Abstract
Background/Aims
To investigate the use of measurements of liver stiffness (LS) by two-dimensional real-time shear wave elastography (SWE) for predicting the development of hepatocellular carcinoma (HCC) in patients with chronic hepatitis B (CHB).
Methods
We retrospectively collected data on 291 enrolled patients with CHB whose LS had been measured using SWE.
Results
The mean age of the patients was 46.8 years; males predominated (67%), and 40 of the patients (14%) had clinical cirrhosis. Among the patients, 165 (56.7%) received antiviral treatment. The median LS value was 7.4 kPa, and the median follow-up period was 35.8 months (range, 3.0 to 52.8 months). During follow-up, HCC developed in 13 patients (4.5%), and the cumulative incidence rates of HCC at 1, 2, and 4 years were 1.1%, 3.6%, and 8.4%, respectively. Based on a multivariate analysis, older age (≥50 years) and higher LS value (≥10 kPa) were independently associated with the risk of developing HCC (hazard ratio [HR], 4.53, p=0.023; and HR, 4.08, p=0.022). The cumulative incidence rate of HCC was significantly higher in patients with higher LS values (≥10 kPa) than in those with lower LS values (<10 kPa) (p=0.001).
Conclusions
Increased LS measured by SWE at any time point regardless of antiviral treatment is associated with an increased risk of HCC in patients with CHB.
Keywords: Elastography, shear wave, Liver stiffness, Hepatitis B, chronic, Carcinoma, hepatocellular
INTRODUCTION
Chronic hepatitis B virus (HBV) infection affected about 248 million individuals globally in 2010, and is the most common cause of end stage liver disease such as liver cirrhosis and hepatocellular carcinoma (HCC).1 Until now, the known risk factors for HCC development were older age, male gender, family history of HCC, alcohol consumption, high HBV DNA level and cirrhosis.2 Of these, cirrhosis was the most important risk factor.2 So, it is important to detect advanced fibrosis or cirrhosis early in patients with chronic hepatitis B (CHB), and these patients should be included in an optimized surveillance program for HCC.
To date, liver biopsy is considered the gold standard for evaluating liver fibrosis and cirrhosis.3 However, biopsy is a costly and invasive procedure that may be accompanied by complications, such as pain, hemorrhage and perforation.4 Hence the opportunity for repeated examinations is limited. Furthermore, liver biopsy is susceptible to sampling error and intra- and interobserver variability in interpretation.5 Because of these limitations, many noninvasive methods have been proposed as substitutes for liver biopsy.6
Over the last decade, elastography, especially transient elastography (TE) has been widely used to assess liver fibrosis and cirrhosis and portal hypertension-related complications in patients with chronic liver disease.7–9 In addition, TE has been shown to be a useful predictor of HCC development in CHB patients.10,11 Recently, Kim et al.12 reported that TE can identify CHB patients with so-called subclinical cirrhosis who are not clinically cirrhotic but have an increased risk of developing HCC.
Second-generation elastography procedures known as acoustic radiation force impulse imaging and two-dimentional real-time shear wave elastography (SWE) are subsequently introduced and actively used worldwide.13–15 Unlike TE, LS measurement by SWE is guided by a B-mode image and assesses precisely the degree of liver fibrosis.16 Several studies have reported that SWE is as good as or better than TE for assessing liver fibrosis.14,17,18 However, there have been few studies using LS measured by SWE to predict HCC development in patients with CHB.2 We therefore performed this study to investigate the potential of SWE for predicting HCC in patients with HBV-related chronic hepatitis or compensated cirrhosis.
