Abstract
Serrated polyps are important contributors to the burden of colorectal cancers (CRC). These lesions were once considered to have no malignant potential, but currently up to 30% of all CRC are recognized to arise from the serrated neoplasia pathway. The primary premalignant lesions are sessile serrated adenomas/polyps (SSA/Ps), although traditional serrated adenomas are relatively uncommon. Compared to conventional adenomas, SSA/Ps are morphologically subtle with indistinct borders, may be difficult to detect endoscopically, are more prevalent than previously thought, are associated with synchronous and metachronous advanced neoplasia, and have a higher risk of incomplete resection. Although many lesions remain “dormant,” progressive disease is associated with the development of dysplasia and more rapid progression to CRC. As a result, SSA/Ps are strongly implicated in the development of interval cancers. These factors represent unique challenges that require a meticulous approach to their management. In this review, we summarize the contemporary literature on the characterization, detection and resection of SSA/Ps.
Keywords: Sessile serrated adenoma, Detection, Endoscopic imaging, Histology, Endoscopic resection
INTRODUCTION
Sessile serrated adenomas/polyps (SSA/Ps), hyperplastic polyps (HPs) and traditional serrated adenomas (TSAs) form a heterogeneous group of lesions known as serrated polyps.1,2 These lesions share a common serrated or ‘saw-toothed’ histological appearance of their epithelial crypts, with each subtype being defined by specific architectural features, location and extent of the proliferative zone. The classification of serrated polyps has evolved over time, reflecting advances in our understanding of their histopathological, morphological and molecular features (Table 1).3,4 Before the turn of the century, virtually all serrated polyps were called HPs, as these lesions were believed to have no risk of malignancy and therefore were of little clinical significance.5,6 We now also know SSA/Ps and TSAs have the potential for dysplasia and subsequent malignant transformation, and account for up to 30% of all colorectal cancers (CRC).2 The World Health Organization (WHO) classification, last updated in 2010, standardized terminology and definitions of serrated lesions.1 However, the detection of SSA/Ps may be hampered due to a range of factors. These polyps are usually found in the right colon where bowel preparation can be poor, have a flat morphology with indistinct borders, pale surface and may be concealed by a mucous cap or stool debris.2,5,7 As a result, SSA/Ps may be easily missed or be inadequately resected, contributing to the development of interval cancers.8–10 Recognizing these lesions as important cancer precursors, knowledge regarding their identification and management is paramount for all endoscopists.
Table 1.
Endoscopic, Histologic, and Molecular Features of Sessile Serrated Adenoma/Polyps
| Endoscopic | Histologic | Molecular | |
|---|---|---|---|
| Nondysplastic | Flat (0-IIa/0-IIb) morphology Pale colour, indistinct borders Mucous cap, surrounding rim of debris/stool Type II-0 pit pattern |
Saw-toothed architecture of crypt epithelium Boot shaped crypts +/− goblet/mucinous cells at base Pseudoinvasion |
BRAF V600E mutation CIMP-high MLH1 promotor methylation KRAS mutations (infrequent) |
| Dysplastic | Transition from flat to nodular, sessile or depressed area Type III–V pit pattern NICE 2, Sano II on NBI |
Adenomatous dysplasia* (most common) Serrated dysplasia† (less common) |
Reduced expression of MLH1 Microsatellite instability Silencing of other tumor suppressor genes‡ |
CIMP, CpG island methylator phenotype; NICE, narrow band imaging (NBI) international colorectal endoscopic classification.
Characterized by elongated penicillate nuclei with hyperchromasia, nuclear pseudostratification and amphophilic cytoplasm;3
Characterized by cells with a more cuboidal shape and eosinophilic cytoplasm, enlarged vesicular nuclei and prominent nucleoli;3
Including p16INK4a, IGFBP7 and MGMT.4
SCALE OF THE PROBLEM: IMPORTANCE OF SESSILE SERRATED ADENOMAS
Previous studies reported SSA/P prevalence of between 0.6% and 5.3%, probably reflecting differences in endoscopic detection and variations in histological definitions.11–15 Recent work, however, suggests the prevalence may be higher. For example, a single center 4-year European study in a screening population of 3,364 patients with 4,251 resected and histologically confirmed polyps found 399 of these lesions were SSA/Ps.16 The prevalence of SSA/Ps overall was 8.2%, increasing to 9.0% in patients older than 50 years. Per-polyp analysis showed the typical SSA/P was sessile or flat, 5 mm in size and located in the right colon. The higher prevalence of SSA/Ps in this study was favored by involvement of an expert pathologist, high adenoma detection rate (ADR) (median 38.5%) amongst endoscopists, and good quality bowel preparation (median Boston Bowel Preparation score, 8; 90% ≥6), signifying the importance of pairing both quality indicators of colonoscopy and pathological expertise in the diagnosis of these lesions.16
Despite an overall improvement in recognition of SSA/Ps, wide variation in SSA/P detection rates amongst endoscopists is reported by some studies.16–18 For example in the aforementioned study by Ijspeert et al.16 the SSA/P detection rate ranged between 2.5% and 13.6%. A similar SSA/P detection range (1% to 18%) was reported in another study involving tertiary center gastroenterologists, with the odds of detecting at least one proximal serrated polyp for individual endoscopists ranging from 0.05 to 0.67 compared to the highest level detector.17 Misclassification of these lesions amongst pathologists could account for some of this difference. In a study of 1,910 average risk patients undergoing screening colonoscopy, the prevalence of SSA/Ps rose from 1.5% to 8.1% after all polyps in the serrated class were reassessed by an expert pathologist.19 A multicenter study of 350 serrated polyps from 5,778 detected lesions, found the number of serrated lesions per colonoscopy ranged between 0.00 and 0.11, with some centers’ pathologists having never identified proximal serrated lesions as SSA/Ps.20 These data suggest enhanced awareness, education and training are necessary for both endoscopists and pathologists alike to improve outcomes in these areas.
SSA/Ps are also associated with synchronous advanced neoplasia in the colon.13,21–25 In an early study of 3,121 asymptomatic patients undergoing screening colonoscopy, those with at least one proximal serrated polyp were more likely than those without, to have synchronous advanced neoplasia (17.3% vs 10.0%; odds ratio [OR], 1.90; 95% confidence interval [CI], 1.33 to 2.70), particularly in patients with serrated polyps ≥10 mm in size (OR, 3.14; 95% CI, 1.59 to 6.20).21 Furthermore detection of proximal serrated polyps at baseline examination was associated with an increased risk of interval neoplasia on subsequent surveillance colonoscopy.21 Other subsequent large studies have reported similar findings.13,22,24 Recently, a systematic review and meta-analysis of nine studies with 34,084 participants and overall serrated polyp prevalence of 15.6% showed that serrated polyps were associated with a more than 2-fold increased risk of detection of synchronous advanced neoplasia (OR, 2.05; 95% CI, 1.38 to 3.04).25 Individuals with proximal and large serrated polyps had the highest risk (OR, 2.77; 95% CI, 1.71 to 4.46 and OR, 4.10; 95% CI, 2.69 to 6.26, respectively).25 Thus, there is strong data supporting carefully searching for synchronous lesions whenever an SSA/P is detected, and particularly if it is large.
