L-18 signaling through MyD88 was essential to induce IFN-γ and control type A F. tularensis infection.
Keywords: tularemia, TLR, caspase-1
Abstract
Francisella tularensis is a highly infectious intracellular bacterium that causes the potentially fatal disease tularemia. We used mice with conditional MyD88 deficiencies to investigate cellular and molecular mechanisms by which MyD88 restricts type A F. tularensis infection. F. tularensis–induced weight loss was predominately dependent on MyD88 signaling in nonhematopoietic cells. In contrast, MyD88 signaling in hematopoietic cells, but not in myeloid and dendritic cells, was essential for control of F. tularensis infection in tissue. Myeloid and dendritic cell MyD88 deficiency also did not markedly impair cytokine production during infection. Although the production of IL-12 or -18 was not significantly reduced in hematopoietic MyD88-deficient mice, IFN-γ production was abolished in these animals. In addition, neutralization studies revealed that control of F. tularensis infection mediated by hematopoietic MyD88 was entirely dependent on IFN-γ. Although IL-18 production was not significantly affected by MyD88 deficiency, IL-18 was essential for IFN-γ production and restricted bacterial replication in an IFN-γ–dependent manner. Caspase-1 was also found to be partially necessary for the production of IL-18 and IFN-γ and for control of F. tularensis replication. Our collective data show that the response of leukocytes to caspase-1–dependent IL-18 via MyD88 is critical, whereas MyD88 signaling in myeloid and dendritic cells is dispensable for IFN-γ–dependent control of type A F. tularensis infection.
Introduction
Francisella tularensis is a highly infectious, Gram-negative, facultative, intracellular bacterium that causes the zoonotic disease, tularemia. The type and severity of tularemia depend on the strain, dose, and route of infection. F. tularensis subspecies tularensis (type A) and holarctica (type B) cause most human cases, with the subspecies tularensis being the most virulent [1]. All forms of tularemia typically present with sudden onset of fever, headaches, chills, sore throat, coryza, and generalized body aches 3–5 d after exposure [2]. Pulmonary infection results in the most severe form of disease, and untreated pulmonary cases of tularemia caused by type A F. tularensis have mortality rates >30% [3]. Cutaneous or ulceroglandular tularemia is the most common form of human disease occurring in 75–85% of patients. A papule appears at the site of the cutaneous infection at about the time of generalized symptoms in ulceroglandular tularemia. The papule becomes a painful pustule and ulcerates within a few days of its appearance. Cutaneous tularemia is rarely fatal, but affected lymph nodes may rupture and become fluctuant. In addition, lymphadenopathy and ulcers may persist for months, even with appropriate antibiotic therapy [2, 4]. Data from experimental models suggest that a more robust immune response to cutaneous rather than pulmonary Francisella infection in part explain the differential host susceptibility to infection by these routes [5, 6]. Despite this differential susceptibility to infection, in mice it has been shown that the control of systemic, rather than pulmonary, bacterial burden is critical to surviving challenge with type A F. tularensis, regardless of the route of infection [7, 8].
A live vaccine strain (LVS) derived from the F. tularensis subspecies holarctica was developed over 50 yr ago; however, questions remain concerning its efficacy and residual virulence, and thus LVS is not licensed for human use [1]. LVS retains dose- and route-dependent virulence in mice [9]; however, it is not as virulent as WT A or B strains of F. tularensis. LVS and a related Francisella species, F. novicida, can cause disease in mice, but do not require BSL3 containment. These strains have been extensively studied as model intracellular pathogens [10, 11]. However, type A strains of F. tularensis possess immune evasion capabilities not observed in model Francisella strains [5, 12–14], and numerous vaccines and immunotherapeutics that confer potent protection against model strains of Francisella have demonstrated little or no efficacy against challenge with type A F. tularensis [5, 11, 15–17]. Therefore, results of studies investigating elicited and protective immune responses against model strains of Francisella are not always applicable to strains of Francisella that cause disease in humans.
MyD88 is an adaptor protein that relays TLR, IL-1R, and IL-18R signaling [18, 19]. MyD88−/− mice are highly susceptible to infection with LVS, F. novicida, and type A F. tularensis [20–22]. Both TLR and IL-1/IL-18 signaling appear to contribute to MyD88-dependent protection against LVS and F. novicida after s.c. or intradermal infection [22–25]. Although MyD88 has been shown to be necessary for protection and inflammatory cytokine production during pulmonary infection with type A F. tularensis, the mechanism underlying this protection remains undefined. However, it is known that TLR4 deficiency does not affect resistance to type A F. tularensis infection in vivo [26] and that type A F. tularensis can suppress TLR2- and -4-dependent immune responses [12, 27, 28]. In addition, TLR agonists confer more potent protection against model strains of Francisella than against type A F. tularensis [11, 15, 29]. These data suggest that TLR signaling plays a larger role in host protection against model Francisella strains than against the strains of F. tularensis that cause disease in humans. As the LD50 of type A F. tularensis infection in mice is <10 CFU by all routes tested [30], we focused on restriction of F. tularensis replication in tissues in this study as a correlate of protection. Using mice with cell-type–specific MyD88 deficiencies, we determined that MyD88 restricts type A F. tularensis infection by responding to IL-18 to induce IFN-γ.
MATERIALS AND METHODS
Bacterial strains, culture conditions, and mice
F. tularensis SchuS4 (acquired from the National Institutes of Health, Rocky Mountain Laboratories, Hamilton, MT, USA) was cultured in modified Mueller-Hinton (MMH) broth (0.025% ferric pyrophosphate, 2% IsoVitaleX, and 0.1% glucose) at 37°C with constant shaking overnight, aliquotted into 1 ml samples, frozen at −80°C, and thawed just before use [31]. Frozen stocks were titered by enumerating viable bacteria from serial dilutions plated on MMH agar (0.025% ferric pyrophosphate, 2% IsoVitaleX, 0.1% glucose, and 0.025% FBS) [32, 33]. The number of viable bacteria in frozen stock vials varied by <5% over a 12 mo period. These stocks were used to generate cultures for F. tularensis SchuS4 infections. All experiments with F. tularensis SchuS4 were performed in biosafety level 3 facilities at the University of Missouri.
Mice
Experiments were conducted in 6–12-wk-old age- and sex-matched mice on a C57BL/6 background. Breeding pairs of C57BL/6, MyD88−/−, Caspase-1/11−/−, Caspase-11−/−, and IL-18−/− mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). To generate mice with conditional MyD88 deficiencies, mice with floxed MyD88 alleles (Jackson stock 008888, MyD88fl/fl) and mice expressing Cre recombinase, under the control of the Vav1 (Jackson stock 008610), LysM (Jackson stock 004781), or CD11c promoters (Jackson stock 008068), were purchased from The Jackson Laboratory and intercrossed to create MyD88fl/fl mice expressing Cre recombinase under the control of these promoters (for reference see Table 1 and https://www.jax.org/research-and-faculty/tools/cre-repository). WT C57BL/6 and MyD88fl/fl mice displayed a similar phenotype after infection and were both used as control animals. After infection, all mice were maintained in individually ventilated cages under high-efficiency particulate air-filtered barrier conditions of 12 h of light and 12 h of darkness in animal BSL-3 facilities, and were provided with sterile food and water. All studies were conducted in accordance with University of Missouri Animal Care and Use Committee guidelines.
TABLE 1.
