Abstract
The hypothalamus is an evolutionarily conserved brain structure that regulates an organism’s basic functions, such as homeostasis and reproduction. Several hypothalamic nuclei and neuronal circuits have been the focus of many studies to understand their role in regulating these basic functions. Within the hypothalamic neuronal populations, the arcuate melanocortin system plays a major role in controlling homeostatic functions. The arcuate pro-opiomelanocortin (POMC) neurons in particular have been shown to be critical regulators of metabolism and reproduction because of their projections to several brain areas both in and outside of the hypothalamus, such as autonomic regions of the brain stem and spinal cord. Here, we review and discuss the current understanding of POMC neurons from their development and intracellular regulators to their physiological functions and pathological dysregulation.
Keywords: POMC, hypothalamus, development, food intake, obesity
INTRODUCTION
The hypothalamus, an evolutionarily conserved brain structure, regulates an organism's basic functions, such as homeostasis and reproduction. Within the hypothalamus many nuclei and neuronal circuitries have been characterized, and their functions have been studied. Research on the hypothalamic arcuate nucleus and its neuronal populations has gained momentum with the discovery of leptin over 20 years ago. This led to the hypothesis that in this nucleus, the so-called melanocortin system previously thought to be involved in food regulation (1) plays a fundamental role in metabolism regulation. The arcuate melanocortin neurons consist of two distinct neuronal populations: the pro-opiomelanocortin (POMC)-expressing neurons and the neuropeptide Y/agouti-related peptide (NPY/AgRP)-expressing neurons. More recent studies using pharmacogenetic and optogenetic techniques (2, 3) have further proved that although POMC neuronal activation reduces food intake and increases energy expenditure, NPY/AgRP neuronal activation induces increased food intake and decreased energy expenditure (4, 5). These opposite functions in metabolism regulation are accomplished by the opposite effects on their target neurons, the melanocortin 4 receptor (MC4R)-expressing neurons, in several brain areas. POMC cells activate MC4R-expressing neurons in the paraventricular nucleus of the hypothalamus (PVH) and in other brain regions, including the brainstem, thus inhibiting food intake and increasing energy expenditure. Conversely, NPY/AgRP neurons antagonize these effects (4). Furthermore, the NPY/AgRP neurons, containing the inhibitory neurotransmitter GABA, project onto POMC neurons (1). Thus, in addition to their action on MC4R-expressing cells, NPY/AgRP/GABA neurons also have a direct inhibitory effect on POMC neuronal activity.
In the last few years, new data have come to light suggesting that the roles of these two neuronal populations in metabolism regulation and their developmental origins are more complex than previously thought. In this review, we highlight the recent discoveries that have expanded our understanding of POMC neurons before and after birth as well as in adults. We discuss new findings that are changing our view of POMC neurons from a homogeneous to heterogeneous population of neurons. Finally, we describe how POMC neurons respond and function to regulate energy balance (metabolism) in different metabolic states.
POMC PROGENITOR/LINEAGE AND ADULT NEUROGENESIS
Embryonic Development
The hypothalamic primordium arises from cells in the ventral neural tube of the diencephalon at embryonic day (E) 9.5 in the mouse. The highly proliferative progenitor cells of the hypothalamic arcuate nucleus (ARC) originate from the hypothalamic ventricular zone (HVZ). These HVZ cells gradually migrate laterally (5), differentiate, and then form each hypothalamic nucleus by E16.5–18.0 (Figure 1a) (6). POMC neurons can be identified as early as E10.5 in the anterobasal area, which also contains neurons of the ventromedial nucleus of the hypothalamus that are marked by steroidogenic factor 1 (7). The transcriptional control of the development and function of the ARC neurons is the focus of several studies. To date, several transcription factors have been identified. The Nkx2-1 homeobox gene, also known as thyroid transcription factor 1 (TTF-1), in ventral hypothalamic progenitor cells is required for the specification of this region (8), as deletion of Nkx2-1 leads to the loss of the neural tube ventral midline (9).
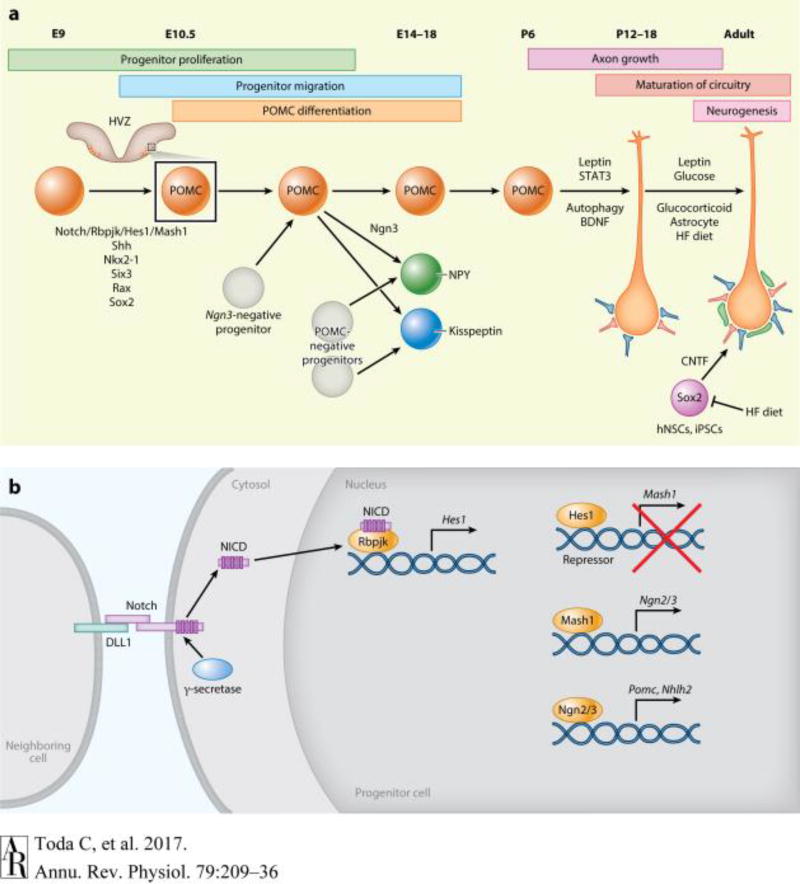
Figure 1.
Development of POMC neurons. (a) Overview of the factors that regulate embryonic POMC development and postnatal maturation of POMC circuitry. Notch signaling, Shh, Nkx2-1, Six3, Rax and Sox2 regulate POMC neurogenesis during embryonic development. Some POMC-positive progenitors become NPY and Kisspeptin neurons. At birth, POMC neuronal projections are immature. Leptin, autophagy, and BDNF regulate POMC axon growth. In the adult stage, leptin, glucose, glucocorticoid, and HFD modulate synaptic input organization onto the POMC neurons (red, excitatory synapses; blue, inhibitory synapses; green, astrocytes). POMC neurogenesis also occurs in adulthood, and it is shown to be induced by the injections of the hNSCs and iPSCs. CNTF and HF diet affect adult POMC neurogenesis. (b) Outline of the Notch signaling pathway regulating POMC neurogenesis. The progenitor cell, via the formation of the Notch/DLL1 complex with a neighboring cell, inhibits its own differentiation to a POMC neuron (lateral inhibition) by activating the transcriptional repressor Hes1, which is induced by NICD/Rbpjk binding. Once the Notch/DLL1 is removed, Mash1 and Ngn2/3 can initiate the differentiation of the cell into a POMC neuron. Abbreviations: BDNF, brain-derived neurotrophic factor; CNTF, ciliary neurotrophic factor; E, embryonic day; HFD, high-fat diet; hNSC, hypothalamic neural stem cell; HVZ, hypothalamic ventricular zone; iPSC, inducible pluripotent stem cell; Ngn 2/3, neurogenin 2/3; NICD, intracellular domain of Notch; NPY, neuropeptide Y; NSC, neural stem cell; POMC, pro-opiomelanocortin.
Sonic hedgehog (Shh), Six3, and retinal anterior neural fold homeobox (Rax) were also identified as critical regulators of HVZ and POMC development (Figure 1a). Shh, which is secreted by prechordal mesoderm at E7.5 and by hypothalamic neuroepithelium at E8.5, plays a critical role in the development of the ventral portion of the neural tube (10). Shh signaling increases Nkx2-1 expression (11), which, as mentioned above, plays a critical role in the development of the neural tube ventral midline (9), and deletion of Shh in mice with the Nkx2-1-Cre driver (12) affects the development of POMC neurons. Six3 is a regulator of forebrain development, including the hypothalamus (13), and is crucial for Shh expression (14). Finally, Rax, a key regulator of eye development, is also important for the formation of the ventral neural tube (15). If Rax is deleted in mice with the Six3-Cre driver, a transient loss of the POMC neurons occurs at E10.5, followed by their reappearance at E17.5 (16).
Mash1, a member of the basic helix-loop-helix family of transcription factors, is also expressed in progenitor cells of the ventral hypothalamus, and its deletion induces hypoplastic ARC due to defects in neurogenesis and apoptosis (7). Mash1 is critical for POMC differentiation through neurogenin 2/3 (Ngn2/3) and nescient helix-loop-helix 2/pro-hormone convertase 1 (Nhlh2/PC1). Mash1 knockout mice fail to express Ngn3 transcript and cannot develop POMC-positive neurons (7). Genetic fate-mapping studies using Ngn3-Cre mice have shown, however, that not all POMC neurons are derived from the Ngn3-positive progenitors (Figure 1a). Knockout mice for Ngn3 exhibit a loss of 60–70% of POMC-positive cells that occurs between E10.5–17.5. As a result, mice with a selective deletion of Ngn3 in Nkx2-1 cells become obese in adulthood (23, 24). In addition, Ngn3 regulates Nhlh2 expression, which is required for the expression of PC1, the enzyme responsible for the proteolytic cleavage of the POMC precursor to α-melanocyte-stimulating hormone (α-MSH) (23), thus affecting the production of α-MSH.
During the early stage of embryonic development, Notch signaling and the so-called lateral inhibition are also critical events in the neurogenesis and differentiation of POMC neurons (Figure 1b). Notch signaling, an evolutionarily conserved signaling pathway, regulates multiple processes during development via cell–cell communication. This pathway is initiated when a transmembrane Notch receptor anchored in a cell interacts with a transmembrane Notch ligand (Delta or Serrate/Jagged) in a neighboring cell. Once the receptor–ligand binding is formed, a series of proteolytic cleavages are triggered that result in the release of the intracellular domain of Notch (NICD). This intracellular domain will then form a nuclear complex with a recombination signal-binding protein for the immunoglobulin kappa J region (RBPJ) that will activate the transcription of target genes (17, 18). Some targets of the NICD/RBPJ complex are the transcriptional repressors Hes (hairy/enhancer of split) and Hey (Hes-related type) genes (19, 20). Hes1 works as a transcriptional repressor of Mash1 and thus prevents the initiation of POMC neuronal differentiation (21). A function of Notch is lateral inhibition, whereby a ligand-expressing cell inhibits the expression of the ligand in the neighboring cells, thus preventing those cells from adopting the same fate and generating a patched cellular pattern (22). Therefore, when Notch is separated from the neighboring cells, the progenitor cells can no longer express the repressor Hes1, and Mash1 can now enhance Ngn2/3, which then initiates POMC neurogenesis (Figure 1b) (23, 24). In support of this, conditional Rbpjk deletion in Nkx2-1 cells (25) leads to fewer progenitors and increased numbers of differentiated POMC neurons (21). By contrast, mice expressing constitutively active NICD in Nkx2-1 cells have a higher number of hypothalamic progenitors but a reduced number of differentiated POMC neurons (21). The mechanisms by which all the above factors and Notch signaling interact and contribute to POMC development remain an ongoing research focus.
Interestingly, POMC mRNA levels are highest at E13 and then gradually decrease by E18. Indeed, studies from the Zeltser group (26, 27) have found that POMC-positive progenitors differentiate to other neuronal populations that reside in the ARC and in other brain regions, and during this process they lose POMC expression. Studies using POMC-Cre and loxP-mediated reporter gene mice show that 25–50% of NPY neurons and approximately 18% of kisspeptin neurons express POMC-Cre lineage traces (26, 28, 29). This suggests that a subset of the NPY and kisspeptin neurons derive from POMC-positive progenitors (Figure 1a). Given that POMC progenitors are regulated by the factors described above, development of NPY and kisspeptin neurons is also influenced by these factors. For example, Rbpjk ablation in Nkx2-1-expressing cells increases both POMC and NPY neurons. On the other hand, expressing constitutively active NICD significantly decreases POMC and NPY neurons (21). Mash1 knockout mice also exhibit a reduction in NPY and POMC neurons, and ectopic expression of Ngn2 in Mash1 null mice partially rescues this phenotype, increasing NPY neurons (7). However, other factors appear more selective. For example, in Ngn3 knockout mice POMC neurons decrease while NPY neurons increase (23). Similarly, Rbpjk ablation in Nkx2-1 cells decreases kisspeptin neurons while increasing POMC neurons (28). Collectively, these data suggest that Ngn3 and Rbpjk might promote NPY/kisspeptin neurogenesis from POMC-negative progenitors.
