Role for mast cell CRF1 in potentiating mast cell degranulation in response to immunological and psychological stressors.
Keywords: anaphylaxis, immune response, intestinal permeability, G protein-coupled receptor, allergy, functional bowel disorders
Abstract
Life stress is a major risk factor in the onset and exacerbation of mast cell–associated diseases, including allergy/anaphylaxis, asthma, and irritable bowel syndrome. Although it is known that mast cells are highly activated upon stressful events, the mechanisms by which stress modulates mast cell function and disease pathophysiology remains poorly understood. Here, we investigated the role of corticotropin-releasing factor receptor subtype 1 (CRF1) in mast cell degranulation and associated disease pathophysiology. In a mast cell–dependent model of IgE-mediated passive systemic anaphylaxis (PSA), prophylactic administration of the CRF1-antagonist antalarmin attenuated mast cell degranulation and hypothermia. Mast cell–deficient KitW-sh/W-sh mice engrafted with CRF1−/− bone marrow–derived mast cells (BMMCs) exhibited attenuated PSA-induced serum histamine, hypothermia, and clinical scores compared with wild-type BMMC-engrafted KitW-sh/W-sh mice. KitW-sh/W-sh mice engrafted with CRF1−/− BMMCs also exhibited suppressed in vivo mast cell degranulation and intestinal permeability in response to acute restraint stress. Genetic and pharmacologic experiments with murine BMMCs, rat RBL-2H3, and human LAD2 mast cells demonstrated that although CRF1 activation did not directly induce MC degranulation, CRF1 signaling potentiated the degranulation responses triggered by diverse mast cell stimuli and was associated with enhanced release of Ca2+ from intracellular stores. Taken together, our results revealed a prominent role for CRF1 signaling in mast cells as a positive modulator of stimuli-induced degranulation and in vivo pathophysiologic responses to immunologic and psychologic stress.
Introduction
MCs are innate immune cells well-known for their critical roles in health and survival, including pathogen defense, immune modulation, and tissue repair. As sentinel cells, MCs are strategically positioned at environmental host interfaces, such as epithelial barriers in the gut, skin, respiratory tract, and bladder, are located in close proximity to blood vessel endothelium and neurons and express receptors for a wide array of Ags, pathogens, cytokines, and neuropeptides, among others. Furthermore, MCs synthesize and store potent mediators, including histamine, proteases, and cytokines within cytoplasmic granules, which are released immediately upon stimuli-induced degranulation. Together, these unique properties of MCs provide a mechanism to sense environmental and host stress cues and to generate a rapid and robust response required for survival and for return to homeostasis. However, excessive MC degranulation is central to the pathogenesis of a number of stress-related MC diseases, including allergy/anaphylaxis, asthma, and functional and inflammatory GI disorders [1–4], but the mechanisms driving MC hyperactivation in these diseases remain poorly defined.
A major risk factor in the onset of MC-associated disorders, including allergy, asthma, and IBS, is life stress [5–7]. Experimental studies in animal models and humans have reported a link between psychologic stressors and increased tissue MC activation and pathophysiology, including inflammation and intestinal permeability [8–13]. Despite the established connection between stress and MC activation, precisely how stress modulates MC activation and its role in MC disease pathogenesis has remained elusive.
The CRF system, composed of CRF and related the family of Ucn (Ucn I–III) [14], and their GPCRs, CRF1 and CRF2 [15], are well-established as a major stress regulatory system in the body. Although most extensively studied in the context of psychosocial stress, HPA axis regulation, and neurobehavioral paradigms [14], the CRF system has also been shown to be active in peripheral immunologic and infectious challenge conditions [16–20], suggesting an important role for the CRF system in regulating a diverse array of stressful stimuli. Specifically, an extensive literature exists about the CRF system regulation of neuronal function, especially within the CNS; however, comparatively, there is little information about the role of the CRF system in regulating specific immune cells and immune-mediated pathophysiology in vivo. Human MCs express CRF1 and CRF2 [21–23], which, upon activation with pharmacologic agonists, induce canonical GPCR signaling pathways and selective cytokine release in vitro [21, 22, 24]. However, the role that CRF receptors have in MC degranulation and the MC-associated diseases in vivo is unknown. The objective of the present studies was to investigate the role of CRF1 receptor signaling in MC degranulation responses and in vivo pathophysiologic responses to immunologic and psychologic stressors.
MATERIALS AND METHODS
Animals
All protocols were approved by the Michigan State University Institutional Animal Care and Use Committee (protocol 03/15-039-00). Founding breeders for all mice strains were obtained from the Jackson Laboratory (Bar Harbor, ME, USA) and were housed in accordance with guidelines from the American Association for Laboratory Animal Science care and research protocols. C57BL/6 (stock 000664) and KitW-sh/W-sh mice (stock 012861) used in this study were derived from homozygous breeders. Heterozygous CRF1+/− mice (B6; 129-Crhr1tm1Klee/J; stock 004454) were purchased and bred to obtain WT (CRF1+/+) and CRF1−/− mice. The generation of CRF1-deficient mice has been described previously [25]. Genotyping of offspring was performed both in house and confirmed commercially (GeneTyper, New York, NY, USA) using PCR protocols published at http://www.jax.org.
PSA model
Mice were sensitized by i.p. injection with 20 µg of IgE monoclonal anti-DNP in 100 µl PBS. The following day, mice were challenged with 1 mg of DNP in 100 µl PBS (i.p.). For CRF1 antagonist experiments, IgE-sensitized WT C57Bl/6 mice were administered the CRF1 antagonist Antalarmin (Sigma-Aldrich, St. Louis, MO, USA) via the tail vein (15 mg/kg), 30 min before DNP-HSA challenge. Rectal temperatures were recorded at 0, 15, 30, 60, and 120 min after DNP challenge (TH-5 Thermalert; Physitemp, Clifton, NJ, USA). In indicated experiments, clinical symptoms were recorded every 10 min and were used to derive clinical scores using the anaphylactic symptom scoring system developed by Sun et al. [26]. Briefly, absence of clinical symptoms was given a score of 0; repetitive facial scratching, a score of 1; reduced activity and facial puffiness, a score of 2; periods of motionlessness for 1 min, a score of 3; and no movement to gentle prodding, a score of 4. Mice were euthanized 120 min after DNP injection, and serum was collected via cardiac puncture and stored at −80°C until serum histamine level was quantified using a commercial EIA kit (Oxford Biomedical Research, Rochester Hills, MI, USA). Tissue MC activation was assessed in mesenteric tissues collected from mice at 120 min after DNP challenge, and tissues were fixed in Carnoy’s fixative and stained with toluidine blue to visualize the MCs and assess degranulation.
BMMC engraftment in KitW-sh/W-sh mice
Female KitW-sh/W-sh mice (8–10 wk old) were injected i.p. with 1 × 107 BMMCs (suspended in 200 µl of sterile 1× PBS) derived from WT or CRF1−/− mice. At 12 wk after engraftment, mice were used for PSA and RS experiments, as described in the Material and Methods section. Successful tissue MC engraftment was confirmed by counting MC numbers in intestinal mesenteric sections stained with toluidine blue.
Restraint stress model
Mice were placed in individual, transparent, 50-ml, plastic conical tubes, which were modified with air holes, for either 1 or 3 h, depending on the experiment. Control (nonstressed) mice remained in their original home cages for 3 h without food and water to avoid confounding effects of water or feed intake between stressed and unstressed animals. After RS, mice were immediately euthanized by CO2 inhalation, and serum and ileal segments were collected for measurement of serum histamine and intestinal permeability, respectively, in Ussing chambers (Physiologic Instruments, San Diego, CA).
Ussing chamber experiments: TER and FD4 flux measurements
Ileum was harvested from mice immediately after euthanasia and was prepared for mounting in Ussing chambers. Ileal segments were opened lengthwise along the antimesenteric border and placed in oxygenated (95% O2, 5% CO2) rodent Ringer solution (154 Na+ mM, 6.3 K+ mM, 137 Cl− mM, 0.3 H2PO3 mM, 1.2 Ca2+ mM, 0.7 Mg2+ mM, and 24 HCO3−; pH 7.4) at 37°C, and then, mounted in a 0.3-cm2 aperture in the Ussing chambers (Physiologic Instruments or World Precision Instruments, Sarasota, FL, USA), as described in previous studies [27, 28]. The tissue was bathed in rodent Ringer solution on each side of the tissue. The serosal bathing solution contained 10 mM glucose, which was balanced with 10 mM mannitol on the mucosal side. Bathing solutions were oxygenated (95% O2, 5% CO2) and maintained at 37°C. The spontaneous potential difference was measured using Ringer-agar bridges connected to calomel electrodes, and the potential difference was short-circuited through Ag-AgCl electrodes using a voltage clamp that corrected for fluid resistance. Tissues were maintained in the short-circuited state, except for brief intervals, to record the open-circuit potential difference. TER (Ω cm2) was calculated from the spontaneous potential difference and short-circuit current. After a 30-min equilibration period in Ussing chambers, TER was recorded at 5-min intervals for a 60-min period and then averaged to derive the basal TER values for a given animal.
After 30 min equilibration in Ussing chambers, FD4 (100 mg/ml; Sigma-Aldrich) was added to the mucosal bathing reservoir of the Ussing chambers. After 15 min equilibration, standards were taken from the serosal side of each chamber, and a 60 min flux period was established by taking 0.5 ml samples from the mucosal compartment. The quantity of FD4 was established by measuring the fluorescence in mucosal reservoir fluid samples in a fluorescence plate reader at 540 nm. Data were presented as the rate of FD4 flux in nanogram of FD4 minutes per square centimeter.
Culture and activation of BMMCs, RBL-2H3 cells, and human LAD2 MCs
Isolated bone marrow progenitor cells were cultured in RPMI 1640 media (with l-glutamine) supplemented with FBS (10%), sodium pyruvate (1 mM), MEM nonessential amino acids (1×), HEPES buffer (10 mM), penicillin (100 U/ml), and streptomycin (100 μg/ml) and with recombinant cytokines [stem cell factor (5 ng/ml) and IL-3 (5 ng/ml)]. After 4 wk, cultures were composed mainly of MCs (98%), as determined by toluidine blue (Sigma-Aldrich) staining, and flow cytometry analysis (LSR II; BD Biosciences, East Lansing, MI, USA) using fluorescently labeled c-Kit (BioLegend, San Diego, CA, USA) and high-affinity IgE receptor (FcεRI) Abs (eBioscience, San Diego, CA, USA) Media and supplements were purchased from Corning Cellgro (Manassas, VA, USA), and recombinant cytokines were purchased from R&D Systems (Minneapolis, MN, USA). The rat basophilic leukemia cell MC line (RBL-2H3) was grown in MEM media with 10% FBS. Human LAD2 cells, a gift provided by Dr. A. Kirshenbaum (U.S. National Institutes of Health, Bethesda, MD, USA), were cultured in StemPro-34 media (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with StemPro-34 nutrient supplement (Thermo Fisher Scientific), l-glutamine (2 mM; Thermo Fisher Scientific), penicillin (100 U/ml), streptomycin (100 μg/ml), and recombinant human stem cell factor (100 ng/ml; PeproTech, Rocky Hill, NJ, USA). Cell density was kept below 0.5 × 106 cells/ml, and one-half of the media was replaced weekly.
