Abstract
Recent reports have shown that antibiotics such as macrolide, aminoglycoside, and tetracyclines have immunomodulatory effects in addition to essential antibiotic effects. These agents may have important effects on the regulation of cytokine and chemokine production. However, the precise mechanism is unknown. This time, we used Multi Plex to measure the production of cytokines and chemokines following tetracycline treatment of lipopolysaccharide (LPS)-induced THP-1 cells. The signaling pathways were investigated with Western blotting analysis. Three tetracyclines significantly suppressed the expression of cytokines and chemokines induced by LPS. Minocycline (50 μg/ml), tigecycline (50 μg/ml), or doxycycline (50 μg/ml) were added after treatment with LPS (10 μg/ml). Tumor necrosis factor-α was downregulated to 16%, 14%, and 8%, respectively, after 60 min compared to treatment with LPS without agents. Interleukin-8 was downregulated to 43%, 32%, and 26% at 60 min. Macrophage inflammatory protein (MIP)-1α was downregulated to 23%, 33%, and 16% at 120 min. MIP-1β was downregulated to 21%, 11%, and 2% at 120 min. Concerning about signaling pathways, the mechanisms of the three tetracyclines might not be the same. Although the three tetracyclines showed some differences in the time course, tetracyclines modulated phosphorylation of the NF-κB pathway, p38 and ERK1/2/MAPK pathways, resulting in inhibition of cytokine and chemokine production. In addition, SB203580 (p38 inhibitor) and U0126 (ERK1/2 inhibitor) significantly suppressed the expression of TNF-α and IL-8 in LPS-stimulated THP-1 cells. And further, the NF-κB inhibitor, BAY11-7082, almost completely suppressed LPS-induced these two cytokines production. Thus, more than one signaling pathway may be involved in tetracyclines downregulation of the expression of LPS-induced cytokines and chemokines in THP-1 cells. And among the three signaling pathways, NF-κB pathway might be the dominant pathway on tetracyclines modification the LPS-induced cytokines production in THP-1 cells. In general, minocycline and doxycycline suppressed the production of cytokines and chemokines in LPS-stimulated THP-1 cell lines via mainly the inhibition of phosphorylation of NF-κB pathways. Tigecycline has the same structure as the other tetracyclines, however, it showed the different properties of cytokine modulation in the experimental time course.
Abbreviations: LPS, lipopolysaccharide; NF-κB, nuclear factor-kappa B; IκBα/β, inhibitor kappa B alpha and beta; IKKα, inhibitor kappa B kinase alpha; IKKβ, inhibitor kappa B kinase beta
Keywords: Lipopolysaccharide (LPS), Cytokine, Chemokine, Tetracyclines, Nuclear factor kappa B (NF-κB), Mitogen-activated protein kinase (MAPK)
Highlights
-
•
Tetracyclines inhibit cytokine and chemokine production in LPS-induced THP-1 cells.
-
•
Tetracyclines inhibit NF-κB, p38 and activate ERK phosphorylation.
-
•
Tetracyclines regulate these pathways and NF-κB pathway has a pivotal role.
1. Introduction
Tetracycline was discovered in the 1940s and has revealed effectiveness against various microorganisms including gram-positive and gram-negative bacteria, chlamydiae, mycoplasmas, rickettsiae and protozoan parasites for more than 60 years. Tetracycline has been used extensively for prophylaxis and therapy in both human and animal infections. However, recent studies have shown that tetracycline and its analogs such as minocycline, doxycycline, and tigecycline, have several non-antibiotic, anti-inflammatory properties including a modulatory effect on immunostimulatory activities in vitro [1], [2], [3].
Doxycycline has a broad-spectrum antibacterial activity and it is useful for both gram-negative and gram-positive microorganisms. It inhibits bacterial protein synthesis by binding to the 30S ribosomal subunit. Doxycycline inhibits T-cell proliferation and production of cytokines and chemokines induced by staphylococcal exotoxins in human peripheral blood mononuclear cells [1]. It also inhibits the production of interleukin (IL)-1β in lipopolysaccharide (LPS)-stimulated corneal epithelial cells [4]. Thus, doxycycline modulates cytokine production in different types of cell lines. Castro et al. [5] showed that doxycycline and tetracycline modulate IL-6, IL-1β, tumor necrosis factor (TNF)-α in patient with dengue hemorrhagic fever. Doxycycline gives more significant effect on modulating cytokine than tetracycline. Both doxycycline and minocycline decreased the production of TNF-α, IL-6, and IL-8 in a dose-dependent manner [6].
Minocycline has also recently been reported to have additional effects besides antimicrobial functions. However, the precise mechanisms still remain unclear. Tai et al. investigated the effects of minocycline on cytokine and chemokine production and the expression levels of intracellular phosphorylated proteins in an in vitro model of LPS-induced cytokine response [7]. Many recent studies have elucidated on non-antibiotic properties of minocycline, including anti-inflammatory and anti-apoptotic activities as well as inhibition of proteolysis, angiogenesis, and tumor metastasis [8].
Tigecycline is the first glycylcycline antibiotic to be approved by the US Food and Drug Administration. Tigecycline derived from minocycline has an activity against many gram-positive and gram-negative organisms including methicillin-resistant Staphylococcus aureus, vancomycin-intermediate and vancomycin-resistant enterococci, and extended-spectrum β-lactamase–producing Escherichia coli and Klebsiella pneumoniae [9]. The efficacy of tigecycline was already reported by Pachon-Ibanez et al. [10]. Pichardo et al. [11] reported the in vitro activities of tigecycline and imipenem against 49 isolates of Acinetobacter baumannii, including those resistant to imipenem. These results showed that tigecycline has efficient activity against A. baumannii, including strains resistant to imipenem. Tigecycline is also active against Acinetobacter spp. and S. maltophilia strains. This agent may play a crucial role in severe respiratory infections of both nosocomial and community origin [12]. In addition, tigecycline significantly attenuates the expression and release of nuclear factor-kappa B (NF-κB), TNF-α, and IL-1β, as well as nitric oxide levels in LPS-induced pheochromocytoma (PC12) cells. Tigecycline modifies cytokine and chemokine production in LPS-induced PC12 cells [13].
