Abstract
Pancreatic cancer (PC) is the third most common cause of cancer-related death in the United States and the 12th most common worldwide. Mortality is high, largely due to late stage of presentation and suboptimal treatment regimens. Approximately 10% of PC cases have a familial basis. The major genetic defect has yet to be identified but may be inherited by an autosomal dominant pattern with reduced penetrance. Several known hereditary syndromes or genes are associated with an increased risk of developing PC and account for approximately 2% of PCs. These syndromes include the hereditary breast-ovarian cancer syndrome, Peutz-Jeghers syndrome, familial atypical multiple mole melanoma, Lynch syndrome, familial polyposis, ataxia-telangiectasia, and hereditary pancreatitis. Appropriate screening using methods such as biomarkers or imaging, with endoscopic ultrasound and magnetic resonance imaging, may assist in the early detection of neoplastic lesions in the high-risk population. If these lesions are detected and treated before the development of invasive carcinoma, PC disease morbidity and mortality may be improved. This review will focus on familial PC and other hereditary syndromes implicated in the increased risk of PC; it will also highlight current screening methods and the future of new screening modalities.
Keywords: Pancreatic neoplasms, Familial pancreatic cancer, Mass screening, High-risk
INTRODUCTION
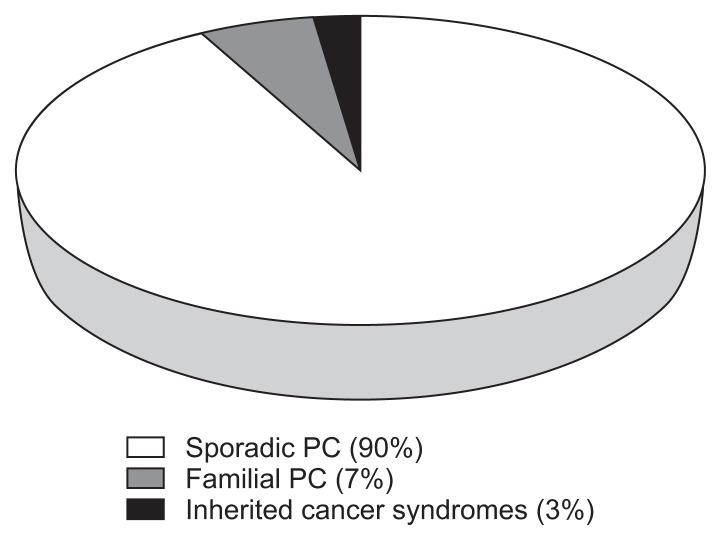
Pancreatic cancer (PC) is the third most common cause of death from cancer in the United States and the 12th most common cancer related death worldwide.1 There were an estimated 49,000 new diagnoses and over 41,000 associated deaths in the United State in 2015 with over 227,000 deaths per year worldwide.2,3 The majority (96%) are cancers that arise from the exocrine pancreas.3 Although diagnostic accuracy and treatments have improved, the survival rates remain dismal with an average 5-year survival of 7%, which can be attributed to the characteristically late stage of the disease at the time of diagnosis.3 Both environmental and genetic risk factors contribute to the disease. PC often develops in three settings: sporadic PC, familial pancreatic cancer (FPC), and inherited cancer syndromes (Fig. 1).4,5 Several reports have estimated that up to 10% of PC cases have a familial basis.4,6 This review will focus on the overview of PC with an emphasis on FPC including risk factors, high-risk screening methods and new screening modalities.
Fig. 1.
Proportions of pancreatic cancer (PC) due to inherited factors.
PANCREATIC CANCER STAGING AND PROGNOSIS
The survival rates of PC are disappointing with 1-year and 5-year survival rates of 29% and 7%, respectively.3 This is in large part due to the lack of symptoms associated with early stages of PC. Patients often present at later stages with weight loss, abdominal discomfort and jaundice.3 Surgical resection is the only curative treatment for PC.7 However, less than 20% of patients are surgical candidates at the time of presentation and the median survival of nonresected patients is 3.5 months.8 Even those patients who are candidates for surgery have a median survival of 12.6 months.8 The absence of symptoms until advanced stages of disease underscores the importance of early detection, both through identification of high-risk individuals and high-risk precursor lesions.
HEREDITARY PANCREATIC CANCER SYNDROMES
Identification of individuals at high-risk of PC based on family history or germline genetic mutations may allow for early detection and therapy. Hereditary pancreatic cancer syndromes include: hereditary breast-ovarian cancer (HBOC), Peutz-Jeghers syndrome, familial atypical multiple mole melanoma (FAMMM), Lynch syndrome (or hereditary nonpolyposis colorectal carcinoma [HNPCC]), familial adenomatous polyposis (FAP), ataxia-telangiectasia (ATM), and hereditary pancreatitis (HP). These hereditary cancer syndromes account for approximately 10% to 15% of hereditary PC cases (Table 1). The genetic etiology of the majority of FPC has yet to be identified.4
Table 1.
Cancer Syndromes and Genes Currently Associated with Pancreatic Cancer
| Syndrome | Gene | Lifetime risk of PC, % | Other associated cancers |
|---|---|---|---|
| Peutz-Jeghers syndrome | STK11/LKB1 | 11–36 | Esophagus, stomach, small intestine, colon, breast, lung, ovary, uterus |
| Familial atypical multiple mole melanoma | p16INK4A (CDKN2A or MTS1) | 17 | Melanoma |
| Hereditary breast cancer | BRCA1 | Increased | Breast, ovarian |
| BRCA2 | RR 3.5–5.9 | ||
| PALB2 | Increased | ||
| ATM | Increased | ||
| Lynch syndrome | HNPCC | 3.7 | Colon, endometrium, ovary, stomach, small intestine, urinary tract, brain, cutaneous sebaceous glands |
| Familial polyposis | APC | 1.7 | Colon, medulloblastoma, papillary thyroid carcinoma, hepatoblastoma, desmoid tumors |
| Hereditary pancreatitis | PRSS1 | 25–40 | None |
PC, pancreatic cancer; RR, relative risk.