SSA/Ps are associated with increased risk for CRC, including interval cancers.2,26–28 Approximately 30% of all CRC is believed to develop along the serrated neoplasia pathway,2,29 and although polypectomy reduces CRC incidence,30,31 the imperfect protection of colonoscopy against right colon CRC32,33 may be accounted for by failed detection or inadequate resection of SSA/Ps.10 A recent Danish case-control study of 272,342 individuals and 2,045 cases of CRC showed those with a history of SSA/Ps had a significantly increased risk of CRC than patients without these polyps.27 The CRC risk was particularly elevated in patients with proximal SSA/Ps (OR, 12.42; 95% CI, 4.88 to 31.58), SSA/Ps with dysplasia (OR, 4.76; 95% CI, 2.59 to 8.73) and females with SSA/Ps (OR, 5.05; 95% CI, 3.05 to 8.37).27 The CRC risk was significantly elevated for those patients with TSAs (OR, 4.84; 95% CI, 2.36 to 9.93) and for conventional adenomas (OR, 2.51; 95% CI, 2.25 to 2.80).27 Further implicating SSA/Ps, interval cancers also commonly occur in the right colon,32,34 have the CpG island methylator phenotype (CIMP) with microsatellite instability (MSI), and activating mutations of the BRAF gene more often than noninterval CRC.35
SERRATED POLYPOSIS SYNDROME
Serrated polyposis syndrome (SPS) is characterized by multiple, large and/or proximal serrated polyps. Diagnosis is based upon satisfaction of one or more of the following WHO criteria (1) at least five serrated polyps proximal to the sigmoid colon, two of which are larger than 10 mm; (2) any number of serrated polyps proximal to the sigmoid colon in a patient with a first degree relative with SPS; or (3) greater than 20 serrated polyps of any size, distributed throughout the colon.36 Patients with SPS have an increased risk of CRC, with a recent study reporting a standard incidence ratio of CRC in patients with SPS of 18.72 (95% CI, 6.87 to 40.74), and a lifetime CRC risk of up to 50%.37,38 Although SPS has features of being a hereditary condition including familial clustering and increased risk of CRC amongst relatives, no germline mutations have been identified. Nonetheless, screening colonoscopy is recommended for first degree relatives, beginning at 40 years of age or 10 years younger than the age at diagnosis of the youngest affected relative.2,39 Management strategies focus on completely resecting all proximal polyps followed by annual surveillance,39 although a consensus on risk-stratified management (e.g., based upon polyp burden, location, histology and presence of dysplasia) of SPS is still pending. Two recent multicenter series from Spanish and Dutch-British cohorts of patients with SPS managed by intensive endoscopic surveillance showed a 1.5% to 1.9% absolute 5-year CRC risk estimate,40,41 less than previously expected. These results indicate that in SPS patients without CRC, early recognition and treatment of serrated polyps is imperative, and that protection from CRC by colonoscopic surveillance in dedicated centers is feasible.42
CHARACTERIZATION OF SESSILE SERRATED ADENOMAS
1. The serrated neoplasia pathway
Carcinogenesis in SSA/Ps is believed to progress through a unique epigenetic pathway. This involves hypermethylation of CpG islands (CIMP) on the promotor regions of tumor suppressor genes, in which a cytosine (C) is followed by a guanine (G) nucleotide linked by a phosphodiester bond (CpG).43 Epigenetic silencing of the DNA mismatch repair (MMR) gene MLH1 through promotor hypermethylation leads to the MSI phenotype, and leaves the cell vulnerable to mutations in genes controlling cell growth.44–46 Notably, although MLH1 methylation occurs in early SSA/Ps, only reduced or loss of gene expression, which requires extensive methylation, is associated with dysplasia and progression to malignancy.3 This is supported by the observation that variably decreased MLH1 expression is seen in dysplastic areas of SSA/Ps, with loss of expression in invasive MSI-high CRC.47 The CIMP status of a lesion can be determined by assessment of a panel of 5 or 6 MMR genes, in which promotor hypermethylation of three or more genes is considered CIMP-high.35 SSA/Ps, especially those with dysplasia, are considered the probable precursors to sporadic CIMP-high, MSI-high CRC given the similarities in their molecular profiles including hypermethylation of MMR genes MLH1, and of other tumor suppressor genes such as p16INK4a, IGFBP7 and MGMT.48–51 Activation of the BRAF oncogene (BRAF V600E mutation) is also a feature of the serrated neoplasia pathway52,53 and is closely associated with CIMP-high CRC.43,54 BRAF regulates cell proliferation, differentiation and survival, and is hypothesized to have a role in early serrated polyp development.4,55
2. Histopathological features
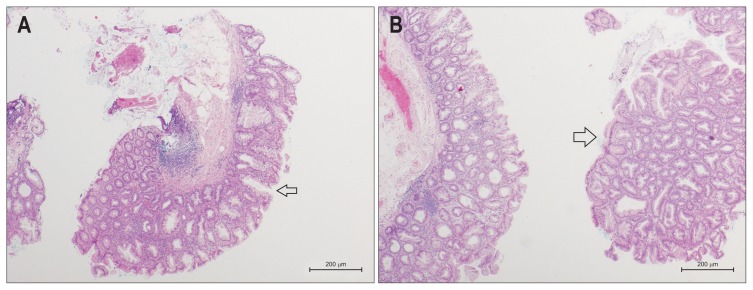
Sporadic serrated polyps are characterised by a serrated architecture of the epithelium that lines the colonic crypts, thought to result from decreased epithelial cell apoptosis.56 The subtypes of serrated lesions may be distinguished by the location and extent of the proliferative zone.29 The specific causes of these changes are presumed to result from epigenetic alterations in genes responsible for cell proliferation and differentiation, as well as genetic changes such as mutations in BRAF.2 SSA/Ps are characterised by distorted crypt growth and dilatation of the crypt base, leading to the formation of ‘boot’ or ‘L’ or ‘anchor’-shaped crypts (Fig. 1).2,6 The basal aspect of the crypt may contain hyper-serration, mature goblet cells and mucinous cells, which are responsible for the excessive mucin frequently seen within the dilated crypts and on the surface of the lesion. Recently, an expert consensus panel recommended that one unequivocal architecturally distorted crypt base was sufficient to diagnose an SSA/P.2 Pseudoinvasion below the muscularis mucosae, also known as displaced crypts, also often occurs in SSA/Ps.57 SSA/Ps are not typically dysplastic, although cytological dysplasia resembling conventional adenoma with frequent loss of expression of MLH1 on immunohistochemistry may develop in some lesions and potentially progress quickly to invasive malignancy.3,14
Fig. 1.
Histologic features of sessile serrated adenomas/polyps (SSA/Ps). (A) A serrated adenoma (SSA/P) without dysplasia showing the classical features of broad bases and dilated crypts (arrow). H&E stained, low power magnification. (B) An SSA/P with mild dysplasia is shown in the right-side specimen (arrow). The glandular architecture and surface epithelium of the dysplastic component resembles a conventional adenoma. The left-sided specimen is nondysplastic. H&E stained, low power magnification.
3. Endoscopic features of sessile serrated adenomas
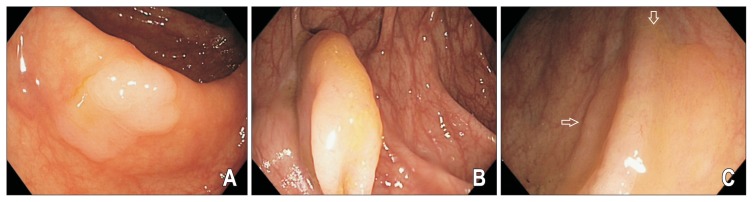
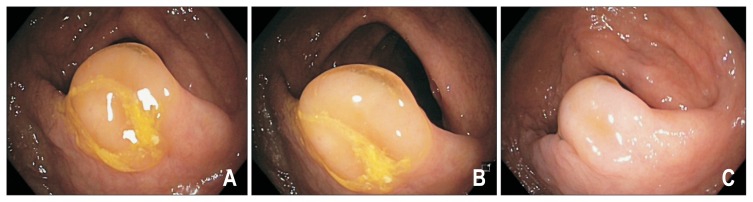
SSA/Ps are most commonly located in the right colon, have a sessile or flat morphology sometimes resembling prominent mucosal folds, pale in colour similar to the surrounding mucosa and with indistinct borders (Fig. 2). About two-thirds of lesions are covered by a tenacious mucous cap (Fig. 3), with less common signs including a rim of stool debris, alteration of fold contour, interrupted underlying mucosal pattern and a dome shaped protuberance.58 Because of these features, the endoscopic appearance of SSA/Ps may be subtle and even large lesions may be missed without careful attention from the endoscopist. It is prudent to note the location of the polyp before washing off the mucous cap as the SSA/P may be difficult to discern afterwards.2 Other factors associated with SSA/Ps include female sex, smokers with more than a 20 pack year history, diabetes and obesity.59,60
Fig. 2.
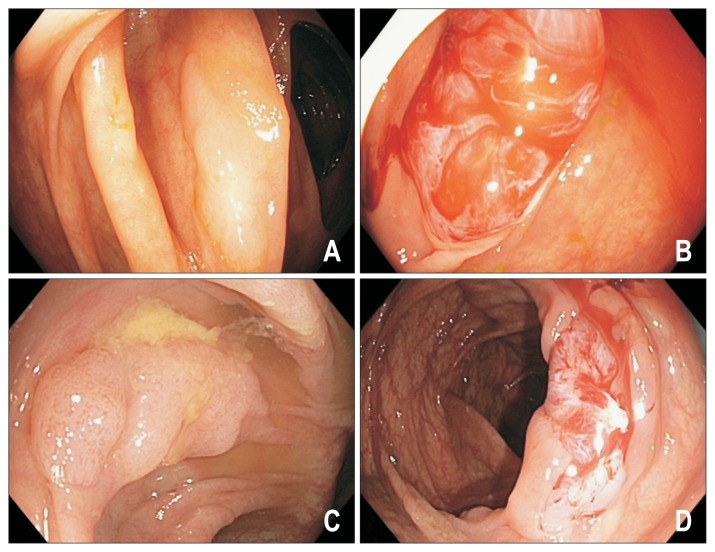
(A–C) Endoscopic appearance of nondysplastic sessile serrated adenomas/polyps (SSA/Ps). SSA/Ps are often found in the right colon, are morphologically flat and pale, have a color similar to the surrounding mucosa and have indistinct borders (arrows). Detection requires good bowel preparation and a high index of suspicion.
Fig. 3.
Sessile serrated adenomas/polyp (SSA/P) before and after cleaning of the mucous cap. This nondysplastic SSA/P is covered by a tenacious mucous cap with a surrounding rim of stool (A, B). The lesion becomes less conspicuous (C) upon cleansing and can potentially be mistaken for a prominent mucosal fold.
