Colonic CD11c+ antigen-presenting cells imprint CD8 T cell homing back to the colon, and not the small intestine, dependent on differential retinoic-acid levels inducing different homing receptors.
Keywords: gut, CCR9, alpha4beta7, RALDH
Abstract
Mechanisms that imprint T cell homing to the small intestine have been well studied; however, those for homing to the colon are poorly understood. Recently, we found that these are distinct subcompartments of the gut mucosal immune system, which implies differential homing. Here, we show that colonic CD11c+ APCs imprint CD8+ T cell preferential homing to the colon, in contrast to those from the small intestine that imprint CD8+ T cell homing to the small intestine, and that the differences are related to the variable ability of APCs to induce α4β7-integrin and CCR9 expression on T cells. Colon APCs also expressed lower levels of retinoic acid–producing enzymes that are known to control the mucosal homing of T cells. These findings are the first to our knowledge to directly demonstrate that colon APCs imprint T cells to selectively home to the large bowel, which is critical for the design of successful T cell–based therapies and vaccines, such as colon cancer immunotherapy and HIV vaccines.
Introduction
Selective homing to tissue-specific sites has already been demonstrated for many locations [1–6]. As the majority of T cell responses are induced in draining (secondary) lymphoid structures that attract migratory DCs from peripheral tissues, it is vital for DCs—while presenting foreign antigens to the T cell—to also deliver the information about the location of the pathogen, so that when the T cell recirculates via the thoracic duct back to the blood stream, it can find the intruder more easily. T cell migration in steady-state conditions to small and large intestine depends on the same types of cell integrins (i.e., on α4β7 and αEβ7); however, the requirement for chemokines in homing to the small and large bowel is differential: CCR9/CCL25 chemokine is needed to mediate the migration of T cells—both CD4+ and CD8+—to the small intestine, but not to the colon, and GPR15—for Foxp3+ T regulatory cells [7] in mice and some other CD4+ T cells in humans [8]—to the large intestine. Chemokines that control the migration of CD8 T cells to the colon are unknown, as is the mechanism of induction of their receptors. Previous studies have shown that the homing of T cells to the small intestine is imprinted by Peyer’s patch DCs [1–3]. This homing was shown to be dependent on α4β7-integrin and CCR9 chemokine receptor expression on the T cells, induced by high levels of retinoic acid expressed by Peyer’s patch DCs [9, 10]. Although we and others have demonstrated that rectal or colonic immunization induces differential homing to large and small bowel tissues, whether colonic APCs are able to induce selective T cell migration to the large bowel remain unanswered, and the mechanisms that control the induction of homing of CD8+ T cells to the large intestine or colorectal mucosa are not well understood.
Recently, using pH-sensitive nanoparticles to selectively deliver Ags to either small or large intestine, we found evidence that, surprisingly, effector and memory T cells do not easily intermix between these compartments, so that there are separate small or large intestinal responses [5]. Such selective responses imply different homing mechanisms, because if the homing receptors were identical, then the 2 subcompartments would always rapidly re-equilibrate, forming a single more homogeneous gut mucosal immune compartment.
Here, we show that CD8+ T cell homing to the large intestine is imprinted by colonic CD11c+ APCs: naive TCR transgenic CD8+ T cells stimulated in vitro with colon-derived Ag-pulsed CD11c+ APCs preferentially migrate to the large intestine, but not the small intestine, which is in contrast to T cells that are cocultured with small intestine–derived CD11c+ APCs that preferentially home to the small intestine, but not the large intestine. CD8 T cell homing to the large intestine depended on α4β7 integrin, but not CCR9, and correlated with the differential expression of retinoic acid by these APCs. Understanding these homing mechanisms may guide the development of strategies to induce selective T cell homing to particular compartments where T cells are necessary to protect against particular infections, such as HIV, the transmission of which is often via colorectal or genital mucosa.
MATERIALS AND METHODS
Mice
The following strains of mice were used in these studies: C57BL/6 (Charles River Laboratories, Wilmington, MA, USA), CCR9 KO and β7 KO on a C57BL/6 background (both from The Jackson laboratory, Bar Harbor, ME, USA), and OT-1 (Taconic Biosciences, Germantown, NY, USA). Mice were housed under specific pathogen–free and Helicobacter-free conditions, and experiments were performed under protocols that were approved by the National Cancer Institute Animal Care and Use Committee.