Early Postnatal Development
In mice, ARC neuronal projections are incomplete at birth but then mature postnatally during lactation (Figure 1a). For example, projections from the ARC to the dorsomedial hypothalamus evolve on postnatal (P) day 6. Projections to the PVH occur on P8–10 and to the other nuclei on P12–16 (30). Leptin, which has a robust surge in circulating levels between P6–14 (31), has a crucial effect on ARC neuronal axon growth. Indeed, leptin knockout (ob/ob) mice display reduced α-MSH-positive fibers in the PVH. Leptin administration on P12, but not on P80, can restore α-MSH fibers (32). In line with this, the long form of leptin receptor (LepRb) starts to be expressed in the ARC between P6–10, and LepRb-null (db/db) mice, similar to the ob/ob mice, show reduced α-MSH projections to the PVH compared to wild-type controls (33). In addition, mice with mutations on Tyr1138 on LepRb that lack LepRb–signal transducer and activator of transcription 3 (STAT3) signal transduction show a significant decrease of α-MSH fibers in the PVH (33), suggesting that STAT3 signaling is critical for POMC axonal growth. Conversely, although mice with Tyr985 mutation in LepRb and defects in the LepRb-extracellular signal-regulated kinase (ERK) pathway show a decrease in POMC–PVH projections at P12, by P80 they display a similar POMC–PVH projection compared to wild-type controls, which suggests that other signaling pathways may compensate to normalize POMC projections (33).
Axonal growth is regulated by a dynamic remodeling of cytosolic structures and requires protein degradation and turnover that lead to replacement of damaged organelles and proteins, referred to as autophagy. Autophagy was recently shown to be an important intracellular mechanism involved in POMC axonal growth. Morphological studies revealed that autophagy occurs in the hypothalamus during development. Coupe and collaborators (34) demonstrated that loss of autophagy-related protein 7 (Atg7), an essential autophagy gene, reduces POMC projections to the PVH as early as P14 without altering POMC cell number (34), suggesting that this process has a specific role on postnatal axonal growth. Mice with selective deletion of Agt7 in POMC neurons displayed increased postweaning body weight and adiposity that were associated with a disruption in the maturation of POMC axonal projections. Because leptin directly promotes POMC axonal growth postnatally (32) and also promotes autophagy (35), it is conceivable that the neurotrophic effects of leptin postnatally may be mediated, at least in part, via autophagy activation. In addition, brain-derived neurotrophic factor (Bdnf) a critical gene in synaptic plasticity, neural circuit development, and energy metabolism regulation (36), plays a role in POMC axonal growth. Deletion of the long 3′UTR Bdnf mRNA transcript leads to a decrease of α-MSH projections into the dorsomedial hypothalamus but not the PVH without affecting POMC cell number (37). Considering that leptin was shown to increase Bdnf mRNA and protein levels in the hypothalamus, BDNF may also play a role in leptin signaling and axonal growth of POMC neurons (38).
Adult POMC Neurogenesis and Artificial POMC-Like Neurogenesis from Pluripotent Stem Cells
Hypothalamic POMC neurogenesis can also occur in adult mice (Figure 1a) (39, 40). In vivo bromodeoxyuridine (BrdU) labeling studies found BrdU incorporation as late as 30 days after its administration in adult mice (39). Despite the caveats of this technique, another fate-mapping study that used lentivirus-expressing Sox2, which marks neural stem cells (NSCs), revealed that Sox2-positive cells differentiate to POMC neurons in mice at three months of age (39). As many as 1,000 new ARC neurons were generated from Sox2-positive cells 80 days after virus infection. These new neurons account for 6% of all neurons in this region, with 10% of these new neurons being POMC neurons, and only 3% being NPY neurons (39).
Several factors affect POMC neurogenesis. For example, intracerebroventricular (ICV) injection of the ciliary neurotrophic factor increases BrdU incorporation in POMC-positive neurons, and many of these newborn POMC cells were responsive to leptin showing phosphorylated STAT3 (pSTAT3) immunoreactivity (40). High-fat diet (HFD) feeding also affects hypothalamic neurogenesis in adult mice. Specifically, the HFD effect on neurogenesis has two phases, an initial phase at days 1–3 during which neurogenesis is enhanced, and a second phase from day 4 when neurogenesis is attenuated (39, 41, 42). Some of these newborn cells become POMC neurons after 2–3 weeks (41). If AraC protein is used to block neurogenesis during HFD, dramatic weight gain ensues (41). Also, ablation of Sox2-positive cells in mice by the thymidine kinase and ganciclovir system resulted in a 10% reduction in POMC cells and an obese/diabetic phenotype (43). Thus, these data suggest that neurogenesis from Sox2-positive NSCs is required for the homeostatic regulation of energy metabolism by the hypothalamus. Although neurogenesis of hypothalamic circuits seems to occur in response to energy supply, it is of interest that a continuous overload of nutrients attenuates neurogenesis, and as a consequence leads to a deterioration of the hypothalamic homeostatic control (42).
Because neural stem cells play a role in the hypothalamic regulation of homeostasis, transplantation of these cells may be beneficial for curing metabolic dysfunctions. Indeed, Czupryn and collaborators (44) found that transplantation of hypothalamic cells collected from E13.5 into the hypothalamus of db/db mice at P0.5–5.5 leads to differentiated POMC-like neurons that are β-endorphin positive, glucose sensing, leptin activated, and insulin suppressed. As a result, these mice showed a decrease in their body weight and an overall improved metabolic phenotype (44), thus suggesting that transplanted embryonic cells can generate functional neuronal circuit(s) in the hypothalamus. However, Li et al. (43) reported that HFD feeding attenuates the survival rate of the transplanted hypothalamic NSCs (hNSCs) or inducible pluripotent stem cells (iPSCs). The mechanism responsible for this attenuation is unclear, but the activation of IκB kinase β (IKKβ) and the nuclear factor-κB (NF-κB) pathways during HFD feeding may be involved. In support of this, ICV injection of a lentivirus encoding constitutively active IKKβ under control of the Sox2 promoter in mice fed a standard chow diet decreased the number of ARC POMC cells and affected neurogenesis; this resulted in increased body weight three months post-ICV injection (39). In addition, suppressing IKKβ by lentivirus-mediated expression of a dominant-negative form of IKKβ restored the survival rate of transplanted hNSCs and iPSCs in HFD-fed mice (43). In this study (43), hNSCs and iPSCs injected through the carotid artery were able to cross the leaky hypothalamic blood-brain barrier (BBB) during HFD feeding, settle in the hypothalamus, and differentiate into POMCergic and GABAergic neurons, inducing an improvement of metabolic phenotypes in obese mice. Differentiation into POMCergic and GABAergic neurons was pivotal for the improved phenotype, as treatment with shRNA against POMC or GABA prevented the beneficial effects of the transplantation on energy metabolism (43). Thus far, these types of studies have been limited to rodent models, but it is possible that stem cell therapy in humans might be targeted to cure severe metabolic disorders. Future investigations into the potential role of hypothalamic neurogenesis in the treatment of obesity are warranted.
THE HETEROGENEITY OF POMC NEURONS
The hypothalamic POMC neurons are generally considered as a homogenous neuronal population. However, their projection fields are shown to be diverse. For example, whereas POMC neurons in the rostral arcuate nucleus project mostly to autonomic areas (45, 46), caudal arcuate POMC neurons project mainly to hypothalamic areas, including the PVH (1, 47), suggesting the possible heterogeneity of these cells.
Recent evidence shows that POMC neurons are indeed heterogeneous in nature in terms of the neurotransmitters and receptors they express. For example, both leptin and serotonin depolarize POMC neurons (48, 49). However, none of the serotonin-responsive POMC neurons are activated by leptin, and leptin-activated POMC cells are located more laterally in the ARC than the serotonin-responsive cells (48). Williams and collaborators (49) reported another example of POMC heterogeneity, showing that leptin and insulin acutely affect anatomically segregated POMC neuronal populations of the ARC (Figure 2a). In addition, subsets of POMC neurons express either glutamate, GABA, or both (50). The percentage of POMC neurons expressing vesicular glutamate transporter 2 (vGlut2) mRNA is highest at P1 and gradually decreases by 8 weeks of age in mice. In contrast, the percentage of neurons that express glutamate decarboxylase 67 (GAD67) mRNA, an enzyme that catalyzes the decarboxylation of glutamate to GABA, is lowest at P1 and increases over time (51). Furthermore, three different subpopulations of POMC neurons were described according to their responses to changing glucose levels: spontaneous excitatory postsynaptic current positive [sEPSC(+)] neurons that decrease their sEPSC when lowering glucose concentrations, the sEPSC(−) neurons that decrease their sEPSC when increasing glucose levels, and the sEPSC(+/−) neurons (52). The sEPSC(+/−) POMC neurons show an acute phase (approximately 10 min) in which they decrease their sEPSC when exposed to low glucose concentrations. They then increase their sEPSC after 25 min of continuously low levels of glucose (52), suggesting that glucose induces excitatory synaptic plasticity. The reasons for POMC neuronal heterogeneity are unclear. Studies looking at the metabolic effects of selective deletion of either leptin receptors or serotonin receptors in POMC neurons show a differential role of these neurons in feeding regulation, energy expenditure, and glucose metabolism. This therefore suggests that distinct POMC neuronal populations in the ARC may influence diverging downstream pathways that ultimately will affect food intake, energy expenditure, or glucose metabolism.
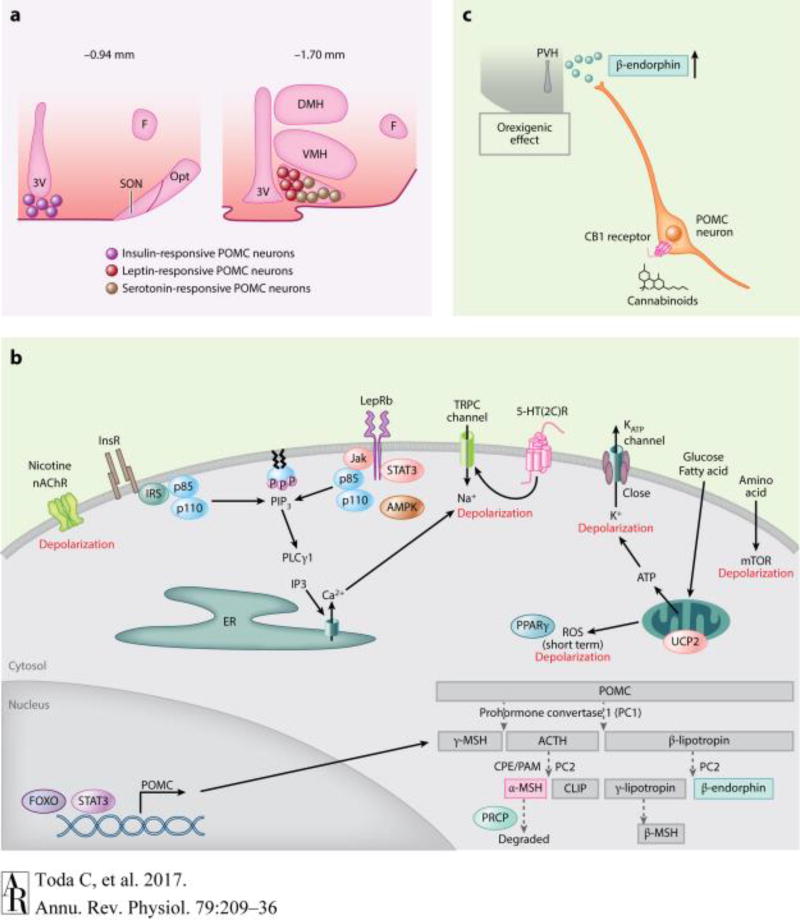
Figure 2.
Hormonal and nutritional regulation of POMC neurons. (a) Localization of insulin-, leptin- and serotonin-responsive POMC neurons in the ARC, modified from (49). (b) POMC protein processing and signaling pathways regulating POMC neuronal activity. Please note that the effects of insulin, leptin, and 5-HT may not occur in the same POMC cell as shown in panel a. (c) The paradoxical orexigenic effect of POMC neuronal activation in response to cannabinoids due to the release of the orexigenic POMC-derived β-endorphin. Abbreviations: 3V, third ventricle; ACTH, adrenocorticotropin; AMPK, 5’-adenosine monophosphate activating kinase; ARC, arcuate nucleus; CLIP, corticotropin-like intermediate lobe peptide; CPE, carboxypepetidase E; DMH, dorsomedial nucleus of the hypothalamus; ER, endoplasmic reticulum; F, fornix; FOXO, forkhead transcription factor; InsR, insulin receptors; IRS, insulin receptor substrate; Jak, Janus kinase; lepRb, leptin receptor; MSH, melanocyte-stimulating hormone; mTOR, mammalian target of rapamycin; nAChR, nicotinic acetylcholine receptor; Opt, optic tract; p85/p110, p85 and p110 subunit of phosphoinositol-3-kinase; PAM, peptidyl α-amidating monooxygenase; PC, prohormone convertase; POMC, pro-opiomelanocortin; PIP3, phosphatidylinositol3,4,5-triphosphate; PLCg1, phospholipase C gamma 1; POMC, pro-opiomelanocortin; PPARg, peroxisome-proliferator activated receptor gamma; PRCP, prolylcarboxypeptidase; PVH, paraventricular nucleus of the hypothalamus; ROS, reactive oxygen species; SON, supraoptic nucleus; STAT3, signal transducer and activator of transcription 3; TRPC, transient receptor potential canonical; UCP2, uncoupling protein 2; VMH, ventromedial nucleus of the hypothalamus.
