Abstract
PURPOSE
We aimed to assess the safety and effectiveness of mechanical recanalization in patients with ischemic stroke in the anterior circulation within 8 h since symptoms onset and with unknown onset time. We compared time intervals <6 h vs. 6–8 h/unknown onset time, as only limited data are available for a time window beyond 6 h.
METHODS
Our cohort included 110 consecutive patients (44 males; mean age, 73.0±11.5 years) with ischemic stroke in the anterior circulation due to the acute occlusion of a large intracranial artery who underwent mechanical recanalization within an 8-hour time window or with unknown onset time. All patients underwent unenhanced computed tomography (CT) of the brain, CT angiography of the cervical and intracranial arteries and digital subtraction angiography. Perfusion CT was performed in patients beyond a 6-hour time window/with unknown onset time. We collected the following data: baseline characteristics, presence of risk factors, neurologic deficit at the time of treatment, time to therapy, recanalization rate, and 3-month clinical outcome. Successful recanalization was defined as Thrombolysis in Cerebral Infarction score of 2b/3 and good clinical outcome as modified Rankin scale value of 0–2 points.
RESULTS
Successful recanalization was achieved in 82 patients (74.5%): in 61 patients treated within 6 h (73.5%), 7 patients treated within 6–8 h (63.6%), and 13 patients with unknown onset time (81.3%). Good 3-month clinical outcome was achieved in 61 patients (55.5%): in 46 patients treated within 6 h (55.4%), 5 patients treated within 6–8 h (45.5%), and 10 patients with unknown onset time (62.5%). Recanalization success or clinical outcome were not significantly different between patients treated at different time windows.
CONCLUSION
Our data confirms the safety and effectiveness of mechanical recanalization performed in carefully selected patients with ischemic stroke in the anterior circulation within 8 h of stroke onset or with unknown onset time in everyday practice.
Until December 2014, intravenous thrombolysis (IVT) applied within a 4.5 h time window represented the only reperfusion treatment with proven efficacy in acute ischemic stroke (1, 2). Nevertheless, this method has some limitations (3, 4). Late recanalization of large occluded arteries leads to tissue reperfusion with development of large infarctions, associated with a high probability of an unfavorable clinical outcome including death (5, 6). Endovascular treatment (EVT) has been developed to achieve better results in these patients.
Results of IMS III (7), SYNTHESIS (8), and MR RESCUE (9) trials published at the beginning of 2013 were disappointing; the clinical outcome was not better in EVT treated patients compared with IVT treated patients, due to a low percentage of successful recanalizations achieved by EVT (10, 11). Studies published since December 2014 (12–16) finally brought evidence of higher effectiveness of EVT of the anterior circulation occlusion when performed within a 6-hour time window from the symptoms onset. A longer time window was applied in the ESCAPE (up to 12 h) and REVASCAT (up to 8 h) trials; however, the number of patients treated after 6 h was rather small in both trials (n=49 and n=20, respectively) (14, 16). Endovascular treatment was indicated only in patients with a small proven ischemic deficit (defined as Alberta Stroke Program Early Computed Tomography Score [ASPECTS] ≥ 6) (15, 17), or the presence of a salvageable tissue (ischemic penumbra) on perfusion CT (13), or of moderate-to-good collateral circulation on multiphase CT angiography (14). All patients had proven occlusion of a large cerebral artery on CT angiography or magnetic resonance angiography before the intervention and, when considering the treatment, the condition of the collateral system was taken into account. A maximal effort put on the speed and efficacy of the diagnostic process and treatment resulted in significant shortening of both the time from onset to treatment and the time to brain tissue reperfusion.
Our aim was to assess the safety and effectiveness of mechanical recanalization in patients with ischemic stroke in the anterior circulation within 8 h since symptoms onset/with unknown onset time, including the comparison of the time intervals up to 6 h vs. 6–8 h/with unknown onset time, as only limited data are available for a time window beyond 6 h.
Methods
Study population
This retrospective study included 110 consecutive patients with acute ischemic stroke in the anterior circulation due to occlusion of a large intracranial artery, who underwent mechanical recanalization in our comprehensive stroke center (CSC) within a 27-month period. Of the patients, 44 (40%) were males; mean age was 73.0±11.5 years, with 33 patients (30%) older than 80 years (Table 1). IVT was used in 69 patients (62.7%) before the EVT. The median baseline National Institutes of Health Stroke Scale (NIHSS) was 14.5 points. Seventy-two patients (65.5%) were primarily transported to the CSC and remaining 38 (34.5%) were secondarily transported from four primary stroke centers. The time of the stroke onset was not known in 21 patients (19.1%).
Table 1.