Cell transfer studies
Mice were either injected s.c. in the flank with 10e5 FLT3L-expressing B16 tumor cells (kindly provided by U. von Andrian, Harvard Medical School, Boston, MA, USA) or treated with FLT3L protein (a gift of Immunex, Seattle, WA, USA) to increase the number of DCs that were present in the tissues. CD11c+ cells were isolated from the spleen, small intestine LP, and colon LP by using magnetic bead separation. In brief, spleens were sterilely mashed in RPMI 1640 and passed through 100-µm strainers (Falcon, Corning, NY, USA), and RBCs were lysed with ACK lysing buffer (Lonza, Walkersville, MD, USA). Colon and small intestinal tissues were washed with PBS and digested for 30 min with RPMI 1640, 10% FBS, penicillin-streptomycin, collagenase, and DNAse I (Roche Diagnostics, Indianapolis, IN, USA). Digested tissues were passed through a cell strainer, washed in PBS, and resuspended in 40% Percoll. Cells were then spun at 800 g for 15 min to remove dead cells. Supernatant was removed and the pellet was resuspended in PBS BSA (1%) and sorted by using magnetic bead-labeled CD11c Ab (Miltenyi Biotec, Auburn, CA, USA). CD11c+ cells were then cocultured 1:1 with purified splenic CD8+ T cells from OT-1 mice for 3.5–4 d in the presence of SIINFEKL (OVA epitope) peptide (PolyPeptide Labs, San Diego, CA, USA) and IL-2 (2 µg/ml) according to the method by Mora et al. [2]. Stimulated T cells (10–20 million) were then CFSE labeled and transferred i.v. into each recipient mouse. In some experiments, CD8+ T cells (2–5 million cells) that were purified from the spleens of naive wild-type or KO mice were labeled with CFSE (CellTrace CFSE Cell Proliferation Kit Catalog; Thermo Fisher Scientific, Waltham, MA, USA) and injected i.v. into recipient C57BL/6 mice with or without treatment (as indicated) of the recipient with 7 µg mLT (R192G; a gift from John Clements, Tulane University, New Orleans, LA, USA) as a mucosal adjuvant. Twenty hours post-transfer, tissues were isolated from recipient mice and analyzed for the presence of CFSE-expressing CD8+ T cells by flow cytometry. Note that the small number of purified CD11c+ APCs that can be obtained from the colon or small intestine of mice limits the number of T cells that can be cocultured at an appropriate T cell:APC ratio (1:1, on the basis of the method by Mora et al [2]), and CFSE labeling results in additional losses and therefore limits the number of CFSE-labeled cultured T cells that can be prepared to transfer to recipient mice. Therefore, the number of recipient mice that can be studied in each experiment is limited. In other experiments, CD8+ T cells after coculture with tissue DCs were analyzed by flow cytometry for expression of integrin α4β7 and chemokine receptors CCR9 and CXCR3. RALDH levels that were present in CD11c+ APCs from different tissues were measured according to manufacturer instructions using ALDEFLUOR kit (StemCell Technologies, Vancouver, BC, Canada).
Flow cytometry
Flow cytometry was carried out on a BD LSR II with 4 lasers or a BD FACSCalibur with 2 lasers (BD, Brea, CA, USA) and analyzed with Flow Jo software (Treestar, Ashland, OR, USA). Fluorescent Abs, all from BioLegend (San Diego, CA, USA), were anti–CD3-Pacific Blue (clone 17A2), anti–CD8a-APC-Cy7 (clone 53-6.7), anti–CXCR3-APC (clone CXCR3-173), anti–CCR9-PE-Cy7 (clone CW-1.2), and anti–integrin β7-PerCPCy5.5 (clone FIB27). CFSE labeling was performed with CellTrace CFSE Cell Proliferation Kit Catalog (Thermo Fisher Scientific) according to manufacturer instructions.
Immunizations
C57BL/6 mice were immunized intrarectally by using a 22 gauge gavage needle or i.p. with the immunodominant SIINFEKL OVA peptide (25 µg; PolyPeptide Labs) and 7 µg mLT emulsified in DOTAP (Roche Diagnostics) adjuvant (10 µg), a cationic lipofection agent that has been demonstrated to protect peptides for intrarectal delivery and that promotes immunogenicity [11]. Ag-specific CD8+ T cells were detected by flow cytometry using APC-labeled SIINFEKL loaded H-2Kb tetramer (National Institutes of Health Tetramer Facility, Atlanta, GA, USA), viability dye, and Abs to CD8, CD3, CXCR3, CCR9, and α4β7.
Quantitative real-time PCR
RNA from cells was isolated by using RNeasy Micro Kit (Qiagen, Germantown, MD, USA). RT-PCR was performed by using Taqman reagents RALDH2 (Aldh1a2, Mm00501306_m1; ABI) and Gapdh (Mm99999915_g1; ABI) using TaqMan Fast Advanced Master Mix (ABI Waltham, MA, USA). Ct values were estimated by using default settings and ΔΔCt values were calculated as described elsewhere.
Statistics
Statistical significance was evaluated by paired or unpaired Student’s t test as appropriate in each case, or by ANOVA together with Dunnett’s Multiple Comparison Test, using Prism (GraphPad Software, La Jolla, CA, USA). A P value of < 0.05 was considered significant.
RESULTS
Colon CD11c+ APCs selectively imprint CD8+ T cell homing to the colonic mucosa
Effector memory T cells infiltrate many tissues in steady-state conditions, especially in mucosa-associated sites. Migration and the subsequent localization of the T cell to those sites depends on a unique combination of integrins and chemokine receptors expressed by a T cell. As it is thought that the induction of the immune response happens in the lymph node, although draining the tissue, but frequently not embedded within it, how T cells manage to acquire the information about where to migrate to during the activation is a long-standing question. Studies have suggested, at least in some cases, that APCs carry information with them to the lymph node that is later on passed on to T cells during the stimulation process. Tissue imprinting was demonstrated for small intestinal APCs [1–3]; however, as small and large intestine T cells seem to have different migratory affinities, the question remained: do colonic APCs have a similar capacity to induce the homing, but to the colon only?