FAMILIAL PANCREATIC CANCER
Hereditary PC syndromes only explain a fraction of the clusters of familial based trends. The term FPC applies to families with two or more first-degree relatives (FDRs) with PC that do not fulfill the criteria of any other inherited tumor syndrome.9 FPC accounts for approximately 80% of PC clustering.6 When compared with the general population, the risk of developing PC in individuals with two FDRs has been estimated at 6.4-fold greater risk with a lifetime risk of 8% to 12%; those with three FDRs have a remarkable 32-fold greater risk and a 40% lifetime risk of developing PC.10 One study, using complex segregation analysis, suggested that a yet unidentified major autosomal dominant inherited gene with reduced penetrance could represent a high risk mutation found in FPC.11
A large European study investigated 106 FPC families and found that from one generation to the next, the age of death from PC was younger with each generation: a phenomenon known as anticipation.12 Subsequent studies performed by the European Registry of Hereditary Pancreatitis (EUROPAC) and German national case collection for FPC (FaPaCa) confirmed this phenomenon, showing an earlier development of PC by approximately 10 years in 59% to 80% of FPC families.12,13
Hereditary breast-ovarian cancer (HBOC) syndrome and other Fanconi anemia genes are inclusive of BRCA1, BRCA2/FANCD1, PALB2/FANCN, FANCC, and FANCG. The HBOC syndrome represents early-onset breast and ovarian cancers stemming from germline mutations in BRCA1 and BRCA2 tumor suppressor genes. The Fanconi anemia genes have well-described involvement in multiple DNA repair mechanisms, one of which includes the BRCA1/BRCA2 pathway.14 BRCA2 mutations are the most frequently identified mutation in FPC, associated with a 3.5- to 10-fold increased risk of PC compared to the general population.15,16 The association between BRCA1 mutations and PC is less well defined but has been reported at an approximate 2.5 to 3 times increased risk.17,18 However, two studies found no link between BRCA1 mutation and PC.19,20 Partner and Localizer of BRCA2 (PALB2), also known as (FANCN), is a protein implicated in the nuclear localization and stability required for some functions of BRCA2.21 Its association with PC was first described using whole genome sequencing performed on FPC patients. PALB2 was postulated to be the second most commonly mutated gene in hereditary PC, accounting for 1% to 3% of FPC individuals22 and conferring up to 8.6-fold increased risk.23 However, these results have not been confirmed in other studies.
Peutz-Jeghers syndrome (PJS) is an autosomal dominant polyposis disease associated with an inherited mutation in the STK11/LKB1 tumor suppressor gene.24,25 The typical characteristics of this mutation are mucocutaneous pigmentations of the lips, buccal mucosa and periorbital areas.26 This germline mutation carries an increased risk of cancer with a cumulative risk for all cancers of 93% from age 15 to 64 years.27 Multiple gastrointestinal cancers have been implicated, including gastric and small bowel adenocarcinoma but also nongastrointestinal cancers, such as breast, ovarian, endometrial, cervical and testicular cancers.27 PJS is associated with a 132-fold27 increased risk of PC alone when compared to the general population, and a lifetime PC risk of 11% to 36%.25
Familial atypical multiple mole melanoma (FAMMM) is an autosomal dominant disorder of a germline mutation in p16INK4A (also known as CDKN2A or MTS1). This mutation is commonly known for atypical nevi and high-risk cutaneous malignant melanomas.28 The p16INK4A has been shown to function as a melanoma tumor-suppressor gene. Studies have confirmed this germline mutation of p16INK4A in some American, European and Australian melanoma-prone kindreds.29–31 Several studies also found increased risk of PC and referred to it as a separate syndrome called FAMMM pancreatic cancer (FAMMM-PC). Individuals with this syndrome have a relative risk of 20% to 34% for the development of PC, and an approximate lifetime risk of 17%.32
Lynch syndrome (or HNPCC) is the most common inherited colorectal cancer syndrome and is associated with multiple mutations in mismatch repair genes including MLH1, MSH2, MSH6, PMS2, and EPCAM.33 In addition to having a predisposition for cancers of the colon, endometrium, ovary, stomach, small intestines, urinary tract, brain and cutaneous sebaceous glands, individuals with mismatch repair mutations have a lifetime risk of 3.7% for developing PC.33,34 There is little evidence to support isolated risk attributed to specific genotypes.
Familial adenomatous polyposis (FAP) is a colorectal cancer syndrome resulting from a mutation in the adenomatous polyposis (APC) gene causing hundreds of adenomatous colorectal polyps which if left untreated will inevitably lead to colorectal cancer. FAP syndrome is associated with a 4.5-fold increase of developing PC and a lifetime risk of 1.7%.35
Ataxia-telangiectasia (ATM) is an autosomal recessive disorder characterized by progressive neurologic symptoms and resulting in a marked predisposition for cancer, particularly lymphoma and leukemia.36,37 Monoallelic mutations in the ATM gene, which is involved in DNA repair, confer an increased risk of cancer, particularly breast cancer in females.38 Monoallelic mutations in the ATM gene also result in at least twice the rate of PC compared with the general population.38 One study analyzed a FPC cohort of 166 patients and found that 2.4% were monoallelic mutation carriers; this proportion increased to 4.6% with three or more affected family members.37
Hereditary pancreatitis (PRSS1) is a rare, autosomal dominant form of chronic pancreatitis which presents as repeated episodes of acute pancreatitis during childhood and adolescence resulting in chronic pancreatitis in early adulthood.39 A majority of HP is caused by germline mutation in PRSS1 which codes for the enzyme trypsinogen.40 PRSS1 mutation carriers have increased risk of PC beginning in the fifth decade of life with a lifetime risk of 25% to 40% in comparison to the general population.41
Palladin (PALLD) was found to be overexpressed in a PC prone family and thought to predispose to FPC in an autosomal dominant fashion.42 After finding overexpression of PALLD mRNA in PC tissue, PALLD was postulated to be a proto-oncogene encoding for a component of the cytoskeleton responsible for cell shape and motility.42,43 Follow-up studies, including sequencing of the entire PC genome, have failed to identify somatic mutations in PALLD.43–45 A postulated role of PALLD in FPC requires further investigation.
IDENTIFICATION OF HIGH-RISK INDIVIDUALS
The first step in identification of FPC is construction of a three-generation pedigree. Family members with a history of cancer are identified, and information such as age of onset, age of death, and tobacco exposure is noted. If multiple PCs are identified, or multiple cancers (particularly young onset cancers) are present, it is reasonable to consider FPC or a hereditary PC syndrome and place a referral to a genetic counselor. Previously, working with a genetic counselor meant identifying high-risk tendencies within a family including characteristics such as Ashkenazi Jewish heritage or clusters of breast and ovarian cancers, which might drive testing for specific germline mutations. With expanding capabilities for high-throughput DNA sequencing and declining costs, commercial panel tests for PC (including analysis of upwards of 13 genes) are now available and widely employed.46
Genetic testing may be most informative when affected family members are able to undergo gene testing. The high mortality rate and short survival time in PC often make this difficult. Additionally, since the genetic basis of the majority of FPC is not fully understood, the utility of genetic testing in the absence of testing an affected family member is often further diminished, and gene test results may be uninformative. Despite these limitations, future FPC management and screening will likely include genome sequencing as a part of standard management. When a hereditary PC syndrome is suspected, gene testing and DNA banking of affected family members should be encouraged soon after diagnosis so that other family members may make informed screening and management decisions. Multiple large academic centers offer risk stratification as described above, along with clinical genetic testing and research protocols that may include DNA banking.
MODIFIABLE RISK FACTORS
Risk factors such as tobacco use, obesity, heavy alcohol consumption, and diabetes mellitus (DM) have all been demonstrated to increase the risk of PC.3 These risk factors, although well-described, have not been extensively studied in hereditary PC.