Baseline characteristics
| Observed parameter | All | ICA (± MCA) occlusion | MCA occlusion |
|---|---|---|---|
| Patients, n (%) | 110 (100) | 37 (33.6) | 73 (66.4) |
|
| |||
| Age (years), mean±SD | 73.0±11.5 (38–93) | 70.1±11.6 (43–88) | 74.4±11.3 (38–93) |
| >80 years, n (%) | 33 (30) | 9 (24.3) | 24 (32.9) |
|
| |||
| Male sex, n (%) | 44 (40) | 17 (15.5) | 27 (24.5) |
|
| |||
| Vascular risk factors, n(%) | |||
| Arterial hypertension | 86 (78.2) | 29 (26.4) | 57 (51.8) |
| Diabetes mellitus | 34 (30.9) | 11 (10) | 23 (20.9) |
| Dyslipidemia | 35 (31.8) | 9 (8.2) | 23 (20.9) |
| Atrial fibrillation | 58 (52.7) | 12 (10.9) | 46 (41.8) |
| Ischemic heart disease | 25 (22.7) | 10 (9.1) | 15 (13.6) |
|
| |||
| NIHSS baseline, median (range) | 14.5 (5–40) | 14.0 (5–25) | 15.0 (5–40) |
|
| |||
| Onset-to-groin puncture time (min), median (range) | 245.5 (35–1220) | 246.0 (105–1220) | 245.0 (35–1150) |
|
| |||
| Recanalization methods | |||
|
| |||
| Stent retrievers, n (%) | 104 (94.5) | 32 (86.5) | 72 (98.6) |
| Stent retriever type, n of devices | 108 | ||
| Solitaire | 87 | ||
| Trevo | 16 | ||
| Eric 4 | 2 | ||
| Catch | 1 | ||
| Preset | 2 | ||
|
| |||
| i. a. Actilyse (± stent retriever), n (%) | 3 (2.7) | 0 (0) | 3 (4.1) |
|
| |||
| Intracranial PTAS, n (%) | 2 (1.8) | 1 (0.9) | 1 (0.9) |
|
| |||
| CAS, n (%) | 18 (16.4) | 18 (48.6) | 0 (0) |
|
| |||
| IVT, n (%) | 69 (62.7) | 24 (64.9) | 45 (61.6) |
ICA, internal carotid artery; MCA, middle cerebral artery; SD, standard deviation; NIHSS, National Institutes of Health Stroke Scale; Solitaire, Solitaire™, Covidien; Trevo, Trevo® ProVue™, Concentric Medical; Eric 4, ERIC® 4 Retrieval Device, MicroVention Terumo Medical; Catch, Catch Device®, Balt Extrusion; Preset, pREset®, Phenox GmbH; rtPA, recombinant tissue plasminogen activator; PTAS, percutaneous transluminal angioplasty with stent placement; CAS, carotid stenting; IVT, intravenous thrombolysis.
This study was approved by the institutional review board. Informed consent for the eligible and available treatment was obtained from all conscious patients.
Computed tomography
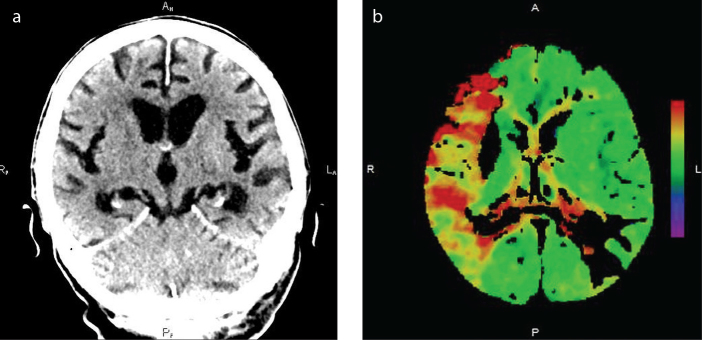
Unenhanced brain CT with the assessment of the ASPECTS and CT angiography of the cervical and intracranial arteries including the evaluation of the collateral system was the standard diagnostic method used in all patients (Fig. 1a). For CT angiography, a nonionic contrast iomeprol (Iomeron 400®, Bracco) was administered intravenously (total volume of 60 mL, speed 4 mL/s). In addition, we carried out perfusion CT in patients treated within 6–8 h or with unknown onset time using 40 mL of the same contrast medium infused at 5 mL/s intravenously. The time to maximum delay >6 s was used for the display of ischemic penumbra; relative cerebral blood flow <30% of that in normal tissue was used for a diagnosis of ischemic core (irreversibly injured brain tissue) (18) (Fig. 1b). A Somatom Definition AS+ (Siemens) scanner was used for CT examinations.
Figure 1. a, b.
Computed tomography. Wake-up stroke in the territory of the right middle cerebral artery displayed on unenhanced (a) and perfusion (time-to-peak) (b) images.
Intravenous thrombolysis
IVT with a standard dose of 0.9 mg/kg (maximum dose 90 mg) of recombinant tissue plasminogen activator (rtPA; Actilyse®, Boehringer Ingelheim) was applied in all patients with known stroke onset time fulfilling the inclusion (treatment within 4.5 h from stroke onset) and exclusion criteria according to the valid guidelines. Ten percent of the rtPA dose was administered as intravenous bolus, followed by a 60 min infusion of the remaining 90% of the dose (19).
