Abstract
PURPOSE
We aimed to investigate residual adipose tissue with whole-body magnetic resonance imaging to differentiate between subtypes of lipodystrophy.
METHODS
A total of 32 patients (12 with congenital generalized lipodystrophy [CGL], 1 with acquired generalized lipodystrophy [AGL], 12 with familial partial lipodystrophy [FPLD], and 7 with acquired partial lipodystrophy [APL]) were included.
RESULTS
Despite generalized loss of metabolically active adipose tissue, patients with CGL1 caused by AGPAT2 mutations had a significant amount of residual adipose tissue in the scalp, earlobes, retro-orbital region, and palms and soles. No residual adipose tissue was noted particularly in the head and neck, palms and soles in CGL2 caused by BSCL2 mutations. CGL4 caused by mutations in the PTRF gene was characterized with well-preserved retro-orbital and bone marrow fat in the absence of any visible residual adipose tissue in other areas. No residual adipose tissue was observed in AGL. Despite loss of subcutaneous fat, periarticular adipose tissue was preserved in the lower limbs of patients with FPLD. Retro-orbital adipose tissue was surprisingly preserved in APL, although they lacked head and neck fat.
CONCLUSION
Lipodystrophies are a heterogeneous group of disorders characterized by generalized or partial loss of adipose tissue, which can be congenital or acquired. Our results suggest that residual adipose tissue characteristics can help distinguish different subtypes of lipodystrophy.
Lipodystrophies are a heterogeneous group of disorders characterized by congenital or acquired loss of adipose tissue (1). Congenital generalized lipodystrophy (CGL) is associated with the near-absence of all body adipose tissue at birth or nearly complete loss of adipose tissue during early infancy. CGL patients develop severe insulin-resistance triggering poorly controlled diabetes (2). CGL is an autosomal recessive disorder. Several responsible genes have been identified: 1-acylglycerol-3-phosphate O-acyltransferase 2 (AGPAT2, CGL1) (3), Berardinelli-Seip congenital lipodystrophy 2 (BSCL2, CGL2) (4), caveolin 1 (CAV1, CGL3) (5), and polymerase I and transcript release factor (PTRF, CGL4) (6, 7). Familial partial lipodystrophy (FPLD) is another rare form of lipodystrophy characterized by selective loss of subcutaneous adipose tissue from the arms, legs, chest, and trunk of most patients (8). However, subcutaneous fat may accumulate in other regions of the body such as the face and neck. FPLD is associated with insulin-resistant diabetes, elevated triglyceride levels, and fatty liver. Several responsible genes have been identified, most of which are autosomal-dominant (8–11). Mutation of lamin A/C (LMNA, the Dunnigan variety) is the most common cause of FPLD (8). Mutations in peroxisome proliferator activated receptor gamma (PPARG), which encodes a key transcription factor regulating adipocyte differentiation and insulin sensitivity, are also relatively common in FPLD patients (12).
Patients with acquired lipodystrophies develop progressive adipose tissue loss at some point during life; the conditions are not caused by genetic mutations. Near-absence loss of body fat is observed in those with acquired generalized lipodystrophy (AGL). Acquired partial lipodystrophy (APL) is characterized by the loss of adipose tissue from only certain areas and often commences during childhood (13). APL usually affects the face first and then progresses to the neck, upper extremities, thorax, and abdomen, sparing the lower extremities (14).
In this study, we attempted to determine residual adipose tissue characteristics in patients with various types of lipodystrophy.
Methods
Patient selection
A total of 13 patients with generalized lipodystrophies (12 patients with CGL: 7 patients with CGL1, 3 patients with CGL2, and 2 patients with CGL4; and 1 patient with AGL) and 19 patients with partial lipodystrophy (12 patients with FPLD and 7 patients with APL) were included between September 2013 and December 2015. Institutional review board approval was granted for this prospective study, and informed consent was obtained from all patients.
CGL was clinically diagnosed based on generalized loss of adipose tissue that was either remarkable at birth or evident early in life. AGL was diagnosed based on acquired generalized fat loss. FPLD was diagnosed based on loss of adipose tissue from selected areas. APL was diagnosed based on acquired fat loss characteristically commencing on the face and progressing downward to the trunk and upper extremities. Each diagnosis was confirmed by whole-body magnetic resonance imaging (MRI) and was supported by subtype-specific clinical and laboratory features.
