Abstract
Microglial cells are the resident tissue macrophages of the CNS and are widely recognized for their immune surveillance of the healthy CNS. In addition to this well-accepted function, recent findings point to major roles for microglia in instructing and regulating the proper function of the neuronal networks in the adult CNS, but these cells are also involved in creating neuronal networks by orchestrating construction of the whole network during development. In this Review, we highlight recent findings about the steady-state functions of microglial cells, the factors that are important for physiological microglial function, and how microglia help to maintain tissue homeostasis in the CNS.
Introduction
The adult CNS consists of billions of neurons, with each neuron creating up to 15,000 interconnections with other neurons (1). This immense neuronal network is delicate and can be only partially replaced; therefore, it is necessary to maintain, support, and guard this fragile system (2–5). In the adult CNS, neuronal maintenance and support are performed by different cell types: glial cells, including astrocytes and oligodendrocytes, often referred to as macroglia, and the resident tissue macrophages of the CNS parenchyma, including the microglia and macrophages of the CNS interfaces such as perivascular, meningeal, and choroid plexus macrophages (6, 7). Historically, the name “glia” is derived from the Greek word for glue, and for a long time, glial cells were just seen as the “cement” that maintains the structure of the CNS. Interestingly, Rudolf Virchow, who first named and described the neuroglia as a connective material between the neurons in 1856, pointed out that understanding the function and behavior of the nervous system requires characterization of the neuroglial biology (8). Following Virchow’s observations, more and more studies were performed to determine the identity of glial cells. Following the characterization of astrocytes, Müller cells, and radial glia, Santiago Ramón y Cajal discovered a new element of the neuroglia, which he claimed was of mesodermal origin (9, 10). In 1919, Pío del Río Hortega showed for the first time that this newly discovered “third element” included both oligodendrocytes and microglia (11). He described the microglial cells as wandering histiocytes with a mesodermal origin, separating them from the other “classical” glial cells with an epidermal origin (12, 13). Nowadays, it is still accepted that microglia are resident tissue macrophages with a mesodermal origin.
Nearly every tissue in the body contains specialized tissue macrophages; however, microglia are a special subset of immune cells, as they are located within an organ that is considered to be immune-privileged (14). During the last hundred years, the aims in the field were to obtain a detailed understanding of microglia under physiological and pathological conditions in the CNS. Microglia were long considered to be in a resting state in the healthy CNS, while inflammation or an infection in the CNS resulted in activation of the microglia to a professional phagocyte that either resolves the inflammation or infection or potentially promotes a pathological process. Nowadays, mounting evidence indicates that microglia are not in a resting state under healthy conditions, but instead play an essential role in maintaining tissue homeostasis under physiological conditions.
In this Review, we summarize the current state of knowledge about the physiological role and function of microglia during brain development and in the healthy adult CNS. We aim to give a detailed view of the physiological tasks microglia cells have in the healthy CNS and further highlight how microglia senescence is created and maintained during development to fulfill these essential tasks in the parenchyma.
Microglial development: from an erythromyeloid progenitor to a specialized tissue macrophage
To fully understand microglial function and properties under steady-state conditions in the CNS, it is important to understand the origins and developmental pathways of these cells. As proposed by Pío del Río Hortega, microglia are of mesodermal origin. In recent decades, it has become accepted that a hematopoietic progenitor enters the CNS early on during embryonic development from a single wave of mesodermal progenitors (6, 15, 16). After several decades of debate as to whether microglia are derived from a single wave of progenitors that enter the CNS only once during development and locally expand and maintain themselves within the CNS throughout an individual’s life, or whether microglial hematopoietic progenitors invade the CNS during early embryogenesis and are also continuously exchanged from a circulating hematopoietic progenitor pool throughout life (17). A number of studies in the last several years have provided detailed insights into microglial development and proven that microglial cells are derived from one single embryonic wave of progenitors (18–21). The first microglia enter the developing neuroectoderm in the mouse embryo at 9.5 days postconception (dpc) (18, 21, 22). These early embryonic microglia are derived from erythromyeloid progenitors (EMPs), which are generated from extraembryonic hematogenic endothelium positive for the angiopoietin receptor Tie2 in the blood islands of the yolk sac around 8.0 dpc. The EMPs leave the yolk sac soon after their generation and colonize the developing fetal liver, eventually giving rise to a plethora of other tissue macrophages such as Langerhans cells in the epidermis or Kupffer cells in the liver (19). EMPs are c-Kit+CD45+CSF1R+CX3CR1–F4/80– progenitor cells that differentiate further into immature macrophage intermediates and then to yolk sac–derived macrophages, which invade the CNS and give rise to the first embryonic microglia (18, 19, 21, 23). Interestingly, the CNS seems to be one of the first tissues to be colonized with tissue macrophages derived from the yolk sac EMPs (18). The differentiation of embryonic microglia from yolk sac EMPs is dependent on two myeloid transcription factors, the ETS-domain transcription factor Pu.1 and IFN regulatory factor 8 (Irf8). However, the yolk sac EMPs and embryonic microglia are independent of the transcription factor c-myb, which is a key transcription factor for the maintenance of hematopoietic stem cells (HSCs) derived from embryonic definitive hematopoiesis microglia as well as perivascular and meningeal macrophages in the CNS. It is also well accepted that adult microglia are derived from a single wave of yolk sac EMPs, with no further contribution of myeloid progenitors from another hematopoietic origin, such as circulating HSC-derived myeloid progenitors from adult bone marrow (20, 21, 23–27). Upon entering the developing neuroectoderm, the embryonic microglial population expands via proliferation throughout development (21, 22, 28). The expansion and differentiation of microglia during development are highly dependent on colony-stimulating factor 1 receptor (Csf1r) expression (18, 29). Csf1r-deficient mice lack microglia and are devoid of the yolk sac–derived EMPs that give rise to embryonic microglia. Interestingly, mice deficient in one of the two known Csf1r ligands, colony-stimulating factor 1 (Csf1) or interleukin-34 (Il34), show only reduced numbers of microglia. Il34-deficient animals have decreased numbers of microglia in several brain regions (30, 31), whereas Csf1-deficient animals are reported to have either no microglia phenotype or a slightly decreased number of microglia (29, 32–34). In a study comparing microRNA expression in adult microglia and other myeloid cells, it was found that microglia are highly dependent on TGF-β signaling during their differentiation. Microglia are absent in the CNS of TGF-β–deficient mice, and they develop defects in glutamate homeostasis and synaptic plasticity (35). It is not yet clear how microglia are distributed throughout the CNS, and several different mechanisms have been proposed for their distribution, such as migration along radial glial cells toward different brain regions or the recruitment of microglia via guidance cues supplied by different brain regions (36). Undisturbed microglial development is tightly coupled to normal physiological function of adult microglia in the CNS, and a slight disturbance in the maturation and expansion of the cell population can have severe effects on the functional properties of the CNS (37). Therefore, it is still necessary to uncover more details of microglial cell development to completely understand the process that establishes a network of adult steady-state microglia during development.
