Abstract
Treatment of a mouse model of oxygen-induced retinopathy (OIR) with recombinant human Norrin (Norrie Disease Protein, gene: NDP) accelerates regrowth of the microvasculature into central ischemic regions of the neural retina, which are generated after treatment with 75% oxygen. While this reduces the average duration and severity of ischemia overall, we do not know if this accelerated recovery of the microvasculature results in any significant survival of retinal ganglion cells (RGCs). The purpose of this study was to investigate ganglion cell survival with and without the intravitreal injection of Norrin in the murine model of oxygen induced retinopathy (OIR), using two strains of mice: C57BL/6J and Thy1-YFP mice. Intravitreal injections of Norrin or vehicle were done after five days of exposure to 75% oxygen from ages P7 to P12. The C57BL/J mice were followed by Spectral-Domain Optical Coherence Tomography (SD-OCT), and the average nerve fiber layer (NFL) and inner-plexiform layer (IPL) thicknesses were measured at twenty-four locations per retina at P42. Additionally, some C57BL/J retinas were flat mounted and immunostained for the RGC marker, Brn3a, to compare the population density of surviving retinal ganglion cells. Using homozygous Thy1-YFP mice, single intrinsically fluorescent RGCs were imaged in live animals with a Micron-III imaging system at ages P21, 28 and P42. The relative percentage of YFP-fluorescent RGCs with dendritic arbors were compared. At age P42, the NFL was thicker in Norrin-injected OIR eyes, 14.4 μm, compared to Vehicle-injected OIR eyes, 13.3 μm (p=0.01). In the superior retina, the average thickness of the IPL was greater in Norrin-injected OIR eyes, 37.7 μm, compared to Vehicle-injected OIR eyes, 34.6 μm (p = 0.04). Retinas from Norrin injected OIR mice had significantly more surviving RGCs (p = 0.03) than vehicle-injected mice. Based upon NFL thickness and counts of RGCs, we conclude that Norrin treatment, early in the ischemic phase, increased the relative population density of surviving RGCs in the central retinas of OIR mice.
Keywords: Oxygen induced retinopathy, Ganglion cell, Retinal ischemia, Neovascular, Norrin, Optical Coherence Tomography, Nerve fiber layer
1. Introduction
Retinal neovascular diseases such as retinopathy of prematurity (ROP) and diabetic retinopathy are characterized by retinal vascular and neural changes that occur in response to ischemia. Ischemia alters the normal biochemical regulation of both vascular growth and neural growth, which results in pathological changes (Fulton et al., 2009). Vascular Endothelial Growth Factor-A (VEGFA) is a key molecular player in the retina’s physiological response to ischemia, when low oxygen increases the expression of VEGFA (Ozaki et al., 1999; Wang et al., 2014). Higher concentrations of VEGFA disorganize the blood-retinal barrier (BRB) leading to vessel tortuosity, neovascularization, hemorrhage, and retinal detachment.
In addition to vascular pathologies, neural dysfunction has been reported in ROP and diabetic retinopathy patients (Barnaby et al., 2007; Fulton et al., 2001; Kim et al., 2012; Park et al., 2011). Some clinical evidence suggests that retinal ganglion cell (RGC) density might be reduced in prematurely born children with ROP and in diabetic patients. Children of a gestational age of 32 weeks or less, with severe ROP (stages 3–4), exhibit significantly reduced retinal nerve fiber layer (RNFL) thickness compared to children born at full term (Åkerblom et al., 2012). Diabetic patients also exhibit RNFL thinning that is proportional to their disease severity (Park et al., 2011). Some studies using rodent models of oxygen induced retinopathy (OIR) have presented evidence that neural abnormalities are manifested in concert with vascular abnormalities (Akula et al., 2007; Chen et al., 2013; Robinson et al., 2001).
The mouse OIR model involves exposing post-natal mice to high oxygen (75%) during retinal maturation. This causes ablation of vascular beds in the central retina that leads to a state of ischemia when the mice are returned to normal room air, which is 21% oxygen (Lange et al., 2009; Scott et al., 2014; Smith et al., 1994). We and others have shown that intravitreal injection of Norrin, a Wnt-signaling ligand, promotes earlier re-vascularization of the OIR retina as well as reduced neovascularization (Ohlmann et al., 2010; Tokunaga et al., 2013). We know that ischemia has a destructive effect upon ganglion cells (Kaur et al., 2008) and Norrin has been shown to be neuroprotective to RGCs in a mouse model of excitotoxic RGC damage (Seitz et al., 2010). Norrin is known to exert its activity by activating the Wnt-β-catenin signaling pathway in retinal endothelial cells. When it binds to its receptor, Fzd4 and co-receptor, LRP5, it inactivates a downstream degradation complex thereby stabilizing β-catenin, a transcription factor (MacDonald et al., 2009; Rao and Kühl, 2010). β-catenin translocates into the nucleus, where it interacts with TCF/LEF transcription factors to activate transcription of genes involved with proliferation, migration and development of the retinal vasculature (Xia et al., 2008; Ye et al., 2010, 2009).
We do not know for certain if treatment with exogenous Norrin increases the final density of surviving ganglion cells in the mouse OIR model. In order to explore Norrin’s potential benefit for RGC survival in the OIR model, we examined morphological changes induced by oxygen induced retinopathy and compared the changes in Norrin-treated OIR eyes to untreated OIR eyes. Our analysis included two in vivo imaging techniques. Spectral-Domain Optical Coherence Tomography (SD-OCT) was used to assess overall structure and to measure retinal layer thicknesses in OIR and normal C57BL/6J mice. Finally, in order to measure surviving retinal ganglion cell density, we used virtual microscopy to image entire flat mounted neural retinas (age P42) after immuno-staining for the Brn3a transcription factor, a nuclear marker of RGCs. This relative population density of surviving RGCs was compared between the central and more peripheral retinal regions within the same retinas. Additionally, intrinsically fluorescent RGCs were imaged, in vivo, in the retinas of OIR and non-OIR transgenic Thy1-YFP mice.
We conclude from our study that Norrin treatment increased the population density of surviving retinal ganglion cells and reduced thinning of the NFL in the oxygen induced retinopathy model.
2. Methods
2.1. Animals
All experiments performed in this study were carried out with the approval of Oakland University’s Animal Care and Use Committee and conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Mice were housed at Oakland University in a facility approved by the Association for Assessment and Accreditation of Laboratory Animal Care International.
C57BL/6J mice were obtained from Charles River Laboratories (Wilmington, MA). Hemizygous Thy-1-YFP transgenic mice (B6.Cg-Tg(Thy1-YFP)HJrs/J) were obtained from the Jackson Laboratory (Bar Harbor, ME). This specific strain of Thy1-YFP mice expresses Yellow Fluorescent Protein (YFP) under the control of the Thy1-gene promoter in a small percentage of retinal ganglion cells, and were previously used in time-lapse studies in experimental glaucomatous optic neuropathy (Feng et al., 2013; Leung et al., 2011; Williams et al., 2013). Using YFP-fluorescence live retinal imaging we found that the offspring of hemizygous (+/Thy1-YFP) breeders exhibited Mendelian-like inheritance with three phenotypes: 25% without any fluorescent RGCs (+/+), 50 % with only a few fluorescent RGCs per retina (+/Thy1-YFP), and 25 % with numerous fluorescent RGCs per retina (Thy1-YFP /Thy1-YFP). The later mice were selectively set aside as breeders for this study and produced litters with 100 % numerous YFP fluorescent ganglion cells.