Digital subtraction angiography and endovascular treatment
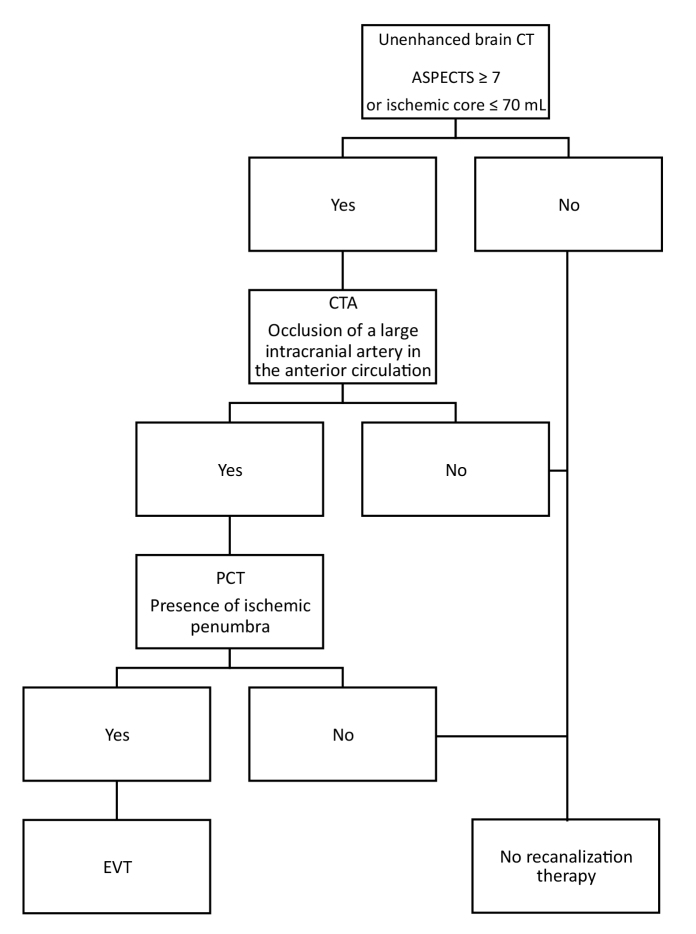
Patients with ASPECTS ≥7 or with perfusion CT showing a small ischemic core (volume threshold ≤70 mL, based on the results of previous studies) (20) and the presence of ischemic penumbra were indicated for EVT. Only patients without mismatch were excluded from therapy (Fig. 2). A poor condition of the collateral system, if representing the only unfavorable prognostic factor, was not a reason for exclusion from EVT. The upper limit for starting the treatment was 8 h from the symptoms onset; the result of perfusion CT was crucial in patients with an unknown symptom onset time. We attempted to start EVT as soon as possible, without waiting for the IVT effect, if applied.
Figure 2.
Indication of endovascular treatment in patients treated within 6–8 h or with unknown onset time. CT, computed tomography; ASPECTS, Alberta Stroke Program Early Computed Tomography Score; CTA, computed tomography angiography; PCT, perfusion computed tomography; EVT, endovascular therapy.
A biplane angio machine (Philips Allura FD 20/20) was used for digital subtraction angiography examination. Nonionic contrast iodixanol (Visipaque 320, GE Healthcare AS) was administered intraarterially using Seldinger technique (total volume of 6 mL, speed 5 mL/s).
Types of EVT are specified in Table 1. In the majority of procedures, a balloon guiding catheter (Merci 8F, Concentric Medical) was placed within the internal carotid artery (ICA); if there was a loop present, the catheter was placed below it. A 0.021-inch microcatheter with stent-retriever was introduced subsequently. The stent-retriever was deployed across the occlusion and after 4 min the stent was slowly retrieved, while flow arrest in the accessing artery was applied by balloon inflation. Manual aspiration was applied through the guiding catheter via sidearm using a 20 cc syringe. General anesthesia was avoided whenever possible. Blood pressure was kept above 140 mm Hg. Intraarterial infusion of rtPA was used as an adjunctive method of recanalization in 3 cases due to persisting thrombus.
Observed parameters
The observed parameters were the same as those in large randomized clinical trials: patient age, neurologic deficit (assessed using the NIHSS score (21)), the ratio of occlusions of M1 segment of the middle cerebral artery (MCA) and of the cervical segment of the ICA, the use of IVT and stent retrievers, and particular time intervals. In patients with unknown stroke onset time, we used the time since the patient was last seen normal (LSN). The effectiveness of recanalization was evaluated using the Thrombolysis in Cerebral Infarction (TICI) score with successful recanalization defined as TICI 2b/3 (22). Modified Rankin scale (mRS) (23) was used for the assessment of 3-month clinical outcome; good outcome was defined as 0–2 points. Clinical outcome was assessed by a certified treating neurologist not blinded to the results of the EVT. A telephone interview with another treating physician or family members was used in patients who were unable to attend a follow-up visit at the CSC.