MATERIALS AND METHODS
1. Patients
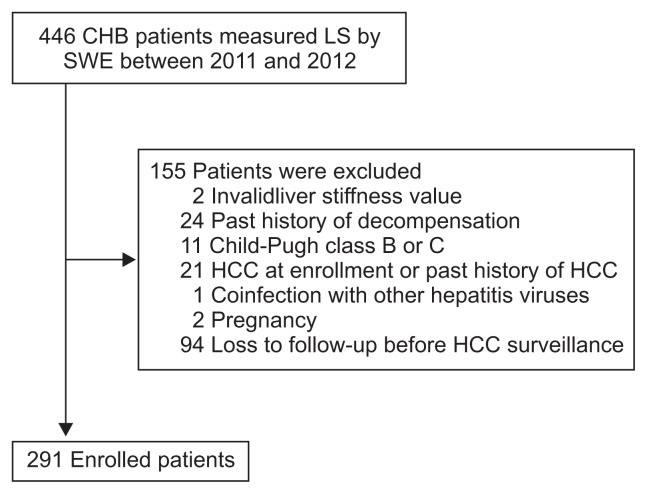
LS was measured by SWE in 446 patients with CHB from January 2011 to December 2012 at Hanyang University Guri Hospital, Guri, Korea. Of these, 155 patients were excluded for the following reasons (Fig. 1): (1) invalid LS value (n=2), (2) past history of decompensation (n=24), (3) Child-Pugh class B or C (n=11), (4) HCC at enrollment, or past history of HCC (n=21), (5) co-infection with other hepatitis viruses (n=1), (6) pregnancy (n=2), and (7) loss to follow-up before HCC surveillance (n=94). Finally, 291 patients with CHB were included in the analysis. According to the Korea association for the Study of the Liver guideline, hepatitis B e antigen (HBeAg)-positive patients with an HBV DNA level of ≥20,000 IU/mL and an alanine transaminase (ALT) level of ≥2×upper normal limit (UNL), HBeAg-negative patients with an HBV DNA level of ≥2,000 IU/mL and an ALT level of ≥2×UNL, and compensated cirrhotic patients with an HBV DNA level of ≥2,000 IU/mL regardless of the ALT level were administered to antiviral agents.19 Our study was approved by the Ethics Committee of Hanyang University Guri Hospital (IRB number: 2015-11-011-001).
Fig. 1.
Flow chart of enrolled patients.
CHB, chronic hepatitis B; LS, liver stiffness; SWE, shear wave elastography; HCC, hepatocellular carcinoma.
2. Clinical and laboratory parameters
We collected the following clinical and laboratory data; age, gender, clinical cirrhosis, previous or ongoing antiviral treatment, alcohol abuse, diabetes mellitus, family history of HCC, body mass index (BMI), platelet (PLT) count, albumin, total bilirubin, prothrombin time (international normalized ratio, INR), aspartate transaminase (AST), ALT, HBsAg, HBeAg, HBeAb, HBV DNA and LS measurement by SWE. Additionally FIB-4 index ([age×AST]/[PLT count (×109/L)×√ALT]) and APRI (AST/upper limit of normal/PLT count [×109/L]×100) were calculated using the same blood. Clinical cirrhosis was defined as (1) a PLT count <100,000/mm2 and ultrasonographic findings suggestive of cirrhosis, including surface nodularity with splenomegaly (>12 cm) and (2) presence of esophageal or gastric varices.11 Alcohol abuse was defined as ingestion of >30 g/day of alcohol in men and >20 g/day in women. All patients underwent measurement of baseline serum laboratory parameters within 3 months of the first elastography imaging, and then every 3 to 6 months. Serum HBV DNA levels were measured by real-time PCR (Roche Diagnostics, Branchburg, NJ, USA; detection range: 20~1×109 IU/mL). In our study, 25 percentile, median, and 75 percentile of LS value were 5.9, 7.4, and 10.2 kPa, respectively. Thus, the patients with LS ≥10 kPa (73.5 percentile) could be regarded as the high risk group. For this, we used 10 kPa as the cutoff value of LS for HCC development risk.
3. Patient follow-up
Patients were screened for HCC every 6 to 12 months by α-fetoprotein assay and ultrasonography, or dynamic computed tomography. A diagnosis of HCC was established according to the Guidelines of the American Association for the Study of Liver Diseases.20 The last follow-up date was June 2015.
4. Liver stiffness measurement by SWE
For LS measurement an Aixplorer ultrasound (US) system (Supersonic Imagine SA, Aix-en-Provence, France) equipped with an elastographic function was used. SWE is based on a two-dimensional elastography technique. All LS measurement were performed after conventional liver US by two abdominal radiologists (Y.K. and W.K.J.) with over 10 years of clinical experience in abdominal radiology and liver US as part of their regular practice. They also had experience of performing LS measurement in more than 100 patients. For the LS measurements, hepatic ultrasonograms were obtained via a right intercostal window, with 5- to 10-repetition per session, and the median value was taken as the LS measurement. Ultrasonograms were acquired at a location more than 2 cm below the hepatic capsule, and the region-of-interest was located away from large vessels to avoid measurement error. Unreliable LS measurement due to noncooperation or a thick subcutaneous layer of fat was considered as invalid LS measurement.