Promoters for generation of cell-type–specific MyD88-deficient mice
| Promoter | Specificity |
|---|---|
| Vav1a | Hematopoietic cells (and progenitors) |
| LyzM | Monocytes, mature macrophages, granulocytes |
| CD11c | 95% of CD11cHI and 50–80% of plasmacytoid dendritic cells. Low levels of expression reported in lymphocytes (<10%) and myeloid cells (<1%). |
Mouse infection
F. tularensis inocula were prepared by the dilution of frozen stock of known titer in PBS. For s.c. infections, mice were anesthetized with isoflurane gas, and a 200 µl volume of PBS containing ∼75 CFU of SchuS4 was then injected into the flank with a 27-gauge needle. For intratracheal infection, mice were anesthetized with isoflurane, and a 22-gauge blunted needle was passed through the oral pharynx into the trachea. A 50 μl volume of PBS containing 75 CFU of SchuS4 then was injected into the lung [5]. In some experiments IFN-γ was depleted in vivo via i.p. administration of 1.0 mg anti-IFN-γ mAb(clone XMG1.2; Bio X Cell, West Lebanon, NH, USA) one day prior to and 0.5 mg anti-IFN-γ three days after infection, whereas control mice received rat IgG. For NK cell depletion, mice received 0.5 mg i.p. of anti-NK1.1 (clone PK136, Bio X Cell) one day prior to, and two days after infection, with control animals receiving rat IgG. Mice receiving IFN-γ were treated daily, starting at the time of infection with 105 U of recombinant IFN-γ i.p. (Shenandoah Biotechnology, Warwick, RI, USA), whereas control animals received PBS, as described by others [34].
CFU and cytokine determination
In mice infected with type A F. tularensis, innate immune activation is undetectable until more than 48 h after infection [20]. Thus, in our study, all mice were euthanized 4 d after infection, which allowed us to measure both cytokines and CFU in the same animal. For CFU determination, mouse organs were removed, weighed, and homogenized in sterile PBS, and homogenates were serially diluted and plated in triplicate on MMH plates, which were then incubated at 37°C for 48 h, at which time CFU were enumerated. Splenic CFU data was calculated as Log10 CFU/spleen, and hepatic CFU data was calculated as Log10 CFU per gram of liver. For cytokine assays, a protease inhibitor cocktail (Thermo Fisher Scientific, Waltham, MA, USA) was added to spleen homogenates, which were then centrifuged for 10 min at 10,000 g and filter sterilized. Blood from the caudal vein was also collected at the time of necropsy. After the blood was allowed to clot for 30 min at room temperature, it was centrifuged for 10 min at 10,000 g. Serum was then collected and filter sterilized. Tissue and serum filtrates were stored at −70°C until analysis. All cytokines except for IFN-γ and IL-18 were measured with a Luminex MagPix instrument with Milliplex magnetic reagents, according to the manufacturer’s instructions (Millipore-Sigma, Billerica, MA, USA). Luminex data were analyzed with Milliplex Analyst Software (Millipore-Sigma). Splenic IFN-γ was measured via ELISA, and serum IL-18 was measured via Luminex, both with reagents from eBioscience (San Diego, CA, USA), according to the manufacturer’s instructions.
Flow cytometry
Spleens were homogenized in sterile PBS. Single-cell suspensions were strained through 80 μm mesh and washed with complete medium (CM; RPMI 1640, 0.1 HEPES, 1 mM sodium pyruvate, 1 mM nonessential amino acids, and 10% FBS). The cells were resuspended in RPMI 1640 medium with 0.1 mM HEPES, and RBCs were lysed. Spleen cells were then washed and resuspended in CM and cultured for 5 h at 37°C/5% CO2 with a cell-stimulation cocktail containing PMA, ionomycin, Brefeldin A, and monensin (eBioscience). Cells were then stained for surface markers with Abs (anti-NK1.1, clone PK136; anti-CD3, clone 17A2; anti-CD4, clone GK1.5; and anti-CD8, clone 2.43), fixed in paraformaldehyde, and permeabilized with 0.2% saponin before intracellular staining for IFN-γ (clone XMG1.2). Fluorescence was acquired on a CyAn ADP Analyzer (Beckman Coulter, Brea, CA, USA). FlowJo software (Ashland, OR, USA) software was used for analysis.
Statistical analysis
SigmaPlot 13.0 (Systat, San Jose, CA, USA) was used for all statistical comparisons. Significant differences between 2 groups were determined with a 2-tailed Student’s t test with significance set at P < 0.05. For comparison between 3 or more groups, analysis was by 1-way ANOVA followed by Tukey’s or Dunnett’s test as described in the figure legends, with significance set at P < 0.05.
RESULTS
Hematopoietic, but not myeloid or dendritic cell, MyD88 signaling is essential for restriction of virulent F. tularensis infection
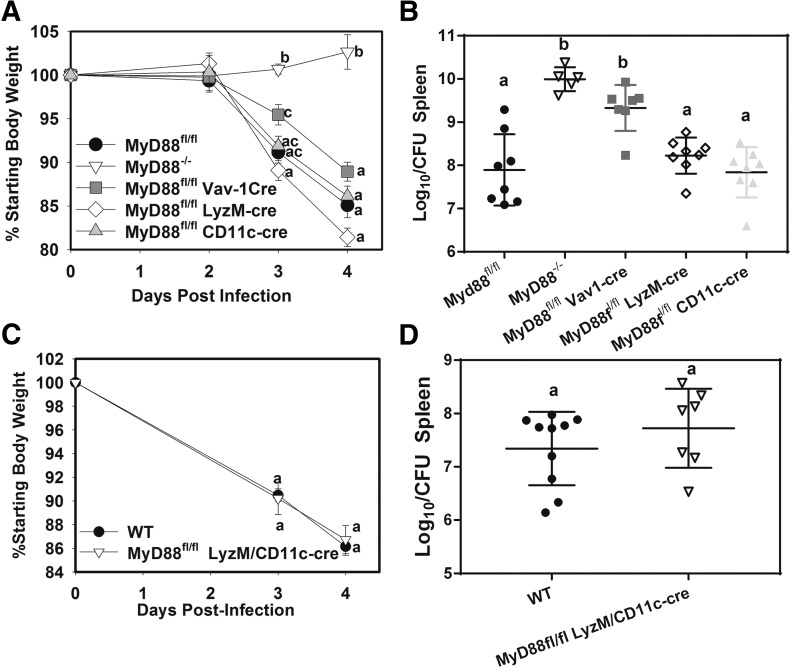
To determine what cell types mediate MyD88-dependent immunity against tularemia, we infected MyD88fl/fl (control), MyD88−/−, hematopoietic MyD88-deficient (MyD88fl/fl Vav1-cre), myeloid MyD88-deficient (MyD88fl/fl LyzM-cre) mice and mice lacking dendritic cell MyD88 (MyD88fl/fl CD11c-cre) with 75 CFU of F. tularensis SchuS4 s.c. (also see Table 1). Body weights (Fig. 1A) were recorded over time, and splenic bacterial burden was determined 4 d after infection (Fig. 1B). Similar to another report of pulmonary infection with type A F. tularensis [20], MyD88−/− mice were resistant to weight loss induced by s.c. Francisella infection (Fig. 1A). However, mice lacking myeloid or dendritic cell MyD88 were not resistant to Francisella-induced weight loss, whereas mice lacking hematopoietic MyD88 displayed slightly less weight loss than control mice at day 3, but not at day 4, after infection. MyD88−/− or hematopoietic MyD88-deficient mice displayed an elevated splenic F. tularensis burden, whereas myeloid and dendritic cell MyD88 was dispensable for control of infection. In other infection models, it has been reported that MyD88 signaling in myeloid or dendritic cells alone is sufficient to induce an immune response [35]. To determine whether a lack of myeloid MyD88 could be compensated for by the presence of dendritic cell MyD88 (and vice versa) we generated mice that lacked MyD88 in both myeloid and dendritic cells (MyD88fl/fl LyzM-cre/CD11c-cre). These mice and WT controls were infected s.c. with F. tularensis SchuS4, weight loss was measured (Fig. 1C), and splenic bacterial burden was assayed 4 d after infection (Fig. 1D). Similar to what we found in mice lacking MyD88 in myeloid or dendritic cells alone, mice lacking MyD88 in both myeloid and dendritic cells were not resistant to Francisella-induced weight loss and displayed a bacterial burden similar to that of control mice (Fig. 1C and D). Taken together, these results show that hematopoietic MyD88 restricts bacterial replication independent of MyD88 signaling in myeloid and dendritic cells, whereas nonhematopoietic MyD88 mediates weight loss during experimental tularemia.