Physiological function of microglia during embryonic and postnatal development
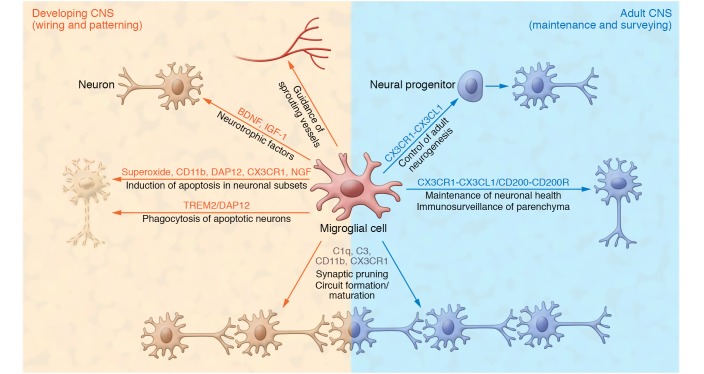
While the first microglial progenitors are entering the neuroectoderm, the first neurons are born in the mouse CNS (38). There is also some evidence that microglial cells appear in CNS regions with the onset of functional neuronal networks (39). This correlative event might indicate that during the development of neurons and neuronal circuit formation, microglia are required in the developing neuroectoderm. Csf1r-deficient animals, which do not have any microglia, exhibit severe defects in brain development with marked structural abnormalities in brain architecture, including olfactory bulb atrophy, expansion of the lateral ventricles, and dramatic thinning of the neocortex (29, 40). Microglia seem to serve several different functions during CNS development particularly as “architects,” orchestrating and coordinating the patterning and wiring in the developing CNS. In the following sections we will further discuss four main functions of microglia during development: the phagocytosis of dead cells, the trophic support of developing neurons in the CNS, the guidance of developing vasculature in the CNS, and the support and refinement of developing neural circuits by synaptic pruning (Figure 1).
Figure 1. Homeostatic function of microglia in the developing and adult CNS.
In addition to their function as a resident immune cell in the CNS parenchyma, microglia display a variety of other functions to maintain tissue homeostasis. Microglia modulate wiring and patterning in the developing CNS by regulating apoptosis of neuronal subpopulations, releasing neurotrophic factors, and guiding sprouting vessels in the parenchyma. They also are important for circuit formation and maturation of neuronal networks, and for regulating adult neurogenesis and maintaining neuronal health in the adult CNS.
Phagocytosis of apoptotic neurons during development.
As professional phagocytes, microglia play a major role in removing apoptotic neurons from the developing CNS. In some regions up to half of all neurons born during development undergo apoptosis prior to adulthood (41, 42). Microglia are found in close proximity to apoptotic neurons in the developing brain, where they remove apoptotic debris (43–45), as well as induce programmed cell death. Microglia induce apoptosis in Purkinje cells of the cerebellum, but also in other brain regions including hippocampus, via release of superoxide ion, similar to the respiratory bursts seen in neutrophils (46–48). Release of superoxide ion is induced by the integrin CD11b and the immune receptor DNAX activation protein of 12 kDa (DAP12) after microglial interaction with the target neuron (48). DAP12 and its receptor, triggering receptor expressed on myeloid cells 2 (TREM2), have been shown to induce removal of apoptotic neurons in vitro and indicate that microglia play a critical role in eliminating apoptotic neurons during development without inducing an inflammatory phenotype (49, 50). Interestingly, mice lacking microglia, such as Pu.1- or Csf1r-deficient animals, have not been found to have major defects in removal of apoptotic neurons during development, suggesting that other cells in the CNS might be involved in the removal of apoptotic neurons.
Trophic support of developing neurons.
In addition to the removal of idle neurons during development, microglia also support and promote neuronal survival in the developing CNS via secretion of different neurotrophic factors. Microglia are critical for the survival of cortical layer V neurons during postnatal development (51). Ablation of microglia or deficiency of the chemokine receptor Cx3cr1 resulted in a decreased survival of the layer V neurons. Interestingly, microglial secretion of insulin-like growth factor 1 (IGF-1) mediated the supportive effect on layer V neurons during development. Microglia also appear to be involved in the induction of programmed cell death via the release of neurotrophins (52). In the chick retina, tropomyosin receptor kinase A–negative (TrKA-negative) neurons are removed by the microglial secretion of nerve growth factor (NGF), which induces apoptosis via binding to low-affinity neuronal growth factor receptor p75 (52). Several in vitro studies showing the effects of microglia-secreted factors on neuronal survival indicate that there may be a variety of factors that can promote or reduce neuronal survival during development. Microglia are also involved in controlling the size of the neural precursor pool in the subventricular zone (SVZ), by eliminating T-box brain protein 2–positive (TBR2+) and paired box protein-6–positive (Pax6+) neural precursors (53). Microglia activation by LPS or microglia inactivation by doxycycline or progesterone modulates neural precursor cell number (53, 54). Overactivation of microglia leads to a decreased number of precursors and a thinning of the SVZ, whereas a decrease in microglial activity results in an increased number of neural precursors (54). However, it should be mentioned here that the treatments used in these studies were not specific to microglia. Also, Pu.1-deficient animals show a decreased number of proliferating cortical progenitors in ex vivo cultures (55). Thus, microglia seem to play a major part in the establishment of the correct number of neurons by regulating cell death and survival in a region-specific manner through the orchestration of prodeath and prosurvival signals.
Guidance of the developing vasculature.
One important event during the development of the CNS from a few neuronal layers to billions of neurons organized in different circuits within distinct brain regions is the vascularization of the CNS to provide nutrients and oxygen. The sprouting of blood vessels into the neuroectoderm starts around 10 dpc in mice (56), shortly after the first microglial progenitors enter the CNS. Microglia in the developing neuroectoderm play an essential role in vascular networking by serving as “tip macrophages” that connect sprouting vessels. In mice and zebrafish, microglia promote the branching of invading sprouting vessels, and a reduction in microglia leads to a decreased number of vascular branching points (57). Moreover, Csf1-deficient animals, which do not have retinal microglia, exhibit decreased branching of the vascular plexus compared with their WT counterparts (58). Vessel growth and density were reduced after microglia depletion, but could be restored after intravitreal injection of microglia (59). Microglia guide the sprouting vessel tip by secretion of soluble guidance factors rather than by direct contact, with bidirectional communication between the sprouting vessel and the microglia (60).
Maturation and refinement of neuronal circuits.