The effectiveness of tetracycline and its derivatives in clinical use in various diseases have been investigated. The other properties of tetracyclines and their possibility for clinical use have been shown in rosacea, bullous dermatoses, neutrophilic diseases, pyoderma gangrenosum, sarcoidosis, cancer metastasis, periodontitis and autoimmune disorders [14]. Tetracyclines have also modulate cytokine production and cytotoxicity [15]. We hypothesized that tetracyclines might inhibit the production of cytokines and chemokines in addition to their conventional antimicrobial effects and may thus control inflammation. The precise mechanism of modulation of the expression of cytokines and chemokines by tetracyclines remains unknown. In this study, we showed that three tetracycline derivatives, minocycline, tigecycline, and doxycycline, have different modulatory effects on extracellular signal-regulated kinase (ERK)1/2, p38/mitogen-activated protein kinase (MAPK), and NF-κB signaling pathways to suppress production of cytokines and chemokines induced in the THP-1 cell line stimulated with LPS.
2. Materials and methods
2.1. Drugs and chemicals
Minocycline, doxycycline, U0126 and BAY11-7082 were purchased from Sigma Chemical Company (St. Louis, MO, USA). SB203580 was purchased from Wako Industrial Company (Osaka, Japan). Tigecycline was a gift from Pfizer Inc. (New York, NY, USA). LPS from Pseudomonas aeruginosa Stereotype 10 (Sigma Chemical Company) was used as a bacterial component that induces cytokine and chemokine production. Tetracyclines were dissolved in nanopure water and stored at −20 °C. LPS was dissolved in nanopure water and stored at −80 °C. Primary antibodies included rabbit polyclonal anti-IκBα and phospho-IκBα, rabbit polyclonal anti-IKKα and phospho-IKKα, rabbit polyclonal anti-IKKβ and phospho-IKKβ, rabbit polyclonal anti-NF-κB and phospho-NF-κB, rabbit polyclonal anti-phospho-ERK1/2, rabbit polyclonal anti-phospho-p38 (all from Cell Signaling Technology, Beverly, MA, USA), and rabbit polyclonal anti-actin antibody (Sigma Chemical Company).
2.2. Cell culture and LPS stimulation
The THP-1 human monocytic leukemia cell line was purchased from RIKEN Cell Bank (Wako, Japan). The cells were cultured in RPMI-1640 medium with 10% FBS at 37 °C in humidified air with 5% CO2. THP-1 (5×105/ml) cells added with LPS were incubated for the indicated time in the presence of different antibiotics. Supernatants were collected to measure cytokine and chemokine production. Cell pellets were used for Western blotting analysis.
After treatment with LPS (10 μg/ml) without tetracyclines, with minocycline (50 μg/ml), with tigecycline (50 μg/ml), or with doxycycline (50 μg/ml), samples were collected 30, 60, 120 or 240 min after treatment.
2.3. Cytokine and chemokine measurements
To measure cytokines and chemokines, we used the Multi Plex Bead Immunoassay (Bio-Plex Suspension Array System, BIO-RAD Laboratories, Inc., Hercules, CA, USA) as previously described [7]. We measured 12 cytokines or chemokines including TNF-α, TNF-β, interferon (IFN)-γ, IL-1α, IL-1β, IL-8, IL-6, IL-12, IL-17, macrophage inflammatory protein (MIP)-1α, MIP-1β, and vascular endothelial growth factor (VEGF). The TNF-α and IL-8 enzyme-linked immunosorbent assay (ELISA) kits were purchased from Invitrogen Corporation (Camerio, CA, USA). ELISA was conducted to confirm the TNF-α and IL-8 production after treatment with different signal pathway inhibitors such as SB203580, U0126 and BAY11-7082 in LPS-stimulated THP-1 cells. The experiments were performed at least three times and the optical density of the samples was measured at 450 nm using an automated ELISA reader (SPECTRA max M5; Tokyo, Japan).