Statistical analysis
We intended to compare outcomes in subgroups of patients treated wihin 6 h vs. within 6–8 h or with unknown onset time. Odds ratio (OR), 95% confidence interval (CI) and significance level were calculated. Fisher exact test was used for comparison of qualitative parameters, while Mann-Whitney U test and Student’s t-test were used for comparison of quantitative ones. Normality was tested by Kolmogorov-Smirnov test. All data were analyzed using Statistics toolbox in MatLab environment (MathWorks).
Results
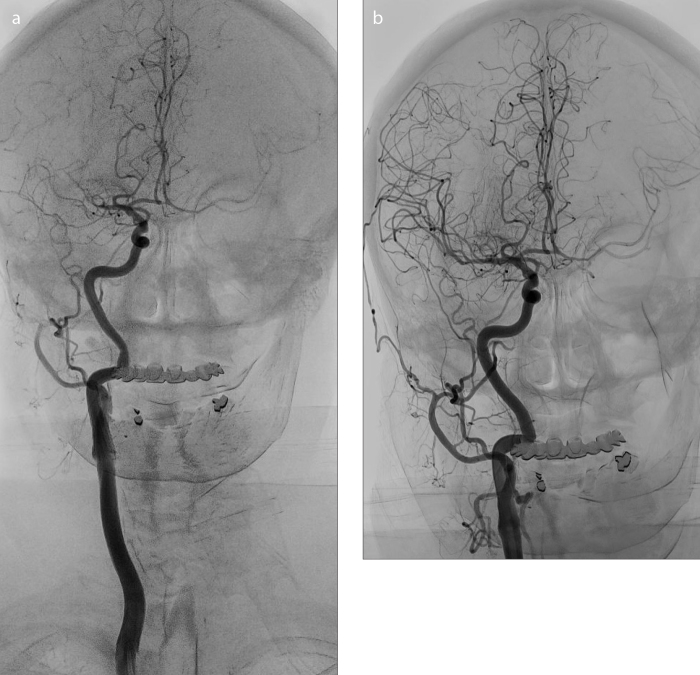
CT angiography revealed the occlusion of a large intracranial artery in the anterior circulation in all patients before the EVT. During the intervention, digital subtraction angiography confirmed an isolated MCA (M1 segment [Fig. 3a] or M2 segment) occlusion in 73 patients (66.4%), while 37 patients (33.6%) had occlusion in the ICA (cervical section or distal segments) including T-occlusions involving distal ICA and proximal MCA and anterior cerebral artery segments (Table 1).
Figure 3. a, b.
Digital subtraction angiography. Occlusion of the right middle cerebral artery (M1 segment) (a) and its recanalization (TICI 3) achieved using a stent retriever (b).
Stent retrievers were used in 104 patients (94.5%). In 18 patients presenting with tandem (ICA and MCA) pathology, carotid stenting was used before intracranial EVT. A small dose (3–20 mg) of rtPA was applied intraarterially during the endovascular intervention in 3 patients (Table 1).
In the whole patient set, median time interval was 143 min (30–1125 min) from stroke onset/LSN to IVT start (if applied), 245 min (120–1220 min) from stroke onset/LSN to groin puncture, and 300 min (165–1284 min) from stroke onset/LSN to recanalization (or to termination of intervention in case of failure). In patients with known onset time, median time interval was 130 min (30–255 min) from stroke onset/LSN to IVT start, 220 min (120–385 min) from stroke onset/LSN to groin puncture, and 265 min (165–473 min) from stroke onset/LSN to recanalization. In patients with primary transport to CSC, the observed median times were 137 min (85–255 min) from stroke onset/LSN to IVT start, 184 min (120–385 min) from stroke onset/LSN to groin puncture, and 235 min (165–456 min) from stroke onset/LSN to recanalization. In the CSC, the median door-to-needle time was 45 min in 42 IVT treated patients; median door-to-groin puncture time was 80 min in the whole set and 76 min in the IVT treated patients. The median IVT-to-groin puncture time was 58 min in the whole set and 47 min in patients primarily transported to the CSC.
A successful recanalization (Fig. 3b) was achieved in 82 patients (74.5%): in 61 patients treated within 6 h (73.5%), 7 patients treated within 6–8 h (63.6%), and 13 patients with unknown onset time (81.3%) (P > 0.05). When comparing patients with primary and secondary transport to the CSC, successful recanalization was achieved in 52 (72.2%) vs. 30 (78.9%). No statistically significant differences were found in baseline characteristics of patients with good vs. none/poor recanalization (Table 2).
Table 2.