5. Statistical analysis
Categorical variables are given as frequencies and percentages. Continuous variables are given as mean values with standard deviations and median values with ranges for parametric and nonparametric variables, respectively. The cumulative incidence rate of HCC was calculated using the Kaplan-Meier method. Time-dependent areas under the receiver operating characteristics (AUROC) using LS measurement was assessed to predict HCC development. To assess risk factors for HCC development, univariate and multivariable Cox proportional hazards models were used. The variables for a multivariable analysis were determined on the basis of their statistical significance in the univariate analysis (p<0.10). A backward conditional stepwise procedure was performed in the multivariable analysis to avoid multicollinearity. Independent risk factors were compared using the log-rank test. The p-values <0.05 in two-sided tests were considered statistically significant. SPSS 18.0 for Windows (SPSS Inc., Chicago, IL, USA) was used for all statistical analysis except time-dependent AUROC. Time-dependent AUROC was calculated using R 3.1.1 (Vienna, Austria; http://www.R-project.org).
RESULTS
1. Baseline characteristics
The baseline characteristics of the 291 patients are summarized in Table 1. Their mean age was 46.8 years and they were predominantly male (n=195, 67%). One hundred sixty-five patients (56.7%) received previous or ongoing antiviral treatment. Clinical cirrhosis was identified in 40 (14%). Thirty-two patients had alcohol abuse and 23 had diabetes mellitus. Mean BMI and ALT were 24.2 kg/m2 and 53.8 U/L, respectively. In addition, the mean values of APRI and FIB-4 index were 0.8 and 2.0, respectively. The median value of LS was 7.4 kPa (range, 3.4 to 36.0 kPa).
Table 1.
Baseline Characteristics of the Enrolled Patients (n=291)
| Variable | Value |
|---|---|
| Age, yr | 46.8±11.4 |
| Sex | |
| Male | 195 (67) |
| Female | 96 (33) |
| Clinical cirrhosis | 40 (14) |
| Antiviral treatment | 165 (57) |
| Alcohol abuse | 32 (11) |
| Diabetes mellitus | 23 (8) |
| Family history of HCC | 45 (16) |
| BMI, kg/m2 | 24.2±3.1 |
| Platelet count, ×103/mm2 | 172.7±53.1 |
| Albumin, g/dL | 4.4±0.4 |
| Total bilirubin, mg/dL | 0.9±1.0 |
| Prothrombin time, INR | 0.9±0.1 |
| AST, U/L | 46.3±124.4 |
| ALT, U/L | 53.8±119.8 |
| HBeAg positivity | 119 (41) |
| HBV DNA, IU/mL | 110.0 (undetectable–170,000,000) |
| APRI | 0.8±2.3 |
| FIB-4 | 2.0±1.9 |
| LS by SWE, kPa | 7.4 (3.4–36.0) |
Data are presented as mean±SD, number (%), or median (range).
HCC, hepatocellular carcinoma; BMI, body mass index; INR, international normalized ratio; AST, aspartate aminotransferase; ALT, alanine aminotransferase; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; APRI, AST to platelet ratio index; FIB-4, fibrosis-4 index; LS, liver stiffness; SWE, shear wave elastography.
2. HCC and liver-related events
The median follow-up period was 35.8 months (range, 3.0 to 52.8 months) (784.9 person-years of follow-up). During follow up, 13 patients (4.5%) developed HCC. According to the modified staging system of the International Union against Cancer, there were four (30.8%), six (46.2%), two (15.4%), and one (7.7%) stage I, II, III and IVa patients, respectively. During the follow-up period, four patients developed liver-related complications other than HCC and all were ascites. The LS values of the four patients who developed ascites were 9.83, 12.15, 12.25 and 13.12 kPa, respectively, and all had clinical cirrhosis. One of these patients developed HCC later.
3. Comparison between patients who did and did not develop HCC
The baseline characteristics of the patients who did and did not develop HCC are shown in Table 2. The mean LS value by SWE was significantly higher in patients who did develop HCC than in those who did not (12.2 kPa vs 7.3 kPa, p=0.007). Age was also significantly higher in patients who did develop HCC than in those who did not (57.2 years vs 46.4 years, p<0.001), but PLT count was lower (123×103/mm2 vs 175×103/mm2, p<0.001).