POMC PROCESSING
POMC mRNA is expressed not only in the hypothalamic arcuate nucleus but also in other brain areas, including the brainstem and pituitary (4). This gene encodes a polypeptide hormone precursor that undergoes tissue-specific proteolysis, thus generating a specific repertoire of biologically active peptides and hormones, including adrenocorticotropin (ACTH), α-, β- and γ-MSHs, and β-endorphin (Figure 2b), all of which can modulate food consumption and energy balance.
These functional peptides are generated through a complex posttranslational process, which allows them to exert a broad spectrum of physiological effects in a tissue-dependent manner. In the corticotrophs of the anterior pituitary, POMC is mostly processed to ACTH, β-lipotropin (β-LPH), and a 16-kDa N-terminal fragment, whereas in the melanotrophs POMC is further metabolized in smaller peptides, including α-MSH (4). This latter neuropeptide is predominantly generated at the hypothalamic level through a cascade of posttranslational reactions, some of which are catabolized by the following enzymes: PC1 and PC2, the carboxypeptidase E (CPE), and the peptidyl α-amidating monooxygenase (PAM) (Figure 2b). These enzymes are required for both the synthesis of the active form of α-MSH and the regulation of all POMC-derived peptides. It is worth noting that expression of PC1 and PC2 drives the tissue-specific posttranslational processing of the POMC precursor protein, whereas CPE and PAM play a key role in regulating the trafficking of the POMC precursor protein from the Golgi to the secretory granules (53, 54). Finally, once α-MSH is synthesized through this complex pathway, it can be degraded by the prolylcarboxypeptidase (PRCP), which by removing its C-terminal valine residue will inactivate this neuropeptide (55, 56). Indeed, PRCP plays a crucial role in regulating the melanocortin system efficacy, as its inhibition or deletion leads to a reduction in food consumption, body weight, and an increase in energy expenditure (57, 58). Besides this pathway, POMC can be cleaved in β-LPH and then converted into β-endorphin, an orexigenic opioid peptide, whose acetylation results in the loss of its opioid activity.
In conclusion, it is now clear that the regulation of POMC processing, which is a pathway that generates several biologically active neuropeptides with opposing effects on feeding behavior and energy expenditure, is essential for energy homeostasis (59). Accordingly, impairment in the POMC posttranslational processing is associated with the development of obesity both in humans and animals (60).
REGULATION OF POMC NEURONS
By conveying information about the metabolic status of the organism, several peripheral signals, such as hormones and nutrients, regulate POMC transcription and processing as well as POMC neuronal activity, synaptic plasticity, and axonal projections (2, 61–64). By integrating these peripheral signals, POMC neurons are, in turn, responsible for the maintenance of energy and glucose homeostasis (Figure 2b). Below, we describe prominent peptide hormones and small signaling molecules that modulate POMC neurons (see Table 1).
Table 1.
POMC (pro-opiomelanocortin) neuronal regulation
| MODULATOR OF POMC NEURONS |
EFFECTS ON POMC NEURONS |
KO animals | PHENOTYPE | REFERENCES |
|---|---|---|---|---|
|
| ||||
| Leptin | Enhancement of POMC function | LepR deletion in POMC neurons | Obese Hyperglycemia Hyperinsulemia hyperleptinemia | 68–72 |
|
| ||||
| Selective re-expression of LepR in LepR-null mice (db/db) | Modest reduction of body weight | 73, 74 | ||
| No effect on food intake | ||||
| Partial normalization of energy expenditure | ||||
| Restored impaired glucose metabolism | ||||
|
| ||||
| LepR deletion in astrocytes | Impaired leptin-induced suppression of feeding | 76 | ||
|
| ||||
| Insulin | Inhibition (slice preparation) | InsR deletion in POMC neurons | No effect on body weight and glucose metabolism | 49, 72, 79 |
|
| ||||
| Activation (slide preparation) | InsR reintroduced in POMC neurons of L1 mice | Increased energy expenditure and hepatic glucose production | 78, 80 | |
|
| ||||
| Ghrelin | Indirect inhibition | None | No ghrelin receptor in POMC | 81, 82, 85, 86, 88 |
|
| ||||
| Glucocorticoids | Inhibition (slice preparation) | ADX animals and ADX mice with corticosterone replacement | Reduced food intake and body weight | 92 |
|
| ||||
| Endocannabinoids | Activation | CB1RKO mice; | CB1R activation promotes POMC neuronal activation via the release of b-endorphin | 2, 98, 99 |
| CB1R KO mice with selective re-expression of CB1R in POMC neurons | ||||
|
| ||||
| Nicotine | Activation | AAV-shRNA targeting β4 nAChR in the vetral hypothalamus | Nicotine-induced hypophagia induces POMC neuronal activation. | 101, 102 |
| POMC knockout mice | Knockdown of β4 acetylcholine receptors in the ventral hypothalamus abolish nicotine-induced hypophagia. | 101, 102 | ||
| Nicotine-induced hypophagia does not occur in POMC KO mice | ||||
|
| ||||
| Serotonin | Activation | 5ht2Cr knockout | Hepatic insulin resistance | 106 |
|
| ||||
| 5ht2Cr expressed only in POMC neurons | Glucoregulatory defects; | 106 | ||
| Obese mice on HFD | ||||
| Selective POMC 5ht2Cr deletion | Normal body weight, hyperinsulemic, hypergluconemic, insulin resistant, hyperphagic, and obese on HFD | 107 | ||
|
| ||||
| Glucose | Activation | Kir6.2 deletion in POMC neurons | Glucose intolerant | 109 |
|
| ||||
| Peroxisome-proliferator-activated receptor gamma (PPARg) delition in neurons and PPARg deletion in POMC neurons | Increased hepatic insulin sensitivity and weight gain induced by TZDs during HFD is abolished in neuron-specific PPARg KO mice. | 110, 111, 113, 114 | ||
| Alteration in hypothalamic expression of PPARg affects energy balance. | ||||
| Mice with selective deletion on PPARgamma in POMC neurons are resistant to HFD | ||||
|
| ||||
| Fatty Acids | Activation dependent on type of fatty acids | SUR1 deleted-POMC neurons | Oleic acid excites POMC neurons | 117 |
| SUR1-deleted POMC neurons are not depolarized by oleic acid | ||||
|
| ||||
| Amino Acids | Activation (acutely) | Tsc1c KO | Acute activation of mTOR by amino acid decreases food intake; chronic activation of mTOR in TSC1 KO increase body weight | 120–122 |
| Inhibition (chronically) | ||||
Abbreviations: ADX, adrenalectomized; CB1R, cannabinoid receptor type 1; HFD, high-fat diet; InsR, insulin receptor; Kir6.2, inward-rectifier potassium ion channel 6.2; KO, knockout; LepRb, leptin receptor; mTOR, mechanistic target of rapamycin; nAChR, nicotinic acetylcholine receptor; POMC, pro-opiomelanocortin; PPARγ, peroxisome proliferator–activated receptor gamma; SUR1, sulfonylurea receptor 1; TSC1, tuberous sclerosis protein 1; TZD, thiazolidinedione.
Leptin
Leptin, an adipocyte-derived hormone, plays a prominent role in the regulation of POMC neurons. As mentioned above, a subpopulation of POMC neurons expresses the long form of LepRb. Via a direct effect on POMC neurons, leptin activates the LepRb-STAT3 pathway and the Jak2-phosphoinositol-3-kinase (PI3K)–transient receptor potential canonical (TRPC) channel pathway. This induces increased POMC mRNA levels and neuronal depolarization that will result in a decrease of food intake. In addition to these signaling pathways, leptin also affects the mammalian target of rapamycin (mTOR) pathway and the activity of AMP-activated protein kinase (AMPK) (65, 66). The inhibition of hypothalamic AMPK is a necessary event for the effect of leptin on feeding and body weight, as constitutively active hypothalamic AMPK blocks its anorexigenic effect (66). In addition, activation of the mTOR pathway affects AMPK phosphorylation, an event necessary for leptin's effects on hypothalamic AMPK activity, hypothalamic neuropeptide expression, food intake, and body weight (65). This suggests that similar to the muscle (67), reciprocal modulation of the activity of mTOR and AMPK also exists in the hypothalamus.
To date, several mouse models with LepRb deletion in POMC neurons have been generated and studied (68–72), showing the important role of POMC neurons in mediating leptin effects on energy homeostasis. Interestingly, however, when LepRbs were reexpressed in POMC neurons of LepRb-null mice (db/db), only a modest reduction in body weight was observed, which was due to partial normalization of energy expenditure; no effect on food intake was found (73, 74). Furthermore, a significant improvement in glucose metabolism was observed (73, 74).
In addition to the direct effect of leptin on POMC neurons via its receptor, leptin also affects the synaptic input organization onto POMC cells (Figure 1a) (75). The effect of leptin on the synaptic input organization onto POMC neurons (75) is mediated by the direct effect of leptin on both specific neuronal populations, such as the NPY/AgRP neurons, and glial cells. For example, by directly inhibiting the neighboring orexigenic NPY/γ-aminobutyric (GABA) neurons (63), leptin will decrease the number of GABAergic inputs onto POMC cells (75). In addition, via its direct effect on glial cells, leptin will affect the glial coverage of POMC neurons. Indeed, selective deletion of LepRb in astrocytes (76) induces alteration in glial morphology and causes a decrease in glial coverage and an increase in synaptic inputs onto POMC neurons. As a result, leptin-induced suppression of feeding is diminished in mice with selective deletion of LepRb in glial cells, suggesting that glial cells play an active role in the initiation of hypothalamic synaptic plasticity and the neuroendocrine control of feeding by leptin.
Insulin
Insulin, a hormone produced by pancreatic β cells (77, 78), modulates POMC neuronal activity in the regulation of glucose and energy metabolism. Remarkably, its effect on POMC neurons is controversial. Two reports demonstrated that insulin suppresses POMC neuronal activity (49, 79), whereas a later study showed that insulin excites POMC neurons (80). This discrepancy could be attributed to the utilization of different insulin formulations. The earlier study by Williams and collaborators (49) used a formulation containing Zn+2, which might promote POMC hyperpolarization (49). Qiu et al. (80) used purified insulin that lacked this cation and found that insulin depolarizes POMC neurons via PI3K-TRPC5 channels (80).
Consistent with Qui et al.'s (80) report, if insulin receptor (InsR) is reintroduced in POMC neurons in L1 mice that lack hypothalamic insulin receptors, inhibitory synaptic contacts onto POMC neurons are reduced concomitant with an increased energy expenditure, exacerbated insulin resistance, and increased hepatic glucose production (HGP) (78). The phenotypes of the POMC InsR versus the AgRP InsR knockin mice were diametrically opposite, suggesting that the insulin signaling in AgRP neurons decreases HGP, whereas insulin signaling in POMC neurons promotes HGP and energy expenditure (78). In support of this, mice with selective deletion of InsR in AgRP neurons fail to suppress hepatic glucose production (81). Interestingly, however, when InsR was selectively ablated in POMC neurons, no effects on either body weight or glucose metabolism were observed (68). Furthermore, although both insulin and leptin activate the same intracellular enzyme, PI3K (81), selective deletion of LepRb in POMC neurons produces mice with increased body weight and impaired glucose metabolism. Selective double deletion of InsR and LepRb in POMC cells does not alter body weight but does impair glucose metabolism (82). Thus, these results further argue in favor of distinct arcuate POMC neuronal subpopulations.
Ghrelin
The gastrointestinal octanoylated peptide hormone ghrelin has well-established orexigenic actions, which are mainly mediated by the arcuate neurons (83–87). A particular subset of these hypothalamic cells (i.e., AgRP/NPY neurons) can be easily reached by the ghrelin due to their peculiar anatomical position and their high expression of the ghrelin receptor (86).
A growing body of evidence demonstrates that circulating ghrelin levels rise after fasting, thus promoting the activation of arcuate AgRP/NPY orexigenic neurons (85, 88–89). The most important consequence of activating these orexigenic neurons is the inhibition of POMC neurons, which leads to a dramatic increase in food intake. Several studies established that the effects of ghrelin on POMC neuronal activity are indirect and mediated by the neighboring AgRP neurons (85, 89). First, POMC neurons do not express ghrelin receptors (86). Second, we and others (85, 87, 89) have shown that ghrelin indirectly inhibits POMC neuronal activity, increasing the miniature inhibitory postsynaptic currents (mIPSCs) on POMC cells. The ghrelin-driven hyperpolarization of POMC neurons is accompanied by a reduction of asymmetric synapses, thus resulting in less excitatory synapses in POMC cells. Consistent with this, ghrelin administration to mice with selective deletion of vesicular GABA transporter in AgRP neurons failed to increase the frequency of inhibitory postsynaptic potentials in POMC neurons, suggesting that ghrelin-stimulated GABAergic input onto POMC neurons is mediated by NPY/AgRP neurons (89).
Finally, our group reported an additional mechanism underpinning the orexigenic effects of ghrelin, showing that both peripheral and central administration of ghrelin increases the mRNA and protein levels of PRCP, the enzyme responsible for the degradation of α-MSH (90). Therefore, by affecting PRCP levels and thus modulating POMC processing, ghrelin can further regulate melanocortin signaling (64).
Glucocorticoids
Among the plethora of factors regulating melanocortin system activity, glucocorticoids secreted from the adrenal cortex play a prominent role. Cortisol or corticosterone (in rodents) synthesis and secretion are controlled by both the hypothalamic releasing factor corticotropin and the pituitary POMC-derived ACTH and play important roles in regulating energy homeostasis, in part by regulating POMC neurons. As reviewed elsewhere (62, 91), glucocorticoids are able to promote positive energy balance by several mechanisms, including direct transcriptional regulation of hypothalamic neuropeptides, alteration of the sensitivity of peptide signaling in the central melanocortin system, and influence on the activity of arcuate neuronal populations. Accordingly, an increase in glucocorticoid levels was found in several genetic and dietary models of obesity, whereas adrenalectomy (ADX) reverses the obese phenotype by reducing food intake and preventing excessive weight gain.