Baseline characteristics of patients stratified by reperfusion grade
| Observed parameter | Good recanalization | None/poor recanalization |
|---|---|---|
| Patients, n (%) | 82 (74.5) | 28 (25.5) |
|
| ||
| Age (years), mean±SD (range) | 72.4±11.9 (39–93) | 73.3±11.4 (38–91) |
|
| ||
| Male sex, n (%) | 51 (62.2) | 15 (53.6) |
|
| ||
| NIHSS baseline, median (range) | 13.0 (5–23) | 16.5 (7–40) |
|
| ||
| Occluded artery, n (%) | ||
| ICA (±MCA) | 27 (32.9) | 10 (35.7) |
| MCA | 55 (67.1) | 18 (64.3) |
|
| ||
| Onset-to-groin puncture time (min), median (range) | 243.0 (35–1220) | 245.5 (120–840) |
|
| ||
| IVT, n (%) | 51 (62.2) | 18 (64.3) |
P > 0.05 in all comparisons.
SD, standard deviation; NIHSS, National Institutes of Health Stroke Scale; ICA, internal carotid artery; MCA, middle cerebral artery; IVT, intravenous thrombolysis.
Five groin hematomas were observed; none of them required a surgical revision and all occurred in patients after IVT and after the use of a closure device (Angio-Seal™ Evolution™, St. Jude Medical). There were no intracranial or extracranial arterial injuries that required stent placement or embolization of the bleeding artery.
At 3-month follow-up, 61 patients (55.5%) were independent (mRS 0–2) and 20 patients (18.2%) died. In subgroup analyses, of 21 patients with an unknown stroke onset time, 13 (61.9%) achieved good clinical outcome after 3 months and 6 (28.6%) died; of 20 patients with tandem occlusions, 11 (55%) achieved good clinical outcome and 5 (25%) died; of 28 patients ≥80 years, 9 (32.1%) achieved good outcome and 8 (28.6%) died. Good clinical outcome rates were not significantly different between patients treated within 6 h (55.4%), within 6–8 h (45.5%), and with unknown onset time (62.5%) (Table 3).
Table 3.
Differences between subgroups of patients treated within 6 h, within 6–8 h, or with unknown onset time
| Observed parameter | EVT ≤6 h | EVT 6–8 h | Unknown onset time |
|---|---|---|---|
| Patients, n (%) | 83 (75.5) | 11 (10) | 16 (14.5) |
|
| |||
| Age (years), mean±SD (range) | 72.9±10.8 (38–93) | 72.3±8.0 (62–83) | 73.8±12.5 (43–88) |
|
| |||
| Male sex, n (%) | 36 (43.4) | 1 (9.1) | 5 (31.3) |
|
| |||
| NIHSS baseline, median (range) | 14.7 (5–28) | 14.4 (8–20) | 14.8 (5–40) |
|
| |||
| Occluded artery, n (%) | |||
| ICA (±MCA) | 29 (34.9) | 3 (27.3) | 4 (25) |
| MCA | 54 (65.1) | 8 (72.7) | 12 (75) |
|
| |||
| Onset-to-groin puncture time (min), median (range) | 205.5 (35–350) | 414.4 (360–470) | N/A |
|
| |||
| Recanalization (TICI 2b/3), n (%) | 61 (73.5) | 7 (63.6) | 13 (81.3) |
|
| |||
| Good clinical outcome (mRS 0–2), n (%) | 46 (55.4) | 5 (45.5) | 10 (62.5) |
EVT, endovascular therapy; SD, standard deviation; NIHSS, National Institutes of Health Stroke Scale; ICA, internal carotid artery; MCA, middle cerebral artery; TICI, thrombolysis in cerebral infarction; mRS, modified Rankin scale; N/A, not applicable.
Discussion
Large randomized clinical trials performed within the last few years brought the evidence of the superiority of EVT (usually performed within a 6-hour time window from the symptoms onset) over the best medical treatment (including IVT) in ischemic stroke in the anterior circulation due to occlusion of a large cerebral artery (12–16, 24, 25). However, limited data are available for a time window beyond 6 h. In the ESCAPE trial, the benefit of EVT in the achievement of a good 3-month clinical outcome was similar in the whole set of patients (23.7%; OR, 3.1; 95% CI, 2.0–4.7; P < 0.05) and in the subgroup of patients treated in 6–12 h (21.4%; OR, 2.5; 95% CI, 1.4–4.5; P > 0.05). In the REVASCAT trial, similar results were observed with 15.5% benefit of EVT over the best medical treatment (OR, 1.70; 95% CI, 1.05–2.8; P < 0.05). In our study, good 3-month clinical outcome was achieved in 55.4% of patients treated within 6 h and in 55.6% of patients treated within 6–8 h/with unknown onset time (OR, 1.27; 95% CI, 0.51–3.14; P = 0.60). Our results are comparable with those reported in the ESCAPE trial for EVT treated patients (53%) and even better than those observed in the REVASCAT trial (43.7%) (14, 16).