Active cigarette smoking dramatically increases risk for PC with an incidence rate that is twice as high in smokers as in nonsmokers and this risk may be more pronounced in the FPC population.47 In subjects with at least one FDR with PC, the standardized incidence ratio (SIR) for PC in those with tobacco exposure was 19.2, compared with a SIR of 6.2 in nonsmokers.10 Smoking has also been shown to lower the age of onset of PC by 10 years.48 This risk is even higher among smokers with hereditary pancreatitis who tend to develop disease 20 years before nonsmokers.49 Heavy alcohol use (>3 drinks per day) has been associated with a 1.22-fold increased risk of PC.50 This may in part be explained by alcohol’s effect on upregulating inflammatory pathways, which leads to pancreatitis and ultimately may result in necrosis and carcinogenesis.51
DM, another well-known risk factor for PC, has a prevalence in the PC population as high as 40%.52 The mechanism of PC development is not clearly understood in long standing DM but could be related to the cellular proliferative effect of hyperglycemia, hyperinsulinemia, and abnormalities in insulin receptor pathways including insulin growth factor, mammalian target of rapamycin or protein kinase B (AKT).53–55 New-onset DM has also been shown to be an early manifestation of PC known as type 3c, or pancreaticogenic, diabetes.53 Therefore, there exists both a causal and consequential effect of DM on the development of PC. A meta-analysis of 36 studies showed that individuals who were recently diagnosed with DM within the last 4 years had a 50% greater risk of malignancy compared with those who had DM for more than 5 years.56,57 Thus, recent development of DM may be a sign of otherwise asymptomatic PC. However, screening for PC in the setting of new-onset DM is not currently recommended.
PANCREATIC CANCER PRECURSOR LESIONS
Several PC precursor lesions have recognizable latent or early stages of disease.58 These lesions include pancreatic intraepithelial neoplasms (PanINs), intraductal papillary mucinous neoplasm (IPMNs) and mucinous cystic neoplasms (MCNs).
PANCREATIC INTRAEPITHELIAL NEOPLASIA
PanINs are microscopic noninvasive, small epithelial neoplasms.59 Grades of PanINs have been classically defined by degree of atypia: PanIN-1A (flat), PanIN-IB (papillary without dysplasia), PanIN-2 (papillary with dysplastic changes), and PanIN-3 (carcinoma in situ).60 In 2015, revised recommendations for classification uses a two-tiered system of low-grade and high-grade lesions.61 PanIN-2 and PanIN-3 lesions are precursors of invasive PC in patients with both sporadic PC and FPC, yet the frequency and rate at which they progress to invasive cancer is unknown.62 It may take a decade or more for an early PanIN to progress to invasive cancer.63 This presents a challenge for screening, making it difficult to establish an appropriate window for early detection. One study investigated pancreatic tissue after surgical removal and found 82% of invasive cancer specimens harbored PanIN lesions compared to just 28% in normal samples.64 While there is a well-established association between PanIN lesions and invasive cancer, autopsy studies have identified PanIN lesions in the pancreata of up to 48% of healthy controls indicating that not all PanIN lesions progress to PC.65,66 Furthermore, the frequency of PanIN lesions increases when comparing normal pancreata to pancreatitis to ductal adenocarcinoma (16%, 60%, and 82%), respectively.64 Since PanIN-1 lesions likely confer little risk and PanIN-3 lesions greater risk, identification of higher grade indicates neoplastic potential and would be a target of interest in a screening and surveillance program.62 PanIN lesions are particularly important to identify in hereditary syndromes as they are found with 2.75-fold increased frequency in familial PC cases.67
PANCREATIC CYSTIC LESIONS: MCNs AND IPMNs
IPMNs are grossly visible, noninvasive, mucin-producing epithelial neoplasms. IPMNs can be classified into three categories which include main duct IPMN (MD-IPMN), branch duct IPMN (BD-IPMN) and mixed type.59 IPMNs affect men slightly more than women and are located most frequently in the pancreatic head.59,68,69 MD-IPMNs have a much higher incidence of malignancy (24% to 45%), while BD-IPMNs are often incidental findings with a lower risk for malignancy managed with close surveillance.70,71 Mixed type IPMN shows features of both MD-IPMN and BD-IPMN.71 BD-IPMNs have been estimated to grow at an average rate of 1.1 mm per year; cysts that grow at a faster rate of more than 2.2 mm per year represent a higher risk for malignancy.72 It takes approximately 5 years from the time of development of an IPMN to progress to an invasive carcinoma.73
MCNs are mucin-producing, cyst-forming epithelial neoplasms with distinctive ovarian-type stroma. They almost always occur in middle aged females (99.7%) and are located predominantly in the body or tail of the pancreas (94.6%).74 Noninvasive MCNs have a 5-year survival approaching 100%, while invasive MCNs have a 5-year survival of 57%.75
RECOMMENDATIONS FOR SCREENING
Widespread cancer screening programs are reserved for populations with high disease prevalence.76 PC has a low population prevalence with approximately 68 out of 100,000 individuals over the age of 55 developing PC yearly77 and a lifetime risk of approximately 1.5% in the general population.78 Given this low incidence, screening for the general population is not recommended. A hypothetical scenario of 100,000 individuals in the general population using a screening test with 100% sensitivity and 98% specificity would result in only 68 true positive test results and nearly 2,000 false positive results. This high false positive rate would lead to unnecessary, expensive and often invasive testing for individuals who have no increased risk for PC. Therefore, a current goal is to identify high risk individuals, like those with FPC or hereditary PC syndromes, who may benefit from PC screening.
As first described by Wilson and Jungner79 in 1968, several important criteria must be met in order to consider screening for a disease: (1) the disease for which one is screening must be an important health issue; (2) precursor lesions must be recognized during a latent or early asymptomatic stage; (3) facilities for diagnosis and management of the PC must be available; (4) treatments are acceptable to patients; and (5) testing for the disease must be suitable to both the medical community and the population to be screened. Additional objectives that need to be addressed in order to achieve early detection and cure of PC include further understanding of the natural history of PC, developing consensus policies on individuals who are candidates for screening, and development of effective and cost-effective screening tools.
PANCREATIC CANCER SCREENING
At some institutions, individuals at risk for PC are considered for screening if they carry a >5% lifetime risk of developing PC compared to the general population.9,76 In an effort to develop more concrete screening and surveillance guidelines, the International Cancer of the Pancreas Screening (CAPS) Consortium, a multidisciplinary panel of 49 experts, convened in 2011 to answer the following questions: who should be screened, how should high-risk individuals be screened and followed, and how to define success from PC screening.76 The individuals who are recommended for screening by CAPS guidelines are listed in Table 2. There was no consensus on what age to initiate screening, although a majority recommended starting at age 50 unless high risk factors are present; for example mutation in the PRSS1 gene, PJS, or individuals who smoke, all of which have well known association with earlier onset of PC.48,49,76
Table 2.
