Abstract
Objective
To investigate the relationship between sleep disturbance, fatigue, and urinary incontinence (UI)/overactive bladder (OAB) symptoms among patients with OAB.
Methods
Patients who were diagnosed with overactive bladder (OAB) and age-matched control subjects without OAB were enrolled. Sleep disturbance and fatigue symptoms were assessed using the PROMIS short forms. UI/OAB symptoms were assessed using the ICIQ-UI, ICIQ-OAB, OAB-q, UDI-6, and IIQ-7 questionnaires. Psychosocial health (depression, anxiety, perceived stress level) was also assessed.
Results
OAB patients reported significantly greater sleep disturbance compared to controls (PROMIS 8b T-scores: 54.3 ± 10.3 vs. 43.8 ± 9.2). OAB patients also reported significantly greater fatigue compared to controls (PROMIS 7a T-scores: 54.7 ± 9.6 vs. 46.0 ± 6.4). After adjusting for nocturia, the differences in sleep disturbance between OAB and controls became insignificant (p=0.21) while the differences in fatigue between OAB and controls remained significant (p=0.014). Among OAB patients, there were positive correlations between sleep disturbance and the severity of OAB symptoms (ICIQ-OAB), poorer health-related quality of life (OAB-q QOL), the severity of urinary incontinence symptoms (ICIQ-UI), greater incontinence impact (IIQ-7) and urinary bother (UDI-6). Positive correlations were also observed between fatigue and worse UI/OAB symptoms and quality of life. Both sleep disturbance and fatigue were associated with poor psychosocial health (depression, anxiety, higher stress level) among OAB patients.
Conclusions
Sleep disturbance and fatigue are present in substantial percentages of OAB patients. Among OAB patients, sleep disturbance and fatigue were associated with more severe UI/OAB symptoms, worse health-related quality of life, and poorer psychosocial health.
Keywords: Sleep, fatigue, overactive bladder, urinary incontinence, nocturia, psychosocial
Introduction
Overactive bladder (OAB) affects one out of six adults in the United States.1 OAB affects physical and psychosocial function. The presence of nocturia – one of the symptoms of OAB – inevitably impacts sleep due to frequent nighttime awakenings. Sleep is recognized as a major component of mental and physical health,2 and sleep disturbance is a significant cardiovascular and metabolic risk factor.3
Even though many studies have reported the relationship between nocturia and sleep, to our knowledge, few studies have examined the relationship between urinary incontinence (UI)/overactive bladder (OAB) and sleep disturbance. Kemmer et al studied men with obstructive sleep apnea (OSA), and showed that patients with moderate to severe OSA presented with a higher incidence of OAB symptoms than patients with milder OSA.4 Tsujimura et al also studied men with OSA, and showed that the negative association between sleep efficiency and urgency – the cardinal symptom of OAB – is mediated independent of the effects of nocturia.5 Scovell et al studied men who were working nonstandard (e.g., night) shifts, and showed an inverse relationship between sleep quality and the severity of male LUTS among these men.6 Coyne et al showed in a national survey study that increasing nocturia is associated with poorer sleep in individuals with and without OAB symptoms.7 There are several weaknesses among these studies: 1) none of the studies have specifically recruited OAB patients, and it is unclear if findings from their study population (e.g., patients with known OSA, male LUTS patients, nonstandard shift workers, or national survey participants) might be applicable to the clinical OAB population; 2) many of the sleep studies did not assess functional outcomes using validated sleep questionnaires.4,5
In this study, we have recruited patients with a clinical diagnosis of OAB, and specifically examined the relationship of urinary incontinence (UI) and overactive bladder (OAB) symptoms with sleep disturbance and fatigue using validated questionnaires from the PROMIS item bank (Patient-Reported Outcomes Measurement Information System).8 We will test the hypothesis that sleep disturbance and fatigue are associated with more severe UI/OAB symptoms, worse health-related quality of life, and poorer psychosocial health in the clinical OAB population, after taking into account their nocturia.
Methods
Subjects
Between October 2012 and July 2014, adult urology patients aged 18 or above diagnosed with OAB, and an age-matched control group without OAB symptoms were recruited into this study that inquired their sleep disturbance, fatigue, and urinary symptoms. For OAB, patients must complain of urinary urgency, with or without urgency incontinence, usually with frequency and nocturia, in the absence of infection or other identifiable causes, in accordance with the 2002 ICS (International Continence Society) definition of OAB.9 The clinical evaluation was performed by one clinician (HL) and followed the published AUA guidelines.10 Subjects with a history of urinary incontinence surgery, prostate surgery, urethral stricture disease, neurogenic bladder, urinary retention, pelvic radiation, tuberculosis cystitis, cyclophosphamide cystitis, genitourinary cancer, urinary stones, a documented positive urine culture in the past 6 weeks, or a post-void residual volume ≥ 150 mL were not eligible. Controls were recruited by advertisement in local commuter train stations and a university-wide volunteers who are willing to be contacted for various research studies at database of Washington University. Controls must have no prior diagnosis of OAB or interstitial cystitis/bladder pain syndrome, no significant lower urinary tract symptoms (AUA symptom index < 7), no bladder or pelvic pain, and no evidence of active urinary infection. The control group was age-matched to the OAB cohort. All subjects signed an informed consent. The Washington University School of Medicine Institutional Review Board approved this study.
