Abstract
Purpose of review:
To provide an overview of the epidemiology of depression in chronic neurologic conditions that can affect individuals throughout the lifespan (epilepsy, migraine, multiple sclerosis [MS]) and examine depression screening tools for adults with these conditions.
Recent findings:
Depression is common in neurologic conditions and can be associated with lower quality of life, higher health resource utilization, and poor adherence to treatment. It affects around 20%–30% of those with epilepsy, migraine, and MS, and evidence for a bidirectional association exists for each of these conditions. Depression screening tools generally perform well in neurologic conditions, but are not without limitations.
Summary:
Depression is a major contributor to poor outcomes in epilepsy, migraine, and MS. Although psychiatric resources are scarce globally, this is no reason to ignore depression in neurologic conditions. Depression screening tools are available in neurology and should be considered in clinical practice.
The burden of depression and neurologic conditions is considerable and their comorbid existence is challenging. Depressive disorders are a leading contributor to the global burden of disease.1 The most common form is major depressive disorder (MDD), which is characterized by persistently diminished mood or loss of interest or pleasure present most of the time, nearly every day for 2 weeks, along with additional depressive symptoms (table 1).2 Similarly to depressive disorders, the burden of neurologic disorders is substantial in part because most neurologic conditions are chronic, can develop at any time in one's, life and few are curable. Furthermore, depression is a common comorbidity in most neurologic disorders. A recent large-scale population-based study found a prevalence of depression ranging from 18% to 30%, depending on the neurologic condition in question.3 An analysis of this dataset confirmed high frequencies of suicidal ideation in association with depression in these populations.4 Depression can magnify neurologic symptoms, diminish adherence to medical management, reduce quality of life, and interfere with self-management.5–7
Table 1.
Symptoms required (at least 5) for the diagnosis of a major depressive episode, in addition to depressed mood or diminished interest2
Depression is often undiagnosed and undertreated in the general population and in those with neurologic conditions
Depression is a stigmatized condition and as a result, people with depression may be reticent to disclose their symptoms. In the general population, studies have consistently reported that a large proportion of people with depressive disorders do not seek help and among those who do, treatment is often inadequate.8 In neurologic settings, they may also be uncertain of the appropriateness of raising emotional concerns with neurologists or staff. Indeed, depression is often undertreated or undetected in neurologic conditions. For example, in a recent Canadian study, 70% of individuals with epilepsy who had depression were not receiving treatment for it.9 Yet there are brief and freely available validated instruments to screen for depression in those with epilepsy, migraine, and multiple sclerosis (MS). In public health applications, screening seeks to identify cases of disease at a preclinical stage, and can improve outcomes if earlier intervention leads to better outcomes. In clinical settings, screening has a broader meaning. It usually implies the application of a measurement strategy either for earlier detection or to detect unknown cases (case-finding) or symptomatic (e.g., poorly managed) cases.
In this narrative review, we examine the epidemiology of depression in 3 chronic neurologic conditions—epilepsy, migraine, and MS—that can develop at any time in one's life. We also present various estimates throughout the article, depending on the study cited, including (1) prevalence (or point prevalence)—the proportion of individuals affected by a condition at a particular point in time; (2) annual prevalence—the proportion of individuals affected by a condition annually; (3) 1-year prevalence—the proportion of individuals affected by a condition over a 1-year period, and (4) crude prevalence—the proportion of individuals affected by a condition at a particular point in time not taking into account adjustment for age or sex (or other important variables that could influence the estimate).
In this article, we also discuss the strengths and limitations of screening for depression and the use of specific screening instruments for adults with neurologic conditions, including epilepsy, migraine, and MS.
The epidemiology of depression in epilepsy
The prevalence of active depression in epilepsy was previously examined in a systematic review of population-based studies, and ranged between 13.2% and 36.5%, with a pooled estimate of 24.1%.10 Those with epilepsy had nearly 3 times the odds of having depression compared to those without epilepsy.10 Estimates were slightly higher in studies based on self-report than in studies using validated depression scales or administrative data. Regardless of the source or method of ascertainment use to detect depression, depression was consistently higher in epilepsy than in the general population.