SSA/Ps are identified endoscopically by a Type II open-shape (II-0) pit pattern (sensitivity 65.5%, specificity 97.3% using magnification and indigo carmine chromoendoscopy).61 On narrow band imaging (NBI), other endoscopic predictors of SSA/Ps include a cloud-like surface, indistinct borders, irregular shape, and dark spots inside the crypts.62 A recent systematic review and meta-analysis assessing the utility of image enhanced endoscopy in differentiating SSA/Ps from nonneoplastic tissue showed 80% sensitivity for magnification-NBI, 60% for NBI, 49% for autofluorescence, and 47% for flexible spectral imaging color enhancement.63 In head to head comparisons with white light endoscopy (WLE), only NBI and magnification-NBI demonstrated significantly greater sensitivity.63 The NBI International Colorectal Endoscopic (NICE) classification based upon lesion colour, vessel appearance and surface pattern distinguishes hyperplastic from adenomatous polyps,64 however, does not accurately diagnose SSA/Ps.65 To improve the endoscopic identification of SSA/Ps using NBI, the NICE classification was combined with the criteria for differentiation of SSA/Ps62 to form the recently proposed Workgroup Serrated Polyps and Polyposis (WASP) classification.66 In the first validation phase using this classification, the accuracy of optical diagnosis for SSA/Ps versus non-SSA/Ps with high confidence amongst a cohort of 10 gastroenterologists was 0.83 (95% CI, 0.75 to 0.91) rising to 0.93 (95% CI, 0.87 to 0.98) after completion of a standardised WASP training module.66 Although promising, further validation of the WASP criteria in prospective trials is awaited before its routine implementation to daily practice. In practice and with experience, the recognition of SSA/Ps and their differentiation from adenomas is usually not challenging.67
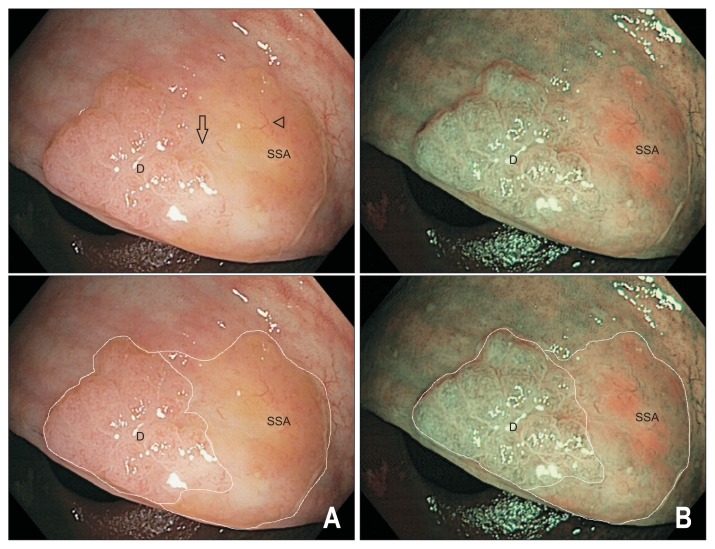
Whereas nondysplastic SSA/Ps have a relatively homogeneous appearance, progression to more advanced lesions with dysplasia (SSA/P-D) is associated with accumulation of aberrant DNA methylation and additional lesion changes resembling that of a conventional adenoma (Fig. 4).61,67,68 The identification of an endoscopically apparent transition point between two differing surface patterns within a lesion should alert the endoscopist to an SSA/P harbouring dysplasia. The dysplastic component is usually a small (1 to 5 mm) centrally or peripherally located nodule, and occasionally minimally elevated or depressed area within the lesion.67 Examination of the surface pit pattern with WLE and NBI often reveals two distinct patterns corresponding to the different histology, with the dysplastic component exhibiting a type III (tubular or roundish pits) or type IV (branched or gyrus-like pits) pattern.67 With NBI, the area of dysplasia is darker due to more abundant and thicker surface capillaries in keeping with a NICE 2 or Sano II vascular pattern, compared with the relatively hypovascular background pattern of the nondysplastic SSA/P.67 Once dysplasia develops, transformation to invasive cancer can be rapid and may occur even when lesions are small.67,69 Large (≥20 mm) SSA/Ps may more frequently harbour dysplasia, and was present in 32.4% of all such lesions referred for endoscopic mucosal resection (EMR) in a prospective multicenter study of large laterally spreading lesions (LSLs).68 Multivariable analysis revealed SSA/P-D were significantly associated with increasing age (OR, 1.69 per decade; 95% CI, 0.19 to 2.40), increasing lesion size (OR, 1.90 per 10 mm; 95% CI, 1.30 to 2.78), an “adenomatous” pit pattern (Kudo III, IV or V) (OR, 3.98; 95% CI, 1.94 to 8.15) and any 0-Is component within an SSA/P (OR, 3.10; 95% CI, 1.19 to 8.12).68
Fig. 4.
Endoscopic appearance of sessile serrated adenomas/polyps (SSA/Ps) with dysplasia. A 20 mm SSA/P-D viewed under white light (A) and narrow band imaging (B) with and without the dysplastic (label D) and nondysplastic (label SSA) components outlined. The lesion has developed a raised, nodular component on the left-hand aspect with a type IV surface pit pattern indicative of dysplastic transformation (label D). The nondysplastic component of the lesion (label SSA) is pale with relatively hypovascular background surface markings and is covered by a thin layer of stool debris (arrowhead). Note there is an obvious transition zone from the nondysplastic flat SSA/P to the area of dysplasia (arrow). The lesion and a rim of normal tissue were removed en bloc by endoscopic mucosal resection; histology confirmed a completely resected SSA/P with mild dysplasia.
DETECTION OF SESSILE SERRATED ADENOMAS
Endoscopic detection of SSA/Ps can be assisted by high definition (HD) endoscopes, chromoendoscopy and/or image enhancement, adoption of quality criteria for colonoscopy and possibly use of ancillary devices. High definition scopes deliver better image quality and brighter illumination, and their use improves the detection of both adenomas and SSA/Ps.70 In another study, the combination of HD colonoscopes with chromoendoscopy (0.4% indigo carmine) during scope withdrawal increased the overall detection rate for adenomas (0.95 vs 0.66 per patient) and serrated lesions (1.19 vs 0.49 per patient) (p<0.001) compared with standard colonoscopy.71 Potential drawbacks included longer procedural times and additional cost of chromoendoscopic dye. The utility of NBI compared with HD-WLE for detecting serrated lesions was assessed in a randomized controlled trial of 800 patients.72 Although more proximal colon serrated lesions were detected by NBI than HD-WLE (204 vs 158), this did not achieve statistical significance.72 Similarly, a randomized multicenter trial found no significant difference in polyp miss rates using HD-WLE or NBI in patients with SPS.73
The detection of SSA/Ps in CRC screening programs was assessed in a multicenter retrospective series of over 70,000 colonoscopies, reporting significant association with caecal intubation rate (OR, 3.75; 95% CI, 2.22 to 6.34), presence of at least one advanced adenoma (OR, 2.08; 95% CI, 1.86 to 2.33) and ADR.74 In this study, no association between faecal immunochemical test (FIT) and detection of SSA/Ps was found.74 Similar outcomes were reported from a prospective population screening study of over 6,000 patients, finding FIT detected SSA/Ps with significantly lower sensitivity than conventional adenomas.75 In a multicenter study of almost 8,000 colonoscopies, serrated polyp detection increased with each minute of withdrawal time above 6 minutes, with maximal benefit at 9 minutes (incident rate ratio, 1.77; 95% CI, 1.15 to 2.72).76 Other studies have also demonstrated the benefits of a longer withdrawal technique,18 including second looks and retroflexion in the right colon,77 with careful cleaning and meticulous mucosal examination for the detection of SSA/Ps.
Adequacy of bowel preparation is well documented for optimising detection of conventional adenomas as well as for SSA/Ps. One study reported overall SSA/P detection of 4.6% versus 12.0% (OR, 0.37; 95% CI, 0.15 to 0.87) and 1.5% versus 7.9% (OR, 0.19; 95% CI, 0.05 to 0.81) in the right colon for intermediate quality preparation versus high quality preparation, respectively.78 This study also showed that any level of preparation below high quality was associated with a significant decrease in SSA/P detection, whereas intermediate quality preparation was still adequate for adenoma detection.78 Split dose bowel preparation improves colonic cleansing and detection of conventional adenomas.79,80 In a prospective randomised trial of 341 patients, split dose bowel preparation also improved SSA/Ps detection relative to single dose regimens (9.9% vs 2.4%, p=0.004), with improved patient tolerance and quality of preparation.81
Ancillary devices used with the aim of improving mucosal examination and polyp detection include disposable attachments to the colonoscope such as transparent caps and Endocuff (ARC Medical Design, Leeds, UK), accessory video processors such as Third Eye® Retroscope® and Third Eye® PanoramicTM (Avantis Medical Systems, Sunnyvale, CA, USA), and specialised colonoscopes such as Full Spectrum Endoscopy® (EndoChoice Inc., Alpharetta, GA, USA), Extra-Wide-Angle-View colonoscope (Olympus, Tokyo, Japan), NaviAidTM G-EYETM balloon colonoscope (SMART Medical Systems Ltd., Ra’anana, Israel).82–84 Distal attachment caps have not demonstrated improved polyp detection,85 whereas the others have shown promise but are either technically intensive and/or associated with significant additional cost. A recent review based on observational data suggested use of Endocuff may result in higher ADR (35.4% to 53.5%), particularly for polyp detection in the right colon.83 However, a multicenter randomised trial did not demonstrate Endocuff identified an increased number of patients with one or more adenomas relative to conventional colonoscopy.86 Taking everything together, meticulous examination technique, high quality bowel preparation and use of HD scopes remain the key to optimising SSA/P detection. Endoscopists with high ADRs are unlikely to gain significant improvements in ADR by using the additional technologies and ancillary devices currently available.
