Abstract
Atypical antipsychotics such as olanzapine often induce excessive weight gain and type 2 diabetes. However, the mechanisms underlying these drug-induced metabolic perturbations remain poorly understood. Here, we used an experimental model that reproduces olanzapine-induced hyperphagia and obesity in female C57BL/6 mice. We found that olanzapine treatment acutely increased food intake, impaired glucose tolerance, and altered physical activity and energy expenditure in mice. Furthermore, olanzapine-induced hyperphagia and weight gain were blunted in mice lacking the serotonin 2C receptor (HTR2C). Finally, we showed that treatment with the HTR2C-specific agonist lorcaserin suppressed olanzapine-induced hyperphagia and weight gain. Lorcaserin treatment also improved glucose tolerance in olanzapine-fed mice. Collectively, our studies suggest that olanzapine exerts some of its untoward metabolic effects via antagonism of HTR2C.
Keywords: Endocrinology, Metabolism
Keywords: Diabetes, Obesity
Introduction
Atypical antipsychotics (AATPs) are second-generation antipsychotics that are currently used to treat a variety of psychiatric conditions, including schizophrenia, bipolar disorder, depression, and autism (1). Despite their documented efficacy and low risks for extrapyramidal symptoms, most AATPs have been linked to drug-induced metabolic side effects, including excessive weight gain, dyslipidemia, and type 2 diabetes (2). Notably, schizophrenic patients have a reduced life span, with obesity-related metabolic syndrome being the leading cause of death (2). Moreover, the incidence of diabetes among AATP users is 4 times higher than in age-, race-, and sex-matched controls (3). While morbid obesity and type 2 diabetes typically take years to unfold in the general population, these conditions manifest acutely (within months) following AATP treatment (4). The rapid disease onset suggests a distinct etiology underlying AATP-induced metabolic syndrome that remains poorly understood.
AATPs bind to multiple monoamine receptors in the brain (5). The beneficial psychotropic effects are thought to be mediated primarily by a combinatorial blockade of serotonin 2a and dopamine D2 receptors (6). However, the molecular mechanisms that underlie AATPs’ untoward metabolic effects remain largely unknown. Both genome-wide association and candidate studies in human patients have suggested the involvement of multiple genes, including those that encode the histamine, α-adrenergic, and serotonin (5-HT) receptors (7–9). Among them, Htr2c encodes the 5-HT 2C receptor, which acts in the brain to regulate food intake, body weight, and glucose metabolism (10, 11). Blockade of HTR2C signaling in mice leads to hyperphagia and obesity (12) that resemble AATP-induced metabolic symptoms in humans. Many AATPs, including olanzapine, antagonize HTR2C (13), raising the possibility that Htr2c antagonism contributes to AATP-induced metabolic syndrome (14, 15). However, previous efforts to test this hypothesis using genetic models have been hindered by the difficulty of replicating AATP-induced metabolic perturbations in mice (16). Although it is one of the most commonly prescribed and effective AATPs, olanzapine causes the most weight gain and metabolic impairments in humans (17). To characterize its metabolic effects, we adopted an olanzapine-supplemented diet to reproduce human plasma olanzapine levels in female C57BL/6 mice (18). In the current study, we investigated the role of Htr2c in olazanpine-induced metabolic impairments in mice. We also assessed whether an HTR2C-specific agonist alleviates olanzapine’s untoward metabolic effects.