Genetic testing
All patients with CGL and FPLD underwent molecular genetic studies of the genes AGPAT2, BSCL2, CAV1, PTRF, LMNA, LMNB2, PPARG, PLIN1, AKT2, and CIDEC that confirmed mutations in AGPAT2, BSCL2, PTRF, LMNA, PPARG loci in most patients. Genomic DNA was isolated from peripheral blood cells using standard techniques. Mutation analyses were performed by sequencing of the coding exons and the exon-intron boundaries of the genes. PCR primers used to amplify the regions of interest can be sent upon request. Sequencing was performed with Miseq V2 chemistry on MiSeq instrument (Illumina Inc.). Analysis was performed with IGV software (Broad Institute).
MRI acquisition
All patients underwent whole-body MRI. The images were acquired using a 1.5 T MRI system with a 6 multichannel body coil and obtained from the top of the head to the end of the toes in axial and coronal planes (Gyroscan Achieva, release 8.1; Philips Medical Systems). The whole-body MRI protocol included coronal and axial T1-weighted turbo spin-echo (TSE) images (time to repetition [TR]/time to echo [TE], 396/17; flip angle, 90°; echo train length [ETL], 6; field of view [FOV], 50×180 cm; slice thickness, 5 mm), coronal T2-weighted TSE images (TR/TE, 3495/90; flip angle, 90°; ETL, 56; FOV, 50×180 cm; ST, 5 mm), and coronal T2-weighted fat-saturated Spectral Presaturation with Inversion Recovery (SPIR) images (TR/TE, 3505/90; flip angle, 90°; ETL, 56; FOV, 50×180 cm; ST, 5 mm).
MRI analysis
Images were evaluated for residual adipose tissue in periarticular, external genital, head and neck regions, palms and soles, scalp, and breasts. The presence of residual adipose tissue in the target tissues was decided with the hyperintense signal on both T1- and T2-weighted TSE images, and signal loss on fat-saturated T2-weighted images. Control images were obtained from age- and gender-matched healthy subjects for each site (Figs. 1a, 1b, 2a, 2b). Images of all patients were interpreted by two radiologists (C.A. and M.S.) in consensus. The residual adipose tissue was assigned as present, reduced, or absent for each area.
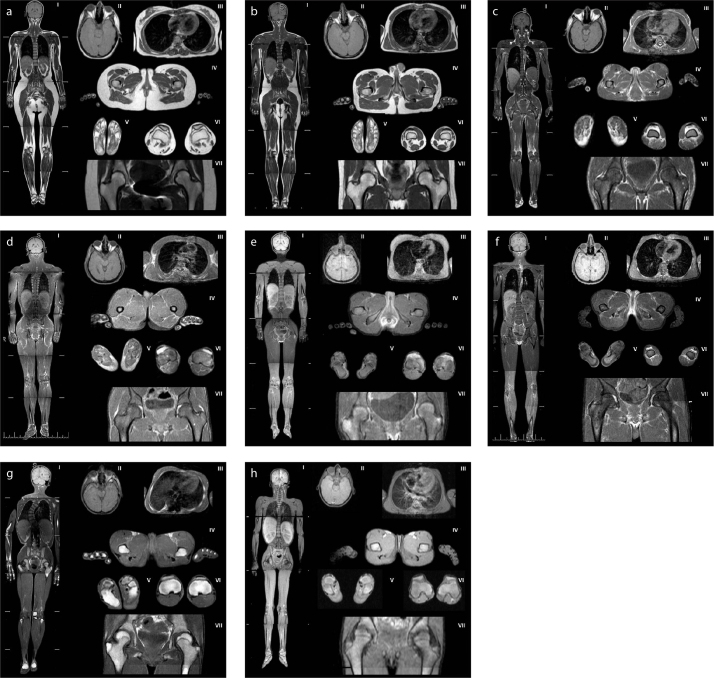
Figure 1. a–h.
Magnetic resonance images of patients with generalized lipodystrophy. Panels (a, b) show control subjects with normal adipose tissue distribution (a, a 28-year-old healthy woman; b, a 26-year-old healthy man). Panels (c, d) show adipose tissue distribution in patients with CGL1: (c), a 30-year-old female (patient 1.1); (d), a 31-year-old male (patient 2.2). Panels (e, f) show adipose tissue distribution in patients with CGL 2: (e), a 25-year-old female (patient 6.1); (f), a 19-year-old male (patient 6.2). Panel (g) shows adipose tissue distribution in a 16-year-old female patient with CGL4 (patient 8.1). Panel (h) shows adipose tissue distribution in an 11-year-old female patient with AGL (patient 10).