In addition to controlling transcript levels of neuropeptides in the arcuate, corticosterone affects POMC neuronal activation via alterations of synaptic input organization onto POMC neurons (92). Indeed, data from our laboratory have demonstrated that ADX leads to a decrease in inhibitory synapses onto POMC neurons, resulting in a decrease in mIPSCs and an increase in miniature EPSCs of POMC neurons (92); these deficits are restored after corticosterone replacement (92).
Endocannabinoids
Endocannabinoids, which have recently been redefined as thrifty lipids and dietary fat sensors, control the rewarding properties of food and stimulate energy intake (93, 94). The endocannabinoid system (ECS) exerts biological effects through activation of the G protein-coupled CB1 and CB2 receptors; both receptors are expressed in POMC neurons.
The ECS regulates the complex behavior of food seeking and the process of energy storage by modulating the activity of extrahypothalamic neurons (95) and regulating other hormones that convey information to the ARC about the whole-body energy status (96). Besides the effects of endocannabinoids on the limbic forebrain and brainstem, their orexigenic actions were recently correlated with an increase in NPY and the POMC-derived β-endorphin (97) and a change in POMC neuronal activity (2, 98, 99).
POMC neurons continuously release endocannabinoids under basal conditions. Interestingly, this constitutive endocannabinoid release and exogenously applied cannabinoid receptor type 1 (CB1R) agonists lead to the retrograde inhibition of the presynaptic GABA release onto POMC neurons (98), suggesting that the activation of CB1R receptors results in a paradoxical increase in the excitatory tone on the anorexigenic POMC neurons. In support of this, a recent study from our group and Horvath's group (2) has shown that hyperphagic stimulation of CB1R results in the activation of POMC neurons and that POMC neuronal activation is key for the increased feeding behavior evoked by CB1R. Although the Pomc gene encodes the anorexigenic peptide α-MSH, it also encodes the orexigenic peptide β-endorphin (100). Indeed, CB1R activation produced a significant increase in PCs, including PC1 and PC2, the latter identified as an indispensable enzyme for the processing of POMC into β-endorphin (2). In fact, hypothalamic β-endorphin levels were increased after CB1R activation. Additionally, the blockage of central μ-opioid receptors prevented CB1R activation-induced feeding (2), suggesting that CB1R-induced acute feeding is evoked by POMC neuronal activation via β-endorphin release and μ-opioid activation (Figure 2c). These findings bring POMC neurons into a new light, suggesting a more complex role in feeding regulation than previously thought.
Nicotine
Smoking-induced nicotine dependence poses a significant global health burden, and smoking is known to affect feeding behavior. Subsequently, smokers are associated with a significantly lower body mass index compared to nonsmokers, and quitting smoking induces weight gain. These effects are attributed to the actions of nicotine at both peripheral and central levels. Centrally, nicotine has a direct effect on POMC neurons (101, 102). It depolarizes them via the activation of α3β4 nicotinic acetylcholine receptors, thus decreasing food intake and body weight (101). In addition, siRNA-mediated knockdown of MC4R in the PVH blunts the effect of nicotine on food intake (101), suggesting that the melanocortin system plays a role in nicotine-induced hypophagia. Interestingly, nicotine also depolarizes the orexigenic NPY/AgRP neurons (102). However, nicotine evokes a greater depolarization in POMC neurons compared to NPY/AgRP neurons. Although it evokes an inhibitory effect on the synaptic release of glutamate onto NPY/AgRP neurons, no effect on synaptic glutamate release was observed in POMC neurons (102). Collectively, these data suggest that nicotine's effects on POMC neurons may be one potential site for selective pharmacological intervention to regulate feeding.
Serotonin
Serotonin, via 5-hydroxytryptamine 2C receptor (5-HT2CR) in the brain, regulates energy balance and glucose homeostasis (48, 103–107). Meta-chlorophenylpiperazine (mCPP), a selective 5-HT2CR agonist, improves insulin resistance and glucose intolerance in diet-induced obesity (DIO) mice via the melanocortin 4 receptor signaling pathway (104). This suggests the involvement of POMC neurons in the central serotonin control of metabolism; POMC neurons indeed express 5-HT2CRs (103) and receive innervation from serotoninergic neurons (108). In addition, 5-HT drugs activate POMC neurons (103), and 5-HT2CR agonists stimulate POMC expression (104). Administration of 5-HT receptor agonists in global 5-HT2CR knockout mice with selective reexpression of 5-HT2CR in POMC neurons induces suppression of feeding (105) and controls glucose metabolism by regulating hepatic insulin sensitivity (106). mCPP has been shown to depolarize approximately 25% of POMC neurons through TRPC channels (48, 106), and mice selectively lacking Htrc2r in POMC neurons have normal body weight but develop glucoregulatory defects including hyperinsulinemia, hyperglucagonemia, and insulin resistance (107). In addition, these mice do not display the anorectic response to serotonergic agents that suppress appetite but develop hyperphagia and obesity when exposed to HFD feeding (107). Thus, these data show that serotonin, through its 5-HT2CR, regulates energy and glucose homeostasis via the direct control of POMC neurons.
Glucose
Glucose is an important modulator of POMC neurons (109). Physiologically, when glucose levels rise (during the postprandial period), POMC neuronal activity increases, thus determining the activation of neuronal circuits that convey the satiety signal as well as hepatic glucose homeostasis and insulin sensitivity.
How does glucose regulate POMC neuronal activation? Whether or not glucose is directly uptaken by POMC neurons, its oxidation by the mitochondria will produce ATP. By binding and closing the ATP-dependent potassium channels (KATP), ATP will in turn depolarize POMC membrane potential, thus increasing POMC electrical activity. In support of this, Parton and collaborators (109) reported that POMC neurons with a mutated KATP channel subunit are unable to sense glucose and alter their electrical activity.
In addition to the production of ATP, mitochondrial glucose oxidation also affects the production of reactive oxygen species (ROS). Indeed, high glucose levels increase ROS in POMC neurons (61, 85, 110), whereas low glucose levels, such as during fasting, reduce POMC ROS levels, and POMC neurons are silent. Thus, ROS may function as modulators of POMC neuronal activity, and their increased levels during positive energy balance may lead to satiety. Indeed, when ROS scavengers are centrally administered, feeding behavior and cellular events associated with negative energy balance are promoted (110), whereas when increased ROS levels are promoted in POMC neurons, feeding behavior and associated cellular events to satiety are observed (110, 111). Thus, it is reasonable to hypothesize that short-term ROS peaks may occur daily and may be critical for the proper behavioral, endocrine, and autonomic response to nutrient intake.
Conversely, if the exposure to hyperglycemia is sustained, such as during HFD feeding, a diet characterized by elevated glucose and fatty acids (FAs) and prolonged and elevated ROS generation may be detrimental, as shown in other glucose-sensing brain areas (112). Therefore, the impaired ability of POMC neurons to respond to circulating signals including leptin might contribute to triggering the so-called leptin resistance (110). In support of this, recent data from several laboratories including ours show that activation of peroxisome proliferator–activated receptor gamma (PPARγ) in POMC neurons during HFD feeding may be involved in the development of leptin resistance (110, 111, 113, 114) by affecting ROS levels in these neurons. But how do ROS regulate POMC neuronal activity? ROS are highly reactive molecules shown to impact several signaling pathways, ion channels, and transports and known to modify kinases and ubiquitination/proteasome systems (115). Several studies have shown that ROS can cause reversible posttranslational protein modifications to regulate protein activity. For example, ROS can oxidize thiol groups on cysteine residues in target proteins, including phosphatase enzymes and ion channels (116), thus altering their enzymatic and activity levels. Therefore, it is conceivable that in POMC neurons, glucose oxidation and promotion of ROS production may affect the activity of ion channels, including KATP, thus altering POMC membrane potential.
Fatty Acids
POMC neurons are able to sense FAs. These acids, and more specifically oleic acid, trigger depolarization of POMC neurons and induce a decrease in food intake (117). Similar to glucose sensing, KATP channels mediate FA sensing in POMC neurons. An increase in ATP production by β-oxidation of FAs closes the KATP channels, thus depolarizing these neurons (117).
In addition to the intracellular effects on POMC neurons, HFD feeding has been associated with changes in the synaptic input organization onto POMC neurons (Figure 1a) (118). Specifically, in DIO animals, the total number of synapses onto POMC neurons decreases due to a significant reduction in inhibitory synapses (118). This phenomenon is associated with increased glial ensheathment around the POMC perikarya (118). Interestingly, organization of synaptic input onto POMC neurons differs between DIO and diet-resistant (DR) animals (118), with DIO animals presenting a higher number of inhibitory synapses onto POMC neurons compared to DR animals (118). Note that POMC-specific deletion of the GABAB receptor protects animals against obesity when fed an HFD (119).
Amino Acids
The mammalian target of rapamycin complex 1 (mTORC1) is known as an amino acid sensor. ICV injection of L-leucine activates mTORC1 signaling and decreases food intake (120). Injection of either rapamycin, an inhibitor of mTOR, or ERK inhibitor restores the leucine-induced decrease in food intake (120, 121). However, long-term activation of the mTOR signaling revealed a different effect. The tuberous sclerosis protein 1/2 (TSC1/2) complex is an inhibitory upstream protein of the mTORC1. Mice with selective deletion of TSC1 in POMC neurons show a higher body weight and food intake (122). Rapamycin treatment of these animals ameliorates the hyperphagia and obesity (122). These effects of mTOR are found to be associated with aging-related obesity. Notably, mTOR signaling in POMC neurons increases during aging, and because of this and an increased KATP channel expression, POMC neurons are electrically silent in aged mice (123). However, ICV infusion of rapamycin to aged mice restores the excitability of POMC neurons and decreases both food intake and body weight (123).
MALNUTRITION AND OVERNUTRITION DURING PREGNANCY
Both POMC neuronal projections and POMC responsiveness to different signals are affected by the nutritional states during pregnancy (Figure 3). During the so-called “Dutch Hunger Winter” at the end of World War II in the Netherlands, babies of mothers who had suffered severe malnutrition during pregnancy were small sized at birth and later exhibited accelerated catch-up growth accompanied with attenuated glucose metabolism and decreased insulin secretion (124, 125). Similar results and higher morbidity of type II diabetes are reported in other populations that experienced gestational famines, such as in Austria, Ukraine, China, and Nigeria (125). Rodent models of prenatal maternal undernutrition also show defects in glucose tolerance and insulin secretion. In rats, the offspring born from 70% food-restricted mothers have an abnormal rhythm of food intake (126). Maternal undernutrition suppresses the surge of leptin secretion between P6–14 and attenuates both POMC neuronal projections and mRNA in neonates (127). Similar to calorie restriction, protein restriction during gestation and lactation induces a defect in POMC neuronal projections to the PVH (128). Interestingly, when protein restriction was stopped at birth and mothers received normal amounts of protein during lactation, POMC projections to the PVH were restored. Additionally, an enhanced leptin surge and a catch-up growth in the body weight were observed in the pups (128).
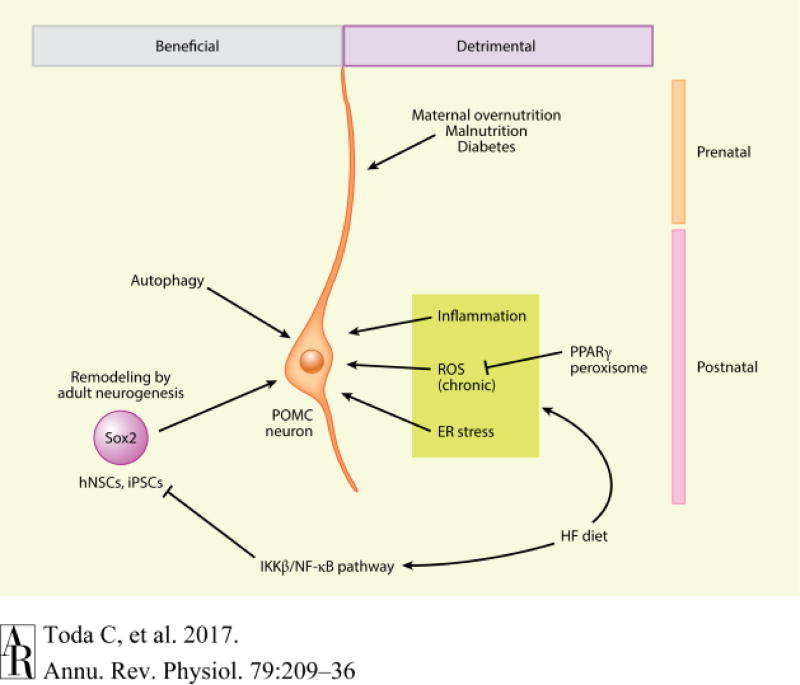
Figure 3.