Recently, microglia were shown to play an important role in the maturation and formation of neuronal circuits, especially during postnatal development. Electron microscopy data revealed that microglia in the healthy CNS are in constant contact with neuronal and astrocytic elements, and it was therefore suggested that microglia are located close to synapses in the healthy brain (61, 62). It was also shown that microglial cells make direct contact with pre- and postsynaptic structures, and these cells have been seen to continuously interact for 4–5 minutes in the healthy CNS (63). However, this contact is greatly prolonged during the status epilepticus in the ischemic CNS (63), and neuronal hyperactivity results in an increase in microglial processes that are recruited toward the active synapse (64). One can conclude from these and other studies that microglia seem to sense synaptic activity, prolong their contact with actively signaling synapses, and also modulate the neuronal activity at the synapse (63–65). Notably, it has not been shown that interactions between microglial processes and synaptic boutons are correlated with the disappearance of these boutons after contact occurs. In the developing brain, the creation of the synapse architecture in neuronal circuits is a critical aspect of CNS maturation (66). Tremblay and colleagues used detailed light and electron microscopy to show that during learning and sensory experiences, microglia are in contact with different synaptic elements, including dendritic spines, axonal terminals, astrocytic processes, and the synaptic cleft (67). Microglia also engulf and remove synaptic elements during sensory experience and are involved in the plasticity of the synapse (67). It was later shown that microglia take up postsynaptic material, as indicated by the presence of postsynaptic density protein 95–positive (PSD95+) puncta in the microglial processes, as well as synaptosomal-associated protein 25–positive (SNAP25+) material, which is derived from presynaptic elements (68). This study further revealed a dynamic role of microglia in synaptic pruning through removal of unused dendritic spines during postnatal phases. Furthermore, synaptic pruning was dependent on microglia number during the postnatal phase. Cx3cr1 deficiency reduces the number of microglia during the postnatal phase, leading to reduced synaptic pruning and impaired neuronal circuit maturation (68). Microglial synaptic pruning is also dependent on the neuronal activity of the dendritic spines as well as the labeling of developing synapses with complement component C3, which leads to phagocytosis via the microglial complement receptor CD11b (also known as CR3) (69, 70). Several studies indicate that microglia play a major role in synaptic pruning of neurons, and we are only starting to understand the detailed mechanisms of this process. More research is needed to further elucidate the role of the synaptic pruning performed by microglial cells compared with synaptic pruning seen in other glial cells such as astrocytes and self-pruning of neurons (70–73).
The developing CNS has many more synapses than the mature adult CNS; thus, the removal of unused synapses and the maintenance of functional interconnections is an important process. Disruptions in this process are thought to be associated with autism spectrum disorders or other behavior defects and learning disorders (74, 75). Methyl CpG-binding protein 2–deficient (Mecp2-deficient) animals or animals with a myeloid-specific deletion of Mecp2 show Rett syndrome–like phenotypes, which can be rescued by reintroduction of WT bone marrow–derived macrophages to the CNS (76). Interestingly, it has been shown that phagocytic activity of the WT bone marrow–derived cells is necessary to rescue the Rett syndrome phenotype. In contrast, other studies investigating the function of microglia in the development of Mecp2 deficiency–induced Rett syndrome revealed enhanced phagocytosis of dendritic spines during synaptic pruning that is not rescued by reintroduction of Mecp2 into microglia (77, 78). These studies suggest a contribution of microglia in disease pathology, but other mechanisms of disease could also be involved. Further investigation needs to be carried out to reveal the precise contribution of microglia to the development of Rett syndrome.
Psychiatric disease syndromes like obsessive-compulsive disorder (OCD) are potentially associated with dysfunctional microglia. For example, homeobox protein B8–deficient (Hoxb8-deficient) mice develop extensive grooming behavior with hair loss and skin lesions similar to the human OCD spectrum disorder trichotillomania (79). Hoxb8-mutant animals also display an altered sensory response to nociceptive and thermal stimuli that results from a defect of interneurons in the dorsal spinal cord (80). HOXB8 in the CNS is expressed by a subpopulation of microglia, and transplantation of WT bone marrow into Hoxb8-mutant mice rescued pathological grooming behavior, but did not rescue the sensory phenotype (81).
Several studies in the last decade have revealed that microglia contribute to the formation and development of the CNS. As summarized above, this contribution includes regulation of circuit maturation processes and the guidance of developing vessels in the brain. Moreover, microglia enter the CNS at an early time point during development and function both as innate immune cells to protect the developing brain and to guide and construct the developing nervous system. These findings suggest we are just beginning to understand the important roles of microglia in CNS development.
Physiological function of microglia in the adult brain
Microglia are distributed over the entire CNS parenchyma and can be found in every region in the healthy adult CNS. The density of microglia differs between anatomical regions and ranges from 5% in the cortex to 12% in the substantia nigra (82). Interestingly, microglia seem to be more abundant in gray matter than in white matter (83). In several studies it was suggested that microglial cells maintain their own population in the adult CNS via endogenous proliferation (20, 27, 28, 84), but only a limited amount of experimental evidence supporting this hypothesis is available so far. A recent investigation revealed that microglial cell number is tightly controlled by temporal and spatial coupling of apoptosis and proliferation within the microglial population, which provides evidence for tight control of microglial cell numbers (85). Using a novel microglia-fate mapping system, it was shown that in the healthy brain regional differences in microglia self-renewal exist and that microglia expansion is a random process during homeostasis that can shift to clonality upon pathology (86).
In each CNS region, microglia occupy distinct nonoverlapping territories. Therefore, each microglial cell has a highly specialized morphology under physiological conditions with a small cell soma containing few organelles and surrounding elongated processes with secondary branching and lamellipodia (87, 88). This delicate morphology was already recognized by Pío del Río Hortega, who described the radial ramified processes of microglia in 1919 (11). Compared with other tissue macrophages, microglia have a large cell surface area; for example, the perimeter of a hippocampal microglial cell is seven times that of a Kupffer cell in the liver (82). While the phenotype of microglia under physiological conditions has long been described as resting and downregulated, there is accumulating evidence that microglia are important for the maintenance of tissue homeostasis and regulate numerous processes in the adult CNS (6, 89). New imaging techniques and genetic tools have allowed visualization of living microglia in vivo and ex vivo. It was also shown that the elongated processes of microglia move continuously, scanning the surrounding area (62, 90). Several ex vivo studies have monitored physiological microglial motility in hippocampal or cortical slice cultures (90, 91). Even though these studies do not reflect the overall physiological behavior of microglia in vivo, they showed that ramified microglia are motile and seem to retract and extend their processes while mediating pinocytosis (90–92). Two-photon microscopy imaging revealed a strict territorial organization of microglia in vivo, with distances of 50–60 μm between each cell soma. The somata of physiological microglia are stable and nonmotile; however, the processes of each microglial cell are highly dynamic, and retract and extend to sample the surrounding CNS (61, 62). The sampling is highly random and has a high turnover rate, such that each microglia can sample the CNS parenchyma once in a few hours. The ramified morphology as well as the motility of microglial processes allows them to serve different physiological functions in the adult CNS.