Current Cancer of the Pancreas Screening Consensus Guidelines for Pancreatic Cancer Screening
| Candidates for pancreatic cancer screening |
|---|
| Individuals with ≥3 affected blood relatives, at least one of who is a FDR |
| Individuals with ≥2 affected FDRs with PC, with at least one affected FDR |
| Individuals with Peutz-Jeghers syndrome |
| Mutation carriers of p16, BRCA2, PALB2 with one affected FDR |
| Mutation carriers of BRCA2 with two affected family members, even if no FDRs |
| Mutation carriers of MMR (Lynch syndrome) with one affected FDR |
FDR, first-degree relative; PC, pancreatic cancer.
TRADITIONAL SCREENING MODALITIES
1. Imaging
Most centers consider endoscopic ultrasonography (EUS) and magnetic resonance imaging (MRI) with magnetic resonance cholangiogram (MRCP) to be the most accurate tools for pancreatic imaging. These modalities are more sensitive at detecting very small pancreatic lesions with the benefit of no ionizing radiation when compared with computed tomography (CT).9,76 MRI/MRCP is often used due to its ability to detect small cystic pancreatic lesions or abnormalities of the pancreatic duct. While MRI is noninvasive and can also detect extrapancreatic lesions, not all patients can tolerate the procedure due to claustrophobia. Furthermore, if a lesion is detected, the patient may require a confirmatory EUS. In a study of high risk individuals, EUS identified pancreatic lesions in 42.6% of participants, MRI/MRCP in 33.3% and CT in 11%.80 EUS is often favored given its ability to detect small solid or cystic lesions <1 cm.81 EUS can also be paired with fine needle aspiration in the event that sampling is necessary, with a sensitivity, specificity, and accuracy of 91%, 100%, and 92%, respectively in diagnosing pancreatic malignancy.82 However, EUS requires gastroenterologists with an advanced level of training, which ultimately results in an operator dependent study with problematic predictive value.83 Furthermore, the procedure is invasive, often requiring anesthesia, with complications of endoscopy that may result in bleeding, infection, or bowel perforation. Data in high-risk individuals suggests that EUS may be superior in detecting solid lesions, while MRI may have improved sensitivity for cystic lesions.80,84 Over-diagnosis is a major concern, particularly given the modest inter-observer agreement between imaging modalities.85,86 The result may be overtreatment of benign lesions, a grave risk when taking into account the morbidity and mortality involved in pancreatic surgery. The strengths and weaknesses of traditional screening methods are summarized in Table 3.
Table 3.
Strengths and Weaknesses of Current Screening and Surveillance Methods and New Methods under Development
| Strength | Weakness | |
|---|---|---|
| Currently available screening method | ||
| Computed tomography | Rapid time interval for diagnosis | Ionizing radiation exposure Limitations with imaging small lesions |
| Magnetic resonance imaging | Accurate No ionizing radiation Can detect extrapancreatic lesions Superior ability to detect small cystic lesions |
Over-diagnosis May have limitations in detecting small solid lesions Some patient have difficulty tolerating imaging test |
| Endoscopic ultrasound | Ability to do fine-needle aspiration Superior ability to detect small solid lesion |
Invasive procedure Operator dependent Requires anesthesia |
| Biomarkers: CEA/CA 19-9 | Measures progression of established disease Measures response to chemotherapy |
Not a reliable screening or surveillance tool May be elevated in nonpancreatic disease |
| Screening methods under development | ||
| Pancreatic juice (TP53, KRAS, GNAS) | May identify specific gene mutations in pancreatic cancer development | Requires endoscopic ultrasound |
| Stool DNA | Noninvasive | Under development May be a complimentary tool to other screening methods Would require confirmatory testing |
| Methylated DNA markers | Potential to discriminate between high-grade and low-grade precursor lesions | Under development May be a complimentary tool to other screening methods Would require confirmatory testing |
| MicroRNA | Helpful diagnostic and prognostic biomarker Reasonable sensitivity and specificity for tumors |
Under development May be a complimentary tool to other screening methods Would require confirmatory testing |
2. Biomarkers
The role of tumor markers is currently limited in the screening of asymptomatic individuals for PC. Several tumor markers have been evaluated including carbohydrate antigen 19-9 (CA 19-9) and carcinoembryonic antigen but none have proven useful as a screening modality.9,76 CA 19-9 is a biomarker that is used in monitoring PC disease recurrence and is therefore most informative when used to measure response to adjuvant chemotherapy or in monitoring after PC resection.87,88 One study compiled data for the use of CA 19-9 screening in the general population and found a median sensitivity of 0.79 (range, 70% to 90%), specificity of 0.82 (68% to 91%), positive predictive value of 72 (41 to 95) and negative predictive value of 81 (65 to 98) in diagnosing PC. Based on this data, CA 19-9 as a screening test cannot be used alone confidently.89,90
NEW SCREENING MODALITIES
Pancreatic juice collected from the duodenum, has recently received interest as a potential source for biomarker measurement. Pancreatic juice is made up of a remarkably rich source of proteins that are shed by the pancreatic ductal cells.91 One study compared the proteins extracted from the pancreatic juice in patients with pancreatitis versus patients with PC; of the 72 proteins isolated, nine proteins were distinctly expressed in PC patients alone.92 Some high risk markers isolated from pancreatic juice include mutations in TP53, KRAS and guanine nucleotide binding protein alpha stimulating (GNAS). The TP53 gene has proven to be highly specific for invasive PC and high grade dysplasia (HGD), found in up to 75% of invasive PCs.93 The concomitantly high proportion of PanIN-3 lesions in this population supports PanIN-3 lesions as a precursor source for TP53 mutations.94 GNAS is another well-known mutation that is highly specific for IPMNs; GNAS has been detected in approximately 64% of individuals with IPMNs and 0% of healthy controls.95 The prevalence of GNAS found in pancreatic juice of patients with IPMNS was similar to that isolated in resected specimens of IPMNs.94 Conversely, KRAS mutations have been isolated in more than 90% of PanIN lesions and a majority of IPMNs and MCNs. KRAS mutations alone cannot reliably distinguish low grade precursors from HGD, as they have been seen in both invasive PCs (73%) but also healthy controls (19%), who likely harbor benign PanIN-1 lesions.94 See Table 3 for a summary of strengths and weaknesses of new screening methods under development.
Stool DNA is a promising, noninvasive approach used to test for DNA in the stool offering an opportunity to analyze excreted exfoliants.96 Initially for colorectal screening, this technique is being explored for PC screening. One study investigated nine targeted genes by real-time methylation specific PCR in PC cases and found that methylated BMP3 alone detected 51% of PCs, mutant KRAS detected 50%, and combination of the two markers detected 67% of PCs.97 Further work in this area is required.