Figure 1. Hematopoietic MyD88 restricts type A F. tularensis infection independent of MyD88 signaling in myeloid or dendritic cells.
(A–D) WT, MyD88fl/fl (control), MyD88−/−, and conditional MyD88-deficient mice (n = 5–10 per group) were infected s.c. with 75 CFU of F. tularensis SchuS4. (A and C) Weight loss was recorded and (B and D) splenic bacterial burden was measured 4 d after infection. Data are representative of at least 2 experiments per mouse strain. Error bars: body weights, sem; CFU, sd. Groups with the same letter are not significantly different from each other. P < 0.05, by ANOVA followed by Tukey’s post hoc test.
Both hematopoietic and nonhematopoietic MyD88 signaling contribute to inflammatory cytokine production during F. tularensis infection
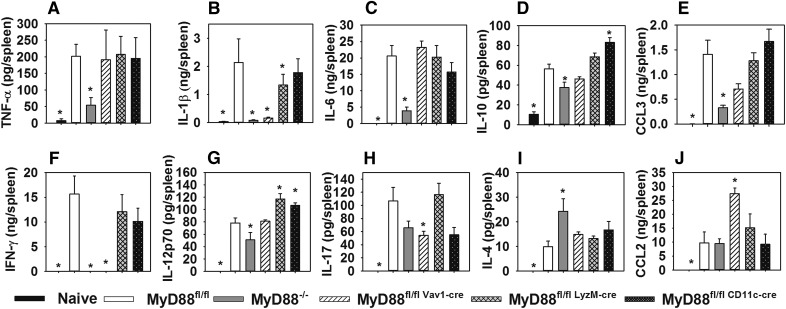
To investigate immunologic mechanisms underlying MyD88-dependent control of infection, we examined cytokine levels via Luminex and ELISA in spleens from control (MyD88fl/fl), MyD88−/−, and conditional MyD88-deficient mice 4 d after s.c. infection with F. tularensis SchuS4. Other than modestly reduced IL-1β level in mice lacking myeloid MyD88, myeloid or dendritic cell MyD88 deficiency did not significantly hinder the production of any cytokines relative to control animals (Fig. 2). In fact, IL-12 production was actually enhanced in mice lacking myeloid or dendritic cell MyD88 (Fig. 2G), and IL-10 level were elevated in the absence of dendritic cell MyD88 (Fig. 2D). In contrast, the production of IL-1β and IFN-γ were markedly reduced in MyD88−/− and hematopoietic MyD88-deficient mice (Fig. 2B and F). TNF-α, CCL3, and IL-6 production appeared to be mediated by nonhematopoietic MyD88, as the levels of these cytokines were markedly reduced in MyD88−/−, but not hematopoietic MyD88-deficient animals (Fig. 2A, C, and E). MyD88−/− mice also had a modest, but statistically significant, reduction in splenic IL-12 level (Fig. 2G), whereas IL-17 production was significantly reduced in mice lacking hematopoietic MyD88 (Fig. 2H). IL-4 was the only cytokine found to be elevated in MyD88−/− mice (Fig. 2I), whereas CCL-2 level was elevated in hematopoietic MyD88-deficient mice (Fig. 2J). In sum, both hematopoietic and nonhematopoietic MyD88 signaling contribute to cytokine production during tularemia, but these effects are largely independent of MyD88 signaling in myeloid and dendritic cells.
Figure 2. Both hematopoietic and nonhematopoietic MyD88 contribute to inflammatory cytokine protection during infection with type A F. tularensis.
MyD88fl/fl (control), MyD88−/−, and conditional MyD88-deficient mice (n = 5–8 per group) were infected s.c. with 75 CFU of F. tularensis SchuS4. Four days after infection, splenic cytokine levels of TNF-α (A), IL-1β (B), IL-6 (C), IL-10 (D), CCL-3 (E), IFN-γ (F), IL12p70 (G), IL17 (H), IL-4 (I), and CCL-2 were assayed via multiplex assay or ELISA. Cytokines were also measured in naïve WT spleens (n = 5). Error bars, sem. *P < 0.05 vs. infected MyD88fl/fl mice, by ANOVA followed by Dunnett’s test.
Hematopoietic MyD88 is essential for IFN-γ–dependent restriction of F. tularensis infection
We and others have found IFN-γ to be critical for protection against pulmonary infection with virulent type A or B F. tularensis [9, 32, 36]. Abolished IFN-γ production correlated with increased bacterial burden in both MyD88−/− and hematopoietic MyD88-deficient mice, and we postulated that IFN-γ is critical for MyD88-dependent restriction of F. tularensis replication. To address this hypothesis, WT and hematopoietic MyD88-deficient mice were treated with IFN-γ-neutralizing Abs or IgG as a control. Mice were then infected s.c. with F. tularensis SchuS4. Four days after infection, splenocytes from infected IgG-treated mice were investigated for IFN-γ production by flow cytometry (Fig. 3A–C) and splenic and hepatic bacterial burden was determined (Fig. 3D). We found that IFN-γ producing NK cells constituted a greater proportion of splenocytes than IFN-γ-producing CD4+ or CD8+ T cells (Fig. 3A) and that hematopoietic MyD88 was critical for NK cell IFN-γ production (Fig. 3B and C). We also found that, although IFN-γ neutralization or hematopoietic MyD88 deficiency alone both resulted in markedly enhanced splenic and hepatic bacterial burden, neutralization of IFN-γ from mice lacking hematopoietic MyD88 did not affect bacterial load in the spleen or liver (Fig. 3D). Thus, the effects of IFN-γ and hematopoietic MyD88 on control of F. tularensis infection are interdependent.
Figure 3. IFN-γ is essential for hematopoietic MyD88-dependent restriction of type A F. tularensis infection.
(A) WT mice (n = 7) were infected with 75 CFU of F. tularensis SchuS4 s.c. Four days after infection, the proportion of IFN-γ–producing NK cells (NK1.1+/CD3-/IFN-γ+), CD4 T cells (CD3+/CD4+/IFN-γ+), and CD8 T cells (CD3+/CD8+/IFN-γ+) among splenocytes were determined via flow cytometry. Error bars, sem. Means with the same letter are not significantly different from each other. P < 0.05, by ANOVA followed by Tukey’s post hoc test. (B and C) Flow cytometry was performed on splenocytes from WT and hematopoietic MyD88-deficient mice 4 d after s.c. infection. (B) Representative flow cytometry plots after gating on NK cells (NK1.1+/CD4−/CD8−/CD3−). (C) Mean percentage of IFN-γ producing cells amongst splenic NK cells. Error bars, sem. Data are representative of 2 independent experiments (n = 5–6 per group). Means with the same letter are not significantly different from each other. P < 0.05, by ANOVA followed by Tukey’s post hoc test. (D) WT and hematopoietic MyD88-deficient mice (MyD88fl/fl Vav1-Cre) were treated with IgG or anti-IFN-γ and infected s.c. with 75 CFU of F. tularensis SchuS4. Four days after infection, bacterial burden was measured in spleen and liver. Data are representative of 2 independent experiments. Error bars, sd (n = 5–6 per group). Groups with the same letter are not significantly different from each other. P < 0.05, by ANOVA followed by Tukey’s post hoc test.
