Abstract
Background
COPD is the third leading cause of death in the United States. Cigarette smoking accelerates the age-related forced expiratory volume in 1 s (FEV1) decline, an important determinant for the genesis of COPD. Hispanic smokers have lower COPD prevalence and FEV1 decline than non-Hispanic whites (NHWs).
Patients and methods
A nutritional epidemiological study was conducted in the Lovelace Smokers cohort (LSC; n=1,829) and the Veterans Smokers cohort (n=508) to identify dietary nutrients (n=139) associated with average FEV1 and its decline and to assess whether nutrient intakes could explain ethnic disparity in FEV1 decline between Hispanics and NHW smokers.
Results
Nutrients discovered and replicated to be significantly associated with better average FEV1 included magnesium, folate, niacin, vitamins A and D, eicosenoic fatty acid (20:1n9), eicosapentaenoic acid (20:5n3), docosapentaenoic acid (DPA; 22:5n3), docosahexaenoic acid (22:6n3), and fiber. In addition, greater intakes of eicosenoic fatty acid and DPA were associated with slower FEV1 decline in the LSC. Among omega 3 polyunsaturated fatty acids, DPA is the most potent nutrient associated with better average FEV1 and slower FEV1 decline. Adverse effect of continuous current smoking on FEV1 decline was completely negated in LSC members with high DPA intake (>20 mg/day). Slower FEV1 decline in Hispanics compared to NHWs may be due to the greater protection of eicosenoic fatty acid and DPA for FEV1 decline rather than greater intake of protective nutrients in this ethnic group.
Conclusion
The protective nutrients for the preservation of FEV1 in ever smokers could lay foundation for designing individualized nutritional intervention targeting “optimal physiological levels” in human to improve lung function in ever smokers. Ethnic disparity in FEV1 decline may be explained by difference in magnitude of protection of dietary intakes of eicosenoic fatty acid and DPA between Hispanics and NHWs.
Keywords: nutrientomics, spirometry, ethnic disparity
Background
COPD, characterized by a progressive and partially irreversible airflow limitation, is the third leading cause of death in the United States. Cigarette smoking, exposure to secondhand smoke, bacterial and viral infections, and indoor and outdoor air pollutants, are common risk factors, which may affect the maximally attained forced expiratory volume in 1 s (FEV1) in early adulthood or accelerate the age-related FEV1 decline at older age, two important determinants in the genesis of COPD.1–3 Several dietary factors and patterns may be protective for obstructive lung diseases. Using cross-sectional or case–control designs, candidate dietary nutrients and food items including vitamins A, C, and E, β-carotene, omega 3 polyunsaturated fatty acids (n-3 PUFAs), magnesium, dietary fiber, and hard fruit such as apple were associated with better FEV1 or lower prevalence of COPD.4–6 A few studies collected longitudinal spirometry data and identified greater intake of vitamin C and fresh fruit and serum carotenoids at baseline or increased consumption or level over time as being associated with slower FEV1 decline, although the results were inconsistent.7–12 In addition, a prudent diet rich in fruit, vegetables, whole-meal cereals, and fish was inversely associated with prevalent COPD.9,13–15
New Mexico (NM) has the highest percentage (47%) of Hispanics of any state with the majority of Hispanics born in the United States.16 The ancestry of NM Hispanics is mainly composed of 63% European and 35% Native American ancestry.1,17 NM Hispanic smokers have lower prevalence of COPD compared to non-Hispanic whites (NHWs) that may be attributed to lower exposure to cigarette smoke, slower FEV1 decline, having Native American ancestry and protective sequence variants that are polymorphic only in Hispanics.1,18,19 The effect of dietary intakes on this ethnic disparity has never been explored before.
In this study, we used the validated Harvard food frequency questionnaire (FFQ) to assess dietary intake of 139 nutrients at study entry in current and former smokers from the Lovelace Smokers cohort (LSC; n=1,829) and the Veteran Smokers cohort (VSC; n=508). Protective dietary nutrients associated with better FEV1 were discovered in the LSC and replicated in the VSC. We further assessed whether the nutrients associated with better FEV1 had any effect on age-related FEV1 decline in the LSC due to the availability of longitudinal spirometry data. Finally, the ethnic disparity of dietary intake of protective nutrients and their potential contribution to the ethnic disparity of FEV1 decline were explored in 327 Hispanics and 1,502 NHWs in the LSC.
Methods
LSC and VSC
Enrollment in the LSC started in 2001 with the goal to conduct longitudinal studies on biomarkers of respiratory diseases, including COPD and lung cancer in biospecimens from smokers.20 Enrollment was restricted to current and former smokers aged 40–74 years with a minimum of 10 pack-years of smoking. Cohort members returned approximately every 18 months, and a spirometry test was conducted at every visit by certified and registered respiratory therapists strictly adhering to the 1994 American Thoracic Society guidelines.21 The VSC began recruitment of smokers in 2000 with enrollment criteria similar to the LSC except that most VSC participants were males (96.7%) and had smoked at least 100 cigarettes during their life time. All participants signed a consent form written in English, and the institutional review boards of the Lovelace Respiratory Research Institute (Western institutional review board) and New Mexico Veteran Health Care System approved all investigations using human tissues and clinical data.
Baseline dietary assessment
The English version of the validated Harvard semiquantitative FFQ was completed at study entry.22 The FFQ collects the consumption frequency and serving size for ~150 food items over the previous 12 months and has excellent coverage for food items of the US Southwestern style.23 Daily estimates of the nutrient intakes are derived by summing over all foods, the products of the reported frequency of each food by the amount of nutrient in a specified (or assumed) serving of that food, based primarily on US Department of Agriculture publications.22 The applicability of this FFQ in New Mexicans is further introduced in Supplementary material. This study focused on 139 nutrients with <40% missing rate in 1,829 LSC and 508 VSC members. A convenient set of 28 LSC cohort members filled the FFQ for a second time at follow-up visitŝ9.4 years after the study entry and these were used to assess the stability of the dietary pattern over time.
Statistical analysis
In discovery analysis, we assessed the association between dietary nutrients and repeated FEV1 measurements collected at multiple visits in the LSC (n=1,829) using linear mixed effects (LME) model with a subject-specific random intercept. A total of 8,468 postbronchodilator FEV1 measures were obtained from 1,829 LSC members over a median follow-up period of 5.3 years (interquartile range [IQR]: 1.5–10 years). The average interval between visits was 1.45 years with an IQR of 1.32–1.61 years. Covariates included baseline variables (eg, age, sex, ethnicity, smoking history [smoking status and pack-years], body mass index, educational level, and height), total calorie intake, and time since enrollment (TSE) at each PFT test. This analysis tested whether higher intake of protective or harmful nutrients was associated with on average better or worse FEV1 across multiple visits. Nutrients associated with repeated spirometry measurements with false discovery rate (FDR) <0.05 in the LSC were further assessed in the replication cohort (VSC, n=508). Because only spirometry data at study entry were available from the VSC, multivariate linear regression was used to assess the association between nutrients and FEV1. Nutrients associated with FEV1 with FDR <0.05 in the VSC were deemed as being replicated. Second, the effect of nutrients on FEV1 decline was assessed in LSC members (n=1,499) with at least one follow-up visit and by including an interaction term between nutrient and TSE at each spirometry test in the LME model. In addition to the variables listed earlier, baseline FEV1 was further included as an independent variable for adjustment. Third, the ethnic disparity in nutrient intakes was assessed using multivariate linear regressions with natural log transformed nutrients as the outcome which had improved normality of the residual and satisfied the homoscedasticity assumption.22 Covariates for adjustment included age, sex, smoking history (smoking status and pack-years), and total calorie intake. Finally, ethnic difference in magnitude of association between nutrients and FEV1 decline was assessed in the LSC by including a three-way interaction term among dietary intake, ethnicity, and TSE in the LME model. All statistical analyses were conducted in SAS 9.4.
