Abstract
Granulocyte colony-stimulating factor (G-CSF), a hematopoietic growth factor, is a standard supportive therapy given during cancer treatment. It induces acceleration in neutrophil recovery through stimulation of mobilization of hematopoietic progenitors. Given that the latter is also induced by chemotherapy itself, the timing of administration of G-CSF post-chemotherapy might impact the resultant overall effects. The present study aimed to determine the optimal timing of G-CSF post chemotherapy to exert its optimal effects on the immune cell recovery and its impact on antigen-specific CD8+ T-cell responses. B6 mice were treated once with cyclophosphamide (4 mg/mouse; CTX) and then daily with G-CSF (5μg/mouse) from Days 1–5, 2–5, or 5–9 post-CTX treatment. The total numbers of various immune cell types were analyzed on Days 7, 9, and 12 post-CTX treatment. To evaluate effects on CD8+ T-cell responses, a pmel-1 transgenic mouse model was used in combination with prime boost peptide vaccination therapy. The total number of white blood cells (WBC), neutrophils, monocytes, lymphocytes, granulocytes, and dendritic cells (DC) were significantly increased after G-CSF treatment in particular when G-CSF was administered from Days 2–5 post-CTX treatment. Application of this timing of G-CSF and CTX treatment after adoptive transfer of T-cells followed by prime-boost vaccination with antigenic peptide did not block the expansion of the donor pmel-1 CD8+ T-cells. In conclusion, adjusting the timing of treatment with G-CSF post chemotherapy can optimize its promoting effects on recovery of myeloid cells without altering the associated antigen-specific immunity.
Keywords: Post-chemotherapy, Cyclophosphamide, G-CSF, antigen-specific immunity
Introduction
The DNA alkylating agent cyclophosphamide (CTX) is a chemotherapeutic drug used to treat various types of cancer and is well known to potentiate the immune response by removing tumor-induced T-regulatory cells and blocking their function (Ghiringhelli et al. 2004; Lutsiak et al. 2005) by removing endogenous cellular elements that act as sinks for cytokines that are capable of augmenting the activity of self/tumor-reactive CD8+ T-cells (Gattinoni et al. 2005). It is transformed via hepatic and intracellular enzymes to active alkylating metabolites and prevents cell division primarily by crosslinking DNA and RNA strands. The removal of host lymphocytes also allows the accumulation of homeostatic cytokines such as interleukin (IL)-7 and IL-15, and thereby promoting the ability of donor T-cells to mediate anti-tumor immunity (Gattinoni et al. 2005). In addition, pre-conditioning a host with CTX can effectively augment the anti-tumor efficacy of adoptively transferred T-cells in response to vaccination (Gattinoni et al. 2005, 2006; Wrzesinski et al. 2005; Paulos et al. 2007; Salem et al. 2007, 2009, 2010; Rubinstein et al. 2013).
Because high doses of CTX lead to significant neutropenia, various therapeutic regimens involving administration of hematopoietic growth factors, in particular such as G-CSF (granulo-cyte colony stimulating factor), have been used to reduce myelosuppression and subsequent neutropenia by enhancing hematopoietic stem cell (HSC) mobilization (Nervi et al. 2006; Winkler and Levesque 2006; Montgomery and Cottler-Fox 2007). Studies previously noted that G-CSF administration for 5 consecutive days after CTX treatment can induce transient increases in numbers of dendritic cells (DC) measured on Days 7 and 9 when administered from Days 5–9 as compared to effects when administered Days 1–5 (Salem et al. 2010). Importantly, G-CSF administration early after CTX treatment neither alter the expansion of myeloid DC in the blood (Salem et al. 2010) nor the expansion of the tumor reactive CD8+ T-cells (Rubinstein et al. 2013).
Cancer immunotherapy based on vaccination has been found to induce significant anti-tumor immunity. For instance, we have found recently that CTX can induce increases in the numbers of DC in the peripheral blood during the time of recovery from leucopenia (Days 9–14). These cells were activated and migrated to lymph nodes after administration of the Toll-like receptor-3 (TLR3) agonist polyinosinic:polycytidylic acid [poly(I:C)] at this phase (Salem et al. 2009). Interestingly, concomitant vaccination with melanoma peptide along with poly(I:C) induced expansion and persistence of adoptively transferred tumor reactive CD8+ T-cells and induced regression of advanced melanoma (Salem et al. 2007). These studies indicate that vaccination with a tumor antigen can enhance tumor-reactive CTL activity and CD8+ T-cells and anti-tumor immunity (Winkler and Levesque 2009).
Given this critical effect of the timing of G-CSF administration on the host responses, the aim of the present study was to compare the effects of three different timing regimens of G-CSF on the expansion of immune cells at different timepoints to determine the optimal timing of its administration for its beneficial effects in the absence or presence of vaccination.
Materials and Methods
Mice
B6.SJL (Ly5.1), C57BL/6 (Ly5.2; B6), pmel-1 TCR transgenic (on B6 background), were purchased from The Jackson Laboratory (Bar Harbor, ME). Pmel-1 (Ly5.2) mice were bred with Ly5.1 wild-type (WT) mice to generate Ly5.1 mice heterozygous for the pmel-1 TCR Vα1/Vβ13 transgene. Pmel-1T cells expressing the Vα1/Vβ13 TCR specifically recognize the H-2Db-restricted human gp10025–33 epitope (KVPRNQDWL: gp10025–33). All mice were held and treated under pathogen-free conditions in facilities maintained at 22 ± 2°C with a 45–50% relative humidity and with a 12-hr light:dark cycle. All mice had ad libitum access to sterilized standard rodent chow and filtered water. All studies were done in accordance with institutional and federal guidelines and were approved by the Medical University of South Carolina IACUC.