There are a number of studies that demonstrate a bidirectional relationship between epilepsy and depression (i.e., epilepsy is associated with depression, and depression is associated with epilepsy), some of which were described in a recent review.11 As in the studies described in the prior paragraph, depression is associated with incident epilepsy and epilepsy with incident depression, regardless of the source or methods of ascertainment used to identify either condition.11
Risk factors for depression in epilepsy have been poorly investigated, as shown in a recent systematic review on the topic in community-based epilepsy cohorts.12 The authors found that most studies do not adequately assess risk factors for depression in epilepsy. Surprisingly, the only possible risk factors identified were sociodemographic factors (e.g., female sex, age, socioeconomic status, relationship status, ethnicity) rather than epilepsy-related factors, and even those were uncertain and not consistently reported as they have been in the general population or other chronic diseases.12 A more recent cross-sectional study by the same authors confirmed that in general, risk factors for epilepsy are similar to those found in the general population, with minimal if any evidence for disease-specific risk factors for depression in persons with epilepsy.13
Screening for depression in epilepsy
Although there are no evidence-based guidelines on the diagnosis and management of depression in epilepsy, consensus and expert-based statements recommend screening for depression in epilepsy annually, though some experts express concerns about the possible resource implications of screening everyone.14 On the other hand, depression in epilepsy is associated with poor outcomes including lower quality of life, poor adherence to treatment, and higher health care utilization.7,15,16 Depression is also strongly associated with suicidal ideation.4 In a recent study, the adjusted odds of suicidal ideation were 21, 14, and 21 for patients with epilepsy, migraine, or MS, respectively, if they had depression.4
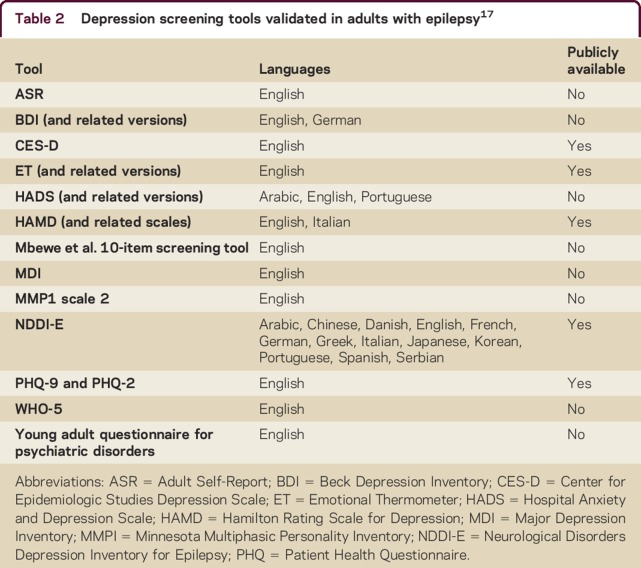
We recently completed a systematic review examining the validity of depression screening tools in persons with epilepsy.17 Out of more than 16,000 abstracts screened in any language, 38 articles met all eligibility criteria, and 16 screening tools were studied in children and adults in 13 languages including Arabic, Chinese, Danish, English, French, German, Greek, Italian, Japanese, Korean, Portuguese, Spanish, and Serbian (table 2).17 Although study quality varied between studies, in general, all of the tools performed relatively well, often with sensitivity, specificity, and positive predictive values that were greater than 80%, but lower negative predictive values (around 50%–60%). The depression screening tool that was most commonly validated in persons with epilepsy was the Neurological Disorders Depression Inventory for Epilepsy (NDDI-E), i.e., 26 studies.17 Currently, the NDDI-E seems most practical as it is publicly available, free to use, easy to score, and available in many languages. However, it has not been validated in the general population, as has the Patient Health Questionnaire–9 (PHQ-9), which is also available in the public domain and has been validated in both the general population and in persons with epilepsy. Ultimately the best tool will be targeted to the setting in which it is used based on resource availability and ease of use for clinical personnel.
Table 2.