Results
Characteristics of the study subjects
A total of 327 Hispanics and 1,502 NHWs from LSC and 164 Hispanics and 344 NHWs from VSC who had complete data for dietary intake and spirometry data were studied (Table 1). VSC members were older and predominantly male and had more former smokers and Hispanics compared to the LSC members. In addition, VSC enrolled light smokers (<10 pack-years) who make up 16.7% of the cohort. The completeness of the FFQ and spirometry data at baseline was comparable between the two cohorts.
Table 1.
Characteristics of LSC and VSC members
| Variable | LSC | VSC |
|---|---|---|
| n | 1,829 | 508 |
| Age (years, | 57.2±9.4 | 62.1±8.5 |
| mean ± SD) | ||
| Sex (male, %) | 22.4 | 96.7 |
| Hispanics (%) | 17.9 | 32.3 |
| Current smokers (%) | 55.7 | 37.0 |
| Packyears (pys, | 35.0 (26.0–49.0) | 32.5 (15.0–58.0) |
| median [Q1–Q3]) | ||
| <10 (%) | 0 | 16.7 |
| 10–29 (%) | 33.4 | 28.8 |
| 29–43 (%) | 33.4 | 17.0 |
| ≥43 (%) | 33.2 | 37.4 |
| Number of unanswered food items | 0 (0–2) | 0 (0–1) |
| Total calorie intake (kcal) | 1,724.7 (1,343.7–2,177.4) | 1,903.4 (1,405.3–2,493.5) |
| Number of spirometry (median [Q1–Q3]) | 4 (2–7) | 1a |
| Spirometry at baseline | ||
| FEV1 (L/s, mean ± SD) | 2.5±0.7 | 2.6±0.6 |
| FVC (L, mean ± SD) | 3.4±0.9 | 3.5±0.7 |
| FEV1/FVC (%, mean ± SD) | 72.8±10.7 | 72.5±11.6 |
Note:
Only spirometry data at study entry in the VSC was available for this study.
Abbreviations: LSC, Lovelace Smokers cohort; VSC, Veteran Smokers cohort; SD, standard deviation; Q, quartile; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity.
Stability of dietary intake pattern
A moderate to high correlation was identified for dietary nutrient intake between baseline and follow-up visits that were 9.4 years apart with a median spearman correlation coefficient >0.60. The median percentage of changes in nutrient levels in the second FFQ relative to the baseline one is 12.2% with an IQR of 6.7%–22.4%. These findings support a relatively stable dietary pattern over a decade for the studied population.
Nutrients affecting FEV1
Thirty-three nutrient measurements were discovered to be associated with FEV1 with FDR <0.05 (not shown) and were further tested in the VSC. Fifteen nutrient measurements that assessed the dietary intakes of one mineral (magnesium), four vitamins (folate, niacin, A, and D), four long-chain unsaturated fatty acids (eicosenoic fatty acid [20:1n9], eicosapentaenoic acid [EPA, 20:5n3], docosapentaenoic acid [DPA; 22:5n3], docosahexaenoic acid [DHA; 22:6n3]), and the Association of Official Agricultural Chemists (AOAC) fiber were significantly associated with greater FEV1, while trans-oleic fatty acid measurement was associated with worse FEV1 in the VSC (FDR <0.05; Table 2). A combinational effect of the protective nutrients (magnesium, folate, niacin, vitamins A and D, long chain N3 fatty acids [EPA + DPA + DHA], and AOAC fiber) was assessed by creating a score that sums the standardized intakes of these nutrients. The differences of FEV1 between cohort members with high (upper quartile) versus low (lower quartile) scores were 183.4±42.2 mL/s (P<0.0001) in the LSC and 307.4±93.4 mL/s (P=0.0011) in the VSC.
Table 2.
Nutrients associated with FEV1 (mL/s) in the LSC and VSCa
| Nutrient (U/day)b | LSC (n=1,829)
|
VSC (n=508)
|
||
|---|---|---|---|---|
| Estimate (SE) | FDR | Estimate (SE) | FDR | |
| Minerals | ||||
| Magnesium (174.7 mg) | 82.2 (20.0) | 0.0027 | 202.6 (55.8) | 0.011 |
| Magnesium (144.7 mg)c | 107.1 (24.2) | 0.0014 | 156.8 (58.1) | 0.031 |
| Vitamins | ||||
| Total folate intake (478.6 μg) | 61.4 (17.7) | 0.010 | 136.1 (42.5) | 0.021 |
| Folic acid (411.0 μg) | 43.5 (16.4) | 0.039 | 112.2 (38.7) | 0.026 |
| Folate equivalents (860.5 μg) | 52.6 (16.8) | 0.022 | 128.1 (40.6) | 0.021 |
| Niacin (24.8 mg) | 24.0 (7.8) | 0.022 | 93.1 (33.2) | 0.027 |
| Vitamin A (9,160.2 IU) | 37.6 (13.5) | 0.030 | 66.7 (29.3) | 0.050 |
| Vitamin D (468.9 IU) | 40.4 (15.2) | 0.039 | 119.6 (41.8) | 0.026 |
| Fatty acids | ||||
| Eicosenoic fatty acid (135 mg) | 34.4 (13.5) | 0.049 | 70.6 (29.7) | 0.045 |
| EPA (100 mg) | 17.9 (6.1) | 0.025 | 34.0 (14.5) | 0.045 |
| DPA (20 mg) | 29.9 (10.2) | 0.025 | 68.0 (22.3) | 0.021 |
| DHA (150 mg) | 27.6 (10.3) | 0.039 | 61.4 (23.7) | 0.033 |
| EPA + DPA + DHA (310 mg) | 28.0 (9.7) | 0.025 | 57.0 (22.3) | 0.033 |
| EPA + DHA (290 mg) | 27.6 (9.6) | 0.025 | 55.8 (22.2) | 0.034 |
| trans-oleic (1.2 g) | −75.9 (29.8) | 0.049 | -112.3 (42.0) | 0.031 |
| AOAC fiber (10.5 g) | 80.9 (20.3) | 0.0032 | 97.8 (41.8) | 0.045 |
Notes: EPA, eicosapentaenoic fatty acid 20:5n3; DPA, docosapentaenoic fatty acid 22:5n3; DHA, docosahexaenoic fatty acid 22:6n3; eicosenoic fatty acid 20:1n9.
IQR is used for calculating the estimate and SE. Association analysis in the LSC was conducted based on longitudinal spirometry data using LME model with adjustment for important covariates. Association analysis in the VSC was conducted based on baseline spirometry data using linear regression with adjustment for important covariates.
Assessment of total folate intake and folate equivalents includes all sources (ie, natural food, supplements, and fortified foods). Folic acid is from supplements and fortified foods. A total of 38% study subjects have missing data for trans-oleic fatty acid.
Estimate of intake without counting supplement.
Abbreviations: FEV1, forced expiratory volume in 1 s; LSC, Lovelace Smokers cohort; VSC, Veteran Smokers cohort; SE, standard error; FDR, false discovery rate; AOAC, the Association of Official Agricultural Chemists; IQR, interquartile range; LME, linear mixed effects.