Based on gene expression and surface protein expression profiles, a microglial cell can easily be identified as a resident tissue macrophage derived from yolk sac EMPs, while all yolk sac EMP-derived tissue macrophages share a core signature with each other (20, 27, 37). However, as a result of their adaptation to the CNS, microglia exhibit decreased expression of hundreds of transcripts compared with other tissue macrophage populations (93). Using the microglial surface marker transmembrane protein 119 (TMEM119), Bennett and colleagues performed gene expression profiling of microglia at different developmental time points and found that these cells undergo a defined maturation process that stabilizes around postnatal day 14 (94). In addition to TMEM119, several other genes were found to be highly expressed by microglia and could potentially be used to separate them from other tissue macrophage populations and bone marrow–derived myeloid cells. Potential microglia-specific markers are the cell surface protein Siglec-H and the transcriptional regulator Sal-like 1 (SALL1) (refs. 93, 95–97, and Figure 2). However, whether these surface markers are shared by nonmicroglia CNS macrophages in the perivascular and meningeal space is currently unclear.
Figure 2. Factors regulating microglial homeostasis.
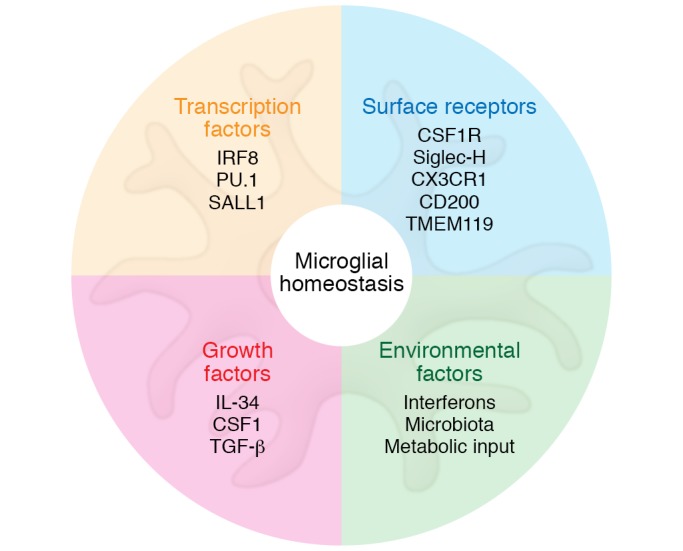
Microglial homeostasis is tightly regulated and maintained by different factors, including cell-intrinsic transcription factors and cell surface receptors. However, external factors also exert effects on microglial function, including growth factors in the CNS parenchyma or factors from outside the CNS such as metabolites, type I interferons or the microbiota, all of which can have a major impact on microglial homeostasis.
Microglia as modulators of synaptic plasticity.
Microglia play a critical role in maintaining synaptic plasticity under physiological conditions and in regulating synaptic properties, especially during learning and circuit maturation (98), which particularly occur during postnatal development and early adulthood. However, it is not yet clear whether microglia play a role in the maintenance of synapse properties in the healthy adult CNS. Depletion of microglia in the postnatal CNS or deletion of brain-derived neurotrophic factor (BDNF) in CX3CR1+ cells, which include microglia, resulted in reduced synaptic structural plasticity associated with learning (99). Depletion of microglia in different postnatal phases led to a decrease in both synapse elimination and formation during learning, indicating that microglia play a role in dendritic spine remodeling during motor skill learning. Interestingly, microglia deletion in early adulthood still resulted in reduced synapse elimination and decreased motor learning (99). Therefore, microglia retain the capacity to regulate learning-induced synapse modification in young adult animals. Surprisingly, microglia deletion via administration of the CSF1R inhibitor PLX3397 in adult animals (2 months old) did not result in behavioral or cognitive defects or changes in synaptic properties, even after 3 weeks of treatment (100).
Adult resident microglia are in continuous contact with neurons to monitor their functional status. This interaction and communication between neurons and microglia are maintained by a variety of receptors and ligands such as CX3CR1/fractalkine (CX3CL1) or CD200/CD200R (Figure 2). The detailed interaction network between microglia and neurons and how this network maintains the physiological status of microglia and the appropriate function of the neuronal network are reviewed in detail elsewhere (101–103).
Regulation of adult neurogenesis by microglia.
Adult microglia regulate postnatal neurogenesis in the hippocampus and SVZ. Whereas aging seems to have a negative effect on microglia, resulting in a decrease in neurogenesis, exercise and environmental enrichment physiologically prime microglia to support adult neurogenesis (104–106). Exercise enhances hippocampal microglial release of the chemokine CX3CL1, which protects neuronal precursor cells (106). Additionally, microglia help maintain the hippocampal neurogenic niche in the dentate gyrus via expression of CX3CR1, which mediates the communication and interaction between microglia and neurons in adult mice (103). In several studies, microglial CX3CR1 was also shown to play a major role in supporting and regulating adult neurogenesis. Deficiency in CX3CR1 or interruption of CX3CR1 signaling resulted in an upregulation of proinflammatory signaling in microglia and a dramatic reduction of adult hippocampal neurogenesis (107, 108). Neuronal progenitors continuously proliferate in the subgranular zone of the dentate gyrus, but only a few of them become integrated in functional neuronal circuits; all other cells undergo apoptosis. Ramified microglia in the hippocampus phagocytose apoptotic cells via terminal branches, building characteristic ultrastructural formations that can be described as “ball and chain” structures (109). Because of their role in the maintenance of the hippocampal neurogenic niche during adulthood, microglia are essential components in learning and memory formation. A recent study also showed that microglia serve important functions in phagocytosing apoptotic cells from the neurogenic niche in the SVZ (110). The Tyro3, Axl, and Mertk (TAM) RTKs Mer and Axl expressed on microglial cells were shown to be detrimental for phagoptosis. Phagoptosis is the phagocytosis of neuronal progenitors that are still viable but already upregulated first apoptotic markers leading to recognition by phagocytes. Therefore, microglia regulate cellular density of neuronal progenitors in the SVZ during adult neurogenesis (110).
Mutations affecting microglial function in the adult CNS.