2.2. Oxygen induced retinopathy model
The oxygen-induced retinopathy (OIR) model using C57BL/6J or Thy1-YFP transgenic mice neonates was used as previously described (Smith et al., 1994; Tokunaga et al., 2014, 2013). This model is well characterized and widely used for experimental studies of retinal ischemia (Smith et al., 1994). Mice were exposed to 75 % oxygen for five days from ages P7–P12. This high oxygen environment suppresses oxygen-regulated growth factors causing inhibition of vessel growth and vessel loss (vaso-obliteration). At age P12, mice were removed from 75 % oxygen chamber and returned to room air (RA). After returning to RA (20 % oxygen), the retina continues to mature and the central, avascular area experiences a rapid increase in the expression of VEGFA (Wang et al., 2014) that causes neovascularization as well as normal vessel re-growth.
2.3. Anesthesia for injections or retinal imaging
Pupils were dilated with tropicamide and phenylepherine drops prior to anesthesia. Mice were anesthesized with an intra-peritoneal injection of a Ketamine HCL (50 mg/kg) and Xylazine (7 mg/kg).
2.4. Norrin treatment analysis with the C57BL/6J OIR model
Mice were anesthetized and their pupils dilated prior to injections. Recombinant carrier free human Norrin (R&D Systems, Minneapolis, MN, USA) was reconstituted to a concentration of 250 μg/ml with 4 mM HCl according to the manufacturer’s instructions and aliquots were diluted to a concentration of 100 ng/μL using Balanced Salt solution (BSS). Depending on the treatment group, 0.5 μL of Norrin (50 ng) or vehicle (1.6 mM HCl in BSS) control was injected into the vitreous cavity of right eyes at age P14 using a nanofil syringe (10 μL) with a 34-gauge triple-beveled needles (World Precision Instruments, Sarasota FL). Following application of 5 % povidone iodine solution to the ocular surface and eyelids, the needle was inserted 0.5 mm posterior to the corneal limbus through the sclera under direct visualization through a surgical microscope. The needle was angled posteriorly to avoid lens trauma. Left eyes were not injected. An equal number of litter mates were injected with Norrin or vehicle when possible (OIR; 8 litters, RA;1 litter). C57BL/6J mice were used for analysis of retinal layer thickness (SD-OCT), ganglion cell density (virtual microscopy) of whole retinal flat mounts, and retinal histology (virtual microscopy) of H&E stained sections.
2.5 Norrin treatment and analysis with Thy1-YFP(homozygous)OIR model
Norrin preparation and use varied slightly for Thy1-YFP mice. Norrin was reconstituted with phosphate buffer solution (PBS) and intravitreal injections (20 ng in 0.5 μL or 100 ng in 1.0 μL) were done at age P12. Norrin, 0.5 μl or 1.0 μl, was injected into the vitreous cavity of right eyes and vehicle (PBS) was injected into the vitreous cavity of left eyes. Thy1-YFP mice were used to compare the relative percentage of YFP-expressing ganglion cells with dendritic arbors using live fluorescence retinal microscopy (Micron III system). Imaging was carried out at ages P21, P28 and P42.
2.6. SD-OCT analysis of retinal layer thickness
To minimize corneal clouding and cataract formation, hydroxypropylmethylcellulose 2.5% (Cornea Coat, Insight Instruments, Inc, Stuart, FL, USA) and artificial tears solutions were applied generously to the corneal surface during imaging. Mice were placed upon a multi-access support and the eye was aligned for scanning. SD-OCT scans were taken using the SD-OCT Envisu R2200 model (Bioptigen, Durham NC). A rectangular scan pattern of 1.4 mm x 1.4 mm was used (1000 A-scans by 100 B-scans). Retinal layers were marked and measured using processed OCT images with InVivoVue Diver 2.0 software (Bioptigen). A fixed 5x5 grid was centered on the optic disc (See Fig-1). Measurements were then taken at the boundaries of the Nerve Fiber Layer and Ganglion Cell layer (NFL/GCL), Inner Plexiform Layer (IPL) and Retinal Pigment Epithelium (RPE). Locations were between 0.4 mm and 0.7 mm from the optic nerve where we had consistently observed the most amount of oxygen induced disruption. NFL/GCL and IPL measurements (24 +/− 4 per eye) were then used to generate an average for each layer per eye. For comparisons of superior versus inferior regions, the five top row and five bottom row locations were averaged, respectively (Fig. 1). Results from 13 Norrin-injected OIR (Nor/OIR) eyes, 9 vehicle-injected OIR (Veh/OIR) mice and 4 room air raised (RA) mice were used for comparisons.
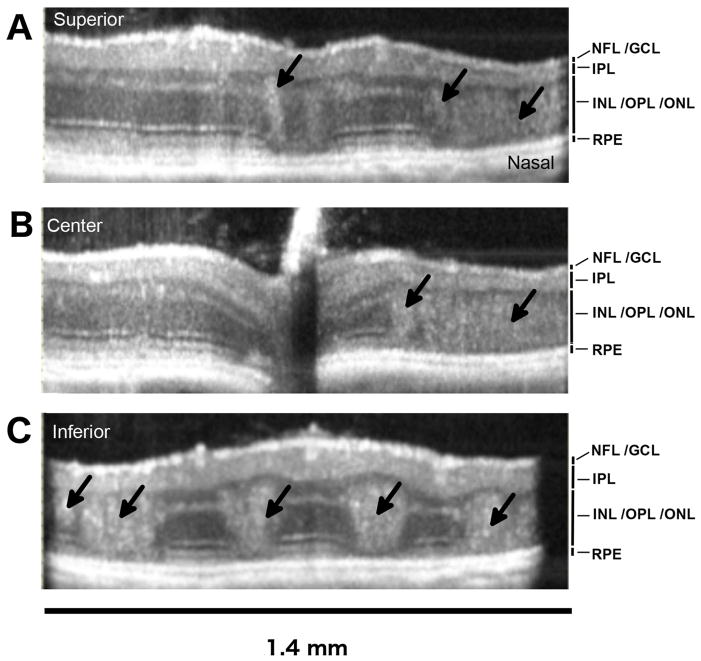
Figure 1. SD-OCT imaging and analysis of the OIR disruption of retinal laminar layers in the OIR mouse retina.
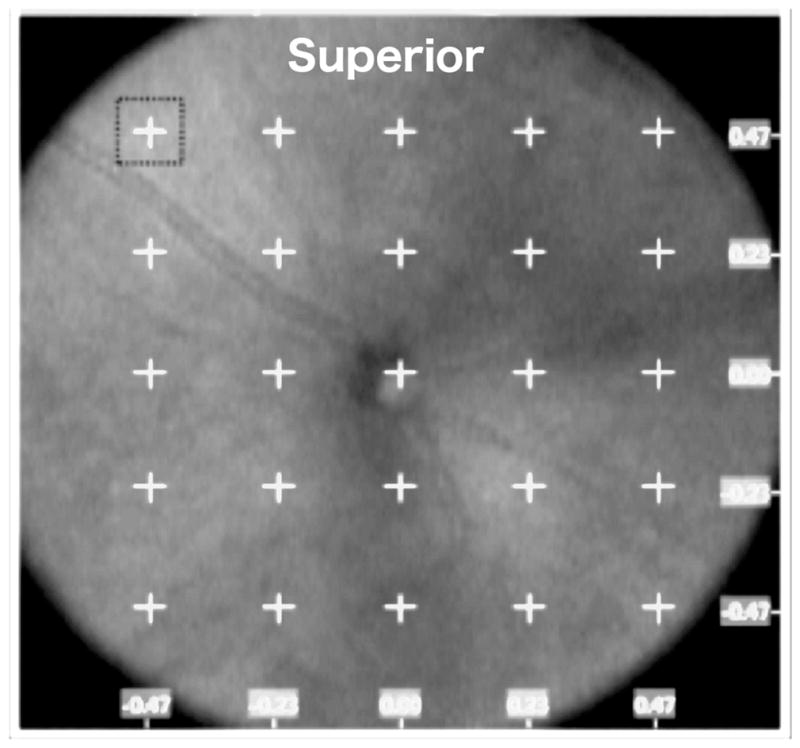
A. SD-OCT en face view (1.41 x 1.41 mm) from a P42 OIR eye, showing a 5 X 5 grid with 0.23 mm interval spacing, centered on the optic disc and used to mark 24 locations for measurements of retinal layer thickness. The horizontal axis (x) and vertical axis (y) coordinates are indicated (mm). Retinal layer measurements were not taken from the central grid location (0,0).