Cell lines, antibodies, and reagents
Anti-CD16/CD32, and FITC-, PE-, or cychrome-conjugated monoclonal antibodies (mAb) against Ly5.1, and anti-CD8 were purchased from BD Pharmingen (San Diego, CA). G-CSF (Neupogen) was purchased from the local pharmacy at the Medical University of South Carolina. Human gp10025–33 epitope (KVPRNQDWL: gp10025–33) (American Peptide Company, Sunny-vale, CA) was dissolved in 10% DMSO (Sigma, St. Louis, MO) and diluted in PBS (phosphate-buffered saline, pH 7.4). Cyclophosphamide (CTX; Sigma) and TLR3 agonist poly(I:C) (Invitrogen, Carlsbad, CA) were each re-constituted in PBS and frozen until use.
Chemotherapy and G-CSF treatment
As previously described (Salem et al. 2007, 2010; Yankelevich et al. 2008), mice were intraperitoneally (IP) injected with PBS or 4 mg CTX/mouse. Mice were then left without further treatment (groups PBS and CTX) or treated with a subcutaneous (SC) injection of 5 μg G-CSF daily for three different treatment cycles: from Days 1 to 5 (groups PBS/G-CSF/d1-5 and CTX/G-CSF/d1-5), from Days 2 to 5 (groups PBS/G-CSF/d2-5 and CTX/G-CSF/d2-5) or from Days 5 to 9 (groups PBS/G-CSF/d5-9 and CTX/G-CSF/d5-9) after the PBS or CTX treatment. Mice were then bled on Day 7 (i.e., 2 d after the end of the Days 1-5 cycle and Days 2–5 cycle and in the middle of the Days 5–9 cycle), Day 9 (i.e., 4 d after the end of the Days 1–5 cycle and Days 2–5 cycle, and on Day 9 of the Days 5–9 cycle), and Day 12 (i.e., 7 d after the end of the Days 1–5 cycle and Days 2–5 cycle and 3 d after the end of the Days 5–9 cycle) after the PBS or CTX treatment to determine the numbers of white blood cells (WBC) in the peripheral blood.
CTX preconditioning, adoptive T cell transfer, and vaccination
Naive Ly5.2 mice were inoculated with 2.5 × 105 B16 melanoma (H2Kb) cells in their right flank, treated 10 days later (timepoint when tumor was established) by IP injection with PBS or 4 mg CTX (Salem et al. 2007), and adoptively transferred 1 day later by lateral tail vein injection with 106 of naive pmel-1 Ly5.1 cells. Mice were left without further manipulation or vaccinated by SC injection of 100 μg gp10025–33 melanoma peptide ± 200 μg poly(I:C) 2 days post PBS/CTX treatment. Control groups of recipient wild type naive Ly5.2 mice were IP injected with PBS or 4 mg CTX and left without further treatment (groups: PBS and CTX). Mice were bled on Day 5 post vaccination (Day 7 post CTX treatment) to analyze frequency/pheno-type of the donor pmel-1 CD8+T cells and DC in their PBL. Donor cells defined as Ly5.1+ and CD8+ T-cells and their phenotypes were analyzed by flow cytometry (BD Biosciences, San Jose, CA). Peripheral blood samples were collected from the retro-orbital plexus at the pre-determined timepoints and the samples then processed for VetScan HM5 Hematology (Abaxis) to assess total peripheral blood leukocyte (PBL) count.
Flow cytometry
For flow cytometry analysis, fresh single-cell suspensions of leukocytes from blood (0.5–1.0 × 106) were treated with anti-CD16/CD32 mAb for 5 min on ice, then stained with the indicated fluorophore-conjugated mAb (at manufacturer-recommended levels), incubated for 30 min on ice, and then immediately analyzed by flow cytometry using a FACS Calibur system (BD Biosciences, San Jose, CA); all data analysis was done with Cell-Quest software (Becton Dickinson, San Jose, CA). A minimum of 50,000 events was acquired for each sample. Total numbers of different cell populations in each compartment were calculated each time.
Statistical analysis
All results are presented as means ± SE. Statistical comparisons between CTX/G-CSF groups and their PBS/G-CSF counterparts were made using a paired Student’s t test. Statistical comparisons among prospective groups were analyzed using a one-way analysis of variance (ANOVA) as part of an SPSS software package (v.16.0 for Windows, 2007; SPSS, Inc., Chicago, IL). Statistical significance was determined by a post-hoc test followed by Dunnett’s multiple comparison tests to compare treatment means vs. respective controls. All p values were paired 2-tailed, with p-values < 0.05 considered significant.
Results
Effect of timing of G-CSF treatment on WBC, lymphocytes, granulocytes, and monocytes
Administration of G-CSF after PBS treatment caused no significant changes in total numbers of peripheral blood WBC when analyzed on Day 7 or Day 9, but significant decreases on Day 12, in the PBS/G-CSF/d1-5 mice as compared to in mice treated with PBS alone (Figure 1). In comparison, G-CSF given after PBS treatment resulted in significant increases in total WBC numbers when measured on Days 7 and 9 in the PBS/G-CSF/d5-9 groups (compared to in mice treated with PBS alone); the effect was no longer apparent on Day 12. With respect to hosts receiving CTX, G-CSF given after CTX treatment induced significant increases in total WBC numbers when analyzed on Day 7 for the CTX/G-CSF/d2-5 and CTX/G-CSF/d5-9 mice and Day 9 for the CTX/G-CSF/d5-9 hosts as compared to CTX-treated control mice that then received no G-CSF (Figure 1). The effect was no longer apparent on Day 12 for any treated group.
Figure 1. Administration of G-CSF post-CTX treatment induced increases in total numbers of WBC.