(I), whole body, coronal T1-weighted imaging; (II), orbital fat and scalp, axial T1-weighted imaging; (III), breasts, axial T1-weighted imaging; (IV), external genital region and palms, axial T1-weighted imaging; (V), soles, axial T1-weighted imaging; (VI), patellar region, axial T1-weighted imaging; (VII), hip region, coronal T1-weighted imaging.
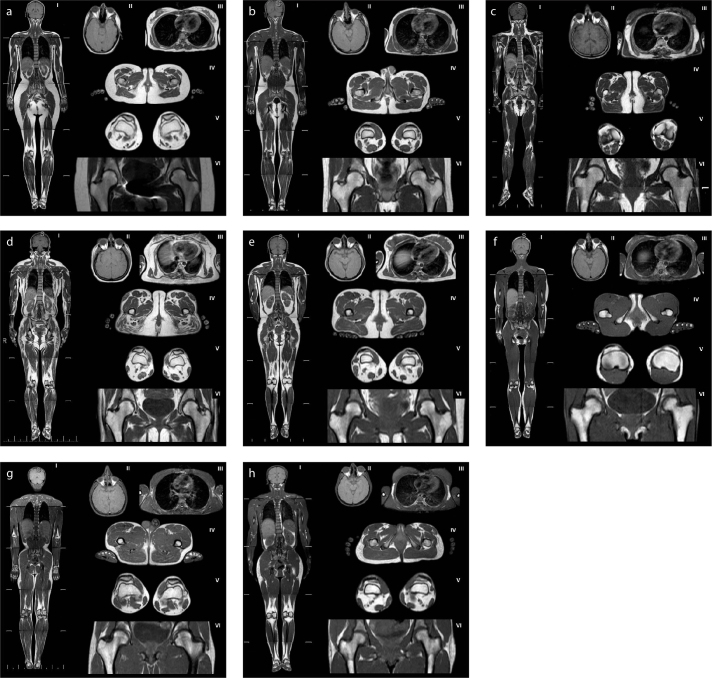
Figure 2. a–h.
Magnetic resonance images of patients with partial lipodystrophy. Panels (a, b) show control subjects with normal adipose tissue distribution (a, a 28-year-old healthy woman; b, a 26-year-old healthy man). Panels (c–f) show adipose distribution in FPLD patients: (c), a 30-year-old female with classical codon 482 LMNA mutation (patient 11.1); (d), a 48-year-old female with non-codon 482 LMNA mutation (patient 16.1); (e), a 28-year-old female with PPARG mutation (patient 17.1); (f), a 34-year-old female, mutation negative (patient 18.1). Panels (g, h) show adipose distribution in patients with APL: (g), a 26-year-old male (patient 23); (h), a 32-year-old female (patient 24).
(I), whole body, coronal T1-weighted imaging; (II), orbital fat and scalp, axial T1-weighted imaging; (III), breasts, axial T1-weighted imaging; (IV), external genital region and palms, axial T1-weighted imaging; (V), soles, axial T1-weighted imaging; (VI), patellar region, axial T1-weighted imaging.
Laboratory measurements
Glucose, triglycerides, and cholesterol levels were measured by standardized methods with appropriate quality control and quality assurance procedures. Direct low-density lipoprotein (LDL) cholesterol measurement was performed. Leptin and adiponectin levels were measured with enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (Boster; Leptin: EK0437, sensitivity <10 pg/mL; Adiponectin: EK0595, sensitivity <60 pg/mL).
Statistical analysis
Statistical analysis was performed using Statistical Package of Social Science (SPSS, IBM Corp.), version 22.0 for Windows. Data were expressed as median and interquartile range (IQR). Categorical variables regarding the residual adipose tissue were reported as present, reduced, or absent for each area. Categorical parameters were analyzed by using chi-square test. Mann-Whitney U test was used to compare continuous parameters. A P value less than 0.05 was considered as statistically significant.
Results
Median age of the patients was 30 years (IQR, 21–38 years; range, 11–63 years). There were 8 males (25%) and 24 females (75%). The clinical and laboratory characteristics of patients are shown in Table 1. Patients with generalized lipodystrophies were younger. As a result of generalized adipose tissue loss, circulating leptin and adiponectin levels were more severely suppressed in patients with generalized lipodystrophies than those with partial lipodystrophies. Table 2 summarizes the distribution of residual adipose tissue in patients with various forms of lipodystrophies.
Table 1.