Beneficial and detrimental factors regulating POMC neurons during prenatal and postnatal periods. POMC neurons maintain their physiological functions by autophagy and adult neurogenesis. However, inflammation, chronic ROS production, and ER stress that are induced by HFD feeding deteriorate POMC functions and projections. Prenatal factors, such as maternal nutritional states, have profound effects on POMC neuronal functions and thus on the whole-body energy and glucose homeostasis. Abbreviations: ER, endoplasmic reticulum; HF, high-fat; hNSC, hypothalamic neural stem cell; IKKβ, IκB kinase β; NF-κB, nuclear factor-κB; iPSC, inducible pluripotent stem cell; POMC, pro-opiomelanocortin; PPARg, peroxisome proliferator-activated receptor gamma; ROS, reactive oxygen species.
Similar to undernutrition, maternal overnutrition during both gestation and lactation also causes obesity in adult offspring (129). Maternal HFD feeding affects the programming of the POMC neurocircuit, predisposing offspring to metabolic disorders. For example, maternal HFD feeding activates astrocytes, which serve as key regulators of glucose and glutamate transport to neurons (130). Maternal HFD feeding induces an increase in glial coverage onto POMC neurons, resulting in decreased inhibitory inputs and an enhancement of POMC glucose sensing (130). Importantly, many of the known modulators of POMC neurons change when mothers are fed an HFD, including FAs, glucose, insulin, and inflammatory cytokines. All of these circulating signals may contribute to the offspring obese phenotype (131).
Steculorum & Bouret (131) unmasked that streptozotocin-induced type I diabetes in dams decreases leptin sensitivity in ARC neurons and decreases α-MSH fibers in the PVH with an increased number of POMC cell bodies in the ARC. In addition to the diet, the timing of exposure also plays an important role in determining the metabolic fate of the offspring. For example, two studies have revealed that maternal HFD feeding during lactation, which corresponds to the last trimester of human pregnancy, leads to increased body weight and attenuated glucose metabolism in the offspring (132, 133). It was found that during lactation, HFD feeding decreases both α-MSH and AgRP projections to hypothalamic targeted areas, including the PVH (133). Interestingly, insulin receptor deletion in POMC neurons of the offspring of lactating HFD-fed mothers prevented the decrease of α-MSH projections to the preautonomic PVH, the altered parasympathetic innervation to pancreatic β-cells, and the impaired glucose-stimulated insulin secretion of the offspring (133). Thus, these data indicate that maternal HFD feeding during this critical time window induces an abnormal insulin signaling in POMC neurons of offspring, which in turn may contribute to the long-term impairment of hypothalamic control of energy and glucose homeostasis.
OBESE STATE
Inflammation
Classically, inflammation is an acute protective response to pathogens and injury to help return the system to normal status. However, overnutrition-induced inflammation in metabolic tissues, which is termed metaflammation, has unique features compared to classic inflammation (134). Unlike classic inflammation, metaflammation is a chronic, low-grade sterile inflammation without any microbial infection. In this atypical inflammation, the hypothalamus, especially the ARC, might be considered as the primary metabolic tissue where inflammatory responses to metabolic changes occur. This is because hypothalamic proinflammatory cytokines (Il1b, Il6, and Tnfα) and NF-κB pathway genes (Ikbkb and Ikbke) are all significantly increased within 1 to 3 days of HFD feeding, whereas inflammation is not yet detected in peripheral organs such as the liver and adipose tissue (135, 136). Therefore, metaflammatory responses occur rapidly in the hypothalamus compared to peripheral tissues.
Several cytokines are able to enter the ARC by crossing the BBB, including tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), interleukin-1α (IL-1α) and interleukin-1β (IL-1β) (137); they can directly influence neurons expressing these cytokine receptors (138). In conditions of nutrient deprivation and excess nutrients, the permeability of the BBB is increased (139). These physiological changes facilitate how ARC neurons sense circulating signals, including cytokines. Several studies established the role of cytokines in central inflammation by assessing the effects of central administration of cytokines and/or after neuron-specific deletion of cytokines and their receptors. For example, central administration of TNF-α rapidly increases cytokines, such as IL-1β, IL-6, and IL-10, and also significantly increases neuropeptide levels in the hypothalamus (140). In addition, acute TNF-α-induced inflammation inhibits food intake by modulating insulin and leptin signaling in the hypothalamus (141, 142) and reduces expression of thermogenic proteins in brown adipose tissue and skeletal muscle (142, 143). Consistent with this, genetically deficient mice for either TNF-α (144) or TNF-α receptors (142, 143) are protected from DIO. Interestingly, although central administration of TNF-α significantly induced IKKβ phosphorylation, particularly in POMC neurons, and the Pomc gene is a downstream target of NF-κB (145), POMC-specific IKKβ ablation is insufficient to prevent HFD-induced obesity but rather prevents obesity-induced hypertension (146). By contrast, AgRP-specific IKKβ-deficient mice are partially protected from HFD-induced obesity (146, 147), thus suggesting that in the ARC the physiological effects of neuronal IKKβ/NF-κB activation are cell-type dependent.
Contrary to TNF-α-deficient mice, whole-body IL-6-deficient mice develop mature-onset obesity, including glucose intolerance and leptin resistance (148), and ICV injection of IL-6 increases energy expenditure and decreases body fat (149). IL-6 is an important inflammatory cytokine mediating the beneficial effects of exercise on leptin and insulin sensitivity (150). In addition to IL-6, hypothalamic IL-10 is necessary to exert the beneficial metabolic effect of exercise and IL-6 (150). POMC and NPY neurons may be directly involved with the anti-inflammatory effects of IL-6 and IL-10 because both neuronal populations express IL-6 and IL-10 receptors (150). Interestingly, whereas physical activity increases POMC mRNA and reduces NPY mRNA levels in obese animals, it does not alter these mRNAs in lean animals (150). Intrahypothalamic injection of recombinant IL-10 reduces food intake and restores insulin and leptin sensitivity in obese mice (150). Consistent with this, systemic IL-10 treatment produces an anorexigenic effect in leptin-deficient (ob/ob) mice (151). Systemic IL-10 treatment to ob/ob mice remarkably induces STAT3 phosphorylation and POMC mRNA expression, suggesting that IL-10 can substitute for leptin in STAT3 activation, particularly in POMC neurons (151).
IL-1β, known as a classic proinflammatory cytokine, also has a beneficial effect similar to IL-6 and IL-10. Central or peripheral administration of IL-1β suppresses food intake and increases body temperature (152). Conversely, central administration of IL-1 receptor antagonist blunts the anorexigenic effect of leptin, and leptin fails to suppress food intake in IL-1 receptor-deficient mice, suggesting that IL-1 is a central mediator of leptin action (152, 153). Indeed, IL-1β induces c-fos activation in POMC neurons and stimulates the release of α-MSH from hypothalamic explants (154, 155). Activation of POMC neurons by IL-1β seems to be mediated by IL-1R expressed in POMC neurons. Indeed, electrophysiological recordings showed that IL-1β increases the frequency of action potentials in POMC neurons (155). It should be noted that although POMC neurons may play a relevant role in central inflammation, the genetic animal models targeting cytokines or cytokine receptors have thus far provided limited direct evidence because most of these animal models do not target specific neuronal populations, such as the POMC neurons.
In addition to cytokines, Toll-like receptors (TLRs), key membrane-bound pattern-recognition receptors involved in innate immune responses, have been implicated in the central development of metabolic disorders. HFD feeding and central lipid infusion activate TLR4 signaling and the downstream IKKβ/NF-κB pathway, resulting in metabolic dysfunctions, such as central leptin resistance, systemic glucose intolerance, increased food intake, and weight gain (147, 156). Similarly, TLR2/4-deficient mice are protected from HFD-induced obesity (157, 158). Furthermore, brain-specific disruption of myeloid differentiation factor 88, which is a downstream signal adaptor for most TLRs (159), protects mice from DIO (156). However, a recent study reported conflicting results and showed that TLR2-deficient mice display mature-onset obesity possibly due to reduced hypothalamic α-MSH levels (160). Future studies targeting TLR deficiency in specific neuronal populations will address the direct involvement of TLR signaling in the central regulation of metabolism.
Endoplasmic Reticulum Stress
The endoplasmic reticulum (ER) is a fundamental organelle for maintaining cellular metabolic homeostasis and preserving systemic metabolic homeostasis. The ER serves many functions, including lipid and carbohydrate metabolism in the smooth ER, and the protein folding and transport of synthesized proteins to the Golgi apparatus in the rough ER. Under normal ER homeostasis, misfolded proteins are delivered to the cytoplasm and undergo proteasomal degradation, which is important for quality control of proteins. Attenuation in ER homeostasis leads to the accumulation of misfolded proteins that in turn activates stress-responsive signaling pathways known as the unfolded protein response (UPR) to maintain metabolic homeostasis at the cellular level (161). Therefore, UPR signaling is essential for cellular adaptation to changes of the cellular nutrient state (161). Many studies have shown that defects in the UPR pathway causes metabolic disorders, including impaired glucose tolerance and insulin and leptin resistance (161, 162). For example, mice with a pan-neuronal deletion of X-box-binding protein 1 (Xbp1), a transcription factor triggered by ER stress, display increased body weight, glucose intolerance, insulin resistance, and leptin resistance on HFD feeding compared to control mice (162). In addition, mice with POMC-specific constitutive expression of Xbp1 are protected against DIO, as they show improved leptin and insulin sensitivity and liver metabolism (163). Further, POMC-specific Xbp1-deficient mice preserved leptin sensitivity and leptin-induced depolarization of POMC by ER stress-induced treatment (163). Besides molecular events triggered by UPR signaling, ER–mitochondria communication is important in maintaining normal leptin sensitivity. Mitofusin 2 (Mfn2), a dynamin-like GTPase protein involved in mitochondrial fusion, is a key molecule for ER–mitochondria contacts (164). POMC-specific Mfn2-deficient mice display a significant decrease in ER–mitochondria contacts (165), which is associated with leptin resistance, hyperphagia, reduced energy expenditure, and defective POMC processing (165). Indeed, we have recently shown that in a transgenic mouse model with improved leptin sensitivity on an HFD, the number of ER–mitochondria contacts in POMC neurons was preserved compared to obese controls (111).
Autophagy
Autophagy, a catabolic cellular process involving the degradation of damaged cellular components, represents a quality control process for organelles such as the ER and mitochondria (166). Several recent studies have demonstrated that defects in hypothalamic autophagy lead to metabolic dysregulation. HFD feeding is associated with decreased autophagy in the mediobasal hypothalamus (167). Moreover, mediobasal hypothalamic inhibition of Agt7, an essential autophagy gene, in mice fed a standard chow diet was shown to increase energy intake and reduce energy expenditure by promoting hypothalamic inflammation (167).
Subsequent studies showed that mice with selective POMC deletion of Atg7 displayed increased body weight, food intake, adiposity, decreased energy expenditure, leptin resistance, and glucose intolerance (34, 168, 169). Furthermore, POMC-derived α-MSH projections to the PVH are diminished in POMC-specific Atg7 knockout mice compared to controls (34). Similar to POMC-specific Atg7 knockout mice, POMC-selective Atg12-deficient mice also displayed a similar obese phenotype (170). Interestingly, a recent study showed that activation of autophagy in POMC neurons in response to cold exposure regulates lipophagy in brown adipose tissue and the liver (171). In contrast to the role of autophagy in POMC neurons, selective deletion of Atg7 in AgRP neurons results in reduced body weight, adiposity, and refeeding response to fasting that is at least partly due to increased α-MSH levels in the hypothalamus. This suggests that inhibition of autophagy in AgRP neurons may downregulate the inhibitory effects of AgRP onto POMC neurons, thus promoting the effect of POMC neurons on energy metabolism (172). In summary, hypothalamic autophagy seems to play a key role in the regulation of energy homeostasis. Furthermore, as autophagy has been shown to decline in aging, this mechanism may also contribute to the altered energy balance observed with age.
FUTURE DIRECTIONS
Much progress has been made in our understanding of the physiological functions of POMC neurons in the regulation of energy and glucose metabolism. However, recent evidence shows that POMC neurons are heterogeneous in nature and can function as orexigenic neurons. This suggests that more studies are needed to identify the molecular profile of the diverse POMC subpopulations and their projection fields and to dissect the role of POMC neurons in regulating energy and glucose metabolism.
SUMMARY POINTS.
The development of POMC neurons and their neuronal circuit is regulated by multiple transcriptional factors, hormonal signals, and nutritional status. Maternal nutrition during gestation also plays a key role in the development of this neuronal population.
POMC neurons are heterogeneous in terms of their responsiveness to different hormones and nutrients.
POMC neurons can act as anorexigenic or orexigenic according to the peptides released.
Neurogenesis of POMC neurons in adulthood is artificially reproducible.
Pathological changes in the function and projection of POMC neurons deteriorate the homeostatic control of energy metabolism, leading to metabolic syndromes (Figure 3).
Acknowledgments
This work was supported by National Institutes of Health grants DK097566, DK105571, and DK107293 (to S.D.) and the Japan Society for the Promotion of Science Postdoctoral Fellowships for Research Abroad (to C.T.).
