Abstract
In the medical era of early detection of diseases and tailored therapies, an accurate characterization and staging of the disease is pivotal for treatment planning. The widespread use of computed tomography (CT)—often with the use of contrast material (CM)—probably represents the most important advance in diagnostic radiology. The result is a marked increase in radiation exposure of the population for medical purposes, with its intrinsic carcinogenic potential, and CM affecting kidney function. The radiologists should aim to minimize patient’s risk by reducing radiation exposure and CM amount, while maintaining the highest image quality. To achieve this goal, it is necessary to perform “patient-centric imaging”. The purpose of this review is to provide radiologists with “tips and tricks” to control radiation dose at CT, summarizing technical artifices in order to reduce image noise and increase image contrast. Also chest CT tailored protocols are supplied, with particular attention to three most common thoracic CT protocols: aortic/cardiac CT angiography (CTA), pulmonary CTA, and routine chest CT.
In recent years, medicine made a tremendous progress mostly based on the early detection of diseases and tailored operative and nonoperative treatments, providing a more favorable clinical outcome for patients.
A case in point is the presymptomatic detection of unsuspected lung cancer in the era of screening programs resulting in high rates of identification for localized or early-stage tumors and reducing the rate of explorative surgical procedures and palliative surgery/treatments.
On the other hand, a targeted treatment can be achieved only through accurate characterization and staging of the disease. In this context, adequate anatomical vascular imaging-based planning is required to guide surgical and interventional procedures.
The recent introduction of mini-invasive surgery and new percutaneous or endovascular procedures provided several advantages over open surgery, including lower intra- or perioperative complications, faster patient recovery, shorter hospital stay, and lower overall costs.
These new techniques require an even superior treatment planning to that obtained for conventional surgery, and diagnostic imaging has the additional purposes of alerting operators to possible difficulties, reducing conversion rate to open surgery and avoiding intraprocedural complications.
The decisive breakthrough in imaging was achieved with the technological development of cross-sectional imaging modalities such as computed tomography (CT). Specifically, multidetector CT (MDCT) has permitted an even quicker and better evaluation of patients for a broad spectrum of medical indications, becoming an essential tool for decision-making and treatment planning. Consequently, increasing growth rate of CT exams performed annually has been registered (1).
The single most significant progress in diagnostic radiology is probably represented by the wide utilization of CT. Nevertheless, CT uses much more elevated doses of ionizing radiation compared with plain-film radiography, causing a considerable rise in radiation exposure of patients for medical purposes, with an intrinsic carcinogenic potential that we cannot ignore (2). CT examinations contribute approximately 49% of the medical radiation dose to the population as a whole (3).
Besides that, use of contrast medium (CM), especially but not only for vascular studies, can impact kidney function, in particular in patients with renal insufficiency (4). Most important risk factors for contrast-induced acute kidney injury are pre-existing chronic kidney diseases (glomerular filtration rate <60 mL/min per 1.73 m2) and diabetes mellitus which may have additive effects on each other (4). A minimum volume of CM should be employed, because increasing CM dose is associated with higher occurrence of nephropathy (5).
CT technique ensures huge advantages in the diagnostic field; however, we need to change our daily practice to achieve the highest cost-benefit ratio in using this modality, in terms of radiation dose and CM amount delivered to the patients.
The aim of this review is to supply radiologists with “tips and tricks” to make diagnostic exams ever more safe. To achieve this goal it is necessary to perform a “patient-centric imaging”, in order to minimize patient’s risk by reducing radiation exposure and CM amount, while maintaining the highest image quality.
Techniques for controlling radiation dose at CT
The expansion of automatic techniques represents a valid approach to reduce exposure to radiation. Automatic exposure control (AEC), available from most important scanner producers, have the major goal of adjusting radiation tube current for different levels of X-ray beam attenuation by body tissues.
Although most multidetector row CT scanners now use AEC techniques to modulate tube current as appropriate for patient attenuation, older scanners and certain low-dose scanning protocols still operate with fixed tube current or tube current-time product. Thus, the radiologists should know how to manually modify scanning parameters in order to obtain radiation dose reduction, considering variations in patient size, region scanned, or clinical indications.
Dose decreases linearly with decreasing tube current-time product (mAs) (6). Moreover, tube current (mA), exposure time (i.e., rotation time), or both could alter the tube current-time product (7). On the other hand, the decrease of tube voltage (kV) is associated with an exponential reduction of radiation exposure (6). Furthermore, dose is also inversely proportional to the pitch. With other scanning factors held constant, higher pitch results in faster scanning, but most important in this context, radiation dose decreases as pitch is increased and vice versa (6).
In summary, to obtain a reduction of radiation dose on CT, it is possible to lower mAs and kV values and increase pitch.
However, radiologists should keep image quality high enough to allow diagnosis and eventual therapeutic planning. Image quality depends on several factors and primarily on contrast-to-noise ratio (8).
Noise reduction
It is generally accepted that low-dose CT scans increase the image noise with a potential reduction of image quality. Scanner manufacturers are elaborating new hardware and software solutions in order to raise our capabilities for dose reduction in CT. While the industry elaborates these new protocols with lower doses, it has become evident that images reconstructed with a filtered back projection (FBP) technique are frequently inadequate. In spite of the fact that FBP images are rapid and robust, they are prone to high noise, streak artifacts, and poor low contrast detectability when using low-dose CT techniques. CT equipment manufacturers have countered this limitation by creating alternative image reconstruction modalities that decrease image noise and try to obtain more information from the data set. These reconstruction modalities use maximum likelihood algorithms and are referred to as iterative reconstructions (IRs). IR algorithms have been recently introduced in clinical practice because they improve image quality and dose reduction (9).
The IRs are based on a statistical computational method, which evaluates the noise amount in the raw data caused by fluctuations in neighboring voxels and subtracts the noise stepwise in several validation loops. The result of the first correction loop is compared with the “master data,” and an updated image is created for the next iteration, determining a noise reduction. Three cycles of iterative process yield an image quality similar to that of FBP, saving 35% to more than 60% of the standard dose (10–12). Several IR techniques are currently available, and they can be categorized as: IR performed from the image space data, IR performed directly from the raw data, and IR performed from both image space and raw data. Compared with FBP images, images reconstructed with IR have a different look and feel. The appearance has been described waxy, plastic, or impressionistic, but the information is essentially the same (Figs. 1, 2). On-going development of IR techniques promises to reach even lower amount of noise, allowing further decrease in X-ray dose.