Depression screening tools validated in adults with epilepsy17
The epidemiology of depression in migraine
A meta-analysis conducted in 2011 analyzed 12 original studies assessing the prevalence of depression in individuals with and without migraine.18 Prevalence estimates of depression ranged from 8.6% to 47.9% in those with migraine and 3.4% to 24.4% in those without migraine.18 Having migraine was associated with more than 2 times the odds of having depression (odds ratio [OR] 2.2, 95% confidence interval [CI] 2.0–2.3). A Canadian population-based study found that the 1-year prevalence of depression in the total study sample (n = 131, 535) was 7.4% (95% CI 7.2–7.6).19 The prevalence of reported migraine was 9.4% (95% CI 9.2–9.7). Individuals with migraine had a 1-year prevalence of 17.6% (95% CI 16.6–18.6) for depression, a 2.6 times greater prevalence compared to those without migraine.19
It has also been shown that the association of migraine and depression is stronger in those who have migraine with aura,20–22 and in those with chronic or daily headache.23,24 One large study found that in patients with frequent disabling migraines, the odds of depression was over 30 times higher (OR 31.8, 95% CI 12.9–78.5) as compared to patients with episodic headaches.24 A population-based study found that patients who were widowed, separated, divorced, or in a lower income category were more likely to have coexisting migraine and depression.25 In addition to the above risk factors, childhood adversities, previous depression, and female sex all increase the likelihood of developing frequent or severe headaches as an adult.26
In several longitudinal studies, it has been observed that the temporal association between migraine and depression is bidirectional, i.e., either condition can develop first and increases the odds of the other condition developing later.20,27–29 However, a recent retrospective cohort study using 12 years of follow-up data from the Canadian National Population Health Survey indicated that migraine occurs first, followed later by depression.30 Long-term prospective studies are needed to clarify this directionality more definitively.
Screening for depression in migraine
Despite the considerable comorbidity of depression and migraine, there are no guidelines to indicate which patients should be screened, which screening instruments are best to use, or how often screening should be performed.31 Some authors believe that formal screening tools should be used in patients with chronic headaches, those refractory to standard migraine therapies, and those referred to subspecialty headache clinics.31 Depression screening tools in patients with migraine, similar to those with other neurologic conditions, have limitations, including false-positive or false-negative results, symptoms that can be present in both disorders, and the need for confirmation of the diagnosis by a physician.
The most appropriate tool to screen for depression in migraine patients has not yet been determined. The PHQ-9 and Hospital Anxiety and Depression Scale (HADS) are commonly used in migraine studies because they are simple and quick to administer in outpatient settings. Other tools commonly used in migraine studies are the Beck Depression Inventory and Hamilton Depression Rating Scale. In general, these instruments have been validated in a number of different populations,32–35 but not specifically in the setting of migraine, with the exception of one study assessing the PHQ-9.36
The PHQ-9 is a self-administered scale containing 9 questions based on DSM-IV criteria. It can be scored by summating the points for each question or using a diagnostic algorithm. In the summation method, each of the 9 questions is scored from 0 to 3, for a total range of scores of 0–27. Once a total score is obtained, a cutoff point is selected. Those scoring above the cutoff point are qualified as having depression and those below the cutoff are qualified as not having depression.34 A meta-analysis performed in 2012 showed that the PHQ-9 had acceptable diagnostic accuracy for diagnosing depression for cutoff scores of 8–11 across a variety of settings.34 A study performed in Korea assessed 132 migraine patients and found an acceptable sensitivity (79.5%) and specificity (81.7%) at a cutpoint of 7 when assessing the PHQ-9 against the Mini International Neuropsychiatric Interview–Plus version 5.0.0, which served as the gold standard in this study.36 This indicates that different cutpoints may need to be considered for patients with migraine as compared to the general population. In addition, this study found that the PHQ-2, which is a validated but shorter self-administered scale, using the first 2 questions of the PHQ-9,37 may also be useful as a screening tool in migraine.