Assessment
Sleep disturbance and fatigue symptoms were assessed using validated questionnaires from the PROMIS item bank (Patient-Reported Outcomes Measurement Information System).8 The PROMIS questionnaires were developed in 2004 in conjunction with the NIH to assess patient-reported outcomes in physical, mental, and social health. Items were tested in the US general populations with good reliability and construct validity. The short-form questionnaires used here respectively contain 8 items to assess sleep disturbance (PROMIS Sleep Disturbance 8b) and fatigue symptoms (PROMIS Fatigue 7a). PROMIS 8b assesses self-reported perceptions of sleep quality, sleep depth, adequacy of and satisfaction with sleep over the past seven days. PROMIS 7a evaluates self-reported symptoms such as feeling tired, extreme exhaustion, and impact on daily activities and normal functioning over the past seven days. The items on the questionnaires are listed in Table 2. Raw scores were re-scaled and reported as T-scores, which have a set mean value of 50 and a standard deviation of 10 in the general population. A higher PROMIS T-score represents more sleep disturbance and fatigue.
Table 2.
Comparison of sleep disturbance and fatigue between OAB and controls
| OAB (n = 51) | Controls (n = 30) | p-value (adjusted for age and sex) | p-value (adjusted for age, sex, AND NOCTURIA) | |
|---|---|---|---|---|
| Sleep measures (over past 7 days) | ||||
| PROMIS-8b (sleep disturbance, T-score) | 54.3 ± 10.3 | 43.8 ± 9.2 | <0.001 | 0.213 |
| % with PROMIS-8b > 50 | 68.6% | 23.3% | <0.001 | 0.053 |
| Odds ratio of having PROMIS-8b > 50 in OAB | OR = 8.5 95% CI [2.8, 25.6] |
|||
| My sleep was restlessa | 3.1 ± 1.3 | 1.7 ± 1.0 | <0.001 | 0.075 |
| I was satisfied with my sleepb | 3.4 ± 1.3 | 2.3 ± 1.3 | <0.001 | 0.190 |
| My sleep was refreshingb | 3.2 ± 1.3 | 2.3 ± 1.3 | 0.005 | 0.612 |
| I had difficulty falling asleepa | 2.5 ± 1.4 | 1.8 ± 1.0 | 0.014 | 0.790 |
| I had trouble staying asleepa | 3.1 ± 1.4 | 1.9 ± 1.0 | <0.001 | 0.627 |
| I had trouble sleepinga | 3.0 ± 1.4 | 1.9 ± 0.9 | <0.001 | 0.543 |
| I got enough sleepb | 3.0 ± 1.2 | 2.1 ± 1.0 | <0.001 | 0.430 |
| My sleep quality wasc | 3.1 ± 1.1 | 2.0 ± 0.9 | <0.001 | 0.104 |
| Fatigue measures (over past 7 days) | ||||
| PROMIS-7a (fatigue, T-score) | 54.7 ± 9.6 | 46.0 ± 6.4 | <0.001 | 0.014 |
| % with PROMIS-7a > 50 | 62.7% | 26.7% | 0.005 | 0.017 |
| Odds ratio of having PROMIS-7a > 50 in OAB | OR = 4.3 95% CI [1.5, 11.8] |
|||
| How often did you feel tired?d | 3.3 ± 1.1 | 2.5 ± 0.7 | 0.002 | 0.040 |
| How often did you experience extreme exhaustion?d | 2.6 ± 1.2 | 1.5 ± 0.6 | <0.001 | 0.039 |
| How often did you run out of energy?d | 2.7 ± 1.1 | 1.8 ± 0.8 | 0.001 | 0.006 |
| How often did your fatigue limit you at work (include work at home)?d | 2.5 ± 1.1 | 1.5 ± 0.8 | <0.001 | 0.001 |
| How often were you too tired to think clearly?d | 2.3 ± 1.1 | 1.4 ± 0.7 | <0.001 | 0.039 |
| How often were you too tired to take a bath or shower?d | 2.0 ± 1.1 | 1.4 ± 0.8 | 0.023 | 0.335 |
| How often did you have enough energy to exercise strenuously?e | 3.6 ± 1.2 | 3.3 ± 1.3 | 0.496 | 0.253 |
1 = Not at all, 2 = A little bit, 3 = Somewhat, 4 = Quite a bit, 5 = Very much
5 = Not at all, 4 = A little bit, 3 = Somewhat, 2 = Quite a bit, 1 = Very much
5 = Very poor, 4 = Poor, 3 = Fair, 2 = Good, 1 = Very good
1 = Never, 2 = Rarely, 3 = Sometimes, 4 = Often, 5 = Always
5 = Never, 4 = Rarely, 3 = Sometimes, 2 = Often, 1 = Always
Urinary incontinence (UI) and overactive bladder (OAB) symptoms were assessed using the following validated questionnaires: 1) International Consultation on Incontinence – Urinary Incontinence Short Form (ICIQ-UI)11, 2) International Consultation on Incontinence – Overactive Bladder (ICIQ-OAB)12, 3) OAB-q Short Form13, 4)
Urogenital Distress Inventory Short Form (UDI-6)14, and 5) Incontinence Impact Questionnaire Short Form (IIQ-7)14. Briefly, ICIQ-UI is a 4-item questionnaire that assesses the frequency, amount and interference of urinary incontinence. ICIQ-OAB is a 4-item questionnaire that inquires about daytime frequency, nighttime frequency, urgency, and urgency incontinence. OAB-q contains two sub-scales that assess symptom bother and health-related quality of life. UDI-6 and IIQ-7 measure urinary distress and incontinence impact.