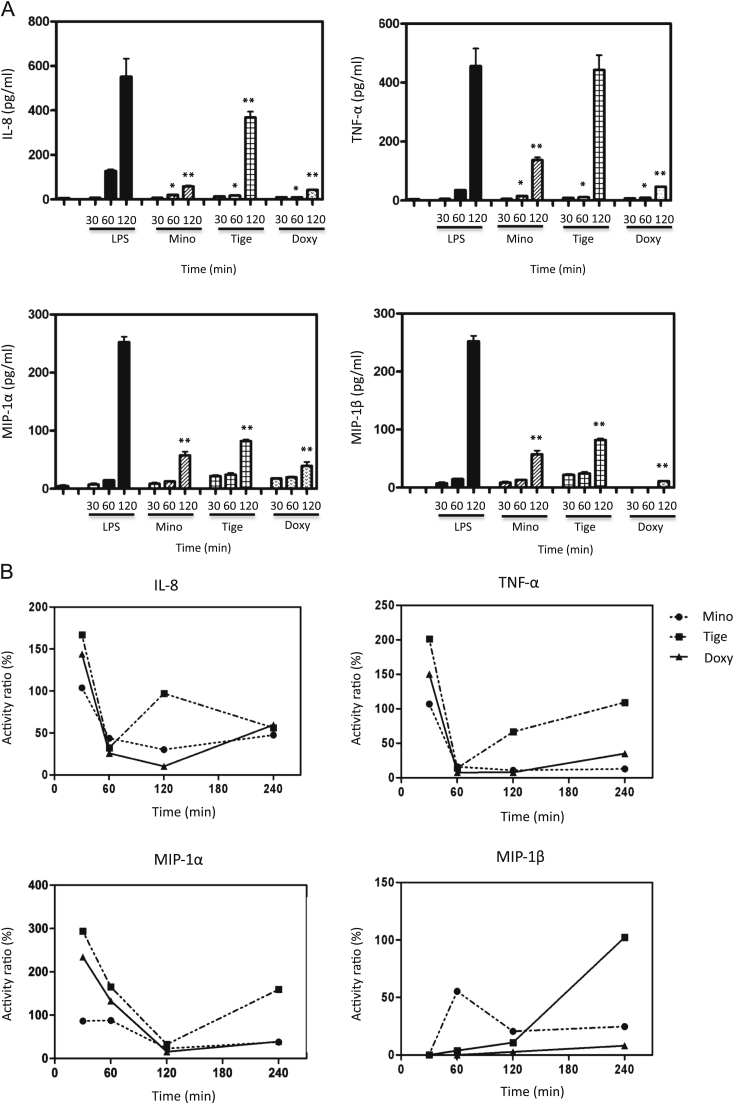
The values of the measured cytokines and chemokines were shown (Fig. 1). Otherwise we calculated the ratio of the value at each point (30 min, 60 min, 120 min, 240 min) to the control value. We used the control value measured in LPS stimulated THP-1 cells without tetracyclines. The values measured after administration of tetracyclines were divided by the control value (Fig. 2).
Fig. 1.
(A) Time-dependent changes in cytokine and chemokine production in LPS-stimulated THP-1 cells. After LPS (10 μg/ml) treatment for 15, 30, 60, or 120 min without any agents and with minocycline (50 μg/ml), tigecycline (50 μg/ml), or doxycycline (50 μg/ml), cytokines and chemokines were measured using Multi Plex according to the manufacturer’s protocols. * p<0.05 compared with LPS only at 60 min. ** p<0.05 compared with LPS only at 120 min. Mino: minocycline, Tige: tigecycline, Doxy: doxycycline. (B) The rate of cytokine and chemokine production in the THP-1 cell line compared to the production of cytokines and chemokines by LPS stimulation without tetracyclines. After LPS treatment (10 μg/ml) for 30, 60, 120 or 240 min without any agents and with minocycline (50 μg/ml), tigecycline (50 μg/ml), or doxycycline (50 mg/ml), cytokines and chemokines were measured with Multi Plex.
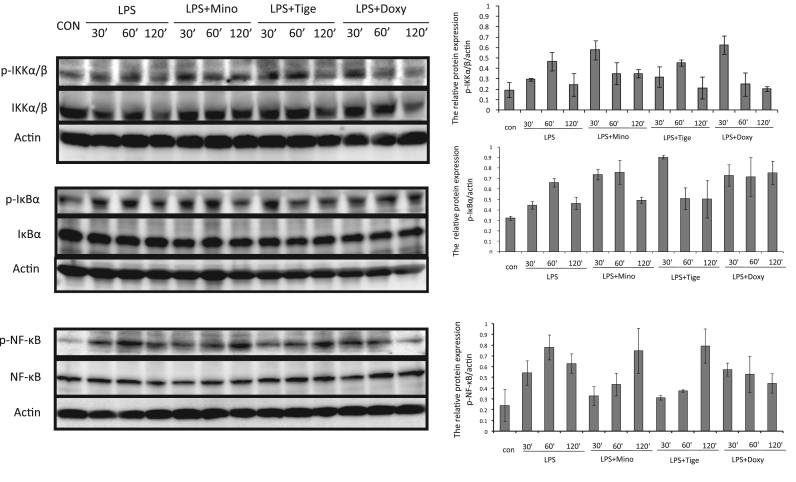
Fig. 2.
Effects of minocycline, doxycycline, and tigecycline on the modulation of NF-κB, phospho-NF-κB, IKKα/β, phospho-IKKα/β, IκBα, and phospho-IκBα in LPS-stimulated THP-1 cells. THP-1 cells were incubated without or with 10 μg/ml LPS, or with LPS plus minocycline (50 μg/ml), doxycycline (50 μg/ml), or tigecycline (50 μg/ml) for 30, 60, or 120 min. NF-κB, phospho-NF-κB, IKKα/β, phospho-IKKα/β, IκBα, and phospho-IκBα were assessed with Western blotting.
2.4. Western blotting analysis
To elucidate modulation of signaling pathways, the protein levels of phospho-ERK1/2 (Thr185/Tyr187), phospho-p38 (Thr180/Tyr182), nuclear factor-κB alpha (IκBα) (Ser32), phospho-IκBα, NF-κB, phospho-NF-κB, IKKα, phospho-IKKα, IKKβ, and phospho-IKKβ were determined with Western blotting. Protein lysates were electrophoretically separated on sodium dodecyl sulfate-polyacrylamide gels and transferred to polyvinylidene difluoride membranes. The membranes were probed with primary antibodies followed by secondary antibodies. 5 μl antibodies were diluted to 5 ml Phosphate Bufferd Salts with Tween. The ratio of dilution was 1:1000. The signal was visualized and quantified using Image Quant LAS4000 mini apparatus (GE Healthcare, Uppsala, Sweden) and Supersignal West Pico Chemiluminescent Substrate (enhanced chemiluminescence) (Thermo Scientific Company, Barrington, IL, USA).