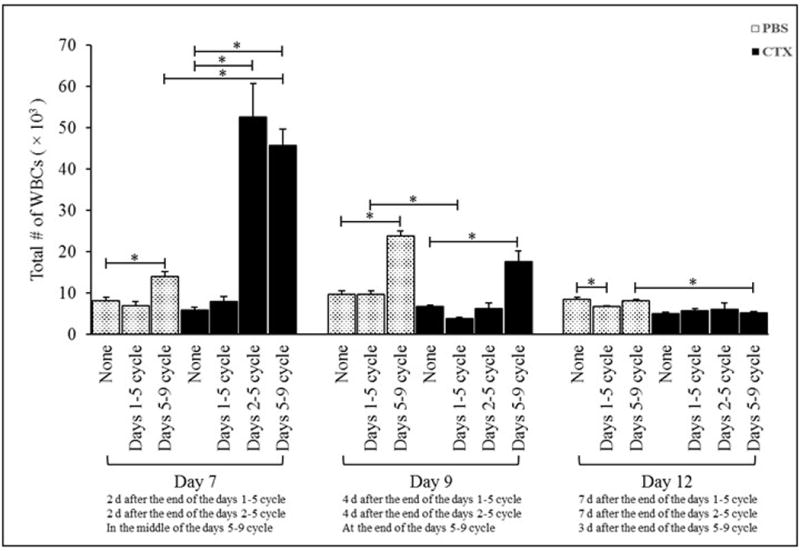
Mice were intraperitoneally (IP) injected with PBS or 4 mg CTX/mouse. Mice were then left without further treatment (groups PBS and CTX) or treated daily with subcutaneous (SC) injections of 5 μg G-CSF in three different cycles: from Days 1-5 (groups PBS/G-CSF/d1-5 and CTX/G-CSF/d1-5), from Days 2-5 (groups PBS/G-CSF/d2-5 and CTX/G-CSF/d2-5), or from Days 5-9 (groups PBS/G-CSF/d5-9 and CTX/G-CSF/d5-9) post-PBS or CTX treatment. Mice were bled on Days 7, 9, and 12 post-PBS and -CTX treatment to determine total numbers of white blood cells (WBC) in the peripheral blood. *p ≤ 0.05 vs. prospective controls.
Daily G-CSF injections on Days 1–5 post-CTX induced significant decreases [relative to levels in G-CSF-treated mice on Days 1–5 post-PBS] in total numbers of peripheral WBC when analyzed on Day 9. G-CSF administered on Days 5–9 post-CTX resulted in significant increases in total WBC on Day 7, but significant decreases on Day 12, relative to values in PBS-treated mice that received G-CSF in the same timeframe (Figure 1). Together, the data indicated timing of G-CSF administration post-chemotherapy (CTX) could alter mobilizing effects on leukocytes. The highest effect appeared when treatments started 2 days after chemotherapy, but remained so even if therapy began 5 days post-CTX. However, again, effects of the G-CSF were short-lived.
In regard to lymphocytes, in PBS-treated mice, G-CSF given on Days 1–5 induced signi-ficant increases in total numbers of lymphocytes when analyzed on Day 7 and induced signifi-cant decrease in the numbers when analyzed on Day 12 post-PBS [compared to in hosts that received no G-CSF]. Additionally, mice given G-CSF on Days 5–9 post-PBS had significant increases on Days 7 and 9 as compared to values in PBS-only counterparts that received no G-CSF (Figure 2), with no effect seen on Day 12. G-CSF given on Days 5-9 post-CTX resulted in significant decreases in total lymphocyte numbers on Days 9 and 12 relative to values in PBS-treated mice that received G-CSF in the same timeframe. However, G-CSF administered on Days 1-5 post-CTX induced a significant decrease in total lymphocyte numbers when analyzed on Days 7, 9, and 12 as compared to in PBS-treated mice that received G-CSF in the same timeframe (Figure 2). Giving G-CSF to CTX-injected hosts on Days 2–5 or Days 5–9 post-CTX treatment resulted in significant increases in total numbers of lymphocytes on Day 7 and on Day 9 for the CTX/G-CSF/d5-9 group only, as compared to in CTX-treated controls. Oddly, among mice that received G-CSF from Days 1–5 or 2–5 post-CTX, lymphocyte levels were actually significantly decreased on Day 12 (relative to levels in hosts receiving CTX only).
Figure 2. Administration of G-CSF post-CTX treatment induced increases in numbers of lymphocytes.
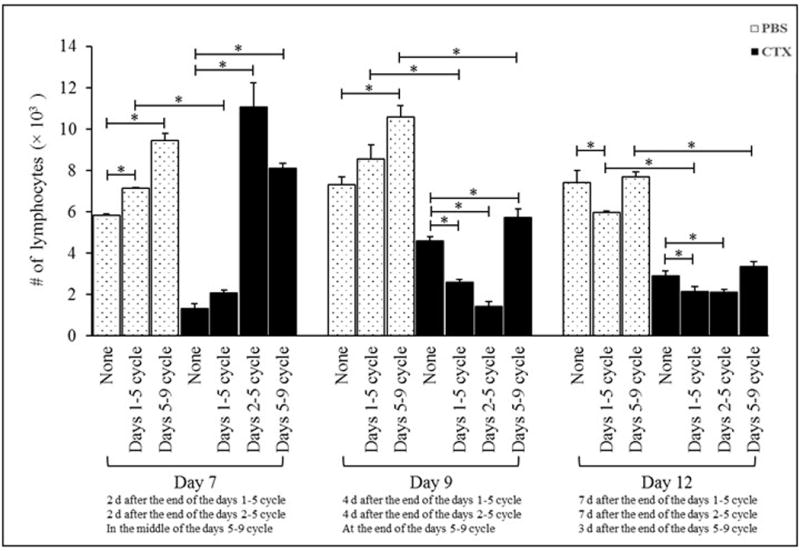
Mice were IP injected with PBS or 4 mg CTX. Mice were then left without further treatment (groups PBS and CTX) or treated daily with SC injections of 5 μg G-CSF in three different treatment cycles: from Days 1-5, from Days 2-5, or from Days 5-9 post-PBS or -CTX treatment. Mice were bled on Days 7, 9, and 12 post-PBS and -CTX treatment to determine total numbers of lymphocytes in peripheral blood. *p ≤ 0.05 vs. prospective controls.