The epidemiology of depression in MS
The prevalence of depression in MS depends on how it is defined. Many studies have used symptom rating scales, assigning a cutpoint to screen for clinically important levels of depression. These studies have reported that approximately 20%–40% of people with MS in clinical settings are depressed.38–42 A recent national survey conducted in Canada using the PHQ-9 reported a depression point prevalence of 26% in patients with MS in the general population.43 The non-MS population prevalence in the general population, using a cutoff score of >10 for cases of depression, is 8.4% in Canada.44
Other studies have assessed major depressive episode using semi-structured diagnostic instruments. Rather than producing symptom ratings, these interviews apply diagnostic criteria to identify depressive disorders, as opposed to elevated symptoms. Studies using such instruments have reported MDD prevalence estimates between 17% and 50%.45–48 It is important to note that MDD criteria identify people who experience recurrent episodes of depression, and do not imply the presence of depressive symptoms at a particular point in time. Annual prevalence, a type of period prevalence, has also been investigated and was 15.7% in one population-based study.49
Some studies have used validated case definitions based on administrative data sources (data collected for administrative purposes, such as hospitalization and physician billing data) to assess prevalence. Using population-based administrative health data, Marrie et al.50 found a crude prevalence of depression of 20.1% in a sample of 44,452 people with MS, which was contrasted against a prevalence of 11.9% in an otherwise comparable general population sample. The prevalence of bipolar disorder is also elevated in MS,46,51 an estimate based on administrative data being 4.7% in MS, more than twice that of the general population.50 Carta et al.52 has reported that this association is evident across the spectrum of bipolar disorders, including cyclothymia.
Screening for depression in MS
There is a consensus that depression is underdetected in MS, so consensus statements often recommended screening.53,54 However, one study that attempted to implement screening and an associated evidence-based intervention in MS clinic settings was disappointing.55 In this study, of 60 patients who screened positively for depression, 32 declined participation in a management program for their depression, either because they did not consider themselves depressed or because they were receiving treatment that they were satisfied with. Similarly, a study that identified 349 patients with positive screens on the Center for Epidemiologic Studies Depression Scale during its recruitment found that nearly one third of them later needed to be excluded because they were already taking antidepressants.56 When combined with other reasons for exclusion, the trial enrolled only 42 participants. Formal screening can be problematic in busy neurologic settings unless systems are in place to obtain, score, and store the ratings. Also, screening is only useful if systems are in place to initiate next steps—usually a referral for additional assessment.
There has been much concern that standard screening instruments may not be valid in MS populations. Cognitive deficits and fatigue are both overlapping symptoms of MS and depression. Generally, scales that minimize physical symptoms, such as the HADS, the Beck Depression Inventory, or the Beck Fast Screen, have been favored in the MS literature, apparently due to this concern. However, this may actually discourage screening since these scales are copyrighted and their application requires licensing fees. Also, nonspecificity when depression rating scales are used in this population has not been confirmed empirically. A study that compared the public domain PHQ-9 to the HADS in MS failed to find evidence that the HADS is better in terms of its diagnostic validity.57 As none of these scales are diagnostic measures, decisions about treatment should be based on clinical assessment of those screening positive. These is currently no evidence that any specific scale is a superior predictor of treatment outcomes.
There are challenges to screening that go beyond valid measurement. For example, a large proportion of people with MS take antidepressants,58 such that many positive screens in clinical settings often represent inadequate outcomes of treatment rather than detection of depression. Also, adjustment disorders (e.g., at illness onset or the stress associated with MS attacks) may produce false-positive results. Low recruitment into a postscreening treatment trial indicates that screen-positive participants are not always eager to pursue additional assessment and treatment.56
There is currently insufficient evidence to recommend routine formal screening for depression in MS, and pros (better detection) and cons (such as logistics and influence on resources) need to be carefully considered in settings that are considering its implementation. Informal approaches such as using the first 2 items of the PHQ-9, the PHQ-2,37 asked verbally during clinical interactions can be surprisingly accurate as a case-finding strategy57,59 and may assist with the detection of depression in this population.
Summary, gaps, and future directions
It is clear that depression is associated with epilepsy, migraine, and MS and that the presence of these comorbid conditions influences outcomes negatively in these populations. What is less certain is whether there are risk factors that are consistently predictive of depression in these populations that can be targeted. If such factors are identified, primary prevention may be possible in the future. For the time being, better detection offers the possibility of better management and improved outcomes. Of course, better detection in the absence of better management will not be an effective strategy. Consequently, integration of mental health and neurologic services should be a priority.