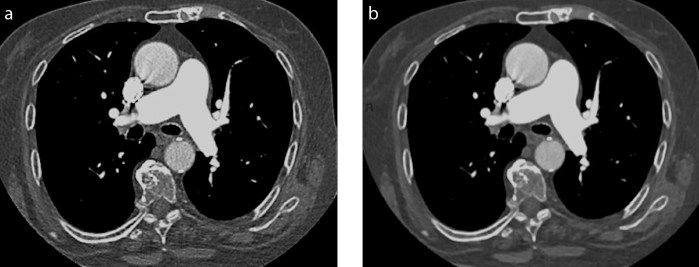
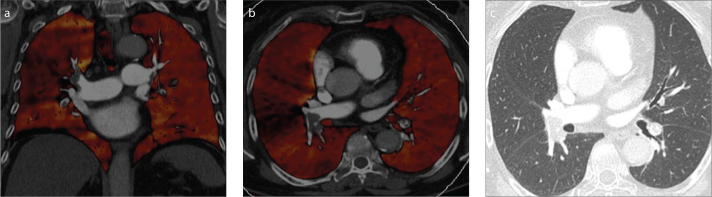
Figure 1. a, b.
Axial computed tomography (CT) images reconstructed with filtered back projection (FBP) (a) or iterative reconstruction (IR) (b) in an oncologic patient with mild dyspnea who underwent CT pulmonary angiography to exclude pulmonary embolism. Axial image reconstructed with IR is waxier; however, the information is essentially the same, excluding pulmonary embolism in the main pulmonary arteries.
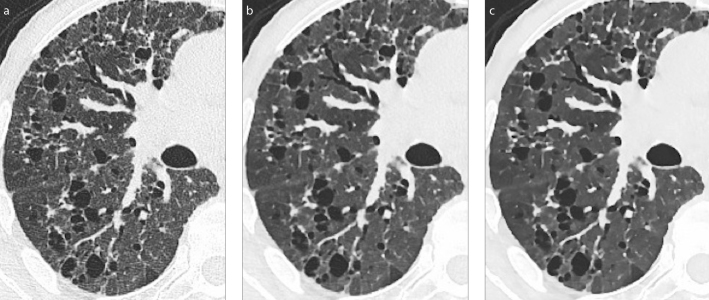
Figure 2. a–c.
Axial CT images in parenchymal window obtained with a Siemens Somatom Definition Flash 128-row, reconstructed with FBP (a), IR with strength 3 (b), or IR with strength 5 (c). The image looks more plastic with increasing number of iterations.
Increasing contrast
If image quality depends on contrast-to-noise ratio and reducing dose results in increased noise, it is logical that to maintain quality while lowering dose, contrast should be raised. The very easy and basic relation expressed as “the higher the iodine application per unit of time, the higher the enhancement” is an accepted simple rule. To achieve this objective we have two possibilities: increasing the injection speed (owing to the issues related to intravenous access this possibility is limited) or using high concentration CM (HCCM).
Adequate arterial enhancement is mandatory to obtain high quality CT studies, particularly in the angiographic ones.
In CT scan, the principal elements affecting contrast enhancement have been grouped into three sections: patient, CM, and CT scan (13). Contrast pharmacokinetics and enhancement are based on the patient and CM characteristics and not on the scan technique. However, scanning elements play a major role by enabling image acquisition at a predetermined time point of contrast enhancement.
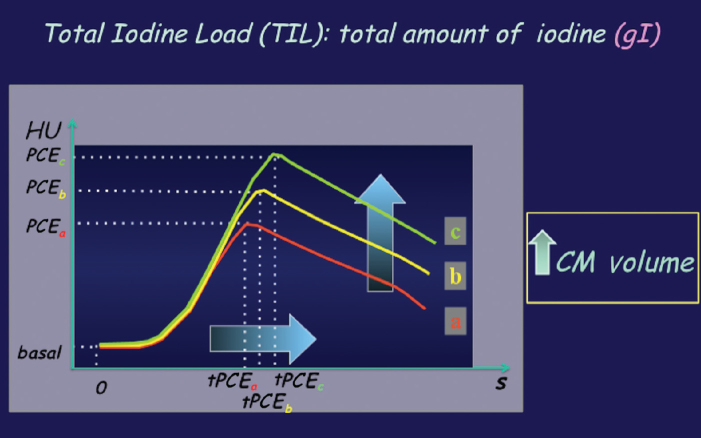
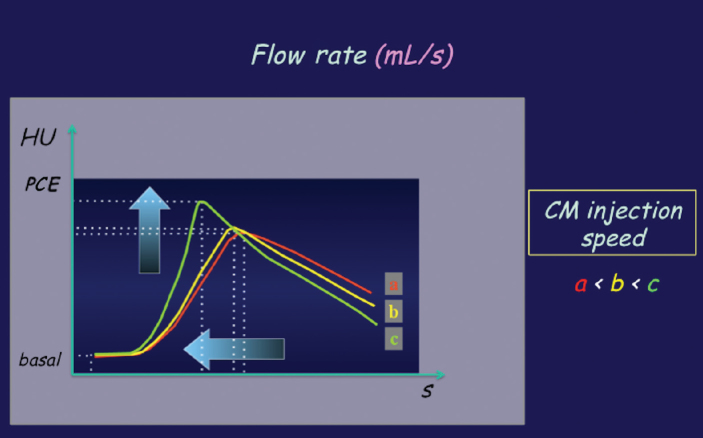
Moreover, vascular enhancement is a function of total iodine load (TIL), which is represented by CM volume (Fig. 3), and of iodine delivery rate (IDR), which is the amount of iodine injected per second (IDR [gI/s] = flow rate [mL/s] × iodine concentration [gI/mL]). Thus IDR can be manipulated by adjusting either flow rate or iodine concentration of CM. When the flow rate is increased, the magnitude of peak enhancement increases but the duration of peak enhancement decreases, as well as the time to achieve the peak enhancement and the injection duration (Fig. 4). Therefore, the temporal window for CT scanning is reduced and a more precise scan timing is needed.
Figure 3.
Contrast material (CM) volume. CM volume increase results in increase of injection duration. The peak enhancement will be delayed (it should be considered in the CT scan timing planning) and its magnitude increased (higher enhancement). HU, Hounsfield units; PCE, peak contrast enhancement; tPCE, time of peak contrast enhancement.