Table 2.
Comparison of the Baseline Characteristics of Chronic Hepatitis B Patients with and without Hepatocellular Carcinoma Development
| Variable | Non-HCC (n=278, 95.5%) | HCC (n=13, 4.5%) | p-value |
|---|---|---|---|
| Age, yr | 46.4±11.3 | 57.2±8.1 | 0.001 |
| Sex | 0.862 | ||
| Male | 186 (67) | 9 (69) | |
| Female | 92 (33) | 4 (31) | |
| Clinical cirrhosis | 34 (12) | 6 (46) | 0.001 |
| Antiviral treatment | 157 (56) | 8 (61) | 0.719 |
| Alcohol abuse | 30 (11) | 2 (15) | 0.609 |
| Diabetes mellitus | 22 (8) | 1 (8) | 0.974 |
| Family history of HCC | 42 (15) | 3 (23) | 0.445 |
| BMI, kg/m2 | 24.1±3.1 | 25.7±3.4 | 0.086 |
| Platelet count, ×103/mm2 | 175.0±52.3 | 123.2±46.8 | 0.001 |
| Albumin, g/dL | 4.4±0.4 | 4.4±0.3 | 0.556 |
| Total bilirubin, mg/dL | 0.9±1.0 | 0.9±0.2 | 0.992 |
| Prothrombin time, INR | 0.9±0.1 | 1.0±0.1 | 0.679 |
| AST, U/L | 46.3±127.2 | 39.1±17.0 | 0.831 |
| ALT, U/L | 54.8±122.4 | 32.1±22.9 | 0.505 |
| HBeAg positivity | 114 (41) | 5 (39) | 0.855 |
| HBV DNA, IU/mL | 110.0 (undetectable–170,000,000) | 32.6 (undetectable–37,100,000) | 0.559 |
| APRI | 0.8±2.3 | 1.1±1.1 | 0.673 |
| FIB-4 | 1.9±1.8 | 4.1±2.9 | 0.019 |
| LS by SWE, kPa | 7.3 (3.4–36.0) | 12.2 (7.1–23.8) | 0.007 |
Data are presented as mean±SD, number (%), or median (range).
HCC, hepatocellular carcinoma; BMI, body mass index; INR, international normalized ratio; AST, aspartate aminotransferase; ALT, alanine aminotransferase; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; APRI, AST to platelet ratio index; FIB-4, fibrosis-4 index; LS, liver stiffness; SWE, shear wave elastography.
4. Independent predictors of HCC development
In univariate analysis, HCC development was significantly associated with older age (≥50 years) (hazard ratio [HR], 5.39; p=0.010), clinical cirrhosis (HR, 4.76; p=0.005), lower PLT counts (<150×103/mm2) (HR, 3.15; p=0.044), higher total bilirubin (≥1.0 mg/dL) (HR, 3.34; p=0.031), FIB-4 ≥3.25 (HR, 7.77; p<0.001) and higher LS values (≥10 kPa) (HR, 5.97; p=0.003) (Table 3). In multivariable analysis, older age (≥50 years) and higher LS value (≥10 kPa) were independently associated with risk of developing HCC (HR, 4.53, p=0.023; and HR, 4.08, p=0.022) (Table 3).
Table 3.