For granulocytes, G-CSF given to PBS-treated mice from Days 5–9 resulted in significant increases in granulocyte numbers on Days 7 and 9, but not Day 12, post-PBS (Figure 3A). There was no impact from G-CSF given on Days 1–5 at any of these three timepoints. In mice given G-CSF from Days 2–5 (on Day 7) and Days 5–9 (on Days 7 and 9) post-CTX, there were significant increases in granulocyte numbers as compared to values in CTX-treated mice given no G-CSF (Figure 3A). Among CTX-treated hosts, G-CSF given on Days 1–5 had no significant impact on granulocyte levels at any of the timepoints. In all cases, no effects were evident among CTX hosts by Day 12 post-CTX when compared to control (CTX-treated) mice. G-CSF given on Days 1–5 or Days 5–9 post-CTX induced significant increases in total granulocyte numbers on Day 7 or Day 12 as compared to in PBS-treated mice given G-CSF in same timeframe (Figure 3A).
Figure 3. Administration of G-CSF post-CTX treatment induced increases in numbers of granulocytes and monocytes.
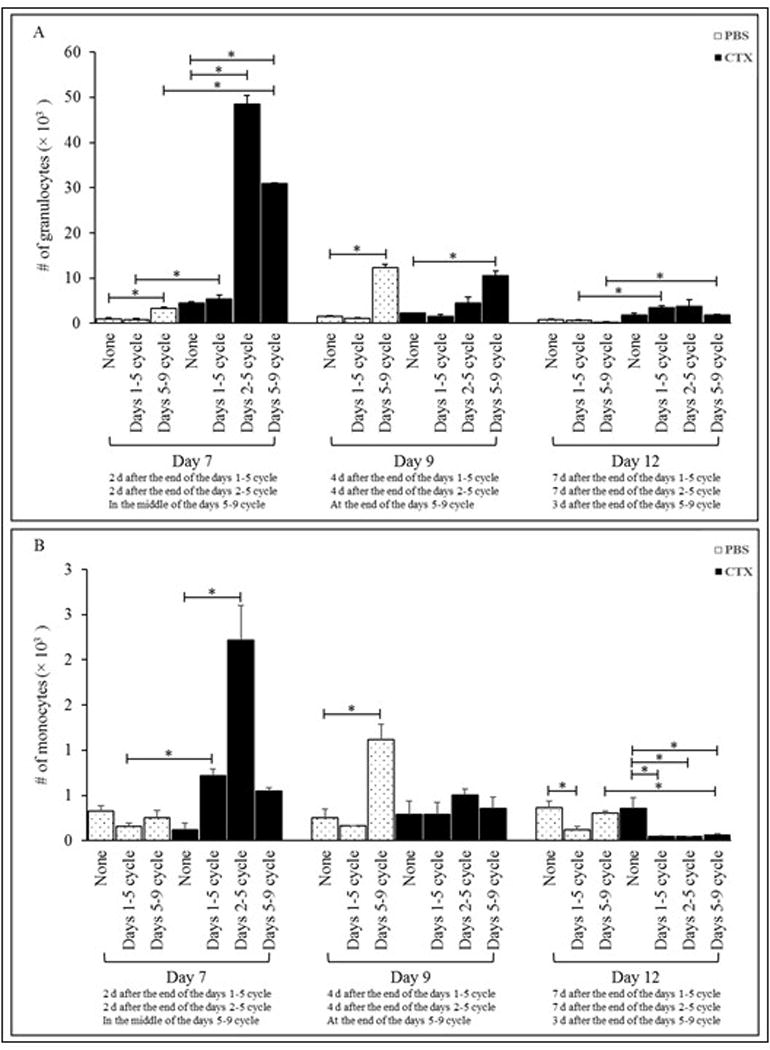
Mice were IP injected with PBS or 4 mg CTX. Mice were then left without further treatment (groups: PBS and CTX) or treated daily with SC injections of 5 μg G-CSF in three different treatment cycles: from Days 1-5, from Days 2-5, or from Days 5-9 post-PBS or -CTX treatment. Mice were bled on Days 7, 9, and 12 post-PBS and -CTX treatment to determine total numbers of (A) granulocytes and (B) monocytes in peripheral blood. *p ≤ 0.05 vs. prospective controls.
G-CSF given to PBS-injected mice from Days 1–5 or Days 5–9 had no effect on blood monocyte numbers at Day 7 post-PBS (Figure 3B). Only on Day 9 did mice in the Days 5–9 cycle present with a significant increase; on Day 12, hosts from the Days 1–5 cycle had a signi-ficant decrease (relative to in PBS-only hosts). Among CTX-treated mice given G-CSF, treatments on Days 1–5, 2–5, or 5–9 had no significant impact on Day 9, but induced significant decreases on Day 12 [relative to levels in CTX-only hosts]. However, among CTX mice in the Days 2–5 regimen, there was a significant effect (increase vs. CTX only) on monocyte levels, but only on Day 7. Interestingly, on Day 12, monocyte levels among all G-CSF-treated CTX mice seemed below those of PBS/G-CSF counterparts; these differences were significantly lower in Days 5–9 G-CSF mice. In contrast, on Day 7, monocytes levels in all G-CSF-treated CTX mice were above those of PBS/G-CSF counterparts; these differences were significantly higher in the Days 1–5 G-CSF hosts. However, among CTX-treated mice given G-CSF, treatments on Days 1–5 or Days 5–9 had no significant impact relative to numbers seen in PBS/G-CSF counterparts on Day 9.