RESECTION OF SESSILE SERRATED ADENOMAS
Complete polyp resection is the fundamental principle governing treatment of SSA/Ps. All serrated lesions except for diminutive rectosigmoid lesions should be removed.2 However, endoscopic detection and resection of SSA/Ps is hampered by their predominantly flat morphology, inconspicuous surface features and indistinct borders. Once detected, these lesions can be excised endoscopically utilizing similar principles to those for resection of conventional adenomas. The Complete Adenoma Resection (CARE) study assessed the incomplete resection rate (IRR) of polyps by immediate biopsy of the resection margins in 1,427 patients undergoing colonoscopy with at least one nonpedunculated polyp. The study found that SSA/Ps were more likely to be incompletely resected than conventional adenomas (31% vs 7.2%, p<0.001), and that the IRR rose to 47.6% for larger (10 to 20 mm) SSA/Ps.10 In this study, the two strongest associations for IRR were increasing polyp size (relative risk [RR], 2.1; 95% CI, 1.13 to 3.86 for lesions 10–20 mm vs 5–9 mm), and SSA/P diagnosis (RR, 3.74; 95% CI, 2.04 to 6.84).10 Significant variation in rates of complete resection were also observed amongst endoscopists,10 indicating that careful attention to polypectomy technique is essential to achieving satisfactory outcomes, and particularly for SSA/Ps.
1. Removal of small sessile serrated adenomas (<10 mm)
Cold snare polypectomy (CSP), when performed correctly, is ideal for removal of diminutive and small SSA/Ps up to 10 mm in size, due to its efficacy and safety.87–89 CSP is superior to cold forceps polypectomy with regard to completeness of excision of small and diminutive polyps.88–90 Hot forceps polypectomy is associated with high rates of deep tissue injury, poor histological specimens, residual tissue, and is now strongly discouraged.91,92 The principle of CSP is to ensure that complete polyp removal is achieved with a 1 to 2 mm margin of normal tissue.92,93 Our recommended approach to CSP is described in Table 2.
Table 2.
Technical Tips for the Removal of SSA/Ps (<10 mm) by Cold Snare Polypectomy
|
SSA/Ps, sessile serrated adenoma/polyps.
The efficacy of CSP using thin wire snares has been assessed by a number of studies, although none have solely included SSA/Ps. In one study, completeness of excision based on endoscopic imaging was significantly higher with thin wire (0.30 mm) than thick wire (0.47 mm) snares (90.2% vs 73.3%, p<0.05), with a trend towards higher complete pathological excision (73.3% vs 65.2%, p=0.4).94 Another prospective randomized controlled trial of 210 lesions resected by CSP found complete pathological resection was significantly greater with thin wire than thick wire snares (91% vs 79%, p=0.015), particularly for polyps 8 to 10 mm in size.95
The risk of complications related to CSP such as perforation and clinically significant bleeding is extremely low.87 The rate of perforation is negligible as the closed snare is unable to cut through muscularis propria, and its occurrence has mostly been associated with lesions removed using electrocautery (hot snare polypectomy, HSP).96,97 Furthermore, compared with HSP, CSP has similar rates of complete polyp resection, shorter procedure time and no increase in clinically significant bleeding.98–100 Protrusions within the cold snare defect occur in approximately one in six cases, and may create concern for incomplete resection, however, these do not contain residual polyp nor are they associated with adverse outcomes.101 Immediate bleeding after CSP is common, but is typically self-limited and without risk of ongoing or delayed bleeding.99
2. Removal of large sessile serrated adenomas (10–20 mm)
EMR is the first-line therapy for LSLs. The supporting data is mostly based on resection of conventional adenomas,92,102,103 however, studies have also shown large (≥10 mm) SSA/Ps can be adequately treated by EMR (Fig. 5).104–107 HSP is highly operator dependant and may be inadequate for resection of SSA/Ps. For example, the CARE study showed wide inter-operator variability in efficacy of HSP with almost half of the lesions 10 to 20 mm in size incompletely resected using this technique.10 Large SSA/Ps are also more likely to harbor dysplasia, which may be subtle, and these lesions should be carefully examined prior to removal, particularly with respect to their surface pattern and peripheral extent, to ensure complete resection. As mentioned above, dysplasia is manifest as a transition point with a change in surface appearance from the usual flat SSA/P morphology to a nodular or minimally elevated or depressed area within the lesion, along with an “adenomatous” (type III–IV) pit pattern.67 The safety and efficacy of endoscopic resection for large SSA/Ps was demonstrated in a 2 center retrospective study of 199 patients with 251 proximal colon SSA/Ps measuring 10 mm or larger removed by EMR.104 After mean follow-up of 17.8±15.4 months, five patients (3.6%; 95% CI, 0.5% to 6.7%) developed local recurrence with a median size of 4 mm.104 The recurrences were all cured endoscopically. There were no complications and no high grade dysplasia or advanced CRC following the index colonoscopy.104
Fig. 5.
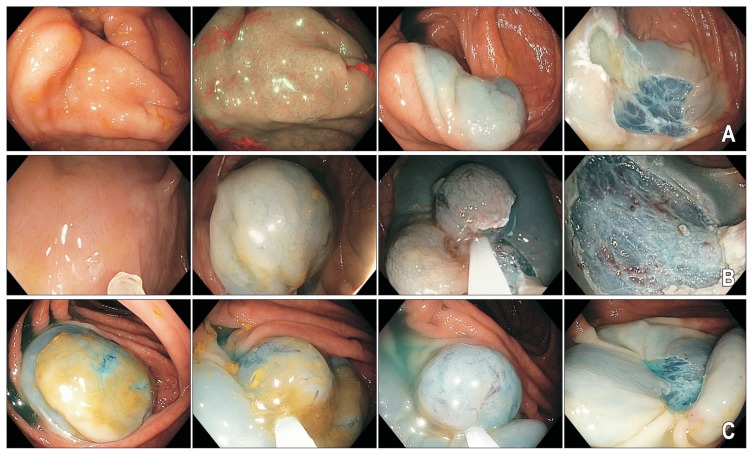
Endoscopic mucosal resection of sessile serrated adenomas/polyps (SSA/Ps). (A–C) Note the inconspicuous appearance of all three lesions despite their larger sizes. Submucosal chromogelofusine injection assists with delineating the peripheral extent of the lesion. A margin of normal tissue should be captured during mucosal resection. Thermal ablation of the resection margins with snare tip soft coagulation (effect 4, 80W; VIO 300D; Erbe) reduces the risk of lesion recurrence.
The median size of large SSA/Ps tend to be smaller than that of adenomatous LSLs. SSA/Ps seem to be relatively loosely attached to the deeper mural layers, usually lift easily and are not associated with submucosal fibrosis.107 As such, SSA/Ps are generally easier to remove by EMR than adenomatous LSLs. Nonetheless, EMR is associated with risks such as perforation (1% to 2%), post polypectomy syndrome (0.5%) and clinically significant post EMR bleeding (6% to 11%).102,108–110 Therefore, it may be beneficial to remove large SSA/Ps by piecemeal CSP, achieving complete excision whilst mitigating many of the adverse effects of EMR (Fig. 6). In a pilot study of 15 patients with adenomatous colonic polyps (mean size, 20 mm; range, 10 to 45 mm) removed by piecemeal CSP using a stiff thin wire snare, technical success was 100%, with no perforation, no post polypectomy syndrome and only one delayed bleeding episode in a patient on warfarin.113 A subsequent study involving 30 sessile colonic polyps ≥10 mm in size treated by piecemeal CSP also found this technique to be feasible, with no significant adverse events.114 At first follow-up after 6 months, 20% of patients had small volume residual tissue, and all cases were treated endoscopically.114 Although piecemeal CSP appears effective and safe for resection of large SSA/Ps, particularly those 10 to 20 mm in size, prospective studies are awaited to determine the long term durability of this technique, and whether risks of complications such as bleeding are truly reduced.
Fig. 6.
Piecemeal cold snare polypectomy of sessile serrated adenomas/polyp (SSA/P). Larger (10 to 15 mm) SSA/Ps (A, C) removed by piecemeal cold snare polypectomy (B, D).