The findings discussed above indicate that microglia play an essential role in maintaining tissue homeostasis in the healthy CNS and therefore the undisturbed function of the neuronal network; however, the entire repertoire of microglial function has not been fully elucidated. Newly developed genetic tools and techniques will help to identify and verify more physiological functions in the future. We would now like to highlight a few examples demonstrating the detrimental role of microglial cells for a physiological and healthy tissue homeostasis in the CNS. The induced deficiency of the microglial signature transcription factor SALL1 in adult microglia resulted in a loss of microglia-specific genes and the expression of genes associated with other tissue macrophages. SALL1 deficiency in microglia and other CNS tissue macrophages resulted in an increase in the number of microglia and a reactive microglia phenotype that was accompanied by a decrease in differentiating doublecortin-positive neuroblasts in the hippocampus (95). Loss of the transcription factor IRF8, which is involved in microglial development, resulted in drastic morphological changes in adult microglia and a maturation defect accompanied by marked changes in the gene expression profile of microglia under physiological conditions (20). Further, IRF8-deficient patients exhibited severe immunodeficiency; however, the impact of IRF8 deficiency on their cognitive function was not studied (111).
Ubiquitin-specific protease 18 (USP18) was recently found to be a critical negative regulator of microglial activation, especially in white matter microglia (112). Loss of USP18 induces an overactivated phenotype in resting microglia and severe CNS pathology. Usp18-deficient microgliopathy resulted from prolonged STAT1 activation and expression of IFN-induced genes in microglial cells. Absence of type 1 IFN receptor α chain (IFNAR1) rescued the hyperactivated microglial phenotype, indicating that there is a tonic IFN signal under physiological conditions in the CNS, as well as an inability of Usp18-deficient microglia to terminate IFN signaling. Therefore, dysregulation of IFN signaling results in a loss of microglial homeostasis in the adult CNS (Figure 2). Notably, the recent identification of Usp18-deficient patients with severe brain abnormalities underscores the essential role of USP18 for normal CNS homeostasis (113).
Microglial interaction with peripheral processes.
Under physiological conditions, microglia are meant to serve as resident immune cells in a highly immune-privileged organ with little contact with the periphery. However, it is now clear that microglia may interact directly or indirectly (through perivascular macrophages and endothelial cells) with the periphery, including with the circulating blood. Microglia are highly sensitive to peripheral metabolic changes, with excess dietary lipids resulting in an immunoreactive phenotype (114). It has also been shown that microglial cells in the hypothalamus exhibit a proinflammatory gene expression profile and activated morphology upon high-fat-diet feeding. Another study demonstrated that microglia are highly dependent on the presence of the gut microbiota and associated metabolites to develop into fully functional mature microglia in the adult CNS (115) (Figure 2). Microglia from germ-free mice or adult mice with depleted gut microbiota showed an immature microglial phenotype with a concomitant impaired immunological response and a drastic change in gene expression (110), as short-chain fatty acids, which are metabolites produced from resident gut microbiota, trigger maturation of adult microglia. These recent data demonstrate an important and unexpected role of the gut microbiota in the maturation and physiological status of microglia in the CNS and suggest studies to further analyze the interaction of the gut microbiota and microglia and the consequences of these interactions for CNS homeostasis.
Summary
The findings summarized in this Review highlight a plethora of novel microglia functions in the CNS under physiological conditions, from the guidance of developing neuronal networks to the maintenance of neuronal circuits in the adult. Unlike a resting immune cell, microglia are responsible for multiple tasks in the physiological CNS to maintain homeostatic function. Furthermore, these cells resemble a dynamic and highly specialized tissue macrophage, equipped with a gene signature imprinted from their yolk sac EMP origin and a highly specialized gene signature acquired upon entry into the CNS. Although we have a good idea where microglial cells originate and when they populate the CNS, it still remains unclear how microglial cells control their cell number and distribution throughout the adult CNS. Based on the recent literature, we can conclude that if microglia cannot develop into their physiological ramified state, the tissue homeostasis of the adult CNS is highly disturbed. During development, microglia are integrated in a complex signaling network and highly adapted to the CNS environment; even small alterations during this time can disturb these cellular interactions and result in dramatic short- or long-term changes. This signaling network of microglial cells with neurons, but also with other neighboring microglial cells, is not fully understood, and we are just beginning to understand the communication and interactions of microglial cells in the healthy CNS. Recent studies have only started to demonstrate the complexity of microglial involvement in neuronal network formation and wiring, and it is still uncertain how many behavioral and psychiatric disorders may result from microglial dysfunction or mutations affecting microglial cells. Therefore, there are still many questions about microglial behavior under steady-state conditions. We believe that all of the findings described in this Review mark only the start of a full understanding of how microglial cells function under steady-state conditions, and that physiological microglial behavior will be uncovered in future studies that employ newly developed tools to study microglia in the healthy CNS.
Acknowledgments
We apologize to colleagues whose work could not be cited because of space constraints. We thank Jessica A. Sharrock for critically reading the manuscript. MP is supported by the Bundesministerium für Bildung und Forschung-funded Competence Network on Multiple Sclerosis (KKNMS), the European Union’s Seventh Framework Program FP7 under grant agreement 607962 (nEUROinflammation), the Sobek-Stiftung and the DFG (SFB 992, SFB1140, SFB/TRR167, Reinhart-Koselleck-Grant), and the Ministry of Science, Research and the Arts, Baden-Wuerttemberg (Sonderlinie “Neuroinflammation”).
Version 1. 07/17/2017
Electronic publication
Version 2. 09/01/2017
Print issue publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information: J Clin Invest. 2017;127(9):3201–3209.https://doi.org/10.1172/JCI90602.
Contributor Information
Katrin Kierdorf, Email: k.kierdorf@imperial.ac.uk.
Marco Prinz, Email: marco.prinz@uniklinik-freiburg.de.