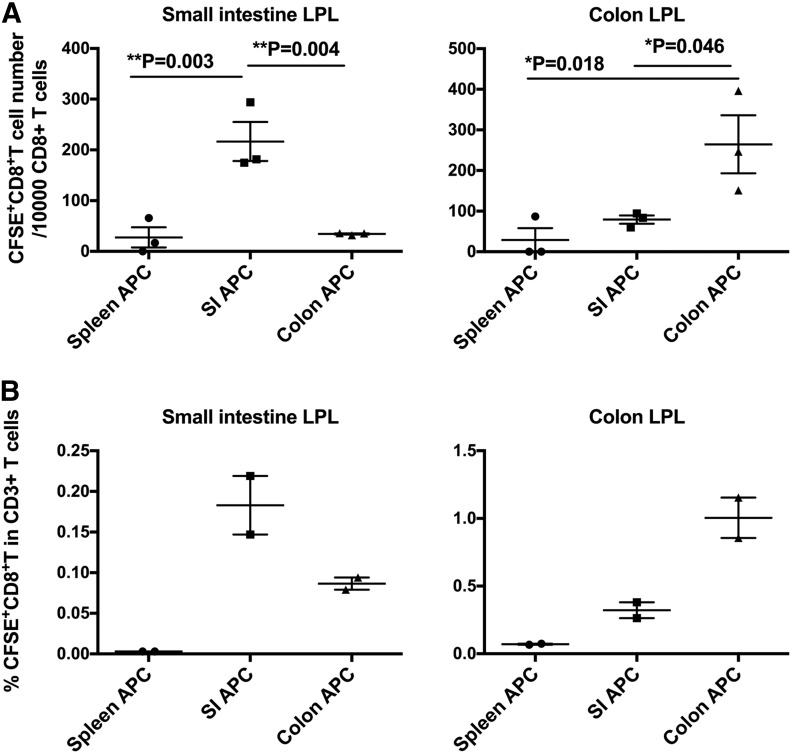
To answer this question, we adopted the approach of T cell imprinting that has been used in other studies [2] by incubating ova peptide–pulsed CD11c+ APCs that were isolated from the colon, small intestine, or spleen as a control with OVA peptide–specific OT-I TCR transgenic CD8+ T cells for 3 d. We then reisolated T cells from these cultures, labeled them with CFSE fluorescent dye, and injected them i.v. into naive recipient C57BL/6 mice. Twenty hours later, we isolated gut mucosal tissues from recipient mice, and quantified the number of labeled CD8+ CD3+ T cells that had homed to either the small or large intestinal LP (Fig. 1). As we gated on CD3+ cells (Supplemental Fig. 1), CFSE-labeled cells that were counted had to be T cells and not phagocytic cells that took up CFSE. In Fig. 1A (left), examining small intestinal LP confirms the previous finding [1–3] that CD11c+ APCs from the small intestine induced homing of specific T cells to the small intestinal LP much more efficiently than did those from the spleen. Of interest, CD11c+ APCs from the colon did not efficiently induce homing to the small intestinal LP (P = 0.002 vs. small intestinal CD11c+ APCs), which was similar to the in vivo priming we observed earlier [5]. When we examined the colon LP, to which CD8+ T cell homing had not been previously investigated, we observed the reciprocal pattern (Fig. 1A, right). CD11c+ APCs from the colon imprinted homing to the colonic LP more effectively than did those from the small intestine (P = 0.02) or spleen. A similar independent experiment confirmed the same results (Fig. 1B). These results confirmed our hypothesis that it was CD11c+ APCs—primarily DCs—from each mucosal subcompartment that imprinted T cell homing back to that subcompartment.
Figure 1. Colonic DCs are more effective than small intestinal (SI) DCs at inducing homing of CD8+ T cells to the colon.
(A and B) OT-I TCR transgenic T cells that were specific for OVA epitope SIINFEKL were stimulated for 3 d with this peptide and DCs from indicated tissues, then labeled with CFSE and transferred i.v. into naive recipient syngeneic C57BL/6 mice. Twenty hours later, small intestinal (left) and colonic (right) LP lymphocytes (LPL) were harvested from mice and analyzed by flow cytometry for CFSE+ cells. (A) Statistical analysis of panel A was performed by ANOVA, showing overall P = 0.0028 (left) and P = 0.0223 (right). Then, Dunnett’s multiple comparison test was performed for the pairwise comparisons in each panel with P values shown. (B) Repeated experiment. Panels A and B are independent experiments, representative of a total of 4 experiments with similar results. Each point represents a different recipient mouse, as described in Materials and Methods.
Differential expression of homing receptors after intrarectal immunization or after culture with CD11c+ APCs from different compartments
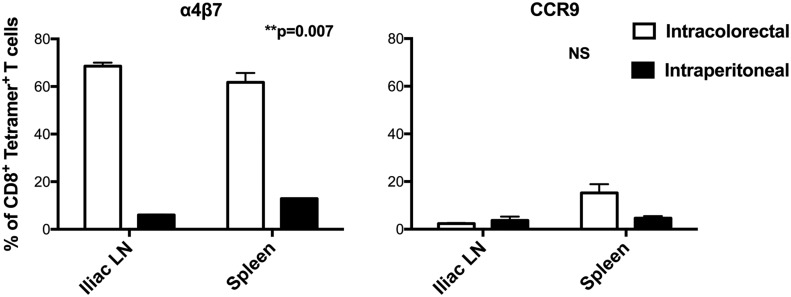
To determine which homing receptors were involved, we immunized mice with the SIINFEKL peptide intrarectally, compared with i.p., and examined the homing receptors that were induced (Fig. 2). Receptors that were preferentially induced by immunization in the colon would be more likely to be involved in homing to those sites. We focused first on receptors that have been previously shown to be involved in homing to the small intestine, integrin α4β7, and chemokine receptor CCR9 [10, 12]. Integrin α4β7 was induced by intracolorectal immunization on SIINFEKL-Kb tetramer–positive T cells in both the iliac lymph nodes, which are reported to be sites for DCs migrating from the distal colon [13], and the spleen, but not by i.p. immunization. Thus, this integrin seems to be induced selectively or imprinted on circulating T cells—in spleen and lymph nodes—by colorectal exposure to Ag. In contrast, CCR9 was not induced by either immunization route at either site. Whereas it remains possible that CCR9 would have been better up-regulated by oral rather than i.p. immunization, it would be difficult to administer short peptides orally without degradation. These results motivated additional exploration of these receptors.