Correct selection of patients contributed to the positive results of this study, e.g., selection of patients with a high probability of good clinical outcome and a small risk of complications. When aiming to use EVT in patients beyond the standard 6-hour time window or in patients with an unknown stroke onset time, it is crucial to assess the actual state of brain tissue before treatment, often using up-to-date imaging methods. In our study, EVT was indicated in patients with ASPECTS ≥7 or with perfusion CT demonstrating a small ischemic core (volume ≤70 mL) and the presence of ischemic penumbra. The same ASPECTS value was used as a threshold also in the REVASCAT trial (16), while in the ESCAPE (14) and SWIFT-PRIME (15) trials ASPECTS ≥6 was applied. Regarding our chosen volume of ischemic core (≤70 mL), Sanak et al. (20) previously identified this threshold value as a clinical outcome predictor in patients with ischemic stroke due to occlusion of the MCA treated by IVT or by intraarterial thrombolysis based on diffusion-weighted images. The same threshold volume of the ischemic core was applied also in the EXTEND-IA trial with patient selection based on perfusion CT imaging, using a RAPID software (26, 27). This software also identified ischemic penumbra; 25% of potential patients were not included in their study based on perfusion CT results (13). In MR CLEAN (12) and SWIFT PRIME (15) trials, perfusion CT was used as a diagnostic method in 65% and 81% of patients, respectively. In the ESCAPE trial, the presence of moderate-to-good collateral circulation on multiphase CT angiography represented one of the main inclusion criteria (14). Nevertheless, in our study, a poor condition of the collateral system, if representing the only unfavorable prognostic factor, was not a reason for patient exclusion from the EVT.
Clinical trials demonstrated that timely treatment represented a strong predictor of achievement of favorable clinical outcome. For example, the ratio of patients with a favorable clinical outcome was lower in MR CLEAN (12) and REVASCAT (16) trials, both having relatively long onset-to-groin puncture times (median, 260 and 269 min, respectively). We achieved a median onset-to-groin puncture time of 245 min, which was longer than the median time in the remaining trials (13–15), but this interval was shorter in patients transfered directly to our CSC (median, 195 min). The interval between the initiation of the IVT and the groin puncture was reported as >70 min in the published trials (12–14); in these trials, EVT could have been delayed due to different IVT efficacy in particular patients and/or due to logistic problems. This time interval was shorter in our study (median, 58 min), as we attempted to start EVT as soon as possible, without waiting for the IVT effect. This is in accordance with the current guidelines (28, 29). Our effort resulted in a median door-to-groin puncture time of 85 min in the whole set and even 76 min in IVT treated patients. Thus, IVT administration did not prolong door-to-groin puncture time in our CSC. However, time intervals since stroke onset/LSN were relatively longer in our study. This was influenced by several factors such as delays during the prehospital phase (caused mainly by the late call to the emergency medical service), a 19% rate of patients with an unknown stroke onset time (whose arrival intervals to the hospital were extremely long), and a 35% rate of patients with secondary transport to our CSC. Our cohort consisted of consecutive patients and unlike the randomized clinical trials none of them was excluded from treatment because of a condition that could prolong the procedure.
A successful recanalization rate of 75% and self-sufficiency rate of 56% achieved in our study are comparable with the published results and better than those presented in the MR CLEAN (12), ESCAPE (14), and REVASCAT (16) trials. Mortality rate of 18% in our study is comparable with that in the MR CLEAN (12) and REVASCAT (16) trials, but higher than the mortality rate in the EXTEND-IA (13), ESCAPE (14), and SWIFT PRIME (15) trials. Mortality could have been influenced by the higher mean age of our patients, including 30% of patients aged >80 years (with 31% mortality).
When comparing particular patient subgroups, the best results were achieved in patients with unknown onset time in terms of both recanalization success (81.3%) and good clinical outcome (62.5%). The same outcome measures were slightly better in patients treated within 6 h than those treated within 6–8 h (recanalization success 73.5% vs. 63.6% and good clinical outcome in 55.4% vs. in 45.5%). While the latter differences are quite predictable (i. e., lower recanalization rate and lower rate of good clinical outcomes in patients treated later than in those treated earlier), the reasons for achievement of best results in patients with unknown onset time are not clear. One may just assume that the mean time from stroke onset to recanalization might be shorter in these patients. Nevertheless, none of these differences was statistically significant, probably due to the small number of patients in each subgroup.
The two main limitations of this study are its retrospective observational character and the low number of patients treated within 6–8 h/with unknown onset time. A third limitation is that the choice of treatment method was dependent on physician decision. On the other hand, our study represents real-world data. For example, slow recruitment was observed in some trials due to the number of restrictive inclusion criteria (e.g., 1.35 patients/month/center in the EXTEND-IA trial and 1.44 patients/month/center in the ESCAPE trial) (13, 14), while in our center, we perform EVT in 4.1 patients/month on average. Fourth, the unknown percentage of patients with a possible fresh stroke among those with unknown onset time may have led to a bias towards a favorable time window and, consequently, better results.