2.7. Live Retinal imaging of intrinsically fluorescent Retinal Ganglion Cells
Using homozygous Thy1-YFP mice, a Micron-III live retinal imaging system (Phoenix Labs Inc, Pleasanton, CA, USA) was used for color photography and yellow fluorescent protein (YFP) imaging. Pupils of mice were first dilated with both tropicamide and phenylephrine eye drops. For imaging, mice were then anesthetized and arranged under a felt blanket on a mouse-warming table (36°C). While under anesthesia, corneas were protected with Genteel eye gel. A color bright-field image of the central retina was captured. Excitation (500 nm) and emission (524 nm) filters for the YFP protein were then dialed into position to image fluorescent YFP-RGCs (Semrock FF01-500/24-25, Semrock FF01-524/27-12.5). This process was repeated for the other eye. Images were captured at ages P21, P28, and P42.
The first two experimental groups compared the eyes of animals raised in room air (RA) to those subjected to high oxygen treatment from ages P7–P12. In total a series of retinal images was obtained from 30 RA eyes and 23 OIR eyes. The third experimental group consisted of OIR mice that had received intravitreal injections of Norrin (20 ng or 100 ng) in the right eye, and PBS alone in the contralateral (left) eye. The contralateral eye was used as control to minimize inter-mouse variability. For the (20 ng) Norrin group, retinal images from 6 pairs of eyes were used for analysis and for the (100 ng) Norrin group, retinal images from 8 pairs of eyes were used for analysis.
Three trained assessors counted the number of YFP-RGC cell bodies per eye, with or without visible dendritic arborization, in a masked fashion. The mean counts per eye were calculated. The number of RGC cell bodies with dendrites was reported as a percentage of the total number of RGC cell bodies (+/− dendrites) per image.
2.8. Virtual Microscopy and retinal histology
Using the C57BL/6J strain, room air (RA) and oxygen induced retinopathy (OIR) mice were sacrificed at p42, their eyes were enucleated and fixed overnight in Davidson’s fixative. After paraffin embedding, the eyes were cut into 5 μm sections and stained with Hematoxylin and Eoisin (H&E). The slides were digitized using an Olympus SL120 Virtual Microscopy Slide Scanner (Olympus, Center Valley, PA) with the 20x objective.
2.9. Immunostaining and measurement of RGC density in total retinal flat-mounts
Using the C57BL/6J strain, room air (RA) control and oxygen induced retinopathy (OIR) mice, three per group, were sacrificed at P42 and their eyes fixed in PBS, 4 % paraformaldehyde overnight at 4° C. The retinas were then isolated, mounted flat and incubated for 30 minutes with 2 % Triton X-100. A brush was then used to remove residual vitreous humor while soaking in PBS. Retinas were treated for 20-minutes at room temperature with Bloxall Blocking solution (Vector Laboratories, Burlingame CA) to inhibit endogenous peroxidase activity. After washing with PBS, retinas were blocked with a solution of Avidin (Vector Laboratories, 1/20 dilution) in PBS, 5 % rabbit serum for 30 minutes at 4°C. Blocked retinas were then incubated overnight at 4°C in a solution of goat anti-Brn3a primary antibody (Santa Cruz Biotechnologies, Dallas TX) diluted (1:100) in PBS, 5 % rabbit serum. The following day, Vectastain-ABC reagents were used with DAB substrate to stain RGC nuclei.
Whole stained flat mounted retinas were digitized with an Olympus SL120 Virtual Microscopy Slide Scanner using the 20x objective. For each retina, 8 “center” (~300–900 μm from optic nerve) and 8 “periphery” (~1400–2000 μm from optic nerve) images were captured. Nikon Elements was used to count the number of Brn3a positive cells in the high-magnification images. The center and periphery counts were then averaged. To account for inter-animal differences, a ratio of the center to periphery RGC counts was used for group comparisons.
2.10. Statistic analysis
Statistical calculations were performed using functions in Microsoft Excel. A two-tailed student’s t-test was used for group comparisons. A paired t-test was used to compare Norrin/OIR and PBS/OIR groups when fellow eyes of Thy1-YFP mice were injected.
3. Results
3.1. OIR Disruption of INL and ONL Organization
In order to evaluate oxygen-induced changes to retinal morphology in vivo, SD-OCT was performed at ages P21, P42 and P56 in 75 % oxygen-treated (OIR) mice. A heterogeneous interspersion of the INL (inner nuclear layer) and ONL (outer nuclear layer) was noted in all OIR mice. The extent of INL/ONL disruption was particularly remarkable in the inferior neural retina compared to the superior neural retina (Fig. 2A–C).
Figure 2. Laminar disruption of the bipolar and photoreceptor cell layers in the OIR mouse retina in vivo.
Examples of horizontal B-scans from the P42, OIR eye shown en face in Figure-1. Laminar disruptions (black arrows) were detected in the living eye, demonstrating that disruptions and intermixing of nuclei between the inner nuclear layer (INL) and outer nuclear layer (ONL), seen in fixed retinal tissue sections, are not artifacts of histology processing. The numbers of larger disruptions were greater in the inferior retina compared to the superior retina. The optical back-scattering density of tissue disruptions was also greater in the inferior retina. Horizontal B-scan locations shown are (A) superior (y = +0.47mm), (B) central (y = 0.0 mm) and (C) inferior (y = −0.47 mm). The retinal layers visible are labeled: NFL/GCL (nerve fiber layer / ganglion cell layer), IPL (inner plexiform layer), INL/OPL/INL (inner nuclear layer / outer plexiform layer / outer nuclear layer), RPE (retinal pigment epithelium).
To confirm that the laminar disruption seen with SD-OCT involved cellular disorganization we also prepared some standard histology of retinal sections (H&E staining) from P42 retinas. To view the entire span of the neural retina from the disc to the periphery, the stained sections were scanned using virtual microscopy (Dailey et al., 2017). While the progression of OIR induced laminar disorganization continued from age P21 to P42, some of the disorganization resolved by age P56 in both Norrin-treated and vehicle-treated OIR retinas (Dailey et al., 2017). Disruption in the laminar structure of the retina was most often located in the central half of the retina, and not the periphery, corresponding with the OIR-induced central avascular areas typical of the mouse OIR model. Disruption of the retinal layers involved displacement and mixing of photoreceptor nuclei with cell bodies of the inner nuclear retina (Dailey et al., 2017).