Figure 4.
CM flow rate. When increasing the flow rate, the magnitude of peak enhancement will be increased but the duration of peak enhancement will be reduced, as well as the time to reach the peak enhancement and the duration of injection. HU, Hounsfield units; PCE, peak contrast enhancement.
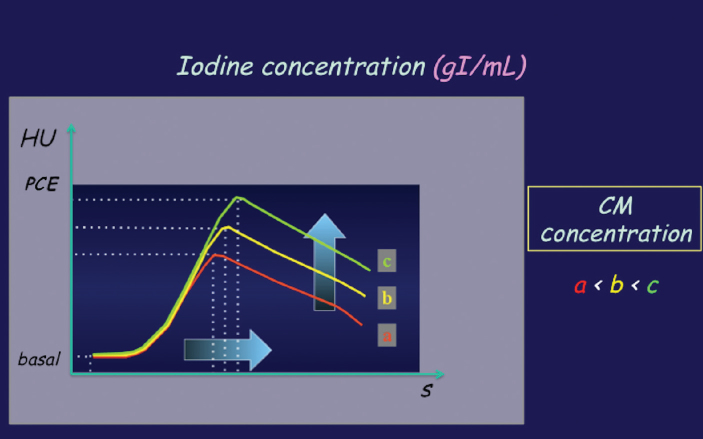
On the other hand, by increasing CM concentration (maintaining the other parameters constant) we administer a larger dose of iodine, resulting in a higher degree of peak enhancement and a wider temporal window for CT scanning (Fig. 5). The main advantage of using HCCM is reducing volume of CM, obtaining an earlier and higher peak enhancement, which is similar in effect to increasing the flow-rate (both lead to a quick iodine delivery). This is helpful when using more rapid MDCT scanners because they need to maximize the arterial enhancement for CTA.
Figure 5.
CM concentration. By increasing CM concentration while maintaining the other parameters constant, a larger dose of iodine is administered. This results in a larger magnitude of peak contrast enhancement and a wider temporal window for CT imaging at a given level of enhancement. HU, Hounsfield units; PCE, peak contrast enhancement.
It is mandatory to perform dedicated acquisition protocols, acquiring images at precise time points of enhancement. The best example is represented by cardiac CT, which nowadays can obtain images of the heart and its vessels within a single heart beat. Spatial and temporal resolutions play a pivotal role in this context.
In a review by Bae et al. (13) it is clearly explained that high-concentration CM enables faster delivery of a larger amount of iodine, resulting in a higher degree of peak contrast enhancement and a wider temporal window for CT imaging. The advantage of using a high-iodine-concentration CM is also scientifically evidenced in CT angiographic applications, such as the study of patients with pulmonary embolism. Langenberger et al. (14) demonstrated that a high-iodine concentration CM allows significantly higher CT attenuation of the pulmonary arterial vasculature, in the main pulmonary arteries and also at segmental and sub-segmental branches, and indicated that the greatest benefit was observed in improving the depiction of emboli in small-caliber vessels.
A study by Rist et al. (15) demonstrated that when a HCCM (400 mgI/mL) is used, it is possible to lower CM volumes and reduce injection flow rates, and still obtain a homogeneous enhancement of the ventricular cavities and coronary arteries equivalent to that achieved using a CM with standard concentration (300 mgI/mL).
In the last years, the use of more highly concentrated CM offered a number of other advantages over a CM with standard iodine concentration. In detail, IDR can be increased without having to increase the CM injection rate. This may be of particular value in elderly patients with poor venous access, in oncologic patients undergoing chemotherapy, or in patients in whom an increased injection rate might be problematic for other reasons, such as increased risk of CM extravasation. Moreover, the overall volume of CM can be reduced. For example, an increase of the CM iodine concentration by 25%, from 300 mgI/mL to 400 mgI/mL, permits an equivalent 25% reduction in the total CM volume administered, from 120 mL to 90 mL. A reduced volume of CM is going to have less impact on kidney function, potentially decreasing the chance of CM-related nephropathy in patients with renal insufficiency (16).
Furthermore, low kV settings, which exponentially reduce radiation exposure as said above, allow an intensification of vessel density due to the increase of photoelectric effect of X-ray attenuation at lower tube voltages for structures of high atomic number, such as iodine (7).
Thus, at a practical level, augmented noise at low radiation doses can be circumvented by raising intravascular contrast enhancement by means of a low kV setting and high-iodine concentration low-volume CM injection (17–19).
Chest CT tailored protocols
Patient-centric imaging is based on age, gender, height, weight, cardiac output, and underlying disease. All these factors should be taken into account in daily practice, in an effort to reduce the patient dose.
Briefly, carcinogenic risk decreases with increasing age (20). Regarding gender, the degree and timing of contrast enhancement are marginally different between men and women, principally due to differences in blood volume. Considering average-sized adults, female patients have 5%–10% less blood volume than males. This can justify the higher contrast enhancement obtained in female patients with the injection of a fixed iodine amount per body weight (13). Moreover, up to the age of 60 years, women are more sensitive to radiation than men of the same age.
The body weight represents the main patient-related element affecting the degree of vascular and parenchymal contrast enhancement, because of the logical association between the body weight and the blood volume. Heavier patients have larger blood volumes, leading to a more dilute CM and reduced iodine concentration in the blood, and consequently lower contrast enhancement.
A work by Bae et al. (21) demonstrated an inverse correlation between aortic attenuation and height. In particular, the authors described a lower aortic attenuation in taller patients when all other variables remain fixed.
In terms of blood flow dynamics, circulation slows when cardiac output decreases. In these cases CM bolus arrives and clears slowly. This results in a retarded CM bolus arrival as well as delayed arterial peak and parenchymal enhancement (13).
A very important factor in which radiologists can significantly act is the choice of protocol according to the underlying disease or, at least, the suspected disease to explore. For example, by reducing the number of CT phases from four (pre-contrast, post-contrast arterial phase, post-contrast portal venous phase, and post-contrast delayed phase) to two, the dose is reduced by 50% (22). Of course, this should be decided on the basis of radiologic/clinical needs.
Putting all these observations together, and considering the key anatomic structures of the chest we will consider three common thoracic CT protocols: aortic/cardiac CT angiography (CTA), pulmonary CTA, and routine chest CT (Table).
Table.