3. Removal of larger sessile serrated adenomas (>20 mm)
EMR of larger SSA/Ps (>20 mm) is safe and effective, with comparable recurrence rates to that seen with similar sized conventional adenomas (8.7% vs 11.1%, p=0.8).105 A recent multicenter, prospective cohort of 2,000 LSLs ≥20 mm (median size 35 mm) comprising 323 SSA/Ps and 1,527 adenomas, showed large SSA/Ps could be successfully removed by EMR in almost all cases.107 The study reported EMR of these lesions compared with adenomatous LSLs, was easier to perform, with less intraprocedural bleeding and similar rates of significant adverse events.107 Cumulative recurrence rates at 6 and 12 months for SSA/Ps was significantly less compared with adenomas (6.3% and 7.0% vs 16.1% and 20.4%, p<0.001, respectively). Subgroup analysis by lesion size revealed an 8-fold increased risk of recurrence for 20 to 25 mm adenomatous LSLs versus SSA/Ps, but no significant difference in risk between lesion types in larger lesion groups.107 The technique of EMR for removal of LSLs including large SSA/Ps has been described,92,115,116 and key aspects are summarized in Table 3.117,118
Table 3.
Technical Tips for the Removal of larger SSA/Ps by Endoscopic Mucosal Resection
|
SSA/Ps, sessile serrated adenoma/polyps; NBI, narrow band imaging; EMR, endoscopic mucosal resection.
SURVEILLANCE
Recommendations for colonoscopy surveillance intervals in patients with SSA/Ps follow similar principles to that of conventional adenomas, and are based upon lesion number, size and histology, albeit with some caveats. Although guidelines exist,2,39,119 these are largely based upon observational data and expert opinion, as prospective, controlled data on the natural history of SSA/Ps is lacking. Major European and North American societal guidelines are largely congruent and recommend the following intervals of colonoscopy surveillance: 5 years for patients with a single SSA/P without dysplasia <10 mm in size, 3 to 5 years for patients with <3 SSA/Ps without dysplasia each <10 mm in size, 3 years for patients with ≥3 SSA/Ps without dysplasia each <10 mm in size, and 3 years for patients with “high risk” lesions (any lesion ≥10 mm in size or with dysplasia).39,119 Lesions removed piecemeal may warrant early follow-up colonoscopy at 6 months given the potential for incomplete resection, although this area requires much further systematic study to optimise techniques and quantitate the risks.
Recent expert consensus guidelines advocate a slightly more aggressive surveillance recommendation, suggesting an interval colonoscopy in 1 to 3 years after resection of any SSA/P with dysplasia or after resection of ≥2 SSA/Ps of ≥10 mm in size.2 These recommendations are based upon the observation that interval CRC are more likely right sided, colonoscopy is less effective at preventing proximal CRC, and the greater variability in detection of SSA/Ps compared with conventional adenomas.2 Other guidelines do not make any specific recommendations with respect to serrated lesions, instead treating such lesions the same as conventional adenomas.120
CONCLUSIONS
As our understanding of the biological behaviour of SSA/Ps improves, we increasingly recognise the clinical significance of these lesions, in particular their potential to progress to CRC and role in development of interval cancers. SSA/Ps can be difficult to identify and use of HD colonoscopes, quality bowel preparation, meticulous mucosal examination and withdrawal technique are the factors most likely to improve their detection. Each lesion should be carefully assessed to determine its peripheral extent and localise any dysplastic areas. Like all adenomatous colorectal polyps, complete endoscopic resection is the key to successful eradication of SSA/Ps. Lesions ≤10 mm in size are suitable for removal by CSP. Lesions 10 to 20 mm in size may be removed by either piecemeal CSP or EMR. Larger lesions are currently best removed by EMR. Improved detection, accurate characterisation and safe and complete resection of SSA/Ps are imperative to optimising patient outcomes and reducing the incidence of CRC.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. Lyon: IARC Press; 2010. [Google Scholar]
- 2.Rex DK, Ahnen DJ, Baron JA, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol. 2012;107:1315–1329. doi: 10.1038/ajg.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang HM, Mitchell JM, Sepulveda JL, Sepulveda AR. Molecular and histologic considerations in the assessment of serrated polyps. Arch Pathol Lab Med. 2015;139:730–741. doi: 10.5858/arpa.2014-0424-RA. [DOI] [PubMed] [Google Scholar]
- 4.IJspeert JE, Vermeulen L, Meijer GA, Dekker E. Serrated neoplasia-role in colorectal carcinogenesis and clinical implications. Nat Rev Gastroenterol Hepatol. 2015;12:401–409. doi: 10.1038/nrgastro.2015.73. [DOI] [PubMed] [Google Scholar]
- 5.Obuch JC, Pigott CM, Ahnen DJ. Sessile serrated polyps: detection, eradication, and prevention of the evil twin. Curr Treat Options Gastroenterol. 2015;13:156–170. doi: 10.1007/s11938-015-0046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crockett SD, Snover DC, Ahnen DJ, Baron JA. Sessile serrated adenomas: an evidence-based guide to management. Clin Gastroenterol Hepatol. 2015;13:11–26. doi: 10.1016/j.cgh.2013.10.035. [DOI] [PubMed] [Google Scholar]
- 7.Bouwens MW, van Herwaarden YJ, Winkens B, et al. Endoscopic characterization of sessile serrated adenomas/polyps with and without dysplasia. Endoscopy. 2014;46:225–235. doi: 10.1055/s-0034-1364936. [DOI] [PubMed] [Google Scholar]
- 8.Burgess NG, Tutticci NJ, Pellise M, Bourke MJ. Sessile serrated adenomas/polyps with cytologic dysplasia: a triple threat for interval cancer. Gastrointest Endosc. 2014;80:307–310. doi: 10.1016/j.gie.2014.03.050. [DOI] [PubMed] [Google Scholar]
- 9.Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795–1803. doi: 10.1056/NEJMoa0907667. [DOI] [PubMed] [Google Scholar]
- 10.Pohl H, Srivastava A, Bensen SP, et al. Incomplete polyp resection during colonoscopy-results of the complete adenoma resection (CARE) study. Gastroenterology. 2013;144:74–80. doi: 10.1053/j.gastro.2012.09.043. [DOI] [PubMed] [Google Scholar]
- 11.Hetzel JT, Huang CS, Coukos JA, et al. Variation in the detection of serrated polyps in an average risk colorectal cancer screening cohort. Am J Gastroenterol. 2010;105:2656–2664. doi: 10.1038/ajg.2010.315. [DOI] [PubMed] [Google Scholar]
- 12.Sanaka MR, Gohel T, Podugu A, et al. Adenoma and sessile serrated polyp detection rates: variation by patient sex and colonic segment but not specialty of the endoscopist. Dis Colon Rectum. 2014;57:1113–1119. doi: 10.1097/DCR.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 13.Hazewinkel Y, de Wijkerslooth TR, Stoop EM, et al. Prevalence of serrated polyps and association with synchronous advanced neoplasia in screening colonoscopy. Endoscopy. 2014;46:219–224. doi: 10.1055/s-0033-1358800. [DOI] [PubMed] [Google Scholar]
- 14.Lash RH, Genta RM, Schuler CM. Sessile serrated adenomas: prevalence of dysplasia and carcinoma in 2139 patients. J Clin Pathol. 2010;63:681–686. doi: 10.1136/jcp.2010.075507. [DOI] [PubMed] [Google Scholar]
- 15.Kumbhari V, Behary J, Hui JM. Prevalence of adenomas and sessile serrated adenomas in Chinese compared with Caucasians. J Gastroenterol Hepatol. 2013;28:608–612. doi: 10.1111/jgh.12100. [DOI] [PubMed] [Google Scholar]
- 16.IJspeert JE, de Wit K, van der Vlugt M, Bastiaansen BA, Fockens P, Dekker E. Prevalence, distribution and risk of sessile serrated adenomas/polyps at a center with a high adenoma detection rate and experienced pathologists. Endoscopy. 2016;48:740–746. doi: 10.1055/s-0042-105436. [DOI] [PubMed] [Google Scholar]
- 17.Kahi CJ, Hewett DG, Norton DL, Eckert GJ, Rex DK. Prevalence and variable detection of proximal colon serrated polyps during screening colonoscopy. Clin Gastroenterol Hepatol. 2011;9:42–46. doi: 10.1016/j.cgh.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 18.de Wijkerslooth TR, Stoop EM, Bossuyt PM, et al. Differences in proximal serrated polyp detection among endoscopists are associated with variability in withdrawal time. Gastrointest Endosc. 2013;77:617–623. doi: 10.1016/j.gie.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 19.Abdeljawad K, Vemulapalli KC, Kahi CJ, Cummings OW, Snover DC, Rex DK. Sessile serrated polyp prevalence determined by a colonoscopist with a high lesion detection rate and an experienced pathologist. Gastrointest Endosc. 2015;81:517–524. doi: 10.1016/j.gie.2014.04.064. [DOI] [PubMed] [Google Scholar]