CRF1 overexpression and knockdown in RBL-2H3 MCs
LentiORF clone of CRF1 (CRHR1), transcript variant 1 (OriGene, Rockville, MD, USA), CRF1 shRNA EGFP-tagged (MSH026851-LvU6; GeneCopoeia, Rockville, MD, USA) and control membrane GFP–tagged plasmid were obtained from OriGene (RC226816L2). DH5α-competent cells were obtained from Thermo Fisher Scientific (18265017). Plasmids were transfected and plated on selective antibiotic-resistance LB plates, according to the manufacturer’s protocol. After overnight incubation at 37°C, a single colony was picked for each individual clone and inoculated on 2.5 ml LB medium containing antibiotics and kept for 8 h at 37°C at 200 rpm. A total of 600 µl of culture inoculum was transferred into 300 ml of LB broth on a sterile 1-L flask with antibiotics and kept at 200 rpm at 37°C overnight. Plasmid was extracted and the concentration was measured according to the manufacturer’s protocol (12662; Qiagen, Germantown, MD, USA).
Human embryonic kidney cell 293Ta lentivirus cells were seeded at a concentration of 1.5 × 106 cells per well (6 wells) and 2.5 µg of CRF1 overexpressed or eGFP plasmid control DNA was transfected using a lentiviral packaging kit (OriGene) according to the manufacturer’s protocol. After overnight incubation at 37°C with 5% CO2, the transfection medium was replaced with normal 5% DMEM media with polybrene (1 µl of 4 mg/ml); 48 h after transfection, the supernatant was collected from the 6-well plate and filtered onto a Nalgene ultracentrifuge tube (Nalge Nunc International, Rochester, NY, USA), and 5 ml of 20% sucrose was added into the bottom of the tube and centrifuged at 25,000 rpm for 2 h at 4°C. Then, the supernatant was discarded and the lentiviral overexpressing CRF1 or CRF1 shRNA plasmid or an EGFP control plasmid pellet was resuspended with 150 µl of 4% lactose and kept on ice for 15 min and centrifuged at a high speed for 1 min. Lentiviral aliquots were stored at −80°C until transfection.
Lentivirus tittering was performed, and RBL-2H3, at a concentration of 2.5 × 105 cells, was plated on a 12-well plate and grown overnight. Lentivirus overexpressing CRHR1 or CRF1 shRNA plasmid or EGFP control at a multiplicity of infection of 40 and polybrene at a final concentration of 8 µg/ml were added to each well. The plate was centrifuged at 300 g for 60 min at 30°C. The plate was then incubated at 37°C overnight, and the medium was replaced with 15% MEM. After 48 h of transfection, the cells were trypsinized and transferred onto T75 flasks. The RBL-2H3 overexpressing CRF1 or CRF1 shRNA or empty plasmid, tagged with GFP, was sorted on the flow cytometry based on the GFP-positive cells and multiplied and the RBL-2H3 cell aliquots were stored at liquid nitrogen for further study.
CRF1 siRNA knockdown in RBL-2H3 cells
RBL-2H3 cells, seeded at a concentration on 5 × 105 in a 6-well plate, were transfected with CRF1 siRNA (NM-030999, Sigma-Aldrich) or scrambled siRNA (SIC00-1, Sigma-Aldrich) of 150 ng using Qiagen Hi-Perfect transfection reagent, according to its manufacturer’s protocol; 6 h after transfection, normal growth medium was added to each well and incubated at 37°C with 5% CO2 for 48 h. After the incubation, transfected cells were sensitized with IgE overnight, stimulated with DNP for 1 h. In select experiments, cells were stimulated with A23187 or c48/80 (non-IgE primed) and β hex, histamine, and Ca2+ mobilization assays were performed, as described below.
β-Hex assay
RBL 2H3 cells and murine BMMCs (2 × 106 cells/ml) were sensitized overnight at 37°C with 1 μg/ml anti–DNP-IgE (Sigma-Aldrich) in complete RPMI 1640 medium with cytokines. The following day, cells were washed and resuspended in Tyrode’s buffer, and BMMCs were seeded in a 96-well plate (0.45 × 106 cells/well). The cells were equilibrated for 1 h and stimulated with the indicated concentrations of DNP (Sigma-Aldrich) for 1 h at 37°C. For experiments with LAD2 cells, cells were sensitized overnight with 100 ng/ml biotinylated human IgE (United States Biological, Salem, MA, USA). The next day, cells were washed with Tyrode’s buffer to remove excess IgE and challenged with streptavidin (500 ng/ml) for 7 min and centrifuged at 450 g for 5 min at 5°C, and 30 μl of supernatant was transferred into a new plate containing 10 μl of PNAG (p-nitrophenyl-N-acetyl-β-d-glucosaminide) (3.4 mg/ml in citrate buffer) and incubated for a further 90 min at 37°C. Excess cell supernatants were removed carefully, and cell pellets were lysed (0.1% Triton X-100 in Tyrode’s buffer), and the residual β-hex was estimated in parallel. The reaction was stopped by adding 100 μl of 0.1 M carbonate/bicarbonate buffer (pH 10) into each well. For A23187 and c48/80 stimulation, unsensitized BMMCs were stimulated for 1 h. β-activity was measured by addition of PNAG in 0.1 M sodium citrate (pH 4.5) for 1 h at 37°C. The reaction was terminated with 0.1 M carbonate buffer (pH 10.0), and the absorbance was recorded at 405 nm. The percentage of degranulation was calculated by dividing the optical absorbance of the supernatant by the sum of the optical absorbance by the supernatant and cell pellet (lysed with 0.1% Triton X-100) and multiplying by 100.
Histamine measurements in BMMC and RBL-2H3 cells
Histamine concentrations were quantified in cell pellets and supernatants from stimulated MC cultures with a histamine EIA kit (Oxford Biomedical Research).
Intracellular calcium measurements
RBL-2H3 cells and murine BMMCs derived from CRF1 WT and CRF1−/− mice were used for this study. On the day of the experiment, cells were washed and resuspended in calcium assay buffer. Changes in intracellular Ca2+ in response to stimulus were detected using the Fluo 4 NW calcium assay kit (Thermo Fisher Scientific), according to manufacturer’s instructions. Briefly, IgE-sensitized MCs were loaded at a concentration of 5 × 104 (RBL-2H3) and 1 × 105 (BMMCs) and incubated at 37°C for 30 min with the fluorescent Ca2+ indicator, Fluo 4 NW, in the presence of probenecid, followed by incubation at room temperature for another 30 min. Changes in fluorescence was measured by stimulating the cells with dose concentrations of DNP/HSA or A23187 or c48/80. Change in fluorescence was measured using the FDSS/μCELL kinetic plate reader (Hamamatsu Photonics, Shizuoka, Japan) or Fluoroskan Ascent FL microplate reader (Thermo Fisher Scientific) at 480 nm excitation and 540 nm emission. The ratio was calculated by the average increase in fluorescence divided by the baseline determined for each time point after the addition of stimulus.
RT-PCR
Total RNA was extracted from RBLs of transiently or permanently transfected cells along with respective controls and 6–10-wk-old BMMCs of CRF1 WT and CRF1−/− cells using Qiagen RNeasy minikit (Qiagen). One microgram of total RNA was used to synthesis the cDNA and amplified using the maxima first strand cDNA synthesis kit (Thermo Fisher Scientific). RT-PCR mix was prepared by using SYBR select master mix (Thermo Fisher Scientific) and real-time PCR was carried out in A&B Fast 7500 Biosystems (Thermo Fisher Scientific) with CRF1-specific primers (KSPQ12012; Sigma-Aldrich) and CRF2 specific primers (KSPQ12012; Sigma-Aldrich). GAPDH forward 5′-TGCACCACCAACTGCTTAG-3′ and reverse 5′-GGATGCAGGGATGATGTTC-3′ was used as normalizing control.
Protein extraction and Western blot analysis
Cellular lysates were prepared by resuspending cells in RIPA buffer (Thermo Fisher Scientific) containing protease and phosphatase inhibitors (Thermo Fisher Scientific) at 4°C. Lysates were sonicated and centrifuged at 21,000 g for 10 min. The protein concentration was measured using DC Protein Assay Kit (Bio-Rad Laboratories, Hercules, CA, USA). Cellular proteins were separated by SDS-PAGE and transferred to a polyvinylidene difluoride membrane. The membranes were blocked with 5% w/v BSA in TBS with 0.1% TBS-T for 1 h at room temperature, washed in TBS-T, incubated with either goat anti-CRF1 antibody (ab77686, 0.3 μg/ml; Abcam, Cambridge, MA, USA) or pERK (5683, 1:1000; Cell Signaling Technology, Danvers, MA, USA,) or β-actin (4970, 1:1000; Cell Signaling Technology) antibody diluted in 5% w/v BSA in TBS-T for overnight at 4°C or 2 h at room temperature and then washed with TBS-T. Subsequently, the membranes were incubated with an HRP-linked goat polyclonal (for CRF1, SC2020, 1:2000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) or rabbit polyclonal secondary antibody (for pERK and β-actin, 7074, 1:2000; Cell Signaling Technology) for 1 h at room temperature, washed with TBS-T and incubated in SuperSignal West Pico chemiluminescent substrate (Thermo Fisher Scientific). Bands were visualized with ChemiDoc MP Imager (Bio-Rad Laboratories) and the Image Lab software (version 4.1; Bio-Rad Laboratories) was used for densitometric analysis.
Immunofluorescent staining and confocal analysis
RBL-2H3 cells were cultured in 8-well chamber slides (Thermo Fisher Scientific) and 6–8-wk-old WT BMMCs were pelleted, washed once with 1× PBS, cytospun onto slides, and fixed with 4% PFA for 20 min at 4°C. Slides were blocked at room temperature for 1 h with blocking buffer (10% normal donkey serum, 0.3% Triton X-100) in 1× PBS, and incubated with goat anti-CRF1 antibody (0.3 μg/ml, ab77686; Abcam) in dilution buffer (1% BSA, 1% normal donkey serum, 0.3% Triton X-100, and 0.01% sodium azide in 1× PBS) for overnight at 4°C. Slides were washed 3 times for 5 min then incubated in anti-goat FITC (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) in dilution buffer (1:1000) for 1 h at room temperature in the dark. Slides were washed 3 times for 5 min with 1× PBS, and one drop of ProLong Diamond Antifade Mountant with DAPI (P36971; Thermo Fisher Scientific) was added with cover slips. Slides were dried at room temperature overnight and stored at 4°C until imaged. Confocal images were obtained with an FV 1000 confocal laser scanning microscope (Olympus America, Center Valley, PA, USA).
cAMP assays in BMMCs
Six-week-old WT mice BMMCs were used for this assay (2 × 106 cells/ ml). The assay was performed in 96-well low-volume plates certified for homogenous time-resolved fluorescence assays (Cisbio, Bedford, MA, USA). The assay was run in triplicate, and isobutylmethylxanthine was purchased from Sigma-Aldrich (I5879). Forskolin was used as positive control (F6886; Sigma-Aldrich). The assay was performed according to manufacturer’s protocol (62 AM4PEB; Cisbio), and the measurements were taken with a homogenous time-resolved fluorescence–compatible time-resolved fluorescence resonance energy transfer reader synergy neo plate reader (US Bio-Tek, Shoreline, WA, USA). Calculations of the ratio of the acceptor and donor emission signals for each individual well were calculated by the ratio of 665/620-nm and expressed as the fold change over vehicle.