Depression screening tools are imperfect, can be falsely positive (e.g., fatigue due to the MS or due to antiepileptic medication side effects in those with epilepsy), and require resources for implementation and for these reasons, their use is uncommon. However, despite some limitations, they provide an opportunity for health care providers to begin exploring the possibility that depression may be present in their patients with epilepsy, migraine, and MS. The implementation of depression screening tools in routine neurologic care will depend on strong information systems and coordination of the follow-up. Randomized controlled trials of formal screening interventions should be conducted to confirm that this strategy is efficacious and cost-effective. Informal screening measures, including the selective use of instruments and interview questions during clinical interactions, can be considered an element of good clinical care.
The management of depression is beyond the scope of this article; however, there is a general paucity of high-quality randomized controlled trials of depression treatment in these conditions. For example, in MS, a Cochrane Review found only 2 small low-quality trials of antidepressant treatment (total n = 70)60 and a recent guideline found only weak evidence for a single antidepressant treatment: telephone-administered cognitive behavioral therapy.54 A less restrictive systematic review found evidence of efficacy both for psychological and pharmacologic treatments.61 This is not unique to depression. Clinical trials of treatments for nonpsychiatric comorbidities are also uncommon. However, this does not provide evidence of a lack of efficacy. In clinical practice, offering standard evidence-based management for depression is usually a more acceptable option than withholding treatment due to uncertainties about generalizability to these populations. Hopefully, over time, the optimal ways to manage depression in persons with neurologic conditions will be identified. It is likely that the optimal treatment protocols will have similarities to standard depression treatment, since many nonspecific aspects of depression (e.g., coping with threats, stressors, and losses, addressing destructive cognitive and behavioral aspects of depression) are likely to be relevant. However, these conditions are also all diseases of the CNS and optimal management of depression is likely also to depend on integrated pathophysiologic and specific clinical connections between these problems. In the meantime, a multipronged approach may be considered (e.g., online educational tools, self-management, counseling, medications) and should be guided using a personalized approach.
Take-home points
Depression can have a serious effect on neurologic conditions, yet patients may not raise this concern with their neurologist.
Those with epilepsy or migraine have more than 2 times the odds of having active depression compared to those without these conditions.
The most widely validated screening tool for depression in epilepsy is the NDDI-E, which is publicly available and has been translated into 13 languages.
The most appropriate tool has not been determined for migraine, but one study shows support for the use of PHQ-9.
Despite theoretical concerns to the contrary, standard public domain scales such as the PHQ-9 perform well in MS.
Footnotes
See editorial, page 96
AUTHOR CONTRIBUTIONS
N. Jetté: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, study supervision. F. Amoozegar: drafting/revising the manuscript, or interpretation of data. Scott B. Patten: drafting/revising the manuscript, study concept or design, analysis or interpretation of data.
STUDY FUNDING
No targeted funding reported.
DISCLOSURES