3.2. Thicker NFL/GCL and IPL in Norrin-Injected OIR eyes
The NFL/GCL (nerve fiber layer/ganglion cell layer), IPL (inner plexiform layer) and total retinal thickness were measured at P42 in OCTs using Bioptigen Diver software. Average width measurements were generated at 12–16 points using a 5x5 grid (Fig. 1). Locations ranged from 0.4–0.7 mm from the optic nerve. Locations within 0.3mm of the optic nerve were excluded and the INL, OPL (outer plexiform layer) and ONL (outer nuclear layer) thicknesses were not measured separately since the laminar disruption in OIR retinas made it difficult to consistently distinguish the boundaries between those layers. The average NFL/GCL widths for RA (room air), Norrin-injected OIR eyes and Vehicle-injected OIR eyes were 15.6 μm, 14.4 μm and 13.3 μm respectively (Table 1). A significantly thicker NFL/GCL was found in the Norrin-injected OIR eyes, 14.4 μm, compared to Vehicle-injected OIR eyes, 13.3 μm, (p = 0.01).
Table 1.
Average NFL/GCL and IPL Thickness Measurements using SD-OCT
| GROUP | Number of Eyes | NFL/GCL Thickness (μm) | IPL Thickness (μm) |
|---|---|---|---|
| Room Air | 4 | 15.6 (±0.2) | 52.6 (±1.3) |
| Norrin-Injected | 13 | 14.4 (±0.9)* | 38.2 (±3.0) |
| Norrin Fellow Eye | 13 | 14.0 (±1.3) | 38.8 (±2.2) |
| Vehicle-Injected | 9 | 13.3 (±0.9) | 37.5 (±2.4) |
| Vehicle Fellow Eye | 9 | 13.7 (±1.2) | 37.5 (±2.2) |
p=0.01 compared to Vehicle-Injected
Averaged across the entire measurement grid, there were no significant differences detected in the IPL thickness. However, since more severe OIR disruption was noted in the inferior portion of the OIR retinas, the measurements were also grouped for analysis according to the superior versus inferior location: superior (5 points located above the optic nerve) and inferior (5 points below the optic nerve). (See Table 2) This comparison revealed an even greater difference in the NFL/GCL thickness between Norrin-injected OIR eyes (15.1 μm) and Vehicle-injected OIR eyes (13.4 μm) in the superior retina (p = 0.02). Additionally, a significant difference could be seen in the superior IPL thickness between Norrin-injected OIR eyes and Vehicle-injected OIR eyes (p = 0.04): 37.7 μm and 34.6 μm, respectively (Table 2). Average NFL/GCL and IPL thicknesses in non-injected fellow eyes of Norrin treated mice was similar to that of vehicle-injected eyes in the superior retina, 13.9 μm and 34.7 μm, respectively.
Table 2.
Superior and Inferior – NFL/GCL and IPL Thickness Measurements
| GROUP | Number of Eyes | INFERIOR (μm) | SUPERIOR (μm) | ||
|---|---|---|---|---|---|
| NFL/GCL | IPL | NFL/GCL | IPL | ||
| Room Air | 4 | 15.4 (±1.0) | 50.6 (±1.0) | 15.7 (±0.9) | 53.1 (±2.1) |
| Norrin-Injected | 13 | 14.0 (±1.1) | 36.1 (±4.0) | 15.1 (±1.9)* | 37.7 (±4.1)** |
| Vehicle-Injected | 9 | 13.8 (±0.9) | 36.9 (±3.6) | 13.4 (±1.4) | 34.6 (±3.6) |
p=0.02 compared to Vehicle-injected.
p=0.04 compared to Vehicle-injected.
3.3. Increased ratio (center / peripheral) of surviving number of RGCs in Norrin-Injected OIR eyes
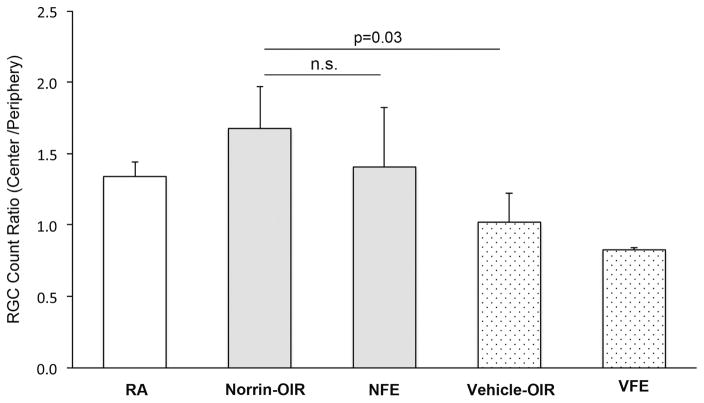
In order to count RGCs, C57BL/6J mouse retinas were flat-mounted and immunostained with an antibody to the RGC-specific transcription factor Brn3a, at age P42. RGC loss is variable for each individual eye in an OIR model because the severity of ischemia depends on the physical patterns of large retinal vessels, which are unique to each eye. The severity of the RGC loss is also variable between mice of slightly different body weights within the same litter, and can also be different between litters. For this reason we evaluated the ratio of the density of surviving RGCs in the most central regions of the retina compared to the adjacent peripheral regions. After staining, two sets of 8 images were collected from each flat mounted retina; one set was closer to the optic disc and termed “Center” (300–900 μm from the disc) and the other set further from the disc termed “Peripheral” (1000–1500 μm from the disc). (See Fig. 3A) For each flat-mounted retina, the number of RGCs was averaged for the Central and Peripheral image sets, and then the counts from the Center were normalized relative to the Periphery as a ratio (center/periphery). This method of normalization was chosen since most disorganization is seen in the central region with little in peripheral retina. The RGC density ratio (center/periphery) was 1.3 for room air (RA) mice. Comparisons revealed a significantly greater RGC count ratio (center/periphery) (n = 3/group, p = 0.03) in the Norrin-injected OIR eyes (1.7) compared to the Vehicle-injected OIR eyes (1.0). The Norrin-injected OIR eyes also had a significantly greater RGC count ratio (p = 0.004) compared to Vehicle-injected fellow eyes (VFE), which had a ratio of 0.8 (Fig. 4).
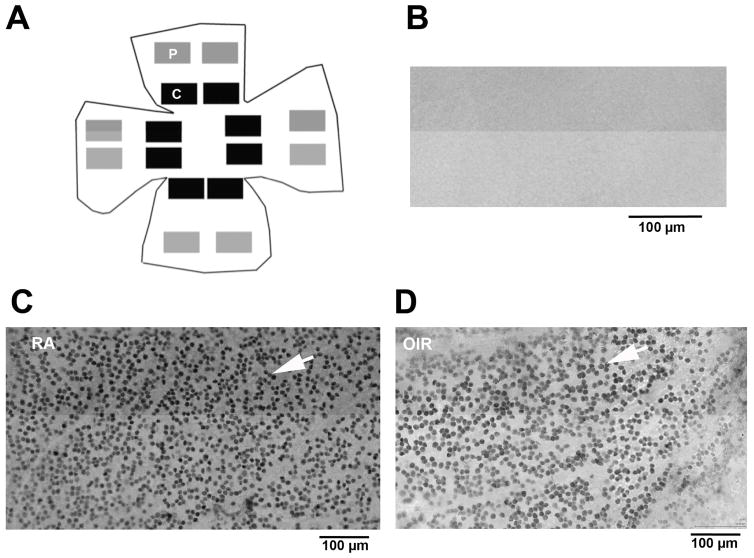
Figure 3. Diagram showing the image field locations used for measurement of RGC density in flat-mounted retinas.