In conclusion, data from our center confirm the safety and effectiveness of mechanical recanalization in patients with acute ischemic stroke in the anterior circulation within 8 h since symptoms onset/with unknown onset time in everyday clinical practice. Suitable patients beyond a 6-hour time window or with unknown stroke onset time should be selected based on assessment of the actual state of brain tissue (which, however, should not delay the initiation of the treatment) and should be treated using up-to-date stent retrievers. The evaluation of ASPECTS and perfusion CT demonstrating a small ischemic core and the presence of ischemic penumbra are important for proper patient selection. The logistics of both prehospital and hospital phase may shorten treatment time. Randomized clinical trials are needed for definite confirmation of EVT safety and effectiveness in acute ischemic stroke due to occlusion of a large cerebral artery beyond standard time intervals.
Main points.
In patients with ischemic anterior circulation stroke treated with mechanical recanalization, only limited data are available for a time window beyond 6 h from stroke onset and for stroke with unknown onset time.
In our comprehensive stroke center, successful recanalization was achieved in 74.5% and good 3-month clinical outcome was achieved in 55.5% of patients who underwent mechanical recanalization of acute occlusion of a large intracranial artery in the anterior circulation.
In our cohort, no statistically significant differences were found between groups of patients treated within a standard 6 h time interval from stroke onset and those treated within a 6–8 h time interval/with unknown onset time, in terms of successful recanalization and achievement of good 3-month clinical outcome.
We report that mechanical recanalization represents a safe and effective treatment method in carefully selected patients with ischemic anterior circulation stroke treated within an 8-hour time window/with unknown onset time in everyday practice.
Footnotes
Financial disclosure
This study was partially supported by the grant projects of the Ministry of Health of the Czech Republic (FN HK 00179906) and of the Charles University in Prague, Czech Republic (PROGRES Q40).
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998;352:1245–1251. doi: 10.1016/s0140-6736(98)08020-9. https://doi.org/10.1016/S0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 2.Lees KR, Bluhmki E, von Kummer R, et al. ECASS, ATLANTIS, NINDS and EPITHET rt-PA Study Group. ECASS, ATLANTIS, NINDS and EPITHET rt-PA Study Group. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375:1695–1703. doi: 10.1016/S0140-6736(10)60491-6. https://doi.org/10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- 3.Christou J, Burgin WS, Alexandrov AV, Grotta JC. Arterial status after intravenous TPA therapy for ischaemic stroke: a need for further investigations. Int Angiol. 2001;20:208–213. [PubMed] [Google Scholar]
- 4.Saqqur M, Uchino K, Demchuk AM, et al. CLOTBUST Investigators. Site of arterial occlusion identified by transcranial doppler predicts the response to intravenous thrombolysis for stroke. Stroke. 2007;38:948–954. doi: 10.1161/01.STR.0000257304.21967.ba. https://doi.org/10.1161/01.STR.0000257304.21967.ba. [DOI] [PubMed] [Google Scholar]
- 5.Alexandrov AV, Molina CA, Grotta JC, et al. CLOTBUST Investigators. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004;351:2170–2178. doi: 10.1056/NEJMoa041175. https://doi.org/10.1056/NEJMoa041175. [DOI] [PubMed] [Google Scholar]
- 6.Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. 2007;38:967–973. doi: 10.1161/01.STR.0000258112.14918.24. https://doi.org/10.1161/01.STR.0000258112.14918.24. [DOI] [PubMed] [Google Scholar]
- 7.Broderick JP, Palesch YY, Demchuk AM, et al. Interventional Management of Stroke (IMS) III Investigators. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013;368:893–903. doi: 10.1056/NEJMoa1214300. https://doi.org/10.1056/NEJMoa1214300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciccone A, Valvassori L, Nichelatti M, et al. SYNTHESIS Expansion Investigators. Endovascular treatment for acute ischemic stroke. N Engl J Med. 2013;368:904–913. doi: 10.1056/NEJMoa1213701. https://doi.org/10.1056/NEJMoa1213701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kidwell CS, Jahan R, Gornbein J, et al. MR RESCUE Investigators. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med. 2013;368:914–923. doi: 10.1056/NEJMoa1212793. https://doi.org/10.1056/NEJMoa1212793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parsons MW, Albers GW. MR RESCUE. Is the glass half-full or half-empty? Stroke. 2013;44:2055–2057. doi: 10.1161/STROKEAHA.113.001443. https://doi.org/10.1161/STROKEAHA.113.001443. [DOI] [PubMed] [Google Scholar]