Factors Predicting Hepatocellular Carcinoma Development in Compensated Patients with Chronic Hepatitis B
| Variable | Univariate HR (95% CI) | p-value | Multivariable HR (95% CI) | p-value |
|---|---|---|---|---|
| Sex | ||||
| Female | 1 | |||
| Male | 1.11 (0.34–3.61) | 0.864 | ||
| Age, yr | ||||
| <50 | 1 | 1 | ||
| ≥50 | 5.39 (1.48–19.81) | 0.010 | 4.53 (1.23–16.67) | 0.023 |
| Clinical cirrhosis | ||||
| Absent | 1 | |||
| Present | 4.76 (1.60–14.19) | 0.005 | ||
| Antiviral treatment | ||||
| No | 1 | |||
| Yes | 1.12 (0.37–3.43) | 0.840 | ||
| Alcohol abuse | ||||
| Absent | 1 | |||
| Present | 1.45 (0.32–6.56) | 0.626 | ||
| Diabetes mellitus | ||||
| Absent | 1 | |||
| Present | 1.11 (0.14–8.54) | 0.921 | ||
| Family history of HCC | ||||
| Absent | 1 | |||
| Present | 1.58 (0.44–5.75) | 0.486 | ||
| BMI, kg/m2 | ||||
| <25 | 1 | |||
| ≥25 | 2.46 (0.81–7.52) | 0.114 | ||
| Platelet count, ×103/mm2 | ||||
| ≥150 | 1 | |||
| <150 | 3.15 (1.03–9.66) | 0.044 | ||
| Albumin, mg/dL | ||||
| ≥4.0 | 1 | |||
| <4.0 | 1.96 (0.43–8.84) | 0.383 | ||
| Total bilirubin, mg/dL | ||||
| <1.0 | 1 | |||
| ≥1.0 | 3.34 (1.11–9.99) | 0.031 | ||
| Prothrombin time, INR | ||||
| <1.0 | 1 | |||
| ≥1.0 | 0.84 (0.19–3.81) | 0.822 | ||
| AST, U/L | ||||
| <40 | 1 | |||
| ≥40 | 2.37 (0.77–7.26) | 0.131 | ||
| ALT, U/L | ||||
| <40 | 1 | |||
| ≥40 | 0.53 (0.12–2.37) | 0.402 | ||
| HBeAg positivity | ||||
| Absent | 1 | |||
| Present | 1.05 (0.34–3.22) | 0.930 | ||
| HBV DNA, IU/mL | ||||
| <2,000 | 1 | |||
| ≥2,000 | 0.93 (0.29–3.04) | 0.907 | ||
| APRI | ||||
| <1.0 | 1 | |||
| ≥1.0 | 2.70 (0.93–9.81) | 0.099 | ||
| FIB-4 index | ||||
| <3.25 | 1 | |||
| ≥3.25 | 7.77 (2.39–25.27) | 0.001 | ||
| LS by SWE, kPa | ||||
| <10 | 1 | 1 | ||
| ≥10 | 5.97 (1.84–19.41) | 0.003 | 4.08 (1.23–13.55) | 0.022 |
HR, hazard ratio; CI, confidence interval; HCC, hepatocellular carcinoma; BMI, body mass index; INR, international normalized ratio; AST, aspartate aminotransferase; ALT, alanine aminotransferase; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; APRI, AST to platelet ratio index; FIB-4, fibrosis-4 index; LS, liver stiffness; SWE, shear wave elastography.
5. Incidence of HCC development according to risk factors
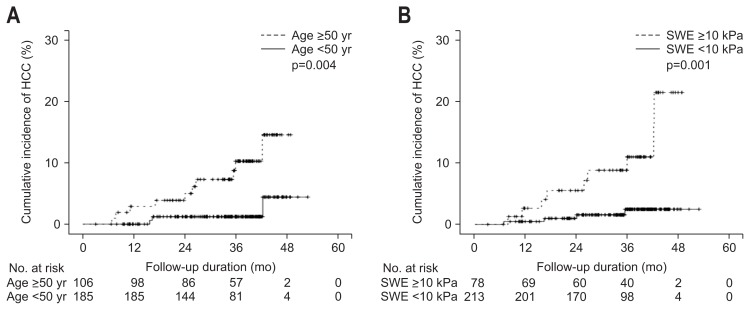
Cumulative rates of HCC at 1-, 2-, and 4-year were 1.1%, 3.6%, and 8.4%, respectively, for all patients. It was significantly higher in patients with higher LS values (≥10 kPa) than those with lower LS values (<10 kPa) (log-rank test, p=0.001) (Fig. 2B). The cumulative rates of HCC at 1-, 2-, and 4-years were 0.5%, 1.6%, and 2.5%, respectively, in patients with LS values <10 kPa, and 2.6%, 5.5%, and 21.5%, respectively, in patients with LS scores ≥10 kPa. The time-dependent AUROCs of LS measurement by SWE for HCC development at 1-, 2-, 3-, 4-, and 5-year were 0.756, 0.712, 0.772, 0.747, and 0.747, respectively.
Fig. 2.