References
- 1.Tang Y, Nyengaard JR, De Groot DM, Gundersen HJ. Total regional and global number of synapses in the human brain neocortex. Synapse. 2001;41(3):258–273. doi: 10.1002/syn.1083. [DOI] [PubMed] [Google Scholar]
- 2.Gage FH. Neurogenesis in the adult brain. J Neurosci. 2002;22(3):612–613. doi: 10.1523/JNEUROSCI.22-03-00612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hagg T. Molecular regulation of adult CNS neurogenesis: an integrated view. Trends Neurosci. 2005;28(11):589–595. doi: 10.1016/j.tins.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- 5.Nottebohm F. Neuronal replacement in adulthood. Ann N Y Acad Sci. 1985;457:143–161. doi: 10.1111/j.1749-6632.1985.tb20803.x. [DOI] [PubMed] [Google Scholar]
- 6.Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci. 2014;15(5):300–312. doi: 10.1038/nrn3722. [DOI] [PubMed] [Google Scholar]
- 7.Prinz M, Erny D, Hagemeyer N. Ontogeny and homeostasis of CNS myeloid cells. Nat Immunol. 2017;18(4):385–392. doi: 10.1038/ni.3703. [DOI] [PubMed] [Google Scholar]
- 8. Virchow R. Die Cellularpathologie in ihrer Begründung auf physiologische und pathologische Gewebelehre. Berlin, Germany(20): August Hirschwald; 1858. [Google Scholar]
- 9.Kettenmann H, Verkhratsky A. Neuroglia: the 150 years after. Trends Neurosci. 2008;31(12):653–659. doi: 10.1016/j.tins.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Somjen GG. Nervenkitt: notes on the history of the concept of neuroglia. Glia. 1988;1(1):2–9. doi: 10.1002/glia.440010103. [DOI] [PubMed] [Google Scholar]
- 11. Del Rio-Hortega P. El “tercer elemento” de los centros nerviosus. I. La microglia en estado normal. II. Intervencion de la microglia en los procesos patologicos (Celulas en bastoncito y cuerpos granuloadiposos). III. Naturaleza probable de la microglia. 1919;9:68–120. [Google Scholar]
- 12. Del Rio-Hortega P. Microglia. In: Wilder P, ed. Cytology and Cellular Pathology of the Nervous System. New York, New York, USA: 1932:483–534. [Google Scholar]
- 13.Rio-Hortega P. The microglia. Lancet. 1939;233(6036):1023–1026. [Google Scholar]
- 14.Carson MJ, Doose JM, Melchior B, Schmid CD, Ploix CC. CNS immune privilege: hiding in plain sight. Immunol Rev. 2006;213:48–65. doi: 10.1111/j.1600-065X.2006.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ginhoux F, Lim S, Hoeffel G, Low D, Huber T. Origin and differentiation of microglia. Front Cell Neurosci. 2013;7:45. doi: 10.3389/fncel.2013.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomez Perdiguero E, Schulz C, Geissmann F. Development and homeostasis of “resident” myeloid cells: the case of the microglia. Glia. 2013;61(1):112–120. doi: 10.1002/glia.22393. [DOI] [PubMed] [Google Scholar]
- 17.Chan WY, Kohsaka S, Rezaie P. The origin and cell lineage of microglia: new concepts. Brain Res Rev. 2007;53(2):344–354. doi: 10.1016/j.brainresrev.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Ginhoux F, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330(6005):841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomez Perdiguero E, et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518(7540):547–551. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hagemeyer N, et al. Transcriptome-based profiling of yolk sac-derived macrophages reveals a role for Irf8 in macrophage maturation. EMBO J. 2016;35(16):1730–1744. doi: 10.15252/embj.201693801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kierdorf K, et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat Neurosci. 2013;16(3):273–280. doi: 10.1038/nn.3318. [DOI] [PubMed] [Google Scholar]
- 22.Alliot F, Godin I, Pessac B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res Dev Brain Res. 1999;117(2):145–152. doi: 10.1016/S0165-3806(99)00113-3. [DOI] [PubMed] [Google Scholar]
- 23.Hoeffel G, et al. C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity. 2015;42(4):665–678. doi: 10.1016/j.immuni.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10(12):1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- 25.Goldmann T, et al. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat Immunol. 2016;17(7):797–805. doi: 10.1038/ni.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mildner A, et al. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci. 2007;10(12):1544–1553. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- 27.Schulz C, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336(6077):86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 28.Hashimoto D, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38(4):792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erblich B, Zhu L, Etgen AM, Dobrenis K, Pollard JW. Absence of colony stimulation factor-1 receptor results in loss of microglia, disrupted brain development and olfactory deficits. PLoS One. 2011;6(10):e26317. doi: 10.1371/journal.pone.0026317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greter M, et al. Stroma-derived interleukin-34 controls the development and maintenance of langerhans cells and the maintenance of microglia. Immunity. 2012;37(6):1050–1060. doi: 10.1016/j.immuni.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, et al. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat Immunol. 2012;13(8):753–760. doi: 10.1038/ni.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blevins G, Fedoroff S. Microglia in colony-stimulating factor 1-deficient op/op mice. J Neurosci Res. 1995;40(4):535–544. doi: 10.1002/jnr.490400412. [DOI] [PubMed] [Google Scholar]
- 33.Kondo Y, Lemere CA, Seabrook TJ. Osteopetrotic (op/op) mice have reduced microglia, no Abeta deposition, and no changes in dopaminergic neurons. J Neuroinflammation. 2007;4:31. doi: 10.1186/1742-2094-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo J, et al. Colony-stimulating factor 1 receptor (CSF1R) signaling in injured neurons facilitates protection and survival. J Exp Med. 2013;210(1):157–172. doi: 10.1084/jem.20120412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butovsky O, et al. Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nat Neurosci. 2014;17(1):131–143. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pont-Lezica L, Béchade C, Belarif-Cantaut Y, Pascual O, Bessis A. Physiological roles of microglia during development. J Neurochem. 2011;119(5):901–908. doi: 10.1111/j.1471-4159.2011.07504.x. [DOI] [PubMed] [Google Scholar]
- 37.Matcovitch-Natan O, et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science. 2016;353(6301):aad8670. doi: 10.1126/science.aad8670. [DOI] [PubMed] [Google Scholar]
- 38.Martynoga B, Drechsel D, Guillemot F. Molecular control of neurogenesis: a view from the mammalian cerebral cortex. Cold Spring Harb Perspect Biol. 2012;4(10):a008359. doi: 10.1101/cshperspect.a008359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rigato C, Buckinx R, Le-Corronc H, Rigo JM, Legendre P. Pattern of invasion of the embryonic mouse spinal cord by microglial cells at the time of the onset of functional neuronal networks. Glia. 2011;59(4):675–695. doi: 10.1002/glia.21140. [DOI] [PubMed] [Google Scholar]
- 40.Nandi S, et al. The CSF-1 receptor ligands IL-34 and CSF-1 exhibit distinct developmental brain expression patterns and regulate neural progenitor cell maintenance and maturation. Dev Biol. 2012;367(2):100–113. doi: 10.1016/j.ydbio.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dekkers MP, Barde YA. Developmental biology. Programmed cell death in neuronal development. Science. 2013;340(6128):39–41. doi: 10.1126/science.1236152. [DOI] [PubMed] [Google Scholar]