Figure 2. Intrarectal immunization induces α4β7, but not CCR9, on Ag-specific CD8+ T cells.
C57BL/6 mice were immunized intracolorectally or i.p. with SIINFEKL OVA peptide, and 6 d later, CD8+ T cells from iliac lymph nodes or spleen were analyzed for staining by the SIINFEKL-H-2Kb tetramer to determine Ag specificity and for α4β7-integrin (left) or CCR9 (right). Bars represent means ± sem. For α4β7, the difference between intracolorectal and i.p. immunization was significant (P = 0.007), whereas there was no significant difference between i.p. and intracolorectal immunization for CCR9. This experiment is representative of 2 experiments with similar results.
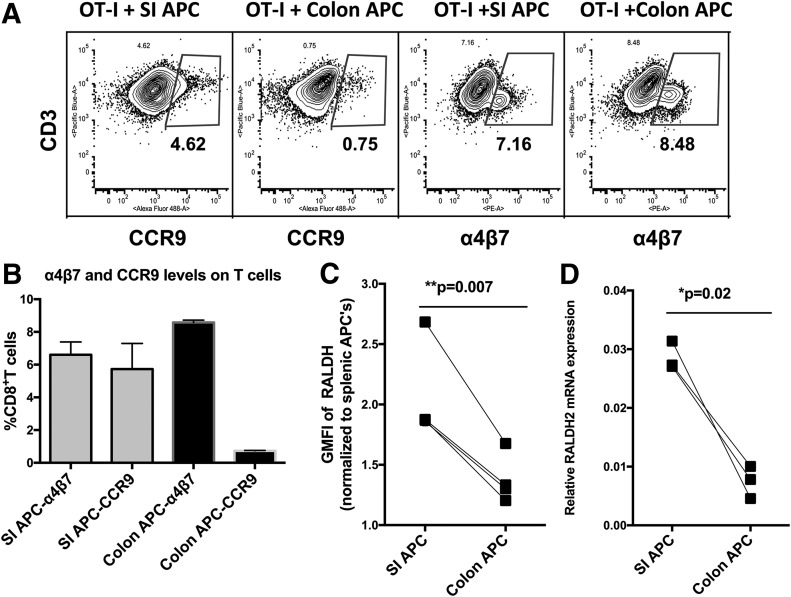
As small and large intestinal DCs have a similar phenotype [14], we wondered whether they might induce similar changes on the surfaces of coincubated primed T cells, despite their different migratory capacities. Therefore, similar to the experiment described earlier, OT-I TCR transgenic CD8+ T cells were cocultured with ova peptide–pulsed CD11c+ APCs from each compartment for 3 d, but instead of using these T cells for adoptive transfer in vivo, we stained them for the expression of homing receptors (Fig. 3A and B). As reported previously [2], CD11c+ APCs from the small intestine induced the up-regulation of both α4β7-integrin and CCR9; however, we found that those from the colon induced the expression of α4β7, but not CCR9 (P = 0.046), which was consistent with their preferential induction of homing to the colon, but not the small intestine, and implied the existence of another alternative way of attracting T cells to colonic tissue. Earlier studies [15] have shown that the expression of CCR9 and α4β7-integrin on T cells depended on a high level of retinoic acid synthesis by DCs, but CCR9 required higher levels than did α4β7. In our hands, although CCR9 was not induced by colon CD11c+ APCs, α4β7-integrin was. To investigate a potential role of retinoic acid in the expression of α4β7-integrin, we compared RALDH protein expression levels by intracellular ALDEFLuor staining with flow cytometry mean fluorescent index in CD11c+ APCs that were isolated from the colon or small intestine in 4 independent experiments (Fig. 3C). Of interest, in 4 independent paired experiments, colon CD11c+ APCs expressed consistently lower levels of RALDH than did small intestine CD11c+ APCs in all 4 experiments (P = 0.007), which is consistent with lower levels being sufficient to induce α4β7 but not CCR9. The experiment was also independently confirmed by RT-PCR for RALDH2 mRNA expression (Fig. 3D) in 3 independent experiments with 3 mice each. Thus, the differential level of RALDH in colonic vs. small intestinal CD11c+ APCs could explain the differential induction of homing receptors, but also raises the intriguing possibility that another mechanism could be responsible for driving the levels of α4β7 on T cells by colonic CD11c+ APCs.
Figure 3. Effect of DCs from colon or small intestine (SI) on T cell homing receptors and relation to retinoic acid production by the DCs.