Fields were selected from virtual microscopy scans of complete flat-mounted retinas (age P42) after immuno-labeling for Brn3a, a transcription factor present in the nuclei of retinal ganglion cells. (A) The number of Brn3a positive RGCs were counted by computer image analysis and averaged in 8 central (C, black boxes) and 8 peripheral (P, grey boxes) images. Examples of immuno-labeling quality from central regions are shown: (B) no-primary anti-body, negative control, (C) Brn3a positive RGC nuclei (white arrow) in a normal, room-air, retina, and (D) Brn3a positive RGC nuclei (white arrow) in an OIR retina.
Figure 4. Norrin increases the number of Brn3a-labeled RGCs in OIR eyes.
The ratio (center/periphery) of the RGC cell density was significantly higher in Norrin-injected OIR eyes (Norrin) compared to vehicle-injected OIR eyes (Vehicle) (p=0.03). Other groups: room-air (RA), fellow non-injected eye of Norrin-treated mice (NFE), fellow non-injected eye of vehicle-treated mice (VFE).
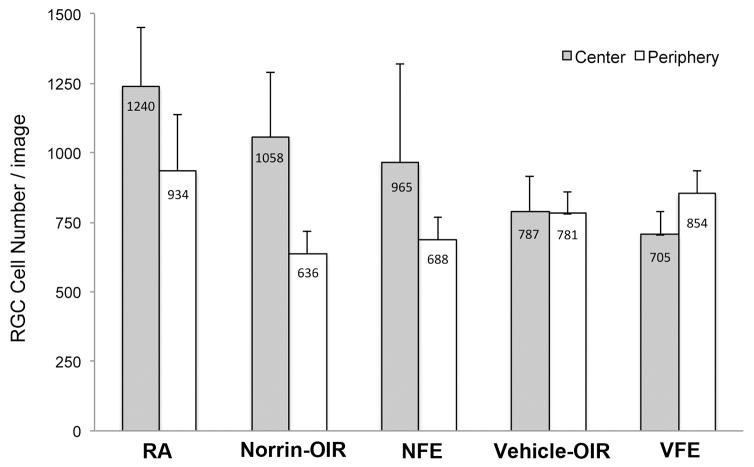
Interestingly, the non-injected fellow eyes of Norrin-injected OIR mice (NFE) had a RGC count ratio (center/periphery) of 1.4. This suggested a possible fellow eye effect since the RGC count ratio of the fellow eyes were not significantly different from the Norrin-injected eyes (p = 0.23). The average central and peripheral counts are shown in Figure-5 for the same groups.
Figure 5. Evidence of a fellow eye effect with Norrin-treatment.
Plot of the average RGC cell numbers counted per image in the Central and Peripheral image sets. Room-air (RA), Norrin-injected OIR eyes (Norrin-OIR), Fellow of Norrin-injected OIR eye (NFE), Vehicle-injected OIR eyes (The number of central cell counts were higher in the Norrin fellow eyes (NFE) compared to vehicle-injected OIR eyes (Vehicle-OIR) or vehicle-injected OIR fellow eyes (VFE).
3.4. Visible RGC dendritic arborization in vivo in Norrin-Treated Thy1-YFP mice
Homozygous Thy1-YFP mice were used to visualize changes in RGCs over time using live retinal imaging (Fig. 6A). In this strain, Yellow Fluorescent Protein (YFP) is expressed in only a small fraction of the total RGC population, driven by the Thy1-gene promoter. Combined with live retinal fluorescent imaging it is possible to visualize individual RGS in vivo. Images were taken of the endogenously fluorescent RGCs on days P21, P28 and P42 using a Micron-III retinal imaging system, equipped with a YFP excitation and emission filter set. On average, 22 ganglion cell bodies were visible per retina in RA images and 19 cell bodies were visible in the OIR images, using the Micron-III system. Images captured a circular field that extended 55% of the distance from the disc to the retinal periphery. The raw number of fluorescent counts can vary, even among litter mates. For this reason, we compared the percentage of RGCs that had visible dendritic arborization rather than raw RGC counts to compare treatment groups.
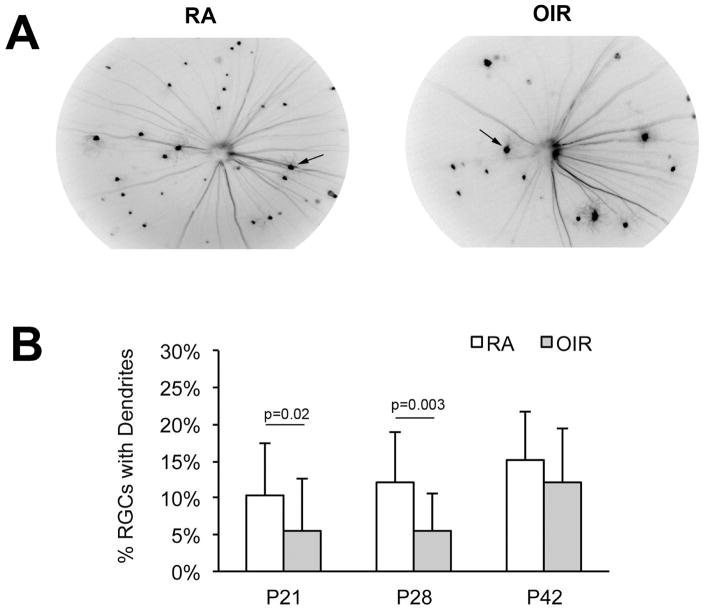
Figure 6. OIR-induced reduction in the percentage of RGC's with dendritic arborization.
(A) Example in vivo images of Thy1-YFP RGCs obtained using a Micron-III imaging system equipped with a YFP excitation and emission filter set. Images captured at P28 in RA (left) and OIR (right) mice. (B) The percentage of RGCs with dendritic arbors was significantly lower in OIR eyes (n=23) compared to RA eyes (n=30) at ages P21 and P28.
We compared OIR and control RA (room air) Thy1-YFP mice, without any Norrin treatment. The percentage of RGC cell bodies with visible dendritic arborization was generally reduced in OIR group mice compared to the RA group (Fig. 6B). This difference was significance at age P21 (p = 0.02), when 10 % of RGC had dendrites in RA mice (n = 30) and 6 % in OIR mice (n = 23), and again at age P28 (p = 0.003) when 12 % of RGC had dendrites in RA mice (n = 18) versus 6% in the OIR mice (n = 18). By age P42, 15 % of fluorescent RGCs in RA mice (n = 20) and 11 % of fluorescent RGCs in OIR mice (n = 13) had visible dendrites (n.s.).
Having established that oxygen exposure reduced the percentage of RGCs with visible dendritic arborization in Thy1-YFP mice during recovery from OIR, we conducted further experiments to investigate Norrin treatment in this model. We did so by injecting Norrin (20 ng or 100 ng) in one eye and PBS in the fellow eyes of Thy1-YFP OIR mice. The percentages of YFP-positive RGCs with visible dendrites were determined at ages P17, P28, and P42. There were no significant differences in the percentages of RGCs with visible dendrites in Norrin-injected eyes at compared to PBS injected eyes. See Table 3.
Table 3.