CRF and Ucn assays in BMMCs
WT BMMCs were sensitized overnight at 37°C with 1 μg/ml anti–DNP-IgE in serum-free BMMC culture media. The following day, BMMCs (3 × 106 cells/ml) were washed and seeded on a 24-well plate (0.5 ml/well) equilibrated in serum-free BMMC culture medium for 1 h and then stimulated with vehicle (serum-free medium), DNP (50 ng/ml), or A23187 (1 μM) for 0, 5, 15, 30, 45, and 60 min at 37°C. Cells were centrifuged, and supernatant was collected. Samples were run in duplicates, and neuropeptide (CRF and Ucn) concentrations in the supernatant were quantified with CRF and Ucn EIA kit (Phoenix Pharmaceuticals, Burlingame, CA, USA), respectively, according to the manufacturer’s protocol.
Statistical analysis
Values are reported as means ± se based on the experimental number (n). Time-course data (body temperature and Ca2+ responses) were analyzed using a 1-way ANOVA on repeated measures, with time and experimental group as the main factors. Comparison among multiple experimental groups was performed using a 1- or 2-way ANOVA, where appropriate, with a Tukey’s posttest. Comparisons between two treatments were analyzed using an unpaired 2-tailed t test. Values of P < 0.05 were considered statistically significant. All statistical analyses and calculations were performed using Prism 5 software (GraphPad Software, San Diego, CA, USA).
RESULTS
CRF1 antagonism attenuates in vivo IgE-mediated MC degranulation and anaphylaxis
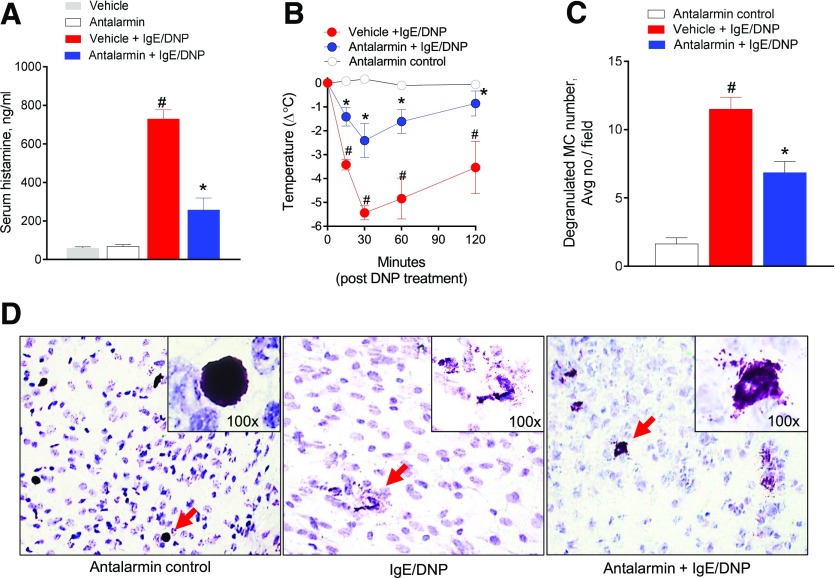
To determine the biologic importance of CRF1 in MC activation and MC-dependent pathophysiology in vivo, we examined that effect of prophylactic treatment with the CRF1 antagonist antalarmin before induction of PSA. Overnight sensitization of mice with anti-DNP IgE, followed by DNP injection, induced a marked anaphylactic response in mice as determined by a 12-fold increase in serum histamine over control animals (Fig. 1A) and a sharp reduction in core body temperature that was most severe at 30 min after DNP injection (−Δ5.4 ± 0.29°C; Fig. 1B). Compared with IgE/DNP controls, mice prophylactically treated with antalarmin markedly attenuated PSA-induced serum histamine levels (by 65%) and hypothermia (by 56%). Histologic assessment of mesenteric tissue MCs revealed a reduction in the number of degranulated MCs in antalarmin-treated mice (Fig. 1C and D).
Figure 1. Prophylactic CRF1 antagonism attenuates IgE-mediated PSA in vivo.
C57B6 WT, male mice (6–8 wk old), sensitized overnight with IgE (20 µg in 100 µl PBS), were administered PBS (vehicle) or the CRF1 antagonist antalarmin (15 mg/kg, i.v.) 30 min before i.p. injection of DNP-HSA to induce PSA. (A) Serum histamine levels in mice 120 min after PSA induction. (B) Change in body temperature after induction of PSA. (C) Average number of degranulated MCs per ×20 field in intestinal mesentery among treatment groups. (D) Representative images of mesenteric MCs stained with toluidine blue (TB) from each treatment group. Arrows indicate TB-stained MCs. Data represent means ± se and were analyzed using a 1-way ANOVA with a post hoc Tukey’s test. *#P < 0.05 vs. other treatment groups.
CRF1 expressed on MCs is critical for IgE-mediated MC degranulation and anaphylaxis
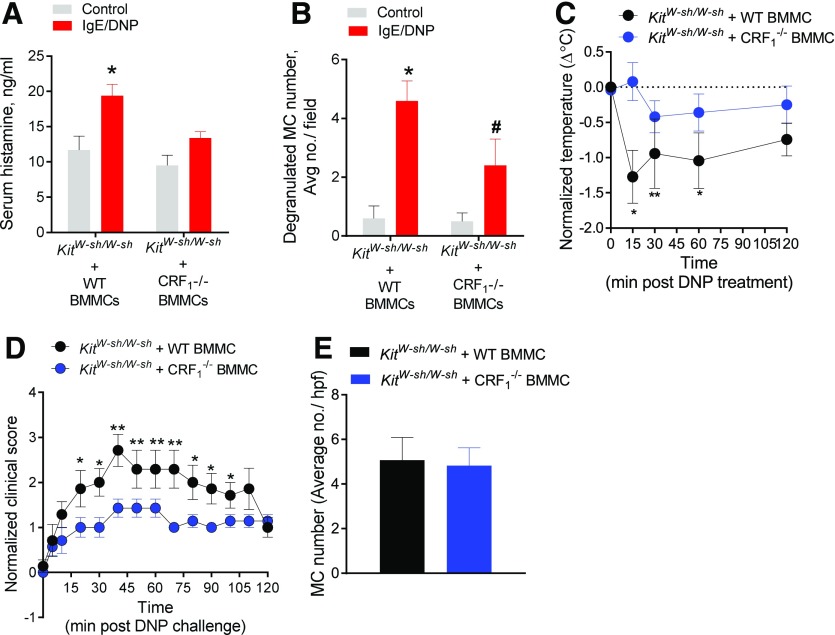
The pharmacologic CRF1 antagonist experiment above revealed an important in vivo contribution of CRF1 to PSA-induced MC degranulation and anaphylaxis. However, because of the broad expression of CRF1 receptors on multiple cell types, other than MCs, which are known to contribute to anaphylactic responses, including enteric neurons, epithelial cells, and immune cells [29–31], we next investigated the importance of CRF1 expression on MCs in IgE-mediated MC degranulation and anaphylaxis. To accomplish this, we used the MC knock-in approach, in which 8-wk-old, MC-deficient KitW-sh/W-sh mice were systemically engrafted (via i.p. injection) with cultured BMMCs derived from either WT (CRF+/+) or CRF1−/− mice. At 12 wk after engraftment, mice were sensitized with anti-IgE as previously described and challenged with DNP antigen to trigger PSA. Induction of PSA in WT BMMC-engrafted KitW-sh/W-sh mice evoked an increase in serum histamine (Fig. 2A) and increased tissue MC degranulation (Fig. 2B), with a corresponding reduction in body temperature (Fig. 2C). In contrast, KitW-sh/W-sh mice engrafted with CRF1−/− BMMCs did not exhibit significantly elevated serum histamine or hypothermic responses to PSA. In line with serum histamine and hypothermic responses, we also showed that CRF1−/− BMMC-engrafted KitW-sh/W-sh mice exhibited less-severe clinical scores associated with PSA (Fig. 2D). Compared with WT C57Bl/6 mice used in the experiment described in Fig. 1, BMMC-engrafted KitW-sh/W-sh mice exhibited lower PSA-induced serum histamine and corresponding hypothermic responses. The reduced responses in engrafted KitW-sh/W-sh mice, compared with WT mice, has been reported by others [32, 33] and likely reflects the limitations in achieving full repletion of tissue MCs to that of WT mice. Regardless, evaluation of MC engraftment efficiency in WT (CRF1+/+) and CRF1−/− BMMC-engrafted KitW-sh/W-sh did confirm the successful and comparable BMMC engraftment rates (Fig. 2E).
Figure 2. CRF1 receptors expressed on MCs promote IgE-mediated MC degranulation and anaphylaxis in vivo.
MC-deficient KitW-sh/W-sh mice were engrafted with BMMCs derived from WT or CRF1−/− mice. AT 12 wk after engraftment, PSA was induced in mice as described in the Materials and Methods section. (A) Serum histamine levels in mice 120 min after PSA induction. (B) Change in body temperature after induction of PSA. (C) Average number of degranulated MCs per ×20 field in intestinal mesentery between treatment groups. (D) Normalized clinical disease. (E) Intestinal mesentery MC counts in KitW-sh/W-sh mice engrafted with BMMCs derived from WT or CRF1−/− mice. Data represent means ± se and were analyzed using a 2-way ANOVA with a post hoc Tukey’s test (A and B) or 2-tailed t test (C–E). *P < 0.05; **P < 0.01; *#P < 0.05 vs. other treatment groups (ANOVA).
CRF1 agonists do not induce MC degranulation in murine BMMCs and RBL-2H3 MCs
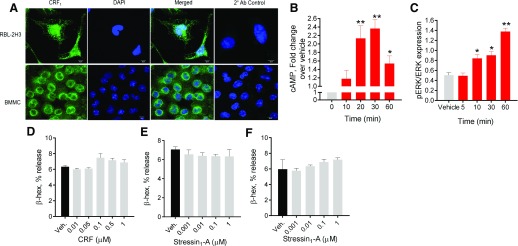
Previous in vitro studies demonstrated that human MCs express CRF receptors, which, upon activation with CRF ligand, induce the release of de novo, synthesized mediators such as vascular endothelial growth factor, IL-6 IL8, and TNF [21, 22, 24]. Although these studies demonstrated the presence of functional CRF receptor signaling in MCs, the precise role of CRF receptor subtypes in MC degranulation remains to be fully elucidated. To address that gap in knowledge, we measured MC degranulation responses to CRF1 agonists CRF and stressin1-A [34] in primary and transformed MC lines. First, we confirmed CRF receptor expression in commonly used rodent MC models, rat RBL-2H3 MCs, and murine BMMCs, via immunofluorescence labeling (Fig. 3A) and via quantitative PCR and/or Western blotting (Supplemental Fig. 1). Stimulation of MCs with CRF1 ligands induced elevations in cAMP and pERK expression in BMMCs, thus confirming that CRF receptors on BMMCs were functional and induced canonical GPCR signaling (Fig. 3B and C). However, CRF1 agonists failed to induce MC degranulation measured as β-hex release (Fig. 3D–F). The range of concentrations used for CRF were based on the affinity of CRF for CRF1 and CRF2 [35, 36] and the ranges used previously in cultured MCs [22, 24, 37] and in tissue physiology experiments in humans and animals [38, 39].
