Abstract
Objective:
Anti-C1q antibodies (Anti-C1q Ab) are seen in hypocomplementemic urticarial vasculitis syndrome (HUVS), infection-associated vasculitis such as hepatitis C virus-related vasculitis and in autoimmune diseases such as rheumatoid vasculitis, polyarteritis nodosa, giant cell arteritis, vascular Behcet’s disease, and cryoglobulin associated vasculitis. Aim of this study is to evaluate the presence of Anti-C1q Ab in vasculitis and to determine if any difference exists between primary and secondary vasculitis in relation to this antibody.
Patients and Methods:
Consecutive patients with diagnosis of either a primary or secondary vasculitis were recruited. Primary vasculitis were diagnosed by the American College of Rheumatology 1990 criteria. Clinical features and serological markers were noted. Anti-C1q Ab was assayed by commercially available ELISA kit (Demeditec Diagnostics GmbH, Germany).
Results:
Sixty-four patients were recruited for the study comprising of 41 primary vasculitis and 23 secondary vasculitis cases. No difference in Anti-C1q Ab levels between primary and secondary vasculitis was noted. Four patients were positive for Anti-C1q Ab out of the 64 patients. Of the four, one patient was diagnosed as HUVS, 2 patients as systemic lupus erythermatosus with vasculitis (16.7%) and another patient was diagnosed as rheumatoid arthritis with vasculitis (14.28%). Anti-C1q Ab negatively correlated with age and C3, but it correlated positively with erythrocyte sedimentation rate (ESR) in vascultic patients.
Conclusion:
Presence of anti-C1q Ab did not differ between the patients with primary and secondary vasculitis. Anti-C1q Ab titers correlated with younger age, high ESR, and low C3 in patients with vasculitis in our study.
Keywords: Anti-C1q antibody, complements, India, vasculitis
Introduction
C1q is specifically bound to early apoptotic belbs of keratinocytes and vascular endothelial cells, which subsequently leads to activation of complement system, thereby facilitating clearance of apoptotic bodies. Anti-C1q antibody (Anti-C1q Ab) develops in disorders characterized by immune complex mediated injury, more commonly in skin and glomerular microvasculature.1 Anti-C1q Ab are IgG antibodies bound to collagen like C1q. Anti-C1q Ab are seen in hypocomplementemic urticarial vasculitis syndrome (HUVS) (100%), mixed connective tissue disorder (94%), felty’s syndrome (76%), systemic lupus erythermatosus (SLE) (30-60%), and rheumatoid vasculitis (32%).2,3 Other than HUVS, Anti-C1q Ab is also seen in vasculitic disorders such as polyarteritis nodosa (PAN), giant cell arteritis,2 vascular Behcet’s disease4 and cryoglobulin associated vasculitis.
Anti-C1q Ab is also seen in patients with vasculitis of infectious etiology, which includes hepatitis C virus related vasculitis5 and toxocariasis.6 Thus, vasculitis due to autoimmune conditions and infections can be associated with elevated Anti-C1q Ab. However, role of Anti-C1q Ab in pathogenesis of vasculitis is not clear. The aim of the present study is to determine if presence of ant-C1q antibody in primary and secondary vasculitic conditions has clinical implications.
Justification of undertaking the study
Ideal marker of disease activity in vasculitic diseases is in scarcity. As vasculitic diseases can be life-threating, study of utlility of Anti-C1q Ab in these diseases is worthwhile in the light of the biological basis explained above.
Patients and Methods
An observational study was carried out between January 2013 and January 2015 in the Department of Clinical immunology and Rheumatology of Christian Medical College, Vellore, India. This study was approved by the Institutional Review Board. Consecutive patients with diagnosis of either a primary or secondary vasculitis were recruited after getting written consent from the participants. Primary vasculitis were diagnosed by the American College of Rheumatology 1990 criteria. Various clinical parameters, laboratory and serological markers were noted at the time of recruitment. Clinical parameters included duration of disease before presentation, presence of organ system involvement (arthritis, skin symptoms, serositis, and central nervous system symptoms), presence of thromboembolic events and major infections.