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Horvath TL, Naftolin F, Kalra SP, Leranth C. Neuropeptide-Y innervation of beta-endorphin-containing cells in the rat mediobasal hypothalamus: a light and electron microscopic double immunostaining analysis. Endocrinology. 1992;131:2461–67. doi: 10.1210/endo.131.5.1425443. [DOI] [PubMed] [Google Scholar]
- 2.Koch M, Varela L, Kim JG, Kim JD, Hernández-Nuño F, et al. Hypothalamic POMC neurons promote cannabinoid-induced feeding. Nature. 2015;519:45–50. doi: 10.1038/nature14260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sternson SM, Atasoy D, Betley JN, Henry FE, Xu S. An emerging technology framework for the neurobiology of appetite. Cell Metab. 2016;23:234–53. doi: 10.1016/j.cmet.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Diano S. New aspects of melanocortin signaling: a role for PRCP in α-MSH degradation. Front. Neuroendocrinol. 2011;32:70–83. doi: 10.1016/j.yfrne.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McClellan KM, Calver AR, Tobet SA. GABAB receptors role in cell migration and positioning within the ventromedial nucleus of the hypothalamus. Neuroscience. 2008;151:1119–31. doi: 10.1016/j.neuroscience.2007.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimada M, Nakamura T. Time of neuron origin in mouse hypothalamic nuclei. Exp. Neurol. 1973;41:163–73. doi: 10.1016/0014-4886(73)90187-8. [DOI] [PubMed] [Google Scholar]
- 7.McNay DEG, Pelling M, Claxton S, Guillemot F, Ang S-L. Mash1 is required for generic and subtype differentiation of hypothalamic neuroendocrine cells. Mol. Endocrinol. 2006;20:1623–32. doi: 10.1210/me.2005-0518. [DOI] [PubMed] [Google Scholar]
- 8.Marin O, Baker J, Puelles L, Rubenstein JL. Patterning of the basal telencephalon and hypothalamus is essential for guidance of cortical projections. Development. 2002;129:761–73. doi: 10.1242/dev.129.3.761. [DOI] [PubMed] [Google Scholar]
- 9.Kimura S, Hara Y, Pineau T, Fernandez-Salguero P, Fox CH, et al. The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 1996;10:60–69. doi: 10.1101/gad.10.1.60. [DOI] [PubMed] [Google Scholar]
- 10.Blaess S, Szabó N, Haddad-Tóvolli R, Zhou X, Álvarez-Bolado G. Sonic hedgehog signaling in the development of the mouse hypothalamus. Front. Neuroanat. 2014;8:156. doi: 10.3389/fnana.2014.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manning L, Ohyama K, Saeger B, Hatano O, Wilson SA, et al. Regional morphogenesis in the hypothalamus: a BMP-Tbx2 pathway coordinates fate and proliferation through Shh downregulation. Dev. Cell. 2006;11:873–85. doi: 10.1016/j.devcel.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 12.Shimogori T, Lee DA, Miranda-Angulo A, Yang Y, Wang H, et al. A genomic atlas of mouse hypothalamic development. Nat. Neurosci. 2010;13:767–75. doi: 10.1038/nn.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lagutin OV, Zhu CC, Kobayashi D, Topczewski J, Shimamura K, et al. Six3 repression of Wnt signaling in the anterior neuroectoderm is essential for vertebrate forebrain development. Genes Dev. 2003;17:368–79. doi: 10.1101/gad.1059403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geng X, Speirs C, Lagutin O, Inbal A, Liu W, et al. Haploinsufficiency of Six3 fails to activate Sonic hedgehog expression in the ventral forebrain and causes holoprosencephaly. Dev. Cell. 2008;15:236–47. doi: 10.1016/j.devcel.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L, Mathers PH, Jamrich M. Function of Rx, but not Pax6, is essential for the formation of retinal progenitor cells in mice. Genesis. 2000;28:135–42. [PubMed] [Google Scholar]
- 16.Lu F, Kar D, Gruenig N, Zhang ZW, Cousins N, et al. Rax is a selector gene for mediobasal hypothalamic cell types. J. Neurosci. 2013;33:259–72. doi: 10.1523/JNEUROSCI.0913-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tamura K, Taniguchi Y, Minoguchi S, Sakai T, Tun T, et al. Physical interaction between a novel domain of the receptor Notch and the transcription factor RBP-Jκ/Su(H) Curr. Biol. 1995;5:1416–23. doi: 10.1016/s0960-9822(95)00279-x. [DOI] [PubMed] [Google Scholar]
- 18.Fortini ME. Notch signaling: the core pathway and its posttranslational regulation. Dev. Cell. 2009;16:633–47. doi: 10.1016/j.devcel.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Jarriault S, Brou C, Logeat F, Schroeter EH, Kopan R, Israel A. Signalling downstream of activated mammalian Notch. Nature. 1995;377:355–58. doi: 10.1038/377355a0. [DOI] [PubMed] [Google Scholar]
- 20.Maier MM, Gessler M. Comparative analysis of the human and mouse Hey1 promoter: Hey genes are new Notch target genes. Biochem. Biophys. Res. Commun. 2000;275:652–60. doi: 10.1006/bbrc.2000.3354. [DOI] [PubMed] [Google Scholar]
- 21.Aujla PK, Naratadam GT, Xu L, Raetzman LT. Notch/Rbpjκ signaling regulates progenitor maintenance and differentiation of hypothalamic arcuate neurons. Development. 2013;140:3511–21. doi: 10.1242/dev.098681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bray SJ. Notch signalling: a simple pathway becomes complex. Nat. Rev. Mol. Cell. Biol. 2006;7:678–89. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 23.Pelling M, Anthwal N, McNay D, Gradwohl G, Leiter AB, et al. Differential requirements for neurogenin 3 in the development of POMC and NPY neurons in the hypothalamus. Dev. Biol. 2011;349:406–16. doi: 10.1016/j.ydbio.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Anthwal N, Pelling M, Claxton S, Mellitzer G, Collin C, et al. Conditional deletion of neurogenin-3 using Nkx2.1iCre results in a mouse model for the central control of feeding, activity and obesity. Dis. Model. Mech. 2013;6:1133–45. doi: 10.1242/dmm.011916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yee CL, Wang Y, Anderson S, Ekker M, Rubenstein JLR. Arcuate nucleus expression of NKX2.1 and DLX and lineages expressing these transcription factors in neuropeptide Y+, proopiomelanocortin+, and tyrosine hydroxylase+ neurons in neonatal and adult mice. J. Comp. Neurol. 2009;517:37–50. doi: 10.1002/cne.22132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Padilla SL, Carmody JS, Zeltser LM. Pomc-expressing progenitors give rise to antagonistic neuronal populations in hypothalamic feeding circuits. Nat. Med. 2010;16:403–5. doi: 10.1038/nm.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Padilla SL, Reef D, Zeltser LM. Defining POMC neurons using transgenic reagents: impact of transient Pomc expression in diverse immature neuronal populations. Endocrinology. 2012;153:1219–31. doi: 10.1210/en.2011-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biehl MJ, Raetzman LT. Rbpj-κ mediated Notch signaling plays a critical role in development of hypothalamic Kisspeptin neurons. Dev. Biol. 2015;406:235–46. doi: 10.1016/j.ydbio.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanz E, Quintana A, Deem JD, Steiner RA, Palmiter RD, McKnight GS. Fertility-regulating Kiss1 neurons arise from hypothalamic Pomc-expressing progenitors. J. Neurosci. 2015;35:5549–56. doi: 10.1523/JNEUROSCI.3614-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouret SG, Draper SJ, Simerly RB. Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. J. Neurosci. 2004;24:2797–805. doi: 10.1523/JNEUROSCI.5369-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahima RS, Prabakaran D, Flier JS. Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. J. Clin. Investig. 1998;101:1020–27. doi: 10.1172/JCI1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304:108–10. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- 33.Bouret SG, Bates SH, Chen S, Myers MG, Simerly RB. Distinct roles for specific leptin receptor signals in the development of hypothalamic feeding circuits. J. Neurosci. 2012;32:1244–52. doi: 10.1523/JNEUROSCI.2277-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coupé B, Ishii Y, Dietrich MO, Komatsu M, Horvath TL, Bouret SG. Loss of autophagy in pro-opiomelanocortin neurons perturbs axon growth and causes metabolic dysregulation. Cell Metab. 2012;15:247–55. doi: 10.1016/j.cmet.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malik SA, Marino G, BenYounes A, Shen S, Harper F, et al. Neuroendocrine regulation of autophagy by leptin. Cell Cycle. 2011;10:2917–23. doi: 10.4161/cc.10.17.17067. [DOI] [PubMed] [Google Scholar]
- 36.Xu B, Goulding EH, Zang K, Cepoi D, Cone RD, et al. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat. Neurosci. 2003;6:736–42. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liao G-Y, Bouyer K, Kamitakahara A, Sahibzada N, Wang C-H, et al. Brain-derived neurotrophic factor is required for axonal growth of selective groups of neurons in the arcuate nucleus. Mol. Metab. 2015;4:471–82. doi: 10.1016/j.molmet.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liao GY, An JJ, Gharami K, Waterhouse EG, Vanevski F, et al. Dendritically targeted Bdnf mRNA is essential for energy balance and response to leptin. Nat. Med. 2012;18:564–71. doi: 10.1038/nm.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J, Tang Y, Cai D. IKKβ/NF-κB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nat. Cell Biol. 2012;14:999–1012. doi: 10.1038/ncb2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kokoeva MV, Yin H, Flier JS. Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science. 2005;310:679–83. doi: 10.1126/science.1115360. [DOI] [PubMed] [Google Scholar]
- 41.Gouazé A, Brenachot X, Rigault C, Krezymon A, Rauch C, et al. Cerebral cell renewal in adult mice controls the onset of obesity. PLOS ONE. 2013;8:e72029. doi: 10.1371/journal.pone.0072029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McNay DEG, Briançon N, Kokoeva MV, Maratos-Flier E, Flier JS. Remodeling of the arcuate nucleus energy-balance circuit is inhibited in obese mice. J. Clin. Investig. 2012;122:142–52. doi: 10.1172/JCI43134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li J, Tang Y, Purkayastha S, Yan J, Cai D. Control of obesity and glucose intolerance via building neural stem cells in the hypothalamus. Mol. Metab. 2014;3:313–24. doi: 10.1016/j.molmet.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Czupryn A, Zhou Y-D, Chen X, McNay D, Anderson MP, et al. Transplanted hypothalamic neurons restore leptin signaling and ameliorate obesity in db/db mice. Science. 2011;334:1133–37. doi: 10.1126/science.1209870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swanson LW, Kuypers HG. A direct projection from the ventromedial nucleus and retrochiasmatic area of the hypothalamus to the medulla and spinal cord of the rat. Neurosci. Lett. 1980;17:307–12. doi: 10.1016/0304-3940(80)90041-5. [DOI] [PubMed] [Google Scholar]
- 46.Elias CF, Lee C, Kelly J, Aschkenasi C, Ahima RS, et al. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron. 1998;21:1375–85. doi: 10.1016/s0896-6273(00)80656-x. [DOI] [PubMed] [Google Scholar]
- 47.Baker RA, Herkenham M. Arcuate nucleus neurons that project to the hypothalamic paraventricular nucleus: neuropeptidergic identity and consequences of adrenalectomy on mRNA levels in the rat. J. Comp. Neurol. 1995;358:518–30. doi: 10.1002/cne.903580405. [DOI] [PubMed] [Google Scholar]
- 48.Sohn J-W, Xu Y, Jones JE, Wickman K, Williams KW, Elmquist JK. Serotonin 2C receptor activates a distinct population of arcuate pro-opiomelanocortin neurons via TRPC channels. Neuron. 2011;71:488–97. doi: 10.1016/j.neuron.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Williams KW, Margatho LO, Lee CE, Choi M, Lee S, et al. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J. Neurosci. 2010;30:2472–79. doi: 10.1523/JNEUROSCI.3118-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jarvie BC, Hentges ST. Expression of GABAergic and glutamatergic phenotypic markers in hypothalamic proopiomelanocortin neurons. J. Comp. Neurol. 2012;520:3863–76. doi: 10.1002/cne.23127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dennison CS, King CM, Dicken MS, Hentges ST. Age-dependent changes in amino acid phenotype and the role of glutamate release from hypothalamic proopiomelanocortin neurons. J. Comp. Neurol. 2016;524:1222–35. doi: 10.1002/cne.23900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu J, Jiang L, Low MJ, Rui L. Glucose rapidly induces different forms of excitatory synaptic plasticity in hypothalamic POMC neurons. PLOS ONE. 2014;9:e105080. doi: 10.1371/journal.pone.0105080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ciccotosto GD, Schiller MR, Eipper BA, Mains RE. Induction of integral membrane PAM expression in AtT-20 cells alters the storage and trafficking of POMC and PC1. J. Cell Biol. 1999;144:459–71. doi: 10.1083/jcb.144.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cool DR, Normant E, Shen F, Chen HC, Pannell L, et al. Carboxypeptidase E is a regulated secretory pathway sorting receptor: genetic obliteration leads to endocrine disorders in Cpefat mice. Cell. 1997;88:73–83. doi: 10.1016/s0092-8674(00)81860-7. [DOI] [PubMed] [Google Scholar]