N. Jetté serves as an Associate Editor for Epilepsia and on the Editorial Board of Neurology; receives research support from Canadian Institutes for Health Research, Alberta Health, Hotchkiss Brain Institute, and the Cumming School of Medicine at the University of Calgary; and holds a Canada Research Chair in Neurologic Health Services Research. F. Amoozegar has received speaker honoraria from Allergan, Tribute Pharmaceuticals Aralez, and EMD Serono and conference travel and accommodations from Teva and receives research support from Amgen, Eli Lilly, Teva, Allergan, and the University of Calgary. S.B. Patten serves as Editor-in-Chief of Canadian Journal of Psychiatry, Senior Associate Editor of Epidemiology & Psychiatric Sciences; and on the Editorial Advisory Boards of Chronic Diseases in Canada and Clinical Practice and Epidemiology in Mental Health; receives publishing royalties for Epidemiology for Canadian Students (Brush Education, 2015); and receives research support from Canadian Institutes for Health Research, Hotchkiss Brain Institute, and Pfizer Canada. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
REFERENCES
- 1.Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med 2013;10:e1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders–5. Arlington, VA: American Psychiatric Association Publishing; 2013. [Google Scholar]
- 3.Bulloch AG, Fiest KM, Williams JV, et al. Depression: a common disorder across a broad spectrum of neurologic conditions: a cross-sectional nationally representative survey. Gen Hosp Psychiatry 2015;37:507–512. [DOI] [PubMed] [Google Scholar]
- 4.Altura KC, Patten SB, Fiest KM, Atta C, Bulloch AG, Jette N. Suicidal ideation in persons with neurologic conditions: prevalence, associations and validation of the PHQ-9 for suicidal ideation. Gen Hosp Psychiatry 2016;42:22–26. [DOI] [PubMed] [Google Scholar]
- 5.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med 2000;160:2101–2107. [DOI] [PubMed] [Google Scholar]
- 6.Wang JL, Reimer MA, Metz LM, Patten SB. Major depression and quality of life in individuals with multiple sclerosis. Int J Psychiatry Med 2000;30:309–317. [DOI] [PubMed] [Google Scholar]
- 7.Ettinger AB, Good MB, Manjunath R, Edward Faught R, Bancroft T. The relationship of depression to antiepileptic drug adherence and quality of life in epilepsy. Epilepsy Behav 2014;36:138–143. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez HM, Vega WA, Williams DR, Tarraf W, West BT, Neighbors HW. Depression care in the United States: too little for too few. Arch Gen Psychiatry 2010;67:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fiest KM, Patten SB, Altura KC, et al. Patterns and frequency of the treatment of depression in persons with epilepsy. Epilepsy Behav 2014;39:59–64. [DOI] [PubMed] [Google Scholar]
- 10.Fiest KM, Dykeman J, Patten SB, et al. Depression in epilepsy: a systematic review and meta-analysis. Neurology 2013;80:590–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hesdorffer DC. Comorbidity between neurologic illness and psychiatric disorders. CNS Spectr 2016;21:230–238. [DOI] [PubMed] [Google Scholar]
- 12.Lacey CJ, Salzberg MR, D'Souza WJ. Risk factors for depression in community-treated epilepsy: systematic review. Epilepsy Behav 2015;43:1–7. [DOI] [PubMed] [Google Scholar]
- 13.Lacey CJ, Salzberg MR, D'Souza WJ. What factors contribute to the risk of depression in epilepsy? Tasmanian Epilepsy Register Mood Study (TERMS). Epilepsia 2016;57:516–522. [DOI] [PubMed] [Google Scholar]
- 14.Kerr MP, Mensah S, Besag F, et al. International consensus clinical practice statements for the treatment of neuropsychiatric conditions associated with epilepsy. Epilepsia 2011;52:2133–2138. [DOI] [PubMed] [Google Scholar]
- 15.Cramer JA, Blum D, Fanning K, Reed M; Epilepsy Impact Project Group. The impact of comorbid depression on health resource utilization in a community sample of people with epilepsy. Epilepsy Behav 2004;5:337–342. [DOI] [PubMed] [Google Scholar]
- 16.Cramer JA, Blum D, Reed M, Fanning K; Epilepsy Impact Project Group. The influence of comorbid depression on quality of life for people with epilepsy. Epilepsy Behav 2003;4:515–521. [DOI] [PubMed] [Google Scholar]
- 17.Gill SJ, Lukmanji S, Fiest KM, Patten SB, Wiebe S, Jette N. Depression screening tools in persons with epilepsy: a systematic review of validated tools. Epilepsia Epub 2017 Jan 8. [DOI] [PubMed]