Percentage of RGCs with Dendritic Arbors (contralateral eyes)
| Group | P17 (%) | P28 (%) | P42 (%) |
|---|---|---|---|
| Norrin 20 ng (n=3) | 1.7 (±2.9) | 5.8 (±5.4) | 10.3 (±10.3) |
| PBS | 0.0 (±0) | 0.0 (±0) | 0.0 (±0) |
| Group | P21 | P28 | P42 |
|
| |||
| Norrin 100 ng (n=6) | 5.8 (±8.0) | 17.9 (±8.4) | 22.0 (±7.4) |
| PBS | 8.6 (±11.5) | 11.2 (±6.3) | 14.9 (±7.0) |
4. Discussion
In the mouse OIR model, capillaries are lost in the central retina during the high oxygen phase from age P7 to P12. The central retina becomes hypoxic upon return to room air and an aggressive, but disorganized, neovascular regrowth ensues. This disorganization is likely the result of a substantial percentage of retinal astrocytes and the fact that surviving astrocytes are impaired in their capacity to guide capillary growth (Bucher et al., 2013). In this environment, treatment with Norrin somewhat tames neovascular response and ultimately accelerates the revascularization of the central retina (Tokunaga et al., 2013). Other treatments have been reported that reduce the severity of aggressive neovascular growth in this model and facilitate re-establishment of normal vascular beds in ablated zones (May, 2012)
4.1. Nerve Fiber Layer thinning In Vivo is ameliorated by Norrin-treatment
Clinically, SD-OCT is becoming a valuable tool to monitor NFL/GCL thickness in humans, where thinning of this layer results from the death and loss of RGCs. Mezu-Ndubuisi et al., demonstrated that SD-OCT can detect an average reduction in total retinal thickness in OIR mouse retinas in vascular ablated zones that were first located by fluorescein angiography (Mezu-Ndubuisi et al., 2014). To achieve the sensitivity required to compare the NFL/GCL thickness, we employed an SD-OCT system equipped with an optical system designed specifically for the mouse eye, and used software to collect many measurements per retina based on a reproducible 5x5 coordinate grid. Averaging from many measurement locations per retina was necessary to make these comparisons possible in mouse eyes. We found that OIR eyes injected with Norrin had increased NFL/GCL and IPL thicknesses, as well as an increased central / periphery ratio of RGC population density.
A correlation between GCL and IPL thinning and poor visual function has been reported in Diabetic Macular Edema (DME) and Glaucoma patients (Bonnin et al., 2015; de A Moura et al., 2012). Thinning reflects degeneration of retinal ganglion cell bodies and axons in the RNFL and dendritic arbors in the IPL. Thinner RNFLs have been reported in humans with ROP and diabetes (Åkerblom et al., 2012; Park et al., 2011). Akerblom et al reported thinner than normal RNFL thicknesses in children, at age 9, who had severe ROP as infants (Åkerblom et al., 2012). Through mapping of the NFL/GCL and IPL thicknesses with SD-OCT, we found that OIR significantly reduced the thicknesses of these layers and Norrin could counteract this thinning. Preservation of these layers was most significant in the superior retina. This may reflect the less severe nature of layer disruptions in the superior retina, making it easier to observe a Norrin therapeutic effect.
4.2. Norrin-treatment increases the ratio of RGC density (central /periphery) in OIR eyes
We wanted to determine if Norrin could increase the final relative density of surviving RGCs in the affected central retina. It should be noted that two previous studies could not detect a reduction in the numbers of RGC in OIR mice using standard retinal cross sections (Mehdi et al., 2014; Nakamura et al., 2012). However, the relatively small numbers of RGCs present in a retinal cross section, combined with the fact that OIR retinal sections can include normal zones, reduces the power to detect RGC loss. We decided that a more extensive survey of mouse retinas would be essential to have sufficient numbers of RGCs for counting, and so we used whole retinal flat-mounts. We found that OIR caused a significant reduction in central/periphery RGC density ratio and that Norrin-treatment could normalize this effect.
Interestingly, the fellow-eyes of Norrin–injected OIR eyes also had an improved central/periphery RGC density ratio. A fellow-eye effect has been demonstrated in multiple clinical studies involving anti-VEGF treatment of Diabetic Macular Edema (DME) (Avery et al., 2006; Bakbak et al., 2013; Hanhart et al., 2014). A possible fellow-eye therapeutic effect was also reported in diabetics, after bevacizumab injection (Avery et al., 2006). Presumably, when the blood retina barrier is disrupted in response to ischemia, intravitreally injected therapies can enter the blood stream. These results indicate that the choice of fellow eyes as controls should be considered carefully when using the mouse OIR model.
Improved ratios of RGC density (central /periphery) in the OIR model from Norrin-treatment is consistent with the fact that Norrin-treatment accelerates the regrowth of the retinal microvasculature into the central avascular region in this model. Thus, accelerated recovery from ischemia could reduce the final numbers of RGCs lost. Alternatively, Norrin may also have a direct neuroprotective effect upon RGCs themselves. LGR4 has been localized to the GCL in retinas of adult mice (Van Schoore et al., 2005) and Norrin has been shown to activate Wnt-signaling upon LGR4 binding in in vitro studies (Deng et al., 2013). However, our experiments cannot estimate such a possible direct effect in the OIR model, because of the extensive involvement of revascularization in this model.
Seitz et al reported an increase of surviving RGC axons and decreased apoptosis in NDMA-injected mice that were simultaneously injected with Norrin (Seitz et al., 2010). Braunger et al reported that Norrin was able to provide protection against light-induced damage to photoreceptors in mice overexpressing Norrin in the RPE cells (Braunger et al., 2013). The investigators suggested that Norrin provides protection to the photoreceptors by increasing the expression of neurotrophic growth factors, specifically BDNF.
4.3. Effects of OIR on Retinal Ganglion Cells in vivo
RGCs are sensitive to hypoxic stress (Kaur et al., 2008; Kergoat et al., 2006) and the loss of RGCs has been reported in conditions causing retinal ischemia, such as diabetes (Kern and Barber, 2008). For this reason, we created a protocol that allowed us to visually follow individual RGCs over time in Thy1-YFP OIR mice. We took advantage of one particular strain where less than one percent of RGCs express the transgenic Yellow Fluorescent Protein. Using a YFP light filter set and a Micron-III camera, we could image individual RGCs in anesthetized mice, including cell bodies, dendrites, and axons. We found that the OIR treatment reduced the percentage of RGCs with YFP-labeled dendritic arborization. Similar reductions in visible dendritic arborization were reported by investigators using Thy-1 YFP mice to explore retinal ischemia in animal models of Glaucoma. Ischemia caused by elevating intraocular pressure has been shown to cause retraction of dendritic arbors (Li et al., 2011). Loss of dendrites and axons occur first followed by cell bodies. Another group of investigators found a similar RGC degeneration when using an optic nerve crush model (Leung et al., 2011). While Norrin treatment increased the central / peripheral RGC density ratio, Norrin did not have a statistically significant effect on the extent of dendritic arborization in YFP-positive RGCs.
4.4. Extra observations on OIR model progression in vivo
While not the primary aim of our investigations, SD-OCT revealed that significant disruptions of the deep retinal architecture can occur in the murine OIR model. These were found to be zones with displacement of significant numbers of photoreceptor nuclei into what is normally the boundary of the INL. The disrupted architecture could partially resolve over several weeks but does persist. (Dailey et al., 2017). This is consistent with ERG observations, where significant decreases in the A-wave and B-wave amplitudes found in 4-week old OIR mice show partial recovery by 8-weeks (Nakamura et al., 2012). Our use of 3-dimensional SD-OCT also unexpectedly revealed that oxygen-induced disruption of the INL and ONL was generally more severe in the inferior retina versus the superior retina.