Results and Discussion
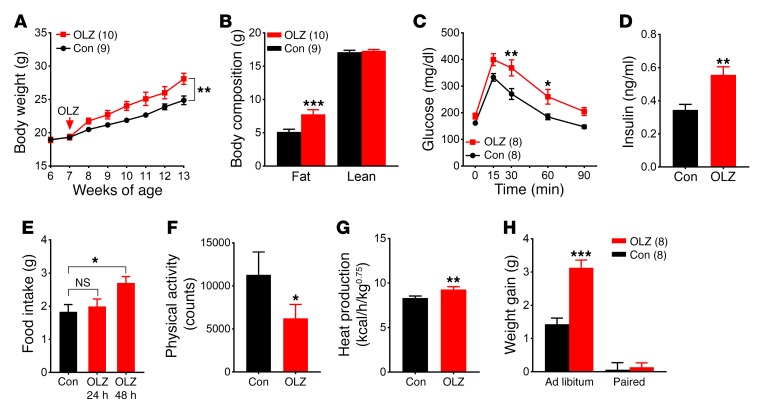
We found that chronic olanzapine exposure of female C57BL/6 mice resulted in excessive weight gain over a 6-week period (Figure 1A). Nuclear magnetic resonance (NMR) analysis revealed an increase in fat mass, but not lean mass, in olanzapine-fed mice (Figure 1B). In addition to causing obesity, chronic olanzapine treatment impaired glucose tolerance (Figure 1C). Moreover, fasting plasma insulin levels were significantly higher in olanzapine-fed mice (Figure 1D). To further characterize olanzapine’s acute effects on energy balance, we assessed another cohort of mice in a TSE Systems metabolic chamber system in which food intake, energy expenditure, and physical activity were monitored for a total of 6 days. Mice were fed a control diet (D09092903; Research Diets Inc., 45 kcal% fat, 35 kcal% carbohydrate, 15 kcal% protein) during acclimation and on the first 3 days in the metabolic cages. Mice were then switched to the olanzapine diet (50 mg olanzapine compounded into 1 kg of the control diet, Research Diets Inc.) for the next 3 days. Olanzapine is associated with food craving and binge eating in humans (19). We found an increase in food consumption during the dark phase that developed within 48 hours of olanzapine exposure and persisted for the remainder of the experiment (Figure 1E and Supplemental Figure 1A; supplemental material available online with this article; https://doi.org/10.1172/JCI93362DS1). In addition to causing hyperphagia, olanzapine has a sedative effect that is thought to contribute to weight gain in humans (20). We found a reduction in physical activity immediately following the dietary switch (Figure 1F). Furthermore, indirect calorimetry analysis revealed an unexpected increase in parameters of energy expenditure, including heat production (Figure 1G), oxygen (O2) consumption, and carbon dioxide (CO2) production following olanzapine exposure (Supplemental Figure 1, B and C). The respiratory exchange ratio remained constant before and after olanzapine treatment (Supplemental Figure 1D). Collectively, our data demonstrate that olanzapine alters food intake and energy homeostasis and that olanzapine treatment in female C57BL/6 mice recapitulates key symptoms of olanzapine-induced metabolic syndrome. Similar analyses in male C57BL/6 mice found that olanzapine treatment led to a similar increase in energy expenditure and a decrease in physical activity. However, olanzapine-induced hyperphagia was less prominent in male compared with female mice. As a result, body weight gain during 6 weeks of olanzapine treatment was less pronounced in C57BL/6 males than in females (Supplemental Figure 2). We tested whether hyperphagia was a primary contributor to weight gain using a pair-feeding paradigm. When fed ab libitum, mice on the olanzapine diet developed hyperphagia (Figure 1E) and gained significantly more weight than those fed the control diet during a 7-day period (Supplemental Figure 1E). In the pair-fed group, hyperphagia was prevented by restricting olanzapine-fed mice to the same amount of food consumed by those fed the control diet. We found that weight gain in the pair-fed olanzapine mice was similar to that in control mice during the same period, suggesting that hyperphagia is required for olanzapine-induced weight gain (Figure 1H).
Figure 1. Olanzapine treatment profoundly alters energy homeostasis in female C57BL/6 mice.
(A) Body weight. (B) Body composition. (C) GTT. (D) Plasma insulin levels. (E–G) Metabolic cage analysis (n = 6) of food intake (E), physical activity (F), and heat production (G). (H) Weight gain in ad libitum– and pair-fed mice. Results are shown as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001 versus other genotypes assessed using either Student’s t test or 2-way ANOVA with Sidak’s multiple comparisons test. OLZ, olanzapine treated; Con, control.