Depression and anxiety symptoms were assessed using the Hospital Anxiety and Depression Scale (HADS).15 The questionnaire contains 7 items for depression (HADS-D) and 7 items for anxiety (HADS-A). Perceived stress was assessed using the validated 10-item perceived stress scale (PSS).16 The PSS measures the degree to which situations are perceived as being unpredictable, uncontrollable and overwhelming during the previous month. Higher PSS scores indicate higher psychological stress.
Statistical analysis
Categorical data were compared using chi-square tests (or Fisher’s exact tests for < 5) and continuous data using Wilcoxon sum-rank tests. Spearman’s correlation was used to examine the relationship between UI/OAB and sleep/fatigue measures, adjusting the p-values for age (e.g., age may affect sleep and LUTS), sex (e.g., gender difference in sleep disturbance has been reported)17, and nocturia. To adjust for nocturia, the number of nighttime urinations was assessed using the nocturia question on the ICIQ- OAB questionnaire (question 4): “During the night, how many times do you have to get up to urinate?” The nocturia variable was entered in multivariate linear regression with T-scores as the response variable. Adjusted p-values were calculated with multivariable linear regression models. p < 0.05 was considered a significant difference. Data were presented as mean ± SD in the tables. All statistical analyses were completed using the open source statistical package R v3.2.2.
Results
Study population
Fifty-one adult OAB patients and 30 age-matched controls participated in this study. Table 1 shows their demographics, UI, OAB and nocturia symptoms. There was no difference in age, sex, or race between the two groups (see Table 1). As expected OAB patients had worse UI/OAB symptoms compared to controls. OAB patients also had a greater number of nighttime urinations compared to controls (2.6 ± 1.1 vs. 0.9 ± 0.7, p<0.001). The odds ratio for having ≥ 1 nighttime urinations was 6.9 in the OAB group (94.0% vs. 76.7%, 95% confidence interval 1.31 to 36.1, p=0.023). The odds ratio for having ≥ 2 nighttime urinations was 41.9 in the OAB group (86.0% vs. 13.3%, 95% confidence interval 10.7 to 164.8, p<0.001). However, OAB patients were not more likely to report nighttime incontinence compared to controls (13.7% vs. 3.3%, odds ratio = 4.5, 95% confidence interval 0.5 to 39.9, p=0.17).
Table 1.
Demographics, UI, OAB & nocturia symptoms
| OAB | Controls | p-value (adjusted for age and sex) | |
|---|---|---|---|
| Demographics | |||
| No. of subjects | 51 | 30 | |
| Age (mean ± SD) | 53.8 ± 11.9 | 54.2 ± 12.3 | 0.984 |
| Sex (% female) | 72.5% | 56.7% | 0.143 |
| Race (% white) | 43.1% | 63.3% | 0.079 |
| UI/OAB measures (over past 4 weeks) (mean ± SD) | |||
| ICIQ-UI (urinary incontinence, 0–21) | 12.0 ± 4.9 | 1.4 ± 2.0 | 0.002 |
| ICIQ-OAB (overactive bladder, 0–16) | 9.3 ± 2.6 | 2.0 ± 1.5 | 0.007 |
| OAB-q symptom bother (6–36) | 18.7 ± 6.7 | 2.2 ± 2.8 | <0.001 |
| OAB-q quality of life (13–78) | 30.2 ± 16.6 | 1.9 ± 2.9 | 0.003 |
| UDI-6 (urogenital distress inventory, 0–24) | 12.7 ± 5.6 | 0.9 ± 1.4 | <0.001 |
| IIQ-7 (incontinence impact questionnaire, 0–28) | 8.8 ± 8.2 | 0.1 ± 0.4 | 0.002 |
| Nocturia measures (over past 4 weeks) | |||
| Number of nighttime urinations (mean ± SD) | 2.6 ± 1.1 | 0.9 ± 0.7 | <0.001 |
| % with nocturia (≥ 1) | 94.0% | 76.7% | 0.023 |
| Odds ratio of having nocturia (≥ 1) in OAB compared to controls | OR = 6.87 95% CI [1.31,36.1] |
0.023 | |
| % with nocturia (≥ 2) | 86.0% | 13.3% | <0.001 |
| Odds ratio of having nocturia (≥ 2) in OAB compared to controls | OR = 41.9 95% CI [10.7, 164.8] |
<0.001 | |
| % with nighttime urinary incontinence | 13.7% | 3.3% | 0.247 |
| Odds ratio of having nighttime UI in OAB compared to controls | OR = 4.5 95% CI [0.5, 39.9] |
0.173 | |
Comparison of Sleep Disturbance and Fatigue Between OAB and Controls
Table 2 compares the sleep disturbance and fatigue between OAB patients (n=51) and controls (n=30). OAB patients reported significantly greater sleep disturbance compared to controls (PROMIS 8b T-scores: 54.3 ± 10.3 vs. 43.8 ± 9.2, p<0.001, after adjusting for age and sex). The difference in mean T-scores was more than 10 points, indicating large significant difference. Greater than two thirds of OAB patients (68.6%) had sleep disturbance worse than the population mean (T-score of 50), compared to 23.3% of controls (p<0.001, odds ratio = 8.5, 95% confidence interval 2.8 to 25.6). Responses to individual items on the sleep questionnaire also showed a significant difference in different aspects of sleep including quality, satisfaction, feeling refreshed, sleep continuity, and ability to fall or stay asleep (all p<0.05).