4.5. Summary
The mouse model of oxygen-induced retinopathy is one good model for studying vascular proliferative mechanisms that are present in disorders such as ROP. It is important to be knowledgeable of disrupted and non-disrupted retinal regions when evaluating measurements in the OIR model. Additionally, only using fellow eyes as controls may sometimes cause investigators to miss a desired treatment effect since there is the possibility of carry-over influence from the injected eye. After taking these factors into consideration for our study, we conclude that Norrin-treatment significantly increased relative RGC survival in the central effected zone of the mouse OIR model. This conclusion was supported by a reduction in thinning of the NFL/GCL, as measured by SD-OCT in vivo, and measurements of the RGC population density ratio (central/peripheral) in whole retinal flat-mount preparations.
Highlights.
Norrin treatment accelerates recovery of the mouse OIR model from ischemic insult.
SD-OCT can compare NFL/GCL (nerve fiber layer/ganglion cell layer) thickness in vivo.
Norrin treatment counters thinning of the NFL/GCL in the mouse OIR model.
Norrin treatment increases the surviving population density of RGCs in OIR retinas.
Acknowledgments
Grant Support:
This study was supported by: Vision Research ROPARD Foundation (KAD, KPM), Special Trustees of Moorfields Eye Hospital, TFC Frost Trust, HCA International (SCW), National Eye Institute - NIH R15EY025089 (KPM).
The authors would like to thank Janet Schofding, Cliff Snitgin and other staff of the Biomedical Research Support Facility for animal care and support, and undergraduate student Jennifer Felisky for assistance with files and submission process for the manuscript. Dr. Drenser is a co-corresponding author for this research. This study was supported by: Vision Research ROPARD Foundation (KAD, KPM), Special Trustees of Moorfields Eye Hospital, TFC Frost Trust, HCA International (SCW), National Eye Institute - NIH R15EY025089 (KPM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Åkerblom H, Holmstrom G, Eriksson U, Larsson E. Retinal nerve fibre layer thickness in school-aged prematurely-born children compared to children born at term. Br J Ophthalmol. 2012;96:956–960. doi: 10.1136/bjophthalmol-2011-301010. [DOI] [PubMed] [Google Scholar]
- Akula JD, Hansen RM, Martinez-Perez ME, Fulton AB. Rod photoreceptor function predicts blood vessel abnormality in retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2007;48:4351–4359. doi: 10.1167/iovs.07-0204. [DOI] [PubMed] [Google Scholar]
- Avery RL, Pearlman J, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ, Wendel R, Patel A. Intravitreal Bevacizumab (Avastin) in the Treatment of Proliferative Diabetic Retinopathy. Ophthalmology. 2006:113. doi: 10.1016/j.ophtha.2006.05.064. [DOI] [PubMed] [Google Scholar]
- Bakbak B, Ozturk BT, Gonul S, Yilmaz M, Gedik S. Comparison of the effect of unilateral intravitreal bevacizumab and ranibizumab injection on diabetic macular edema of the fellow eye. J Ocul Pharmacol Ther. 2013;29:728–732. doi: 10.1089/jop.2013.0049. [DOI] [PubMed] [Google Scholar]
- Barnaby AM, Hansen RM, Moskowitz A, Fulton AB. Development of scotopic visual thresholds in retinopathy of prematurity. Investig Ophthalmol Vis Sci. 2007;48:4854–4860. doi: 10.1167/iovs.07-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnin S, Tadayoni R, Erginay A, Massin P, Dupas B. Correlation between ganglion cell layer thinning and poor visual function after resolution of diabetic macular edema. Investig Ophthalmol Vis Sci. 2015;56:978–982. doi: 10.1167/iovs.14-15503. [DOI] [PubMed] [Google Scholar]
- Braunger BM, Ohlmann A, Koch M, Tanimoto N, Volz C, Yang Y, Bösl MR, Cvekl A, Jägle H, Seeliger MW, Tamm ER. Constitutive overexpression of Norrin activates Wnt/β-catenin and endothelin-2 signaling to protect photoreceptors from light damage. Neurobiol Dis. 2013;50:1–12. doi: 10.1016/j.nbd.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Bucher F, Stahl A, Agostini HT, Martin G. Hyperoxia causes reduced density of retinal astrocytes in the central avascular zone in the mouse model of oxygen-induced retinopathy. Mol Cell Neurosci. 2013;56:225–233. doi: 10.1016/j.mcn.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Chen J, Michan S, Juan AM, Hurst CG, Hatton CJ, Pei DT, Joyal JS, Evans LP, Cui Z, Stahl A, Sapieha P, Sinclair DA, Smith LEH. Neuronal sirtuin1 mediates retinal vascular regeneration in oxygen-induced ischemic retinopathy. Angiogenesis. 2013;16:985–992. doi: 10.1007/s10456-013-9374-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey W, Drenser K, Wong S, Cheng M, Vercellone J, Roumayah K, Fenney E, Deshpande M, Guzman A, Trese M, Mitton K. Disruption of the Retina’s Laminar Structure in a Mouse Model of Oxygen Induced Retinopathy. Data Br. 2017 doi: 10.1016/j.dib.2017.09.075. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de A Moura AL, Raza AS, Lazow MA, De Moraes CG, Hood DC. Retinal ganglion cell and inner plexiform layer thickness measurements in regions of severe visual field sensitivity loss in patients with glaucoma. Eye. 2012;26:1188–1193. doi: 10.1038/eye.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C, Reddy P, Cheng Y, Luo CW, Hsiao CL, Hsueh AJW. Multi-functional norrin is a ligand for the LGR4 receptor. J Cell Sci. 2013;126:2060–8. doi: 10.1242/jcs.123471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Zhao Y, Yoshida M, Chen H, Yang JF, Kim TS, Cang J, Troy JB, Liu X. Sustained ocular hypertension induces dendritic degeneration of mouse retinal ganglion cells that depends on cell type and location. Investig Ophthalmol Vis Sci. 2013;54:1106–1117. doi: 10.1167/iovs.12-10791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton AB, Akula JD, Mocko JA, Hansen RM, Benador IY, Beck SC, Fahl E, Seeliger MW, Moskowitz A, Harris ME. Retinal degenerative and hypoxic ischemic disease. Doc Ophthalmol. 2009;118:55–61. doi: 10.1007/s10633-008-9127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton AB, Hansen RM, Petersen RA, Vanderveen DK. The rod photoreceptors in retinopathy of prematurity: an electroretinographic study. Arch Ophthalmol (Chicago, Ill 1960) 2001;119:499–505. doi: 10.1001/archopht.119.4.499. [pii] [DOI] [PubMed] [Google Scholar]
- Hanhart J, Tiosano L, Averbukh E, Banin E, Hemo I, Chowers I. Fellow eye effect of unilateral intravitreal bevacizumab injection in eyes with diabetic macular edema. Eye (Lond) 2014;28:646–53. doi: 10.1038/eye.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur C, Foulds WS, Ling EA. Hypoxia-ischemia and retinal ganglion cell damage. Clin Ophthalmol. 2008;2:879–89. doi: 10.2147/OPTH.S3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kergoat H, Hérard MÈve, Lemay M. RGC sensitivity to mild systemic hypoxia. Investig Ophthalmol Vis Sci. 2006;47:5423–5427. doi: 10.1167/iovs.06-0602. [DOI] [PubMed] [Google Scholar]