- 11.Campbell BCV, Oxley TJ, Chapot R. Acute ischemic stroke. Time, penumbra, and reperfusion. Stroke. 2014;45:640–644. doi: 10.1161/STROKEAHA.113.003798. https://doi.org/10.1161/STROKEAHA.113.003798. [DOI] [PubMed] [Google Scholar]
- 12.Berkhemer OA, Fransen PS, Beumer D, et al. MR CLEAN Investigators. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. https://doi.org/10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 13.Campbell BC, Mitchell PJ, Kleinig TJ, et al. EXTEND-IA Investigators. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–1018. doi: 10.1056/NEJMoa1414792. https://doi.org/10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 14.Goyal M, Demchuk AM, Menon BK, et al. ESCAPE Trial Investigators. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–1030. doi: 10.1056/NEJMoa1414905. https://doi.org/10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 15.Saver JL, Goyal M, Bonafe A, et al. SWIFT PRIME Investigators. SolitaireTM with the Intention for Thrombectomy as Primary Endovascular Treatment for Acute Ischemic Stroke (SWIFT PRIME) trial: protocol for a randomized, controlled, multicenter study comparing the Solitaire revascularization device with IV tPA with IV tPA alone in acute ischemic stroke. Int J Stroke. 2015;10:439–448. doi: 10.1111/ijs.12459. https://doi.org/10.1111/ijs.12459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jovin TG, Chamorro A, Cobo E, et al. REVASCAT Trial Investigators. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–2306. doi: 10.1056/NEJMoa1503780. https://doi.org/10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 17.Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. Lancet. 2000;355:1670–1674. doi: 10.1016/s0140-6736(00)02237-6. https://doi.org/10.1016/S0140-6736(00)02237-6. [DOI] [PubMed] [Google Scholar]
- 18.Campbell BC, Christensen S, Levi CR, et al. Cerebral blood flow is the optimal CT perfusion parameter for assessing infarct core. Stroke. 2011;42:3435–3440. doi: 10.1161/STROKEAHA.111.618355. https://doi.org/10.1161/STROKEAHA.111.618355. [DOI] [PubMed] [Google Scholar]
- 19.Neumann J, Tomek A, Školoudík D, et al. Doporučený postup pro intravenózní trombolýzu v léčbě akutního mozkového infarktu – verze 2014. Cesk Slov Neurol. 2014;77/110:381–385. [Google Scholar]
- 20.Sanák D, Nosál’ V, Horák D, et al. Impact of diffusion-weighted MRI-measured initial cerebral infarction volume on clinical outcome in acute stroke patients with middle cerebral artery occlusion treated by thrombolysis. Neuroradiology. 2006;48:632–639. doi: 10.1007/s00234-006-0105-0. https://doi.org/10.1007/s00234-006-0105-0. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein LB, Samsa GP. Reliability of the National Institute of Health Stroke Scale. Extension to non-neurologists in the kontext of a clinical trial. Stroke. 1997;28:307–310. doi: 10.1161/01.str.28.2.307. https://doi.org/10.1161/01.STR.28.2.307. [DOI] [PubMed] [Google Scholar]
- 22.Yoo AJ, Simonsen CZ, Prabhakaran S, et al. Cerebral Angiographic Revascularization Grading Collaborators. Refining angiographic biomarkers of revascularization. Improving outcome prediction after intra-arterial therapy. Stroke. 2013;44:2509–2512. doi: 10.1161/STROKEAHA.113.001990. https://doi.org/10.1161/STROKEAHA.113.001990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong KS, Saver JL. Quantifying the value of stroke disability outcomes. WHO Global Burden of Disease Project disability weights for each level of the modified Rankin scale. Stroke. 2009;40:3828–3833. doi: 10.1161/STROKEAHA.109.561365. https://doi.org/10.1161/STROKEAHA.109.561365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pierot L, Derdeyn C. Interventionalist perspective on the new endovascular trials. Stroke. 2015;46:1440–1446. doi: 10.1161/STROKEAHA.115.008416. https://doi.org/10.1161/STROKEAHA.115.008416. [DOI] [PubMed] [Google Scholar]
- 25.Grotta JC, Hacke W. Stroke neurologist’s perspective on the new endovascular trials. Stroke. 2015;46:1447–1452. doi: 10.1161/STROKEAHA.115.008384. https://doi.org/10.1161/STROKEAHA.115.008384. [DOI] [PubMed] [Google Scholar]
- 26.Campbell BC, Yassi N, Ma H, et al. Imaging selection in ischemic stroke: feasibility of automated CT-perfusion analysis. Int J Stroke. 2015;10:51–54. doi: 10.1111/ijs.12381. https://doi.org/10.1111/ijs.12381. [DOI] [PubMed] [Google Scholar]
- 27.Menon BJ, Campbell BCV, Levi Ch, Goral M. Role of imaging in current acute ischemic stroke workflow for endovascular therapy. Stroke. 2015;46:1453–1461. doi: 10.1161/STROKEAHA.115.009160. https://doi.org/10.1161/STROKEAHA.115.009160. [DOI] [PubMed] [Google Scholar]
- 28.Jauch EC, Saver JL, Adams HP, Jr, et al. American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a. https://doi.org/10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 29.Šaňák D, Neumann J, Tomek A, et al. Doporučení pro rekanalizační léčbu akutního mozkového infarktu – verze 2016. Cesk Slov Neurol. 2016;79/112:231–234. https://doi.org/10.14735/amcsnn2016231. [Google Scholar]