- 18.Antonaci F, Nappi G, Galli F, Manzoni GC, Calabresi P, Costa A. Migraine and psychiatric comorbidity: a review of clinical findings. J Headache Pain 2011;12:115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molgat CV, Patten SB. Comorbidity of major depression and migraine: a Canadian population-based study. Can J Psychiatry 2005;50:832–837. [DOI] [PubMed] [Google Scholar]
- 20.Breslau N, Merikangas K, Bowden CL. Comorbidity of migraine and major affective disorders. Neurology 1994;44:S17–S22. [PubMed] [Google Scholar]
- 21.Peterlin BL, Katsnelson MJ, Calhoun AH. The associations between migraine, unipolar psychiatric comorbidities, and stress-related disorders and the role of estrogen. Curr Pain Headache Rep 2009;13:404–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oedegaard KJ, Neckelmann D, Mykletun A, et al. Migraine with and without aura: association with depression and anxiety disorder in a population-based study. The HUNT Study. Cephalalgia 2006;26:1–6. [DOI] [PubMed] [Google Scholar]
- 23.Buse DC, Silberstein SD, Manack AN, Papapetropoulos S, Lipton RB. Psychiatric comorbidities of episodic and chronic migraine. J Neurol 2013;260:1960–1969. [DOI] [PubMed] [Google Scholar]
- 24.Tietjen GE, Brandes JL, Digre KB, et al. High prevalence of somatic symptoms and depression in women with disabling chronic headache. Neurology 2007;68:134–140. [DOI] [PubMed] [Google Scholar]
- 25.Jette N, Patten S, Williams J, Becker W, Wiebe S. Comorbidity of migraine and psychiatric disorders: a national population-based study. Headache 2008;48:501–516. [DOI] [PubMed] [Google Scholar]
- 26.Lee S, Tsang A, Von Korff M, et al. Association of headache with childhood adversity and mental disorder: cross-national study. Br J Psychiatry 2009;194:111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Breslau N, Davis GC. Migraine, physical health and psychiatric disorder: a prospective epidemiologic study in young adults. J Psychiatr Res 1993;27:211–221. [DOI] [PubMed] [Google Scholar]
- 28.Breslau N, Lipton RB, Stewart WF, Schultz LR, Welch KM. Comorbidity of migraine and depression: investigating potential etiology and prognosis. Neurology 2003;60:1308–1312. [DOI] [PubMed] [Google Scholar]
- 29.Breslau N, Schultz LR, Stewart WF, Lipton RB, Lucia VC, Welch KM. Headache and major depression: is the association specific to migraine? Neurology 2000;54:308–313. [DOI] [PubMed] [Google Scholar]
- 30.Modgill G, Jette N, Wang JL, Becker WJ, Patten SB. A population-based longitudinal community study of major depression and migraine. Headache 2012;52:422–432. [DOI] [PubMed] [Google Scholar]
- 31.Maizels M, Smitherman TA, Penzien DB. A review of screening tools for psychiatric comorbidity in headache patients. Headache 2006;46(suppl 3):S98–S109. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell AJ. Clinical utility of screening for clinical depression and bipolar disorder. Curr Opin Psychiatry 2012;25:24–31. [DOI] [PubMed] [Google Scholar]
- 33.Maurer DM. Screening for depression. Am Fam Physician 2012;85:139–144. [PubMed] [Google Scholar]
- 34.Manea L, Gilbody S, McMillan D. Optimal cut-off score for diagnosing depression with the Patient Health Questionnaire (PHQ-9): a meta-analysis. CMAJ 2012;184:E191–E196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale: an updated literature review. J Psychosom Res 2002;52:69–77. [DOI] [PubMed] [Google Scholar]
- 36.Seo JG, Park SP. Validation of the Patient Health Questionnaire–9 (PHQ-9) and PHQ-2 in patients with migraine. J Headache Pain 2015;16:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire–2: validity of a two-item depression screener. Med Care 2003;41:1284–1292. [DOI] [PubMed] [Google Scholar]
- 38.Amtmann D, Kim J, Chung H, et al. Comparing CESD-10, PHQ-9, and PROMIS depression instruments in individuals with multiple sclerosis. Rehabil Psychol 2014;59:220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patten SB, Fridhandler S, Beck CA, Metz LM. Depressive symptoms in a treated multiple sclerosis cohort. Mult Scler 2003;9:616–620. [DOI] [PubMed] [Google Scholar]
- 40.Patten SB, Lavorato DH, Metz LM. Clinical correlates of CES-D depressive symptom ratings in an MS population. Gen Hosp Psychiatry 2005;27:439–445. [DOI] [PubMed] [Google Scholar]