Administration of G-CSF during prime-boost vaccination had no effect on antigen-specific CD8+ T-cell responses
Given that G-CSF treatment from Days 2–5 had the greatest effect in enhancing WBC numbers (on Day 7 post-CTX treatment), the study evaluated effects of this regimen/timing on CD8+ T-cell responses. Immunization with gp10025–33 alone did not induce significant pmel-1 cell expansion in PBS- or CTX-treated hosts as indicated by little change in numbers of the cells in the blood (Figure 4). In comparison, addition of poly(I:C) to this immunization of PBS- or CTX-treated mice (gp10025–33/poly(I:C) and gp10025–33/poly(I:C)/CTX, respectively) significantly enhanced expansion of pmel-1 cells in both PBS or CTX-treated mice compared with PBS- or CTX-treated controls; the latter showed greater expansion than former.
Figure 4. Effect of G-CSF post-CTX treatment on pmel-1 CD8+T cell expansion in response to vaccination.
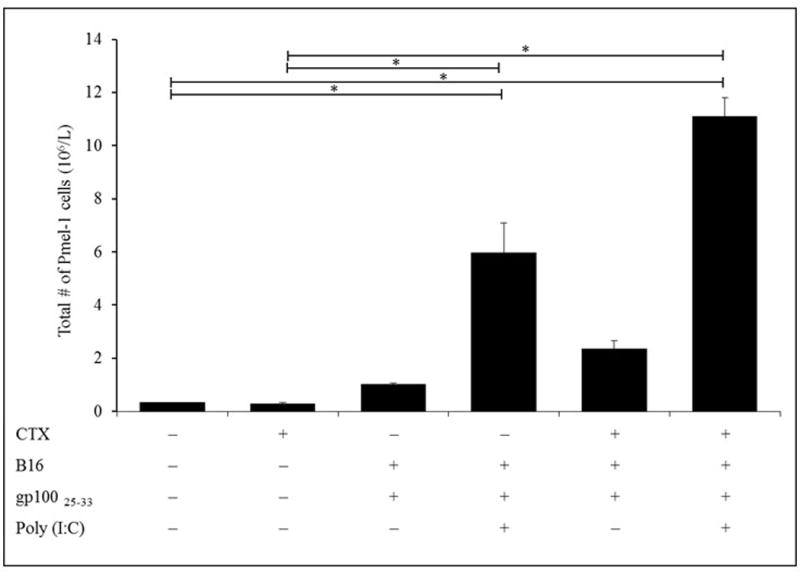
Naive Ly5.2 mice were inoculated with 2.5 ×105 B16 cells in right flank and IP injected 10 days later (timepoint when tumor was established) with PBS or CTX and adoptively transferred with 106 un-fractionated naïve spleen cells from Ly5.1 donor pmel-1 Tg mice. Mice were left without further manipulation or SC vaccinated with gp10025–33 ± poly(I:C) 5 days post-PBS or -CTX treatment. Mice were injected 1 day post-PBS or -CTX treatment with G-CSF from Days 2-5. Mice were then bled on Day 5 post-vaccination (Day 7 post-CTX treatment) to assess total numbers of pmel-1 CD8+ T-cells in PBL by flow cytometry. *p ≤ 0.05 vs. prospective controls.
Vaccination with gp10025–33 alone after pmel-1 cell adoptive transfer significantly increased the numbers of DC in the blood of both PBS- and CTX-treated hosts relative to those in non-vaccinated hosts (Figure 5). The effect was optimal among PBS mice that received vaccine alone; addition of poly(I:C) appeared to significantly mitigate the effect. Vaccination with gp10025–33 alone, gp10025–33/poly(I:C), gp10025–33/CTX or gp10025–33/poly(I:C) of PBS- or CTX-treated-mice induced significant increases in DC numbers among the PBL 5 days post-vaccination compared to values in PBS- treated mice. Of note, DC levels were greater after addition of poly(I:C) in particular only in CTX-treated mice. Since all vaccinated mice were injected also with G-CSF from Days 2–5, these results indicated that increases in CD8+ T-cell numbers induced by G-CSF seemed to not interfere with resultant antigen-specific CD8+ T-cell responses or with expansion of DC populations in situ.
Figure 5. Effect of G-CSF on numbers of DC in gp10025–33 peptide-vaccinated recipient mice.
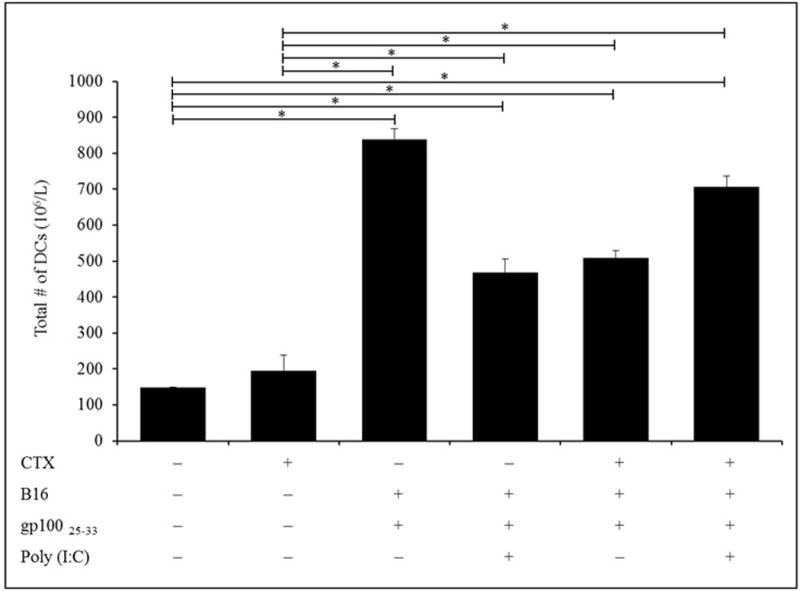
Naive Ly5.2 mice were inoculated with 2.5 ×105 B16 cells in right flank and IP injected 10 days later (timepoint when tumor was established) with PBS or CTX and adoptively transferred with 106 un-fractionated naïve spleen cells from Ly5.1 donor pmel-1 Tg mice. Mice were left without further manipulation or SC vaccinated with gp10025–33 ± poly(I:C) 5 days post-PBS or -CTX treatment. Mice were injected 1 day post-PBS or -CTX treatment with G-CSF from Days 2-5. Mice were then bled on Day 5 post-vaccination (Day 7 post-CTX treatment) to assess total numbers of DC in peripheral blood by flow cytometry. *p ≤ 0.05 vs. prospective controls.