Clinical characteristics of patients with lipodystrophies
| All subjects (n=32 ) | Generalized lipodystrophy (n=13) | Partial lipodystrophy (n=19) | P (generalized vs. partial) | |
|---|---|---|---|---|
| Age (years) | 30 (21–38) | 21 (15–30) | 34 (28–45) | 0.01 |
| Gender (F/M) | 24/8 | 9/4 | 15/4 | 0.68 |
| Glucose (mg/dL) | 143 (114–202) | 144 (108–224) | 142 (116–206) | 0.67 |
| HbA1c (%) | 8.2 (6.2–9.5) | 7.0 (6.0–10.7) | 8.4 (6.2–9.3) | 0.98 |
| Triglyceride (mg/dL) | 372 (223–659) | 581 (310–1268) | 342 (210–544) | 0.47 |
| Total cholesterol (mg/dL) | 181 (159–235) | 169 (145–257) | 195 (164–230) | 0.15 |
| LDL cholesterol (mg/dL) | 98 (68.2–117) | 91 (60–107) | 104 (72–126) | 0.47 |
| HDL cholesterol (mg/dL) | 28 (22–37) | 27 (19–31) | 32 (24–40) | 0.25 |
| Creatinin (mg/dL) | 0.6 (0.5–0.9) | 0.5 (0.4–0.7) | 0.7 (0.6–0.9) | 0.031 |
| ALT (IU/L) | 24 (18–44) | 29 (18–52) | 23 (18–38) | 0.56 |
| Leptin (ng/mL) | 1.2 (0.4–4.5) | 0.3 (0.1–0.7) | 3.4 (1.5–6.6) | 0.001 |
| Adiponectin (μg/mL) | 2.7 (0.9–5.3) | 0.7 (0.5–3.8) | 3.4 (1.8–8.6) | 0.031 |
Data are presented as median (IQR).
F, female; M, male; HbA1c, glycated hemoglobin A1c; LDL, low-density lipoprotein; HDL, high-density lipoprotein; ALT, alanine aminotransferase.
Table 2.
Residual adipose tissue characteristics of patients with various subtypes of lipodystrophy
| Periarticular region | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||||||
| Head and neck | Knee | Hip | |||||||||||||||
|
|
|
|
|||||||||||||||
| Scalp | Retro-orbital | Buccal | Masticator | Para-pharyngeal | Ear lobe | Palms | Soles | Breasts | External genital | İnfra-patellar | Supra-patellar | Popliteal | Acetabular roof | Extra-articular | |||
| Generalized lipodystrophies | CGL | CGL1 | + | + | R | + | R | + | R | + | R | R | + | + | R | + | + |
| CGL2 | − | − | − | − | − | − | − | − | − | R | R | R | R | + | − | ||
| CGL4 | − | + | R | R | R | − | − | − | − | − | R | − | R | − | − | ||
| AGL | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | ||
|
| |||||||||||||||||
| Partial lipodystrophies | FPLD | LMNA | + | + | + | + | + | + | + | + | +/R | + | + | + | + | + | + |
| PPARG | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||
| Negative | + | + | + | + | + | + | R | R | R | + | + | R | − | − | − | ||
| APL | − | + | − | − | − | − | − | R | − | R | + | + | + | + | + | ||
R, reduced; CGL, congenital generalized lipodystrophy; AGL, acquired generalized lipodystrophy; FPLD, familial partial lipodystrophy; LMNA, lamin A/C; PPARG, peroxisome proliferator-activated receptor gamma; APL, acquired partial lipodystrophy.
We evaluated 7 CGL1 patients with mutations in the AGPAT2 gene, all of whom exhibited nearly complete loss of adipose tissue. However, well-preserved adipose tissue was evident in the retro-orbital area, earlobes, and scalp (Fig. 1cII, 1dII). Adipose tissue was present, but reduced in extent, in the buccal and para-pharyngeal areas. Adipose tissue was also preserved in the palms and soles (Fig. 1cIV–V, 1dIV–V). Histopathologically, fat cells were evident in the soles of a CGL1 patient who underwent surgery to treat a diabetic foot ulcer. Adipose tissue was present, but reduced in extent, in the breasts and external genitalia. Intra- and peri-articular fatty deposits were preserved throughout the body, but the extent of popliteal fat was reduced compared with that of controls (Fig.1cVI, 1dVI).