2.5. Statistical analysis
All graphs were generated with GraphPad, Prism software (GraphPad Software, Inc., San Diego, CA, USA). Data were presented as means±standard deviations (SD) and p values were calculated using unpaired Student’s t-test with two-tailed analysis. All statistical analyses were performed using Microsoft Excel (Microsoft Corporation, Redmond, WA, USA). A value of p<0.05 was considered significant.
3. Results
3.1. Tetracycline modification of LPS-induced cytokine and chemokine production in THP-1 cells
Using the Multi Plex kit, we confirmed that LPS induced cytokine and chemokine production in THP-1 cell lines. TNF-β, IFN-γ, IL-1α, IL-1β, IL-6, IL-12, IL-17, and VEGF were not significantly increased following LPS treatment. After treatment with LPS, cytokines (TNF-α, IL-8) and chemokines (MIP-1α, MIP-1β) increased rapidly beginning at 60 to 120 min and then reached stable levels. Tetracyclines downregulated the release of cytokines and chemokines. Minocycline inhibited TNF-α to 16.0% and 10.7% at 60 and 120 min, respectively. Tigecycline inhibited TNF-α to 14.0% and 66.6%, and doxycycline inhibited to 7.6% and 7.8%, respectively (Fig. 1A), compared to control levels.
At 60 and 120 min, minocycline inhibited IL-8 to 43.6% and 30.1%, tigecycline inhibited the cytokine to 32.2% and 97.1%, and doxycycline inhibited IL-8 to 25.9% and 10.3%, respectively, compared to control levels. For MIP-1α at 60 and 120 min, minocycline induced 87.4% and 22.8% of control levels, tigecycline induced 165.0% and 32.6% of control levels, and doxycycline induced 132.9% and 15.6% of control levels, respectively. For MIP-1β at 60 and 120 min, minocycline induced 58.2% and 20.6% of control levels, tigecycline induced 3.8% and 10.9% of control levels, and doxycycline induced 0% and 2.7% of control levels, respectively (Fig. 1B). It was true that the ratios at 30 min were rather too high and that were caused the data at 30 min were unstable and within rather small range, the calculated values might possibly give the misunderstanding. The production of cytokines and chemokines increased gradually and reached the stable level at 4–12 h after LPS stimulation [7]. Thereby the significance of the value at 30 min has not been clarified previously. The ratio showed the high values seemingly.
After treatment with LPS followed by tetracyclines, the production of TNF-α and IL-8 was downregulated. Tigecycline inhibited production of both TNF-α and IL-8 at 60 min. Levels were restored to control levels at 120 min. Minocycline and doxycycline suppressed the production of TNF-α and IL-8 at 120 and 240 min. For the production of MIP-1α and MIP-1β, significant suppression by minocycline and doxycycline was observed. Tigecycline did not significantly inhibit MIP-1β at 240 min. Thus, tetracyclines downregulated cytokines and chemokines production in LPS-stimulated THP-1 cells.
3.2. Effect of tetracyclines on the NF-κB signaling pathway in LPS-stimulated THP-1 cells
To confirm the effect of tetracyclines on the NF-κB signaling pathway in LPS-stimulated THP-1 cells, phospho-NF-κB, NF-κB, phospho-IKKα/β, IKKα/β, phospho-IκBα, and IκBα were assessed with Western blotting. Phospho-NF-κB was significantly increased by LPS stimulation of THP-1 cells, suppressed by minocycline and tigecycline at 30 min, and not suppressed after 120 min compared with LPS alone. Doxycycline suppressed phosphorylation at 60 and 120 min. As regards, phosphorylation of IKKα/β, a similar result was shown to phosphorylation of NF-κB in LPS-stimulated THP-1 cells. Minocyclin suppressed phospho-IKKα/β at 60 min and doxycycline at 60 and 120 min compared with LPS alone. Phosphorylation of IκBα was upregulated by LPS and downregulated by tigecycline at 60 min. However, minocycline and doxycycline did not significantly suppress phospho-IκBα. Although the three tetracyclines showed some differences in time dependent course, tetracyclines modulated phosphorylation of the IKKα/β, IκBα, and NF-κB pathways, resulting in inhibition of cytokine and chemokine production (Fig. 2).
3.3. Effect of tetracyclines on the p38 and ERK/MAPK pathways in LPS-stimulated THP-1 cells
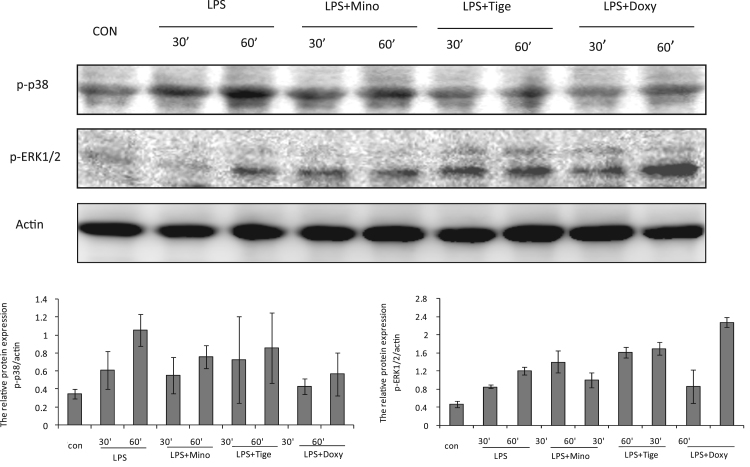
To investigate whether the MAPK signaling pathway was involved in tetracycline modulation of LPS-induced cytokine and chemokine release in THP-1 cells, phospho-ERK1/2 and phospho-p38 were measured in the presence or absence of tetracyclines after 30 and 60 min incubation periods. p38 phosphorylation was significantly activated by LPS stimulation in THP-1 cells. Among the three tetracyclines, minocycline and doxycycline significantly suppressed p38 phosphorylation, but tigecycline did not, compared with LPS alone. For the ERK/MAPK pathway, signal activation was induced by LPS stimulation of THP-1 cells after 60 min. Compared with LPS only, minocycline induced an increase in phospho-ERK1/2 activation at 30 min and the activation was decreased at 60 min after treatment. However, phosphorylation was greater following treatment with the other two tetracyclines (tigecycline and doxycycline) compared with minocycline 60 min after treatment (Fig. 3).