MicroRNA has been isolated in the serum, plasma, saliva, stool, and pancreatic juice and consists of small noncoding RNAs that are cleaved from 70 to 100 nucleotide hairpin pre-microRNA precursors into mature forms of 19 to 25 nucleotides.98 Because microRNAs act as essential posttranscriptional regulators of gene expression, they are important diagnostic and prognostic markers for many solid cancers.99,100 MicroRNA distinctive to PC could serve as an important diagnostic biomarker.98 While many studies of microRNAs in PC have been performed with varying results, miR-21, miR-155, miR-196, and miR-210 have been consistently dysregulated in PC. Additional dysregulation of miR-21, miR-155, and miR-196 has also been noted in IPMNs and PanIN lesions.101 One study isolated 38 distinct microRNAs from whole blood that were found to be significantly dysregulated in patients with PC compared with healthy controls.102 Two microRNA panels were formulated, one comprising four microRNAs (miR-145, miR-150, miR-223, and miR-636) and another with 10 microRNAs (miR-26b, miR-34a, miR-122, miR-126, miR-145, miR-150, miR-223, miR-505, miR-636, and miR-885.5p). These panels have shown promise as a test for stage IA-IIB PC, especially in combination with CA 19-9 with performance measured as the area under the curve (AUC) resulting in an AUC of 0.83 and 0.91 for each panel respectively.102 Later work investigating microRNA in the plasma as an adjunct to CA-19-9 distinguished PC from non-PC tissue with a sensitivity of 92.0% and specificity of 95.6%.99 The combined diagnostic method was most effective at diagnosing early stage 1 tumors (85.2%) and therefore could serve as a complementary tool for early PC diagnosis.99
Methylated DNA Markers have recently been used in discerning high-grade precursor lesions (IPMNs with HGD, PanIN-3, or invasive cancers) from low-grade precursor lesions (IPMNs with low grade dysplasia, PanIN-1, or PanIN-2).103,104 DNA methylation events unique to tumor type and site can be advantageous in isolating high risk pancreatic lesions. A recent study used reduced bisulfate sequencing (RRBS) on DNA from normal frozen pancreatic tissue and neoplastic tissue to identify a panel of markers (TBX15, VWC2, PRKCB, CLEC11A, EMX1, ELM01, DLX 4, ABCB1, ST8SIA1, and SP9) that had strong discrimination between high grade precursor lesions and low-grade precursor lesions with an AUC of >0.85.103,104 The panel detected 89%, 87%, 77%, and 74% of cases at respective specificities of 85%, 90%, 95%, and 100%.96 Several of the RRBS-discovered markers have been found on genes known to be important in tumorigenesis, cell signaling, and epithelial-to-mesenchymal transition.104
CONCLUSIONS
Although treatments of PC has improved, the survival rates remain dismal with an average 5-year survival of 7%. Because of the low incidence of PC in the general population, population-based screening is not recommended. Therefore, recognition of high-risk individuals including those with FPC and other hereditary syndromes is imperative to identify early stages of disease. By recognizing premalignant lesions, we may identify individuals who are candidates for screening and possibly resection of early stage disease. The clinical importance of precursor lesions (PanINs and IPMNs) is becoming better understood as their natural history is defined. This further permits the application of evidence based strategies to develop guidelines for management of these lesions. Increased accessibility of genome sequencing will enable more accurate identification of high risk genes and new targeted gene therapies.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Cancer Society. Cancer facts & figures 2016. Atlanta: American Cancer Society; 2016. [Google Scholar]
- 4.Hruban RH, Canto MI, Goggins M, Schulick R, Klein AP. Update on familial pancreatic cancer. Adv Surg. 2010;44:293–311. doi: 10.1016/j.yasu.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chari ST, Kelly K, Hollingsworth MA, et al. Early detection of sporadic pancreatic cancer: summative review. Pancreas. 2015;44:693–712. doi: 10.1097/MPA.0000000000000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartsch DK, Gress TM, Langer P. Familial pancreatic cancer: current knowledge. Nat Rev Gastroenterol Hepatol. 2012;9:445–453. doi: 10.1038/nrgastro.2012.111. [DOI] [PubMed] [Google Scholar]
- 7.Ni X, Yang J, Li M. Imaging-guided curative surgical resection of pancreatic cancer in a xenograft mouse model. Cancer Lett. 2012;324:179–185. doi: 10.1016/j.canlet.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bilimoria KY, Bentrem DJ, Ko CY, et al. Validation of the 6th edition AJCC pancreatic cancer staging system: report from the national cancer database. Cancer. 2007;110:738–744. doi: 10.1002/cncr.22852. [DOI] [PubMed] [Google Scholar]
- 9.Brand RE, Lerch MM, Rubinstein WS, et al. Advances in counselling and surveillance of patients at risk for pancreatic cancer. Gut. 2007;56:1460–1469. doi: 10.1136/gut.2006.108456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klein AP, Brune KA, Petersen GM, et al. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res. 2004;64:2634–2638. doi: 10.1158/0008-5472.CAN-03-3823. [DOI] [PubMed] [Google Scholar]
- 11.Klein AP, Beaty TH, Bailey-Wilson JE, Brune KA, Hruban RH, Petersen GM. Evidence for a major gene influencing risk of pancreatic cancer. Genet Epidemiol. 2002;23:133–149. doi: 10.1002/gepi.1102. [DOI] [PubMed] [Google Scholar]
- 12.McFaul CD, Greenhalf W, Earl J, et al. Anticipation in familial pancreatic cancer. Gut. 2006;55:252–258. doi: 10.1136/gut.2005.065045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneider R, Slater EP, Sina M, et al. German national case collection for familial pancreatic cancer (FaPaCa): ten years experience. Fam Cancer. 2011;10:323–330. doi: 10.1007/s10689-010-9414-x. [DOI] [PubMed] [Google Scholar]
- 14.D’Andrea AD, Grompe M. The Fanconi anaemia/BRCA pathway. Nat Rev Cancer. 2003;3:23–34. doi: 10.1038/nrc970. [DOI] [PubMed] [Google Scholar]
- 15.Breast Cancer Linkage Consortium. Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst. 1999;91:1310–1316. doi: 10.1093/jnci/91.15.1310. [DOI] [PubMed] [Google Scholar]
- 16.van Asperen CJ, Brohet RM, Meijers-Heijboer EJ, et al. Cancer risks in BRCA2 families: estimates for sites other than breast and ovary. J Med Genet. 2005;42:711–719. doi: 10.1136/jmg.2004.028829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson D, Easton DF Breast Cancer Linkage Consortium. Cancer incidence in BRCA1 mutation carriers. J Natl Cancer Inst. 2002;94:1358–1365. doi: 10.1093/jnci/94.18.1358. [DOI] [PubMed] [Google Scholar]