Recommended protocols for aortic/cardiac CTA, pulmonary CTA, and routine chest CT, in terms of technical and contrast medium parameters
| Aortic/cardiac CTA | Pulmonary CTA | Routine chest CT | |
|---|---|---|---|
| Scanning technique | Dual-source CT | Dual-source CT | Dual-source CT |
| Tube current/tube voltage | Automatic techniques (low kV) | Automatic techniques (low kV) | Automatic techniques (low kV) |
| Imaging reconstructions | Iterative reconstructions | Iterative reconstructions | Iterative reconstructions |
| CM injection duration | 5/10/15/20 s: 15 s | 15 s + ½ acquisition time | Arterial phase: 35–40 s Delayed phase: 55–70 s |
| Iodine delivery rate | 1.6–2 gI/s | 1.4–1.6 gI/s | - |
| Iodine load | 20–25 gI | 20–25 gI | Total iodine load: 0.3–0.4 gI/kg |
CTA, computed tomography angiography; CT, computed tomography; CM, contrast material.
Aortic/cardiac CTA
Scanning technique: Prefer dual-source CT vs. single. Temporal resolution is constantly improved thanks to the appearance and development of dual-source CT systems and IRs, leading to a notable decrease in the ionizing radiation dose (23).
Imaging mode: Prefer prospective electrocardiography (ECG) triggering for cardiac CT. To employ this technique, a low and stable heart rate (<60 beats/min) is required (24).
Tube current/tube voltage: To decrease radiation exposure in coronary CTA it is possible to use automated attenuation-based selection of individualized tube parameters, which are superior to BMI-based selections with expert oversight. It has been demonstrated that image quality is maintained, with an overall dose reduction of 1.4 mSv vs. 2.3 mSv (25). However, BMI remains an important factor. Feuchtner et al. (26) asserted that for patients with a BMI <25 kg/m2 and a low calcium load, the use of 100 kV protocol decreases the dose in cardiac CTA while maintaining high image quality. Moreover Cao et al. (27) showed that in patients with a BMI <23 kg/m2 and a low calcium load, the 80 kV protocol significantly reduces radiation dose and CM amount in cardiac CTA with the image quality maintained.
Prefer iterative reconstructions, if possible. Model-based type of IR technique can assure better image quality in prospectively gated coronary CTA using a low-tube-voltage (28). The introduction of dual-source CT systems provided an entirely novel imaging technique, called prospectively ECG triggered high-pitch spiral mode, that is in between spiral and sequential, delivering an effective dose <0.1 mSv (29). The estimated low-dose coronary CTA can supply sufficient image quality in selected patients by combining high-pitch spiral acquisition and raw data based IRs.
CM injection protocol: The calculation of optimal injection duration for arterial or CTA enhancement is more complicated than that for visceral parenchymal enhancement and is mainly related to the type of CT scanner available. Based on a review of published studies on cardiac and coronary CTA enhancement, it is possible to evaluate the volume of CM and injection rate to obtain a diagnostically adequate aortic (250–300 HU attenuation) and coronary artery enhancement (300–350 HU attenuation) for a 70 kg patient: (1) 45 gI injected at 1.2 gI/s (i.e., 130 mL of 350 mgI/mL concentration at 3.5 mL/s) over 40 s for 4-row MDCT, (2) 35 gI injected at 1.4 gI/s (i.e., 100 mL of 350 mgI/mL at 4 mL/s) over 25 s for 16-row MDCT, and (3) 25 gI injected at 1.6 gI/s (i.e., 60–75 mL of ≥350 mgI/mL at 4–5 mL/s) over 15 s for 64-row MDCT, followed by a saline flush (30).
The high-iodine concentration CM of 400 mgI/mL obtained significant advantages in terms of coronary arterial enhancement compared with the iso-osmolar CM of 320 mgI/mL, when administered at identical flow rates and volumes for coronary dual-source CTA (31). Higher contrast enhancement reduced the number of inadequately visualized segments. The mean heart rate changes observed after intravenous CM injection were generally low without significant differences between the two contrast agents (31). Moreover, differently iodinated CMs demonstrated analogous effect on the attenuation profiles of a plaque, thus different CMs could be similarly used for the delineation of coronary atherosclerotic plaques (32).
In summary, based on the literature review, using a last generation MDCT scanner, an optimal CM injection would be: injection duration, 15 s; IDR, 1.6–2 gI/s; iodine load, 20–25 gI, meaning 60–70 mL HCCM (>350 mgI) at 4–5 mL/s (+ bolus chaser) (Fig. 6).
Figure 6.
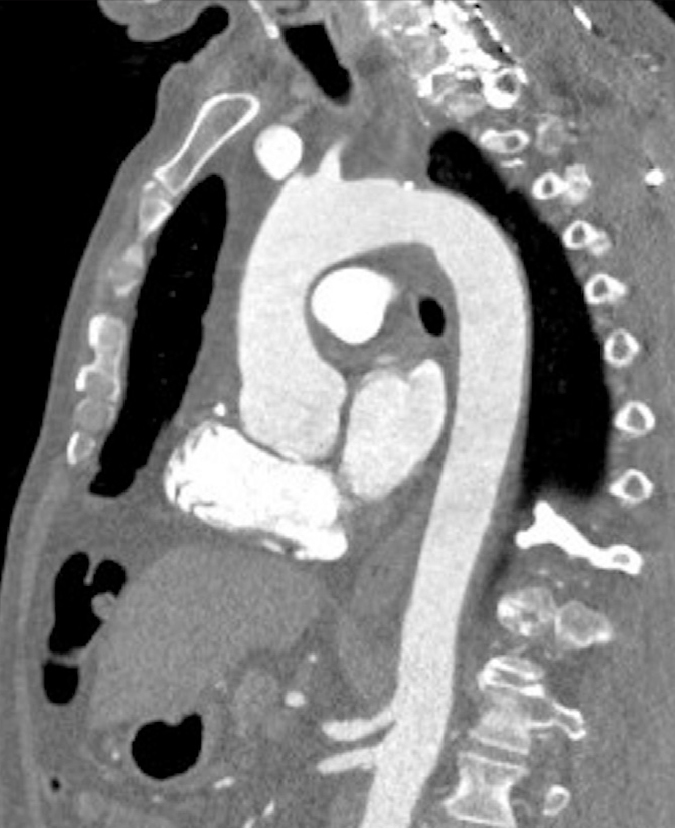
Sagittal contrast-enhanced iterative reconstructed CT image of the aorta in a 67-year-old man with BMI <23 kg/m2, using Siemens Somatom Definition Flash 128-row, pitch of 3.2 and activation of both radiation tubes of the machine. CM used: 60 mL HCCM (400 mgI) at 4 mL/s. CT protocol: automatic exposure control with 80 kV was used in this patient. Dose length product for the study of the aorta was 67 mGy.cm. The effective dose administered for the study of the aorta was 0.9 mSv.