Fig. 3.
Effects of tetracyclines (minocycline, doxycycline, and tigecycline) on the activation of phospho-ERK1/2 and phospho-p38 in LPS-stimulated THP-1 cells. THP-1 cells were incubated without or with 10 μg/ml LPS, or with LPS plus minocycline (50 μg/ml), doxycycline (50 μg/ml), or tigecycline (50 μg/ml) for 30 or 60 min. Phospho-p38 and phospho-ERK1/2 were assessed with Western blotting.
3.4. Tetracyclines suppressed LPS-induced cytokines via both MAPK and NF-κB pathways in THP-1 cells
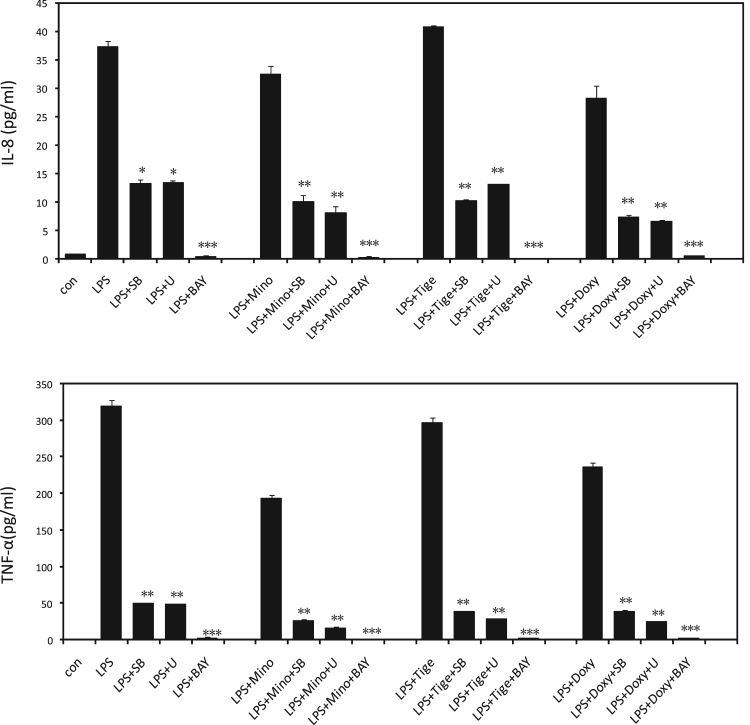
To further confirm the effect of different signal pathway on cytokines production, after pre-incubation with U0126 (ERK/MAPK inhibitor), SB203580 (p38/MAPK inhibitor) and BAY11-7082 (NF-κB inhibitor), we detected the cytokines of TNF-α and IL-8 production in LPS-stimulated THP-1 cells in present or absent of tetracyclines. As shown in Fig. 4, SB203580 and U0126 significantly suppressed the production of TNF-α and IL-8 in LPS-stimulated THP-1 cells. And these inhibitory effects were emphasized by treatment with tetracyclines. It suggested that both ERK/MAPK and p38/MAPK pathways were involved in tetracyclines modification the production of LPS-induced cytokines in THP-1 cells. In addition, the NF-κB inhibitor, BAY11-7082, almost completely suppressed the LPS-induced TNF-α and IL-8 production in THP-1 cell. It suggested that NF-κB signaling pathway might be the dominant pathway on tetracyclines modification the production of LPS-induced cytokines in THP-1 cells (Fig. 4).
Fig. 4.
SB203580, U0126 and BAY11-7082 suppressed TNF-α and IL-8 production in LPS-stimulated THP-1 cells on treatment with or without tetracyclines. THP-1 cells were pre-incubated by SB203580 (10 μM), U0126 (5 μM) and BAY11-7082 (5 μM) for 30 min, followed treatment without or with LPS (10 μg/ml), or with LPS (10 μg/ml) plus minocycline (50 μg/ml), doxycycline (50 μg/ml), or tigecycline (50 μg/ml) for 60 min. TNF-α were measured with ELISA. *p<0.05, **p<0.01, ***p<0.001 compared to the measurement without the inhibitor in the same group. Abbreviation; SB: SB203580, U: U0126, BAY: BAY11-7082.