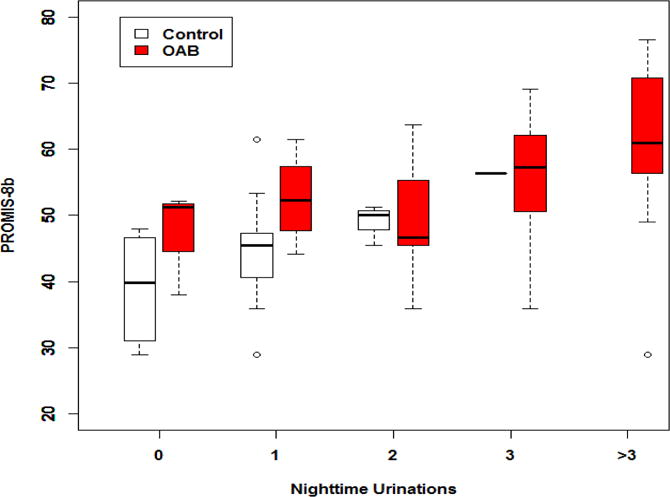
To examine the impact of nocturia on these differences, the comparisons were further adjusted for the number of nighttime urinations. The differences in sleep disturbance between OAB and controls became insignificant after adjusting for nocturia (see the last column of Table 2). Appendix 1 shows the relationships between sleep disturbance and number of nighttime urinations among OAB patients and controls (box plots).
With respect to fatigue, as shown in Table 2, OAB patients also reported significantly greater fatigue compared to controls (PROMIS 7a T-scores: 54.7 ± 9.6 vs. 46.0 ± 6.4, p<0.001, after adjusting for age and sex). More than half of OAB patients (62.7%) had fatigue worse than the population mean (T-score of 50), compared to 26.7% of controls (p=0.005, odds ratio = 4.3, 95% confidence interval 1.5 to 11.8). With the exception of a single item (“How often did you have enough energy to exercise strenuously?”), response to individual items on the fatigue questionnaire also showed a significant difference in different domains such as extreme exhaustion, feeling too tired to work, to think clearly, or to take a bath or shower (all p<0.05). To examine the impact of nocturia on these differences, the comparisons were further adjusted for the number of nighttime urinations. The differences in fatigue between OAB and controls remained statistically significant after adjusting for nocturia (see the last column of Table 2).
Correlation of sleep disturbance to UI/OAB symptoms, health-related quality of life, and psychosocial health among OAB patients
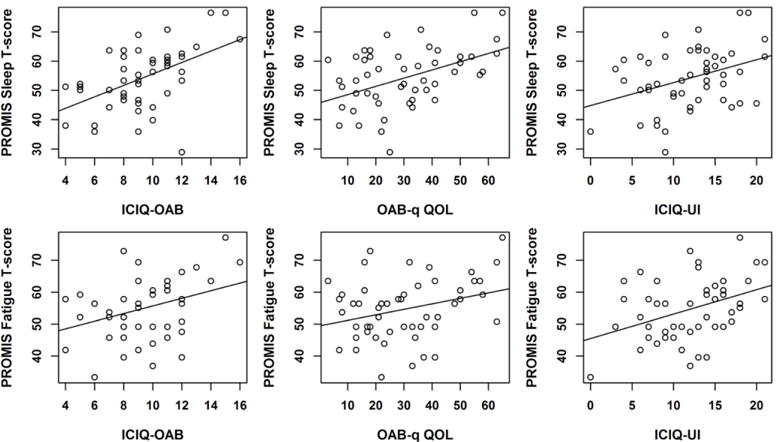
Next we examined the relationships between sleep disturbance/fatigue and UI/OAB among OAB patients Table 3 shows the Spearman’s correlations between sleep disturbance and UI/OAB among OAB patients. Since there was a positive correlation between sleep disturbance and the numbers of nocturia episodes (correlation coefficient = 0.425, p=0.002), the p-values were adjusted for the number of nighttime urinations in addition to age and sex in these analyses. As shown in Table 3, there were positive correlations between sleep disturbance (PROMIS 8b) and the severity of OAB symptoms (ICIQ-OAB, correlation coefficient = 0.45, p=0.012), poorer health-related quality of life (OAB-q QOL, correlation coefficient = 0.40, p=0.009), the severity of urinary incontinence symptoms (ICIQ-UI, correlation coefficient = 0.32, p=0.015), greater incontinence impact (IIQ-7, correlation coefficient = 0.30, p=0.003) and urinary bother (UDI-6, correlation coefficient = 0.25, p=0.027). Appendix 2 shows representative scatterplots (sleep T-scores vs. ICIQ-OAB, OAB-q QOL, ICIQ-UI). Sleep disturbance was also associated with higher depression scores (HADS-D, correlation coefficient = 0.37, p=0.001), higher anxiety scores (HADS-A, correlation coefficient = 0.38, p=0.007), and higher perceived stress levels (PSS, correlation coefficient = 0.36, p=0.004).
