809–816. doi: 10.1002/jgm.534. [DOI] [PubMed] [Google Scholar]
- 45.McMahon J.M., Wells K.E., Bamfo J.E., Cartwright M.A., Wells D.J. Inflammatory responses following direct injection of plasmid DNA into skeletal muscle. Gene Ther. 1998;5:1283–1290. doi: 10.1038/sj.gt.3300718. [DOI] [PubMed] [Google Scholar]
- 46.Vicat J.M., Boisseau S., Jourdes P., Laine M., Wion D., Bouali-Benazzouz R., Benabid A.L., Berger F. Muscle transfection by electroporation with high-voltage and short-pulse currents provides high-level and long-lasting gene expression. Hum. Gene Ther. 2000;11:909–916. doi: 10.1089/10430340050015518. [DOI] [PubMed] [Google Scholar]
- 47.Hojman P., Zibert J.R., Gissel H., Eriksen J., Gehl J. Gene expression profiles in skeletal muscle after gene electrotransfer. BMC Mol. Biol. 2007;8:56. doi: 10.1186/1471-2199-8-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gronevik E., von Steyern F.V., Kalhovde J.M., Tjelle T.E., Mathiesen I. Gene expression and immune response kinetics using electroporation-mediated DNA delivery to muscle. J. Gene Med. 2005;7:218–227. doi: 10.1002/jgm.650. [DOI] [PubMed] [Google Scholar]
- 49.Kreiss P., Bettan M., Crouzet J., Scherman D. Erythropoietin secretion and physiological effect in mouse after intramuscular plasmid DNA electrotransfer. J. Gene Med. 1999;1:245–250. doi: 10.1002/(SICI)1521-2254(199907/08)1:4<245::AID-JGM49>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 50.Deleuze V., Scherman D., Bureau M.F. Interleukin-10 expression after intramuscular DNA electrotransfer: kinetic studies. Biochem. Biophys. Res. Commun. 2002;299:29–34. doi: 10.1016/s0006-291x(02)02580-9. [DOI] [PubMed] [Google Scholar]
- 51.Bettan M., Emmanuel F., Darteil R., Caillaud J.M., Soubrier F., Delaere P., Branelec D., Mahfoudi A., Duverger N., Scherman D. High-level protein secretion into blood circulation after electric pulse-mediated gene transfer into skeletal muscle. Mol. Ther. 2000;2:204–210. doi: 10.1006/mthe.2000.0117. [DOI] [PubMed] [Google Scholar]
- 52.Kos S., Tesic N., Kamensek U., Blagus T., Cemazar M., Kranjc S., Lavrencak J., Sersa G. Improved specificity of gene electrotransfer to skin using pDNA under the control of collagen tissue-specific promoter. J. Membr. Biol. 2015;248:919–928. doi: 10.1007/s00232-015-9799-4. [DOI] [PubMed] [Google Scholar]
- 53.Fabre E.E., Bigey P., Orsini C., Scherman D. Comparison of promoter region constructs for in vivo intramuscular expression. J. Gene Med. 2006;8:636–645. doi: 10.1002/jgm.878. [DOI] [PubMed] [Google Scholar]