4. Discussion
Inflammation is a defensive response to numerous stimuli such as injury, radiation, and pathogens, and occurs through various inflammatory mediators such as cytokines and chemokines, which coordinate host defense and repair [7]. Appropriate cytokine and chemokine production is essential for the host and may involve various immune-mediated processes, leading to protection of host organs against pathogen invasion. However, uncontrolled inflammatory responses can harm the host. Muroya et al. [15] revealed that inflammatory cytokines exert cytotoxicity in the human alveolar epithelial cell line A549. A mixture of IL-1β, TNF-α, and IFN-γ, designated as a “cytomix”, shows augmented cytotoxicity compared with the effects of each individual cytokine. Therefore, over-expression of cytokines and chemokines may lead to secondary damage and a systemic disorder in the organism such as septic shock. LPS, the major component of the outer membrane of gram-negative bacteria, is the main factor responsible for microglial activation [16]. Cytokine and chemokine production induced by LPS both in vivo and in vitro has been reported in previous studies [17], [18]. In the present study, cytokines (TNF-α, IL-8) and chemokines (MIP-1α, MIP-1β) increased rapidly beginning at 60 min after LPS (10 μg/ml) stimulation of THP-1 cells. Therefore, we used this concentration of LPS as the experimental model for further evaluating the immunomodulatory effects of tetracyclines. Minocycline and doxycycline showed the effect on the suppression of the production more than 4 h after LPS administration, but as for tigecycline, the production recovered at 2–4 h.
Tetracyclines show various activities besides of antimicrobial activity [8], [14],19,20]. These non-antibiotic, anti-inflammatory properties suggest that tetracyclines may provide additional clinical benefits for the treatment of some non-bacterial diseases, such as allergic asthma [21], rickettsial infections [22], [23], rheumatoid arthritis [24], [25], neurodegenerative diseases [26], [27] and malignant tumors [28], [29]. Due to the pleiotropic effects of tetracyclines, the immunostimulatory effect on monocytes may contradict its useful effects for the treatment of several kinds of chronic inflammation. However, the precise mechanism of modulation of the production of cytokines and chemokines by tetracyclines is still unknown. In addition, as far as we know, no report has shown a comparison among three tetracyclines. Accordingly, we investigated the different mechanisms of the immunomodulatory effects on cytokines and chemokines by three different tetracyclines in THP-1 cells.
NF-κB is a key regulator of the transcription of many inflammatory cytokines [30]. NF-κB translocation into the nucleus is preceded by the phosphorylation of IκBα, a protein that normally sequesters the NF-κB complex in the cytosol in an inactive form. Following inflammatory stimuli, phosphorylation and degradation of IκBα allow the NF-κB heterodimer to rapidly move into the nucleus [30], [31]. MAPKs are important factors of inflammatory and stress-induced signal pathways which regulated cell survival and death. The ERK signal pathway is induced primarily by mitogenic stimuli and growth factors; otherwise the p38 signal pathway is induced primarily by various stresses including inflammation [32]. Previous reports also showed that minocycline decreases the production of multiple cytokines and chemokines by inhibiting LPS-induced IKKα/β phosphorylation in THP-1 cells [7]. In this study, we found that minocycline induced an increase in phosoho-ERK1/2 activation and suppressed phospho-NF-κB at 30 min. Clinically speaking, most patients with tsutsugamushi disease in Japan, a rickettsial infection disease, show antipyretic and recover quickly when treated with minocycline [33]. This clinical effect may be partly due to rapid modification of signaling pathways by minocycline followed by suppression of cytokine and chemokine production induced by pathogens. In addition, minocycline not only modulates the NF-κB pathway but also suppresses p38 phosphorylation and activates ERK1/2 phosphorylation. Thus, more than one signaling pathway is involved in minocycline downregulation of the expression of LPS-induced cytokines and chemokines in human THP-1 cells. Doxycycline was recently shown to upregulate the expression of the cytokines IL-6 and granulocyte/macrophage colony-stimulating factor via MAPK/ERK and NF-κB pathways in mouse thymic epithelial cells [33]. Doxycycline prevents LPS-induced endothelial barrier dysfunction by inhibiting the activation of the p38/MAPK pathway in human umbilical vein endothelial cells [34]. Here, we evaluated that doxycycline activated phospho-ERK1/2 and suppressed phospho-p38 and phospho-NF-κB 60 min after treatment. Therefore, our results demonstrated that doxycycline can modify both MAPK (p38 and ERK) and NF-κB pathways in THP-1 cells. As shown above, tigecycline suppresses the expression of LPS-induced TNF-α and IL-1β in rat PC12 cells via NF-κB signaling pathways [13]. However, in present study, tigecycline also downregulated the expression of LPS-induced cytokines and chemokines not only by suppressing phosphorylation of NF-κB, but also by suppressing phosphorylation of p38 and activation of the ERK1/2 pathway. Sheth et al. reported that p38 inhibition by SB203580 enhances ERK activity during endotoxemia. They suggested that interaction between the ERK and p38/MAPK pathways induced the apoptotic potential of polymorphonuclear neutrophils in inflammatory states [35]. In our study, SB203580 (p38 inhibitor) and U0126 (ERK1/2 inhibitor) significantly suppressed the production of TNF-α and IL-8 in LPS-stimulated THP-1 cells. And cytokines were further suppressed by treatment of tetracyclines, indicating that the MAPKs are partially associated with the cytokine production. In addition, BAY11-7082 (NF-κB inhibitor) almost completely suppressed LPS-induced cytokine production. Furthermore, despite the phosphorylation levels of upstream signaling molecules, our data showed that p-NF-κB levels were finally suppressed by the three independent tertacyclines at 60 min after LPS stimulation. These findings suggested that NF-κB pathway would be the most striking target on tetracyclines modification due to the LPS-induced cytokine productions in THP-1 cells.
In conclusion, the production of LPS-induced cytokines (TNF-α, IL-8) and chemokines (MIP-1α, MIP-1β) was suppressed by three tetracycline derivatives, minocycline, tigecycline, and doxycycline, in THP-1 cells. However, the mechanisms of action of the three tetracyclines were different. More than one cell signaling pathway may be involved in downregulation of the expression of LPS-induced cytokines and chemokines by tetracyclines in THP-1 cells. Among the three signaling pathways, NF-κB pathway might be the dominant pathway. The effects of tetracyclines on cytokine and chemokine production may be expected for the treatment of the cytokine storm in bacterial infectious diseases. It is necessary to consider about the difference between tigecycline and others in clinical use.