This study also evaluated the therapeutic anti-tumor efficacy of the treatment regimens, i.e., CTX pre-conditioning, adoptive transfer of naive pmel-1 cells, and prime-boost vaccination with gp10025–33 ± poly(I:C), on leukocyte counts. As compared to in PBS-treated non-vaccinated hosts, administration of gp10025–33 alone resulted in significant increases in total WBC numbers (Figure 6A), and in total numbers of monocytes (Figure 6C) and granulocytes (Figure 6D). Treatment of mice with the gp10025–33/Poly (I:C) combination induced no significant effects on all analyzed parameters when compared to values from the control PBS hosts. Compared to in PBS-treated non-vaccinated mice, boosting with the gp10025–33/CTX combination resulted in significant decreases in numbers of lymphocytes (Figure 6B) and increases in monocytes (Figure 6C) and granulocytes (Figure 6D). gp10025–33/poly(I:C)/CTX administration induced significant decrease in lymphocytes and monocytes, but a significant increase in granulocytes in the PBL compared with levels noted in PBS-treated mice.
Figure 6. Effect of G-CSF on differential numbers of WBC in gp10025–33-vaccinated mice.
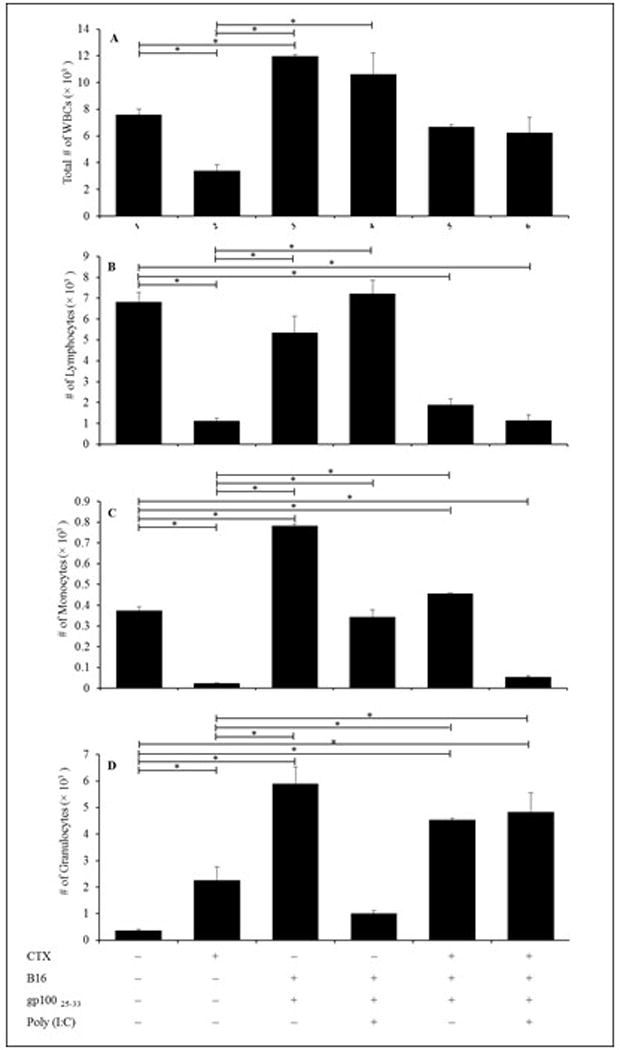
Naive Ly5.2 mice were inoculated with 2.5 ×105 B16 cells in right flank and IP injected 10 days later (timepoint when tumor was established) with PBS or CTX and adoptively transferred with 106 un-fractionated naïve spleen cells from Ly5.1 donor pmel-1 Tg mice. Mice were injected one day post-PBS or -CTX treatment with G-CSF from Days 2-5. Mice were left without further manipulation or SC vaccinated with gp10025–33 ± poly(I:C) 5 days post-PBS or CTX treatment. Mice were then bled on Day 5 post-vaccination (Day 7 post-CTX treatment) to assess total numbers of (A) WBC, (B) lymphocytes, (C) monocytes, and (D) granulocytes in the peripheral blood via flow cytometry. *p ≤ 0.05 vs. prospective controls.
Treatment of Ly5.2 mice with CTX alone resulted in significant decreases in total WBC (Figure 6A) and of lymphocytes and monocytes, and a significant increase in granulocytes (Figure 6D) compared to in PBS-treated mice. Compared to values in CTX-treated non-vaccinated mice there were significant increases in WBC numbers (groups given gp10025–33 alone or gp10025–33/poly(I:C); Figure 6A), lymphocytes (groups boosted with gp10025–33 alone or gp10025–33/poly (I:C); Figure 6B), monocytes (groups administrated gp10025–33 alone, gp10025–33/poly (I:C) or gp10025–33/CTX; Figure 6C), and granulocytes (groups boosted with gp10025–33 alone, gp10025–33/CTX or gp10025–33/Poly (I:C)/CTX; Figure 6D). Since all vaccinated mice were injected also with G-CSF from Days 2–5, these results indicated G-CSF did not interfere with resultant antigen-specific CD8+ T-cell responses or DC expansion.