- 55.Jeong JK, Diano S. Prolyl carboxypeptidase mRNA expression in the mouse brain. Brain Res. 2014;1542:85–92. doi: 10.1016/j.brainres.2013.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jeong JK, Szabo G, Raso GM, Meli R, Diano S. Deletion of prolyl carboxypeptidase attenuates the metabolic effects of diet-induced obesity. Am. J. Physiol. Endocrinol. Metab. 2012;302:E1502–10. doi: 10.1152/ajpendo.00544.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wallingford N, Perroud B, Gao Q, Coppola A, Gyengesi E, et al. Prolylcarboxypeptidase regulates food intake by inactivating α-MSH in rodents. J. Clin. Investig. 2009;119:2291–303. doi: 10.1172/JCI37209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jeong JK, Szabo G, Kelly K, Diano S. Prolyl carboxypeptidase regulates energy expenditure and the thyroid axis. Endocrinology. 2012;153:683–89. doi: 10.1210/en.2011-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wardlaw SL. Hypothalamic proopiomelanocortin processing and the regulation of energy balance. Eur. J. Pharmacol. 2011;660:213–19. doi: 10.1016/j.ejphar.2010.10.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jackson RS, Creemers JW, Ohagi S, Raffin-Sanson ML, Sanders L, et al. Obesity and impaired prohormone processing associated with mutations in the human prohormone convertase 1 gene. Nat. Genet. 1997;16:303–6. doi: 10.1038/ng0797-303. [DOI] [PubMed] [Google Scholar]
- 61.Diano S, Horvath TL. Mitochondrial uncoupling protein 2 (UCP2) in glucose and lipid metabolism. Trends Mol. Med. 2012;18:52–58. doi: 10.1016/j.molmed.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 62.Kim JD, Leyva S, Diano S. Hormonal regulation of the hypothalamic melanocortin system. Front. Physiol. 2014;5:480. doi: 10.3389/fphys.2014.00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Toda C, Diano S. Mitochondrial UCP2 in the central regulation of metabolism. Best Pract. Res. Clin. Endocrinol. Metab. 2014;28:757–64. doi: 10.1016/j.beem.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 64.Jeong JK, Diano S. Prolyl carboxypeptidase and its inhibitors in metabolism. Trends Endocrinol. Metab. 2013;24:61–67. doi: 10.1016/j.tem.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dagon Y, Hur E, Zheng B, Wellenstein K, Cantley LC, Kahn BB. p70S6 kinase phosphorylates AMPK on serine 491 to mediate leptin's effect on food intake. Cell Metab. 2012;16:104–12. doi: 10.1016/j.cmet.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Minokoshi Y, Alquier T, Furukawa N, Kim Y-B, Lee A, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–74. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 67.Aguilar V, Alliouachene S, Sotiropoulos A, Sobering A, Athea Y, et al. S6 kinase deletion suppresses muscle growth adaptations to nutrient availability by activating AMP kinase. Cell Metab. 2007;5:476–87. doi: 10.1016/j.cmet.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 68.Shi H, Sorrell JE, Clegg DJ, Woods SC, Seeley RJ. The roles of leptin receptors on POMC neurons in the regulation of sex-specific energy homeostasis. Physiol. Behav. 2010;100:165–72. doi: 10.1016/j.physbeh.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chun SK, Jo YH. Loss of leptin receptors on hypothalamic POMC neurons alters synaptic inhibition. J. Neurophysiol. 2010;104:2321–28. doi: 10.1152/jn.00371.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.do Carmo JM, da Silva AA, Cai Z, Lin S, Dubinion JH, Hall JE. Control of blood pressure, appetite, and glucose by leptin in mice lacking leptin receptors in proopiomelanocortin neurons. Hypertension. 2011;57:918–26. doi: 10.1161/HYPERTENSIONAHA.110.161349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–84. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 72.Könner AC, Janoschek R, Plum L, Jordan SD, Rother E, et al. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 2007;5:438–49. doi: 10.1016/j.cmet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 73.Huo L, Gamber K, Greeley S, Silva J, Huntoon N, et al. Leptin-dependent control of glucose balance and locomotor activity by POMC neurons. Cell Metab. 2009;9:537–47. doi: 10.1016/j.cmet.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Berglund ED, Vianna CR, Donato J, Jr, Kim MH, Chuang JC, et al. Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. J. Clin. Investig. 2012;122:1000–9. doi: 10.1172/JCI59816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, et al. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304:110–15. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- 76.Kim JG, Suyama S, Koch M, Jin S, Argente-Arizon P, et al. Leptin signaling in astrocytes regulates hypothalamic neuronal circuits and feeding. Nat. Neurosci. 2014;17:908–10. doi: 10.1038/nn.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Konner AC, Janoschek R, Plum L, Jordan SD, Rother E, et al. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 2007;5:438–49. doi: 10.1016/j.cmet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 78.Lin HV, Plum L, Ono H, Gutiérrez-Juárez R, Shanabrough M, et al. Divergent regulation of energy expenditure and hepatic glucose production by insulin receptor in agouti-related protein and POMC neurons. Diabetes. 2010;59:337–46. doi: 10.2337/db09-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Plum L, Ma X, Hampel B, Balthasar N, Coppari R, et al. Enhanced PIP3 signaling in POMC neurons causes KATP channel activation and leads to diet-sensitive obesity. J. Clin. Investig. 2006;116:1886–901. doi: 10.1172/JCI27123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Qiu J, Zhang C, Borgquist A, Nestor CC, Smith AW, et al. Insulin excites anorexigenic pro-opiomelanocortin neurons via activation of canonical transient receptor potential channels. Cell Metab. 2014;19:682–93. doi: 10.1016/j.cmet.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Belgardt BF, Husch A, Rother E, Ernst MB, Wunderlich FT, et al. PDK1 deficiency in POMC-expressing cells reveals FOXO1-dependent and -independent pathways in control of energy homeostasis and stress response. Cell Metab. 2008;7:291–301. doi: 10.1016/j.cmet.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 82.Hill JW, Elias CF, Fukuda M, Williams KW, Berglund ED, et al. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab. 2010;11:286–97. doi: 10.1016/j.cmet.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cone RD, Cowley MA, Butler AA, Fan W, Marks DL, Low MJ. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int. J. Obes. Relat. Metab. Disord. 2001;25(Suppl. 5):S63–67. doi: 10.1038/sj.ijo.0801913. [DOI] [PubMed] [Google Scholar]
- 84.Riediger T, Traebert M, Schmid HA, Scheel C, Lutz TA, Scharrer E. Site-specific effects of ghrelin on the neuronal activity in the hypothalamic arcuate nucleus. Neurosci. Lett. 2003;341:151–55. doi: 10.1016/s0304-3940(02)01381-2. [DOI] [PubMed] [Google Scholar]
- 85.Andrews ZB, Liu Z-W, Walllingford N, Erion DM, Borok E, et al. UCP2 mediates ghrelin's action on NPY/AgRP neurons by lowering free radicals. Nature. 2008;454:846–51. doi: 10.1038/nature07181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Willesen MG, Kristensen P, Romer J. Co-localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology. 1999;70:306–16. doi: 10.1159/000054491. [DOI] [PubMed] [Google Scholar]
- 87.Cowley MA, Smith RG, Diano S, Tschöp M, Pronchuk N, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–61. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 88.Yang Y, Atasoy D, Su HH, Sternson SM. Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop. Cell. 2011;146:992–1003. doi: 10.1016/j.cell.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tong Q, Ye C-P, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat. Neurosci. 2008;11:998–1000. doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kwon Jeong J, Dae Kim J, Diano S. Ghrelin regulates hypothalamic prolyl carboxypeptidase expression in mice. Mol. Metab. 2013;2:23–30. doi: 10.1016/j.molmet.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dallman MF, Akana SF, Strack AM, Scribner KS, Pecoraro N, et al. Chronic stress-induced effects of corticosterone on brain: direct and indirect. Ann. N.Y. Acad. Sci. 2004;1018:141–50. doi: 10.1196/annals.1296.017. [DOI] [PubMed] [Google Scholar]
- 92.Gyengesi E, Liu Z-W, D'Agostino G, Gan G, Horvath TL, et al. Corticosterone regulates synaptic input organization of POMC and NPY/AgRP neurons in adult mice. Endocrinology. 2010;151:5395–402. doi: 10.1210/en.2010-0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.DiPatrizio NV, Piomelli D. The thrifty lipids: endocannabinoids and the neural control of energy conservation. Trends Neurosci. 2012;35:403–11. doi: 10.1016/j.tins.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.DiPatrizio NV, Piomelli D. Intestinal lipid-derived signals that sense dietary fat. J. Clin. Investig. 2015;125:891–98. doi: 10.1172/JCI76302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Parsons LH, Hurd YL. Endocannabinoid signalling in reward and addiction. Nat. Rev. Neurosci. 2015;16:579–94. doi: 10.1038/nrn4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tasker JG. Rapid glucocorticoid actions in the hypothalamus as a mechanism of homeostatic integration. Obesity. 2006;14(Suppl. 5):S259–65. doi: 10.1038/oby.2006.320. [DOI] [PubMed] [Google Scholar]
- 97.Bakkali-Kassemi L, El Ouezzani S, Magoul R, Merroun I, Lopez-Jurado M, Errami M. Effects of cannabinoids on neuropeptide Y and β-endorphin expression in the rat hypothalamic arcuate nucleus. Br. J. Nutr. 2011;105:654–60. doi: 10.1017/S0007114510004095. [DOI] [PubMed] [Google Scholar]
- 98.Hentges ST, Low MJ, Williams JT. Differential regulation of synaptic inputs by constitutively released endocannabinoids and exogenous cannabinoids. J. Neurosci. 2005;25:9746–51. doi: 10.1523/JNEUROSCI.2769-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ho J, Cox JM, Wagner EJ. Cannabinoid-induced hyperphagia: correlation with inhibition of proopiomelanocortin neurons? Physiol. Behav. 2007;92:507–19. doi: 10.1016/j.physbeh.2007.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dutia R, Meece K, Dighe S, Kim AJ, Wardlaw SL. β-Endorphin antagonizes the effects of α-MSH on food intake and body weight. Endocrinology. 2012;153:4246–55. doi: 10.1210/en.2012-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mineur YS, Abizaid A, Rao Y, Salas R, DiLeone RJ, et al. Nicotine decreases food intake through activation of POMC neurons. Science. 2011;332:1330–32. doi: 10.1126/science.1201889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huang H, Xu Y, van den Pol AN. Nicotine excites hypothalamic arcuate anorexigenic proopiomelanocortin neurons and orexigenic neuropeptide Y neurons: similarities and differences. J. Neurophysiol. 2011;106:1191–202. doi: 10.1152/jn.00740.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Heisler LK, Cowley MA, Tecott LH, Fan W, Low MJ, et al. Activation of central melanocortin pathways by fenfluramine. Science. 2002;297:609–11. doi: 10.1126/science.1072327. [DOI] [PubMed] [Google Scholar]
- 104.Zhou L, Sutton GM, Rochford JJ, Semple RK, Lam DD, et al. Serotonin 2C receptor agonists improve type 2 diabetes via melanocortin-4 receptor signaling pathways. Cell Metab. 2007;6:398–405. doi: 10.1016/j.cmet.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xu Y, Jones JE, Kohno D, Williams KW, Lee CE, et al. 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate energy homeostasis. Neuron. 2008;60:582–89. doi: 10.1016/j.neuron.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xu Y, Berglund ED, Sohn J-W, Holland WL, Chuang J-C, et al. 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate insulin sensitivity in liver. Nat. Neurosci. 2010;13:1457–59. doi: 10.1038/nn.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Berglund ED, Liu C, Sohn J-W, Liu T, Kim MH, et al. Serotonin 2C receptors in pro-opiomelanocortin neurons regulate energy and glucose homeostasis. J. Clin. Investig. 2013;123:5061–70. doi: 10.1172/JCI70338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kiss J, Leranth C, Halasz B. Serotoninergic endings on VIP-neurons in the suprachiasmatic nucleus and on ACTH-neurons in the arcuate nucleus of the rat hypothalamus. A combination of high resolution autoradiography and electron microscopic immunocytochemistry. Neurosci Lett. 1984;44:119–24. doi: 10.1016/0304-3940(84)90068-5. [DOI] [PubMed] [Google Scholar]
- 109.Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, et al. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449:228–32. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- 110.Diano S, Liu Z-W, Jeong JK, Dietrich MO, Ruan H-B, et al. Peroxisome proliferation-associated control of reactive oxygen species sets melanocortin tone and feeding in diet-induced obesity. Nat. Med. 2011;17:1121–27. doi: 10.1038/nm.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Long L, Toda C, Jeong JK, Horvath TL, Diano S. PPARγ ablation sensitizes proopiomelanocortin neurons to leptin during high-fat feeding. J. Clin. Investig. 2014;124:4017–27. doi: 10.1172/JCI76220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Campanucci V, Krishnaswamy A, Cooper E. Diabetes depresses synaptic transmission in sympathetic ganglia by inactivating nAChRs through a conserved intracellular cysteine residue. Neuron. 2010;66:827–34. doi: 10.1016/j.neuron.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 113.Lu M, Sarruf DA, Talukdar S, Sharma S, Li P, et al. Brain PPAR-γ promotes obesity and is required for the insulin-sensitizing effect of thiazolidinediones. Nat. Med. 2011;17:618–22. doi: 10.1038/nm.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ryan KK, Li B, Grayson BE, Matter EK, Woods SC, Seeley RJ. A role for central nervous system PPAR-γ in the regulation of energy balance. Nat. Med. 2011;17:623–26. doi: 10.1038/nm.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell. 2012;48:158–67. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Finkel T. From sulfenylation to sulfhydration: what a thiolate needs to tolerate. Sci. Signal. 2012;5:10. doi: 10.1126/scisignal.2002943. [DOI] [PubMed] [Google Scholar]