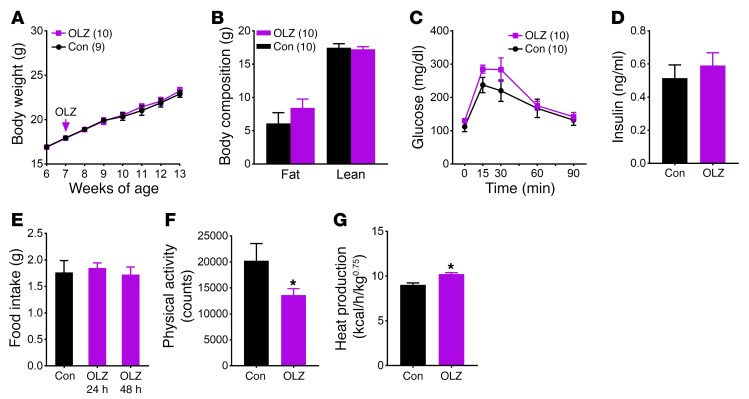
The olanzapine-induced hyperphagia and weight gain allowed us to use genetically modified mice to investigate candidate genes and pathways that underlie these metabolic perturbations. To determine whether olanzapine acts on Htr2c to affect energy balance, we used mice lacking Htr2c (Htr2c-null mice, maintained on a C57BL/6 background). Notably, we found that olanzapine’s effect on weight gain was significantly blunted in Htr2c-null mice (Figure 2A). In contrast with WT mice, olanzapine-fed Htr2c-null mice had body weight and body composition comparable to those fed the control diet (Figure 2, A and B). Furthermore, olanzapine treatment did not significantly alter glucose tolerance or fasting plasma insulin levels in Htr2c-null mice (Figure 2, C and D). We next repeated the metabolic cage analysis in Htr2c-null mice and found that hyperphagia did not develop in Htr2c-null mice following acute olanzapine exposure (Figure 2E and Supplemental Figure 3A). In contrast, olanzapine’s effects on physical activity and energy expenditure persisted in these mice. Similarly to WT mice, Htr2c-null mice exhibited reduced activity (Figure 2F) and an increase in heat production (Figure 2G), O2 consumption, and CO2 production (Supplemental Figure 3, B–D) after the dietary switch. Collectively, our findings suggest that olanzapine’s effects on food intake and weight gain require Htr2c, whereas its effects on physical activity and energy expenditure likely involve additional receptors. Consistent with this observation, Chee and colleagues recently reported that melanin-concentrating hormone (MCH) is necessary for olanzapine suppression of locomotor activity (21).
Figure 2. Olanzapine’s effect on food intake and body weight is mediated by HTR2C.
(A) Body weight. (B) Body composition. (C) GTT. (D) Plasma insulin levels. (E–G) Metabolic cage analysis (n = 6) of food intake (E), physical activity (F), and heat production (G). Results are shown as the mean ± SEM. *P < 0.05 versus other genotypes assessed using either Student’s t test or 2-way ANOVA with Sidak’s multiple comparisons test.
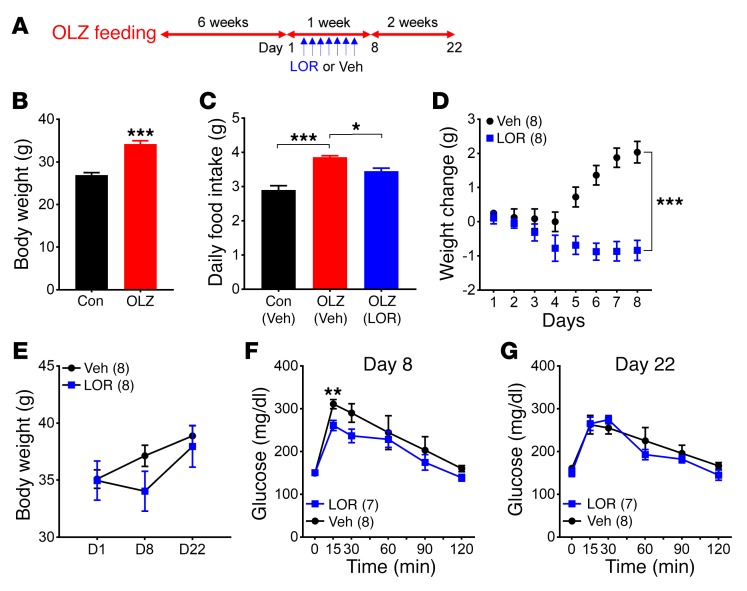
Our finding that Htr2c antagonism contributes to olanzapine-induced hyperphagia and weight gain raises the possibility that agonists for HTR2C may be useful in alleviating these untoward effects. To this end, we fed female C57BL/6 mice the olanzapine diet for 6 weeks. These mice became obese compared with those fed the control diet (Figure 3B). We then treated mice with lorcaserin, an FDA-approved specific HTR2C agonist, or a vehicle for 7 days while still feeding the olanzapine diet (Figure 3A). We used an anorexigenic dose (10 mg/kg) that suppressed food intake in overnight-fasted mice (Supplemental Figure 4). We measured food intake and body weight daily during the treatment as well as 2 weeks after the last dose of lorcaserin or vehicle (Figure 3A). Notably, hyperphagia was evident in olanzapine-fed mice, but was attenuated by lorcaserin treatment (Figure 3C). Consistent with the reduction in food intake, we found that lorcaserin acutely suppressed weight gain in olanzapine-fed mice (Figure 3D). Importantly, we observed a significant improvement in glucose tolerance (day 8, Figure 3F). Nevertheless, the attenuation of weight gain and improvement in glucose tolerance did not persist after cessation of lorcaserin treatment (Figure 3, E and G, day 22).