Table 3.
Correlation of sleep disturbance and fatigue to UI/OAB symptoms, health-related quality of life, and psychosocial health
| Correlation to sleep disturbance | Spearman’s correlation coefficient to sleep disturbance (PROMIS-8b) | p-value (adjusted for age and sex) | p-value (adjusted for age and sex AND NOCTURIAa) |
|---|---|---|---|
| ICIQ-UI (urinary incontinence, 0–21) | 0.32 | 0.008 | 0.015 |
| ICIQ-OAB (overactive bladder, 0–16) | 0.45 | <0.001 | 0.012 |
| OAB-q symptom bother (6–36) | 0.26 | 0.025 | 0.094 |
| OAB-q quality of life (13–78) | 0.40 | 0.001 | 0.009 |
| UDI-6 (urogenital distress inventory, 0–24) | 0.25 | 0.022 | 0.027 |
| IIQ-7 (incontinence impact questionnaire, 0–28) | 0.30 | 0.006 | 0.003 |
| HADS-D (depression, 0–21) | 0.37 | <0.001 | 0.001 |
| HADS-A (anxiety, 0–21) | 0.38 | 0.003 | 0.007 |
| PSS (stress level, 0–40) | 0.36 | 0.008 | 0.004 |
| Correlation to fatigue | Spearman’s correlation coefficient to fatigue (PROMIS-7a) | p-value (adjusted for age and sex) | p-value (adjusted for age and sex AND NOCTURIAa) |
| ICIQ-UI (urinary incontinence, 0–21) | 0.37 | 0.008 | 0.012 |
| ICIQ-OAB (overactive bladder, 0–16) | 0.26 | 0.015 | 0.003 |
| OAB-q symptom bother (6–36) | 0.20 | 0.052 | 0.075 |
| OAB-q quality of life (13–78) | 0.25 | 0.012 | 0.017 |
| UDI-6 (urogenital distress inventory, 0–24) | 0.22 | 0.052 | 0.062 |
| IIQ-7 (incontinence impact questionnaire, 0–28) | 0.36 | <0.001 | <0.001 |
| HADS-D (depression, 0–21) | 0.54 | <0.001 | <0.001 |
| HADS-A (anxiety, 0–21) | 0.50 | <0.001 | <0.001 |
| PSS (stress level, 0–40) | 0.45 | <0.001 | <0.001 |
Adjusted for response to number of nighttime urinations
Correlation of fatigue to UI/OAB symptoms, health-related quality of life, and psychosocial health among OAB patients
Table 3 also shows the Spearman’s correlations between fatigue symptoms and UI/OAB among OAB patients. There were positive correlations between fatigue (PROMIS 7a) and the severity of OAB symptoms (ICIQ-OAB, correlation coefficient = 0.26, p=0.003), poorer health-related quality of life (OAB-q QOL, correlation coefficient = 0.25, p=0.017), the severity of urinary incontinence symptoms (ICIQ-UI, correlation coefficient = 0.37, p=0.008), and greater incontinence impact (IIQ-7, correlation coefficient = 0.36, p<0.001). Appendix 2 shows representative scatterplots (fatigue T-scores vs. ICIQ-OAB, OAB-q QOL, ICIQ-UI). Fatigue was also associated with higher depression scores (HADS-D, correlation coefficient = 0.54, p<0.001), higher anxiety scores (HADS-A, correlation coefficient = 0.50, p<0.001), and higher perceived stress levels (PSS, correlation coefficient = 0.45, p<0.001).
Discussion
Previous studies have demonstrated a strong correlation between sleep disturbance and nocturia. However there are many potential etiologies of nocturia (e.g., global polyuria, nocturnal polyuria, obstructive sleep apnea), and not all causes are urologic in nature or related to OAB. The relationship between UI/OAB symptoms and sleep disturbance remains unclear. Surprisingly, to our knowledge none of the studies have recruited OAB patients and examined the relationship between UI/OAB and sleep/fatigue symptoms. Here we have recruited patients with a clinical diagnosis of OAB, and specifically examined the relationship between UI/OAB symptoms to sleep disturbance and fatigue using validated questionnaires from PROMIS. We show that: 1) sleep disturbance and fatigue are present in a substantial percentages of OAB patients; 2) the difference in sleep disturbance (but not fatigue) between OAB patients and controls is driven primarily by nocturia; 3) however, among OAB patients, sleep disturbance and fatigue are associated with more severe UI/OAB symptoms, worse health-related quality of life and poorer psychosocial health, even after adjusting for nocturia.