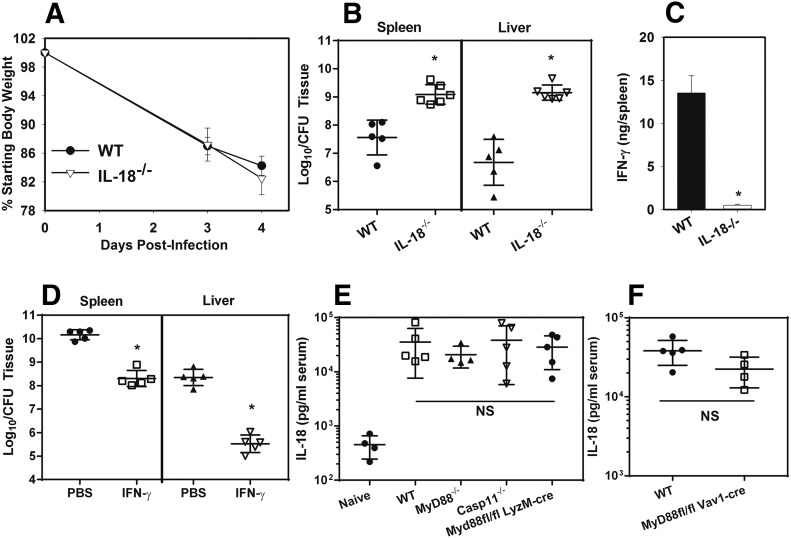
IL-18 is critical for IFN-γ production and restriction of type A F. tularensis infection
IL-12 level was not significantly diminished in mice lacking hematopoietic MyD88 (Fig. 2G), and so we questioned whether IL-18 is critical for MyD88/IFN-γ–dependent immunity against F. tularensis. MyD88 is necessary for IL-18 signaling and can also mediate IL-18 production [18, 37, 38]. Therefore, we infected WT and IL-18−/− mice s.c. with F. tularensis SchuS4. Weight loss was assessed over time, and 4 d after infection, bacterial burden in the spleen and liver were measured along with splenic IFN-γ production. We found that IL-18 was dispensable for F. tularensis-induced weight loss, but critical for controlling bacterial burden and IFN-γ production (Fig. 4A–C). To determine whether the effect of IL-18 is IFN-γ–dependent, we treated IL-18−/− mice with PBS or recombinant IFN-γ and measured tissue bacterial loads 4 d after s.c. infection with F. tularensis SchuS4. IFN-γ treatment significantly reduced bacterial load in the spleen and livers of IL-18−/− mice (Fig. 4D). We then questioned whether MyD88 is essential for IL-18 production by measuring the serum level of IL-18 4 d after s.c. infection with F. tularensis. We found that IL-18 production was not significantly impaired in MyD88−/− mice, Caspase-11−/− mice, or animals lacking myeloid or hematopoietic MyD88 (Fig. 4E and F). These results suggest that MyD88 is dispensable for IL-18 production, but critical for the response to IL-18 in inducing IFN-γ production and control of F. tularensis.
Figure 4. IL-18 is essential for IFN-γ production and protection against type A F. tularensis.
(A–C) WT and IL-18−/− mice were infected with 75 CFU of F. tularensis SchuS4 s.c. Weight loss was recorded (A), and, on day 4 after infection, bacterial burden (B) and splenic IFN-γ production (C) were assayed (n = 5–6 per group). Data are representative of 2 independent experiments. Error bars: body weights and IFN-γ, sem; CFU, sd. *P < 0.05 vs. WT mice as determined via t test. (D) IL-18−/− mice were infected with 75 CFU of F. tularensis SchuS4 s.c and treated daily with 105 U of IFN-γ or with PBS i.p. On day 4 after infection, the bacterial burden was assayed. Error bars, sd (n = 5 per group). *P < 0.05 vs. PBS-treated mice as determined via t test. (E and F) Serum IL-18 levels were measured in naïve WT animals and also in WT, MyD88−/−, conditional MyD88-deficient, and Caspase-11−/− mice 4 d after infection with 75 CFU of F. tularensis SchuS4 s.c. Error bar, sd (n = 4–5/group). No significant differences were detected among infected groups via ANOVA.
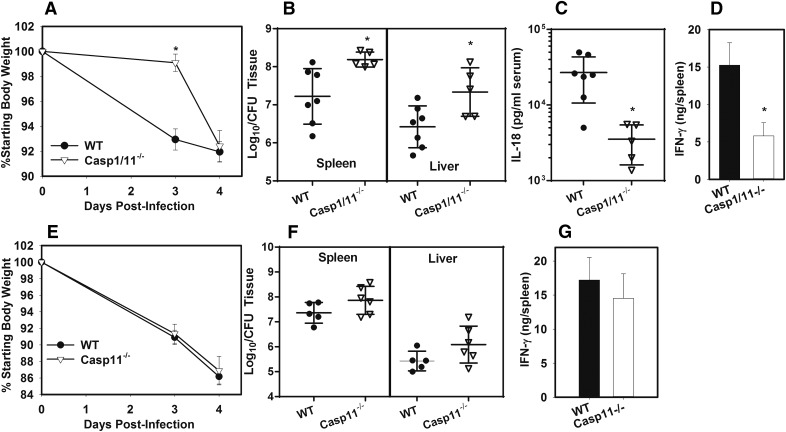
Caspase-1 is partially necessary for control of type A F. tularensis and production of IL-18 and IFN-γ
Processing of bioactive IL-18 most often requires caspase-1 [37]. F. novicida and F. tularensis LVSs have been widely used as tools to study caspase-1 activation [39–45], but reports are conflicting on whether type A F. tularensis activates caspase-1 in vitro [46–48], and the effect of caspase-1 on bacterial burden in vivo has not been studied during infection with virulent type A F. tularensis. Therefore, we investigated whether caspase-1 is necessary for IL-18/IFN–dependent control of type A F. tularensis. WT and caspase1/11−/− mice were infected s.c. with F. tularensis SchuS4. Body weights were measured (Fig. 5A) over time, and 4 d after infection, bacterial burden was assayed in spleen and liver (Fig. 5B), and serum IL-18 (Fig. 5C) and splenic IFN-γ production (Fig. 5D) were assayed. Caspase1/11−/− mice displayed a significant delay in weight loss relative to WT mice (Fig. 5A). Splenic and hepatic bacterial burden was nearly 10-fold higher in caspase1/11−/− mice (Fig. 5B), whereas serum IL-18 level was nearly 10-fold lower in caspase1/11−/− mice relative to control animals (Fig. 5C). IFN-γ production was also significantly reduced in caspase1/11−/− mice (Fig. 5D). To confirm the effect that we observed in caspase1/11−/− was related to caspase-1 and not caspase-11, we next infected WT and Caspase-11−/− mice s.c. with F. tularensis and measured weight loss (Fig. 5E), bacterial burden (Fig. 5F), and splenic IFN-γ production (Fig. 5G). We found that caspase-11 deficiency did not significantly affect weight loss, bacterial burden, or splenic IFN-γ production (Fig. 5E–G), which corresponds with our earlier data showing that caspase-11 did not affect IL-18 production (Fig. 4B). Thus, caspase-1, rather than caspase-11, is necessary for restriction of type A F. tularensis infection.
Figure 5. Caspase-1 is partially required for production of IL-18, IFN-γ, and restriction of type A F. tularensis.
WT and caspase-1/11−/− mice were infected with 75 CFU of F. tularensis SchuS4 s.c. Weight loss was recorded (A), and on day 4 after infection bacterial burden (B), serum IL-18 level (C), and splenic IFN-γ production (D) were assayed. Data are representative of 3 independent experiments. Error bars: body weights and IFN-γ, sem; IL-18 and CFU, sd (n = 5–7 per group) . *P < 0.05, vs. WT mice as determined via t test. (E–G) WT and Caspase-11−/− mice were infected with 75 CFU of F. tularensis SchuS4 s.c. Weight loss (E), bacterial burden (F), and splenic IFN-γ production (G) were measured on day 4 after infection. Data are representative of 2 independent experiments. Error bars: body weights and IFN-γ, sem; CFU, sd (n = 5–6/group). No significant differences were detected between the groups.