Acknowledgment
This work was supported in part by a Grant-in-Aid for Scientific Research (KAKENHI) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (24591478, 2014). We are grateful to M. Sugano for her technical assistance.
Footnotes
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j. bbrep.2015.11.003.
Appendix A. Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
References
- 1.Krakauer T., Buckley M. Doxycycline is anti-inflammatory and inhibits staphylococcal exotoxin-induced cytokines and chemokines. Antimicrob. Agents Chemother. 2003;47:3630–3633. doi: 10.1128/AAC.47.11.3630-3633.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bostanci N., Akgül B., Tsakanika V., Allaker R.P., Hughes F.J., McKay I.J. Effects of low-dose doxycycline on cytokine secretion in human monocytes stimulated with Aggregatibacter actinomycetemcomitans. Cytokine. 2011;56:656–661. doi: 10.1016/j.cyto.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 3.Iwasaki H., Inoue H., Mitsuke Y., Badran A., Ikegaya S., Ueda T. Doxycycline induces apoptosis by way of caspase-3 activation with inhibition of matrix metalloproteinase in human T-lymphoblastic leukemia CCRF-CEM cells. J. Lab. Clin. Med. 2002;140:382–386. doi: 10.1067/mlc.2002.129308. [DOI] [PubMed] [Google Scholar]
- 4.Solomon A., Rosenblatt M., Li D.Q., Liu Z., Monroy D., Ji Z., Lokeshwar B.L., Pflugfelder S.C. Doxycycline inhibition of interleukin-1 in the corneal epithelium. Investig. Ophthalmol. Vis. Sci. 2000;41:2544–2557. [PubMed] [Google Scholar]
- 5.Castro J.E., Vado-Solis I., Perez-Osorio C., Fredeking T.M. Fredeking clinical study: modulation of cytokine and cytokine receptor/antagonist by treatment with doxycycline and tetracycline in patients with dengue fever. Clin. Dev. Immunol. 2011;2011:370872. doi: 10.1155/2011/370872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernardino A.L., Kaushal D., Philipp M.T. The antibiotics doxycycline and minocycline inhibit the inflammatory responses to the lyme disease spirochete Borrelia burgdorferi. J. Infect. Dis. 2009;199:1379–1388. doi: 10.1086/597807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tai K., Iwasaki H., Ikegaya S., Ueda T. Minocycline modulates cytokine and chemokine production in lipopolysaccharide- stimulated THP-1 monocytic cells by inhibiting IκB kinase α/β phosphorylation. Transl. Res. 2013;161:99–109. doi: 10.1016/j.trsl.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Garrido-Mesa N., Zarzuelo A., Gálvez J. Minocycline: far beyond an antibiotic. Br. J. Pharmacol. 2013;169:337–352. doi: 10.1111/bph.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stein G.E., Craig W.A. Tigecycline: a critical analysis. Clin. Infect. Dis. 2006;43:518–524. doi: 10.1086/505494. [DOI] [PubMed] [Google Scholar]
- 10.Pachón-Ibánez M.E., Jiménez-Mejías M.E., Pichardo C., Llanos A.C., Pachón J. Activity of tigecycline (GAR-936) against Acinetobacter baumannii Strains, including those resistant to Imipenem. Antimicrob. Agents Chemother. 2004;48:4479–4481. doi: 10.1128/AAC.48.11.4479-4481.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pichardo C., Pachón-Ibanez M.E., Docobo-Perez F., López-Rojas R., Jiménez-Mejías M.E., Garcia-Curiel A., Pachon J. Efficacy of tigecycline vs. imipenem in the treatment of experimental Acinetobacter baumannii murine pneumonia. Eur. J. Clin. Microbiol. Infect. Dis. 2010;29:527–531. doi: 10.1007/s10096-010-0890-6. [DOI] [PubMed] [Google Scholar]
- 12.Fritsche T.R., Sader H.S., Stilwell M.G., Dowzicky M.J., Jones R.N. Antimicrobial activity of tigecycline tested against organisms causing community-acquired respiratory tract infection and nosocomial pneumonia. Diagn. Microbiol. Infect. Dis. 2005;52:187–193. doi: 10.1016/j.diagmicrobio.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Yagnik R.M., Benzeroual K.E. Tigecycline prevents LPS-induced release of pro-inflammatory and apoptotic mediators in neuronal cells. Toxicol. Vitr. 2013;27:686–693. doi: 10.1016/j.tiv.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 14.Sapadin A.N., Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J. Am. Acad. Dermatol. 2006;54:258–265. doi: 10.1016/j.jaad.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Muroya M., Chang K., Uchida K., Bougaki M., Yamada Y. Analysis of cytotoxicity induced by proinflammatory cytokines in the human alveolar epithelial cell line A549. Biosci. Trends. 2012;6:70–80. [PubMed] [Google Scholar]