Figure 3. Influence of CRF1 agonists on cAMP, pERK signaling, and β-hex release in BMMCs and RBL-2H3 MCs.
(A) RBL-2H3 MCs and BMMCs were immunostained for CRF1 expression. (B) WT BMMCs were stimulated with CRF (0.5 µM) for indicated times, and cellular cAMP levels were measured. (C) Densitometry analysis for ERK phosphorylation, relative to total ERK in WT BMMCs stimulated with CRF (0.5 µM) for indicated times. (D and E) β-Hex release in WT BMMC cells stimulated for 1 h with dose concentrations of CRF (D) or stressin1-A (E). (F) β-Hex release in RBL-2H3 cells stimulated for 1 h with dose concentrations of stressin1-A. Data shown are the means ± se and are representative of 2 (B and C) or 3 (D–F) independent experiments performed in triplicate. Data were analyzed using a 1-way ANOVA with a post hoc Tukey’s test. *P < 0.05; **P < 0.01.
CRF1 acts as a positive modulator of MC degranulation stimuli
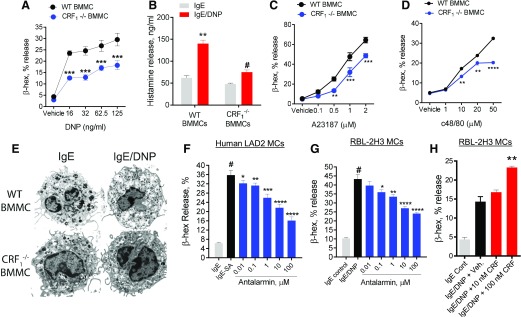
The CRF1 agonist experiments suggested that CRF1 alone was insufficient to induce MC degranulation in vitro. Therefore, we next investigated whether CRF1 could modulate MC degranulation induced by other MC degranulation stimuli. WT and CRF1−/− BMMCs (derived from CRF1−/− mice) were sensitized overnight with IgE–anti-DNP, followed by DNP to induce degranulation. Compared with WT BMMCs, CRF1−/− BMMCs exhibited suppressed degranulation responses, measured as β-hex and histamine release (Fig. 4A and B). To determine whether the dampened degranulation responses in CRF1−/− BMMCs were specific to IgE-FcεR1–mediated signaling pathways, WT and CRF1−/− BMMCs were also stimulated with MC stimuli, which use IgE-independent pathways to induce MC degranulation, including the Ca2+ ionophore A23187 and the Mrgprb2 GPCR agonist c48/80 [40]. These experiments demonstrated a suppressed β-hex release in CRF1−/− BMMCs after c48/80 and A23187 stimulation (Fig. 4C and D). Transmission electron microscopy confirmed degranulation responses, with CRF1−/− BMMCs exhibiting a less-activated appearance compared with WT BMMCs upon IgE/DNP stimulation (Fig. 4E). WT and CRF1−/− BMMCs were shown to contain similar levels of β-hex in cell pellets and were phenotypically similar to WT BMMCs, as determined by toluidine blue staining and expression of c-kit and FcεR1 by flow cytometry (Supplemental Fig. 1). Furthermore, we confirmed the reduced expression of CRF1 in CRF1−/− BMMCs and confirmed that CRF1 deficiency did not significantly alter the expression of the other CRF receptor subtype, CRF2 (Supplemental Fig. 1). Together these data provide support for attenuated degranulation responses observed in CRF1−/− BMMCs not resulting from reduced intracellular mediator content or aberrant phenotype of BMMCs. Experiments conducted with human LAD2 MCs and rat RBL-2H3 MCs treated with dose concentrations of antalarmin showed similar results to genetic CRF1−/− deficiency, with a dose-dependent suppression of IgE-mediated β-hex release (Fig. 4F and G). In contrast, costimulation of IgE/DNP with CRF (100 nM) enhanced β-hex release (Fig. 4H).
Figure 4. CRF1 deficiency or pharmacologic blockade suppresses stimuli-induced degranulation in rodent and human MCs.
(A) BMMCs derived from WT and CRF1−/− mice were sensitized overnight with IgE and stimulated with indicated concentrations of DNP-HSA for 1 h; then, β-hex release was measured as a marker of degranulation. (B) Histamine levels in supernatants from WT and CRF1−/− BMMCs stimulated with IgE/DNP. (C and D) β-Hex release from WT and CRF1−/− BMMCs that were stimulated for 1 h with A23187 (C) or c48/80 (D) at indicated concentrations. (E) Representative transmission electron micrographs of IgE-sensitized WT and CRF1−/− BMMCs +/− after 1 h of stimulation with DNP-HSA (31.5 ng/ml). (F) β-Hex release from IgE-sensitized human LAD2 MCs pretreated with indicated concentrations of antalarmin and stimulated streptavidin (SA). (G and H) β-Hex release of IgE-sensitized RBL-2H3 MCs pretreated with indicated concentrations of antalarmin (G) or CRF (H) and stimulated with DNP for 1 h. Data shown are the means ± se and are representative of 2 (B, F, and H) or 4 (A, C, and D) independent experiments performed in triplicate. Data were analyzed using either a t test (A, C, and D) or ANOVA (B and F–H) with a post hoc Tukey’s test. *P < 0.05; **P < 0.001; ***P < 0.0001; *# P < 0.05 vs. other treatment groups (ANOVA).
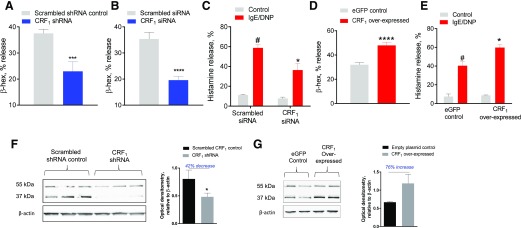
To further define the role of CRF1 expression as a modulator of MC degranulation, CRF1 expression was stably and transiently knocked down in RBL-2H3 MCs with CRF1-shRNA and CRF1-siRNA approaches. RBL-2H3 cells were also transfected with CRF1 overexpressing plasmid to enhance CRF1 expression. These experiments showed that knockdown of CRF1 with shRNA and siRNA resulted in the attenuation of IgE-FcεR1–evoked β-hex (Fig. 5A and B) and histamine release (Fig. 5C), whereas, in contrast, CRF1 overexpression in RBL-2H3 MCs amplified the degranulation response (Fig. 5D and E). We confirmed that CRF1-shRNA down-regulated CRF1 protein expression by 42% in RBL-2H3 MCs by Western blot analyses (Fig. 5F). Specifically, the CRF1 Ab used recognized 2 major protein bands in RBL-2H3 MCs at ∼55 and 37 kDa, which are consistent with the unprocessed form (55 kDa) and the deglycosylated form (37 kDa) of the receptor [41] and with previous reports by our group in the porcine intestine [18] and by others in the rodent intestine [42, 43]. In addition, we confirmed that lentiviral-mediated CRF1 overexpression resulted in a 76% increase in CRF1 protein (Fig. 5G). Together, experiments in cultured MCs demonstrated that CRF1 expression on MCs is a positive modulator of stimuli-induced MC degranulation.
Figure 5. Influence of CRF1 knockdown and overexpression in RBL-2H3 MCs on IgE-mediated MC degranulation.
(A and B) IgE/DNP induced β-hex percentage of release from RBL-2H3 cells transfected with CRF1 shRNA (A) or siRNA (B) (and scrambled controls). (C) IgE/DNP-induced histamine percentage of release into cell culture supernatants from RBL-2H3 cells transfected with siRNA. (D and E) IgE/DNP-induced β-hex (D) and histamine (E) percentage of release from RBL-2H3 cells transfected with lentiviral CRF1 overexpressing plasmid (and lentiviral empty plasmid control). (F) Western blots showing reduction in CRF1 protein bands at ∼55 and 37 kDa in CRF1-shRNA RBL-2H3 MCs and combined densitometric analysis (55 and 37 kDa bands) of CRF1 expression (relative to β-actin expression) presented as a histogram. (G) Western blot analysis showing enhanced expression of CRF1 in CRF1-overexpressed RBL-2H3 MCs and associated densitometric analysis. Data shown are the means ± se and are representative of 3 independent experiments performed in triplicate. Data were analyzed using either a t test (A and C) or 2-way ANOVA (B and D) with a post hoc Tukey’s test. *P < 0.05; **P < 0.001; ***P < 0.0001; *# P < 0.05 vs. other treatment groups (ANOVA).
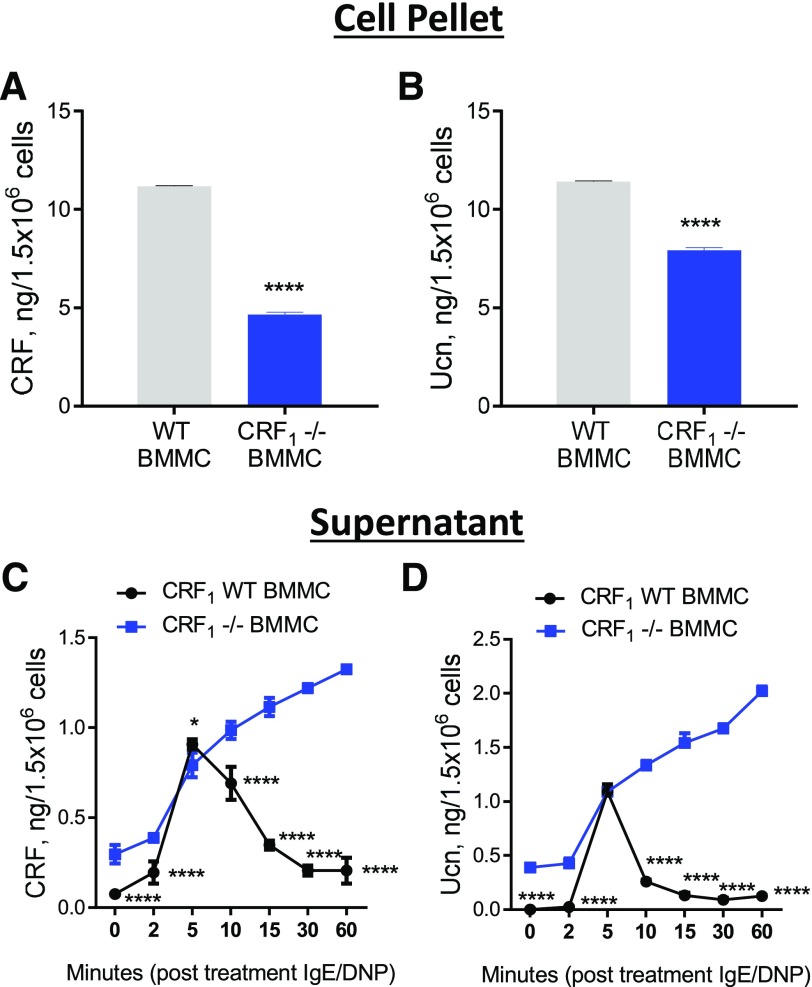
BMMCs store and rapidly release CRF and Ucn in a CRF1-dependant manner
Because the effects of CRF1-expression cultured MCs were observed in the absence of exogenous CRF ligands, we hypothesized that MC CRF1 activation and signaling during degranulation could be mediated by endogenous CRF1 ligands. We, therefore, measured the expression and release of CRF1/2 ligands CRF and Ucn under basal (nonstimulated) and IgE-stimulated conditions in mouse BMMCs (Fig. 6). These experiments showed that BMMCs store CRF and Ucn protein, as measured in cell pellets (Fig. 6A and B), and that CRF and Ucn are present in the supernatant from unstimulated BMMC cultures (Fig. 6C and D). Furthermore, upon stimulation with IgE/DNP in BMMCs, elevated concentrations of CRF and Ucn were detected in the supernatant within 2–5 min of DNP stimulation and reached peak values at 5 min, followed by a rapid decline in WT BMMCs (Fig. 6C and D). CRF1−/− BMMCs exhibited a different pattern of expression and supernatant levels than WT BMMCs did. CRF1−/− BMMCs had reduced concentrations of CRF and Ucn in cell pellets, which corresponded with higher basal levels (time 0) in unstimulated supernatants. After DNP stimulation, CRF1−/− BMMCs exhibited a similar rapid release of CRF and Ucn measured at 5 min; however, in contrast to WT BMMCs, CRF and Ucn supernatant concentrations did not decline and continued to elevate during the 60-min experiment.