- 18.Brose MS, Rebbeck TR, Calzone KA, Stopfer JE, Nathanson KL, Weber BL. Cancer risk estimates for BRCA1 mutation carriers identified in a risk evaluation program. J Natl Cancer Inst. 2002;94:1365–1372. doi: 10.1093/jnci/94.18.1365. [DOI] [PubMed] [Google Scholar]
- 19.Moran A, O’Hara C, Khan S, et al. Risk of cancer other than breast or ovarian in individuals with BRCA1 and BRCA2 mutations. Fam Cancer. 2012;11:235–242. doi: 10.1007/s10689-011-9506-2. [DOI] [PubMed] [Google Scholar]
- 20.Ford D, Easton DF, Bishop DT, Narod SA, Goldgar DE. Risks of cancer in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Lancet. 1994;343:692–695. doi: 10.1016/S0140-6736(94)91578-4. [DOI] [PubMed] [Google Scholar]
- 21.Rahman N, Seal S, Thompson D, et al. PALB2, which encodes a BRCA2-interacting protein, is a breast cancer susceptibility gene. Nat Genet. 2007;39:165–167. doi: 10.1038/ng1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tischkowitz MD, Sabbaghian N, Hamel N, et al. Analysis of the gene coding for the BRCA2-interacting protein PALB2 in familial and sporadic pancreatic cancer. Gastroenterology. 2009;137:1183–1186. doi: 10.1053/j.gastro.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones S, Hruban RH, Kamiyama M, et al. Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science. 2009;324:217. doi: 10.1126/science.1171202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenne DE, Reimann H, Nezu J, et al. Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat Genet. 1998;18:38–43. doi: 10.1038/ng0198-38. [DOI] [PubMed] [Google Scholar]
- 25.van Lier MG, Wagner A, Mathus-Vliegen EM, Kuipers EJ, Steyerberg EW, van Leerdam ME. High cancer risk in Peutz-Jeghers syndrome: a systematic review and surveillance recommendations. Am J Gastroenterol. 2010;105:1258–1264. doi: 10.1038/ajg.2009.725. [DOI] [PubMed] [Google Scholar]
- 26.Tomlinson IP, Houlston RS. Peutz-Jeghers syndrome. J Med Genet. 1997;34:1007–1011. doi: 10.1136/jmg.34.12.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giardiello FM, Brensinger JD, Tersmette AC, et al. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology. 2000;119:1447–1453. doi: 10.1053/gast.2000.20228. [DOI] [PubMed] [Google Scholar]
- 28.Goldstein AM, Chan M, Harland M, et al. Features associated with germline CDKN2A mutations: a GenoMEL study of melanoma-prone families from three continents. J Med Genet. 2007;44:99–106. doi: 10.1136/jmg.2006.043802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamb A, Shattuck-Eidens D, Eeles R, et al. Analysis of the p16 gene (CDKN2) as a candidate for the chromosome 9p melanoma susceptibility locus. Nat Genet. 1994;8:23–26. doi: 10.1038/ng0994-22. [DOI] [PubMed] [Google Scholar]
- 30.Hussussian CJ, Struewing JP, Goldstein AM, et al. Germline p16 mutations in familial melanoma. Nat Genet. 1994;8:15–21. doi: 10.1038/ng0994-15. [DOI] [PubMed] [Google Scholar]
- 31.Goldstein AM, Dracopoli NC, Engelstein M, Fraser MC, Clark WH, Jr, Tucker MA. Linkage of cutaneous malignant melanoma/dysplastic nevi to chromosome 9p, and evidence for genetic heterogeneity. Am J Hum Genet. 1994;54:489–496. [PMC free article] [PubMed] [Google Scholar]
- 32.Goldstein AM, Fraser MC, Struewing JP, et al. Increased risk of pancreatic cancer in melanoma-prone kindreds with p16INK4 mutations. N Engl J Med. 1995;333:970–974. doi: 10.1056/NEJM199510123331504. [DOI] [PubMed] [Google Scholar]
- 33.Kastrinos F, Stoffel EM. History, genetics, and strategies for cancer prevention in Lynch syndrome. Clin Gastroenterol Hepatol. 2014;12:715–727. doi: 10.1016/j.cgh.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Win AK, Young JP, Lindor NM, et al. Colorectal and other cancer risks for carriers and noncarriers from families with a DNA mismatch repair gene mutation: a prospective cohort study. J Clin Oncol. 2012;30:958–964. doi: 10.1200/JCO.2011.39.5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galiatsatos P, Foulkes WD. Familial adenomatous polyposis. Am J Gastroenterol. 2006;101:385–398. doi: 10.1111/j.1572-0241.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- 36.Swift M, Chase CL, Morrell D. Cancer predisposition of ataxia-telangiectasia heterozygotes. Cancer Genet Cytogenet. 1990;46:21–27. doi: 10.1016/0165-4608(90)90004-T. [DOI] [PubMed] [Google Scholar]
- 37.Roberts NJ, Jiao Y, Yu J, et al. ATM mutations in patients with hereditary pancreatic cancer. Cancer Discov. 2012;2:41–46. doi: 10.1158/2159-8290.CD-11-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swift M, Morrell D, Massey RB, Chase CL. Incidence of cancer in 161 families affected by ataxia-telangiectasia. N Engl J Med. 1991;325:1831–1836. doi: 10.1056/NEJM199112263252602. [DOI] [PubMed] [Google Scholar]
- 39.Férec C, Raguénès O, Salomon R, et al. Mutations in the cationic trypsinogen gene and evidence for genetic heterogeneity in hereditary pancreatitis. J Med Genet. 1999;36:228–232. [PMC free article] [PubMed] [Google Scholar]
- 40.LaRusch J, Whitcomb DC. Genetics of pancreatitis. Curr Opin Gastroenterol. 2011;27:467–474. doi: 10.1097/MOG.0b013e328349e2f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lowenfels AB, Maisonneuve P, DiMagno EP, et al. Hereditary pancreatitis and the risk of pancreatic cancer. J Natl Cancer Inst. 1997;89:442–446. doi: 10.1093/jnci/89.6.442. [DOI] [PubMed] [Google Scholar]
- 42.Pogue-Geile KL, Chen R, Bronner MP, et al. Palladin mutation causes familial pancreatic cancer and suggests a new cancer mechanism. PLoS Med. 2006;3:e516. doi: 10.1371/journal.pmed.0030516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klein AP, Borges M, Griffith M, et al. Absence of deleterious palladin mutations in patients with familial pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:1328–1330. doi: 10.1158/1055-9965.EPI-09-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones S, Zhang X, Parsons DW, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Slater E, Amrillaeva V, Fendrich V, et al. Palladin mutation causes familial pancreatic cancer: absence in European families. PLoS Med. 2007;4:e164. doi: 10.1371/journal.pmed.0040164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ambry Genetics. PancNext [Internet] Aliso Viejo: Ambry Genetics; c2016. [cited 2017 Jan 11]. Available from: http://www.ambrygen.com/tests/pancnextambrygen.com/tests/pancnext. [Google Scholar]