- 20.Payne SR, Church TR, Wandell M, et al. Endoscopic detection of proximal serrated lesions and pathologic identification of sessile serrated adenomas/polyps vary on the basis of center. Clin Gastroenterol Hepatol. 2014;12:1119–1126. doi: 10.1016/j.cgh.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 21.Schreiner MA, Weiss DG, Lieberman DA. Proximal and large hyperplastic and nondysplastic serrated polyps detected by colonoscopy are associated with neoplasia. Gastroenterology. 2010;139:1497–1502. doi: 10.1053/j.gastro.2010.06.074. [DOI] [PubMed] [Google Scholar]
- 22.Alvarez C, Andreu M, Castells A, et al. Relationship of colonoscopy-detected serrated polyps with synchronous advanced neoplasia in average-risk individuals. Gastrointest Endosc. 2013;78:333–341. doi: 10.1016/j.gie.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Li D, Jin C, McCulloch C, et al. Association of large serrated polyps with synchronous advanced colorectal neoplasia. Am J Gastroenterol. 2009;104:695–702. doi: 10.1038/ajg.2008.166. [DOI] [PubMed] [Google Scholar]
- 24.Ng SC, Ching JY, Chan VC, et al. Association between serrated polyps and the risk of synchronous advanced colorectal neoplasia in average-risk individuals. Aliment Pharmacol Ther. 2015;41:108–115. doi: 10.1111/apt.13003. [DOI] [PubMed] [Google Scholar]
- 25.Gao Q, Tsoi KK, Hirai HW, et al. Serrated polyps and the risk of synchronous colorectal advanced neoplasia: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110:501–509. doi: 10.1038/ajg.2015.49. [DOI] [PubMed] [Google Scholar]
- 26.Vu HT, Lopez R, Bennett A, Burke CA. Individuals with sessile serrated polyps express an aggressive colorectal phenotype. Dis Colon Rectum. 2011;54:1216–1223. doi: 10.1097/DCR.0b013e318228f8a9. [DOI] [PubMed] [Google Scholar]
- 27.Erichsen R, Baron JA, Hamilton-Dutoit SJ, et al. Increased risk of colorectal cancer development among patients with serrated polyps. Gastroenterology. 2016;150:895–902. doi: 10.1053/j.gastro.2015.11.046. [DOI] [PubMed] [Google Scholar]
- 28.Holme O, Bretthauer M, Eide TJ, et al. Long-term risk of colorectal cancer in individuals with serrated polyps. Gut. 2015;64:929–936. doi: 10.1136/gutjnl-2014-307793. [DOI] [PubMed] [Google Scholar]
- 29.Snover DC. Update on the serrated pathway to colorectal carcinoma. Hum Pathol. 2011;42:1–10. doi: 10.1016/j.humpath.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy: the National Polyp Study Workgroup. N Engl J Med. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 31.Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh H, Nugent Z, Demers AA, Kliewer EV, Mahmud SM, Bernstein CN. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology. 2010;139:1128–1137. doi: 10.1053/j.gastro.2010.06.052. [DOI] [PubMed] [Google Scholar]
- 33.Brenner H, Chang-Claude J, Seiler CM, Rickert A, Hoffmeister M. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med. 2011;154:22–30. doi: 10.7326/0003-4819-154-1-201101040-00004. [DOI] [PubMed] [Google Scholar]
- 34.Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150:1–8. doi: 10.7326/0003-4819-150-1-200901060-00306. [DOI] [PubMed] [Google Scholar]
- 35.Arain MA, Sawhney M, Sheikh S, et al. CIMP status of interval colon cancers: another piece to the puzzle. Am J Gastroenterol. 2010;105:1189–1195. doi: 10.1038/ajg.2009.699. [DOI] [PubMed] [Google Scholar]
- 36.Snover D. Serrated polyps of the colon and rectum and serrated polyposis. 4th ed. Lyon: IARC; 2010. [Google Scholar]
- 37.Edelstein DL, Cruz-Correa M, Soto-Salgado M, et al. Risk of colorectal and other cancers in patients with serrated polyposis. Clin Gastroenterol Hepatol. 2015;13:1697–1699. doi: 10.1016/j.cgh.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edelstein DL, Axilbund JE, Hylind LM, et al. Serrated polyposis: rapid and relentless development of colorectal neoplasia. Gut. 2013;62:404–408. doi: 10.1136/gutjnl-2011-300514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844–857. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 40.IJspeert JE, Rana SA, Atkinson NS, et al. Clinical risk factors of colorectal cancer in patients with serrated polyposis syndrome: a multicenter cohort analysis. Gut. 2017;66:278–284. doi: 10.1136/gutjnl-2015-310630. [DOI] [PubMed] [Google Scholar]
- 41.Carballal S, Rodríguez-Alcalde D, Moreira L, et al. Colorectal cancer risk factors in patients with serrated polyposis syndrome: a large multicenter study. Gut. 2016;65:1829–1837. doi: 10.1136/gutjnl-2015-309647. [DOI] [PubMed] [Google Scholar]
- 42.Hassan C, Repici A, Rex DK. Serrated polyposis syndrome: risk stratification or reduction? Gut. 2016;65:1070–1072. doi: 10.1136/gutjnl-2015-311357. [DOI] [PubMed] [Google Scholar]
- 43.Weisenberger DJ, Siegmund KD, Campan M, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38:787–793. doi: 10.1038/ng1834. [DOI] [PubMed] [Google Scholar]
- 44.Ionov Y, Peinado MA, Malkhosyan S, Shibata D, Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993;363:558–561. doi: 10.1038/363558a0. [DOI] [PubMed] [Google Scholar]
- 45.Fujiwara T, Stolker JM, Watanabe T, et al. Accumulated clonal genetic alterations in familial and sporadic colorectal carcinomas with widespread instability in microsatellite sequences. Am J Pathol. 1998;153:1063–1078. doi: 10.1016/S0002-9440(10)65651-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goel A, Nagasaka T, Arnold CN, et al. The CpG island methylator phenotype and chromosomal instability are inversely correlated in sporadic colorectal cancer. Gastroenterology. 2007;132:127–138. doi: 10.1053/j.gastro.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 47.Sheridan TB, Fenton H, Lewin MR, et al. Sessile serrated adenomas with low- and high-grade dysplasia and early carcinomas: an immunohistochemical study of serrated lesions “caught in the act”. Am J Clin Pathol. 2006;126:564–571. doi: 10.1309/C7JE8BVL8420V5VT. [DOI] [PubMed] [Google Scholar]
- 48.Wong JJ, Hawkins NJ, Ward RL. Colorectal cancer: a model for epigenetic tumorigenesis. Gut. 2007;56:140–148. doi: 10.1136/gut.2005.088799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50:113–130. doi: 10.1111/j.1365-2559.2006.02549.x. [DOI] [PubMed] [Google Scholar]
- 50.Kriegl L, Neumann J, Vieth M, et al. Up and downregulation of p16(Ink4a) expression in BRAF-mutated polyps/adenomas indicates a senescence barrier in the serrated route to colon cancer. Mod Pathol. 2011;24:1015–1022. doi: 10.1038/modpathol.2011.43. [DOI] [PubMed] [Google Scholar]
- 51.Suzuki H, Igarashi S, Nojima M, et al. IGFBP7 is a p53-responsive gene specifically silenced in colorectal cancer with CpG island methylator phenotype. Carcinogenesis. 2010;31:342–349. doi: 10.1093/carcin/bgp179. [DOI] [PubMed] [Google Scholar]
- 52.Deng G, Bell I, Crawley S, et al. BRAF mutation is frequently present in sporadic colorectal cancer with methylated hMLH1, but not in hereditary nonpolyposis colorectal cancer. Clin Cancer Res. 2004;10(1 Pt 1):191–195. doi: 10.1158/1078-0432.CCR-1118-3. [DOI] [PubMed] [Google Scholar]
- 53.Spring KJ, Zhao ZZ, Karamatic R, et al. High prevalence of sessile serrated adenomas with BRAF mutations: a prospective study of patients undergoing colonoscopy. Gastroenterology. 2006;131:1400–1407. doi: 10.1053/j.gastro.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 54.Kambara T, Simms LA, Whitehall VL, et al. BRAF mutation is associated with DNA methylation in serrated polyps and cancers of the colorectum. Gut. 2004;53:1137–1144. doi: 10.1136/gut.2003.037671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kolch W. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem J. 2000;351(Pt 2):289–305. doi: 10.1042/bj3510289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carragher LA, Snell KR, Giblett SM, et al. V600EBraf induces gastrointestinal crypt senescence and promotes tumour progression through enhanced CpG methylation of p16INK4a. EMBO Mol Med. 2010;2:458–471. doi: 10.1002/emmm.201000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aust DE, Baretton GB Members of the Working Group GI-Pathology of the German Society of Pathology. Serrated polyps of the colon and rectum (hyperplastic polyps, sessile serrated adenomas, traditional serrated adenomas, and mixed polyps)-proposal for diagnostic criteria. Virchows Arch. 2010;457:291–297. doi: 10.1007/s00428-010-0945-1. [DOI] [PubMed] [Google Scholar]