Figure 3. Lorcaserin treatment suppresses olanzapine-induced hyperphagia and weight gain.
(A) Schematic of the experiment design. Female C57BL/6 mice (n = 16) were initially fed the olanzapine diet for 6 weeks so that they gained significantly more weight than those fed the control diet (n = 8). They were then separated into 2 groups with equal numbers and treated with either vehicle (VEH) or lorcaserin (LOR) for 7 days. Following drug treatment, mice were fed the olanzapine diet for 2 more weeks. (B) Body weight. (C) Daily food intake. (D) Weight gain during vehicle or lorcaserin treatment. (E) Body weight before and after drug treatment. (F and G) GTTs. Results are shown as the mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001 versus other genotypes assessed using either Student’s t test or 2-way ANOVA with Sidak’s multiple comparisons test.
In summary, our findings support the model that olanzapine acts on multiple receptors and pathways to alter different aspects of energy homeostasis. Notably, our data suggest that olanzapine-induced hyperphagia is the primary cause of weight gain in mice and that olanzapine’s effect on food intake is mediated by Htr2c. Furthermore, we demonstrate that olanzapine-induced hyperphagia is reduced by a HTR2C-specific agonist treatment in olanzapine-fed mice, accompanied by an improvement in glucose homeostasis. Currently, there is no medication specifically targeting AATP-induced metabolic syndrome. Moreover, existing antiobesity and antidiabetic medications only provide limited relief (22, 23). Therefore, our findings suggest that available HTR2C-specific agonists, such as lorcaserin, may be useful in preventing some of the metabolic side effects associated with the use of olanzapine and other AATPs.
Methods
All experiments were repeated with at least 2 independent cohorts of mice.
Mice.
All mice were housed in a temperature-controlled room with a 12-hour light/12-hour dark cycle (lights on at 06:00 am, lights off at 6:00 pm) in the animal facility of the UT Southwestern Medical Center. Mice were provided standard chow (no. 2016; Harlan Teklad) as well as water ad libitum unless otherwise noted. C57BL/6 mice were purchased from the UT Southwestern Rodent Breeding Core Facility. Htr2c-null mice were maintained on a C57BL/6 background. Htr2c-null mice were generated by inserting a transcriptional blocker cassette into the endogenous Htr2c allele. These mice are phenotypically identical to conventional Htr2c-null mice, as we previously described (11).
Body weight and composition.
Body weight was monitored weekly between weaning (at 4 weeks) and up to 20 weeks of age. Body composition was assessed using the Bruker Minispec mq10 NMR analyzer.
Metabolic cage studies.
These studies were conducted in the UT Southwestern Metabolic Phenotyping Core Facility. Data for food intake, energy expenditure, and physical activities were collected using a combined indirect calorimetry system (TSE Systems). Experimental animals were individually acclimated in the metabolic chambers for 5 days before data collection. During data collection, mice were initially maintained on the control diet for the first 3 days and switched to the olanzapine diet for the next 3 days in the metabolic cages.
Glucose tolerance test, glucose, and plasma insulin levels.
For glucose tolerance tests (GTTs), mice were fasted for 6 hours with water provided ad libitum from 9 am on the experimental day. Baseline glucose and insulin levels were collected 2 hours prior to GTT. During GTT, blood glucose levels were monitored at 15, 30, 60, 90, and 120 minutes after an i.p. dose of glucose (1.0 g/kg body weight). Blood glucose was analyzed using a Contour Glucometer (Bayer Pharma AG). Plasma insulin levels were measured using an ELISA kit designed for use in mice (Crystal Chem Inc.).
Feeding studies.