(A and B) OT-I CD8+ TCR transgenic T cells that were specific for the OVA peptide SIINFEKL were stimulated with this peptide and CD11c+ APCs from small intestine or colon as in Fig. 1. Four days later, T cells were stained for α4β7-integrin and CCR9 chemokine receptor and analyzed by flow cytometry. Both sources of DCs up-regulated α4β7, but only small intestinal DCs up-regulated CCR9. Representative flow plot (gates based on isotype controls; A), and means and ranges for 2 experiments using colon or SI CD11c+ APCs pooled from 3 mice each (B). (C) RALDH levels in DCs from small intestine or colon were measured by intracellular staining using an ALDEFluor assay (Materials and Methods), and geometric mean fluorescent index (GMFI) is plotted for 4 independent matched pairs of tissues from 4 independent experiments (P = 0.007). (D) RALDH mRNA levels by RT-PCR showing SI vs. colon CD11c+ APC pairs from 3 independent experiments of 3 mice each (P = 0.02).
(α4)β7-Integrin, but not CCR9, is required for T cell homing to the colon
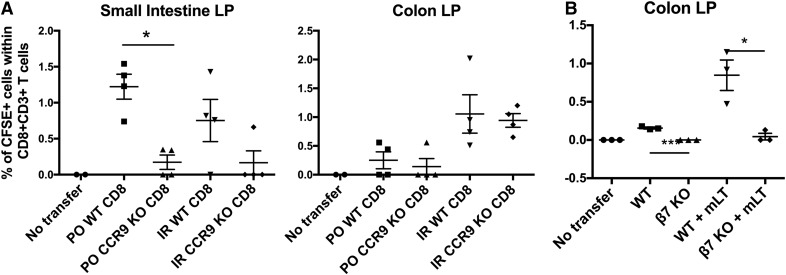
Because colonic CD11c+ APCs selectively induced α4β7, but not CCR9, on CD8+ T cells, we asked whether that selective induction corresponded to a functional requirement for the former, but not the latter, in homing to the colon. α4β7, along with its ligand (MADCAM-1), are known to be critical components of mediating the migration to the small intestine [16–18]. To assess the level of dependency of T cells to be able to migrate to the colon on α4β7-integrin in our model, we used β7 KO mice, which, in addition to α4β7, also lack another important homing molecule, αEβ7. To validate our findings that colon APC-primed T cells, despite a lack of CCR9, can migrate to the colon, we compared CCR9 KO with wild-type mice. In both cases, to induce the homing of CD8+ T cells from these mice to the small or large intestine, we gave the recipient mice mLT (R192G; a gift of John Clements, Tulane University), which has been widely used as a mucosal adjuvant by us and others [11, 19–21]. We delivered the mLT via oral or intrarectal routes to study homing to both the small and large intestine. Eight hours later, CFSE-labeled purified CD8+ T cells from WT or KO spleens were transferred i.v. to the recipient mLT-treated mice, and 20 h later, tissues were harvested and analyzed by flow cytometry for CD3+CD8+CFSE+ T cells. In contrast to T cells from wild-type mice, those from CCR9 KO mice were deficient in homing to the small intestinal LP (Fig. 4A, left), as expected, but, in contrast, were found here to home normally to the colon (Fig. 4A, right); however, using T cells from integrin β7 KO mice, we found that this integrin is needed for homing to the colonic LP (Fig. 4B). These results confirm the hypothesis that α4β7, but not CCR9, is required for CD8+ T cell homing to the colonic LP.
Figure 4. CD8+ T cells require β7-integrin, but not CCR9, to migrate to the colon.
(A) CD8+ T cells do not require CCR9 to migrate to the colon as they do to the small intestine. Wild-type (WT) C57BL/6 mice received 7 µg of mLT intracolorecally (IR) or orally (PO). Eight hours later, CD8+ T cells from the spleens of WT or CCR9 KO mice were purified, labeled with CFSE, and transferred i.v. to WT mice that had been treated with mLT. Twenty hours later, small intestinal or colonic LP cells were harvested and analyzed by flow cytometry for the presence for CD3+CD8+CFSE+ T cells. (B) CD8+ T cells require β7-integrin to migrate to the colon. As in panel A, WT B6 mice were treated or not with mLT intracolorectally, and 8 h later, purified CD8 T cells from WT or β7 KO mice, labeled with CSFE, were transferred i.v. After 20 h, colonic LP cells were analyzed by flow cytometry for CFSE+ CD8+ T cells. These results are representative of 2–3 experiments each with comparable results.
DISCUSSION
It has not been widely appreciated that the gut mucosal immune system is not all one compartment. Whereas it is expected that there should be some crosstalk, we recently found that we could immunize mice orally to selectively deliver Ag to the small or large intestine [5]. Doing so surprisingly induced preferential T cell responses in the small or large intestine, which indicated that these segments of the gut form separate mucosal immune compartments. A corollary to this result is that the homing receptors for T cells to traffic to these compartments must be different and determined by where the T cells were primed. If they were the same, then T cells that were primed in either segment of the gut would traffic to both, making the gut one homogeneous mucosal immune compartment. Our data, as well as that from other studies, suggested that different pathways are involved in the recruitment of lymphocytes to different anatomic parts of the intestine tracts [22]. It was recently reported that cecal patch DCs induce more CCL27 binding to IgA+ plasma cells than do Peyer’s patch DCs, which suggests a role in homing of plasma cells [23]. Whereas much was already known about T cell trafficking to the small intestine, little was known about trafficking to the colon. Because the site of priming seemed to matter, and because it was previously shown that Peyer’s patch DCs induced homing of T cells to the small intestine [1–3], we hypothesized that colonic DCs might imprint homing back to the colon. Indeed, we report here, for the first time to our knowledge, that that is indeed the case, and that CD11c+ APCs from the colon and small intestine can differentially imprint T cells for selective migration and differentially up-regulate the relevant homing receptors on those T cells.