- 58.Tadepalli US, Feihel D, Miller KM, et al. A morphologic analysis of sessile serrated polyps observed during routine colonoscopy (with video) Gastrointest Endosc. 2011;74:1360–1368. doi: 10.1016/j.gie.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 59.Wallace K, Grau MV, Ahnen D, et al. The association of lifestyle and dietary factors with the risk for serrated polyps of the colorectum. Cancer Epidemiol Biomarkers Prev. 2009;18:2310–2317. doi: 10.1158/1055-9965.EPI-09-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anderson JC, Rangasamy P, Rustagi T, et al. Risk factors for sessile serrated adenomas. J Clin Gastroenterol. 2011;45:694–699. doi: 10.1097/MCG.0b013e318207f3cf. [DOI] [PubMed] [Google Scholar]
- 61.Kimura T, Yamamoto E, Yamano HO, et al. A novel pit pattern identifies the precursor of colorectal cancer derived from sessile serrated adenoma. Am J Gastroenterol. 2012;107:460–469. doi: 10.1038/ajg.2011.457. [DOI] [PubMed] [Google Scholar]
- 62.Hazewinkel Y, López-Cerón M, East JE, et al. Endoscopic features of sessile serrated adenomas: validation by international experts using high-resolution white-light endoscopy and narrow-band imaging. Gastrointest Endosc. 2013;77:916–924. doi: 10.1016/j.gie.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 63.Parikh ND, Chaptini L, Njei B, Laine L. Diagnosis of sessile serrated adenomas/polyps with image-enhanced endoscopy: a systematic review and meta-analysis. Endoscopy. 2016;48:731–739. doi: 10.1055/s-0042-107592. [DOI] [PubMed] [Google Scholar]
- 64.Hewett DG, Kaltenbach T, Sano Y, et al. Validation of a simple classification system for endoscopic diagnosis of small colorectal polyps using narrow-band imaging. Gastroenterology. 2012;143:599–607. doi: 10.1053/j.gastro.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 65.Kumar S, Fioritto A, Mitani A, Desai M, Gunaratnam N, Ladabaum U. Optical biopsy of sessile serrated adenomas: do these lesions resemble hyperplastic polyps under narrow-band imaging? Gastrointest Endosc. 2013;78:902–909. doi: 10.1016/j.gie.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.IJspeert JE, Bastiaansen BA, van Leerdam ME, et al. Development and validation of the WASP classification system for optical diagnosis of adenomas, hyperplastic polyps and sessile serrated adenomas/polyps. Gut. 2016;65:963–970. doi: 10.1136/gutjnl-2014-308411. [DOI] [PubMed] [Google Scholar]
- 67.Nanda KS, Tutticci N, Burgess N, Sonson R, McLeod D, Bourke MJ. Caught in the act: endoscopic characterization of sessile serrated adenomas with dysplasia. Gastrointest Endosc. 2014;79:864–870. doi: 10.1016/j.gie.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 68.Burgess NG, Pellise M, Nanda KS, et al. Clinical and endoscopic predictors of cytological dysplasia or cancer in a prospective multicentre study of large sessile serrated adenomas/polyps. Gut. 2016;65:437–446. doi: 10.1136/gutjnl-2014-308603. [DOI] [PubMed] [Google Scholar]
- 69.Goldstein NS. Serrated pathway and APC (conventional)-type colorectal polyps: molecular-morphologic correlations, genetic pathways, and implications for classification. Am J Clin Pathol. 2006;125:146–153. doi: 10.1309/87BD0C6UCGUG236J. [DOI] [PubMed] [Google Scholar]
- 70.Kamiński MF, Hassan C, Bisschops R, et al. Advanced imaging for detection and differentiation of colorectal neoplasia: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2014;46:435–449. doi: 10.1055/s-0034-1365348. [DOI] [PubMed] [Google Scholar]
- 71.Pohl J, Schneider A, Vogell H, Mayer G, Kaiser G, Ell C. Pancolonic chromoendoscopy with indigo carmine versus standard colonoscopy for detection of neoplastic lesions: a randomised two-centre trial. Gut. 2011;60:485–490. doi: 10.1136/gut.2010.229534. [DOI] [PubMed] [Google Scholar]
- 72.Rex DK, Clodfelter R, Rahmani F, et al. Narrow-band imaging versus white light for the detection of proximal colon serrated lesions: a randomized, controlled trial. Gastrointest Endosc. 2016;83:166–171. doi: 10.1016/j.gie.2015.03.1915. [DOI] [PubMed] [Google Scholar]
- 73.Hazewinkel Y, Tytgat KM, van Leerdam ME, et al. Narrow-band imaging for the detection of polyps in patients with serrated polyposis syndrome: a multicenter, randomized, back-to-back trial. Gastrointest Endosc. 2015;81:531–538. doi: 10.1016/j.gie.2014.06.043. [DOI] [PubMed] [Google Scholar]
- 74.Zorzi M, Senore C, Da Re F, et al. Detection rate and predictive factors of sessile serrated polyps in an organised colorectal cancer screening programme with immunochemical faecal occult blood test: the EQuIPE study (Evaluating Quality Indicators of the Performance of Endoscopy) Gut. 2017;66:1233–1240. doi: 10.1136/gutjnl-2015-310587. [DOI] [PubMed] [Google Scholar]
- 75.Chang LC, Shun CT, Hsu WF, et al. Fecal immunochemical test detects sessile serrated adenomas and polyps with a low level of sensitivity. Clin Gastroenterol Hepatol. 2017;15:872–879.e1. doi: 10.1016/j.cgh.2016.07.029. [DOI] [PubMed] [Google Scholar]
- 76.Butterly L, Robinson CM, Anderson JC, et al. Serrated and adenomatous polyp detection increases with longer withdrawal time: results from the New Hampshire Colonoscopy Registry. Am J Gastroenterol. 2014;109:417–426. doi: 10.1038/ajg.2013.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hewett DG, Rex DK. Miss rate of right-sided colon examination during colonoscopy defined by retroflexion: an observational study. Gastrointest Endosc. 2011;74:246–252. doi: 10.1016/j.gie.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 78.Clark BT, Laine L. High-quality bowel preparation is required for detection of sessile serrated polyps. Clin Gastroenterol Hepatol. 2016;14:1155–1162. doi: 10.1016/j.cgh.2016.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.El Sayed AM, Kanafani ZA, Mourad FH, et al. A randomized single-blind trial of whole versus split-dose polyethylene glycol-electrolyte solution for colonoscopy preparation. Gastrointest Endosc. 2003;58:36–40. doi: 10.1067/mge.2003.318. [DOI] [PubMed] [Google Scholar]
- 80.Gurudu SR, Ramirez FC, Harrison ME, Leighton JA, Crowell MD. Increased adenoma detection rate with system-wide implementation of a split-dose preparation for colonoscopy. Gastrointest Endosc. 2012;76:603–608. doi: 10.1016/j.gie.2012.04.456. [DOI] [PubMed] [Google Scholar]
- 81.Horton N, Garber A, Hasson H, Lopez R, Burke CA. Impact of single- vs. split-dose low-volume bowel preparations on bowel movement kinetics, patient inconvenience, and polyp detection: a prospective trial. Am J Gastroenterol. 2016;111:1330–1337. doi: 10.1038/ajg.2016.273. [DOI] [PubMed] [Google Scholar]
- 82.Gralnek IM. Emerging technological advancements in colonoscopy: Third Eye® Retroscope® and Third Eye® Panoramic(TM), Fuse® Full Spectrum Endoscopy® colonoscopy platform, Extra-Wide-Angle-View colonoscope, and NaviAid(TM) G-EYE(TM) balloon colonoscope. Dig Endosc. 2015;27:223–231. doi: 10.1111/den.12382. [DOI] [PubMed] [Google Scholar]
- 83.Patil R, Ona MA, Ofori E, Reddy M. Endocuff-assisted colonoscopy-a novel accessory in improving adenoma detection rate: a review of the literature. Clin Endosc. 2016;49:533–538. doi: 10.5946/ce.2016.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moriyama T, Uraoka T, Esaki M, Matsumoto T. Advanced technology for the improvement of adenoma and polyp detection during colonoscopy. Dig Endosc. 2015;27( Suppl 1):40–44. doi: 10.1111/den.12428. [DOI] [PubMed] [Google Scholar]
- 85.de Wijkerslooth TR, Stoop EM, Bossuyt PM, et al. Adenoma detection with cap-assisted colonoscopy versus regular colonoscopy: a randomised controlled trial. Gut. 2012;61:1426–1434. doi: 10.1136/gutjnl-2011-301327. [DOI] [PubMed] [Google Scholar]
- 86.van Doorn SC, van der Vlugt M, Depla A, et al. Adenoma detection with Endocuff colonoscopy versus conventional colonoscopy: a multicentre randomised controlled trial. Gut. 2017;66:438–445. doi: 10.1136/gutjnl-2015-310097. [DOI] [PubMed] [Google Scholar]
- 87.Repici A, Hassan C, Vitetta E, et al. Safety of cold polypectomy for <10mm polyps at colonoscopy: a prospective multicenter study. Endoscopy. 2012;44:27–31. doi: 10.1055/s-0031-1291387. [DOI] [PubMed] [Google Scholar]