Pulmonary CTA
1) Scanning technique: Prefer dual-source CT vs. single (33). Nowadays, in the context of suspected pulmonary embolism (PE), dual-source CT can provide both anatomical and functional information of the whole lung, by matching CT pulmonary angiography (CTPA) and the evaluation of pulmonary iodine map in a unique volumetric contrast-enhanced CT acquisition.
Dual-source CT employs X-ray tubes and detectors mounted perpendicularly at each other. As each tube can be set at a different tube potential (80 kVp/140 kVp), dual-source CT permits concurrent dual-energy CT acquisition. Iodine attenuates X-ray spectra differently at the different kVp settings. In order to define the quantity of iodinated CM in any voxel, the dual-energy CT data is post-processed and a mathematic algorithm is used to obtain an iodine map that is based on the known attenuation range values for iodine at 80 kVp and at 140 kVp and to measure its relative contribution to each voxel. In the specific case of pulmonary parenchyma, the calculation of iodine distribution, which depends on the microvascular circulation in the lung, can create color-coded pulmonary blood volume (PBV) maps that can be considered as a surrogate indicator of pulmonary perfusion (34). Furthermore, evaluation of morphologic features and anatomic structures is obtained merging image data from both energy levels to generate a hybrid image corresponding to a standard acquisition at 120 kVp. Dual-energy CT technology has the potential to improve diagnostic accuracy thanks to a complete evaluation of pulmonary perfusion and vascular opacification achieved during a single contrast-enhanced CT acquisition obtained with dual-energy modality. PBV images may be employed to discover perfusion defects due to PE. Normal PBV images show homogeneous symmetric lung iodine distribution. PBV perfusion defects due to PE are mainly peripherally located, wedge-shaped and characterized by segmental or lobar distribution (35) (Fig. 7).
Figure 7. a–c.
A 69-year-old woman with bilateral pulmonary embolism. Fused dual-energy CT angiographic image/iodine perfusion maps (a, b) simultaneously show bilateral arterial filling defects, larger on the right with proximal extension in the lobar arteries and interlobar artery, and concordant wedge-shaped perfusion defects in the right upper lobe. Axial CT image (c) at the same level as (b) does not show any abnormality in the corresponding lung parenchyma.
2) Tube current/tube voltage: Prefer automatic techniques that differ according to the scanner type, and use low kV setting. According to a study by Sodickson and Weiss (36), when compared to 120 kVp, 100 kVp pulmonary CTA reduced both radiation exposure and intravenous contrast amount by 33%, without reducing image quality. In particular, they evaluated image quality and radiation exposure as a function of patient size for pulmonary CTA performed at reduced tube voltage and reduced intravenous CM dose (36).
A work by Viteri-Ramírez et al. (37) compared two groups of patients suspected of having PE, weighting ≤80 kg; patients were studied with two different CTPA protocols (group A: 80 kV/60 mL; group B: 100 kV/80 mL). Image quality parameters and effective radiation dose were evaluated. The study showed that CTPA protocol evaluated in group A yielded an image quality analogous to that of group B while decreasing radiation exposure by 60% and CM volume by 25% (37).
3) CM injection protocol: With MDCT scanners, the acquisition of a CTPA lasts only few seconds, therefore it is pivotal to choose injection strategies in order to obtain the major vessel enhancement during scanning time and to avoid running out of CM at the end of the scan. The rule “injection duration equals scanning duration” cannot be used. It would be useful to arbitrarily use as injection duration of a fixed contrast transit time of 15 s plus half the acquisition time to ensure an optimal arterial enhancement without increasing the total CM volume. Finally, to obtain an adequate vascular enhancement during the fast CT acquisitions, it is needed to increase the iodine delivery rate (38–40).
Based on these assumptions, an optimal CM injection would be: injection duration, 15 s + ½ acquisition time; IDR, 1.4–1.6 gI/s; iodine load, 20–25 gI, meaning 50–60 mL HCCM (>350 mgI) at 4–5 mL/s (+ bolus chaser) (Fig. 8).
Figure 8.
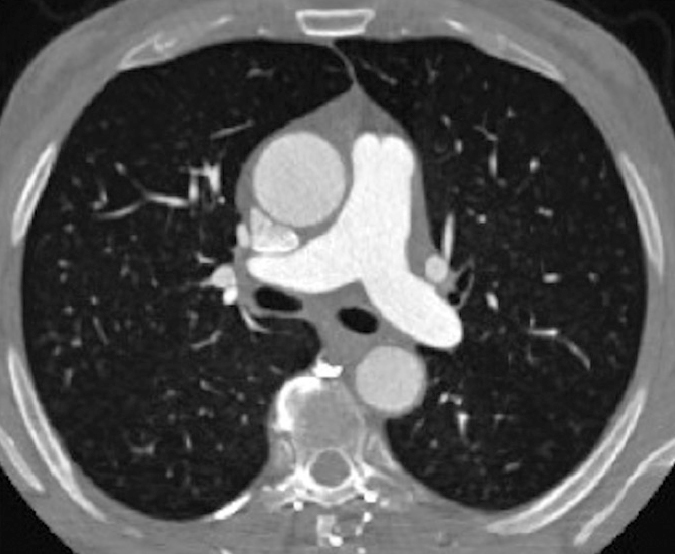
Axial contrast-enhanced CT image (iterative reconstruction with Siemens Somatom Definition Flash 128-row) of the pulmonary artery in a 51-year-old man. CM used: 60 mL HCCM (400 mgI) at 4 mL/s. Technical parameters: dual-source CT; pitch, 3.2; automatic exposure control (80 kV). The effective dose administered was <1 mSv.