- 42.Dekkers MP, Nikoletopoulou V, Barde YA. Cell biology in neuroscience: Death of developing neurons: new insights and implications for connectivity. J Cell Biol. 2013;203(3):385–393. doi: 10.1083/jcb.201306136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ashwell K. Microglia and cell death in the developing mouse cerebellum. Brain Res Dev Brain Res. 1990;55(2):219–230. doi: 10.1016/0165-3806(90)90203-B. [DOI] [PubMed] [Google Scholar]
- 44.Brockhaus J, Möller T, Kettenmann H. Phagocytozing ameboid microglial cells studied in a mouse corpus callosum slice preparation. Glia. 1996;16(1):81–90. doi: 10.1002/(SICI)1098-1136(199601)16:1<81::AID-GLIA9>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 45.Witting A, Müller P, Herrmann A, Kettenmann H, Nolte C. Phagocytic clearance of apoptotic neurons by microglia/brain macrophages in vitro: involvement of lectin-, integrin-, and phosphatidylserine-mediated recognition. J Neurochem. 2000;75(3):1060–1070. doi: 10.1046/j.1471-4159.2000.0751060.x. [DOI] [PubMed] [Google Scholar]
- 46.Marín-Teva JL, Dusart I, Colin C, Gervais A, van Rooijen N, Mallat M. Microglia promote the death of developing Purkinje cells. Neuron. 2004;41(4):535–547. doi: 10.1016/S0896-6273(04)00069-8. [DOI] [PubMed] [Google Scholar]
- 47.Peri F, Nüsslein-Volhard C. Live imaging of neuronal degradation by microglia reveals a role for v0-ATPase a1 in phagosomal fusion in vivo. Cell. 2008;133(5):916–927. doi: 10.1016/j.cell.2008.04.037. [DOI] [PubMed] [Google Scholar]
- 48.Wakselman S, Béchade C, Roumier A, Bernard D, Triller A, Bessis A. Developmental neuronal death in hippocampus requires the microglial CD11b integrin and DAP12 immunoreceptor. J Neurosci. 2008;28(32):8138–8143. doi: 10.1523/JNEUROSCI.1006-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takahashi K, Rochford CD, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med. 2005;201(4):647–657. doi: 10.1084/jem.20041611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takahashi K, Prinz M, Stagi M, Chechneva O, Neumann H. TREM2-transduced myeloid precursors mediate nervous tissue debris clearance and facilitate recovery in an animal model of multiple sclerosis. PLoS Med. 2007;4(4):e124. doi: 10.1371/journal.pmed.0040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ueno M, et al. Layer V cortical neurons require microglial support for survival during postnatal development. Nat Neurosci. 2013;16(5):543–551. doi: 10.1038/nn.3358. [DOI] [PubMed] [Google Scholar]
- 52.Frade JM, Barde YA. Microglia-derived nerve growth factor causes cell death in the developing retina. Neuron. 1998;20(1):35–41. doi: 10.1016/S0896-6273(00)80432-8. [DOI] [PubMed] [Google Scholar]
- 53.Cunningham CL, Martínez-Cerdeño V, Noctor SC. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J Neurosci. 2013;33(10):4216–4233. doi: 10.1523/JNEUROSCI.3441-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tronnes AA, Koschnitzky J, Daza R, Hitti J, Ramirez JM, Hevner R. Effects of lipopolysaccharide and progesterone exposures on embryonic cerebral cortex development in mice. Reprod Sci. 2016;23(6):771–778. doi: 10.1177/1933719115618273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Antony JM, Paquin A, Nutt SL, Kaplan DR, Miller FD. Endogenous microglia regulate development of embryonic cortical precursor cells. J Neurosci Res. 2011;89(3):286–298. doi: 10.1002/jnr.22533. [DOI] [PubMed] [Google Scholar]
- 56.Risau W. Development and differentiation of endothelium. Kidney Int Suppl. 1998;67:S3–S6. doi: 10.1046/j.1523-1755.1998.06701.x. [DOI] [PubMed] [Google Scholar]
- 57.Fantin A, et al. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116(5):829–840. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kubota Y, et al. M-CSF inhibition selectively targets pathological angiogenesis and lymphangiogenesis. J Exp Med. 2009;206(5):1089–1102. doi: 10.1084/jem.20081605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Checchin D, Sennlaub F, Levavasseur E, Leduc M, Chemtob S. Potential role of microglia in retinal blood vessel formation. Invest Ophthalmol Vis Sci. 2006;47(8):3595–3602. doi: 10.1167/iovs.05-1522. [DOI] [PubMed] [Google Scholar]
- 60.Rymo SF, Gerhardt H, Wolfhagen Sand F, Lang R, Uv A, Betsholtz C. A two-way communication between microglial cells and angiogenic sprouts regulates angiogenesis in aortic ring cultures. PLoS One. 2011;6(1):e15846. doi: 10.1371/journal.pone.0015846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davalos D, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8(6):752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 62.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 63.Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29(13):3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eyo UB, Peng J, Swiatkowski P, Mukherjee A, Bispo A, Wu LJ. Neuronal hyperactivity recruits microglial processes via neuronal NMDA receptors and microglial P2Y12 receptors after status epilepticus. J Neurosci. 2014;34(32):10528–10540. doi: 10.1523/JNEUROSCI.0416-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li Y, Du XF, Liu CS, Wen ZL, Du JL. Reciprocal regulation between resting microglial dynamics and neuronal activity in vivo. Dev Cell. 2012;23(6):1189–1202. doi: 10.1016/j.devcel.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 66.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274(5290):1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 67.Tremblay MÈ, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010;8(11):e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Paolicelli RC, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333(6048):1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 69.Schafer DP, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74(4):691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stevens B, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131(6):1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 71.Allen NJ, Barres BA. Signaling between glia and neurons: focus on synaptic plasticity. Curr Opin Neurobiol. 2005;15(5):542–548. doi: 10.1016/j.conb.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 72.Stettler DD, Yamahachi H, Li W, Denk W, Gilbert CD. Axons and synaptic boutons are highly dynamic in adult visual cortex. Neuron. 2006;49(6):877–887. doi: 10.1016/j.neuron.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 73.Tasdemir-Yilmaz OE, Freeman MR. Astrocytes engage unique molecular programs to engulf pruned neuronal debris from distinct subsets of neurons. Genes Dev. 2014;28(1):20–33. doi: 10.1101/gad.229518.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marín O. Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci. 2012;13(2):107–120. doi: 10.1038/nrn3155. [DOI] [PubMed] [Google Scholar]
- 75.Schafer DP, Lehrman EK, Stevens B. The “quad-partite” synapse: microglia-synapse interactions in the developing and mature CNS. Glia. 2013;61(1):24–36. doi: 10.1002/glia.22389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Derecki NC, et al. Wild-type microglia arrest pathology in a mouse model of Rett syndrome. Nature. 2012;484(7392):105–109. doi: 10.1038/nature10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schafer DP, et al. Microglia contribute to circuit defects in Mecp2 null mice independent of microglia-specific loss of Mecp2 expression. Elife. 2016;5:e15224. doi: 10.7554/eLife.15224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang J, et al. Wild-type microglia do not reverse pathology in mouse models of Rett syndrome. Nature. 2015;521(7552):E1–E4. doi: 10.1038/nature14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Greer JM, Capecchi MR. Hoxb8 is required for normal grooming behavior in mice. Neuron. 2002;33(1):23–34. doi: 10.1016/S0896-6273(01)00564-5. [DOI] [PubMed] [Google Scholar]