Three patients had CGL2 caused by mutations in the BSCL2 gene and exhibited a total lack of metabolically active adipose tissue. These patients had no visible adipose tissue in the head and neck region, palms, soles, or breasts (Fig. 1eII–V, 1fII–V). The external genitalia did contain some residual adipose tissue, but the extent thereof was less than that in controls (Fig. 1eIV, 1fIV). Adipose tissue was present, but reduced in extent, in the knee joint. Some adipose tissue was evident in the acetabular roof of the hip joints, but not in the extra-articular regions (Fig. 1eVII, 1fVII).
Two patients had CGL4 caused by PTRF mutations. Despite the nearly complete lack of fat, adipose tissue was clearly preserved in the retro-orbital region (Fig. 1gII). Very small amounts of adipose tissue were evident in the buccal, masticator, para-pharyngeal, infrapatellar, and popliteal regions. No residual adipose tissue was evident at any other site. Interestingly, bone marrow fat was well preserved in these patients but completely absent from the other CGL patients described above (Fig. 1gVII).
Only one patient had AGL. We could not detect any residual adipose tissue at any site, including the head and neck region, palms, soles, breasts, external genitalia, and periarticular region (Fig. 1h).
Of the FPLD patients, eight had mutations in LMNA and one had FPLD caused by a PPARG mutation. We also included three sisters with the FPLD phenotype who were negative for mutations in the genes sequenced. Although subcutaneous fat loss was remarkable in the lower extremities in all patients with FPLD caused by LMNA and PPARG mutations, periarticular adipose tissue was preserved. Reduced breast fat was noticed in patients with the classical codon 482 LMNA mutation (patients 11.1, 12.1, 13.1, 13.2 and 13.3; Fig. 2cIII). However, the three mutation-negative sisters lacked adipose tissue in most periarticular regions. Popliteal fat was totally absent, but infrapatellar fat was very well preserved, and some adipose tissue was also evident in suprapatellar regions (Fig. 2fV). Adipose tissue was present, but reduced in extent, in the palms, soles, and breasts. The external genitalia apparently contained normal amounts of adipose tissue (Fig. 2cIV, 2dIV, 2eIV, 2fIV).
We evaluated seven patients with APL. Adipose tissue was absent from the scalp, masticator, para-pharyngeal regions, and earlobes of all patients and was significantly reduced in extent in the buccal region. However, retro-orbital fat was strikingly preserved (Fig. 2gII, 2hII). All patients lacked adipose tissue in the palms and breasts. Adipose tissue was present, but reduced in extent, in the soles and external genitalia. Periarticular fat was well preserved. In the hip, the acetabular roofs of five patients lacked adipose tissue, although the extra-articular fat pads were normal in extent (Fig. 2gVI, 2hVI).
Discussion
Adipose tissue has several different functions in the body. First, it is a form of energy storage. Second, adipose tissue metabolism regulates body temperature. Third, adipose tissue has mechanical functions. Adipose tissue normally envelops all bodily organs. Adipose tissue protects against injuries (15), and serves as a structural template for various organs. In the retro-orbital region, for example, the orbital structures, with adjacent supporting tissues, are embedded within such adipose tissue (16). All joints feature regions where adipose tissue projects into the bursal cavities as fatty pads; these pads are particularly evident in the shoulder, knee, and hip joints. When a joint is moved, the fatty pads contribute to internal streamlining of the joint cavity.
In the present study, lipodystrophy patients exhibited different patterns of adipose tissue loss. Generalized lipodystrophy was associated with near absence of body adipose tissue. On the other hand, partial lipodystrophy was characterized by selective loss of subcutaneous adipose tissue. Patients with FPLD had selective adipose tissue loss that was predominantly observed in the arms and legs, and chest and trunk regions. Subcutaneous adipose tissue was accumulated in the face and neck in patients with FPLD caused by LMNA mutations. In contrast, APL was characterized by selective loss of adipose tissue, which affected the face and neck, upper extremities, and upper part of the trunk, sparing adipose tissue in the lower extremities. Leptin and adiponectin levels were more severely suppressed in patients with generalized lipodystrophy than in those with partial lipodystrophy, reflecting the severity of adipose tissue loss.