Nutrients affecting FEV1 decline
The effect of dietary nutrients on FEV1 decline was assessed by including an interaction term between each individual nutrient and TSE in the LME model in 1,499 LSC members with ≥2 spirometry tests. This analysis was conducted for nutrients significantly associated with FEV1 (Table 2) to minimize the number of comparisons and it found eicosenoic fatty acid and DPA significantly associated with slower FEV1 decline (P<0.05, Table 3).
Table 3.
The association between long chain unsaturated fatty acid and FEV1 decline (mL/s) in the LSC (n=1,499)a
| Nutrient (U/day) | Time
|
Nutrients
|
Time × nutrients
|
||
|---|---|---|---|---|---|
| Estimate (SE)b | Estimate (SE) | P-value | Estimate (SE) | P-value | |
| Eicosenoic fatty acid (135 mg) | −24.0 (1.1) | 3.2 (5.3) | 0.55 | 1.6 (0.6) | 0.0064 |
| EPA (100 mg) | −22.0 (0.7) | 2.4 (2.5) | 0.33 | 0.3 (0.3) | 0.28 |
| DPA (20 mg) | −23.1 (0.9) | 3.5 (4.1) | 0.40 | 1.2 (0.5) | 0.022 |
| DHA (150 mg) | −22.6 (0.9) | 2.7 (4.2) | 0.52 | 0.9 (0.5) | 0.073 |
| EPA + DPA + DHA (310 mg) | −22.3 (0.8) | 3.2 (3.9) | 0.41 | 0.7 (0.5) | 0.14 |
| EPA + DHA (290 mg) | −22.3 (0.8) | 3.2 (3.9) | 0.40 | 0.7 (0.5) | 0.15 |
Notes: EPA, eicosapentaenoic fatty acid 20:5n3; DPA, docosapentaenoic fatty acid 22:5n3; DHA, docosahexaenoic fatty acid 22:6n3; eicosenoic fatty acid 20:1n9.
IQR is used for calculating the estimate and SE. Association analysis in the LSC was conducted based on longitudinal spirometry data using linear mixed effects model with adjustment for important covariates.
P<0.0001.
Abbreviations: FEV1, forced expiratory volume in 1 s; LSC, Lovelace Smokers cohort; SE, standard error; IQR, interquartile range.
Antagonism of nutrients against cigarette smoking induced FEV1 decline
Cohort members were classified into continuous current smokers (n=507), continuous abstainers (n=620), quitters (current smokers at baseline who quit during follow-up visits and maintained the abstinence status afterward, n=215), and relapsers (n=157). Compared to continuous current smokers (−27.1±1.1 mL/s per year), continuous abstainers (−18.9 mL/s per year, P<0.0001), quitters (−22 mL/s per year, P=0.0069), and relapsers (−19.2 mL/s per year, P=0.0003) had a significantly reduced FEV1 decline. Eicosenoic fatty acid and DPA were converted into binary variables based on the median levels seen in the LSC to facilitate the assessment of three-way interactions among nutrients, smoking behavior change, and TSE using the LME model (Table 4). Continuous abstainers, quitters, and relapsers were combined into one group as noncontinuous current smokers because their FEV1 decline rates were similar and the effect of nutrients on FEV1 decline showed no difference among these three subgroups (all P>0.65). Significant three-way interaction was identified among DPA intake status (>20 versus ≤20 mg/day), continuous current smoking, and TSE (6.4±3.0, P=0.031, Table 4). Stratified analysis by DPA intake status identified that the adverse effect of continuous current smoking on FEV1 decline was completely negated in LSC members with higher DPA intake status (P=0.26, Table 4). The association between continuous smoking and FEV1 decline was independent of intake status of eicosenoic fatty acid.
Table 4.
Antagonism of nutrients against cigarette smoking induced FEV1 decline (mL/s) in the LSC (n=1,499)a
| Nutrient (U/day) | Time
|
Continuous smoking
|
Time × continuous smoking
|
||
|---|---|---|---|---|---|
| Estimate (SE)b | Estimate (SE) | P-value | Estimate (SE) | P-value | |
| All | −19.6 (0.7) | 8.5 (12.5) | 0.50 | −7.5 (1.3) | <0.0001 |
| DPAc | |||||
| >20 mg | −19.7 (1.0) | −5.2 (23.5) | 0.82 | −2.8 (2.5) | 0.26 |
| ≤20 mg | −19.5 (0.9) | 16.0 (14.9) | 0.28 | −9.2 (1.6) | <0.0001 |
| Eicosenoic fatty acidd | |||||
| >135 mg | −18.7 (1.0) | 24.6 (19.3) | 0.20 | −7.5 (2.1) | 0.0004 |
| ≤135 mg | −20.3 (0.9) | −0.3 (16.3) | 0.99 | −7.4 (1.7) | <0.0001 |
Notes: DPA, docosapentaenoic fatty acid 22:5n3.
Association analysis in the LSC was conducted based on longitudinal spirometry data using linear mixed effects model with adjustment for important covariates.
P<0.0001.
P-value for three-way interaction among DPA, continuous smoking, and TSE =0.031.
P-value for three-way interaction among eicosenoic fatty acid, continuous smoking, and TSE =0.99.
Abbreviations: FEV1, forced expiratory volume in 1 s; LSC, Lovelace Smokers cohort; SE, standard error; TSE, time since enrollment.
Effect of dietary intakes of eicosenoic fatty acid and DPA on the ethnic disparity of FEV1 decline
Our previous study identified that Hispanics smokers had significantly lower COPD prevalence than NHWs in the LSC and this ethnic disparity may be due to the slower FEV1 decline in Hispanics.1 We hypothesized that ethnic disparity in FEV1 decline may be partially explained by the dietary intakes of eicosenoic fatty acid and DPA. We first assessed whether Hispanic smokers consume more protective nutrients. Surprisingly, although the total calorie intake was quite comparable between Hispanics and NHWs, Hispanics consume 44 and 28% less DPA than NHWs in LSC and VSC, respectively (P<0.01, Table 5). The difference of consumption of eicosenoic fatty acid between Hispanics and NHWs is very minimal in both cohorts (Table 5). Second, we assessed whether protective nutrients have a stronger favorable association with FEV1 decline in Hispanics compared to NHWs. A significant three-way interaction among DPA intake status, ethnicity, and TSE was identified (9.3±3.6, P=0.0095, Table 6), while the three-way interaction among eicosenoic fatty acid intake status, ethnicity, and TSE was of borderline significance (5.7±3.3, P=0.082, Table 6). Most importantly, the associations between greater intake status of DPA and eicosenoic fatty acid and reduced FEV1 decline were 8.8- and 5.7-fold greater in Hispanics compared to NHWs for DPA and eicosenoic fatty acid, respectively (Table 6). Thus, the ethnic disparity in FEV1 decline may be due to the greater protective effects on FEV1 decline seen in Hispanics versus NHWs.
Table 5.
Ethnic disparity in dietary intakes of eicosenoic fatty acid and DPA in the LSC and VSCa
| Cohort | Nutrient (U/day) | NHWs | Hispanics | P-value |
|---|---|---|---|---|
| LSC | n=1,502 | n=327 | ||
| Eicosenoic fatty acid (mg) | 173.3 (120.1–250.1) | 169.3 (120.1–260.1) | 0.41 | |
| DPA (mg) | 14.3 (10.1–30.1) | 8.0 (10.1–30.1) | 1.6×10-5 | |
| VSC | n=344 | n=164 | ||
| Eicosenoic fatty acid (mg) | 212.9 (140.1–320.1) | 200.7 (130.1–305.0) | 0.0016 | |
| DPA (mg) | 12.1 (10.1–30.1) | 8.7 (10.1–20.1) | 0.0092 |
Notes: DPA, docosapentaenoic fatty acid 22:5n3.