We observed that CD11c+ APCs from the small and large intestine reciprocally imprint homing of T cells back to the compartment from which they came. This difference in CD11c+ APCs correlated with a difference in their ability to up-regulate the expression of α4β7-integrin and the CCR9 chemokine receptor on T cells: colonic APC induced the up-regulation of α4β7, but not CCR9. That regulation, in turn, correlated with different levels of the enzyme, RALDH, required for retinoic acid production. The difference in RALDH expression was recently also found in a study of lymph nodes draining the small and large intestine [24].
Accordingly, we found that CD11c+ APCs from the colon induced α4β7-integrin expression in T cells, but not CCR9, and that, correspondingly, α4β7, but not CCR9, was required for T cells to home to the colon in contrast to the small intestine, which requires both [10]. This result could explain the lack of homing to the small intestine by T cells that are primed on colonic CD11c+ APCs; however, it left us with the question of whether another chemokine receptor was needed instead of CCR9 to home to the colon. We investigated the role of several chemokine receptors that might promote selective CD8 T cell migration to the colon, including CXCR3, as it was found earlier to promote homing to the vaginal mucosa [25] and because there is evidence for T cell crosstalk between the colorectal and vaginal immune systems [4, 5]. Indeed, we found that, compared with the small intestine, the colon has a higher expression level of CXCR3 ligands in the steady state (data not shown); however, when using CXCR3 KO mice for adoptive transfer experiments, we observed that CXCR3 was involved in the homing of CD8 T cells to the colon only under inflammatory, not steady-state conditions (data not shown). In addition, when we tried to examine the up-regulation of chemokine receptors for which we could stain on T cells stimulated by colonic vs. small intestinal CD11c+ APCs, we could not find any consistent selective up-regulation by colonic CD11c+ APCs of CXCR3, CCR6, CCR7, CXCR5, CX3CR1, or CXCR4. Therefore, it remains to be determined whether any chemokine receptor is the primary unique chemokine receptor for colon CD8+ T cell homing. Furthermore, chemokines/chemokine receptors are complicated systems with redundancy. For example, blocking of CXCL12-CXCR4 or CCL20-CCR6 systems have been found to be able to inhibit LP lymphocyte adhesion in the ileum and colon [26], whereas an earlier study identified that only CCR9 is necessary and sufficient to guide T cell homing to small intestine, but CXCR4 and CCR6 were not sufficient for T cell homing to the small intestine [12].
Most of the T cell homing studies focused on CD4+ T cells. In addition to chemokines, several other molecules have been demonstrated to play crucial roles in T cell trafficking. For example, SEW2871, a selective sphingosine-1-phosphate type 1 receptor agonist, has been shown to reduce the homing of T cells into colon LP in an experimental colitis mouse model [27]. Lymphocyte function-associated Ag-1 has been demonstrated to be required for the trafficking of T cells to the mesenteric LNs (MLNs) and homing of colitogenic effector cells to the colon in the induction of chronic colitis [28]. It was also recently reported that GPR15 is a chemokine receptor that is necessary for homing of T regulatory cells to large intestinal mucosa [7]. Although CD8+ T cells were not studied in detail, it did not seem that GPR15 was necessary for CD8+ T cell homing to the colon. Because no Ab is available to stain GPR15 on mouse lymphocytes (S. V. Kim, personal communication, July 26, 2016), they used GPR15-GFP knock-in mice to examine the expression of GPR15 in gut-homing T regulatory cells; however, for that reason, appropriate reagents were not available for us to study the effect of colonic DCs on the expression of GPR15 to rule out (or in) a possible role for this receptor, and no Ab we tested could detect this molecule on cells. A recent study also demonstrated that GPR15 plays an important role in the homing of murine dendritic epidermal T cells from the thymus to the skin [29]. Taken together, these data suggest that GPR15 might contribute to lymphocyte homing to diverse epithelial sites.
A number of important viral infectious diseases, such as HIV, hepatitis B virus, hepatitis C virus, and others, are transmitted via the colorectal mucosa. For this reason, focusing an Ag-specific CD8+ T cell response on the colorectal mucosa would be valuable in designing a vaccine to protect against the mucosal transmission of these viruses. Guiding the Ag-specific CD8+ T cell homing to the colorectal mucosa would be crucial for the prevention and eradication of colorectal cancers. There is precedent in the case of the lung, as intranasal immunization has been found to induce CD8+ T cells that express the mucosal integrin Ly49a that induced homing of T cells to the lung, where they were more effective in treating lung tumors [30]. Indeed, and also in this case, DCs from the lung were involved in inducing such homing. Because it seems that differential expression of retinoic acid by DCs is a key determinant of their imprinting of T cell homing to the colon, approaches to regulate retinoic acid production when T cells are primed could allow the manipulation of this homing. Thus, we believe that learning how to target T cells to the site of disease or disease transmission—in our case, the colorectal mucosa—could allow more effective vaccines to be developed to prevent or treat such diseases.