Figure 6. Concentrations of stored and released CRF and Ucn in WT and CRF1−/− BMMCs.
Concentration of CRF (A) and Ucn (B) in WT and CRF1−/− BMMC pellets, as determined by ELISA. Concentrations of CRF (C) and Ucn (D) in cell supernatants at indicated times after IgE/DNP stimulation. Data shown are the means ± se and are representative of 2 independent experiments performed in triplicate. Data were analyzed using either a t test (A and B) or 2-way ANOVA (C and D) with a post hoc Tukey’s test. *P < 0.05; **P < 0.001; ***P < 0.0001; ****P < 0.00001; *# P < 0.05 vs. other treatment groups (ANOVA).
Expression of CRF1 by MCs is required for MC degranulation and intestinal permeability induced by psychologic RS
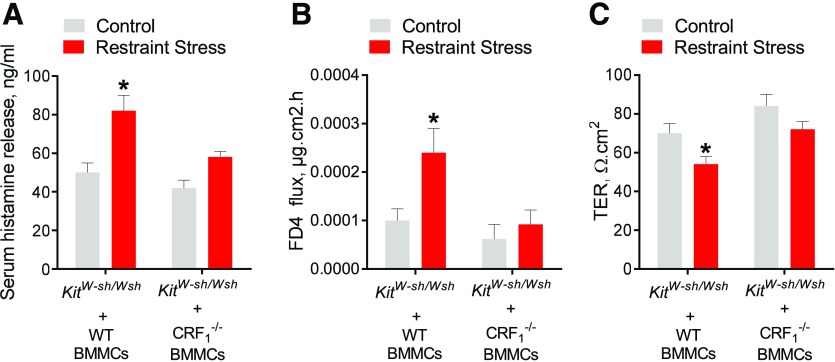
Our in vitro MC culture experiments demonstrated that CRF1 amplified degranulation responses to diverse MC degranulation stimuli, including IgE-FcεR1 cross-linking, c48/80, and A12387, suggesting that CRF1 acts as a global modulator of MC degranulation. We, therefore, hypothesized that CRF1 expression on MCs would have an important role modulating MC degranulation and associated pathophysiology in a non-IgE–dependent MC response in vivo. To test that hypothesis, we examined the role of MC CRF1 on MC degranulation and MC-dependent intestinal permeability in a RS model of psychologic stress. KitW-sh/W-sh mice were engrafted with WT or CRF1−/− BMMCs and then at 12 wk after engraftment, engrafted mice were subjected to acute RS. Compared with nonstressed controls, WT BMMC-engrafted KitW-sh/W-sh mice exhibited a significant increase in serum histamine (by 1.6-fold; Fig. 7A). In contrast, RS-induced histamine responses were absent in CRF1−/− BMMC-engrafted KitW-sh/W-sh mice. Release of MC mediators, including proteases, TNF-α, and histamine have been shown to increase intestinal permeability [39, 44], which is a central pathophysiologic mechanism in GI diseases, such as IBS and IBD [45–48]. Therefore, we measured intestinal permeability in WT- and CRF1−/−-engrafted KitW-sh/W-sh mice after RS. In accordance with MC degranulation responses, KitW-sh/W-sh mice engrafted with WT BMMCs exhibited increased ileal permeability, measured as increased FD4 flux rates (Fig. 7B) and decreased TER values (Fig. 7C) in Ussing chambers, whereas that permeability defect was absent in KitW-sh/W-sh mice engrafted with CRF1−/− BMMCs. Together, these experiments demonstrated that CRF1 expressed on MCs is a central pathophysiologic mechanism of stress-induced MC activation and intestinal permeability.
Figure 7. CRF1 expressed on MCs is critical for in vivo MC degranulation and intestinal permeability induced by acute RS.
MC-deficient KitW-sh/W-sh mice engrafted with BMMCs derived from WT or CRF1−/− mice were subjected to RS for 1 (A) or 3 (B and C) was described in the Materials and Methods section. (A) Serum histamine levels in mice after 1 h of RS. (B and C) FD4 flux rates (B) and TER (C) in colon mounted in Ussing chambers, as described in the experimental methods. Data represent means ± se for n = 5–6 animals/experimental group. Data were analyzed using a 2-way ANOVA (A–C) with a post hoc Tukey’s test (A and B). *P < 0.05.
CRF1 potentiates stimuli-induced intracellular Ca2+ mobilization
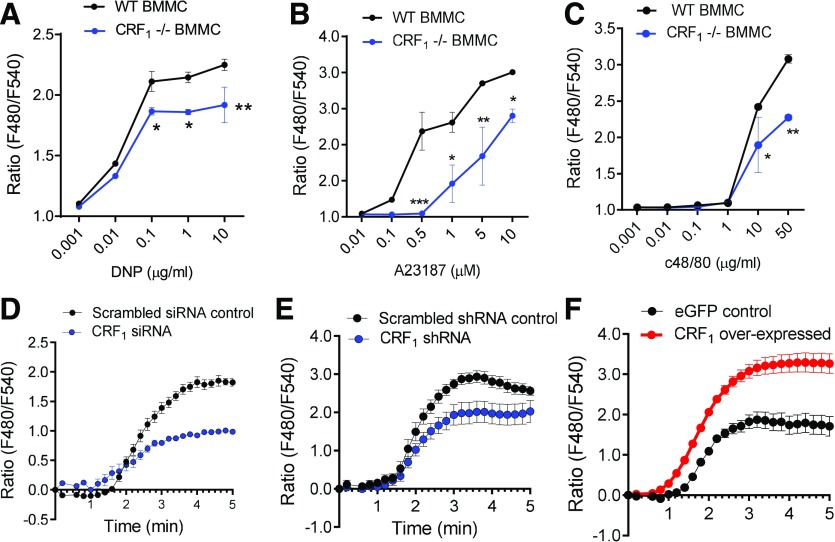
The in vitro and in vivo experiments in the present study collectively demonstrated an essential role for CRF1 on MCs in amplifying MC degranulation responses. We next explored how CRF1 positively modulated MC degranulation. Given that CRF1 was shown to potentiate MC degranulation triggered by various upstream, receptor-dependent stimuli (IgE-FcεR1 cross-linking and GPCR-triggered c48/80) and receptor-independent pathways (e.g., A23187), we hypothesized that CRF1 was modulating a common downstream target in MC degranulation. It is well established that elevations in intracellular cytosolic Ca2+ concentrations from release of intracellular stores and entry via store-operated Ca2+ entry membrane channels is a central mechanism mediating MC granule exocytosis and mediator release [49]. Using Fura-2 Ca2+ fluorescence imaging in BMMCs, we showed that CRF1−/− BMMCs exhibited attenuated intracellular Ca2+ mobilization in response to IgE/DNP, c48/80, and A23187 (Fig. 8A–C). Ca2+ responses were also diminished in RBL-2H3 MCs in which CRF1 expression was knocked down with siRNA (Fig. 8D) or shRNA (Fig. 8E), whereas, in contrast, CRF1-overexpressing RBL-2H3 MCs exhibited exacerbated IgE/DNP-induced by Ca2+ mobilization (Fig. 8F). To distinguish whether the Ca2+-potentiating effects of CRF1 were due to intracellular (endoplasmic reticulum) Ca2+ release vs. extracellular Ca2+ entry mechanisms, we conducted experiments with CRF1-overexpressing RBL-2H3 MCs with the sarco/endoplasmic reticulum Ca2+-ATPase inhibitor thapsigargin, in the presence and absence of extracellular Ca2+. Under Ca2+-replete conditions, thapsigargin induced elevations in cytosolic Ca2+, with a greater response observed in CRF1-overexpressed RBL-2H3 MCs. Removal of extracellular Ca2+ significantly diminished the thapsigargin-induced Ca2+ responses in control and in CRF1-overexpressing RBL MCs; however, an elevated Ca2+ response in CRF1-overexpressing RBL MCs was still evident in Ca2+-free conditions (Fig. 9), therefore, suggesting that CRF1 potentiates Ca2+ release from intracellular stores.
Figure 8. CRF1 potentiates stimuli-induced Ca2+ mobilization in MCs.
RBL-2H3 MCs and BMMCs were loaded with the Fluo 4 Ca2+ indicator and stimulated with the indicated MC degranulation stimuli, as described in the Material and Methods section. (A–C) Intracellular Ca2+ mobilization in response to indicated concentrations of IgE/DNP (A), A23187 (B), and c48/80 (C). (D and E) IgE/DNP-induced intracellular Ca2+ mobilization in RBL-2H3 cells transfected with CRF1-siRNA (D) or CRF1-shRNA (E) and respective scrambled controls. (F) IgE/DNP-induced intracellular Ca2+ mobilization in RBL-2H3 cells stably transfected with lentiviral CRF1-overexpressed plasmid or lentiviral empty plasmid control. Data shown are the means ± se and are representative of 3 independent experiments performed in triplicate. Data were analyzed using a t test *P < 0.05; **P < 0.001; ***P < 0.0001.
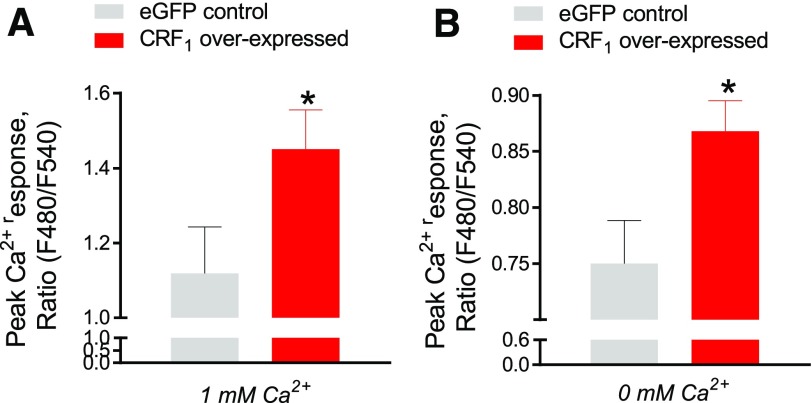
Figure 9. CRF1 potentiates Ca2+ mobilization from intracellular stores in MCs.