- Kern TS, Barber AJ. Retinal ganglion cells in diabetes. J Physiol. 2008;586:4401–4408. doi: 10.1113/jphysiol.2008.156695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Woo S, Ahn J, Park K, Chung H. Long-Term Temporal Changes of Peripapillary Retinal Nerve Fiber Layer Thickness Before and After Panretinal Photocoagulation in Severe Diabetic Retinopathy. Retina. 2012;32:2052–2060. doi: 10.1097/IAE.0b013e3182562000. [DOI] [PubMed] [Google Scholar]
- Lange C, Ehlken C, Stahl A, Martin G, Hansen L, Agostini HT. Kinetics of retinal vaso-obliteration and neovascularisation in the oxygen-induced retinopathy (OIR) mouse model. Graefe’s Arch Clin Exp Ophthalmol. 2009;247:1205–1211. doi: 10.1007/s00417-009-1116-4. [DOI] [PubMed] [Google Scholar]
- Leung CKS, Weinreb RN, Li ZW, Liu S, Lindsey JD, Choi N, Liu L, Cheung CY, Ye C, Qiu K, Chen LJ, Yung WH, Crowston JG, Pu M, So KF, Pang CP, Lam DSC. Long-term in vivo imaging and measurement of dendritic shrinkage of retinal ganglion cells. Investig Ophthalmol Vis Sci. 2011;52:1539–1547. doi: 10.1167/iovs.10-6012. [DOI] [PubMed] [Google Scholar]
- Li ZW, Liu S, Weinreb RN, Lindsey JD, Yu M, Liu L, Ye C, Cui Q, Yung WH, Pang CP, Lam DSC, Leung CKS. Tracking dendritic shrinkage of retinal ganglion cells after acute elevation of intraocular pressure. Investig Ophthalmol Vis Sci. 2011;52:7205–7212. doi: 10.1167/iovs.10-6868. [DOI] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X. Wnt/β-Catenin Signaling: Components, Mechanisms, and Diseases. Dev Cell. 2009 doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May CA. The influence of triamcinolone on endostatin-like proteins in oxygen-induced retinopathy of prematurity. Exp Eye Res. 2012;100:86–87. doi: 10.1016/j.exer.2012.04.017. [DOI] [PubMed] [Google Scholar]
- Mehdi MKIM, Sage-Ciocca D, Challet E, Malan A, Hicks D. Oxygen-induced retinopathy induces short-term glial stress and long-term impairment of photoentrainment in mice. Graefe’s Arch Clin Exp Ophthalmol. 2014;252:595–608. doi: 10.1007/s00417-014-2579-5. [DOI] [PubMed] [Google Scholar]
- Mezu-Ndubuisi OJ, Wanek J, Chau FY, Teng Pyu, Blair NP, Reddy NM, Raj JU, Reddy SP, Shahidi M. Correspondence of retinal thinning and vasculopathy in mice with oxygen-induced retinopathy. Exp Eye Res. 2014;122:119–122. doi: 10.1016/j.exer.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Imai S, Ogishima H, Tsuruma K, Shimazawa M, Hara H. Morphological and functional changes in the retina after chronic oxygen-induced retinopathy. PLoS One. 2012;7:e32167. doi: 10.1371/journal.pone.0032167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlmann A, Seitz R, Braunger B, Seitz D, Bosl MR, Tamm ER, Bösl MR, Tamm ER. Norrin promotes vascular regrowth after oxygen-induced retinal vessel loss and suppresses retinopathy in mice. J Neurosci. 2010;30:183–193. doi: 10.1523/JNEUROSCI.3210-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki H, Yu AY, Della N, Ozaki K, Luna JD, Yamada H, Hackett SF, Okamoto N, Zack DJ, Semenza GL, Campochiaro PA. Hypoxia inducible factor-1alpha is increased in ischemic retina: temporal and spatial correlation with VEGF expression. Invest Ophthalmol Vis Sci. 1999;40:182–189. [PubMed] [Google Scholar]
- Park HYL, Kim IT, Park CK. Early diabetic changes in the nerve fibre layer at the macula detected by spectral domain optical coherence tomography. Br J Ophthalmol. 2011;95:1223–8. doi: 10.1136/bjo.2010.191841. [DOI] [PubMed] [Google Scholar]
- Rao TP, Kühl M. An updated overview on wnt signaling pathways: A prelude for more. Circ Res. 2010 doi: 10.1161/CIRCRESAHA.110.219840. [DOI] [PubMed] [Google Scholar]
- Robinson GS, Ju M, Shih SC, Xu X, McMahon G, Caldwell RB, Smith LE. Nonvascular role for VEGF: VEGFR-1, 2 activity is critical for neural retinal development. Faseb J. 2001;15:1215–1217. doi: 10.1096/fj.00. [DOI] [PubMed] [Google Scholar]
- Scott A, Powner MB, Fruttiger M. Quantification of vascular tortuosity as an early outcome measure in oxygen induced retinopathy (OIR) Exp Eye Res. 2014;120:55–60. doi: 10.1016/j.exer.2013.12.020. [DOI] [PubMed] [Google Scholar]
- Seitz R, Hackl S, Seibuchner T, Tamm ER, Ohlmann A. Norrin Mediates Neuroprotective Effects on Retinal Ganglion Cells via Activation of the Wnt/β-Catenin Signaling Pathway and the Induction of Neuroprotective Growth Factors in Muller Cells. J Neurosci. 2010;30:5998–6010. doi: 10.1523/JNEUROSCI.0730-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LE, Wesolowski E, McLellan A, Kostyk SK, D’Amato R, Sullivan R, D’Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- Tokunaga CC, Chen YH, Dailey W, Cheng M, Drenser KA. Retinal vascular rescue of oxygen-induced retinopathy in mice by norrin. Invest Ophthalmol Vis Sci. 2013;54:222–229. doi: 10.1167/iovs.12-10127. [DOI] [PubMed] [Google Scholar]
- Tokunaga CC, Mitton KP, Dailey W, Massoll C, Roumayah K, Tarabishy N, Cheng M, Guzman E, Drenser KA. Effect of Anti-VEGF Treatment on Developing Retina Following Oxygen-Induced Retinopathy. Invest Ophthalmol Vis Sci. 2014;55:1884–1892. doi: 10.1167/iovs.13-13397. [DOI] [PubMed] [Google Scholar]
- Van Schoore G, Mendive F, Pochet R, Vassart G. Expression pattern of the orphan receptor LGR4/GPR48 gene in the mouse. Histochem Cell Biol. 2005;124:35–50. doi: 10.1007/s00418-005-0002-3. [DOI] [PubMed] [Google Scholar]
- Wang L, Shi P, Xu Z, Li J, Xie Y, Mitton K, Drenser K, Yan Q. Up-regulation of VEGF by retinoic acid during hyperoxia prevents retinal neovascularization and retinopathy. Investig Ophthalmol Vis Sci. 2014;55:4276–4286. doi: 10.1167/iovs.14-14170. [DOI] [PubMed] [Google Scholar]
- Williams PA, Howell GR, Barbay JM, Braine CE, Sousa GL, John SWM, Morgan JE. Retinal ganglion cell dendritic atrophy in DBA/2J glaucoma. PLoS One. 2013;8:e72282. doi: 10.1371/journal.pone.0072282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia CH, Liu H, Cheung D, Wang M, Cheng C, Du X, Chang B, Beutler B, Gong X. A model for familial exudative vitreoretinopathy caused by LPR5 mutations. Hum Mol Genet. 2008;17:1605–1612. doi: 10.1093/hmg/ddn047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Wang Y, Cahill H, Yu M, Badea TC, Smallwood PM, Peachey NS, Nathans J. Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell. 2009;139:285–298. doi: 10.1016/j.cell.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Wang Y, Nathans J. The Norrin/Frizzled4 signaling pathway in retinal vascular development and disease. Trends Mol Med. 2010 doi: 10.1016/j.molmed.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]