The determination of adipose tissue loss pattern by physical examination and imaging was sufficient to distinguish patients with generalized lipodystrophy and partial lipodystrophy, and also those with FPLD and APL. However, we hypothesized that residual adipose tissue characteristics, which can be determined by MRI, could help distinguish subgroups of patients with generalized and partial lipodystrophy. For this aim, we first studied residual adipose tissue characteristics in patients with generalized lipodystrophy. We were able to determine some residual adipose tissue in certain patients with generalized lipodystrophy, although metabolically active fat was completely absent. Adipose tissue was preserved (at normal levels) in the scalp, earlobes, retro-orbital region, palms, and soles of CGL1 patients caused by AGPAT2 mutations. Intra- and peri-articular adipose tissue was also well preserved in this subgroup of patients with generalized lipodystrophy. Some adipose tissue was evident in the breasts, buccal, masticator, and para-pharyngeal areas. In contrast, CGL2 caused by BSCL2 mutation was associated with more extensive loss of adipose tissue, which was completely absent from the head and neck region, palms, and soles. CGL4 caused by PTRF mutation was characterized by very well-preserved retro-orbital and bone marrow fat in the absence of any significant amount of adipose tissue elsewhere. Previously, Simha et al. (17) reported that some adipose tissue was preserved in the palms and soles of CGL1 patients, but not in CGL2 patients (17). Earlier MRI and postmortem studies found that some adipose tissue was preserved in certain body regions of CGL1 patients (3, 18, 19). Our data suggest that analysis of residual adipose tissue characteristics can readily identify generalized lipodystrophies to the subtype, before moving to genetic tests. Thus, CGL1 should be considered if adipose tissue is well preserved in the palms, soles, scalp, and retro-orbital region. CGL4 is the most likely diagnosis when retro-orbital and bone marrow fat are well preserved in patients otherwise exhibiting generalized loss of adipose tissue. CGL2 is characterized by severe loss of adipose tissue, which is associated with total absence of adipose tissue from the head and neck (including the scalp) and the retro-orbital region. We observed no residual adipose tissue in our single patient with AGL. However, AGL is characterized by progressive fat loss commencing at any time during life. Thus, it is possible that some residual adipose tissue remained present during the early stages of the disorder. As far as patients with partial lipodystrophy are concerned, the dramatic preservation of retro-orbital fat in APL patients is of considerable interest; such patients almost completely lack head and neck fat.
As shown previously (17–19), we also found that CGL2 patients suffered more severe loss of body fat than did those with CGL1, although the reason remains unclear. It is not clear why some patients with generalized fat loss retain relatively normal amounts of adipose tissue in certain body areas such as the retro-orbital region, scalp, palms, and soles, despite the fact that metabolically active adipose tissue is almost completely lacking. Adipose tissue is generally regarded as being of mesodermal origin (20). Mesenchymal stem cells can differentiate into adipocytes (21). However, it is not clear if different adipose depots arise from distinct types of adipoblasts or pre-adipocytes. It has been suggested that the adipocytes of various fat depots exhibit different properties that may, in turn, be associated with distinct types of metabolic complications (22, 23). Expression profiling has revealed significant gene expression differences among different fat depots (24–27), suggesting that the developmental origins (i.e., the cellular precursors) of the deposits vary. Fat depots differ in terms of developmental chronology (20); this is also true for pre-adipocytes that generate the depots. Pre-adipocytes isolated from various body regions differ in terms of gene expression patterns and differentiation capacity (25, 28, 29). Recent studies have shown that adipocytes and pre-adipocytes may be heterogeneous even within the same fat depot (30–32).
Our study had several limitations. We were not able to confirm the residual adipose characteristics in repeated images from different subjects in selected subtypes of lipodystrophy because of rarity of these syndromes. Adipose tissue characteristics were studied only in one patient with AGL, two patients with CGL4 and one patient with FPLD caused by PPARG pathogenic variant. The study had a cross-sectional design. No prospective data was reported. In this design, we cannot rule out the possibility of progressive adipose tissue atrophy that would affect the residual fat depots in our study population.
In conclusion, our data shows that residual adipose tissue characteristics allow various lipodystrophy subtypes to be distinguished. Various fat depots are differentially affected in lipodystrophy patients. Pathophysiologically, adipose tissue is complex. Further work is needed to understand how adipose tissue is preserved in certain body regions of some individuals with generalized or partial lipodystrophies.
Main points.
Lipodystrophies are a heterogeneous group of disorders characterized by congenital or acquired loss of adipose tissue.
Whole-body MRI is essential to determine any residual adipose tissue in patients with different subtypes of generalized lipodystrophy.