Discussion
Granulocyte colony-stimulating factor (G-CSF), a hematopoietic growth factor, is a standard supportive therapy given during cancer treatment. It induces acceleration in immune cells recovery through stimulation of mobilization of hematopoietic progenitors. Given the latter are also induced by chemotherapy itself, the timing of G-CSF administration post-chemotherapy might impact on the resultant overall effects (Winkler and Levesque 2006; Montgomery and Cottler-Fox 2007). Indeed, our previous work (Salem et al. 2010) showed that G-CSF administration for 5 consecutive days after CTX treatment could induce transient increases in the numbers of DC measured on Days 7 and 9 when administered from Days 5–9 as compared to effects when administered in the Days 1–5 cycle.
The new concept evaluated in the current study was to compare two different post CTX treatment regimens of G-CSF administration (i.e., Days 2–5 and Days 5–9 cycles) with another regimen of G-CSF administration (Days 1–5 cycle) that had been established in previous work (Salem et al., 2010) to determine an optimal timing for G-CSF to exert optimal effects on the immune cell recovery/mobilization and impact on antigen-specific immune responses. The rationale behind testing these two post-CTX G-CSF administration regimens was to investigate if a delay in G-CSF administration would induce mobilization of progenitor cells in bone marrow. As a result, the highest effect appeared when treatments started beginning 2 days after CTX chemotherapy, but remained so even if therapy began at 5 days. Here, G-CSF treatment on Days 2–5 showed the highest effect on recovery of immune cells (Day 7 post-CTX treatment). Additionally, G-CSF did not interfere with resultant antigen-specific CD8+ T-cell responses or with the expansion of DC populations. However, the effects were short-lived/non-persistent. Still, importantly, granulocytes that had become mobilized as a result of G-CSF treatments inter-fered neither with expansion of DC in the treated hosts nor with antigen-specific CD8+ T-cell responses in situ. Such data are important with regard the clinical application of combinational chemotherapy and immunonotherapy.
The most common regimen of G-CSF therapy that has been applied either in pre-clinical or clinical settings is based on injection of G-CSF from Days 1-5 post-CTX treatment. Because CTX itself induces alteration in the host bone marrow microenvironment and the timing of G-CSF administration post-chemotherapy differs, it was thought that the timing of injection of G-CSF post-CTX treatment was critical to optimize any mobilizing effects of immune cells into circulation. As such, this study compared three different G-CSF administration regimens after CTX treatment to determine the most effective one on immune cell mobilization.
Here, there were marked increases in total WBC in the blood that coincided with increases in granulocyte levels on Day 7 post-CTX treatment, that gradually diminished there-after. Such results were consistent with those of Liu et al. (1996) and Welte et al. (1987) who noted G-CSF administration promoted generation of the granulocytic lineage (mainly neutron-phils) and mobilized hematopoietic stems cells from bone marrow into the peripheral circulation. Though it is not clear how G-CSF treatment augmented post-CTX of immune cell expansion, it could be suggested G-CSF acted on residual progenitors in the bone marrow after CTX treat-ment; immature progenitors were previously seen to re-appear in murine bone marrow by 24 hr after CTX treatment (Yankelevich et al. 2008). This notion was consistent with results of clinical studies that reported mobilization of immune cells into peripheral blood of cancer patients after CTX + G-CSF treatment (Ferrari et al. 2003). Analyses of effects of three different G-CSF post-CTX treatment regimens on granulocytes, monocytes, and lymphocytes showed there was significant impact on numbers of these cell types in the blood during or only shortly after the G-CSF regimen concluded. These results were consistent with those from Demetri and Griffin (1991) who noted G-CSF administration induced increases in WBC counts by acting as a potent hematopoietic growth factor, particularly for cells of the granulocytic lineage.
Past studies from our laboratories also showed that CTX induced increases in circulating levels of DC during recovery from leukopenia, and that administration of the toll-like receptor 3 (TLR3) agonist poly(I:C) during the peak of DC expansion induced activation/migration of DC to lymph nodes (Salem et al. 2009). Furthermore, concomitant administration of poly(I:C) with vaccination with an MHC Class I melanoma self-tumor gp100 peptide at the peak of post-CTX DC expansion resulted in therapeutically effective anti-tumor T-cell responses against advanced melanoma. Any role of the post-CTX-expanded DC in the beneficial effects of CTX was directly confirmed by abrogation of the augmented T-cell responses after depletion of expanded DC before vaccination. The data from this current study showed that vaccination of pre-conditioned recipient mice under treatment of G-CSF (Days 2-5) did not interfere with the increased expansion of DC or antigen-specific CD8+ T-cell responses. Vaccination in the presence of poly(I:C) resulted in lower numbers of DC in the blood - an outcome that could be explained by induced maturation of these cells and their consequent migration to lymph nodes as previously reported (Salem et al. 2010). Moreover, activation of post-CTX expanded DC appeared to enhance the activity of vaccine primed pmel-1 cells better than naive cells, suggesting that a vaccination regimen would be an effective approach to exploit any significant CTX-induced DC expansion.
Conclusions
A large pool of expanded granulocytes can be mobilized by administration of G-CSF at certain timepoints after CTX therapy. These cells do not interfere with the quantity of the tumor-specific responses of CD8+ T-cells. These results provide a useful foundation for a rationale design of anti-cancer immunotherapy regimens based on G-CSF administration.