- 41.Chwastiak L, Ehde DM, Gibbons LE, Sullivan M, Bowen JD, Kraft GH. Depressive symptoms and severity of illness in multiple sclerosis: epidemiological study of a large community sample. Am J Psychiatry 2002;159:1862–1868. [DOI] [PubMed] [Google Scholar]
- 42.Sacco R, Santangelo G, Stamenova S, et al. Psychometric properties and validity of Beck Depression Inventory II in multiple sclerosis. Eur J Neurol 2016;23:744–750. [DOI] [PubMed] [Google Scholar]
- 43.Viner R, Fiest KM, Bulloch AG, et al. Point prevalence and correlates of depression in a national community sample with multiple sclerosis. Gen Hosp Psychiatry 2014;36:352–354. [DOI] [PubMed] [Google Scholar]
- 44.Patten SB, Schopflocher D. Longitudinal epidemiology of major depression as assessed by the Brief Patient Health Questionnaire (PHQ-9). Compr Psychiatry 2009;50:26–33. [DOI] [PubMed] [Google Scholar]
- 45.Minden SL, Orav J, Reich P. Depression in multiple sclerosis. Gen Hosp Psychiatry 1987;9:426–434. [DOI] [PubMed] [Google Scholar]
- 46.Joffe RT, Lippert GP, Gray TA, Sawa G, Horvath Z. Mood disorders and multiple sclerosis. Arch Neurol 1987;44:376–378. [DOI] [PubMed] [Google Scholar]
- 47.Sadovnick AD, Remick RA, Allen J, et al. Depression and multiple sclerosis. Neurology 1996;46:628–632. [DOI] [PubMed] [Google Scholar]
- 48.Feinstein A, Feinstein K. Depression associated with multiple sclerosis: looking beyond diagnosis to symptom expression. J Affect Disord 2001;66:193–198. [DOI] [PubMed] [Google Scholar]
- 49.Patten SB, Beck CA, Williams JVA, Barbui C, Metz L. Major depression in multiple sclerosis: a population-based perspective. Neurology 2003;61:1524–1527. [DOI] [PubMed] [Google Scholar]
- 50.Marrie RA, Fisk JD, Tremlett H, et al. Differences in the burden of psychiatric comorbidity in MS vs the general population. Neurology 2015;85:1972–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Minden SL, Schiffer RB. Affective disorders in multiple sclerosis: review and recommendations for clinical research. Arch Neurol 1990;47:98–104. [DOI] [PubMed] [Google Scholar]
- 52.Carta MG, Moro MF, Lorefice L, et al. The risk of bipolar disorders in multiple sclerosis. J Affect Disord 2014;155:255–260. [DOI] [PubMed] [Google Scholar]
- 53.Goldman Consensus Group. The Goldman Consensus statement on mood disorders in multiple sclerosis. Mult Scler 2005;11:328–337. [DOI] [PubMed] [Google Scholar]
- 54.Minden SL, Feinstein A, Kalb RC, et al. Evidence-based guideline: assessment and management of psychiatric disorders in individuals with MS: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2014;82:174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patten SB, Newman S, Becker M, Riddell C, Metz L. Disease management for depression in an MS clinic. Int J Psychiatry Med 2007;37:459–473. [DOI] [PubMed] [Google Scholar]
- 56.Ehde DM, Kraft GH, Chwastiak L, et al. Efficacy of paroxetine in treating major depressive disorder in persons with multiple sclerosis. Gen Hosp Psychiatry 2008;30:40–48. [DOI] [PubMed] [Google Scholar]
- 57.Patten SB, Burton JM, Fiest KM, et al. Validity of four screening scales for major depression in MS. Mult Scler 2015;21:1064–1071. [DOI] [PubMed] [Google Scholar]
- 58.Patten SB, Williams JV, Metz LM. Anti-depressant use in association with interferon and glatiramer acetate treatment in multiple sclerosis. Mult Scler 2008;14:406–411. [DOI] [PubMed] [Google Scholar]
- 59.Mohr DC, Hart SL, Julian L, Tasch ES. Screening for depression among patients with multiple sclerosis: two questions may be enough. Mult Scler 2007;13:215–219. [DOI] [PubMed] [Google Scholar]
- 60.Koch MW, Glazenborg A, Uyttenboogaart M, Mostert J, De Keyser J. Pharmacologic treatment of depression in multiple sclerosis. Cochrane Database Syst Rev 2011:CD007295. [DOI] [PubMed] [Google Scholar]
- 61.Fiest KM, Walker JR, Bernstein CN, et al. Systematic review and meta-analysis of interventions for depression and anxiety in persons with multiple sclerosis. Mult Scler Relat Disord 2016;5:12–26. [DOI] [PubMed] [Google Scholar]