DISCUSSION
MyD88 and TLRs have been investigated in host protection to Francisella infection; however, most of this work was limited to model strains of Francisella that do not cause disease in humans. In addition, precise mechanisms by which MyD88 confers protection against model or human pathogenic strains of F. tularensis have not been defined. In this study, we investigated cell-type–specific mechanisms of MyD88-dependent restriction of type A F. tularensis infection. Myeloid and dendritic cells are major targets of F. tularensis infection [49] and are the cell types most studied when investigating TLR responses to infection. Surprisingly, we found that MyD88 signaling in LyzM-Cre expressing myeloid and CD11c-Cre-expressing dendritic cells was dispensable for control of type A F. tularensis infection in the spleen (Fig. 1). We also found that hematopoietic, but not myeloid or dendritic cell MyD88, significantly restricted F. tularensis infection in the lung after i.t infection (Supplemental Fig. S1A and B). In addition, we found that beyond a modestly reduced IL-1β level in mice lacking myeloid MyD88, myeloid or dendritic cell MyD88 deficiency did not diminish cytokine production during type A F. tularensis infection. During infection with LVS, IL-1β mediates protection by augmenting IgM production by B1a cells [24]. However, type A F. tularensis degrades host plasmin to evade Ab-mediated responses [14], and B1a cells actually enhance susceptibility to type A F. tularensis [50], which may explain why we found that reduced IL-1β production in mice lacking myeloid MyD88 did not affect susceptibility to infection. Taken together, the data indicate that MyD88-dependent TLR signaling in myeloid and dendritic cells is not essential for restriction of type A F. tularensis infection in vivo.
In contrast to our findings with myeloid and dendritic cell MyD88, mice lacking hematopoietic MyD88 were highly susceptible to infection. Bacterial load was not significantly different between MyD88−/− and hematopoietic MyD88-deficient mice, indicating that the protective effect of MyD88 against F. tularensis was mainly attributable to hematopoietic cells other than myeloid and dendritic cells. Despite a similar splenic bacterial load, MyD88−/− mice were resistant to F. tularensis-induced weight loss, whereas hematopoietic MyD88-deficient mice displayed weight loss similar to control animals. The liver is a major target of Francisella infection, and F. tularensis is known to invade hepatocytes [51, 52]. In other infection models, hepatocytes have been shown to produce inflammatory cytokines in an MyD88-dependent manner [53], and liver inflammation and damage correlate with weight loss in some infections [54–56]. However, when we measured the serum level of alanine transaminase (ALT) and aspartate transaminase (AST) as correlates of liver damage [57] in MyD88fl/fl (control), MyD88−/−, and hematopoietic MyD88-deficient mice 4 d after s.c. infection with F. tularensis SchuS4, no significant differences were observed between mouse strains (Fig. S1C and D). Thus, liver damage does not appear to correlate with MyD88-dependent weight loss during tularemia. In studies in which LPS administration was used as a model of sepsis, others have found that MyD88 signaling in myeloid and hematopoietic cells are critical in anorexia and weight loss [58]. These findings are in contrast to our results and suggest that the model system used may determine what cell types mediate MyD88-dependent weight loss. TNF-α, IL-1β, and IL-6 are the main cytokines associated with anorexia and cachexia during infection [59]. TNF-α and IL-6 production was markedly reduced in MyD88−/−, but not in hematopoietic MyD88-deficient mice, suggesting a possible role for these cytokines in F. tularensis-induced weight loss. The IL-1β level was reduced in the spleens of both MyD88−/− and hematopoietic MyD88-deficient mice suggesting that either IL-1β is dispensable in our model or that IL-1β production in another tissue is responsible for weight loss. Indeed, we found that IL-1R−/−/IL-18−/−, but not IL-18−/− mice, were highly resistant to F. tularensis-induced weight loss (Fig. 4A and unpublished observation), and that mice lacking caspase-1 (which is most often required for IL-1β processing) display a significant delay in weight loss (Fig. 5A), thus suggesting a role for IL-1 signaling in nonhematopoietic cells in F. tularensis–induced weight loss. Others have suggested that weight loss as an indicator of clinical disease during type A F. tularensis infection is associated with the inflammation, rather than direct effects of the bacteria [20]. Our results are in agreement with these findings, as we found that both MyD88−/− and caspase-1/11−/− mice display some degree of resistance to F. tularensis-induced weight loss, but have higher bacterial burden than control animals.
In our evaluation of splenic cytokine production, the most striking finding was the lack of IFN-γ production in mice lacking MyD88 or hematopoietic MyD88. IFN-γ is critical for protection against both model and human pathogenic strains of Francisella [9, 60], and we found that the effects of hematopoietic MyD88 and IFN-γ on bacterial burden were entirely interdependent. Intracellular cytokine staining revealed that NK cells were a major producer of IFN-γ in the spleen. We had demonstrated that human NK cells can be stimulated to produce IFN-γ to enhance clearance of F. tularensis from cocultured Mϕs [33]. However, others have shown that depletion of NK cells from mice infected i.t. with type A F. tularensis does not enhance susceptibility to infection [61]. We did find that depletion of NK cells from control MyD88fl/fl mice, but not from mice lacking hematopoietic MyD88, resulted in a modest, but statistically significant increase in bacterial loads in the livers of mice infected s.c. with F. tularensis (Fig. S1E). These data indicate that NK cells may contribute partially to MyD88-dependent protection. However, other cell types, such as T cells, presumably respond to IL-18 to produce IFN-γ and compensate for NK cell deficiency in restricting F. tularensis infection. We also found that hematopoietic MyD88 was needed for IL-17 production. However, we had already demonstrated that IL-17 is protective against LVS, but is dispensable for protection against type A F. tularensis [32], and therefore the role of IL-17 in MyD88-dependent protection was not further investigated.
IL-18 is essential for IFN-γ production and protection against LVS [24], and in the current study, we examined the role of IL-18 in MyD88-dependent immunity against type A F. tularensis. IL-18 production was not significantly impaired in MyD88−/− mice, indicating that MyD88-dependent TLRs are not essential for IL-18 production. Although IL-18 production was MyD88-independent, hematopoietic MyD88 was critical for the response to IL-18, to induce IFN-γ production and control of type A F. tularensis infection. In experiments in which both IL-18−/− mice and hematopoietic MyD88-deficient mice were infected concurrently, IL-18 deficiency alone resulted in an elevated F. tularensis burden that was similar to that seen in hematopoietic MyD88 deficiency (data not shown). These data further indicate that the protective role of MyD88 is largely independent of MyD88-dependent TLR signaling; however, additional experimentation would be necessary to definitively rule out a protective role for TLRs in type A F. tularensis infection. Although TLR2, which must have MyD88 for signaling [62], is critical for protection against LVS and F. novicida [22], lipids derived from type A F. tularensis, but not LVS or F. novicida, actually suppress host immune responses via TLR2 [12, 27]. In addition, MyD88-dependent TLR agonists confer much more efficacious protection against F. novicida and LVS than against type A F. tularensis [5]. Thus, TLR signaling may be more critical to the protective role of MyD88 in response to LVS or F. novicida, than to protection against type A F. tularensis. The MyD88-independent mechanism by which type A F. tularensis triggers IL-18 production in vivo is currently being investigated. In vitro, Propionibacterium LPS has been shown to induce TLR4/TRIF-dependent, but MyD88-independent, IL-18 production [63]. Francisella produces the heat shock protein DnaK, which can activate dendritic cells in vitro through TLR4/TRIF and could induce IL-18 [64]. In addition, type A Francisella infection also results in the release of damage-associated molecular patterns from host cells, including those known to signal through TRIF [65, 66], and thus could trigger IL-18 production. Alternatively, Francisella is known to be recognized by cytosolic DNA sensors [67, 68], which could also play a role in IL-18 production.