Whole-body MRI characteristics can differentiate between lipodystrophy subtypes before moving to genetic testing.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Garg A. Lipodystrophies. Am J Med. 2000;108:143–152. doi: 10.1016/s0002-9343(99)00414-3. https://doi.org/10.1016/S0002-9343(99)00414-3. [DOI] [PubMed] [Google Scholar]
- 2.Patni N, Garg A. Congenital generalized lipodystrophies-new insights into metabolic dysfunction. Nat Rev Endocrinol. 2015;11:522–534. doi: 10.1038/nrendo.2015.123. https://doi.org/10.1038/nrendo.2015.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agarwal AK, Arioglu E, De Almeida S, et al. AGPAT2 is mutated in congenital generalized lipodystrophy linked to chromosome 9q34. Nat Genet. 2002;31:21–23. doi: 10.1038/ng880. https://doi.org/10.1038/ng880. [DOI] [PubMed] [Google Scholar]
- 4.Magre J, Delepine M, Khallouf E, et al. Identification of the gene altered in Berardinelli-Seip congenital lipodystrophy on chromosome 11q13. Nat Genet. 2001;28:365–370. doi: 10.1038/ng585. https://doi.org/10.1038/ng585. [DOI] [PubMed] [Google Scholar]
- 5.Kim CA, Delepine M, Boutet E, et al. Association of a homozygous nonsense caveolin-1 mutation with Berardinelli-Seip congenital lipodystrophy. J Clin Endocrinol Metab. 2008;93:1129–1134. doi: 10.1210/jc.2007-1328. https://doi.org/10.1210/jc.2007-1328. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi YK, Matsuda C, Ogawa M, et al. Human PTRF mutations cause secondary deficiency of caveolins resulting in muscular dystrophy with generalized lipodystrophy. J Clin Invest. 2009;119:2623–2633. doi: 10.1172/JCI38660. https://doi.org/10.1172/JCI38660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shastry S, Delgado MR, Dirik E, et al. Congenital generalized lipodystrophy, type 4 (CGL4) associated with myopathy due to novel PTRF mutations. Am J Med Genet. 2010;152A:2245–2253. doi: 10.1002/ajmg.a.33578. https://doi.org/10.1002/ajmg.a.33578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peters JM, Barnes R, Bennett L, et al. Localization of the gene for familial partial lipodystrophy (Dunnigan variety) to chromosome 1q21-22. Nat Genet. 1998;18:292–295. doi: 10.1038/ng0398-292. https://doi.org/10.1038/ng0398-292. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal AK, Garg A. A novel heterozygous mutation in peroxisome proliferator-activated receptor-gamma gene in a patient with familial partial lipodystrophy. J Clin Endocrinol Metab. 2002;87:408–411. doi: 10.1210/jcem.87.1.8290. https://doi.org/10.1210/jcem.87.1.8290. [DOI] [PubMed] [Google Scholar]
- 10.Gandotra S, Le Dour C, Bottomley W, et al. Perilipin deficiency and autosomal dominant partial lipodystrophy. N Engl J Med. 2011;364:740–748. doi: 10.1056/NEJMoa1007487. https://doi.org/10.1056/NEJMoa1007487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.George S, Rochford JJ, Wolfrum C, et al. A family with severe insulin resistance and diabetes due to a mutation in AKT2. Science. 2004;304:1325–1328. doi: 10.1126/science.1096706. https://doi.org/10.1126/science.1096706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barroso I, Gurnell M, Crowley VE, et al. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402:880–883. doi: 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- 13.Garg A. Clinical review: Lipodystrophies: genetic and acquired body fat disorders. J Clin Endocrinol Metab. 2011;96:3313–3325. doi: 10.1210/jc.2011-1159. https://doi.org/10.1210/jc.2011-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akinci B, Koseoglu FD, Onay H, et al. Acquired partial lipodystrophy is associated with increased risk for developing metabolic abnormalities. Metabolism. 2015;64:1086–1095. doi: 10.1016/j.metabol.2015.06.004. https://doi.org/10.1016/j.metabol.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11:11–18. doi: 10.1111/j.1467-789X.2009.00623.x. https://doi.org/10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 16.Dozio E, Corsi MM, Ruscica M, et al. Adipokine actions on cartilage homeostasis. Adv Clin Chem. 2011;55:61–79. doi: 10.1016/b978-0-12-387042-1.00004-6. https://doi.org/10.1016/B978-0-12-387042-1.00004-6. [DOI] [PubMed] [Google Scholar]