Age, sex, smoking history (smoking status and pack-years), BMI, educational level, and total calorie intake were adjusted in multivariate linear regression with natural log transformed nutrients as the outcome. Data are shown as geometric means with lower and upper quartiles. Total calorie intake was 1,890.2±716.1 versus 1,796.4±619.1 for Hispanics and NHWs (Wilcoxon test, P=0.067) in the LSC and 2,184.3±1,247.6 versus 2,001.3±793.4 for Hispanics and NHWs (Wilcoxon test, P=0.65) in the VSC.
Abbreviations: LSC, Lovelace Smokers cohort; VSC, Veteran Smokers cohort; NHWs, non-Hispanic whites; BMI, body mass index.
Table 6.
Magnitude of association between dietary nutrients and FEV1 decline (mL/s) in Hispanics and NHWs in the LSC (n=1,499)a
| Nutrientb | Ethnicity | Time
|
Nutrient
|
Time × nutrient
|
||
|---|---|---|---|---|---|---|
| Estimate (SE)c | Estimate (SE) | P-value | Estimate (SE) | P-value | ||
| DPAd | Hispanics | −22.3 (1.8) | 4.2 (26.4) | 0.87 | 10.5 (3.4) | 0.0020 |
| NHWs | −22.5 (0.8) | 17.2 (10.8) | 0.11 | 1.2 (1.3) | 0.35 | |
| Eicosenoic fatty acide | Hispanics | −22.0 (1.9) | −29.1 (26.5) | 0.27 | 6.8 (3.1) | 0.028 |
| NHWs | -22.5 (0.8) | 17.6 (11.6) | 0.13 | 1.2 (1.3) | 0.34 | |
Notes: DPA, docosapentaenoic fatty acid 22:5n3.
Association analysis in the LSC was conducted based on longitudinal spirometry data using linear mixed effects model with adjustment for important covariates.
Eicosenoic fatty acid and DPA were converted into binary variables based on the median levels seen in the LSC (Table 4).
P<0.0001.
P-value for three-way interaction among DPA, ethnicity, and TSE =0.0095.
P-value for three-way interaction among eicosenoic fatty acid, ethnicity, and TSE =0.082.
Abbreviations: FEV1, forced expiratory volume in 1 s; NHWs, non-Hispanic whites; LSC, Lovelace Smokers cohort; SE, standard error; TSE, time since enrollment.
Discussion
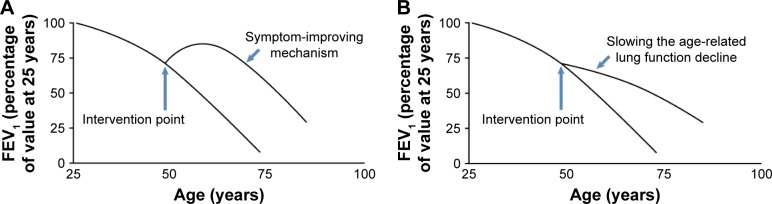
Our comprehensive nutritional study through assessing dietary intake of 139 nutrients in 2,367 smokers from NM identified magnesium, folate, niacin, vitamins A and D, and long chain unsaturated fatty acids (eicosenoic fatty acid and n-3 PUFAs), and dietary fiber as associated with on average better FEV1 in chronic smokers. In addition, the effect of these protective nutrients on lung function showed an obstructive pattern as their associations with FEV1/FVC were significant as well (Table S1).24 The differences of FEV1 between cohort members consuming high (upper quartile) versus low (lower quartile) levels of individual protective nutrients ranged from 17.9 to 107.1 mL/s with a combinational effect of 183.4 mL/s in the LSC. The protective effects became even greater in an older population comprising of predominantly males (VSC). The factors contributing to the observed greater effects in the VSC compared to the LSC are largely unknown and may include sample size, sex difference, age, smoking status, etc. These differences are of substantial public health impact because normal FEV1 loss per year in the LSC is ~22 mL/s per year and the FEV1 difference between current and former smokers is 82 mL/s. Our study is also the first to identify two long chain unsaturated fatty acids (ie, eicosenoic fatty acid and DPA) associated with on average better FEV1 through reducing age-related FEV1 decline in moderate and heavy smokers. In addition, the effect of these two nutrients on lung function decline showed an obstructive pattern as their associations with FEV1/FVC were significant as well (Table S2). Our findings together with others7–9,11,12 suggest that protective dietary nutrients may improve lung function through two hypothetical patterns: symptom-improving pattern for magnesium, folate, niacin, vitamins A and D, EPA, DHA, and dietary fiber (Figure 1A) and slowing the age-related lung function decline pattern for eicosenoic fatty acid and DPA (Figure 1B).
Figure 1.
Two hypothetical models for the effect of dietary intervention on lung function.
Notes: (A) The nutrient supplementation at optimal physiological level may improve lung function that in turn offsets the age-related decline over time. In this pattern, the onset of the intervention improves the lung function without affecting the decline slope. (B) The intervention could directly slow down the age-related lung function decline as reflected by a less steep slope.
Abbreviation: FEV1, forced expiratory volume in 1 s.
Among n-3 PUFAs, daily intake of n-3 DPA is <20% of EPA and DHA in the LSC. Similar dietary intake pattern was also observed in 13,000 Dutch adults.25 However, the protective effect of DPA for average FEV1 is the most potent, as per 100 mg increase in daily intake FEV1 increases by 149.5 mL/s for DPA, 17.9 mL/s for EPA, and 18.4 mL/s for DHA. Furthermore, DPA is the only one that has a statistically significant association with a slower FEV1 decline (P=0.022). Among the three n-3 PUFAs, a more potent effect associated with DPA intake has also been seen in the Edinburgh Artery Study in which DPA was the only n-3 PUFA that reduced the likelihood of developing atherosclerosis.26 In addition, in a multiethnic cohort of 2,837 American adults, dietary intake of DPA was the most potent n-3 PUFA associated with reduced risk for incident cardiovascular disease and coronary heart disease, and plasma phospholipid DPA had the strongest inverse correlation with systemic inflammation markers (ie, IL-6 and CRP).27 The mechanism underlying the protective effect of DPA on lung function may be related to its anti-inflammatory, antiproteolytic, and antioxidative ability. EPA supplementation in macrophages exerts anti-inflammatory effects indirectly through its elongation to DPA that inhibited the proinflammatory mediators derived from cyclooxygenase metabolism.28 In a rat model of pulmonary hypertension, oral administration of DPA for 3 weeks decreased NF-κB and p38 MAPK activation, leading to a reduction in MMP-2, MMP-9, and VEGF expression levels in lung tissue homogenates.29 The degradation of the extracellular matrix by specialized proteolytic enzymes such as matrix metalloproteinases has been shown to play a key pathogenic role in the development of important COPD phenotype emphysema.30 In aged rats, DPA-supplemented diet restored the neuronal function through blocking oxidative changes as quantified by measuring 8-OHdG in the hippocampus and its subsequent activation of sphingomyelinase and caspase 3 activity.31 8-OHdG is an indicator of reactive oxygen species-induced oxidative DNA damage, and compared to healthy smokers, smokers with COPD had significantly elevated levels of 8-OHdG in peripheral blood DNA, a biomarker highly correlated with 8-OHdG in the lung and reduced FEV1.32 The antioxidant role of DPA was further supported by the finding that a significant interaction between DPA intake and continuous smoking on FEV1 decline was identified and the greater intake of DPA <20 mg/day completely negated the effect of continuous smoking on FEV1 decline.