RBL-2H3 cells stably transfected with lentiviral CRF1-overexpressed plasmid or empty control plasmid cells were stimulated with the sarco/endoplasmic reticulum Ca2+-ATPase inhibitor thapsigargin in the presence or absence of Ca2+, as described in the Materials and Methods section. (A) Peak Ca2+ responses (ΔF480/F540 ratio) induced by thapsigargin in RBL-2H3 cells under Ca2+-replete (1 mM Ca2+) (A) or Ca2+-free (B) media. Data shown are the means ± se and are representative of 4 independent experiments performed in triplicate. Data were analyzed using a 2-tailed t test. *P < 0.05.
DISCUSSION
MCs play a critical role in host defense and are among the earliest immune cell responders to environmental, immunologic, and infectious stressors [2, 50]. To perform that role, MCs express a myriad of receptors to sense and integrate stress cues from the microenvironment and, in turn, elicit a quick and powerful immune response via degranulation. Stress is known to modulate MC degranulation and exacerbate MC-associated disorders [5, 7, 51]. Although many stress mediators, such as CRF substance P and catecholamines, have been shown to influence MC activity, the precise mechanisms by which stress modulates MC degranulation and disease susceptibility in vivo has remained elusive. Using genetic and pharmacologic approaches in culture of rodent and human MCs and MC-dependent animal models of anaphylaxis and psychologic stress, we demonstrated a novel role for the MC CRF system via CRF1 receptors in the potentiation of MC degranulation responses and downstream disease pathophysiology.
In the present study, prophylactic administration of the CRF1-antagonist antalarmin significantly attenuated IgE-mediated MC degranulation and anaphylaxis, thus demonstrating the importance of CRF1 as an important and potentially therapeutic target in IgE-mediated anaphylactic responses. Furthermore, engraftment studies in which MC-deficient KitW-sh-Wsh mice were engrafted with WT or CRF1−/− BMMCs highlighted the biologic significance of MC-specific CRF1 receptors in that response. When exploring the functional role of CRF1 in cultured primary MCs and MC lines, we found that, although direct activation of CRF1 alone with CRF1 agonists induced an up-regulation of intracellular signaling pathways, including induction of cAMP and pERK, it did not trigger MC degranulation. The lack of a degranulation response by CRF1 agonists was consistent across all species of MCs used in the present study and is in line with what has been previously reported with CRF receptor ligands in cultured human MCs [22, 24]. However, a compound exocytosis/endocytosis event and 5HT release induced by CRF has been described previously in mouse peritoneal and intestinal MCs [37]. The lack of degranulation response in the present study suggests that the GPCR signaling pathways induced upon CRF1 activation may not directly trigger significant MC degranulation; however, the possibility that in vitro conditions or in vivo tissue factors have a role cannot be ruled out. Despite the inability of CRF1 activation to induce MC degranulation, we showed that activation or inhibition of CRF1 via genetic and pharmacologic approaches resulted in an enhancement or attenuation of stimuli-induced MC degranulation, respectively, induced by a broad array of potent MC stimuli, including IgE/FcƐR1 cross-linking, c48/80, and A23187. These findings were consistent across mouse, rat, and human MCs, supporting the conserved role of MC CRF1 across species. Together, these data demonstrated that, although CRF1 may not directly induce MC degranulation, it exerts a positive, global modulation on MC degranulation.
In the present study, CRF1 expression or pharmacologic blockade in MCs was able to modulate stimuli-induced MC degranulation in the absence of exogenous CRF1 ligands. Experiments measuring the cellular content and the time course release of CRF and Ucn in activated BMMCs revealed that CRF and Ucn are both stored as proteins in the MC and are constitutively released under basal conditions. Furthermore, upon activation with IgE/DNP, a rapid release of CRF and Ucn was observed, which was measurable within 2 min of stimulation, peaked at 5 min, and then rapidly declined thereafter. Together these data provide evidence under basal and activated conditions that CRF receptors are continually exposed active ligands, which conceivably, can rapidly modulate responses to MC stimuli in an autocrine manner. The reason for the decline in supernatant levels of CRF and Ucn after 5 min of stimulation is unclear at this time but could represent a rapid reuptake or retrieval of CRF and Ucn by MCs. Released granule mediator recycling is known to occur rapidly after induction of exocytosis in MCs [52]. Interestingly, we found that CRF1−/− BMMCs exhibited different levels and kinetics for CRF and Ucn release. First, CRF1−/− BMMCs exhibited reduced cellular content of CRF and Ucn and higher basal (unstimulated) levels of CRF and Ucn, compared with WT BMMCs. Second, although the initial IgE/DNP-induced release of CRF and Ucn into the supernatant by WT and CRF1−/− BMMCs was similar, concentrations of CRF and Ucn from CRF1−/− BMMCs did not decline like WT BMMC cultures did and continued to elevate over time. These findings suggest that MC CRF1 could be involved in regulating CRF and Ucn recycling. This is further supported by the reduced pellet CRF and Ucn and higher basal release of CRF and Ucn observed in CRF1−/− BMMCs. Overall, these data provide new insight into a potential mechanism, via CRF1, by which MC CRF receptor ligands are regulated. The precise role of autocrine ligand/CRF1 interactions in mediating the potentiating effects of MC CRF1 on degranulation remains to be elucidated. Such a mechanism could provide a pathway by which MCs are able to be repeatedly activated and release granule mediators, which may be further exacerbated during times of stress.
The present experiments revealed that CRF1 expression and signaling potentiated MC degranulation in responses to a broad array of MC stimuli that use upstream, receptor-dependent (e.g., IgE-FcεR1 and c48/80) and downstream, receptor-independent (e.g., A23187) mechanisms. We were particularly surprised with the ability of CRF1 expression to modulate A23187 actions on MC degranulation. Initially, this finding prompted us to consider an extracellular entry event targeted by CRF1. However, Ca2+-free media did not completely ablate the effect of CRF1 overexpression, suggesting that CRF1 was targeting an intracellular (e.g., ER)-Ca2+ mobilization mechanism. In turn, the enhanced intracellular Ca2+ mobilization by CRF1 would enhance extracellular Ca2+ entry via plasma-membrane CRAC and transient receptor potential cation channels, which drive MC granule exocytosis during degranulation [49, 53]. It is possible that CRF1 effects on A23187-mediated Ca2+ and degranulation could reflect the activation of an intracellular, stored Ca2+ release mechanism modulated by A23187. Although classically recognized for its effect on extracellular Ca2+ entry, evidence has emerged for additional components of A23187-mediated Ca2+ mobilization via upstream activation of PLC and PKC. Dedkova et al. [54] demonstrated that a component of Ca2+ mobilization by A23187 in several tumor and immune cells, originated from a PLC-dependent mobilization of Ca2+ from intracellular stores, whereas an additional influx of Ca2+ into the cells is mediated by a store-regulated mechanism. Kim et al. [55] also showed that A23187 signaling in murine BMMCs can rapidly phosphorylate and activate PKC and Iκ B kinase, which, in turn, enhanced SNARE complex formation and degranulation. Other potential mechanisms for global regulation of Ca2+ mobilization and degranulation by CRF1 might be explained by the following: 1) the influence of CRF1 signaling could be multipronged, regulating both intracellular stores via ER release and extracellular Ca2+ entry mechanisms via CRAC; 2) constitutively active CRF1 signaling, which is supported by the basal production and release of CRF and Ucn shown in this study, could modify the formation STIM-Ori/CRAC complexes in the plasma membrane, thus allowing more-rapid Ca2+ influx with A23187; and 3) CRF1 signaling could modify cytoskeletal biology and dynamics that are required for exocytosis by all stimuli.
Precisely how initiation of upstream CRF1 signaling leads to a potentiation of stimuli-induced intracellular Ca2+ release and degranulation remains to be elucidated. CRF1 and CRF2 receptors are class B1 (secretin-like) GPCRs with complex signaling mechanisms that can couple to different G proteins in a cell-type–specific manner to produce an array of downstream signaling. Although both receptors are reported to activate Gαs and cAMP signaling and Gq and Ca2+ signaling [56, 57], there is also clear evidence for coupling to Gq/11 and Gi/o proteins [30, 58–60]. The latter 2 contribute to activating ERK [58, 61]. The downstream effector mechanisms of CRF1 and CRF2 receptors may also be modulated by cell-type and/or coexpressed scaffolding proteins [30, 60, 61]. GPCRs, such as EP- and sphingosine 1 phosphate-adenosine receptors, can modulate IgE antigen-mediated Ca2+ mobilization and degranulation via G protein–dependent mechanisms [62–69]. It remains unclear whether CRF1 uses similar mechanisms. Given that Ca2+ mobilization represents a common downstream mechanism for degranulation induced by many MC stimuli, these findings implicate a likely mechanism by which CRF1 exerts a global influence on MC degranulation in response to broad range of MC stimuli.
The ability of CRF1 to modulate a diverse array of MC degranulation stimuli and downstream Ca2+ mobilization lead us to hypothesize that MC CRF1 acts a global modulator of MC degranulation and thus would influence non-IgE–dependent MC degranulation and disease pathophysiology. Supporting that hypothesis, MC-deficient KitW-sh-Wsh mice engrafted with CRF1−/− BMMCs exhibited suppressed MC degranulation and associated downstream pathophysiology in response to both IgE-mediated PSA and psychologic RS. Specifically, in the present study, we demonstrated that MC-specific CRF1 expression has an important role in modulating stress-induced intestinal permeability. Although a link between the CRF system, MC activation, and intestinal permeability has been shown in animal models and in humans [10, 38, 39], the precise mechanisms have remained controversial. This new finding has implications for the understanding of highly prevalent GI conditions associated with impaired intestinal barrier function, such as IBS and IBD.
The broad potentiating influence of CRF1 in response to various MC stimuli and in different disease models demonstrated in the present study could provide unique insight into a common pathogenesis for a wide range of MC disorders in which disease flares are triggered by stress, including IBS, chronic pain disorders, arthritis, and asthma. For example, dysregulation of a common stress pathway in the MC, potentially via the MC-CRF1 system, could result in an overall hyperreactive MC state, which, in turn, would lead to exacerbated MC responses and disease. Although such a system may have evolved as a protective mechanism to up-regulate MC activity and host defense in the face of an acute, stressful challenge, dysregulation of this system could contribute to chronic MC disease through the persistent, heightened MC activity. Further elucidation of the mechanisms by which CRF1 amplifies MC responses and under which disease states (e.g., acute vs. chronic, psychologic vs. infections, etc.) will be important to identify potential downstream targets aimed at suppressing MC degranulation and disease. Alternatively, knowledge of how CRF1 enhances MC function could also be applied to situations in which augmenting MC responses would be beneficial to the host, such as for the clearance of pathogens [70, 71] or as vaccine adjuvants [72, 73].