F. novicida and LVS have been used extensively as model organisms to study caspase-1 activation. However, various results have been published on the ability of type A F. tularensis to activate caspase-1 in vitro, which may be related to the particular cell types or conditions employed in these studies [46–48]. In this study, caspase-1 played a partial role in IL-18 production and control of type A F. tularensis in vivo. As mice lacking caspase-1, but not MyD88, had reduced IL-18 level, activation of caspase-1 in vivo by type A F. tularensis does not appear to need MyD88-dependent TLR signaling. Although caspase-1 deficiency led to a ∼10-fold increase in splenic and hepatic bacterial burden, IL-18 deficiency resulted in bacterial burden that increased ∼30- to ∼300-fold. IL-18 can be processed independent of caspase-1 in a manner dependent on the inflammasome adapter protein apoptosis-associated speck-like protein containing CARD (ASC) [69], which could be a mechanism that explains the higher bacterial load we see in mice lacking IL-18 relative to caspase-1–deficient animals during infection with type A F. tularensis. In contrast to our findings, others have found that mice lacking caspase-1 have a higher bacterial burden during F. novicida infection than do mice in which the functions of IL-1 and -18 were blocked [25]. In vivo, type A F. tularensis has been shown to induce caspase-3–dependent cell death [10], whereas LVS and F. novicida induce caspase-1–dependent cell death [10, 23, 25]. This finding could indicate that IL-1/IL-18-independent functions of caspase-1, such as pyroptotic cell death, mediate protection against F. novicida, whereas the role of caspase-1 in response to type A F. tularensis is limited to IL-18 production. We have found that caspase-3 is essential for live vaccine–mediated immunity against type A F. tularensis infection (unpublished observation), suggesting that both caspase-1 and -3 can play a role in protection against tularemia.
Caspase-11 is a noncanonical inflammasome that recognizes cytosolic LPS [70]. Francisella replicates in the host cell cytosol; however, others have found that F. novicida evades detection by caspase-11 because of the tetra-acylation of its lipid A structure [71]. Although there are variations in LPS biosynthesis and structure among Francisella strains, tetra-acylation is a common attribute of all Francisella [72]. In the current study, we did not detect a significant role for caspase-11 in control of type A F. tularensis infection, indicating that the ability to evade caspase-11 detection is conserved among Francisella species.
In sum, we found that hematopoietic MyD88 restricts type A F. tularensis infection by mediating IL-18–dependent IFN-γ production. MyD88-dependent TLR signaling does not appear to be essential for this effect, indicating that type A F. tularensis (or host cell damage caused by infection) is recognized to induce IL-18 in an MyD88-independent manner. Therefore, identification of MyD88-independent pathways that are able to induce IL-18 during type A F. tularensis infection could lead to immunotherapeutic or adjuvant strategies that will enhance the protective efficacy of vaccination or antibiotic therapy against tularemia.
AUTHORSHIP
J.A.S. conceived the project, designed and performed experiments, and wrote the paper. C.A.L. performed experiments and contributed to experimental design and writing of the manuscript.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported in part by the Rocky Mountain Regional Center for Excellence in Bioterrorism and Emerging Infectious Diseases [U.S. National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases Grant U54AI-65357] (to J.A.S.) and funding from the University of Missouri College of Veterinary Medicine and Research Board (Columbia, MO, USA).
Glossary
- ASC
apoptosis-associated speck-like protein containing CARD
- BSL3
biosafety level 3
- i.t.
intratracheal
- CARD
C-terminal caspase-recruitment domain
- CM
complete medium
- LVS
live vaccine strain
- MMH
modified Mueller Hinton
- WT
wild-type
- TRIF
toll-like/interleukin receptor interferon domain-containing adapter-inducing interferon-β
Footnotes
The online version of this paper, found at www.jleukbio.org, contains supplemental information.
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Oyston P. C. (2008) Francisella tularensis: unravelling the secrets of an intracellular pathogen. J. Med. Microbiol. 57, 921–930. [DOI] [PubMed] [Google Scholar]
- 2.Aquino L. L., Wu J. J. (2011) Cutaneous manifestations of category A bioweapons. J. Am. Acad. Dermatol. 65, 1213.e1–1213.e15. [DOI] [PubMed] [Google Scholar]
- 3.Allen L. A., McCaffrey R. L. (2007) To activate or not to activate: distinct strategies used by Helicobacter pylori and Francisella tularensis to modulate the NADPH oxidase and survive in human neutrophils. Immunol. Rev. 219, 103–117. [DOI] [PubMed] [Google Scholar]
- 4.Dennis D. T., Inglesby T. V., Henderson D. A., Bartlett J. G., Ascher M. S., Eitzen E., Fine A. D., Friedlander A. M., Hauer J., Layton M., Lillibridge S. R., McDade J. E., Osterholm M. T., O’Toole T., Parker G., Perl T. M., Russell P. K., Tonat K.; Working Group on Civilian Biodefense (2001) Tularemia as a biological weapon: medical and public health management. JAMA 285, 2763–2773. [DOI] [PubMed] [Google Scholar]
- 5.Skyberg J. A. (2013) Immunotherapy for tularemia. Virulence 4, 859–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woolard M. D., Hensley L. L., Kawula T. H., Frelinger J. A. (2008) Respiratory Francisella tularensis live vaccine strain infection induces Th17 cells and prostaglandin E2, which inhibits generation of gamma interferon-positive T cells. Infect. Immun. 76, 2651–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson R. V., Crane D. D., Bosio C. M. (2010) Long lived protection against pneumonic tularemia is correlated with cellular immunity in peripheral, not pulmonary, organs. Vaccine 28, 6562–6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conlan J. W., Chen W., Shen H., Webb A., KuoLee R. (2003) Experimental tularemia in mice challenged by aerosol or intradermally with virulent strains of Francisella tularensis: bacteriologic and histopathologic studies. Microb. Pathog. 34, 239–248. [DOI] [PubMed] [Google Scholar]
- 9.Crane D. D., Scott D. P., Bosio C. M. (2012) Generation of a convalescent model of virulent Francisella tularensis infection for assessment of host requirements for survival of tularemia. PLoS One 7, e33349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parmely M. J., Fischer J. L., Pinson D. M. (2009) Programmed cell death and the pathogenesis of tissue injury induced by type A Francisella tularensis. FEMS Microbiol. Lett. 301, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Troyer R. M., Propst K. L., Fairman J., Bosio C. M., Dow S. W. (2009) Mucosal immunotherapy for protection from pneumonic infection with Francisella tularensis. Vaccine 27, 4424–4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ireland R., Wang R., Alinger J. B., Small P., Bosio C. M. (2013) Francisella tularensis SchuS4 and SchuS4 lipids inhibit IL-12p40 in primary human dendritic cells by inhibition of IRF1 and IRF8. J. Immunol. 191, 1276–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bosio C. M. (2011) The subversion of the immune system by Francisella tularensis. Front. Microbiol. 2, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crane D. D., Warner S. L., Bosio C. M. (2009) A novel role for plasmin-mediated degradation of opsonizing antibody in the evasion of host immunity by virulent, but not attenuated, Francisella tularensis. J. Immunol. 183, 4593–4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rozak D. A., Gelhaus H. C., Smith M., Zadeh M., Huzella L., Waag D., Adamovicz J. J. (2010) CpG oligodeoxyribonucleotides protect mice from Burkholderia pseudomallei but not Francisella tularensis Schu S4 aerosols. J. Immune Based Ther. Vaccines 8, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rotem S., Cohen O., Bar-Haim E., Bar-On L., Ehrlich S., Shafferman A. (2014) Protective immunity against lethal F. tularensis holarctica LVS provided by vaccination with selected novel CD8+ T cell epitopes. PLoS One 9, e85215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skyberg J. A. (2014) Immunopotentiation for bacterial biodefense. Curr. Top. Med. Chem. 14, 2115–2126. [DOI] [PubMed] [Google Scholar]