Laboratory and serological markers
Laboratory and serologicalmarkers including routine hematology, ESR, complements C3 and C4, urine protein/urine creatinine ratio (UP/UC), presence of autoantibodies (dsDNA antibody, RF, and antiphospholipid antibodies), and histopathology reports were noted as done by some earlier workers.7 When these laboratory results were not available at the precise date of Anti-C1q measurement, values within 15 days of the measurement of Anti-C1q Ab were acceptable.7
Composite indices
Disease activity was assessed at the time of Anti-C1q estimation, by birmingham vasculitis activity score (BVAS).8 Vasculitis damage index (VDI) was also recorded.9
Estimation of Anti-C1q Ab
Blood samples for Anti-C1q Ab were collected during their hospital visits irrespective of disease duration or dose of immunosuppressant. Anti-C1q IgG antibody was assayed by commercially available ELISA kit (Demeditec Diagnostics GmbH, Germany). Results were expressed as unit/ml (U/ml) and serum Anti-C1q Ab level more than or equal to 10 U/ml (cutoff value), as recommended by the manufacturer, was considered to be a positive test value.
Statistical analysis
Sample size was calculated for the study assuming a 25% difference in positivity of Anti-C1q Ab between primary and secondary vasculitis. We, therefore needed a sample size of 40 primary and 20 secondary vasculitis participants with 80% power, 5% error, and an allocation ratio of 2 primary: 1 secondary vasculitis case.
The statistical analysis of data was done using STATA 13.1 I/C (StataCorp LP, Texas, USA, Version 13) statistical package. To test the normality of data distribution, K-S (Kolmogorov–Smirnov) test was done. Mann–Whitney t-test was used to compare Anti-C1q Ab between the groups. Correlation between Anti-C1q Ab and clinical features as well as laboratory markers were done by spearman correlation test. A value of P< 0.05 was considered as significance.
Results
Sixty-four patients were recruited for the study. Baseline characteristics of patients were shown in Table 1. Anti-C1q Ab did not differ between primary and secondary vasculitis. Anti-C1q Ab titer (>10 IU/ml) was elevated only in four patients in the total cohort of 64 patients. Three patients were diagnosed as secondary vasculitis and one was diagnosed as primary vasculitis. To be precise there was 1 patient with the diagnosis of HUVS (Primary vasculitis), 2 patients had SLE with vasculitis (16.7%) and the 4th patient with C1q antibody positivity was diagnosed as rheumatoid arthritis (RA) with vasculitis (14.28%). Serological markers of these four patients were shown in Figure 1.
Table 1.
Baseline characteristics of primary and secondary vasculitis patients
Figure 1.
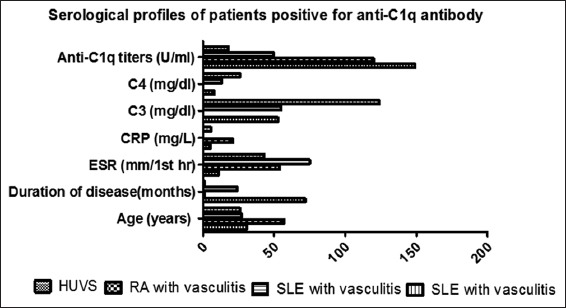
Serological profiles of patients positive for Anti-C1q antibody
Looking at the whole cohort, one patient out of 41 (2.4%) patients with primary vasculitis and 3 patients out of 23 (13%) patients with secondary vasculitis were found to be positive for the Anti-C1q Ab. However, this difference did not reach statistical significance (P = 0.09). Anti-C1q Ab, however, correlated with age (r = −0.3952; P = 0.0013), ESR (r = 0.4628; P = 0.0001), and C3 (r = −0.360; P = 0.019), which was shown in Figure 2. No other significant correlation was observed between the other parameters and Anti-C1q Ab.
Figure 2.
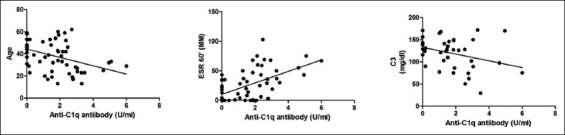
Correlation of Anti-C1q antibody and clinical as well as serological profiles of 63 patients. Anti-C1q antibodies correlated with age (r = −0.3952; P = 0.0013), erythrocyte sedimentation rate (r = 0.4628; P = 0.0001), and C3 (r = −0.360; P = 0.019)
Discussion
The present study found only four patients positive for Anti-C1q Ab out of 64 patients with vasculitis. The positivity of Anti-C1q Ab was higher in secondary vasculitis (13%) as compared to primary vasculitis (2.4%); however, this difference did not reach statistical significance. No definitive conclusion can be drawn due to small sample size.