The difference in hepatocellular carcinoma (HCC) incidence according to age (A) and liver stiffness based on shear wave elastography (SWE) (B).
HCC development differed significantly according to age (log-rank test, p=0.004) (Fig. 2A). The cumulative rates of HCC at 1-, 2-, and 4-year were 0%, 1.2%, and 4.4%, respectively, in patients with age <50 years, and 2.9%, 5.0%, and 14.5%, respectively, in patients with age ≥50 years.
DISCUSSION
We have investigated the potential of LS measured by SWE as a new shear wave-based elastography technique for predicting HCC development in patients with HBV-related chronic hepatitis or compensated cirrhosis. We have shown that the risk of HCC development increased up to 4-fold in CHB patients with LS ≥10 kPa by SWE compared to those with LS <10 kPa, and the incidence of HCC in CHB patients with LS ≥10 kPa was significantly higher than in those with LS <10 kPa. These results show that increased LS values assessed by SWE at any time point regardless of antiviral treatment are associated with an increased risk of HCC, and suggest that SWE is useful as a new method for evaluating the risk of HCC in compensated patients with CHB.
In recent years TE has been used as a popular noninvasive method to predict degrees of liver fibrosis and cirrhosis.7,21 Also many studies have reported that LS measurement by TE is a useful test for HCC development in patient with CHB.11,12,22 However, because of several limitations LS measurement by TE cannot be performed in all patients. Also LS measurement by TE can give unreliable results because of the bulky external vibrator.18 On the other hand, when LS measurement by SWE is used, the shear modulus of the shear wave generated by the acoustic radiation force impulses without requiring an external vibrator, and has good reliability and reproducibility.18 Also, SWE provides information on shear wave propagation over a larger area than TE.16 Furthermore, unlike SWE, TE cannot measure LS in patients with severe obesity, subcutaneous fat, or ascites because of poor penetration.23
Cirrhosis is the most important predictor of HCC development in CHB patients.2 Cirrhosis is commonly diagnosed from radiologic and laboratory findings, or from clinical findings such as varices, ascites, jaundice and hepatic encephalopathy.24 But if the findings are not typical it is difficult to diagnose cirrhosis. Also, since the findings are dichotomized, it is difficult to detect early cirrhosis or advanced fibrosis in patients with CHB. Hence an accurate surrogate for diagnosing cirrhosis is needed and it should be a quantitative measure. Jung et al.11 suggested that LS measurement by TE might be a more reliable method for diagnosing compensated cirrhosis than other clinical findings. As expected, our study showed that the risk of HCC development increased up to 4-fold in CHB patients with LS ≥10 kPa by SWE compared to those with LS <10 kPa when we applied 10 kPa as the cutoff value. The reasons why we determined the 10 cutoff value of LS were as follows: First, as aforementioned 75 percentile of LS value was 10.2 kPa, so the patients with LS ≥10 kPa (73.5 percentile) could be regarded as the high risk group. Second, when AUROC were used to calculate the optimal LS cutoff value for HCC development risk, the LS cutoff value was actually 9.4 kPa which was closely with 10 kPa, and AUROC was 0.783 (95% CI, 0.692 to 0.875; p=0.001; sensitivity, 76.9%; specificity, 73.0%). Furthermore, the cutoff values of LS measured by SWE for liver cirrhosis by biopsy-proven histology in patients with CHB were 10.1 and 11.7 kPa in two recent studies.18,25 These implied that the patients with LS ≥10 kPa in our study might have advanced fibrosis or cirrhosis. However, clinical cirrhosis was identified in only 40 among 78 patients (51.3%) with a high LS value (≥10 kPa) in our study (Fig. 2B). These results indicated that SWE could help distinguish patients with a high risk of HCC earlier before clinical diagnosis of cirrhosis. Therefore we suggest that, like TE, SWE is a more reliable method than clinical criteria for predicting HCC development.