However, fixed protocols may result in attenuation values below the diagnostic level for heavier patients whereas for low-weight patients, attenuation may exceed required levels. Recently, Hendriks et al. (41) used protocols adjusted for patient weight and scan duration, employing automated software that calculates CM amount and flow rate and showed a substantial CM volume reduction in low-weight subjects compared with standard “fixed” protocols. Therefore, a solution could possibly be found in individualizing contrast application for each patient. The type of available scanner should also be considered. As a general rule, by increasing CT rows shorter scan duration and less CM volume could be achieved.
Routine chest CT
Scanning technique: Prefer dual-source CT vs. single source. Regarding the standard protocol for routine chest CT, dual-source scanning is preferred to single because the greater temporal resolution and higher pitch reduce the acquisition time to about 1 s for the entire length of the thorax. The chance to employ dual-source CT technique, however, depends on the circumference of the patient’s chest since the field of the second tube is narrower than that of the first tube, covering only 33 cm (42).
Tube current/tube voltage: Prefer automatic techniques (43), and use low kV setting. Kim et al. (44) asserted that the employment of low kV protocols decreases X-ray exposure at chest CT significantly, while maintaining image quality superimposable to that of standard protocols. The authors examined three different groups: A) the standard protocol, 120 kV, 100 mAs; B) 100 kV, 140 mAs; and C) 80 kV, 180 mAs. Compared with the standard protocol, 100 kV protocol obtained similar results in terms of qualitative score analysis whereas only borderline significance (P = 0.054) was reported for 80 kV protocol. When the patients of 80 kV protocol were subcategorized according to BMI, the image quality scores did not significantly differ from those of the standard protocol, for all BMI subgroups. Compared with the standard protocol, the dose length products of the 100 kV and 80 kV protocols were decreased by 15.36% and 43.57%, respectively.
CM injection protocol: Scan delay, early (40 s) or late (55–70 s); iodine load, 0.3–0.4 gI/kg. Use 60–70 mL HCCM (>350 mgI) at 2–3 mL/s (optional bolus chaser).
In the context of routine chest CT, scan delay depends on clinical diagnostic necessities and target thoracic organs to be studied. Commonly, the scan should start early for the study of thoracic vessels, while it should begin later to obtain better enhancement of all-around soft-tissue structures of the chest. Nowadays, the very fast MDCT allows multiple acquisitions at different and precise phases of contrast enhancement with high spatial and temporal resolution. To fully realize the benefits of MDCT, radiologists should be able to modify parameters for CM administration and scan timing based on specific clinical indications as well as characteristics of different scanners available.
Conclusion
Radiologists should adopt strategies to decrease radiation dose, adapt CT protocols according to diagnostic necessities, and minimize adverse effects. A possible system to overcome the augmented noise issue at low doses of radiation could involve increasing intravascular contrast enhancement using a low kV setting with the injection of a high-iodine concentration CM. Computer modeling and intelligent software will play a crucial role in solving imaging issues, facilitating the interaction between the CT scanner and the injector, and integrating the patient’s clinical data with scan and injection parameters.
Main points.
To achieve the highest cost-benefit ratio in using CT, radiologists should aim at reducing radiation exposure and contrast material (CM) amount, while maintaining the highest image quality.
To save radiation exposure on CT it is possible to use “automatic exposure control,” otherwise to lower mAs and kV values and to increase pitch.
Iterative reconstructions allow image noise reduction.
The use of CM with high iodine concentration allows CM volume reduction and increase of iodine delivery rate, without increasing the injection rate.
In the effort to reduce patient dose, factors such as age, gender, height, weight, cardiac output, and underlying disease should be taken into account in the daily practice.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Mayo-Smith WW, Hara AK, Mahesh M, Sahani DV, Pavlicek W. How I do it: managing radiation dose in CT. Radiology. 2014;273:657–672. doi: 10.1148/radiol.14132328. https://doi.org/10.1148/radiol.14132328. [DOI] [PubMed] [Google Scholar]
- 2.Brenner DJ, Hall EJ. Computed tomography — an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. https://doi.org/10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 3.Mettler FA, Jr, Bhargavan M, Faulkner K, et al. Radiologic and nuclear medicine studies in the United States and worldwide: frequency, radiation dose, and comparison with other radiation sources—1950–2007. Radiology. 2009;253:520–531. doi: 10.1148/radiol.2532082010. https://doi.org/10.1148/radiol.2532082010. [DOI] [PubMed] [Google Scholar]
- 4.Ozkok S, Ozkok A. Contrast-induced acute kidney injury: A review of practical points. World J Nephrol. 2017;6:86–99. doi: 10.5527/wjn.v6.i3.86. https://doi.org/10.5527/wjn.v6.i3.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mujicic E, Kevric E, Rasic S, et al. Contrast media injector technology - renal safety during coronarography. Acta Inform Med. 2015;23:273–275. doi: 10.5455/aim.2015.23.273-275. https://doi.org/10.5455/aim.2015.23.273-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalra MK, Sodickson AD, Mayo-Smith WW. CT radiation: key concepts for gentle and wise use. Radiographics. 2015;35:1706–1721. doi: 10.1148/rg.2015150118. https://doi.org/10.1148/rg.2015150118. [DOI] [PubMed] [Google Scholar]
- 7.McNitt-Gray MF. AAPM/RSNA physics tutorial for residents: topics in CT. Radiation dose in CT. Radiographics. 2002;22:1541–1553. doi: 10.1148/rg.226025128. https://doi.org/10.1148/rg.226025128. [DOI] [PubMed] [Google Scholar]