- 17.Simha V, Garg A. Phenotypic heterogeneity in body fat distribution in patients with congenital generalized lipodystrophy caused by mutations in the AGPAT2 or seipin genes. J Clin Endocrinol Metab. 2003;88:5433–5437. doi: 10.1210/jc.2003-030835. https://doi.org/10.1210/jc.2003-030835. [DOI] [PubMed] [Google Scholar]
- 18.Garg A, Fleckenstein JL, Peshock RM, et al. Peculiar distribution of adipose tissue in patients with congenital generalized lipodystrophy. J Clin Endocrinol Metab. 1992;75:358–361. doi: 10.1210/jcem.75.2.1639935. https://doi.org/10.1210/jc.75.2.358. [DOI] [PubMed] [Google Scholar]
- 19.Chandalia M, Garg A, Vuitch F, et al. Postmortem findings in congenital generalized lipodystrophy. J Clin Endocrinol Metab. 1995;80:3077–3081. doi: 10.1210/jcem.80.10.7559900. https://doi.org/10.1210/jc.80.10.3077. [DOI] [PubMed] [Google Scholar]
- 20.Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. https://doi.org/10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Cignarelli A, Perrini S, Ficarella R, et al. Human adipose tissue stem cells: relevance in the pathophysiology of obesity and metabolic diseases and therapeutic applications. Expert Rev Mol Med. 2012;14:e19. doi: 10.1017/erm.2012.13. https://doi.org/10.1017/erm.2012.13. [DOI] [PubMed] [Google Scholar]
- 22.Lafontan M, Berlan M. Do regional differences in adipocyte biology provide new pathophysiological insights? Trends Pharmacol Sci. 2003;24:276–283. doi: 10.1016/S0165-6147(03)00132-9. https://doi.org/10.1016/S0165-6147(03)00132-9. [DOI] [PubMed] [Google Scholar]
- 23.Smith U. Regional differences in adipocyte metabolism and possible consequences in vivo. Int J Obes. 1985;9( Suppl 1):145–148. [PubMed] [Google Scholar]
- 24.Masuzaki H, Ogawa Y, Isse N, et al. Human obese gene expression. Adipocyte-specific expression and regional differences in the adipose tissue. Diabetes. 1995;44:855–858. doi: 10.2337/diab.44.7.855. https://doi.org/10.1073/pnas.0601752103. [DOI] [PubMed] [Google Scholar]
- 25.Gesta S, Bluher M, Yamamoto Y, et al. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc Natl Acad Sci USA. 2006;103:6676–6681. doi: 10.1073/pnas.0601752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vohl MC, Sladek R, Robitaille J, et al. A survey of genes differentially expressed in subcutaneous and visceral adipose tissue in men. Obes Res. 2004;12:1217–1222. doi: 10.1038/oby.2004.153. https://doi.org/10.1038/oby.2004.153. [DOI] [PubMed] [Google Scholar]
- 27.Vidal H. Gene expression in visceral and subcutaneous adipose tissues. Ann Med. 2001;33:547–555. doi: 10.3109/07853890108995965. https://doi.org/10.3109/07853890108995965. [DOI] [PubMed] [Google Scholar]
- 28.Tchkonia T, Giorgadze N, Pirtskhalava T, et al. Fat depot-specific characteristics are retained in strains derived from single human preadipocytes. Diabetes. 2006;55:2571–2578. doi: 10.2337/db06-0540. https://doi.org/10.2337/db06-0540. [DOI] [PubMed] [Google Scholar]
- 29.Tchkonia T, Lenburg M, Thomou T, et al. Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns. Am J Physiol Endocrinol Metab. 2007;292:E298–307. doi: 10.1152/ajpendo.00202.2006. https://doi.org/10.1152/ajpendo.00202.2006. [DOI] [PubMed] [Google Scholar]
- 30.Bluher M, Patti ME, Gesta S, et al. Intrinsic heterogeneity in adipose tissue of fat-specific insulin receptor knock-out mice is associated with differences in patterns of gene expression. J Biol Chem. 2004;279:31891–31901. doi: 10.1074/jbc.M404569200. https://doi.org/10.1074/jbc.M404569200. [DOI] [PubMed] [Google Scholar]
- 31.Fortier M, Soni K, Laurin N, et al. Human hormone-sensitive lipase (HSL): expression in white fat corrects the white adipose phenotype of HSL-deficient mice. J Lipid Res. 2005;46:1860–1867. doi: 10.1194/jlr.M500081-JLR200. https://doi.org/10.1194/jlr.M500081-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Jernas M, Palming J, Sjoholm K, et al. Separation of human adipocytes by size: hypertrophic fat cells display distinct gene expression. FASEB J. 2006;20:1540–1542. doi: 10.1096/fj.05-5678fje. https://doi.org/10.1096/fj.05-5678fje. [DOI] [PubMed] [Google Scholar]