As high LS values, older age (>50 years) was a risk factor for HCC development. This result is consistent with previous reports.12,22 However, a high HBV DNA load, which was considered an important risk factor for HCC development before the era of antiviral therapy,26 was not a risk factor in our study. For the last few years antiviral agents with high potency and low resistance such as entecavir and tenofovir have been extensively administered to CHB patients, and high viral loads were easily suppressed.27,28 Hence high viral load may no longer be a risk factor for HCC development in CHB patients who are regularly followed up.29
In our study four patients developed liver-related complications other than HCC and all were ascites. HCC was the leading liver-related complication of compensated liver cirrhosis with CHB receiving antiviral treatment in several studies.29,30 Recently Lampertico et al.30 followed up 107 HBeAg-negative compensated cirrhotic patients during a median of 12 years of antiviral therapy and no patient experienced liver-related complications other than HCC. So we think that HCC may be the most common liver-related complication of compensated patients with CHB in the era of antiviral therapy of high potency and low resistance. Therefore it becomes more important to identify those CHB patients without clinically apparent cirrhosis who have an increased risk of HCC development. Our study indicates that, like TE, SWE is useful as a method of evaluating the risk of HCC development in those patients.
This study had several limitations. First, it was a retrospective study involving a relatively small number of patients in a single center. As a result, we could not demonstrate significant correlations between HCC development and other risk factors besides older age and high LS value. Second, since liver biopsy data were not available upon enrollment in most patients, we could not examine any correlations between LS values and detailed histologic findings such as fibrosis, inflammation and steatosis. We believe that a large and long-term prospective study is needed to confirm a robust association between HCC development and LS measured by SWE in patients with CHB.
In conclusion, we found that high LS values measured by SWE at any time point regardless of antiviral treatment are associated with an increased risk of HCC development in compensated patients with CHB. We therefore suggest that SWE could have an important role in evaluating the risk of HCC development in patients with CHB.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546–1555. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 2.Wong VW, Janssen HL. Can we use HCC risk scores to individualize surveillance in chronic hepatitis B infection? J Hepatol. 2015;63:722–732. doi: 10.1016/j.jhep.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 3.Dienstag JL. The role of liver biopsy in chronic hepatitis C. Hepatology. 2002;36(5 Suppl 1):S152–S160. doi: 10.1053/jhep.2002.36381. [DOI] [PubMed] [Google Scholar]
- 4.Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495–500. doi: 10.1056/NEJM200102153440706. [DOI] [PubMed] [Google Scholar]
- 5.Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449–1457. doi: 10.1016/j.hep.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 6.Yoshioka K, Hashimoto S. Can non-invasive assessment of liver fibrosis replace liver biopsy? Hepatol Res. 2012;42:233–240. doi: 10.1111/j.1872-034X.2011.00928.x. [DOI] [PubMed] [Google Scholar]
- 7.Friedrich-Rust M, Ong MF, Martens S, et al. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134:960–974. doi: 10.1053/j.gastro.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 8.Robic MA, Procopet B, Métivier S, et al. Liver stiffness accurately predicts portal hypertension related complications in patients with chronic liver disease: a prospective study. J Hepatol. 2011;55:1017–1024. doi: 10.1016/j.jhep.2011.01.051. [DOI] [PubMed] [Google Scholar]
- 9.Pang JX, Zimmer S, Niu S, et al. Liver stiffness by transient elastography predicts liver-related complications and mortality in patients with chronic liver disease. PLoS One. 2014;9:e95776. doi: 10.1371/journal.pone.0095776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fung J, Lai CL, Seto WK, Wong DK, Yuen MF. Prognostic significance of liver stiffness for hepatocellular carcinoma and mortality in HBeAg-negative chronic hepatitis B. J Viral Hepat. 2011;18:738–744. doi: 10.1111/j.1365-2893.2010.01355.x. [DOI] [PubMed] [Google Scholar]
- 11.Jung KS, Kim SU, Ahn SH, et al. Risk assessment of hepatitis B virus-related hepatocellular carcinoma development using liver stiffness measurement (FibroScan) Hepatology. 2011;53:885–894. doi: 10.1002/hep.24121. [DOI] [PubMed] [Google Scholar]