Eicosenoic fatty acid is a monounsaturated fatty acid with fatty fish as the main food source. Its average intake in human diet is 207.0 mg/day. Using the lipopolysaccharide (LPS)-induced RAW 264.7 macrophages model of inflammation, preincubation of macrophages with eicosenoic fatty acid significantly reduced LPS-induced inducible nitric oxide synthase (iNOS) levels.33 iNOS plays an important role in determining nitrosative stress in the lung, as it produces large amounts of nitric oxide (NO) in response to many endogenous (such as chemokines and cytokines) and exogenous stimuli (such as bacterial toxins, virus infection, allergens, environmental pollutants [ozone, oxidative stress, and silica], hypoxia, and tumors).34 NO reacts with superoxides to form the highly reactive peroxynitrites that have been shown to further produce airway inflammation and cause airway remodeling.35–37 However, cigarette smoking has been shown to reduce the proinflammatory factors induced iNOS expression in lung epithelial cells and exhaled NO levels among smokers.38,39 Interestingly, we did not find any interaction between eicosenoic fatty acid and continuous smoking on FEV1 decline in our study. Taken together, our results suggest that greater intake of eicosenoic fatty acid may reduce the FEV1 decline through alleviating iNOS-mediated nitrosative stress in a cigarette smoke-independent manner.
In this study, we provided the first evidence that instead of greater intakes of these two protective nutrients, it was the much larger protective effect of eicosenoic fatty acid and DPA on FEV1 decline seen in Hispanics that may partially explain why Hispanics had slower FEV1 decline. The underlying mechanism for dietary intake of DPA-mediated ethnic disparity in FEV1 decline may be related to the ethnic disparity in the metabolism of DPA. Only weak associations were identified between fish consumption and plasma phospholipid DPA, suggesting that endogenous metabolism influences circulating DPA concentrations, for example, by chain elongation and desaturation of EPA.27,40,41 In addition, although average dietary intake of DPA in Hispanics was ~75% of that seen in NHWs, plasma phospholipid DPA level was very similar between the two ethnic groups.27 Sequence variants of enzymes involved in this metabolic conversion are associated with DPA levels with several clearly showing ethnic differences in minor allele frequency between HapMap populations of European and Hispanic ancestry.42 For example, C allele of rs3734398 in elongase gene ELOVL2 was associated with higher levels of EPA and DPA and lower levels of DHA, suggesting that C allele decreases the conversion of EPA and DPA to DHA. Frequency of the C allele of rs3734398 was 0.44 and 0.71 in HapMap populations of European and Hispanic ancestry, respectively. Thus, greater C allele frequency in Hispanics may potentially contribute to greater circulating DPA concentrations, which in turn exaggerate the health effects of DPA. Thus, the larger protective effect of dietary intake of DPA on FEV1 decline seen in Hispanics compared to NHWs may be due to greater metabolism favoring DPA accumulation and its subsequent lung effects in Hispanics.
This study has several strengths. First, this is one of the first studies taking an unbiased approach to identify nutrients associated with longitudinal spirometry measurements in moderate and heavy smokers. Second, a rigorous analytical plan that incorporated a discovery and replication approach and FDR correction was taken to minimize the chance of false-positive findings. Finally, NM populations provided a unique opportunity to reliably assess the ethnic disparity in lung function. NM Hispanics are distinct from Hispanic or Latino populations living in other states because they mainly include descendants of Spanish colonists who have settled the area of NM and Southern Colorado since the 1600s. Thus, our findings of ethnic disparity are not likely to be affected by immigration-related factors such as acculturation status, healthy migrant effect, and salmon bias. However, this may be an indicator that the study results may not be generalized to the overall Hispanic populations across the United States.
This study has two limitations. First, our studies and others have shown that the dietary pattern in adults is reasonably stable over several years.22 However, because the FFQ was not implemented in follow-up visits, we are not able to identify nutrients whose changes over time will affect lung function decline. The implementation of the FFQ in follow-up visits in the future would make this test possible. Second, the average lung function in the LSC members is relatively healthy with only 25% of subjects with COPD disease defined by Global Initiative for Chronic Obstructive Lung Disease criteria, most of whom have mild-to-moderate COPD. Thus, whether our findings could be generalized to COPD patients is uncertain.
Conclusion
Through an unbias nutrientomics study, we identified magnesium, folate, niacin, vitamins A and D, long chain unsaturated fatty acids (eicosenoic fatty acid and n-3 PUFA), and dietary fiber associated with on average better FEV1 in chronic smokers. In addition, dietary intakes of eicosenoic fatty acid and DPA were associated with reduced FEV1 decline. These findings could lay foundation for the design of an individualized nutritional intervention targeting “optimal physiological levels” in human to improve lung function in ever smokers. Furthermore, slower FEV1 decline in Hispanics versus NHWs may be partially due to greater protective effect of eicosenoic fatty acid and DPA on age-related FEV1 decline.
Supplementary materials
Methods
Baseline dietary assessment
Cohort members completed the adult English version of validated Harvard Food Frequency Questionnaire (FFQ), a self-administered instrument that includeŝ150 food items distributed within the eight major dietary categories, at the study entry.1 The FFQ collects the consumption frequency and serving size of each specified food item during the past 12 months and has good coverage for food items of the US Southwestern style. The FFQ also has open-ended questions that collect use of food items consumed at least once per week but not listed in the eight major dietary categories. The validity of the application of the FFQ in New Mexico Hispanics was further supported by the results from a previous study that compared the energy and nutrient source between elderly Hispanics and non-Hispanic whites (NHWs) in New Mexico and identified no exclusive pattern for consumption of Southwestern regional foods in Hispanics compared to NHWs.2 In addition, the estimated consumption of vitamins A and C in the current study was highly comparable to the data from a study that itemized all chili-containing traditional Southwestern foods commonly consumed in New Mexico, suggesting the sufficiency of Harvard FFQ in capturing the chili-derived nutrients.2 Furthermore, serum vitamins B12 and C and folate levels were reported to be significantly lower in Hispanics versus NHWs in a previous study of an elderly New Mexican population.3 These three vitamins also showed the ethnic difference in the dietary assessment (not shown). Estimates of daily nutrient intakes are derived by summing over all foods the products of the reported frequency of each food by the amount of nutrient in a specified (or assumed) serving of that food, based primarily on US Department of Agriculture publications.1 Individual nutrient intakes were estimated with and without taking supplement use into consideration, and two values were provided. Because the number of nutrients in the output varied over time (from 104 to 254 nutrients from 2005 to 2014), a total of 139 nutrients with <40% missing rate was included in this study. Cohort members with extremely low or high total caloric intake were excluded.4 None of our participants were removed due to having >70 missing items on the FFQ. A total of 1,829 Lovelace Smokers cohort and 508 Veteran Smokers cohort members completed the FFQ that passed the quality check. A convenient set of 28 cohort members completed an FFQ for a second time at follow-up visitŝ9.4 years after the study entry. Spearman correlation analysis was conducted for 102 nutrients with no missing data for these 28 cohort members to assess the stability of the dietary pattern over time.
Table S1.