For the refeeding experiment, mice were fasted for 18 hours (from 3 pm to 9 am) before being given an i.p. dose of saline or lorcaserin (MedChem Express, HY-153685, 5 or 10 mg/kg dissolved in saline). Thirty minutes after the injection, a chow pellet was given to singly housed mice. Food consumption was monitored at 30, 60, 120, and 240 minutes. For the pair-feeding experiment, 30 mice were individually housed and fed the control diet for a week. Food intake was monitored to calculate the average daily intake for each mouse. Sixteen mice that had similar amounts of daily intake (~2.9 g of the control diet) were used for the pair-feeding experiment. Mice were randomized into 2 groups with equal numbers and body weight. Mice in the control group continued to be fed the control diet, whereas those in the olanzapine group were fed the olanzapine diet for the next 7 days. In both groups, individual mice were fed the exact same amount of daily intake they consumed in the previous week. The other 14 mice were used as ad libitum controls. They were randomized into 2 groups and fed either the control diet or the olanzapine diet for 7 days. All mice remained individually housed during the pair-feeding period.
Statistics.
Data were analyzed with either Student’s t test or 2-way repeated-measures ANOVA, as appropriate, followed by Sidak’s multiple comparisons test using Prism 7.0 (GraphPad). Statistical significance was established at P < 0.05.
Study approval.
All experimental procedures were approved by the IACUC at UT Southwestern Medical Center (animal protocol no. 2015-101099; principal investigator, C. Liu).
Author contributions
CCL, JKE, and CL designed the experiments. CCL, SCW, RW, CMC, NA, DM, and CL collected data. CCL, SCW, JKE, and CL analyzed the data. CCL, SL, JKE, and CL wrote the manuscript.
Supplementary Material
Acknowledgments
The authors would like to thank the staff of the UT Southwestern Metabolic Phenotyping Core. We thank the NIH for its support (R01 DK088423 and R37 DK053301 to JKE; R01 DK114036 to CL; T32 DK007307 to SCW; and F32 DK103449 to CCL). We thank the American Heart Association for its support (16BGIA27260023 and 16SDG27260001 to CL). This work was also supported by a research start-up fund from the UT Southwestern Medical Center (to CL).
Version 1. 08/14/2017
Electronic publication
Version 2. 09/01/2017
Print issue publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information: J Clin Invest. 2017;127(9):3402–3406.https://doi.org/10.1172/JCI93362.
Contributor Information
Rong Wan, Email: rong.wan@utsouthwestern.edu.
Newaz Ahmed, Email: newaz.ahmed@utsouthwestern.edu.
Dias Mathew, Email: rennymathew60@gmail.com.
Syann Lee, Email: syann.lee@utsouthwestern.edu.
Chen Liu, Email: Chen.Liu@UTSouthwestern.edu.
Joel K. Elmquist, Email: joel.elmquist@utsouthwestern.edu.
References
- 1.Keck PE, McElroy SL, Strakowski SM, Soutullo CA. Antipsychotics in the treatment of mood disorders and risk of tardive dyskinesia. J Clin Psychiatry. 2000;61(Suppl 4):33–38. [PubMed] [Google Scholar]
- 2.Newcomer JW. Antipsychotic medications: metabolic and cardiovascular risk. J Clin Psychiatry. 2007;68(Suppl 4):8–13. [PubMed] [Google Scholar]
- 3.Goff DC, et al. A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophr Res. 2005;80(1):45–53. doi: 10.1016/j.schres.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Rojo LE, et al. Metabolic syndrome and obesity among users of second generation antipsychotics: A global challenge for modern psychopharmacology. Pharmacol Res. 2015;101:74–85. doi: 10.1016/j.phrs.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 5.Bymaster FP, et al. Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology. 1996;14(2):87–96. doi: 10.1016/0893-133X(94)00129-N. [DOI] [PubMed] [Google Scholar]
- 6.Meltzer HY, Huang M. In vivo actions of atypical antipsychotic drug on serotonergic and dopaminergic systems. Prog Brain Res. 2008;172:177–197. doi: 10.1016/S0079-6123(08)00909-6. [DOI] [PubMed] [Google Scholar]