- 16.Bi X.L., Yang J.Y., Dong Y.X., Wang J.M., Cui Y.H., Ikeshima T., Zhao Y.Q., Wu C.F. Resveratrol inhibits nitric oxide and TNF-alpha production by lipopolysaccharide-activated microglia. Int. Immunopharmacol. 2005;5:185–193. doi: 10.1016/j.intimp.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Gorczynski R.M., Alexander C., Bessler W., Fournier K., Hoffmann P., Mach J.P., Manuel J., Ramakrishna V., Rietschel E.T., Song L., Waelli T., Westphal O., Zahringer U. Characterization of an interaction between fetal hemoglobin and lipid A of LPS resulting in augmented induction of cytokine production in vivo and in vitro. Int. Immunopharmacol. 2004;4:1859–1872. doi: 10.1016/j.intimp.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Dumitru C.D., Ceci J.D., Tsatsanis C., Kontoyiannis D., Stamatakis K., Lin J.H., Patriotis C., Jenkins N.A., Copeland N.G., Kollias G., Tsichlis P.N. TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell. 2000;103:1071–1083. doi: 10.1016/s0092-8674(00)00210-5. [DOI] [PubMed] [Google Scholar]
- 19.Federici T.J. The non-antibiotic properties of tetracyclines: Clinical potential in ophthalmic disease. Pharmacol. Res. 2011;64:614–623. doi: 10.1016/j.phrs.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 20.Monk E., Shalita A., Siegel D.M. Clinical applications of non-antimicrobial tetracyclines in dermatology. Pharmacol. Res. 2011;63:130–145. doi: 10.1016/j.phrs.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Joks R., Durkin H.G. Non-antibiotic properties of tetracyclines as anti-allergy and asthma drugs. Pharmacol. Res. 2011;64:602–609. doi: 10.1016/j.phrs.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Iwasaki H., Inoue H., Takada N., Mahara F., Ueda T. Cytokine modulation induced by minocycline in Tsutsugamushi disease. Kansenshogaku Zasshi. J. Jpn. Assoc. Infect. Dis. 2000;74:598–600. doi: 10.11150/kansenshogakuzasshi1970.74.598. [DOI] [PubMed] [Google Scholar]
- 23.Iwasaki H., Mizoguchi J., Takada N., Tai K., Ikegaya S., Ueda T. Correlation between the concentrations of tumor necrosis factoralpha and the severity of disease in patients infected with Orientia tsutsugamushi. Int. J. Infect. Dis. 2010;14 doi: 10.1016/j.ijid.2009.06.002. E328–33. [DOI] [PubMed] [Google Scholar]
- 24.Tilley B.C., Alarcón G.S., Heyse S.P. Minocycline in rheumatoid arthritis. A 48-week, double-blind, placebo-controlled trial. MIRA Trial Group. Ann. Intern Med. 1995;122:81–89. doi: 10.7326/0003-4819-122-2-199501150-00001. [DOI] [PubMed] [Google Scholar]
- 25.Langevitz P., Livneh A., Bank I., Pras M. Benefits and risks of minocycline in rheumatoid arthritis. Drug Saf. 2000;22:405–414. doi: 10.2165/00002018-200022050-00007. [DOI] [PubMed] [Google Scholar]
- 26.Costa R., Speretta E., Crowther D.C., Cardoso I. Testing the therapeutic potential of doxycycline in a Drosophila melanogaster model of Alzheimer disease. J. Biol. Chem. 2011;86:41647–41655. doi: 10.1074/jbc.M111.274548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garwood C.J., Cooper J.D., Hanger D.P., Noble W. Anti-inflammatory impact of minocycline in a mouse model of tauopathy. Front. Psychiatry. 2010;1:136. doi: 10.3389/fpsyt.2010.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ataie-Kachoie P., Badar S., Morris D.L., Pourgholami M.H. Minocycline targets the NF-κB Nexus through suppression of TGF-β1-TAK1-IκB signaling in ovarian cancer. Mol. Cancer Res. 2013;11:1279–1291. doi: 10.1158/1541-7786.MCR-13-0239. [DOI] [PubMed] [Google Scholar]
- 29.Connelly L., Barham W., Onishko H.M., Sherrill T., Chodosh L.A., Blackwell T.S., Yull F.E. Inhibition of NF-kappa B activity in mammary epithelium increases tumor latency and decreases tumor burden. Oncogene. 2011;30:1402–1412. doi: 10.1038/onc.2010.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang Y., Li R., Chen X., Zhuo Y., Jin R., Qian X.P., Jiang Y.Q., Zeng Z.H., Zhang Y., Shao Q.X. Doxycycline up-regulates the expression of IL-6 and GM-CSF via MAPK/ERK and NF-κB pathways in mouse thymic epithelial cells. Int. Immunopharmacol. 2011;11:1143–1149. doi: 10.1016/j.intimp.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 31.Hayden M.S., Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 32.Lee J.C., Laydon J.T., McDonnell P.C., Gallagher T.F., Kumar S., Green D. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 33.Murai K., Okayama A., Horinouchi H., Oshikawa T., Tachibana N., Tsubouchi H. Eradication of Rickettsia tsutsugamushi from patients’ blood by chemotherapy, as assessed by the polymerase chain reaction. Am. J. Trop. Med. Hyg. 1995;52:325–327. doi: 10.4269/ajtmh.1995.52.325. [DOI] [PubMed] [Google Scholar]
- 34.Xia J.L., Wang L.Q., Wu L.L., Huang Q.B. Doxycycline Hyclate Protects Lipopolysaccharide-Induced EndothelialBarrier Dysfunction by Inhibiting the Activation of p38 Mitogen-Activated Protein Kinase. Biol. Pharm. Bull. 2014;37:1882–1890. doi: 10.1248/bpb.b14-00298. [DOI] [PubMed] [Google Scholar]
- 35.Sheth K., Friel J., Nolan B., Bankey P. Inhibition of p38 mitogen activated protein kinase increases lipopolysaccharide induced inhibition of apoptosis in neutrophils by activating extracellular signalregulated kinase. Surgery. 2001;130:242–248. doi: 10.1067/msy.2001.115902. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material