- 47.Bosetti C, Lucenteforte E, Silverman DT, et al. Cigarette smoking and pancreatic cancer: an analysis from the International Pancreatic Cancer Case-Control Consortium (Panc4) Ann Oncol. 2012;23:1880–1888. doi: 10.1093/annonc/mdr541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rulyak SJ, Lowenfels AB, Maisonneuve P, Brentnall TA. Risk factors for the development of pancreatic cancer in familial pancreatic cancer kindreds. Gastroenterology. 2003;124:1292–1299. doi: 10.1016/S0016-5085(03)00272-5. [DOI] [PubMed] [Google Scholar]
- 49.Lowenfels AB, Maisonneuve P, Whitcomb DC, Lerch MM, DiMagno EP. Cigarette smoking as a risk factor for pancreatic cancer in patients with hereditary pancreatitis. JAMA. 2001;286:169–170. doi: 10.1001/jama.286.2.169. [DOI] [PubMed] [Google Scholar]
- 50.Tramacere I, Scotti L, Jenab M, et al. Alcohol drinking and pancreatic cancer risk: a meta-analysis of the dose-risk relation. Int J Cancer. 2010;126:1474–1486. doi: 10.1002/ijc.24936. [DOI] [PubMed] [Google Scholar]
- 51.Duell EJ. Epidemiology and potential mechanisms of tobacco smoking and heavy alcohol consumption in pancreatic cancer. Mol Carcinog. 2012;51:40–52. doi: 10.1002/mc.20786. [DOI] [PubMed] [Google Scholar]
- 52.Chari ST, Leibson CL, Rabe KG, et al. Pancreatic cancer-associated diabetes mellitus: prevalence and temporal association with diagnosis of cancer. Gastroenterology. 2008;134:95–101. doi: 10.1053/j.gastro.2007.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cui Y, Andersen DK. Diabetes and pancreatic cancer. Endocr Relat Cancer. 2012;19:F9–F26. doi: 10.1530/ERC-12-0105. [DOI] [PubMed] [Google Scholar]
- 54.Gong J, Robbins LA, Lugea A, Waldron RT, Jeon CY, Pandol SJ. Diabetes, pancreatic cancer, and metformin therapy. Front Physiol. 2014;5:426. doi: 10.3389/fphys.2014.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bao B, Wang Z, Li Y, et al. The complexities of obesity and diabetes with the development and progression of pancreatic cancer. Biochim Biophys Acta. 2011;1815:135–146. doi: 10.1016/j.bbcan.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pannala R, Leirness JB, Bamlet WR, Basu A, Petersen GM, Chari ST. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology. 2008;134:981–987. doi: 10.1053/j.gastro.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huxley R, Ansary-Moghaddam A, Berrington de González A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92:2076–2083. doi: 10.1038/sj.bjc.6602619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Andreotti G, Silverman DT. Occupational risk factors and pancreatic cancer: a review of recent findings. Mol Carcinog. 2012;51:98–108. doi: 10.1002/mc.20779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hruban RH, Maitra A, Kern SE, Goggins M. Precursors to pancreatic cancer. Gastroenterol Clin North Am. 2007;36:831–849. doi: 10.1016/j.gtc.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Distler M, Aust D, Weitz J, Pilarsky C, Grützmann R. Precursor lesions for sporadic pancreatic cancer: PanIN, IPMN, and MCN. Biomed Res Int. 2014;2014:474905. doi: 10.1155/2014/474905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Basturk O, Hong SM, Wood LD, et al. A revised classification system and recommendations from the Baltimore Consensus Meeting for neoplastic precursor lesions in the pancreas. Am J Surg Pathol. 2015;39:1730–1741. doi: 10.1097/PAS.0000000000000533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sipos B, Frank S, Gress T, Hahn S, Klöppel G. Pancreatic intraepithelial neoplasia revisited and updated. Pancreatology. 2009;9:45–54. doi: 10.1159/000178874. [DOI] [PubMed] [Google Scholar]
- 63.Yachida S, Jones S, Bozic I, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–1117. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Andea A, Sarkar F, Adsay VN. Clinicopathological correlates of pancreatic intraepithelial neoplasia: a comparative analysis of 82 cases with and 152 cases without pancreatic ductal adenocarcinoma. Mod Pathol. 2003;16:996–1006. doi: 10.1097/01.MP.0000087422.24733.62. [DOI] [PubMed] [Google Scholar]
- 65.Pour PM, Sayed S, Sayed G. Hyperplastic, preneoplastic and neoplastic lesions found in 83 human pancreases. Am J Clin Pathol. 1982;77:137–152. doi: 10.1093/ajcp/77.2.137. [DOI] [PubMed] [Google Scholar]
- 66.Cubilla AL, Fitzgerald PJ. Morphological lesions associated with human primary invasive nonendocrine pancreas cancer. Cancer Res. 1976;36:2690–2698. [PubMed] [Google Scholar]
- 67.Shi C, Klein AP, Goggins M, et al. Increased prevalence of precursor lesions in familial pancreatic cancer patients. Clin Cancer Res. 2009;15:7737–7743. doi: 10.1158/1078-0432.CCR-09-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crippa S, Fernández-Del Castillo C, Salvia R, et al. Mucin-producing neoplasms of the pancreas: an analysis of distinguishing clinical and epidemiologic characteristics. Clin Gastroenterol Hepatol. 2010;8:213–219. doi: 10.1016/j.cgh.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Werner J, Fritz S, Büchler MW. Intraductal papillary mucinous neoplasms of the pancreas: a surgical disease. Nat Rev Gastroenterol Hepatol. 2012;9:253–259. doi: 10.1038/nrgastro.2012.31. [DOI] [PubMed] [Google Scholar]
- 70.Farrell JJ, Fernández-del Castillo C. Pancreatic cystic neoplasms: management and unanswered questions. Gastroenterology. 2013;144:1303–1315. doi: 10.1053/j.gastro.2013.01.073. [DOI] [PubMed] [Google Scholar]
- 71.Ferrone CR, Correa-Gallego C, Warshaw AL, et al. Current trends in pancreatic cystic neoplasms. Arch Surg. 2009;144:448–454. doi: 10.1001/archsurg.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kang MJ, Jang JY, Kim SJ, et al. Cyst growth rate predicts malignancy in patients with branch duct intraductal papillary mucinous neoplasms. Clin Gastroenterol Hepatol. 2011;9:87–93. doi: 10.1016/j.cgh.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 73.Sohn TA, Yeo CJ, Cameron JL, et al. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg. 2004;239:788–797. doi: 10.1097/01.sla.0000128306.90650.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goh BK, Tan YM, Chung YF, et al. A review of mucinous cystic neoplasms of the pancreas defined by ovarian-type stroma: clinicopathological features of 344 patients. World J Surg. 2006;30:2236–2245. doi: 10.1007/s00268-006-0126-1. [DOI] [PubMed] [Google Scholar]