- 8.Oberhofer N, Compagnone G, Moroder E. Use of CNR as a metric to optimisation in digital radiology. In: Dössel O, Schlege WC, editors. World Congress on Medical Physics and Biomedical Engineering 7–12 September 2009. Vol. 25. Munich: Springer; 2009. p. 296. https://doi.org/10.1007/978-3-642-03879-2_84. [Google Scholar]
- 9.Paruccini N, Villa R, Pasquali C, et al. Evaluation of a commercial Model Based Iterative reconstruction algorithm in computed tomography. Phys Med. 2017;41:58–70. doi: 10.1016/j.ejmp.2017.05.066. https://doi.org/10.1016/j.ejmp.2017.05.066. [DOI] [PubMed] [Google Scholar]
- 10.Pontana F, Pagniez J, Flohr T, et al. Chest computed tomography using iterative reconstruction vs filtered back projection (Part 1): Evaluation of image noise reduction in 32 patients. Eur Radiol. 2011;21:627–635. doi: 10.1007/s00330-010-1990-5. https://doi.org/10.1007/s00330-010-1991-4. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Tan B, Zhao B, Liang C, Xu Z. Raw-data-based iterative reconstruction versus filtered back projection: image quality of low-dose chest computed tomography examinations in 87 patients. Clin Imaging. 2013;37:1024–1032. doi: 10.1016/j.clinimag.2013.06.004. https://doi.org/10.1016/j.clinimag.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Yamada Y, Jinzaki M, Hosokawa T, et al. Dose reduction in chest CT: comparison of the adaptive iterative dose reduction 3D, adaptive iterative dose reduction, and filtered back projection reconstruction techniques. Eur J Radiol. 2012;81:4185–4195. doi: 10.1016/j.ejrad.2012.07.013. https://doi.org/10.1016/j.ejrad.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Bae KT. Intravenous contrast medium administration and scan timing at CT: considerations and approaches. Radiology. 2010;256:32–61. doi: 10.1148/radiol.10090908. https://doi.org/10.1148/radiol.10090908. [DOI] [PubMed] [Google Scholar]
- 14.Langenberger H, Friedrich K, Plank C, et al. MDCT angiography for detection of pulmonary emboli: Comparison between equi-iodine doses of iomeprol 400 mgI/mL and iodixanol 320 mgI/mL. Eur J Radiol. 2009;70:579–588. doi: 10.1016/j.ejrad.2008.01.058. https://doi.org/10.1016/j.ejrad.2008.01.058. [DOI] [PubMed] [Google Scholar]
- 15.Rist C, Nikolaou K, Kirchin MA, et al. Contrast bolus optimization for cardiac 16-slice computed tomography: comparison of contrast medium formulations containing 300 and 400 milligrams of iodine per milliliter. Invest Radiol. 2006;41:460–467. doi: 10.1097/01.rli.0000208239.34723.5d. https://doi.org/10.1097/01.rli.0000208239.34723.5d. [DOI] [PubMed] [Google Scholar]
- 16.Thomsen HS, Morcos SK, Erley CM, et al. Investigators in the Abdominal Computed Tomography: IOMERON 400 Versus VISIPAQUE 320 Enhancement (ACTIVE) Study. The ACTIVE Trial: comparison of the effects on renal function of iomeprol-400 and iodixanol-320 in patients with chronic kidney disease undergoing abdominal computed tomography. Invest Radiol. 2008;43:170–178. doi: 10.1097/RLI.0b013e31815f3172. https://doi.org/10.1097/RLI.0b013e31815f3172. [DOI] [PubMed] [Google Scholar]
- 17.Lu GM, Luo S, Meinel FG, et al. High-pitch computed tomography pulmonary angiography with iterative reconstruction at 80 kVp and 20 mL contrast agent volume. Eur Radiol. 2014;24:3260–3268. doi: 10.1007/s00330-014-3365-9. https://doi.org/10.1007/s00330-014-3365-9. [DOI] [PubMed] [Google Scholar]
- 18.McLaughlin PD, Liang T, Homiedan M, et al. High pitch, low voltage dual source CT pulmonary angiography: assessment of image quality and diagnostic acceptability with hybrid iterative reconstruction. Emerg Radiol. 2015;22:117–123. doi: 10.1007/s10140-014-1230-4. https://doi.org/10.1007/s10140-014-1230-4. [DOI] [PubMed] [Google Scholar]
- 19.Mourits MM, Nijhof WH, van Leuken MH, et al. Reducing contrast medium volume and tube voltage in CT angiography of the pulmonary artery. Clin Radiol. 2016;71:615.e7–615.e13. doi: 10.1016/j.crad.2016.03.005. https://doi.org/10.1016/j.crad.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Shuryak I, Hahnfeldt P, Hlatky L, Sachs RK, Brenner DJ. A new view of radiation-induced cancer: integrating short- and long-term processes. Part I: approach. Radiat Environ Biophys. 2009;48:263–274. doi: 10.1007/s00411-009-0230-3. https://doi.org/10.1007/s00411-009-0230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bae KT, Seeck BA, Hildebolt CF, et al. Contrast enhancement in cardiovascular MDCT: effect of body weight, height, body surface area, body mass index, and obesity. AJR Am J Roentgenol. 2008;190:777–784. doi: 10.2214/AJR.07.2765. https://doi.org/10.2214/AJR.07.2765. [DOI] [PubMed] [Google Scholar]
- 22.Tamm EP, Rong XJ, Cody DD, Ernst RD, Fitzgerald NE, Kundra V. Quality initiatives: CT radiation dose reduction: how to implement change without sacrificing diagnostic quality. Radiographics. 2011;31:1823–1832. doi: 10.1148/rg.317115027. https://doi.org/10.1148/rg.317115027. [DOI] [PubMed] [Google Scholar]
- 23.Fink C, Krissak R, Henzler T, et al. Radiation dose at coronary CT angiography: second-generation dual-source CT versus single-source 64-MDCT and first-generation dual-source CT. AJR Am J Roentgenol. 2011;196:W550–557. doi: 10.2214/AJR.10.5153. https://doi.org/10.2214/AJR.10.5153. [DOI] [PubMed] [Google Scholar]