AUTHORSHIP
A.D., A.H., Y.S., and J.A.B. conceived the study, contributed the hypotheses, designed and analyzed experiments, and wrote the manuscript. A.D., A.H., and S.S.-M. performed most of the experiments. H.Y., B.F., and Y.W. helped with some of the experiments and interpretation of data.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by National Cancer Institute intramural funds under Project Z01-SC-004020 (to J.A.B.). We thank Ulrich von Andrian (Harvard University) for the FLT3L-expressing B16 melanoma cells, John Clements (Tulane University) for the mLT, and the U.S. National Institutes of Health (NIH) Tetramer facility for MHC tetramers. Opinions expressed are those of the authors and not the U.S. Federal Government.
Glossary
- DC
dendritic cell
- KO
knockout
- LP
lamina propria
- mLT
mutant Escherichia coli labile toxin
- LN
lymph node
- RALDH
retinal dehydrogenase
Footnotes
The online version of this paper, found at www.jleukbio.org, contains supplemental information.
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Kelsall B. L., Rescigno M. (2004) Mucosal dendritic cells in immunity and inflammation. Nat. Immunol. 5, 1091–1095. [DOI] [PubMed] [Google Scholar]
- 2.Mora J. R., Bono M. R., Manjunath N., Weninger W., Cavanagh L. L., Rosemblatt M., Von Andrian U. H. (2003) Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature 424, 88–93. [DOI] [PubMed] [Google Scholar]
- 3.Johansson-Lindbom B., Svensson M., Wurbel M. A., Malissen B., Márquez G., Agace W. (2003) Selective generation of gut tropic T cells in gut-associated lymphoid tissue (GALT): requirement for GALT dendritic cells and adjuvant. J. Exp. Med. 198, 963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu Q., Thomson C. W., Rosenthal K. L., McDermott M. R., Collins S. M., Gauldie J. (2008) Immunization with adenovirus at the large intestinal mucosa as an effective vaccination strategy against sexually transmitted viral infection. Mucosal Immunol. 1, 78–88. [DOI] [PubMed] [Google Scholar]
- 5.Zhu Q., Talton J., Zhang G., Cunningham T., Wang Z., Waters R. C., Kirk J., Eppler B., Klinman D. M., Sui Y., Gagnon S., Belyakov I. M., Mumper R. J., Berzofsky J. A. (2012) Large intestine-targeted, nanoparticle-releasing oral vaccine to control genitorectal viral infection. Nat. Med. 18, 1291–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soler D., Humphreys T. L., Spinola S. M., Campbell J. J. (2003) CCR4 versus CCR10 in human cutaneous TH lymphocyte trafficking. Blood 101, 1677–1682. [DOI] [PubMed] [Google Scholar]
- 7.Kim S. V., Xiang W. V., Kwak C., Yang Y., Lin X. W., Ota M., Sarpel U., Rifkin D. B., Xu R., Littman D. R. (2013) GPR15-mediated homing controls immune homeostasis in the large intestine mucosa. Science 340, 1456–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen L. P., Pan J., Dinh T. T., Hadeiba H., O’Hara E. III, Ebtikar A., Hertweck A., Gökmen M. R., Lord G. M., Jenner R. G., Butcher E. C., Habtezion A. (2015) Role and species-specific expression of colon T cell homing receptor GPR15 in colitis. Nat. Immunol. 16, 207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mora J. R., von Andrian U. H. (2004) Retinoic acid: an educational “vitamin elixir” for gut-seeking T cells. Immunity 21, 458–460. [DOI] [PubMed] [Google Scholar]
- 10.Iwata M., Hirakiyama A., Eshima Y., Kagechika H., Kato C., Song S. Y. (2004) Retinoic acid imprints gut-homing specificity on T cells. Immunity 21, 527–538. [DOI] [PubMed] [Google Scholar]
- 11.Belyakov I. M., Ahlers J. D., Clements J. D., Strober W., Berzofsky J. A. (2000) Interplay of cytokines and adjuvants in the regulation of mucosal and systemic HIV-specific CTL. J. Immunol. 165, 6454–6462. [DOI] [PubMed] [Google Scholar]
- 12.Zabel B. A., Agace W. W., Campbell J. J., Heath H. M., Parent D., Roberts A. I., Ebert E. C., Kassam N., Qin S., Zovko M., LaRosa G. J., Yang L. L., Soler D., Butcher E. C., Ponath P. D., Parker C. M., Andrew D. P. (1999) Human G protein-coupled receptor GPR-9-6/CC chemokine receptor 9 is selectively expressed on intestinal homing T lymphocytes, mucosal lymphocytes, and thymocytes and is required for thymus-expressed chemokine-mediated chemotaxis. J. Exp. Med. 190, 1241–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veenbergen S., van Berkel L. A., du Pré M. F., He J., Karrich J. J., Costes L. M., Luk F., Simons-Oosterhuis Y., Raatgeep H. C., Cerovic V., Cupedo T., Mowat A. M., Kelsall B. L., Samsom J. N. (2016) Colonic tolerance develops in the iliac lymph nodes and can be established independent of CD103+ dendritic cells. Mucosal Immunol. 9, 894–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rivollier A., He J., Kole A., Valatas V., Kelsall B. L. (2012) Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. J. Exp. Med. 209, 139–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang C., Kang S. G., HogenEsch H., Love P. E., Kim C. H. (2010) Retinoic acid determines the precise tissue tropism of inflammatory Th17 cells in the intestine. J. Immunol. 184, 5519–5526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagner N., Löhler J., Kunkel E. J., Ley K., Leung E., Krissansen G., Rajewsky K., Müller W. (1996) Critical role for beta7 integrins in formation of the gut-associated lymphoid tissue. Nature 382, 366–370. [DOI] [PubMed] [Google Scholar]