In summary, the present study highlights a novel role for CRF1 receptors expressed on MCs as positive, global modulators of MC degranulation in the acute responses to immunologic and psychologic stress. Given the critical role of MCs as immune sentinels that rapidly respond to diverse stressful stimuli, the MC CRF system may represent a sensing mechanism by the MC to integrate environmental and host stress cues and rapidly amplify degranulation responses, which are critical for survival and the return to homeostasis. However, at the same time, dysregulation or chronic activation of this system could also contribute to mechanisms of chronic disease.
AUTHORSHIP
All authors contributed to generating that data shown in this manuscript. S.A., A.J.G., S.D., and A.J.M. wrote the manuscript, which was reviewed by all authors.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health [Grants R01 HD072968 (to A.J.M.), DK097462 (to A.J.M.), and R03 DK097462 (to A.J.M.)]. A.J.M. is the Matilda R. Wilson Endowed Chair of Large Animal Clinical Sciences at Michigan State University, College of Veterinary Medicine. Stressin-1A and CRF were a kind gift of Dr. Jean Rivier, Sentia Medical Sciences, Inc., Salk Institute for Biological Studies. The authors gratefully acknowledge the assistance of Agnella Izzo Matic, AIM Biomedical LLC, in editing the manuscript. The authors gratefully acknowledge Tom Dexheimer, Manager of the Assay Development and Drug Repurposing Core (ADDRC) at Michigan State University for his advice and technical expertise in conducting the Ca2+ fluorescence analyses.
Glossary
- β-Hex
β-hexosaminidase
- BMMC
bone marrow–derived mast cell
- CRAC
calcium release-activated channels
- CRF
corticotropin-releasing factor
- CRF1/2
corticotropin-releasing factor receptor subtype 1/2
- EGFP
enhanced GFP
- EIA
enzyme immunoassay
- GI
gastrointestinal
- GPCR
G protein-coupled receptor
- HPA
hypothalamic-pituitary-adrenal
- IBD
inflammatory bowel disease
- IBS
irritable bowel syndrome
- LAD2
leukocyte adhesion deficiency-2
- LB
Luria broth
- MC
mast cell
- pERK
phosphorylated extracellular signal-regulated kinase
- PKC
protein kinase C
- PLC
phospholipase C
- PSA
passive systemic anaphylaxis
- RBL
rat basophilic leukemia
- RS
restraint stress
- shRNA
short hairpin RNA
- siRNA
small interfering RNA
- TER
transepithelial electrical resistance
- Ucn
urocortin
- WT
wild-type
Footnotes
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
SEE CORRESPONDING EDITORIAL ON PAGE 1284
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Galli S. J., Tsai M. (2012) IgE and mast cells in allergic disease. Nat. Med. 18, 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galli S. J., Tsai M., Piliponsky A. M. (2008) The development of allergic inflammation. Nature 454, 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bischoff S. C. (2016) Mast cells in gastrointestinal disorders. Eur. J. Pharmacol. 778, 139–145. [DOI] [PubMed] [Google Scholar]
- 4.Boeckxstaens G. E., Wouters M. M. (2017) Neuroimmune factors in functional gastrointestinal disorders: a focus on irritable bowel syndrome. Neurogastroenterol. Motil. 29, e13007. [DOI] [PubMed] [Google Scholar]
- 5.Heffner K. L., Kiecolt-Glaser J. K., Glaser R., Malarkey W. B., Marshall G. D. (2014) Stress and anxiety effects on positive skin test responses in young adults with allergic rhinitis. Ann. Allergy Asthma Immunol. 113, 13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andrae D. A., Patrick D. L., Drossman D. A., Convington P. S. (2013) Evaluation of the irritable bowel syndrome quality of life (IBS-QOL) questionnaire in diarrhea-predominant irritable bowel syndrome patients. Health Qual Life Outcomes 11, 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Priftis K. N., Papadimitriou A., Nicolaidou P., Chrousos G. P. (2009) Dysregulation of the stress response in asthmatic children. Allergy 64, 18–31. [DOI] [PubMed] [Google Scholar]
- 8.Santos J., Benjamin M., Yang P. C., Prior T., Perdue M. H. (2000) Chronic stress impairs rat growth and jejunal epithelial barrier function: role of mast cells. Am. J. Physiol. Gastrointest. Liver Physiol. 278, G847–G854. [DOI] [PubMed] [Google Scholar]
- 9.Söderholm J. D., Yang P. C., Ceponis P., Vohra A., Riddell R., Sherman P. M., Perdue M. H. (2002) Chronic stress induces mast cell-dependent bacterial adherence and initiates mucosal inflammation in rat intestine. Gastroenterology 123, 1099–1108. [DOI] [PubMed] [Google Scholar]
- 10.Wallon C., Yang P. C., Keita A. V., Ericson A. C., McKay D. M., Sherman P. M., Perdue M. H., Söderholm J. D. (2008) Corticotropin-releasing hormone (CRH) regulates macromolecular permeability via mast cells in normal human colonic biopsies in vitro. Gut 57, 50–58. [DOI] [PubMed] [Google Scholar]
- 11.Santos J., Saunders P. R., Hanssen N. P., Yang P. C., Yates D., Groot J. A., Perdue M. H. (1999) Corticotropin-releasing hormone mimics stress-induced colonic epithelial pathophysiology in the rat. Am. J. Physiol. 277, G391–G399. [DOI] [PubMed] [Google Scholar]
- 12.Lennon E. M., Maharshak N., Elloumi H., Borst L., Plevy S. E., Moeser A. J. (2013) Early life stress triggers persistent colonic barrier dysfunction and exacerbates colitis in adult IL-10-/- mice. Inflamm. Bowel Dis. 19, 712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moeser A. J., Ryan K. A., Nighot P. K., Blikslager A. T. (2007) Gastrointestinal dysfunction induced by early weaning is attenuated by delayed weaning and mast cell blockade in pigs. Am. J. Physiol. Gastrointest. Liver Physiol. 293, G413–G421. [DOI] [PubMed] [Google Scholar]
- 14.Bale T. L., Vale W. W. (2004) CRF and CRF receptors: role in stress responsivity and other behaviors. Annu. Rev. Pharmacol. Toxicol. 44, 525–557. [DOI] [PubMed] [Google Scholar]
- 15.Coin I., Katritch V., Sun T., Xiang Z., Siu F. Y., Beyermann M., Stevens R. C., Wang L. (2013) Genetically encoded chemical probes in cells reveal the binding path of urocortin-I to CRF class B GPCR. Cell 155, 1258–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.la Fleur S. E., Wick E. C., Idumalla P. S., Grady E. F., Bhargava A. (2005) Role of peripheral corticotropin-releasing factor and urocortin II in intestinal inflammation and motility in terminal ileum. Proc. Natl. Acad. Sci. USA 102, 7647–7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y., Fang X., Yuan J., Sun Z., Li C., Li R., Li L., Zhu C., Wan R., Guo R., Jin L., Li S. (2014) The role of corticotropin-releasing hormone receptor 1 in the development of colitis-associated cancer in mouse model. Endocr. Relat. Cancer 21, 639–651. [DOI] [PubMed] [Google Scholar]
- 18.Boyer P. E., D’Costa S., Edwards L. L., Milloway M., Susick E., Borst L. B., Thakur S., Campbell J. M., Crenshaw J. D., Polo J., Moeser A. J. (2015) Early-life dietary spray-dried plasma influences immunological and intestinal injury responses to later-life Salmonella Typhimurium challenge. Br. J. Nutr. 113, 783–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahajan S., Liao M., Barkan P., Takahashi K., Bhargava A. (2014) Urocortin 3 expression at baseline and during inflammation in the colon: corticotropin releasing factor receptors cross-talk. Peptides 54, 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taché Y., Kiank C., Stengel A. (2009) A role for corticotropin-releasing factor in functional gastrointestinal disorders. Curr. Gastroenterol. Rep. 11, 270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asadi S., Alysandratos K. D., Angelidou A., Miniati A., Sismanopoulos N., Vasiadi M., Zhang B., Kalogeromitros D., Theoharides T. C. (2012) Substance P (SP) induces expression of functional corticotropin-releasing hormone receptor-1 (CRHR-1) in human mast cells. J. Invest. Dermatol. 132, 324–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao J., Cetrulo C. L., Theoharides T. C. (2006) Corticotropin-releasing hormone induces vascular endothelial growth factor release from human mast cells via the cAMP/protein kinase A/p38 mitogen-activated protein kinase pathway. Mol. Pharmacol. 69, 998–1006. [DOI] [PubMed] [Google Scholar]
- 23.Papadopoulou N. G., Oleson L., Kempuraj D., Donelan J., Cetrulo C. L., Theoharides T. C. (2005) Regulation of corticotropin-releasing hormone receptor-2 expression in human cord blood-derived cultured mast cells. J. Mol. Endocrinol. 35, R1–R8. [DOI] [PubMed] [Google Scholar]
- 24.Cao J., Papadopoulou N., Kempuraj D., Boucher W. S., Sugimoto K., Cetrulo C. L., Theoharides T. C. (2005) Human mast cells express corticotropin-releasing hormone (CRH) receptors and CRH leads to selective secretion of vascular endothelial growth factor. J. Immunol. 174, 7665–7675. [DOI] [PubMed] [Google Scholar]
- 25.Smith G. W., Aubry J. M., Dellu F., Contarino A., Bilezikjian L. M., Gold L. H., Chen R., Marchuk Y., Hauser C., Bentley C. A., Sawchenko P. E., Koob G. F., Vale W., Lee K. F. (1998) Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron 20, 1093–1102. [DOI] [PubMed] [Google Scholar]
- 26.Sun J., Arias K., Alvarez D., Fattouh R., Walker T., Goncharova S., Kim B., Waserman S., Reed J., Coyle A. J., Jordana M. (2007) Impact of CD40 ligand, B cells, and mast cells in peanut-induced anaphylactic responses. J. Immunol. 179, 6696–6703. [DOI] [PubMed] [Google Scholar]
- 27.Smith F., Clark J. E., Overman B. L., Tozel C. C., Huang J. H., Rivier J. E., Blikslager A. T., Moeser A. J. (2010) Early weaning stress impairs development of mucosal barrier function in the porcine intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 298, G352–G363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medland J. E., Pohl C. S., Edwards L. L., Frandsen S., Bagley K., Li Y., Moeser A. J. (2016) Early life adversity in piglets induces long-term upregulation of the enteric cholinergic nervous system and heightened, sex-specific secretomotor neuron responses. Neurogastroenterol. Motil. 28, 1317–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gourcerol G., Wu S. V., Yuan P. Q., Pham H., Miampamba M., Larauche M., Sanders P., Amano T., Mulak A., Im E., Pothoulakis C., Rivier J., Taché Y., Million M. (2011) Activation of corticotropin-releasing factor receptor 2 mediates the colonic motor coping response to acute stress in rodents. Gastroenterology 140, 1586–1596.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tao J., Zhang Y., Huang H., Jiang X. (2009) Activation of corticotropin-releasing factor 2 receptor inhibits Purkinje neuron P-type calcium currents via Goα-dependent PKC epsilon pathway. Cell. Signal. 21, 1436–1443. [DOI] [PubMed] [Google Scholar]