- 75.Crippa S, Salvia R, Warshaw AL, et al. Mucinous cystic neoplasm of the pancreas is not an aggressive entity: lessons from 163 resected patients. Ann Surg. 2008;247:571–579. doi: 10.1097/SLA.0b013e31811f4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Canto MI, Harinck F, Hruban RH, et al. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut. 2013;62:339–347. doi: 10.1136/gutjnl-2012-303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wolfgang CL, Herman JM, Laheru DA, et al. Recent progress in pancreatic cancer. CA Cancer J Clin. 2013;63:318–348. doi: 10.3322/caac.21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975–2012 [Internet] Bethesda: National Cancer Institute; c2015. [cited 2017 Jan 11]. Available from: http://seer.cancer.gov/csr/1975_2012/ [Google Scholar]
- 79.Wilson JM, Jungner G. Principles and practice of screening for disease. Geneva: World Health Organization; 1968. [Google Scholar]
- 80.Canto MI, Hruban RH, Fishman EK, et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology. 2012;142:796–804. doi: 10.1053/j.gastro.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kimmey MB, Bronner MP, Byrd DR, Brentnall TA. Screening and surveillance for hereditary pancreatic cancer. Gastrointest Endosc. 2002;56:S82–S86. doi: 10.1016/S0016-5107(02)70092-8. [DOI] [PubMed] [Google Scholar]
- 82.Raut CP, Grau AM, Staerkel GA, et al. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration in patients with presumed pancreatic cancer. J Gastrointest Surg. 2003;7:118–126. doi: 10.1016/S1091-255X(02)00150-6. [DOI] [PubMed] [Google Scholar]
- 83.Chang MC, Wong JM, Chang YT. Screening and early detection of pancreatic cancer in high risk population. World J Gastroenterol. 2014;20:2358–2364. doi: 10.3748/wjg.v20.i9.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Harinck F, Konings IC, Kluijt I, et al. A multicenter comparative prospective blinded analysis of EUS and MRI for screening of pancreatic cancer in high-risk individuals. Gut. 2016;65:1505–1513. doi: 10.1136/gutjnl-2014-308008. [DOI] [PubMed] [Google Scholar]
- 85.Al-Sukhni W, Borgida A, Rothenmund H, et al. Screening for pancreatic cancer in a high-risk cohort: an eight-year experience. J Gastrointest Surg. 2012;16:771–783. doi: 10.1007/s11605-011-1781-6. [DOI] [PubMed] [Google Scholar]
- 86.Topazian M, Enders F, Kimmey M, et al. Interobserver agreement for EUS findings in familial pancreatic-cancer kindreds. Gastrointest Endosc. 2007;66:62–67. doi: 10.1016/j.gie.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 87.Fong ZV, Winter JM. Biomarkers in pancreatic cancer: diagnostic, prognostic, and predictive. Cancer J. 2012;18:530–538. doi: 10.1097/PPO.0b013e31827654ea. [DOI] [PubMed] [Google Scholar]
- 88.Datta J, Vollmer CM., Jr Investigational biomarkers for pancreatic adenocarcinoma: where do we stand? South Med J. 2014;107:256–263. doi: 10.1097/SMJ.0000000000000088. [DOI] [PubMed] [Google Scholar]
- 89.Steinberg W. The clinical utility of the CA 19-9 tumor-associated antigen. Am J Gastroenterol. 1990;85:350–355. [PubMed] [Google Scholar]
- 90.Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007;33:266–270. doi: 10.1016/j.ejso.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 91.Chen R, Pan S, Yi EC, et al. Quantitative proteomic profiling of pancreatic cancer juice. Proteomics. 2006;6:3871–3879. doi: 10.1002/pmic.200500702. [DOI] [PubMed] [Google Scholar]
- 92.Chen R, Pan S, Cooke K, et al. Comparison of pancreas juice proteins from cancer versus pancreatitis using quantitative proteomic analysis. Pancreas. 2007;34:70–79. doi: 10.1097/01.mpa.0000240615.20474.fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kanda M, Sadakari Y, Borges M, et al. Mutant TP53 in duodenal samples of pancreatic juice from patients with pancreatic cancer or high-grade dysplasia. Clin Gastroenterol Hepatol. 2013;11:719–730. doi: 10.1016/j.cgh.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Eshleman JR, Norris AL, Sadakari Y, et al. KRAS and guanine nucleotide-binding protein mutations in pancreatic juice collected from the duodenum of patients at high risk for neoplasia undergoing endoscopic ultrasound. Clin Gastroenterol Hepatol. 2015;13:963–969. doi: 10.1016/j.cgh.2014.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kanda M, Knight S, Topazian M, et al. Mutant GNAS detected in duodenal collections of secretin-stimulated pancreatic juice indicates the presence or emergence of pancreatic cysts. Gut. 2013;62:1024–1033. doi: 10.1136/gutjnl-2012-302823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Osborn NK, Ahlquist DA. Stool screening for colorectal cancer: molecular approaches. Gastroenterology. 2005;128:192–206. doi: 10.1053/j.gastro.2004.10.041. [DOI] [PubMed] [Google Scholar]
- 97.Kisiel JB, Yab TC, Taylor WR, et al. Stool DNA testing for the detection of pancreatic cancer: assessment of methylation marker candidates. Cancer. 2012;118:2623–2631. doi: 10.1002/cncr.26558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bloomston M, Frankel WL, Petrocca F, et al. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA. 2007;297:1901–1908. doi: 10.1001/jama.297.17.1901. [DOI] [PubMed] [Google Scholar]
- 99.Liu J, Gao J, Du Y, et al. Combination of plasma microRNAs with serum CA19-9 for early detection of pancreatic cancer. Int J Cancer. 2012;131:683–691. doi: 10.1002/ijc.26422. [DOI] [PubMed] [Google Scholar]
- 100.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 101.Hernandez YG, Lucas AL. MicroRNA in pancreatic ductal adenocarcinoma and its precursor lesions. World J Gastrointest Oncol. 2016;8:18–29. doi: 10.4251/wjgo.v8.i1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schultz NA, Dehlendorff C, Jensen BV, et al. MicroRNA biomarkers in whole blood for detection of pancreatic cancer. JAMA. 2014;311:392–404. doi: 10.1001/jama.2013.284664. [DOI] [PubMed] [Google Scholar]
- 103.Majumder S, Taylor WR, Yab TC, et al. Detection of pancreatic high-grade dysplasia and cancer using novel methylated dna markers: discovery and tissue validation. Gastroenterology. 2016;150(4 Suppl 1):S120–S121. doi: 10.1016/S0016-5085(16)30513-3. [DOI] [Google Scholar]
- 104.Kisiel JB, Raimondo M, Taylor WR, et al. New DNA methylation markers for pancreatic cancer: discovery, tissue validation, and pilot testing in pancreatic juice. Clin Cancer Res. 2015;21:4473–4481. doi: 10.1158/1078-0432.CCR-14-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]