- 12.Kim MN, Kim SU, Kim BK, et al. Increased risk of hepatocellular carcinoma in chronic hepatitis B patients with transient elastography-defined subclinical cirrhosis. Hepatology. 2015;61:1851–1859. doi: 10.1002/hep.27735. [DOI] [PubMed] [Google Scholar]
- 13.Friedrich-Rust M, Nierhoff J, Lupsor M, et al. Performance of acoustic radiation force impulse imaging for the staging of liver fibrosis: a pooled meta-analysis. J Viral Hepat. 2012;19:e212–e219. doi: 10.1111/j.1365-2893.2011.01537.x. [DOI] [PubMed] [Google Scholar]
- 14.Ferraioli G, Tinelli C, Dal Bello B, et al. Accuracy of real-time shear wave elastography for assessing liver fibrosis in chronic hepatitis C: a pilot study. Hepatology. 2012;56:2125–2133. doi: 10.1002/hep.25936. [DOI] [PubMed] [Google Scholar]
- 15.Singh S, Venkatesh SK, Wang Z, et al. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: a systematic review and meta-analysis of individual participant data. Clin Gastroenterol Hepatol. 2015;13:440–451.e6. doi: 10.1016/j.cgh.2014.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piscaglia F, Marinelli S, Bota S, et al. The role of ultrasound elastographic techniques in chronic liver disease: current status and future perspectives. Eur J Radiol. 2014;83:450–455. doi: 10.1016/j.ejrad.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Deffieux T, Gennisson JL, Bousquet L, et al. Investigating liver stiffness and viscosity for fibrosis, steatosis and activity staging using shear wave elastography. J Hepatol. 2015;62:317–324. doi: 10.1016/j.jhep.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 18.Leung VY, Shen J, Wong VW, et al. Quantitative elastography of liver fibrosis and spleen stiffness in chronic hepatitis B carriers: comparison of shear-wave elastography and transient elastography with liver biopsy correlation. Radiology. 2013;269:910–918. doi: 10.1148/radiol.13130128. [DOI] [PubMed] [Google Scholar]
- 19.Korean Association for the Study of the Liver. KASL clinical practice guidelines: management of chronic hepatitis B. Clin Mol Hepatol. 2016;22:18–75. doi: 10.3350/cmh.2016.22.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruix J, Sherman M American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu X, Su Y, Song R, et al. Performance of transient elastography assessing fibrosis of single hepatitis B virus infection: a systematic review and meta-analysis of a diagnostic test. Hepatol Int. 2015;9:558–566. doi: 10.1007/s12072-015-9643-z. [DOI] [PubMed] [Google Scholar]
- 22.Wong GL, Chan HL, Wong CK, et al. Liver stiffness-based optimization of hepatocellular carcinoma risk score in patients with chronic hepatitis B. J Hepatol. 2014;60:339–345. doi: 10.1016/j.jhep.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 23.Jeong WK, Lim HK, Lee HK, Jo JM, Kim Y. Principles and clinical application of ultrasound elastography for diffuse liver disease. Ultrasonography. 2014;33:149–160. doi: 10.14366/usg.14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee HS, Kim JK, Cheong JY, et al. Prediction of compensated liver cirrhosis by ultrasonography and routine blood tests in patients with chronic viral hepatitis. Korean J Hepatol. 2010;16:369–375. doi: 10.3350/kjhep.2010.16.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeng J, Liu GJ, Huang ZP, et al. Diagnostic accuracy of two-dimensional shear wave elastography for the non-invasive staging of hepatic fibrosis in chronic hepatitis B: a cohort study with internal validation. Eur Radiol. 2014;24:2572–2581. doi: 10.1007/s00330-014-3292-9. [DOI] [PubMed] [Google Scholar]
- 26.Chen CJ, Yang HI, Su J, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 27.Chang TT, Lai CL, Kew Yoon S, et al. Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology. 2010;51:422–430. doi: 10.1002/hep.23327. [DOI] [PubMed] [Google Scholar]
- 28.Marcellin P, Gane E, Buti M, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468–475. doi: 10.1016/S0140-6736(12)61425-1. [DOI] [PubMed] [Google Scholar]
- 29.Lee HW, Yoo EJ, Kim BK, et al. Prediction of development of liver-related events by transient elastography in hepatitis B patients with complete virological response on antiviral therapy. Am J Gastroenterol. 2014;109:1241–1249. doi: 10.1038/ajg.2014.157. [DOI] [PubMed] [Google Scholar]
- 30.Lampertico P, Invernizzi F, Viganò M, et al. The long-term benefits of nucleos(t)ide analogs in compensated HBV cirrhotic patients with no or small esophageal varices: a 12-year prospective cohort study. J Hepatol. 2015;63:1118–1125. doi: 10.1016/j.jhep.2015.06.006. [DOI] [PubMed] [Google Scholar]