- 18.Adachi O., Kawai T., Takeda K., Matsumoto M., Tsutsui H., Sakagami M., Nakanishi K., Akira S. (1998) Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9, 143–150. [DOI] [PubMed] [Google Scholar]
- 19.Lacey C. A., Mitchell W. J., Brown C. R., Skyberg J. A. (2017) Temporal role for MyD88 in a model of Brucella-induced arthritis and musculoskeletal inflammation. Infect. Immun. 85, e00961–e009616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russo B. C., Brown M. J., Nau G. J. (2013) MyD88-dependent signaling prolongs survival and reduces bacterial burden during pulmonary infection with virulent Francisella tularensis. Am. J. Pathol. 183, 1223–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collazo C. M., Sher A., Meierovics A. I., Elkins K. L. (2006) Myeloid differentiation factor-88 (MyD88) is essential for control of primary in vivo Francisella tularensis LVS infection, but not for control of intra-macrophage bacterial replication. Microbes Infect. 8, 779–790. [DOI] [PubMed] [Google Scholar]
- 22.Abplanalp A. L., Morris I. R., Parida B. K., Teale J. M., Berton M. T. (2009) TLR-dependent control of Francisella tularensis infection and host inflammatory responses. PLoS One 4, e7920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones C. L., Weiss D. S. (2011) TLR2 signaling contributes to rapid inflammasome activation during F. novicida infection. PLoS One 6, e20609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.del Barrio L., Sahoo M., Lantier L., Reynolds J. M., Ceballos-Olvera I., Re F. (2015) Production of anti-LPS IgM by B1a B cells depends on IL-1β and is protective against lung infection with Francisella tularensis LVS. PLoS Pathog. 11, e1004706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mariathasan S., Weiss D. S., Dixit V. M., Monack D. M. (2005) Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J. Exp. Med. 202, 1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen W., KuoLee R., Shen H., Bùsa M., Conlan J. W. (2004) Toll-like receptor 4 (TLR4) does not confer a resistance advantage on mice against low-dose aerosol infection with virulent type A Francisella tularensis. Microb. Pathog. 37, 185–191. [DOI] [PubMed] [Google Scholar]
- 27.Crane D. D., Ireland R., Alinger J. B., Small P., Bosio C. M. (2013) Lipids derived from virulent Francisella tularensis broadly inhibit pulmonary inflammation via toll-like receptor 2 and peroxisome proliferator-activated receptor α. Clin. Vaccine Immunol. 20, 1531–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bosio C. M., Bielefeldt-Ohmann H., Belisle J. T. (2007) Active suppression of the pulmonary immune response by Francisella tularensis Schu4. J. Immunol. 178, 4538–4547. [DOI] [PubMed] [Google Scholar]
- 29.Cole L. E., Mann B. J., Shirey K. A., Richard K., Yang Y., Gearhart P. J., Chesko K. L., Viscardi R. M., Vogel S. N. (2011) Role of TLR signaling in Francisella tularensis-LPS-induced, antibody-mediated protection against Francisella tularensis challenge. J. Leukoc. Biol. 90, 787–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cowley S. C., Elkins K. L. (2011) Immunity to Francisella. Front. Microbiol. 2, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bosio C. M., Dow S. W. (2005) Francisella tularensis induces aberrant activation of pulmonary dendritic cells. J. Immunol. 175, 6792–6801. [DOI] [PubMed] [Google Scholar]
- 32.Skyberg J. A., Rollins M. F., Samuel J. W., Sutherland M. D., Belisle J. T., Pascual D. W. (2013) IL-17 protects against the Francisella tularensis live vaccine strain, but not against a virulent F. tularensis type A strain. Infect. Immun. 81, 3099–3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skyberg J. A., Rollins M. F., Holderness J. S., Marlenee N. L., Schepetkin I. A., Goodyear A., Dow S. W., Jutila M. A., Pascual D. W. (2012) Nasal Acai polysaccharides potentiate innate immunity to protect against pulmonary Francisella tularensis and Burkholderia pseudomallei infections. PLoS Pathog. 8, e1002587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goodyear A., Troyer R., Bielefeldt-Ohmann H., Dow S. (2012) MyD88-dependent recruitment of monocytes and dendritic cells required for protection from pulmonary Burkholderia mallei infection. Infect. Immun. 80, 110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berod L., Stüve P., Swallow M., Arnold-Schrauf C., Kruse F., Gentilini M. V., Freitag J., Holzmann B., Sparwasser T. (2014) MyD88 signalling in myeloid cells is sufficient to prevent chronic mycobacterial infection. Eur. J. Immunol. 44, 1399–1409. [DOI] [PubMed] [Google Scholar]
- 36.Chen W., KuoLee R., Shen H., Conlan J. W. (2004) Susceptibility of immunodeficient mice to aerosol and systemic infection with virulent strains of Francisella tularensis. Microb. Pathog. 36, 311–318. [DOI] [PubMed] [Google Scholar]
- 37.Miao E. A., Andersen-Nissen E., Warren S. E., Aderem A. (2007) TLR5 and Ipaf: dual sensors of bacterial flagellin in the innate immune system. Semin. Immunopathol. 29, 275–288. [DOI] [PubMed] [Google Scholar]
- 38.Seki E., Tsutsui H., Nakano H., Tsuji N., Hoshino K., Adachi O., Adachi K., Futatsugi S., Kuida K., Takeuchi O., Okamura H., Fujimoto J., Akira S., Nakanishi K. (2001) Lipopolysaccharide-induced IL-18 secretion from murine Kupffer cells independently of myeloid differentiation factor 88 that is critically involved in induction of production of IL-12 and IL-1beta. J. Immunol. 166, 2651–2657. [DOI] [PubMed] [Google Scholar]
- 39.Meunier E., Wallet P., Dreier R. F., Costanzo S., Anton L., Rühl S., Dussurgey S., Dick M. S., Kistner A., Rigard M., Degrandi D., Pfeffer K., Yamamoto M., Henry T., Broz P. (2015) Guanylate-binding proteins promote activation of the AIM2 inflammasome during infection with Francisella novicida. Nat. Immunol. 16, 476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ulland T. K., Buchan B. W., Ketterer M. R., Fernandes-Alnemri T., Meyerholz D. K., Apicella M. A., Alnemri E. S., Jones B. D., Nauseef W. M., Sutterwala F. S. (2010) Cutting edge: mutation of Francisella tularensis mviN leads to increased macrophage absent in melanoma 2 inflammasome activation and a loss of virulence. J. Immunol. 185, 2670–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fernandes-Alnemri T., Yu J. W., Juliana C., Solorzano L., Kang S., Wu J., Datta P., McCormick M., Huang L., McDermott E., Eisenlohr L., Landel C. P., Alnemri E. S. (2010) The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat. Immunol. 11, 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones J. W., Kayagaki N., Broz P., Henry T., Newton K., O’Rourke K., Chan S., Dong J., Qu Y., Roose-Girma M., Dixit V. M., Monack D. M. (2010) Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc. Natl. Acad. Sci. USA 107, 9771–9776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rathinam V. A., Jiang Z., Waggoner S. N., Sharma S., Cole L. E., Waggoner L., Vanaja S. K., Monks B. G., Ganesan S., Latz E., Hornung V., Vogel S. N., Szomolanyi-Tsuda E., Fitzgerald K. A. (2010) The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 11, 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Henry T., Brotcke A., Weiss D. S., Thompson L. J., Monack D. M. (2007) Type I interferon signaling is required for activation of the inflammasome during Francisella infection. J. Exp. Med. 204, 987–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weiss D. S., Henry T., Monack D. M. (2007) Francisella tularensis: activation of the inflammasome. Ann. N. Y. Acad. Sci. 1105, 219–237. [DOI] [PubMed] [Google Scholar]