- 24.Chinnaiyan KM, Bilolikar AN, Walsh E, et al. CT dose reduction using prospectively triggered or fast-pitch spiral technique employed in cardiothoracic imaging (the CT dose study) J Cardiovasc Comput Tomogr. 2014;8:205–214. doi: 10.1016/j.jcct.2014.04.001. https://doi.org/10.1016/j.jcct.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Layritz C, Muschiol G, Flohr T, et al. Automated attenuation-based selection of tube voltage and tube current for coronary CT angiography: reduction of radiation exposure versus a BMI-based strategy with an expert investigator. J Cardiovasc Comput Tomogr. 2013;7:303–310. doi: 10.1016/j.jcct.2013.08.010. https://doi.org/10.1016/j.jcct.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 26.Feuchtner GM, Jodocy D, Klauser A, et al. Radiation dose reduction by using 100-kV tube voltage in cardiac 64-slice computed tomography: a comparative study. Eur J Radiol. 2010;75:e51–56. doi: 10.1016/j.ejrad.2009.07.012. https://doi.org/10.1016/j.ejrad.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 27.Cao JX, Wang YM, Lu JG, Zhang Y, Wang P, Yang C. Radiation and contrast agent doses reductions by using 80-kV tube voltage in coronary computed tomographic angiography: a comparative study. Eur J Radiol. 2014;83:309–314. doi: 10.1016/j.ejrad.2013.06.032. https://doi.org/10.1016/j.ejrad.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 28.Oda S, Weissman G, Vembar M, Weigold WG. Iterative model reconstruction: improved image quality of low-tube-voltage prospective ECG-gated coronary CT angiography images at 256-slice CT. Eur J Radiol. 2014;83:1408–1415. doi: 10.1016/j.ejrad.2014.04.027. https://doi.org/10.1016/j.ejrad.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 29.Schuhbaeck A, Achenbach S, Layritz C, et al. Image quality of ultra-low radiation exposure coronary CT angiography with an effective dose <0.1 mSv using high-pitch spiral acquisition and raw data-based iterative reconstruction. Eur Radiol. 2013;23:597–606. doi: 10.1007/s00330-012-2656-2. https://doi.org/10.1007/s00330-012-2656-2. [DOI] [PubMed] [Google Scholar]
- 30.Nakaura T, Awai K, Yauaga Y, et al. Contrast injection protocols for coronary computed tomography angiography using a 64-detector scanner: comparison between patient weight-adjusted- and fixed iodine-dose protocols. Invest Radiol. 2008;43:512–519. doi: 10.1097/RLI.0b013e3181727505. https://doi.org/10.1097/RLI.0b013e3181727505. [DOI] [PubMed] [Google Scholar]
- 31.Becker CR, Vanzulli A, Fink C, et al. Multicenter comparison of high concentration contrast agent iomeprol-400 with iso-osmolar iodixanol-320: contrast enhancement and heart rate variation in coronary dual-source computed tomographic angiography. Invest Radiol. 2011;46:457–464. doi: 10.1097/RLI.0b013e31821c7ff4. https://doi.org/10.1097/RLI.0b013e31821c7ff4. [DOI] [PubMed] [Google Scholar]
- 32.La Grutta L, Galia M, Gentile G, et al. Comparison of iodinated contrast media for the assessment of atherosclerotic plaque attenuation values by CT coronary angiography: observations in an ex vivo model. Br J Radiol. 2013;86:20120238. doi: 10.1259/bjr.20120238. https://doi.org/10.1259/bjr.20120238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pontana F, Moureau D, Schmidt B, et al. CT pulmonary angiogram with 60% dose reduction: Influence of iterative reconstructions on image quality. Diagn Interv Imaging. 2015;996:487–493. doi: 10.1016/j.diii.2014.08.006. https://doi.org/10.1016/j.diii.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Ameli-Renani S, Rahman F, Nair A, et al. Dual-energy CT for imaging of pulmonary hypertension: challenges and opportunities. Radiographics. 2014;34:1769–1790. doi: 10.1148/rg.347130085. https://doi.org/10.1148/rg.347130085. [DOI] [PubMed] [Google Scholar]
- 35.Fink C, Johnson TR, Michaely HJ, et al. Dual-energy CT angiography of the lung in patients with suspected pulmonary embolism: initial results. Rofo. 2008;180:879–883. doi: 10.1055/s-2008-1027724. https://doi.org/10.1055/s-2008-1027724. [DOI] [PubMed] [Google Scholar]
- 36.Sodickson A, Weiss M. Effects of patient size on radiation dose reduction and image quality in low-kVp CT pulmonary angiography performed with reduced IV contrast dose. Emerg Radiol. 2012;19:437–445. doi: 10.1007/s10140-012-1046-z. https://doi.org/10.1055/s-2008-1027724. [DOI] [PubMed] [Google Scholar]
- 37.Viteri-Ramírez G, García-Lallana A, Simón-Yarza I, et al. Low radiation and low-contrast dose pulmonary CT angiography: Comparison of 80 kVp/60 mL and 100 kVp/80 mL protocols. Clin Radiol. 2012;67:833–839. doi: 10.1016/j.crad.2011.11.016. https://doi.org/10.1016/j.crad.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 38.Kerl JM, Lehnert T, Schell B, et al. Intravenous contrast material administration at high-pitch dual-source CT pulmonary angiography: test bolus versus bolus-tracking technique. Eur J Radiol. 2012;81:2887–2891. doi: 10.1016/j.ejrad.2011.09.018. https://doi.org/10.1016/j.ejrad.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 39.Napoli A, Fleischmann D, Chan FP, et al. Computed tomography angiography: state-of-the-art imaging using multidetector-row technology. J Comput Assist Tomogr. 2004;28( Suppl 1):S32–S45. doi: 10.1097/01.rct.0000120859.80935.10. https://doi.org/10.1097/01.rct.0000120859.80935.10. [DOI] [PubMed] [Google Scholar]
- 40.Bae KT, Tao C, Gürel S, et al. Effect of patient weight and scanning duration on contrast enhancement during pulmonary multidetector CT angiography. Radiology. 2007;242:582–589. doi: 10.1148/radiol.2422052132. https://doi.org/10.1148/radiol.2422052132. [DOI] [PubMed] [Google Scholar]
- 41.Hendriks BM, Kok M, Mihl C, Bekkers SC, Wildberger JE, Das M. Individually tailored contrast enhancement in CT pulmonary angiography. Br J Radiol. 2016;89:20150850. doi: 10.1259/bjr.20150850. https://doi.org/10.1259/bjr.20150850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Broucker T, Pontana F, Santangelo T, et al. Single- and dual-source chest CT protocols: Levels of radiation dose in routine clinical practice. Diagn Interv Imaging. 2012;93:852–858. doi: 10.1016/j.diii.2012.07.009. https://doi.org/10.1016/j.diii.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 43.Schimmöller L, Lanzman RS, Dietrich S, et al. Evaluation of automated attenuation-based tube potential selection in combination with organ-specific dose reduction for contrast-enhanced chest CT examinations. Clin Radiol. 2014;69:721–726. doi: 10.1016/j.crad.2014.02.008. https://doi.org/10.1016/j.crad.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 44.Kim MJ, Park CH, Choi SJ, Hwang KH, Kim HS. Multidetector computed tomography chest examinations with low-kilovoltage protocols in adults: effect on image quality and radiation dose. J Comput Assist Tomogr. 2009;33:416–421. doi: 10.1097/RCT.0b013e318181fab5. https://doi.org/10.1097/RCT.0b013e318181fab5. [DOI] [PubMed] [Google Scholar]