- 7.Kim SF, Huang AS, Snowman AM, Teuscher C, Snyder SH. From the Cover: Antipsychotic drug-induced weight gain mediated by histamine H1 receptor-linked activation of hypothalamic AMP-kinase. Proc Natl Acad Sci U S A. 2007;104(9):3456–3459. doi: 10.1073/pnas.0611417104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellingrod VL, et al. Weight gain associated with the -759C/T polymorphism of the 5HT2C receptor and olanzapine. Am J Med Genet B Neuropsychiatr Genet. 2005;134B(1):76–78. doi: 10.1002/ajmg.b.20169. [DOI] [PubMed] [Google Scholar]
- 9.Kroeze WK, et al. H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology. 2003;28(3):519–526. doi: 10.1038/sj.npp.1300027. [DOI] [PubMed] [Google Scholar]
- 10.Berglund ED, et al. Serotonin 2C receptors in pro-opiomelanocortin neurons regulate energy and glucose homeostasis. J Clin Invest. 2013;123(12):5061–5070. doi: 10.1172/JCI70338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Y, et al. 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate energy homeostasis. Neuron. 2008;60(4):582–589. doi: 10.1016/j.neuron.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tecott LH, et al. Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature. 1995;374(6522):542–546. doi: 10.1038/374542a0. [DOI] [PubMed] [Google Scholar]
- 13.Bymaster FP, et al. Antagonism by olanzapine of dopamine D1, serotonin2, muscarinic, histamine H1 and alpha 1-adrenergic receptors in vitro. Schizophr Res. 1999;37(1):107–122. doi: 10.1016/S0920-9964(98)00146-7. [DOI] [PubMed] [Google Scholar]
- 14.Kirk SL, Glazebrook J, Grayson B, Neill JC, Reynolds GP. Olanzapine-induced weight gain in the rat: role of 5-HT2C and histamine H1 receptors. Psychopharmacology (Berl) 2009;207(1):119–125. doi: 10.1007/s00213-009-1639-8. [DOI] [PubMed] [Google Scholar]
- 15.Meltzer HY, Roth BL. Lorcaserin and pimavanserin: emerging selectivity of serotonin receptor subtype-targeted drugs. J Clin Invest. 2013;123(12):4986–4991. doi: 10.1172/JCI70678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper GD, Pickavance LC, Wilding JP, Harrold JA, Halford JC, Goudie AJ. Effects of olanzapine in male rats: enhanced adiposity in the absence of hyperphagia, weight gain or metabolic abnormalities. J Psychopharmacol. 2007;21(4):405–413. doi: 10.1177/0269881106069637. [DOI] [PubMed] [Google Scholar]
- 17.Lett TA, Wallace TJ, Chowdhury NI, Tiwari AK, Kennedy JL, Müller DJ. Pharmacogenetics of antipsychotic-induced weight gain: review and clinical implications. Mol Psychiatry. 2012;17(3):242–266. doi: 10.1038/mp.2011.109. [DOI] [PubMed] [Google Scholar]
- 18.Morgan AP, et al. The antipsychotic olanzapine interacts with the gut microbiome to cause weight gain in mouse. PLoS ONE. 2014;9(12):e115225. doi: 10.1371/journal.pone.0115225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kluge M, et al. Clozapine and olanzapine are associated with food craving and binge eating: results from a randomized double-blind study. J Clin Psychopharmacol. 2007;27(6):662–666. doi: 10.1097/jcp.0b013e31815a8872. [DOI] [PubMed] [Google Scholar]
- 20.Wichniak A, et al. Actigraphic monitoring of activity and rest in schizophrenic patients treated with olanzapine or risperidone. J Psychiatr Res. 2011;45(10):1381–1386. doi: 10.1016/j.jpsychires.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Chee MJ, et al. Melanin-concentrating hormone is necessary for olanzapine-inhibited locomotor activity in male mice. Eur Neuropsychopharmacol. 2015;25(10):1808–1816. doi: 10.1016/j.euroneuro.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baptista T, et al. Rosiglitazone in the assistance of metabolic control during olanzapine administration in schizophrenia: a pilot double-blind, placebo-controlled, 12-week trial. Pharmacopsychiatry. 2009;42(1):14–19. doi: 10.1055/s-0028-1085438. [DOI] [PubMed] [Google Scholar]
- 23.Cavazzoni P, Tanaka Y, Roychowdhury SM, Breier A, Allison DB. Nizatidine for prevention of weight gain with olanzapine: a double-blind placebo-controlled trial. Eur Neuropsychopharmacol. 2003;13(2):81–85. doi: 10.1016/S0924-977X(02)00127-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.