Nutrients associated with FEV1/FVC ratio (%) in the LSC and VSCa
| Nutrient (U/day)b | LSC (n=1,829)
|
VSC (n=508)
|
||
|---|---|---|---|---|
| Estimate (SE) | FDR | Estimate (SE) | FDR | |
| Minerals | ||||
| Magnesium (174.7 mg) | 0.732 (0.396) | 0.074 | 2.421 (1.024) | 0.023 |
| Magnesium (144.7 mg)c | 1.256 (0.481) | 0.025 | 2.161 (1.063) | 0.045 |
| Vitamins | ||||
| Total folate intake (478.6 μg) | 1.019 (0.349) | 0.025 | 2.231 (0.775) | 0.009 |
| Folic acid (411.0 μg) | 0.671 (0.325) | 0.048 | 1.858 (0.706) | 0.013 |
| Folate equivalents (860.5 μg) | 0.857 (0.333) | 0.025 | 2.144 (0.741) | 0.009 |
| Niacin (24.8 mg) | 0.374 (0.154) | 0.025 | 1.861 (0.605) | 0.009 |
| Vitamin A (9,160.2 IU) | 0.754 (0.266) | 0.025 | 1.245 (0.534) | 0.023 |
| Vitamin D (468.9 IU) | 0.454 (0.300) | 0.139 | 1.337 (0.765) | 0.081 |
| Fatty acids | ||||
| Eicosenoic fatty acid (135 mg) | 0.638 (0.267) | 0.025 | 1.541 (0.540) | 0.009 |
| EPA (100 mg) | 0.290 (0.121) | 0.025 | 0.740 (0.263) | 0.009 |
| DPA (20 mg) | 0.513 (0.202) | 0.025 | 1.393 (0.405) | 0.009 |
| DHA (150 mg) | 0.456 (0.205) | 0.034 | 1.319 (0.432) | 0.009 |
| EPA + DPA + DHA (310 mg) | 0.460 (0.192) | 0.025 | 1.229 (0.405) | 0.009 |
| EPA + DHA (290 mg) | 0.452 (0.190) | 0.025 | 1.207 (0.404) | 0.009 |
| trans-oleic (1.2 g) | −0.716 (0.559) | 0.20 | −1.847 (0.767) | 0.022 |
| AOAC fiber (10.5 g) | 1.075 (0.403) | 0.025 | 2.018 (0.761) | 0.013 |
Notes: EPA, eicosapentaenoic fatty acid 20:5n3; DPA, docosapentaenoic fatty acid 22:5n3; DHA, docosahexaenoic fatty acid 22:6n3; eicosenoic fatty acid 20:1n9.
IQR is used for calculating the estimate and 95% CI. Association analysis in the LSC was conducted based on longitudinal spirometry data using linear mixed effects model with adjustment for important covariates. Association analysis in the VSC was conducted based on baseline spirometry data using linear regression with adjustment for important covariates.
Assessment of total folate intake and folate equivalents includes all sources (ie, natural food, supplements, and fortified foods). Folic acid is from supplements and fortified foods. A total of 38% study subjects have missing data for trans-oleic fatty acid.
Estimate of intake without counting supplement.
Abbreviations: FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; LSC, Lovelace Smokers cohort; VSC, Veteran Smokers cohort; SE, standard error; FDR, false discovery rate; AOAC, the Association of Official Agricultural Chemists; IQR, interquartile range.
Table S2.
The association between long chain unsaturated fatty acid and FEV1/FVC decline (%) in the LSC (n=1,499)a
| Nutrient (U/day) | Time
|
Nutrients
|
Time × nutrients
|
||
|---|---|---|---|---|---|
| Estimate (SE)b | Estimate (SE) | P-value | Estimate (SE) | P-value | |
| Eicosenoic fatty acid (135 mg) | −0.47 (0.02) | −0.048 (0.11) | 0.67 | 0.032 (0.012) | 0.0081 |
| EPA (100 mg) | −0.44 (0.01) | 0.010 (0.052) | 0.85 | 0.019 (0.006) | 0.0030 |
| DPA (20 mg) | −0.46 (0.02) | −0.003 (0.087) | 0.98 | 0.032 (0.011) | 0.0028 |
| DHA (150 mg) | −0.46 (0.02) | −0.037 (0.088) | 0.67 | 0.031 (0.011) | 0.0031 |
| EPA + DPA + DHA (310 mg) | −0.45 (0.02) | −0.007 (0.083) | 0.94 | 0.030 (0.010) | 0.0028 |
| EPA + DHA (290 mg) | −0.45 (0.02) | −0.006 (0.082) | 0.94 | 0.030 (0.010) | 0.0028 |
Notes: EPA, eicosapentaenoic fatty acid 20:5n3; DPA, docosapentaenoic fatty acid 22:5n3; DHA, docosahexaenoic fatty acid 22:6n3; eicosenoic fatty acid 20:1n9.
IQR is used for calculating the estimate and SE. Association analysis in the LSC was conducted based on longitudinal spirometry data using linear mixed effects model with adjustment for important covariates.
P<0.0001.
Abbreviations: FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; LSC, Lovelace Smokers cohort; SE, standard error; IQR, interquartile range.
References
- 1.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self- administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. Discussion 1127–1136. [DOI] [PubMed] [Google Scholar]
- 2.Pareo-Tubbeh SL, Romero LJ, Baumgartner RN, Garry PJ, Lindeman RD, Koehler KM. Comparison of energy and nutrient sources of elderly Hispanics and non-Hispanic whites in New Mexico. J Am Diet Assoc. 1999;99(5):572–582. doi: 10.1016/S0002-8223(99)00141-8. [DOI] [PubMed] [Google Scholar]
- 3.Lindeman RD, Romero LJ, Koehler KM, et al. Serum vitamin B12, C and folate concentrations in the New Mexico elder health survey: correlations with cognitive and affective functions. J Am Coll Nutr. 2000;19(1):68–76. doi: 10.1080/07315724.2000.10718916. [DOI] [PubMed] [Google Scholar]
- 4.Stidley CA, Picchi MA, Leng S, et al. Multivitamins, folate, and green vegetables protect against gene promoter methylation in the aerodigestive tract of smokers. Cancer Res. 2010;70(2):568–574. doi: 10.1158/0008-5472.CAN-09-3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
Acknowledgments
We thank Ms Laura Sampson, MS RD, at Harvard School of Public Health for assisting result interpretation. We thank Ms Xiequn Zhang, MS, at Lovelace Respiratory Research Institute for processing dietary data. We thank Ms Elise Calvillo for scientific editing of the figures. We thank the staff from Lovelace Scientific Resources for recruiting and enrolling study subjects and collecting lung function data. We thank the NM residents who participated in this study. This research was supported by NCI grant R01 CA097356, the State of New Mexico, as a direct appropriation from the Tobacco Settlement Fund and NIH/NCI P30 CA118100.
Footnotes
Disclosure
The authors report no financial and nonfinancial competing interests in this work.