From a pathophysiology standpoint, precisely how more severe UI/OAB symptoms may impact sleep/fatigue or vice versa among OAB patients is not well explored. A substantial proportion of our OAB cohort had significant nocturia (94% had ≥1 nighttime urination, 86% had ≥2 nighttime urinations). The frequent nighttime awakenings to urinate may impact sleep quality and lead to fatigue. We speculate that sleep disturbance and fatigue have negative impact on physical and mental health, and may lead to issues such as anxiety, depression, and higher psychological stress, which may in turn impact UI/OAB symptoms. In this study, we observed positive correlations between poor sleep/fatigue and depression, anxiety, and higher stress levels. We have also previously shown that higher anxiety symptoms, depressive symptoms, and psychological stress levels are associated with more severe UI/OAB symptoms.18–20 Feeling sleep deprived and exhausted may disrupt daily functioning, decrease daytime alertness, impair coping skills and cognitive function, making it more difficult for UI/OAB patients to plan their daily routine to mitigate the effects of UI/OAB (e.g., bathroom mapping, pelvic floor exercises). Sleep disturbance has also been shown to be a significant risk factor for cardiovascular and metabolic disorders (e.g., obesity, diabetes),3 and the literature available to date appears to support a link between metabolic disorders and OAB.21
Another potential link between sleep disturbance/fatigue and OAB is found in obstructive sleep apnea (OSA). OSA has been linked to nocturia through atrial natrinuretic peptide (ANP) secretion in response to airway obstruction, leading to nocturnal polyuria.22 Correspondingly, treatment of OSA with continuous positive airway pressure (CPAP) has been shown to reduce nocturia.23 OSA has also been linked to other OAB symptoms such as urgency.4,5,24 This suggests that the urological effects of OSA may extend beyond nocturnal polyuria, involving such mechanisms as OSA-induced hypoxia.5 Recent rat studies showed that OSA-induced hypoxia was associated with bladder oxidative stress, altered cell signaling, and structural damage. These conditions led to increased urinary frequency and involuntary bladder contractions.25 Accordingly, CPAP therapy has been shown to improve OAB in patients with OSA.26
Figure 1 presents our conceptual framework linking sleep disturbance and fatigue to UI/OAB. Since this study was cross-sectional in nature, it would not be possible to examine causality or bi-directionality using our data. The bi-directional relationship between sleep disturbance and UI/OAB has not been studied extensively in the literature. In the longitudinal BACH (Boston Area Community Health) Survey, the presence of poor sleep quality at baseline was correlated with the development of new nocturia, storage and obstructive LUTS symptoms five years later.27 However, the presence of storage and obstructive LUTS symptoms at baseline did not predict the development of poor sleep five years later. Nocturia at baseline predicted the development of sleep issues at follow up. OAB therapy also has been shown to improve sleep quality, including anticholinergics such as oxybutynin,28 and sacral neuromodulation.29 While most OAB treatments presumably improve sleep by reducing nocturia, one study showed that the improvement of sleep with solifenacin treatment was significantly correlated with the decrease in urinary urgency, but not with nocturia.30
Figure 1.
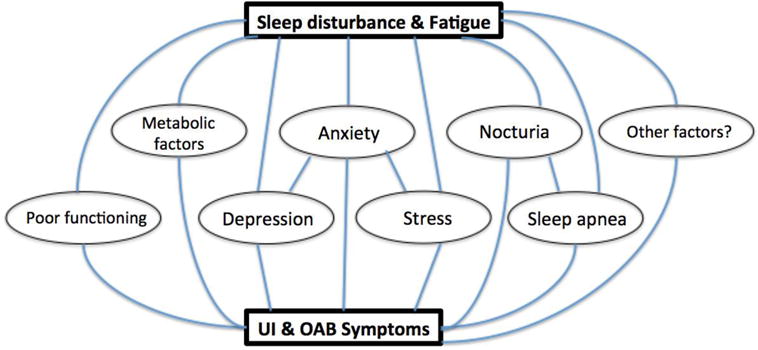
Conceptual framework linking sleep disturbance and fatigue to UI/OAB.
There are some potential weaknesses of this study: First, this was a single institutional study with small sample size. For example, we attempted to do correlation analyses on each of the questions on the various urinary questionnaires. However we were not able to identify a consistent relationship between any single urinary symptom and sleep disturbance (e.g., studying frequency and urgency symptoms separately in the correlation analysis, data not shown). We think the study lacked adequate power for these kinds of detailed analyses. Second, we were not able to completely untangle the impact of nocturia from OAB in the sleep and fatigue analyses. Our OAB cohort had severe nocturia (e.g., 86% had ≥2 nighttime urinations), since they were recruited from a specialized incontinence clinic in an academic medical center. We have not collected data regarding OSA and voiding diary. Recruiting an OAB patient cohort with less severe nocturia (e.g., recruitment from community urology or primary care office) would permit further dissection of the relationships between sleep/fatigue, UI/OAB, and nocturia.