One out of 4 patients with HUVS had high titer Anti-C1q Ab in our study. The presence of high titer of Anti-C1q Ab in patients with HUVS is consistent with earlier findings reported in the literature.2 Studies also documented positivity of Anti-C1q Ab in 21% of patients with antineutrophil cytoplasmic antibody (ANCA) associated vasculitis and 27% of patients with classic PAN.10,11 None of our ANCA-associated vasculitis or classic PAN patients had this antibody. This may be due to our small sample size.
Anti-C1q Ab was ealier reported in 5% and 16% of RA and rheumatoid vasculitis patients, respectively.12 In our study, 14% of patients with rheumatoid vasculitis had this antibody, thus matching with literature. The prevalence of Anti-C1q Ab levels in SLE varies between 30% and 70%. Anti-C1q Ab, however, is most commonly used in clinical practice to predict nephritis flare in SLE patients. In addition, raised Anti-C1q Ab is reported in lupus pneumonitis and nervous system involvement in patients with SLE.13
Our cohort also had one SLE patient presenting with vasculitis, peripheral nervous system involvement, and gangrene who was positive for anti C1q antibody. Yet another patient with lupus having urticarial vasculitis and renal involvement also tested positive for Anti-C1q Ab. Both lupus patients had low titers of anti-dsDNA antibody at time of Anti-C1q Ab measurement. Reason for elevated anti-C1 Ab in these patients cannot be explained by vasculitis per se; is it likely that this antibody may be a marker of current or future development of lupus nephritis?
While studying our primary objectives, we have noted a few clinical correlations of Anti-C1q Ab in vasculitis, which are discussed below. Siegert et al. reported Anti-C1q IgG Ab to be more prevalent in younger patients with SLE; on the other hand, Anti-C1q IgG Ab titer increases with age and more frequent among healthy individuals in older age.14 Orbai et al. also demonstrated Anti-C1q Ab more commonly in younger individuals with lupus as compared to older patients using a cutoff age of 30 years.15 Thus, most of the studies mentioned above including our study demonstrated higher prevalence of Anti-C1q IgG Ab in younger patients with rheumatic diseases.
Anti-C1q Ab also positively correlated with ESR in our study, similar to observations in published reports involving patients with Behcet’s disease with vascular involvement. This correlation may implicate increased disease activity in vasculitic patients similar to lupus nephritis. Positive correlation of low C3 with Anti-C1q antibody reflects activation of complements in vasculitic patients in our study. Complement system is continuously activated by different autoantibodies in SLE patients which causes depletion of C3 and C4 in circulation.16 Anti-C1q Ab, therefore, negatively correlate with C3 and C4 in SLE patients17-19 and HUVS as noted in the present study.
Conclusion
This study found no difference in Anti-C1q Ab levels between our patients with primary and secondary vasculitis. Anti-C1q Ab titers correlated with younger age, high ESR, and low C3 in patients with vasculitis in our study. Therefore, Anti-C1q Ab may act as a potential marker of disease activity in a select subset of younger patients with vasculitis disorders. Therefore, Anti-C1q Ab is likely to have meaningful clinical implications.
Acknowledgments
We thank Ms. Hindumathi for technical assistance for measuring Anti-C1q antibody. This work was supported by funding from Christian Medical College (CMC) fluid research grant.