References
- 1.Bruse S, Sood A, Petersen H, et al. New Mexican Hispanic smokers have lower odds of chronic obstructive pulmonary disease and less decline in lung function than non-Hispanic whites. Am J Respir Crit Care Med. 2011;184(11):1254–1260. doi: 10.1164/rccm.201103-0568OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sood A, Petersen H, Blanchette CM, et al. Wood smoke exposure and gene promoter methylation are associated with increased risk for COPD in smokers. Am J Respir Crit Care Med. 2010;182(9):1098–1104. doi: 10.1164/rccm.201002-0222OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lange P, Celli B, Agusti A, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med. 2015;373(2):111–122. doi: 10.1056/NEJMoa1411532. [DOI] [PubMed] [Google Scholar]
- 4.Hanson C, Rutten EP, Wouters EF, Rennard S. Influence of diet and obesity on COPD development and outcomes. Int J Chron Obstruct Pulmon Dis. 2014;9:723–733. doi: 10.2147/COPD.S50111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romieu I, Trenga C. Diet and obstructive lung diseases. Epidemiol Rev. 2001;23(2):268–287. doi: 10.1093/oxfordjournals.epirev.a000806. [DOI] [PubMed] [Google Scholar]
- 6.Fonseca Wald EL, van den Borst B, Gosker HR, Schols AM. Dietary fibre and fatty acids in chronic obstructive pulmonary disease risk and progression: a systematic review. Respirology. 2014;19(2):176–184. doi: 10.1111/resp.12229. [DOI] [PubMed] [Google Scholar]
- 7.Carey IM, Strachan DP, Cook DG. Effects of changes in fresh fruit consumption on ventilatory function in healthy British adults. Am J Respir Crit Care Med. 1998;158(3):728–733. doi: 10.1164/ajrccm.158.3.9712065. [DOI] [PubMed] [Google Scholar]
- 8.Guenegou A, Leynaert B, Pin I, Le Moel G, Zureik M, Neukirch F. Serum carotenoids, vitamins A and E, and 8 year lung function decline in a general population. Thorax. 2006;61(4):320–326. doi: 10.1136/thx.2005.047373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKeever TM, Lewis SA, Cassano PA, et al. Patterns of dietary intake and relation to respiratory disease, forced expiratory volume in 1 s, and decline in 5-y forced expiratory volume. Am J Clin Nutr. 2010;92(2):408–415. doi: 10.3945/ajcn.2009.29021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bentley AR, Kritchevsky SB, Harris TB, et al. Health ABC Study Dietary antioxidants and forced expiratory volume in 1 s decline: the Health, Aging and Body Composition study. Eur Respir J. 2012;39(4):979–984. doi: 10.1183/09031936.00190010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butland BK, Fehily AM, Elwood PC. Diet, lung function, and lung function decline in a cohort of 2512 middle aged men. Thorax. 2000;55(2):102–108. doi: 10.1136/thorax.55.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKeever TM, Scrivener S, Broadfield E, Jones Z, Britton J, Lewis SA. Prospective study of diet and decline in lung function in a general population. Am J Respir Crit Care Med. 2002;165(9):1299–1303. doi: 10.1164/rccm.2109030. [DOI] [PubMed] [Google Scholar]
- 13.Shaheen SO, Jameson KA, Syddall HE, et al. Hertfordshire Cohort Study Group The relationship of dietary patterns with adult lung function and COPD. Eur Respir J. 2010;36(2):277–284. doi: 10.1183/09031936.00114709. [DOI] [PubMed] [Google Scholar]
- 14.Varraso R, Fung TT, Barr RG, Hu FB, Willett W, Camargo CA., Jr Prospective study of dietary patterns and chronic obstructive pulmonary disease among US women. Am J Clin Nutr. 2007;86(2):488–495. doi: 10.1093/ajcn/86.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varraso R, Fung TT, Hu FB, Willett W, Camargo CA. Prospective study of dietary patterns and chronic obstructive pulmonary disease among US men. Thorax. 2007;62(9):786–791. doi: 10.1136/thx.2006.074534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pew Hispanic Center [webpage on the Internet] Demographic Profile of Hispanics in New Mexico, 2011. 2016. [Accessed Feburary 3, 2016]. Available from: http://www.pewhispanic.org/states/state/nm/
- 17.Leng S, Liu Y, Thomas CL, et al. Native American ancestry affects the risk for gene methylation in the lungs of Hispanic smokers from New Mexico. Am J Respir Crit Care Med. 2013;188(9):1110–1116. doi: 10.1164/rccm.201305-0925OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen W, Brehm JM, Boutaoui N, et al. Native American ancestry, lung function, and COPD in Costa Ricans. Chest. 2014;145(4):704–710. doi: 10.1378/chest.13-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen W, Brehm JM, Manichaikul A, et al. A genome-wide association study of chronic obstructive pulmonary disease in Hispanics. Ann Am Thorac Soc. 2015;12(3):340–348. doi: 10.1513/AnnalsATS.201408-380OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leng S, Stidley CA, Willink R, et al. Double-strand break damage and associated DNA repair genes predispose smokers to gene methylation. Cancer Res. 2008;68(8):3049–3056. doi: 10.1158/0008-5472.CAN-07-6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Standardization of spirometry, 1994 update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152(3):1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 22.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self- administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. Discussion 1127–1136. [DOI] [PubMed] [Google Scholar]
- 23.Pareo-Tubbeh SL, Romero LJ, Baumgartner RN, Garry PJ, Lindeman RD, Koehler KM. Comparison of energy and nutrient sources of elderly Hispanics and non-Hispanic whites in New Mexico. J Am Diet Assoc. 1999;99(5):572–582. doi: 10.1016/S0002-8223(99)00141-8. [DOI] [PubMed] [Google Scholar]
- 24.Occhipinti M, Larici AR, Bonomo L, Incalzi RA. Aging airways: between normal and disease. A multidimensional diagnostic approach by combining clinical, functional, and imaging data. Aging Dis. 2017;8(4):471–485. doi: 10.14336/AD.2016.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKeever TM, Lewis SA, Cassano PA, et al. The relation between dietary intake of individual fatty acids, FEV1 and respiratory disease in Dutch adults. Thorax. 2008;63(3):208–214. doi: 10.1136/thx.2007.090399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leng GC, Horrobin DF, Fowkes FG, et al. Plasma essential fatty acids, cigarette smoking, and dietary antioxidants in peripheral arterial disease. A population-based case–control study. Arterioscler Thromb. 1994;14(3):471–478. doi: 10.1161/01.atv.14.3.471. [DOI] [PubMed] [Google Scholar]
- 27.de Oliveira Otto MC, Wu JH, Baylin A, et al. Circulating and dietary omega-3 and omega-6 polyunsaturated fatty acids and incidence of CVD in the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2013;2(6):e000506. doi: 10.1161/JAHA.113.000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Norris PC, Dennis EA. Omega-3 fatty acids cause dramatic changes in TLR4 and purinergic eicosanoid signaling. Proc Natl Acad Sci U S A. 2012;109(22):8517–8522. doi: 10.1073/pnas.1200189109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morin C, Hiram R, Rousseau E, Blier PU, Fortin S. Docosapentaenoic acid monoacylglyceride reduces inflammation and vascular remodeling in experimental pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2014;307(4):H574–H586. doi: 10.1152/ajpheart.00814.2013. [DOI] [PubMed] [Google Scholar]