Conclusion
Sleep disturbance and fatigue are present in substantial percentages of OAB patients. Among OAB patients, sleep disturbance and fatigue are associated with more severe UI/OAB symptoms, worse health-related quality of life and poorer psychosocial health.
Supplementary Material
Acknowledgments
The study was partly supported by the National Institutes of Health grants P20-DK-097798 and K08-DK-094964. We would like to thank all the subjects who participated in the study, Vivien Gardner for recruiting the subjects, Alexandra Kim for protocol development, and Alethea Paradis for data management (Division of Urologic Surgery). The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
Abbreviations
- LUTS
lower urinary tract symptoms
- OAB
overactive bladder
- OSA
obstructive sleep apnea
- PROMIS
Patient-Reported Outcomes Measurement Information System
- UI
urinary incontinence
Appendix 1
Impact of nocturia on sleep disturbance among OAB patients and controls (box plots). The bottom line is the minimum, the bottom of the box is the 1st quartile, the middle line inside the box is the median the top of the box is the 3rd quartile, and the top line is the maximum. Outliers are donated as circles (more than 1.5 interquartile range below the 1st quartile or above the 3rd quartile).
Appendix 2: Scatterplots of sleep disturbance and fatigue among OAB patients
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stewart WF, Van Rooyen JB, Cundiff GW, Abrams P, Herzog AR, Corey R, Hunt TL, Wein AJ. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20:327–36. doi: 10.1007/s00345-002-0301-4. [DOI] [PubMed] [Google Scholar]
- 2.Strine TW, Chapman DP. Associations of frequent sleep insufficiency with health-related quality of life and health behaviors. Sleep Med. 2005;6:23–7. doi: 10.1016/j.sleep.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Grandner MA, Jackson NJ, Pak VM, Gehrman PR. Sleep disturbance is associated with cardiovascular and metabolic disorders. J Sleep Res. 2012;21:427–33. doi: 10.1111/j.1365-2869.2011.00990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kemmer H, Mathes AM, Dilk O, Groschel A, Grass C, Stockle M. Obstructive sleep apnea syndrome is associated with overactive bladder and urgency incontinence in men. Sleep. 2009;32:271–5. doi: 10.1093/sleep/32.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsujimura A, Takao T, Miyagawa Y, Yamamoto K, Fukuhara S, Nakayama J, Kiuchi H, Suganuma N, Nakamura T, Kumano-Go T, et al. Urgency is an independent factor for sleep disturbance in men with obstructive sleep apnea. Urology. 2010;76:967–70. doi: 10.1016/j.urology.2010.01.070. [DOI] [PubMed] [Google Scholar]
- 6.Scovell JM, Pastuszak AW, Slawin J, Badal J, Link RE, Lipshultz LI. Impaired Sleep Quality is Associated With More Significant Lower Urinary Tract Symptoms in Male Shift Workers. Urology. 2017;99:197–202. doi: 10.1016/j.urology.2016.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coyne KS, Zhou Z, Bhattacharyya SK, Thompson CL, Dhawan R, Versi E. The prevalence of nocturia and its effect on health-related quality of life and sleep in a community sample in the USA. BJU Int. 2003;92:948–54. doi: 10.1111/j.1464-410x.2003.04527.x. [DOI] [PubMed] [Google Scholar]
- 8.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse D, Choi S, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63:1179–94. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, van Kerrebroeck P, Victor A, Wein A. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21:167–78. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- 10.Gormley EA, Lightner DJ, Burgio KL, Chai TC, Clemens JQ, Culkin DJ, Das AK, Foster HE, Jr, Scarpero HM, Tessier CD, et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline. J Urol. 2012;188:2455–63. doi: 10.1016/j.juro.2012.09.079. [DOI] [PubMed] [Google Scholar]
- 11.Avery K, Donovan J, Peters TJ, Shaw C, Gotoh M, Abrams P. ICIQ: a brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol Urodyn. 2004;23:322–30. doi: 10.1002/nau.20041. [DOI] [PubMed] [Google Scholar]
- 12.Jackson S, Donovan J, Brookes S, Eckford S, Swithinbank L, Abrams P. The Bristol Female Lower Urinary Tract Symptoms questionnaire: development and psychometric testing. Br J Urol. 1996;77:805–12. doi: 10.1046/j.1464-410x.1996.00186.x. [DOI] [PubMed] [Google Scholar]
- 13.Coyne K, Revicki D, Hunt T, Corey R, Stewart W, Bentkover J, Kurth H, Abrams P. Psychometric validation of an overactive bladder symptom and health-related quality of life questionnaire: the OAB-q. Qual Life Res. 2002;11:563–74. doi: 10.1023/a:1016370925601. [DOI] [PubMed] [Google Scholar]