References
- 1.Wisnieski JJ, Jones SM. IgG autoantibody to the collagen-like region of Clq in hypocomplementemic urticarial vasculitis syndrome, systemic lupus erythematosus, and 6 other musculoskeletal or rheumatic diseases. J Rheumatol. 1992;19:884–8. [PubMed] [Google Scholar]
- 2.Potlukova E, Kralikova P. Complement component c1q and anti-c1q antibodies in theory and in clinical practice. Scand J Immunol. 2008;67:423–30. doi: 10.1111/j.1365-3083.2008.02089.x. [DOI] [PubMed] [Google Scholar]
- 3.Stojan G, Petri M. Anti-C1q in systemic lupus erythematosus. Lupus. 2016;25:873–7. doi: 10.1177/0961203316645205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bassyouni IH, Gamal S, Talaat RM, Siam I. Autoantibodies against complement C1q in patients with Behcet’s disease: Association with vascular involvement. Mod Rheumatol. 2014;24:316–20. doi: 10.3109/14397595.2013.854071. [DOI] [PubMed] [Google Scholar]
- 5.Saadoun D, Sadallah S, Trendelenburg M, Limal N, Sene D, Piette JC, et al. Anti-C1q antibodies in hepatitis C virus infection. Clin Exp Immunol. 2006;145:308–12. doi: 10.1111/j.1365-2249.2006.03153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Boysson H, Martin Silva N, Comoz F, Boutemy J, Bienvenu B. Vasculitis secondary to anti-C1q antibodies induced by Toxocariasis. Infection. 2015;43:755–8. doi: 10.1007/s15010-015-0766-x. [DOI] [PubMed] [Google Scholar]
- 7.Bock M, Heijnen I, Trendelenburg M. Anti-C1q antibodies as a follow-up marker in SLE patients. PLoS One. 2015;10:e0123572. doi: 10.1371/journal.pone.0123572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luqmani RA, Bacon PA, Moots RJ, Janssen BA, Pall A, Emery P, et al. Birmingham vasculitis activity score (BVAS) in systemic necrotizing vasculitis. QJM. 1994;87:671–8. [PubMed] [Google Scholar]
- 9.Exley AR, Bacon PA, Luqmani RA, Kitas GD, Gordon C, Savage CO, et al. Development and initial validation of the vasculitis damage index for the standardized clinical assessment of damage in the systemic vasculitides. Arthritis Rheum. 1997;40:371–80. doi: 10.1002/art.1780400222. [DOI] [PubMed] [Google Scholar]
- 10.Braun A, Sis J, Max R, Mueller K, Fiehn C, Zeier M, et al. Anti-chromatin and anti-C1q antibodies in systemic lupus erythematosus compared to other systemic autoimmune diseases. Scand J Rheumatol. 2007;36:291–8. doi: 10.1080/03009740701218717. [DOI] [PubMed] [Google Scholar]
- 11.Siegert CE, Kazatchkine MD, Sjöholm A, Würzner R, Loos M, Daha MR. Autoantibodies against C1q: View on clinical relevance and pathogenic role. Clin Exp Immunol. 1999;116:4–8. doi: 10.1046/j.1365-2249.1999.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coremans IE, Daha MR, Van Der Voort E, Siegert CE, Breedveld FC. Subclass distribution of IgA and IgG antibodies against Clq in patients with rheumatic diseases. Scand J Immunol. 1995;41:391–7. doi: 10.1111/j.1365-3083.1995.tb03583.x. [DOI] [PubMed] [Google Scholar]
- 13.Monova D, Monov S, Rosenova K, Argirova T. Autoantibodies against C1q: View on association between systemic lupus erythematosus disease manifestation and C1q autoantibodies. Ann Rheum Dis. 2002;61:563–4. doi: 10.1136/ard.61.6.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siegert CE, Daha MR, Swaak AJ, van der Voort EA, Breedveld FC. The relationship between serum titers of autoantibodies to C1q and age in the general population and in patients with systemic lupus erythematosus. Clin Immunol Immunopathol. 1993;67:204–9. doi: 10.1006/clin.1993.1066. [DOI] [PubMed] [Google Scholar]
- 15.Orbai AM, Truedsson L, Sturfelt G, Nived O, Fang H, Alarcón GS, et al. Anti-C1q antibodies in systemic lupus erythematosus. Lupus. 2015;24:42–9. doi: 10.1177/0961203314547791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holers VM. Anti-C1q autoantibodies amplify pathogenic complement activation in systemic lupus erythematosus. J Clin Invest. 2004;114:616–9. doi: 10.1172/JCI22820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siegert C, Daha M, Westedt ML, van der Voort E, Breedveld F. IgG autoantibodies against C1q are correlated with nephritis, hypocomplementemia, and dsDNA antibodies in systemic lupus erythematosus. J Rheumatol. 1991;18:230–4. [PubMed] [Google Scholar]
- 18.Marto N, Bertolaccini ML, Calabuig E, Hughes GR, Khamashta MA. Anti-C1q antibodies in nephritis: Correlation between titres and renal disease activity and positive predictive value in systemic lupus erythematosus. Ann Rheum Dis. 2005;64:444–8. doi: 10.1136/ard.2004.024943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Hewala A, Nageeb GS, El-shahawy EE, Sharaf DM, Omran AA, El-Messallamy FA, et al. Anti-C1q and anti-dsDNA antibodies in systemic lupus erythematosus: Relationship with disease activity and renal involvement in Sharkia governorate, Egypt. Egypt Rheumatol. 2011;33:203–8. [Google Scholar]