- 30.Houghton AM. Mechanistic links between COPD and lung cancer. Nat Rev Cancer. 2013;13(4):233–245. doi: 10.1038/nrc3477. [DOI] [PubMed] [Google Scholar]
- 31.Kelly L, Grehan B, Chiesa AD, et al. The polyunsaturated fatty acids, EPA and DPA exert a protective effect in the hippocampus of the aged rat. Neurobiol Aging. 2011;32(12):.e2311–.e2315. doi: 10.1016/j.neurobiolaging.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Yang S, Wu H, Zhao J, et al. Feasibility of 8-OHdG formation and hOGG1 induction in PBMCs for assessing oxidative DNA damage in the lung of COPD patients. Respirology. 2014;19(8):1183–1190. doi: 10.1111/resp.12378. [DOI] [PubMed] [Google Scholar]
- 33.Pereira DM, Correia-da-Silva G, Valentao P, Teixeira N, Andrade PB. Anti-inflammatory effect of unsaturated fatty acids and Ergosta-7,22-dien-3-ol from Marthasterias glacialis: prevention of CHOP-mediated ER-stress and NF-kappaB activation. PLoS One. 2014;9(2):e88341. doi: 10.1371/journal.pone.0088341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ricciardolo FL, Sterk PJ, Gaston B, Folkerts G. Nitric oxide in health and disease of the respiratory system. Physiol Rev. 2004;84(3):731–765. doi: 10.1152/physrev.00034.2003. [DOI] [PubMed] [Google Scholar]
- 35.Ricciardolo FL, Di Stefano A, Sabatini F, Folkerts G. Reactive nitrogen species in the respiratory tract. Eur J Pharmacol. 2006;533(1–3):240–252. doi: 10.1016/j.ejphar.2005.12.057. [DOI] [PubMed] [Google Scholar]
- 36.Gabazza EC, Taguchi O, Tamaki S, et al. Role of nitric oxide in airway remodelling. Clin Sci. 2000;98(3):291–294. [PubMed] [Google Scholar]
- 37.Prado CM, Leick-Maldonado EA, Yano L, et al. Effects of nitric oxide synthases in chronic allergic airway inflammation and remodeling. Am J Respir Cell Mol Biol. 2006;35(4):457–465. doi: 10.1165/rcmb.2005-0391OC. [DOI] [PubMed] [Google Scholar]
- 38.Kougias M, Vardavas CI, Anagnostopoulos N, et al. The acute effect of cigarette smoking on the respiratory function and FENO production among young smokers. Exp Lung Res. 2013;39(8):359–364. doi: 10.3109/01902148.2013.830654. [DOI] [PubMed] [Google Scholar]
- 39.Brindicci C, Kharitonov SA, Ito M, et al. Nitric oxide synthase isoenzyme expression and activity in peripheral lung tissue of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181(1):21–30. doi: 10.1164/rccm.200904-0493OC. [DOI] [PubMed] [Google Scholar]
- 40.Mozaffarian D, Lemaitre RN, King IB, et al. Plasma phospholipid long-chain omega-3 fatty acids and total and cause-specific mortality in older adults: a cohort study. Ann Intern Med. 2013;158(7):515–525. doi: 10.7326/0003-4819-158-7-201304020-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilk JB, Tsai MY, Hanson NQ, Gaziano JM, Djousse L. Plasma and dietary omega-3 fatty acids, fish intake, and heart failure risk in the Physicians’ Health Study. Am J Clin Nutr. 2012;96(4):882–888. doi: 10.3945/ajcn.112.042671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lemaitre RN, Tanaka T, Tang W, et al. Genetic loci associated with plasma phospholipid n-3 fatty acids: a meta-analysis of genome-wide association studies from the CHARGE Consortium. PLoS Genet. 2011;7(7):e1002193. doi: 10.1371/journal.pgen.1002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Nutrients associated with FEV1/FVC ratio (%) in the LSC and VSCa
| Nutrient (U/day)b | LSC (n=1,829)
|
VSC (n=508)
|
||
|---|---|---|---|---|
| Estimate (SE) | FDR | Estimate (SE) | FDR | |
| Minerals | ||||
| Magnesium (174.7 mg) | 0.732 (0.396) | 0.074 | 2.421 (1.024) | 0.023 |
| Magnesium (144.7 mg)c | 1.256 (0.481) | 0.025 | 2.161 (1.063) | 0.045 |
| Vitamins | ||||
| Total folate intake (478.6 μg) | 1.019 (0.349) | 0.025 | 2.231 (0.775) | 0.009 |
| Folic acid (411.0 μg) | 0.671 (0.325) | 0.048 | 1.858 (0.706) | 0.013 |
| Folate equivalents (860.5 μg) | 0.857 (0.333) | 0.025 | 2.144 (0.741) | 0.009 |
| Niacin (24.8 mg) | 0.374 (0.154) | 0.025 | 1.861 (0.605) | 0.009 |
| Vitamin A (9,160.2 IU) | 0.754 (0.266) | 0.025 | 1.245 (0.534) | 0.023 |
| Vitamin D (468.9 IU) | 0.454 (0.300) | 0.139 | 1.337 (0.765) | 0.081 |
| Fatty acids | ||||
| Eicosenoic fatty acid (135 mg) | 0.638 (0.267) | 0.025 | 1.541 (0.540) | 0.009 |
| EPA (100 mg) | 0.290 (0.121) | 0.025 | 0.740 (0.263) | 0.009 |
| DPA (20 mg) | 0.513 (0.202) | 0.025 | 1.393 (0.405) | 0.009 |
| DHA (150 mg) | 0.456 (0.205) | 0.034 | 1.319 (0.432) | 0.009 |
| EPA + DPA + DHA (310 mg) | 0.460 (0.192) | 0.025 | 1.229 (0.405) | 0.009 |
| EPA + DHA (290 mg) | 0.452 (0.190) | 0.025 | 1.207 (0.404) | 0.009 |
| trans-oleic (1.2 g) | −0.716 (0.559) | 0.20 | −1.847 (0.767) | 0.022 |
| AOAC fiber (10.5 g) | 1.075 (0.403) | 0.025 | 2.018 (0.761) | 0.013 |
Notes: EPA, eicosapentaenoic fatty acid 20:5n3; DPA, docosapentaenoic fatty acid 22:5n3; DHA, docosahexaenoic fatty acid 22:6n3; eicosenoic fatty acid 20:1n9.
IQR is used for calculating the estimate and 95% CI. Association analysis in the LSC was conducted based on longitudinal spirometry data using linear mixed effects model with adjustment for important covariates. Association analysis in the VSC was conducted based on baseline spirometry data using linear regression with adjustment for important covariates.
Assessment of total folate intake and folate equivalents includes all sources (ie, natural food, supplements, and fortified foods). Folic acid is from supplements and fortified foods. A total of 38% study subjects have missing data for trans-oleic fatty acid.
Estimate of intake without counting supplement.
Abbreviations: FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; LSC, Lovelace Smokers cohort; VSC, Veteran Smokers cohort; SE, standard error; FDR, false discovery rate; AOAC, the Association of Official Agricultural Chemists; IQR, interquartile range.
Table S2.
The association between long chain unsaturated fatty acid and FEV1/FVC decline (%) in the LSC (n=1,499)a
| Nutrient (U/day) | Time
|
Nutrients
|
Time × nutrients
|
||
|---|---|---|---|---|---|
| Estimate (SE)b | Estimate (SE) | P-value | Estimate (SE) | P-value | |
| Eicosenoic fatty acid (135 mg) | −0.47 (0.02) | −0.048 (0.11) | 0.67 | 0.032 (0.012) | 0.0081 |
| EPA (100 mg) | −0.44 (0.01) | 0.010 (0.052) | 0.85 | 0.019 (0.006) | 0.0030 |
| DPA (20 mg) | −0.46 (0.02) | −0.003 (0.087) | 0.98 | 0.032 (0.011) | 0.0028 |
| DHA (150 mg) | −0.46 (0.02) | −0.037 (0.088) | 0.67 | 0.031 (0.011) | 0.0031 |
| EPA + DPA + DHA (310 mg) | −0.45 (0.02) | −0.007 (0.083) | 0.94 | 0.030 (0.010) | 0.0028 |
| EPA + DHA (290 mg) | −0.45 (0.02) | −0.006 (0.082) | 0.94 | 0.030 (0.010) | 0.0028 |
Notes: EPA, eicosapentaenoic fatty acid 20:5n3; DPA, docosapentaenoic fatty acid 22:5n3; DHA, docosahexaenoic fatty acid 22:6n3; eicosenoic fatty acid 20:1n9.
IQR is used for calculating the estimate and SE. Association analysis in the LSC was conducted based on longitudinal spirometry data using linear mixed effects model with adjustment for important covariates.
P<0.0001.
Abbreviations: FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; LSC, Lovelace Smokers cohort; SE, standard error; IQR, interquartile range.