- 117.Jo Y-H, Su Y, Gutierrez-Juarez R, Chua S. Oleic acid directly regulates POMC neuron excitability in the hypothalamus. J. Neurophysiol. 2009;101:2305–16. doi: 10.1152/jn.91294.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Horvath TL, Sarman B, García-Cáceres C, Enriori PJ, Sotonyi P, et al. Synaptic input organization of the melanocortin system predicts diet-induced hypothalamic reactive gliosis and obesity. PNAS. 2010;107:14875–80. doi: 10.1073/pnas.1004282107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ito Y, Banno R, Shibata M, Adachi K, Hagimoto S, et al. GABA type B receptor signaling in pro-opiomelanocortin neurons protects against obesity, insulin resistance, and hypothalamic inflammation in male mice on a high-fat diet. J. Neurosci. 2013;33:17166–73. doi: 10.1523/JNEUROSCI.0897-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cota D, Proulx K, Smith KAB, Kozma SC, Thomas G, et al. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–30. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 121.Blouet C, Jo Y-H, Li X, Schwartz GJ. Mediobasal hypothalamic leucine sensing regulates food intake through activation of a hypothalamus-brainstem circuit. J. Neurosci. 2009;29:8302–11. doi: 10.1523/JNEUROSCI.1668-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mori H, Inoki K, Münzberg H, Opland D, Faouzi M, et al. Critical role for hypothalamic mTOR activity in energy balance. Cell Metab. 2009;9:362–74. doi: 10.1016/j.cmet.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang S-B, Tien A-C, Boddupalli G, Xu AW, Jan YN, Jan LY. Rapamycin ameliorates age-dependent obesity associated with increased mTOR signaling in hypothalamic POMC neurons. Neuron. 2012;75:425–36. doi: 10.1016/j.neuron.2012.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ravelli AC, van der Meulen JH, Michels RP, Osmond C, Barker DJ, et al. Glucose tolerance in adults after prenatal exposure to famine. Lancet. 1998;351:173–77. doi: 10.1016/s0140-6736(97)07244-9. [DOI] [PubMed] [Google Scholar]
- 125.de Rooij SR, Roseboom TJ, Painter RC. Famines in the last 100 years: implications for diabetes. Curr. Diab. Rep. 2014;14:536. doi: 10.1007/s11892-014-0536-7. [DOI] [PubMed] [Google Scholar]
- 126.Breton C, Lukaszewski M-A, Risold P-Y, Enache M, Guillemot J, et al. Maternal prenatal undernutrition alters the response of POMC neurons to energy status variation in adult male rat offspring. Am. J. Physiol. Endocrinol. Metab. 2009;296:E462–72. doi: 10.1152/ajpendo.90740.2008. [DOI] [PubMed] [Google Scholar]
- 127.Delahaye F, Breton C, Risold P-Y, Enache M, Dutriez-Casteloot I, et al. Maternal perinatal undernutrition drastically reduces postnatal leptin surge and affects the development of arcuate nucleus proopiomelanocortin neurons in neonatal male rat pups. Endocrinology. 2008;149:470–75. doi: 10.1210/en.2007-1263. [DOI] [PubMed] [Google Scholar]
- 128.Coupé B, Amarger V, Grit I, Benani A, Parnet P. Nutritional programming affects hypothalamic organization and early response to leptin. Endocrinology. 2010;151:702–13. doi: 10.1210/en.2009-0893. [DOI] [PubMed] [Google Scholar]
- 129.Chen H, Morris MJ. Differential responses of orexigenic neuropeptides to fasting in offspring of obese mothers. Obesity. 2009;17:1356–62. doi: 10.1038/oby.2009.56. [DOI] [PubMed] [Google Scholar]
- 130.Fuente-Martín E, García-Cáceres C, Granado M, de Ceballos ML, Sánchez-Garrido MÁ, et al. Leptin regulates glutamate and glucose transporters in hypothalamic astrocytes. J. Clin. Investig. 2012;122:3900–13. doi: 10.1172/JCI64102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Steculorum SM, Bouret SG. Maternal diabetes compromises the organization of hypothalamic feeding circuits and impairs leptin sensitivity in offspring. Endocrinology. 2011;152:4171–79. doi: 10.1210/en.2011-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sun B, Purcell RH, Terrillion CE, Yan J, Moran TH, Tamashiro KLK. Maternal high-fat diet during gestation or suckling differentially affects offspring leptin sensitivity and obesity. Diabetes. 2012;61:2833–41. doi: 10.2337/db11-0957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vogt MC, Paeger L, Hess S, Steculorum SM, Awazawa M, et al. Neonatal insulin action impairs hypothalamic neurocircuit formation in response to maternal high-fat feeding. Cell. 2014;156:495–509. doi: 10.1016/j.cell.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J. Clin. Investig. 2005;115:1111–19. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Thaler JP, Yi C-X, Schur EA, Guyenet SJ, Hwang BH, et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Investig. 2012;122:153–62. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Waise TMZ, Toshinai K, Naznin F, NamKoong C, Md Moin AS, et al. One-day high-fat diet induces inflammation in the nodose ganglion and hypothalamus of mice. Biochem. Biophys. Res. Commun. 2015;464:1157–62. doi: 10.1016/j.bbrc.2015.07.097. [DOI] [PubMed] [Google Scholar]
- 137.Banks WA. Anorectic effects of circulating cytokines: role of the vascular blood-brain barrier. Nutrition. 2001;17:434–37. doi: 10.1016/s0899-9007(01)00507-x. [DOI] [PubMed] [Google Scholar]
- 138.Szelényi J. Cytokines and the central nervous system. Brain Res. Bull. 2001;54:329–38. doi: 10.1016/s0361-9230(01)00428-2. [DOI] [PubMed] [Google Scholar]
- 139.Langlet F, Levin BE, Luquet S, Mazzone M, Messina A, et al. Tanycytic VEGF-A boosts blood-hypothalamus barrier plasticity and access of metabolic signals to the arcuate nucleus in response to fasting. Cell Metab. 2013;17:607–17. doi: 10.1016/j.cmet.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Amaral ME, Barbuio R, Milanski M, Romanatto T, Barbosa HC, et al. Tumor necrosis factor-α activates signal transduction in hypothalamus and modulates the expression of pro-inflammatory proteins and orexigenic/anorexigenic neurotransmitters. J. Neurochem. 2006;98:203–12. doi: 10.1111/j.1471-4159.2006.03857.x. [DOI] [PubMed] [Google Scholar]
- 141.Romanatto T, Cesquini M, Amaral ME, Roman EA, Moraes JC, et al. TNF-α acts in the hypothalamus inhibiting food intake and increasing the respiratory quotient—effects on leptin and insulin signaling pathways. Peptides. 2007;28:1050–58. doi: 10.1016/j.peptides.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 142.Arruda AP, Milanski M, Coope A, Torsoni AS, Ropelle E, et al. Low-grade hypothalamic inflammation leads to defective thermogenesis, insulin resistance, and impaired insulin secretion. Endocrinology. 2011;152:1314–26. doi: 10.1210/en.2010-0659. [DOI] [PubMed] [Google Scholar]
- 143.Romanatto T, Roman EA, Arruda AP, Denis RG, Solon C, et al. Deletion of tumor necrosis factor-alpha receptor 1 (TNFR1) protects against diet-induced obesity by means of increased thermogenesis. J. Biol. Chem. 2009;284:36213–22. doi: 10.1074/jbc.M109.030874. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 144.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-α function. Nature. 1997;389:610–14. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 145.Shi X, Wang X, Li Q, Su M, Chew E, et al. Nuclear factor κB (NF-κB) suppresses food intake and energy expenditure in mice by directly activating the Pomc promoter. Diabetologia. 2013;56:925–36. doi: 10.1007/s00125-013-2831-2. [DOI] [PubMed] [Google Scholar]
- 146.Purkayastha S, Zhang G, Cai D. Uncoupling the mechanisms of obesity and hypertension by targeting hypothalamic IKK-β and NF-κB. Nat. Med. 2011;17:883–87. doi: 10.1038/nm.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Milanski M, Degasperi G, Coope A, Morari J, Denis R, et al. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J. Neurosci. 2009;29:359–70. doi: 10.1523/JNEUROSCI.2760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wallenius V, Wallenius K, Ahrén B, Rudling M, Carlsten H, et al. Interleukin-6-deficient mice develop mature-onset obesity. Nat. Med. 2002;8:75–79. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- 149.Wallenius K, Wallenius V, Sunter D, Dickson SL, Jansson J-O. Intracerebroventricular interleukin-6 treatment decreases body fat in rats. Biochem. Biophys. Res. Commun. 2002;293:560–65. doi: 10.1016/S0006-291X(02)00230-9. [DOI] [PubMed] [Google Scholar]
- 150.Ropelle ER, Flores MB, Cintra DE, Rocha GZ, Pauli JR, et al. IL-6 and IL-10 anti-inflammatory activity links exercise to hypothalamic insulin and leptin sensitivity through IKKβ and ER stress inhibition. PLOS Biol. 2010;8:e1000465. doi: 10.1371/journal.pbio.1000465. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 151.Nakata M, Yamamoto S, Okada T, Gantulga D, Okano H, et al. IL-10 gene transfer upregulates arcuate POMC and ameliorates hyperphagia, obesity and diabetes by substituting for leptin. Int. J. Obes. 2016;40:425–33. doi: 10.1038/ijo.2015.201. [DOI] [PubMed] [Google Scholar]
- 152.Luheshi GN, Gardner JD, Rushforth DA, Loudon AS, Rothwell NJ. Leptin actions on food intake and body temperature are mediated by IL-1. PNAS. 1999;96:7047–52. doi: 10.1073/pnas.96.12.7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wisse BE, Ogimoto K, Schwartz MW. Role of hypothalamic interleukin-1β (IL-1β) in regulation of energy homeostasis by melanocortins. Peptides. 2006;27:265–73. doi: 10.1016/j.peptides.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 154.Reyes TM, Sawchenko PE. Involvement of the arcuate nucleus of the hypothalamus in interleukin-1-induced anorexia. J. Neurosci. 2002;22:5091–99. doi: 10.1523/JNEUROSCI.22-12-05091.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Scarlett JM, Jobst EE, Enriori PJ, Bowe DD, Batra AK, et al. Regulation of central melanocortin signaling by interleukin-1β. Endocrinology. 2007;148:4217–25. doi: 10.1210/en.2007-0017. [DOI] [PubMed] [Google Scholar]
- 156.Kleinridders A, Schenten D, Könner AC, Belgardt BF, Mauer J, et al. MyD88 signaling in the CNS is required for development of fatty acid-induced leptin resistance and diet-induced obesity. Cell Metab. 2009;10:249–59. doi: 10.1016/j.cmet.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ehses JA, Meier DT, Wueest S, Rytka J, Boller S, et al. Toll-like receptor 2-deficient mice are protected from insulin resistance and beta cell dysfunction induced by a high-fat diet. Diabetologia. 2010;53:1795–806. doi: 10.1007/s00125-010-1747-3. [DOI] [PubMed] [Google Scholar]
- 158.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Investig. 2006;116:3015–25. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–22. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 160.Shechter R, London A, Kuperman Y, Ronen A, Rolls A, et al. Hypothalamic neuronal Toll-like receptor 2 protects against age-induced obesity. Sci. Rep. 2013;3:1254. doi: 10.1038/srep01254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cnop M, Foufelle F, Velloso LA. Endoplasmic reticulum stress, obesity and diabetes. Trends Mol. Med. 2012;18:59–68. doi: 10.1016/j.molmed.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 162.Ozcan L, Ergin AS, Lu A, Chung J, Sarkar S, et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9:35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 163.Williams KW, Liu T, Kong X, Fukuda M, Deng Y, et al. Xbp1s in Pomc neurons connects ER stress with energy balance and glucose homeostasis. Cell Metab. 2014;20:471–82. doi: 10.1016/j.cmet.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–10. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 165.Schneeberger M, Dietrich MO, Sebastián D, Imbernón M, Castaño C, et al. Mitofusin 2 in POMC neurons connects ER stress with leptin resistance and energy imbalance. Cell. 2013;155:172–87. doi: 10.1016/j.cell.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Klionsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat. Rev. Mol. Cell. Biol. 2007;8:931–37. doi: 10.1038/nrm2245. [DOI] [PubMed] [Google Scholar]
- 167.Meng Q, Cai D. Defective hypothalamic autophagy directs the central pathogenesis of obesity via the IκB kinase β (IKKβ)/NF-κB pathway. J. Biol. Chem. 2011;286:32324–32. doi: 10.1074/jbc.M111.254417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Quan W, Kim H-K, Moon E-Y, Kim SS, Choi CS, et al. Role of hypothalamic proopiomelanocortin neuron autophagy in the control of appetite and leptin response. Endocrinology. 2012;153:1817–26. doi: 10.1210/en.2011-1882. [DOI] [PubMed] [Google Scholar]
- 169.Kaushik S, Arias E, Kwon H, Lopez NM, Athonvarangkul D, et al. Loss of autophagy in hypothalamic POMC neurons impairs lipolysis. EMBO Rep. 2012;13:258–65. doi: 10.1038/embor.2011.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Malhotra R, Warne JP, Salas E, Xu AW, Debnath J. Loss of Atg12, but not Atg5, in pro-opiomelanocortin neurons exacerbates diet-induced obesity. Autophagy. 2015;11:145–54. doi: 10.1080/15548627.2014.998917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Martinez-Lopez N, Garcia-Macia M, Sahu S, Athonvarangkul D, Liebling E, et al. Autophagy in the CNS and periphery coordinate lipophagy and lipolysis in the brown adipose tissue and liver. Cell Metab. 2016;23:113–27. doi: 10.1016/j.cmet.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kaushik S, Rodriguez-Navarro JA, Arias E, Kiffin R, Sahu S, et al. Autophagy in hypothalamic AgRP neurons regulates food intake and energy balance. Cell Metab. 2011;14:173–83. doi: 10.1016/j.cmet.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]