- 88.Lee CK, Shim JJ, Jang JY. Cold snare polypectomy vs. cold forceps polypectomy using double-biopsy technique for removal of diminutive colorectal polyps: a prospective randomized study. Am J Gastroenterol. 2013;108:1593–1600. doi: 10.1038/ajg.2013.302. [DOI] [PubMed] [Google Scholar]
- 89.Kim JS, Lee BI, Choi H, et al. Cold snare polypectomy versus cold forceps polypectomy for diminutive and small colorectal polyps: a randomized controlled trial. Gastrointest Endosc. 2015;81:741–747. doi: 10.1016/j.gie.2014.11.048. [DOI] [PubMed] [Google Scholar]
- 90.Raad D, Tripathi P, Cooper G, Falck-Ytter Y. Role of the cold biopsy technique in diminutive and small colonic polyp removal: a systematic review and meta-analysis. Gastrointest Endosc. 2016;83:508–515. doi: 10.1016/j.gie.2015.10.038. [DOI] [PubMed] [Google Scholar]
- 91.Metz AJ, Moss A, McLeod D, et al. A blinded comparison of the safety and efficacy of hot biopsy forceps electrocauterization and conventional snare polypectomy for diminutive colonic polypectomy in a porcine model. Gastrointest Endosc. 2013;77:484–490. doi: 10.1016/j.gie.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 92.Burgess NG, Bahin FF, Bourke MJ. Colonic polypectomy (with videos) Gastrointest Endosc. 2015;81:813–835. doi: 10.1016/j.gie.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 93.Hewett DG. Cold snare polypectomy: optimizing technique and technology (with videos) Gastrointest Endosc. 2015;82:693–696. doi: 10.1016/j.gie.2015.04.028. [DOI] [PubMed] [Google Scholar]
- 94.Din S, Ball AJ, Riley SA, Kitsanta P, Johal S. Cold snare polypectomy: does snare type influence outcomes? Dig Endosc. 2015;27:603–608. doi: 10.1111/den.12431. [DOI] [PubMed] [Google Scholar]
- 95.Horiuchi A, Hosoi K, Kajiyama M, Tanaka N, Sano K, Graham DY. Prospective, randomized comparison of 2 methods of cold snare polypectomy for small colorectal polyps. Gastrointest Endosc. 2015;82:686–692. doi: 10.1016/j.gie.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 96.Chukmaitov A, Bradley CJ, Dahman B, Siangphoe U, BouHaidar D, Warren JL. Polypectomy techniques, endoscopist characteristics, and serious gastrointestinal adverse events. J Surg Oncol. 2014;110:207–213. doi: 10.1002/jso.23615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ko CW, Riffle S, Michaels L, et al. Serious complications within 30 days of screening and surveillance colonoscopy are uncommon. Clin Gastroenterol Hepatol. 2010;8:166–173. doi: 10.1016/j.cgh.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fujiya M, Sato H, Ueno N, et al. Efficacy and adverse events of cold vs hot polypectomy: a meta-analysis. World J Gastroenterol. 2016;22:5436–5444. doi: 10.3748/wjg.v22.i23.5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Paspatis GA, Tribonias G, Konstantinidis K, et al. A prospective randomized comparison of cold vs hot snare polypectomy in the occurrence of postpolypectomy bleeding in small colonic polyps. Colorectal Dis. 2011;13:e345–e348. doi: 10.1111/j.1463-1318.2011.02696.x. [DOI] [PubMed] [Google Scholar]
- 100.Ichise Y, Horiuchi A, Nakayama Y, Tanaka N. Prospective randomized comparison of cold snare polypectomy and conventional polypectomy for small colorectal polyps. Digestion. 2011;84:78–81. doi: 10.1159/000323959. [DOI] [PubMed] [Google Scholar]
- 101.Tutticci N, Burgess NG, Pellise M, Mcleod D, Bourke MJ. Characterization and significance of protrusions in the mucosal defect after cold snare polypectomy. Gastrointest Endosc. 2015;82:523–528. doi: 10.1016/j.gie.2015.01.051. [DOI] [PubMed] [Google Scholar]
- 102.Moss A, Bourke MJ, Williams SJ, et al. Endoscopic mucosal resection outcomes and prediction of submucosal cancer from advanced colonic mucosal neoplasia. Gastroenterology. 2011;140:1909–1918. doi: 10.1053/j.gastro.2011.02.062. [DOI] [PubMed] [Google Scholar]
- 103.Ferrara F, Luigiano C, Ghersi S, et al. Efficacy, safety and outcomes of ‘inject and cut’ endoscopic mucosal resection for large sessile and flat colorectal polyps. Digestion. 2010;82:213–220. doi: 10.1159/000284397. [DOI] [PubMed] [Google Scholar]
- 104.Rao AK, Soetikno R, Raju GS, et al. Large sessile serrated polyps can be safely and effectively removed by endoscopic mucosal resection. Clin Gastroenterol Hepatol. 2016;14:568–574. doi: 10.1016/j.cgh.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 105.Rex KD, Vemulapalli KC, Rex DK. Recurrence rates after EMR of large sessile serrated polyps. Gastrointest Endosc. 2015;82:538–541. doi: 10.1016/j.gie.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 106.Liang J, Kalady MF, Church J. Snaring large serrated polyps. Surg Endosc. 2013;27:1622–1627. doi: 10.1007/s00464-012-2640-6. [DOI] [PubMed] [Google Scholar]
- 107.Pellise M, Burgess NG, Tutticci N, et al. Endoscopic mucosal resection for large serrated lesions in comparison with adenomas: a prospective multicentre study of 2000 lesions. Gut. 2017;66:644–653. doi: 10.1136/gutjnl-2015-310249. [DOI] [PubMed] [Google Scholar]
- 108.Cha JM, Lim KS, Lee SH, et al. Clinical outcomes and risk factors of post-polypectomy coagulation syndrome: a multicenter, retrospective, case-control study. Endoscopy. 2013;45:202–207. doi: 10.1055/s-0032-1326104. [DOI] [PubMed] [Google Scholar]
- 109.Burgess NG, Metz AJ, Williams SJ, et al. Risk factors for intraprocedural and clinically significant delayed bleeding after wide-field endoscopic mucosal resection of large colonic lesions. Clin Gastroenterol Hepatol. 2014;12:651–661. doi: 10.1016/j.cgh.2013.09.049. [DOI] [PubMed] [Google Scholar]
- 110.Burgess NG, Williams SJ, Hourigan LF, et al. A management algorithm based on delayed bleeding after wide-field endoscopic mucosal resection of large colonic lesions. Clin Gastroenterol Hepatol. 2014;12:1525–1533. doi: 10.1016/j.cgh.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 111.Pattullo V, Bourke MJ, Tran KL, et al. The suction pseudopolyp technique: a novel method for the removal of small flat nonpolypoid lesions of the colon and rectum. Endoscopy. 2009;41:1032–1037. doi: 10.1055/s-0029-1215294. [DOI] [PubMed] [Google Scholar]
- 112.Din S, Ball AJ, Riley SA, Kitsanta P, Johal S. A randomized comparison of cold snare polypectomy versus a suction pseudopolyp technique. Endoscopy. 2015;47:1005–1010. doi: 10.1055/s-0034-1392533. [DOI] [PubMed] [Google Scholar]
- 113.Choksi N, Elmunzer BJ, Stidham RW, Shuster D, Piraka C. Cold snare piecemeal resection of colonic and duodenal polyps ≥1 cm. Endosc Int Open. 2015;3:E508–E513. doi: 10.1055/s-0034-1392214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Muniraj T, Sahakian A, Ciarleglio MM, Deng Y, Aslanian HR. Cold snare polypectomy for large sessile colonic polyps: a single-center experience. Gastroenterol Res Pract. 2015;2015:175959. doi: 10.1155/2015/175959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Klein A, Bourke MJ. Advanced polypectomy and resection techniques. Gastrointest Endosc Clin N Am. 2015;25:303–333. doi: 10.1016/j.giec.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 116.Holt BA, Bourke MJ. Wide field endoscopic resection for advanced colonic mucosal neoplasia: current status and future directions. Clin Gastroenterol Hepatol. 2012;10:969–979. doi: 10.1016/j.cgh.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 117.Buchner AM, Guarner-Argente C, Ginsberg GG. Outcomes of EMR of defiant colorectal lesions directed to an endoscopy referral center. Gastrointest Endosc. 2012;76:255–263. doi: 10.1016/j.gie.2012.02.060. [DOI] [PubMed] [Google Scholar]
- 118.Swan MP, Bourke MJ, Alexander S, Moss A, Williams SJ. Large refractory colonic polyps: is it time to change our practice? A prospective study of the clinical and economic impact of a tertiary referral colonic mucosal resection and polypectomy service (with videos) Gastrointest Endosc. 2009;70:1128–1136. doi: 10.1016/j.gie.2009.05.039. [DOI] [PubMed] [Google Scholar]
- 119.Hassan C, Quintero E, Dumonceau JM, et al. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2013;45:842–851. doi: 10.1055/s-0033-1344548. [DOI] [PubMed] [Google Scholar]
- 120.Cancer Council Australia Colonoscopy Surveillance Working Party. Clinical practice guidelines for surveillance colonoscopy-in adenoma follow-up: following curative resection of colorectal cancer; and for cancer surveillance in inflammatory bowel disease. Sydney: Cancer Council Australia; 2011. [Google Scholar]