- 14.Uebersax JS, Wyman JF, Shumaker SA, McClish DK, Fantl JA. Short forms to assess life quality and symptom distress for urinary incontinence in women: the Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Continence Program for Women Research Group. Neurourol Urodyn. 1995;14:131–9. doi: 10.1002/nau.1930140206. [DOI] [PubMed] [Google Scholar]
- 15.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 16.Cohen S, Williamson G. Perceived stress in a probability sample of the United States. In: Spacapan S, Oskamp S, editors. The social psychology of health: Claremont symposium on applied social psychology. Newbury Parl, CA: Sage; 1988. [Google Scholar]
- 17.Krishnan V, Collop NA. Gender differences in sleep disorders. Curr Opin Pulm Med. 2006;12:383–9. doi: 10.1097/01.mcp.0000245705.69440.6a. [DOI] [PubMed] [Google Scholar]
- 18.Lai H, Gardner V, Vetter J, Andriole GL. Correlation between psychological stress levels and the severity of overactive bladder symptoms. BMC Urol. 2015;15:14. doi: 10.1186/s12894-015-0009-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai HH, Shen B, Rawal A, Vetter J. The relationship between depression and overactive bladder/urinary incontinence symptoms in the clinical OAB population. BMC Urol. 2016;16:60. doi: 10.1186/s12894-016-0179-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai HH, Rawal A, Shen B, Vetter J. The Relationship Between Anxiety and Overactive Bladder or Urinary Incontinence Symptoms in the Clinical Population. Urology. 2016;98:50–57. doi: 10.1016/j.urology.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bunn F, Kirby M, Pinkney E, Cardozo L, Chapple C, Chester K, Cruz F, Haab F, Kelleher C, Milsom I, et al. Is there a link between overactive bladder and the metabolic syndrome in women? A systematic review of observational studies. Int J Clin Pract. 2015;69:199–217. doi: 10.1111/ijcp.12518. [DOI] [PubMed] [Google Scholar]
- 22.Umlauf MG, Chasens ER, Greevy RA, Arnold J, Burgio KL, Pillion DJ. Obstructive sleep apnea, nocturia and polyuria in older adults. Sleep. 2004;27:139–44. doi: 10.1093/sleep/27.1.139. [DOI] [PubMed] [Google Scholar]
- 23.Miyazato M, Tohyama K, Touyama M, Nakamura H, Oshiro T, Ueda S, Saito S. Effect of continuous positive airway pressure on nocturnal urine production in patients with obstructive sleep apnea syndrome. Neurourol Urodyn. 2017;36:376–379. doi: 10.1002/nau.22936. [DOI] [PubMed] [Google Scholar]
- 24.Lowenstein L, Kenton K, Brubaker L, Pillar G, Undevia N, Mueller ER, FitzGerald MP. The relationship between obstructive sleep apnea, nocturia, and daytime overactive bladder syndrome in women. Am J Obstet Gynecol. 2008;198:598 e1–5. doi: 10.1016/j.ajog.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 25.Witthaus MW, Nipa F, Yang JH, Li Y, Lerner LB, Azadzoi KM. Bladder oxidative stress in sleep apnea contributes to detrusor instability and nocturia. J Urol. 2015;193:1692–9. doi: 10.1016/j.juro.2014.11.055. [DOI] [PubMed] [Google Scholar]
- 26.Ipekci T, Cetintas G, Celik O, Ekin RG, Sarac S, Tunckiran A, Ilbey YO. Continuous positive airway pressure therapy is associated with improvement in overactive bladder symptoms in women with obstructive sleep apnea syndrome. Cent European J Urol. 2016;69:78–82. doi: 10.5173/ceju.2016.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Araujo AB, Yaggi HK, Yang M, McVary KT, Fang SC, Bliwise DL. Sleep related problems and urological symptoms: testing the hypothesis of bidirectionality in a longitudinal, population based study. J Urol. 2014;191:100–6. doi: 10.1016/j.juro.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yokoyama O, Yamaguchi A, Yoshida M, Yamanishi T, Ishizuka O, Seki N, Takahashi S, Yamaguchi O, Higo N, Minami H, et al. Once-daily oxybutynin patch improves nocturia and sleep quality in Japanese patients with overactive bladder: Post-hoc analysis of a phase III randomized clinical trial. Int J Urol. 2015;22:684–8. doi: 10.1111/iju.12755. [DOI] [PubMed] [Google Scholar]
- 29.Siegel S, Noblett K, Mangel J, Griebling TL, Sutherland SE, Bird ET, Comiter C, Culkin D, Bennett J, Zylstra S, et al. Results of a prospective, randomized, multicenter study evaluating sacral neuromodulation with InterStim therapy compared to standard medical therapy at 6-months in subjects with mild symptoms of overactive bladder. Neurourol Urodyn. 2015;34:224–30. doi: 10.1002/nau.22544. [DOI] [PubMed] [Google Scholar]
- 30.Takao T, Tsujimura A, Yamamoto K, Fukuhara S, Nakayama J, Matsuoka Y, Miyagawa Y, Nonomura N. Solifenacin may improve sleep quality in patients with overactive bladder and sleep disturbance. Urology. 2011;78:648–52. doi: 10.1016/j.urology.2011.04.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