- 31.Yuan P. Q., Wu S. V., Elliott J., Anton P. A., Chatzaki E., Million M., Taché Y. (2012) Expression of corticotropin releasing factor receptor type 1 (CRF1) in the human gastrointestinal tract and upregulation in the colonic mucosa in patients with ulcerative colitis. Peptides 38, 62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cyphert J. M., Kovarova M., Koller B. H. (2011) Unique populations of lung mast cells are required for antigen-mediated bronchoconstriction. Clin. Exp. Allergy 41, 260–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu M., Tsai M., Tam S. Y., Jones C., Zehnder J., Galli S. J. (2006) Mast cells can promote the development of multiple features of chronic asthma in mice. J. Clin. Invest. 116, 1633–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rivier J., Gulyas J., Kunitake K., DiGruccio M., Cantle J. P., Perrin M. H., Donaldson C., Vaughan J., Million M., Gourcerol G., Adelson D. W., Rivier C., Taché Y., Vale W. (2007) Stressin1-A, a potent corticotropin releasing factor receptor 1 (CRF1)-selective peptide agonist. J. Med. Chem. 50, 1668–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dautzenberg F. M., Kilpatrick G. J., Hauger R. L., Moreau J. (2001) Molecular biology of the CRH receptors-- in the mood. Peptides 22, 753–760. [DOI] [PubMed] [Google Scholar]
- 36.Hauger R. L., Risbrough V., Brauns O., Dautzenberg F. M. (2006) Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: new molecular targets. CNS Neurol. Disord. Drug Targets 5, 453–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balseiro-Gomez S., Flores J. A., Acosta J., Ramirez-Ponce M. P., Ales E. (2015) Identification of a new exo-endocytic mechanism triggered by corticotropin-releasing hormone in mast cells. J. Immunol. 195, 2046–2056. [DOI] [PubMed] [Google Scholar]
- 38.Wallon C., Söderholm J. D. (2009) Corticotropin-releasing hormone and mast cells in the regulation of mucosal barrier function in the human colon. Ann. N. Y. Acad. Sci. 1165, 206–210. [DOI] [PubMed] [Google Scholar]
- 39.Overman E. L., Rivier J. E., Moeser A. J. (2012) CRF induces intestinal epithelial barrier injury via the release of mast cell proteases and TNF-α. PLoS One 7, e39935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McNeil B. D., Pundir P., Meeker S., Han L., Undem B. J., Kulka M., Dong X. (2015) Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 519, 237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slominski A., Zbytek B., Pisarchik A., Slominski R. M., Zmijewski M. A., Wortsman J. (2006) CRH functions as a growth factor/cytokine in the skin. J. Cell. Physiol. 206, 780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lakshmanan J., Magee T. R., Richard J. D., Liu G. L., Salido E., Sugano S. K., Ferrini M., Ross M. G. (2008) Localization and gestation-dependent pattern of corticotrophin-releasing factor receptor subtypes in ovine fetal distal colon. Neurogastroenterol. Motil. 20, 1328–1339. [DOI] [PubMed] [Google Scholar]
- 43.O’Malley D., Dinan T. G., Cryan J. F. (2010) Alterations in colonic corticotropin-releasing factor receptors in the maternally separated rat model of irritable bowel syndrome: differential effects of acute psychological and physical stressors. Peptides 31, 662–670. [DOI] [PubMed] [Google Scholar]
- 44.Groschwitz K. R., Ahrens R., Osterfeld H., Gurish M. F., Han X., Abrink M., Finkelman F. D., Pejler G., Hogan S. P. (2009) Mast cells regulate homeostatic intestinal epithelial migration and barrier function by a chymase/Mcpt4-dependent mechanism. Proc. Natl. Acad. Sci. USA 106, 22381–22386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou Q., Zhang B., Verne G. N. (2009) Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain 146, 41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Camilleri M., Lasch K., Zhou W. (2012) Irritable bowel syndrome: methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 303, G775–G785. [DOI] [PubMed] [Google Scholar]
- 47.Issenman R. M., Jenkins R. T., Radoja C. (1993) Intestinal permeability compared in pediatric and adult patients with inflammatory bowel disease. Clin. Invest. Med. 16, 187–196. [PubMed] [Google Scholar]
- 48.Vanuytsel T., van Wanrooy S., Vanheel H., Vanormelingen C., Verschueren S., Houben E., Salim Rasoel S., Tόth J., Holvoet L., Farré R., Van Oudenhove L., Boeckxstaens G., Verbeke K., Tack J. (2014) Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut 63, 1293–1299. [DOI] [PubMed] [Google Scholar]
- 49.Holowka D., Wilkes M., Stefan C., Baird B. (2016) Roles for Ca2+ mobilization and its regulation in mast cell functions: recent progress. Biochem. Soc. Trans. 44, 505–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abraham S. N., St John A. L. (2010) Mast cell-orchestrated immunity to pathogens. Nat. Rev. Immunol. 10, 440–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bennett E. J., Tennant C. C., Piesse C., Badcock C. A., Kellow J. E. (1998) Level of chronic life stress predicts clinical outcome in irritable bowel syndrome. Gut 43, 256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Balseiro-Gomez S., Flores J. A., Acosta J., Ramirez-Ponce M. P., Ales E. (2016) Transient fusion ensures granule replenishment to enable repeated release after IgE-mediated mast cell degranulation. J. Cell Sci. 129, 3989–4000. [DOI] [PubMed] [Google Scholar]
- 53.Suzuki R., Liu X., Olivera A., Aguiniga L., Yamashita Y., Blank U., Ambudkar I., Rivera J. (2010) Loss of TRPC1-mediated Ca2+ influx contributes to impaired degranulation in Fyn-deficient mouse bone marrow-derived mast cells. J. Leukoc. Biol. 88, 863–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dedkova E. N., Sigova A. A., Zinchenko V. P. (2000) Mechanism of action of calcium ionophores on intact cells: ionophore-resistant cells. Membr. Cell Biol. 13, 357–368. [PubMed] [Google Scholar]
- 55.Kim D. Y., Kang T. B., Shim D. W., Sun X., Han J. W., Ji Y. E., Kim T. J., Koppula S., Lee K. H. (2014) Emodin attenuates A23187-induced mast cell degranulation and tumor necrosis factor-α secretion through protein kinase C and IκB kinase 2 signaling. Eur. J. Pharmacol. 723, 501–506. [DOI] [PubMed] [Google Scholar]
- 56.Dautzenberg, F. M., Grigoriadis D. E., Hauger R. L., Steckler T., Vale W. W. (2016) Corticotropin-releasing factor receptors: CRF1 receptor—IUPHAR/BPS guide to pharmacology. Available at: http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=212. Accessed September 19, 2016.
- 57.Dautzenberg, F. M. Grigoriadis D. E., Hauger R. L., Steckler T., Vale W. W. (2016) Corticotropin-releasing factor receptors: CRF2 receptor. IUPHAR/BPS guide to pharmacology. Available at: http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=213. Accessed September 19, 2016.
- 58.Punn A., Chen J., Delidaki M., Tang J., Liapakis G., Lehnert H., Levine M. A., Grammatopoulos D. K. (2012) Mapping structural determinants within third intracellular loop that direct signaling specificity of type 1 corticotropin-releasing hormone receptor. J. Biol. Chem. 287, 8974–8985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brailoiu G. C., Deliu E., Tica A. A., Chitravanshi V. C., Brailoiu E. (2012) Urocortin 3 elevates cytosolic calcium in nucleus ambiguus neurons. J. Neurochem. 122, 1129–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Makarewich C. A., Troupes C. D., Schumacher S. M., Gross P., Koch W. J., Crandall D. L., Houser S. R. (2015) Comparative effects of urocortins and stresscopin on cardiac myocyte contractility. J. Mol. Cell. Cardiol. 86, 179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walther C., Caetano F. A., Dunn H. A., Ferguson S. S. (2015) PDZK1/NHERF3 differentially regulates corticotropin-releasing factor receptor 1 and serotonin 2A receptor signaling and endocytosis. Cell. Signal. 27, 519–531. [DOI] [PubMed] [Google Scholar]
- 62.Gilfillan A. M., Peavy R. D., Metcalfe D. D. (2009) Amplification mechanisms for the enhancement of antigen-mediated mast cell activation. Immunol. Res. 43, 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuehn H. S., Gilfillan A. M. (2007) G protein-coupled receptors and the modification of FcεRI-mediated mast cell activation. Immunol. Lett. 113, 59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feng C., Beller E. M., Bagga S., Boyce J. A. (2006) Human mast cells express multiple EP receptors for prostaglandin E2 that differentially modulate activation responses. Blood 107, 3243–3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kay L. J., Yeo W. W., Peachell P. T. (2006) Prostaglandin E2 activates EP2 receptors to inhibit human lung mast cell degranulation. Br. J. Pharmacol. 147, 707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kuehn H. S., Beaven M. A., Ma H. T., Kim M. S., Metcalfe D. D., Gilfillan A. M. (2008) Synergistic activation of phospholipases Cγ and Cβ: a novel mechanism for PI3K-independent enhancement of FcεRI-induced mast cell mediator release. Cell. Signal. 20, 625–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Serra-Pages M., Olivera A., Torres R., Picado C., de Mora F., Rivera J. (2012) E-prostanoid 2 receptors dampen mast cell degranulation via cAMP/PKA-mediated suppression of IgE-dependent signaling. J. Leukoc. Biol. 92, 1155–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang X. S., Lau H. Y. (2006) Prostaglandin E potentiates the immunologically stimulated histamine release from human peripheral blood-derived mast cells through EP1/EP3 receptors. Allergy 61, 503–506. [DOI] [PubMed] [Google Scholar]
- 69.Nguyen M., Solle M., Audoly L. P., Tilley S. L., Stock J. L., McNeish J. D., Coffman T. M., Dombrowicz D., Koller B. H. (2002) Receptors and signaling mechanisms required for prostaglandin E2-mediated regulation of mast cell degranulation and IL-6 production. J. Immunol. 169, 4586–4593. [DOI] [PubMed] [Google Scholar]
- 70.Choi H. W., Abraham S. N. (2015) Mast cell mediator responses and their suppression by pathogenic and commensal microorganisms. Mol. Immunol. 63, 74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Choi H. W., Bowen S. E., Miao Y., Chan C. Y., Miao E. A., Abrink M., Moeser A. J., Abraham S. N. (2016) Loss of bladder epithelium induced by cytolytic mast cell granules. Immunity 45, 1258–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McGowen A. L., Hale L. P., Shelburne C. P., Abraham S. N., Staats H. F. (2009) The mast cell activator compound 48/80 is safe and effective when used as an adjuvant for intradermal immunization with Bacillus anthracis protective antigen. Vaccine 27, 3544–3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McLachlan J. B., Shelburne C. P., Hart J. P., Pizzo S. V., Goyal R., Brooking-Dixon R., Staats H. F., Abraham S. N. (2008) Mast cell activators: a new class of highly effective vaccine adjuvants. Nat. Med. 14, 536–541. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.