- 80.Holstege JC, et al. Loss of Hoxb8 alters spinal dorsal laminae and sensory responses in mice. Proc Natl Acad Sci U S A. 2008;105(17):6338–6343. doi: 10.1073/pnas.0802176105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen SK, et al. Hematopoietic origin of pathological grooming in Hoxb8 mutant mice. Cell. 2010;141(5):775–785. doi: 10.1016/j.cell.2010.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39(1):151–170. doi: 10.1016/0306-4522(90)90229-W. [DOI] [PubMed] [Google Scholar]
- 83.Perry VH, Hume DA, Gordon S. Immunohisto chemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience. 1985;15(2):313–326. doi: 10.1016/0306-4522(85)90215-5. [DOI] [PubMed] [Google Scholar]
- 84.Lawson LJ, Perry VH, Gordon S. Turnover of resident microglia in the normal adult mouse brain. Neuroscience. 1992;48(2):405–415. doi: 10.1016/0306-4522(92)90500-2. [DOI] [PubMed] [Google Scholar]
- 85.Askew K, et al. Coupled proliferation and apoptosis maintain the rapid turnover of microglia in the adult brain. Cell Rep. 2017;18(2):391–405. doi: 10.1016/j.celrep.2016.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tay TL, et al. A new fate mapping system reveals context-dependent random or clonal expansion of microglia. Nat Neurosci. 2017;20(6):793–803. doi: 10.1038/nn.4547. [DOI] [PubMed] [Google Scholar]
- 87.Perry VH, Gordon S. Macrophages and microglia in the nervous system. Trends Neurosci. 1988;11(6):273–277. doi: 10.1016/0166-2236(88)90110-5. [DOI] [PubMed] [Google Scholar]
- 88.Perry VH, Gordon S. Macrophages and the nervous system. Int Rev Cytol. 1991;125:203–244. doi: 10.1016/s0074-7696(08)61220-6. [DOI] [PubMed] [Google Scholar]
- 89.Prinz M, Priller J. The role of peripheral immune cells in the CNS in steady state and disease. Nat Neurosci. 2017;20(2):136–144. doi: 10.1038/nn.4475. [DOI] [PubMed] [Google Scholar]
- 90.Stence N, Waite M, Dailey ME. Dynamics of microglial activation: a confocal time-lapse analysis in hippocampal slices. Glia. 2001;33(3):256–266. [PubMed] [Google Scholar]
- 91.Glenn JA, Booth PL, Thomas WE. Pinocytotic activity in ramified microglia. Neurosci Lett. 1991;123(1):27–31. doi: 10.1016/0304-3940(91)90150-R. [DOI] [PubMed] [Google Scholar]
- 92.Booth PL, Thomas WE. Evidence for motility and pinocytosis in ramified microglia in tissue culture. Brain Res. 1991;548(1–2):163–171. doi: 10.1016/0006-8993(91)91118-k. [DOI] [PubMed] [Google Scholar]
- 93.Gautier EL, et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13(11):1118–1128. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bennett ML, et al. New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci U S A. 2016;113(12):E1738–E1746. doi: 10.1073/pnas.1525528113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Buttgereit A, et al. Sall1 is a transcriptional regulator defining microglia identity and function. Nat Immunol. 2016;17(12):1397–1406. doi: 10.1038/ni.3585. [DOI] [PubMed] [Google Scholar]
- 96.Koso H, et al. Conditional rod photoreceptor ablation reveals Sall1 as a microglial marker and regulator of microglial morphology in the retina. Glia. 2016;64(11):2005–2024. doi: 10.1002/glia.23038. [DOI] [PubMed] [Google Scholar]
- 97.Lavin Y, et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159(6):1312–1326. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tremblay MÈ, Stevens B, Sierra A, Wake H, Bessis A, Nimmerjahn A. The role of microglia in the healthy brain. J Neurosci. 2011;31(45):16064–16069. doi: 10.1523/JNEUROSCI.4158-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Parkhurst CN, et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155(7):1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Elmore MR, et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron. 2014;82(2):380–397. doi: 10.1016/j.neuron.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Biber K, Neumann H, Inoue K, Boddeke HW. Neuronal ‘On’ and ‘Off’ signals control microglia. Trends Neurosci. 2007;30(11):596–602. doi: 10.1016/j.tins.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 102.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10(11):1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 103.Kierdorf K, Prinz M. Factors regulating microglia activation. Front Cell Neurosci. 2013;7:44. doi: 10.3389/fncel.2013.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Choi SH, et al. Non-cell-autonomous effects of presenilin 1 variants on enrichment-mediated hippocampal progenitor cell proliferation and differentiation. Neuron. 2008;59(4):568–580. doi: 10.1016/j.neuron.2008.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gebara E, Sultan S, Kocher-Braissant J, Toni N. Adult hippocampal neurogenesis inversely correlates with microglia in conditions of voluntary running and aging. Front Neurosci. 2013;7:145. doi: 10.3389/fnins.2013.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vukovic J, Colditz MJ, Blackmore DG, Ruitenberg MJ, Bartlett PF. Microglia modulate hippocampal neural precursor activity in response to exercise and aging. J Neurosci. 2012;32(19):6435–6443. doi: 10.1523/JNEUROSCI.5925-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bachstetter AD, et al. Fractalkine and CX 3 CR1 regulate hippocampal neurogenesis in adult and aged rats. Neurobiol Aging. 2011;32(11):2030–2044. doi: 10.1016/j.neurobiolaging.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sellner S, et al. Microglial CX3CR1 promotes adult neurogenesis by inhibiting Sirt 1/p65 signaling independent of CX3CL1. Acta Neuropathol Commun. 2016;4(1):102. doi: 10.1186/s40478-016-0374-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sierra A, et al. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7(4):483–495. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fourgeaud L, et al. TAM receptors regulate multiple features of microglial physiology. Nature. 2016;532(7598):240–244. doi: 10.1038/nature17630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hambleton S, et al. IRF8 mutations and human dendritic-cell immunodeficiency. N Engl J Med. 2011;365(2):127–138. doi: 10.1056/NEJMoa1100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Goldmann T, et al. USP18 lack in microglia causes destructive interferonopathy of the mouse brain. EMBO J. 2015;34(12):1612–1629. doi: 10.15252/embj.201490791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Meuwissen ME, et al. Human USP18 deficiency underlies type 1 interferonopathy leading to severe pseudo-TORCH syndrome. J Exp Med. 2016;213(7):1163–1174. doi: 10.1084/jem.20151529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Baufeld C, Osterloh A, Prokop S, Miller KR, Heppner FL. High-fat diet-induced brain region-specific phenotypic spectrum of CNS resident microglia. Acta Neuropathol. 2016;132(3):361–375. doi: 10.1007/s00401-016-1595-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Erny D, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18(7):965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]