- 46.Bauler T. J., Chase J. C., Bosio C. M. (2011) IFN-β mediates suppression of IL-12p40 in human dendritic cells following infection with virulent Francisella tularensis. J. Immunol. 187, 1845–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wickstrum J. R., Bokhari S. M., Fischer J. L., Pinson D. M., Yeh H. W., Horvat R. T., Parmely M. J. (2009) Francisella tularensis induces extensive caspase-3 activation and apoptotic cell death in the tissues of infected mice. Infect. Immun. 77, 4827–4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Periasamy S., Le H. T., Duffy E. B., Chin H., Harton J. A. (2016) Inflammasome-independent NLRP3 restriction of a protective early neutrophil response to pulmonary tularemia. PLoS Pathog. 12, e1006059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hall J. D., Woolard M. D., Gunn B. M., Craven R. R., Taft-Benz S., Frelinger J. A., Kawula T. H. (2008) Infected-host-cell repertoire and cellular response in the lung following inhalation of Francisella tularensis Schu S4, LVS, or U112. Infect. Immun. 76, 5843–5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crane D. D., Griffin A. J., Wehrly T. D., Bosio C. M. (2013) B1a cells enhance susceptibility to infection with virulent Francisella tularensis via modulation of NK/NKT cell responses. J. Immunol. 190, 2756–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Law H. T., Lin A. E., Kim Y., Quach B., Nano F. E., Guttman J. A. (2011) Francisella tularensis uses cholesterol and clathrin-based endocytic mechanisms to invade hepatocytes. Sci. Rep. 1, 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Conlan J. W., North R. J. (1992) Early pathogenesis of infection in the liver with the facultative intracellular bacteria Listeria monocytogenes, Francisella tularensis, and Salmonella typhimurium involves lysis of infected hepatocytes by leukocytes. Infect. Immun. 60, 5164–5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee J., Tian Y., Chan S. T., Kim J. Y., Cho C., Ou J. H. (2015) TNF-α induced by hepatitis C virus via TLR7 and TLR8 in hepatocytes supports interferon signaling via an autocrine mechanism. PLoS Pathog. 11, e1004937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ran L., Yu Q., Zhang S., Xiong F., Cheng J., Yang P., Xu J. F., Nie H., Zhong Q., Yang X., Yang F., Gong Q., Kuczma M., Kraj P., Gu W., Ren B. X., Wang C. Y. (2015) Cx3cr1 deficiency in mice attenuates hepatic granuloma formation during acute schistosomiasis by enhancing the M2-type polarization of macrophages. Dis. Model. Mech. 8, 691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rani R., Jordan M. B., Divanovic S., Herbert D. R. (2012) IFN-γ-driven IDO production from macrophages protects IL-4Rα-deficient mice against lethality during Schistosoma mansoni infection. Am. J. Pathol. 180, 2001–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hölscher C., Mohrs M., Dai W. J., Köhler G., Ryffel B., Schaub G. A., Mossmann H., Brombacher F. (2000) Tumor necrosis factor alpha-mediated toxic shock in Trypanosoma cruzi-infected interleukin 10-deficient mice. Infect. Immun. 68, 4075–4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu H. Z., Wang W., Feng D. M., Sai Y., Xue J. L. (2006) Conditional gene modification in mouse liver using hydrodynamic delivery of plasmid DNA encoding Cre recombinase. FEBS Lett. 580, 4346–4352. [DOI] [PubMed] [Google Scholar]
- 58.Ruud J., Wilhelms D. B., Nilsson A., Eskilsson A., Tang Y. J., Ströhle P., Caesar R., Schwaninger M., Wunderlich T., Bäckhed F., Engblom D., Blomqvist A. (2013) Inflammation- and tumor-induced anorexia and weight loss require MyD88 in hematopoietic/myeloid cells but not in brain endothelial or neural cells. FASEB J. 27, 1973–1980. [DOI] [PubMed] [Google Scholar]
- 59.Rao S., Schieber A. M., O’Connor C. P., Leblanc M., Michel D., Ayres J. S. (2017) Pathogen-mediated inhibition of anorexia promotes host survival and transmission. Cell 168, 503–516.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Elkins K. L., Rhinehart-Jones T. R., Culkin S. J., Yee D., Winegar R. K. (1996) Minimal requirements for murine resistance to infection with Francisella tularensis LVS. Infect. Immun. 64, 3288–3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schmitt D. M., O’Dee D. M., Brown M. J., Horzempa J., Russo B. C., Morel P. A., Nau G. J. (2013) Role of NK cells in host defense against pulmonary type A Francisella tularensis infection. Microbes Infect. 15, 201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Underhill D. M., Ozinsky A., Smith K. D., Aderem A. (1999) Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc. Natl. Acad. Sci. USA 96, 14459–14463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Imamura M., Tsutsui H., Yasuda K., Uchiyama R., Yumikura-Futatsugi S., Mitani K., Hayashi S., Akira S., Taniguchi S., Van Rooijen N., Tschopp J., Yamamoto T., Fujimoto J., Nakanishi K. (2009) Contribution of TIR domain-containing adapter inducing IFN-beta-mediated IL-18 release to LPS-induced liver injury in mice. J. Hepatol. 51, 333–341. [DOI] [PubMed] [Google Scholar]
- 64.Ashtekar A. R., Zhang P., Katz J., Deivanayagam C. C., Rallabhandi P., Vogel S. N., Michalek S. M. (2008) TLR4-mediated activation of dendritic cells by the heat shock protein DnaK from Francisella tularensis. J. Leukoc. Biol. 84, 1434–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sharma J., Mares C. A., Li Q., Morris E. G., Teale J. M. (2011) Features of sepsis caused by pulmonary infection with Francisella tularensis Type A strain. Microb. Pathog. 51, 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tsai S. Y., Segovia J. A., Chang T. H., Morris I. R., Berton M. T., Tessier P. A., Tardif M. R., Cesaro A., Bose S. (2014) DAMP molecule S100A9 acts as a molecular pattern to enhance inflammation during influenza A virus infection: role of DDX21-TRIF-TLR4-MyD88 pathway. PLoS Pathog. 10, e1003848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Storek K. M., Gertsvolf N. A., Ohlson M. B., Monack D. M. (2015) cGAS and Ifi204 cooperate to produce type I IFNs in response to Francisella infection. J. Immunol. 194, 3236–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Man S. M., Karki R., Malireddi R. K., Neale G., Vogel P., Yamamoto M., Lamkanfi M., Kanneganti T. D. (2015) The transcription factor IRF1 and guanylate-binding proteins target activation of the AIM2 inflammasome by Francisella infection. Nat. Immunol. 16, 467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pierini R., Perret M., Djebali S., Juruj C., Michallet M. C., Förster I., Marvel J., Walzer T., Henry T. (2013) ASC controls IFN-γ levels in an IL-18-dependent manner in caspase-1-deficient mice infected with Francisella novicida. J. Immunol. 191, 3847–3857. [DOI] [PubMed] [Google Scholar]
- 70.Aachoui Y., Leaf I. A., Hagar J. A., Fontana M. F., Campos C. G., Zak D. E., Tan M. H., Cotter P. A., Vance R. E., Aderem A., Miao E. A. (2013) Caspase-11 protects against bacteria that escape the vacuole. Science 339, 975–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hagar J. A., Powell D. A., Aachoui Y., Ernst R. K., Miao E. A. (2013) Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science 341, 1250–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Okan N. A., Kasper D. L. (2013) The atypical lipopolysaccharide of Francisella. Carbohydr. Res. 378, 79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.