- 17.Schweighoffer T., Tanaka Y., Tidswell M., Erle D. J., Horgan K. J., Luce G. E., Lazarovits A. I., Buck D., Shaw S. (1993) Selective expression of integrin alpha 4 beta 7 on a subset of human CD4+ memory T cells with Hallmarks of gut-trophism. J. Immunol. 151, 717–729. [PubMed] [Google Scholar]
- 18.Williams M. B., Butcher E. C. (1997) Homing of naive and memory T lymphocyte subsets to Peyer’s patches, lymph nodes, and spleen. J. Immunol. 159, 1746–1752. [PubMed] [Google Scholar]
- 19.Dickinson B. L., Clements J. D. (1995) Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect. Immun. 63, 1617–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chong C., Friberg M., Clements J. D. (1998) LT(R192G), a non-toxic mutant of the heat-labile enterotoxin of Escherichia coli, elicits enhanced humoral and cellular immune responses associated with protection against lethal oral challenge with Salmonella spp. Vaccine 16, 732–740. [DOI] [PubMed] [Google Scholar]
- 21.Belyakov I. M., Hel Z., Kelsall B., Kuznetsov V. A., Ahlers J. D., Nacsa J., Watkins D. I., Allen T. M., Sette A., Altman J., Woodward R., Markham P. D., Clements J. D., Franchini G., Strober W., Berzofsky J. A. (2001) Mucosal AIDS vaccine reduces disease and viral load in gut reservoir and blood after mucosal infection of macaques. Nat. Med. 7, 1320–1326. [DOI] [PubMed] [Google Scholar]
- 22.Hart A. L., Ng S. C., Mann E., Al-Hassi H. O., Bernardo D., Knight S. C. (2010) Homing of immune cells: role in homeostasis and intestinal inflammation. Inflamm. Bowel Dis. 16, 1969–1977. [DOI] [PubMed] [Google Scholar]
- 23.Masahata K., Umemoto E., Kayama H., Kotani M., Nakamura S., Kurakawa T., Kikuta J., Gotoh K., Motooka D., Sato S., Higuchi T., Baba Y., Kurosaki T., Kinoshita M., Shimada Y., Kimura T., Okumura R., Takeda A., Tajima M., Yoshie O., Fukuzawa M., Kiyono H., Fagarasan S., Iida T., Ishii M., Takeda K. (2014) Generation of colonic IgA-secreting cells in the caecal patch. Nat. Commun. 5, 3704. [DOI] [PubMed] [Google Scholar]
- 24.Houston S. A., Cerovic V., Thomson C., Brewer J., Mowat A. M., Milling S. (2016) The lymph nodes draining the small intestine and colon are anatomically separate and immunologically distinct. Mucosal Immunol. 9, 468–478. [DOI] [PubMed] [Google Scholar]
- 25.Shin H., Iwasaki A. (2012) A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature 491, 463–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oyama T., Miura S., Watanabe C., Hokari R., Fujiyama Y., Komoto S., Tsuzuki Y., Hosoe N., Nagata H., Hibi T. (2007) CXCL12 and CCL20 play a significant role in mucosal T-lymphocyte adherence to intestinal microvessels in mice. Microcirculation 14, 753–766. [DOI] [PubMed] [Google Scholar]
- 27.Dong J., Wang H., Wu G., Zhao J., Zhang L., Zuo L., Zhu W., Gong J., Li Y., Gu L., Li J. (2014) Oral treatment with SEW2871, a sphingosine-1-phosphate type 1 receptor agonist, ameliorates experimental colitis in interleukin-10 gene deficient mice. Clin. Exp. Immunol. 177, 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koboziev I., Karlsson F., Ostanin D. V., Gray L., Davidson M., Zhang S., Grisham M. B. (2012) Role of LFA-1 in the activation and trafficking of T cells: implications in the induction of chronic colitis. Inflamm. Bowel Dis. 18, 2360–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lahl K., Sweere J., Pan J., Butcher E. (2014) Orphan chemoattractant receptor GPR15 mediates dendritic epidermal T-cell recruitment to the skin. Eur. J. Immunol. 44, 2577–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sandoval F., Terme M., Nizard M., Badoual C., Bureau M. F., Freyburger L., Clement O., Marcheteau E., Gey A., Fraisse G., Bouguin C., Merillon N., Dransart E., Tran T., Quintin-Colonna F., Autret G., Thiebaud M., Suleman M., Riffault S., Wu T. C., Launay O., Danel C., Taieb J., Richardson J., Zitvogel L., Fridman W. H., Johannes L., Tartour E. (2013) Mucosal imprinting of vaccine-induced CD8+ T cells is crucial to inhibit the growth of mucosal tumors. Sci. Transl. Med. 5, 172ra20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.