Acknowledgments
This work was supported by the NIH Grant R01 CA133503A. The authors would like also to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding this research group NO (RG-1435-019).
Footnotes
Declaration of interest
The authors declare no conflicts of interest. The authors alone are responsible for the content of this manuscript.
References
- Demetri GD, Griffin JD. Granulocyte colony-stimulating factor and its receptor. Blood. 1991;78:2791–808. [PubMed] [Google Scholar]
- Ferrari S, Rovati B, Porta C, Alessandrino P, Bertolini A, Collova E, Riccardi A, Danova M. Lack of dendritic cell mobilization into peripheral blood of cancer patients following standard- or high-dose chemotherapy plus granulocyte-colony stimulating factor. Cancer Immunol Immunother. 2003;52:359–366. doi: 10.1007/s00262-002-0365-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzke A, Piao W, Lauber J, Gatzlaff P, Konecke C, Hansen W, Schmitt-Thomsen A, Hertenstein B, Buer J, Ganser A. G-CSF as immune regulator in T-cells expressing G-CSF receptor: Implications for transplantation and autoimmune diseases. Blood. 2003;102:734–739. doi: 10.1182/blood-2002-04-1200. [DOI] [PubMed] [Google Scholar]
- Gattinoni L, Finkelstein S, Klebanoff C, Antony P, Palmer D, Spiess P, Hwang L, Yu Z, Wrzesinski C, Heimann D, et al. Removal of homeostatic cytokine sinks by lympho-depletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T-cells. J Exp Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L, Powell DJ, Rosenberg S, Restifo NP. Adoptive immunotherapy for cancer: Building on success. Nat Rev Immunol. 2006;6:383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, Chauffert B, Solary E, Bonnotte B, Martin F. CD4+CD25+ regulatory T-cells suppress tumor immunity but are sensitive to cyclophosphamide that allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336–344. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- Liu F, Wu H, Wesselschmidt R, Kornaga T, Link D. Impaired production and increased apoptosis of neutrophils in granulocyte colony-stimulating factor receptor-deficient mice. Immunity. 1996;5:491–501. doi: 10.1016/s1074-7613(00)80504-x. [DOI] [PubMed] [Google Scholar]
- Lutsiak M, Semnani R, De Pascalis R, Kashmiri S, Schlom J, Sabzevari H. Inhibition of CD4+CD25+ T-regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862–2868. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- Montgomery M, Cottler-Fox M. Mobilization and collection of autologous hemato-poietic progenitor/stem cells. J Clin Adv Hematol Oncol. 2007;5:127–123. [PubMed] [Google Scholar]
- Morikawa K, Morikawa S, Nakamura M, Miyawaki T. Characterization of granulocyte colony-stimulating factor receptor expressed on human lymphocytes. Br J Hematol. 2002;118:296–304. doi: 10.1046/j.1365-2141.2002.03574.x. [DOI] [PubMed] [Google Scholar]
- Nervi B, Link D, Di Persio J. Cytokines and hematopoietic stem cell mobilization. J Cell Biochem. 2006;99:690–705. doi: 10.1002/jcb.21043. [DOI] [PubMed] [Google Scholar]
- Paulos C, Wrzesinski C, Kaiser A, Hinrichs C, Chieppa M, Cassard L, Palmer D, Boni A, Muranski P, Yu Z, et al. Microbial translocation augments function of adoptively transferred self/tumor-specific CD8+ T-cells via TLR4 signaling. J Clin Invest. 2007;117:2197–2204. doi: 10.1172/JCI32205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein M, Salem M, Doedens A, Moore C, Chiuzan C, Rivell G, Cole D, Goldrath A. G-CSF/anti-G-CSF antibody complexes drive potent recovery and expansion of CD11b+Gr-1+ myeloid cells without compromising CD8+ T-cell immune responses. J Hematol Oncol. 2013;1:6–75. doi: 10.1186/1756-8722-6-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem M, Kadima A, El-Naggar S, Rubinstein M, Chen Y, Gillanders WE, Cole D. Defining the ability of cyclophosphamide pre-conditioning to enhance antigen-specific CD8+ T-cell response to peptide vaccination: Creation of a beneficial host microenvironment involving Type I IFN and myeloid cells. J Immunother. 2007;30:40–53. doi: 10.1097/01.cji.0000211311.28739.e3. [DOI] [PubMed] [Google Scholar]
- Salem M, Diaz-Montero C, Al-Khami A, El-Naggar S, Naga O, Montero A, Khafagy A, Cole D. Recovery from cyclophosphamide-induced lymphopenia results in expansion of immature dendritic cells that can mediate enhanced prime-boost vaccination anti-tumor responses in vivo when stimulated with TLR3 agonist poly(I:C) J Immunol. 2009;182:2030–2040. doi: 10.4049/jimmunol.0801829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem M, Al-Khami A, El-Naggar S, Diaz-Montero C, Chen Y, Cole D. Cyclophos-phamide induces dynamic alterations in host microenvironments resulting in a Flt3 ligand-dependent expansion of dendritic cells. J Immunol. 2010;184:1737–1747. doi: 10.4049/jimmunol.0902309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welte K, Bonilla M, O’reilly R, Souza L. Recombinant human granulocyte colony-stimulating factor. Effects on hematopoiesis in normal and cyclophosphamide-treated primates. J Exp Med. 1987;165:941–948. doi: 10.1084/jem.165.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler I, Levesque J. Mechanisms of hematopoietic stem cell mobilization: When innate immunity assails cells that make blood and bone. Exp Hematol. 2006;34:996–1009. doi: 10.1016/j.exphem.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Wrzesinski C, Paulos C, Gattinoni L, Palmer D, Kaiser A, Yu Z, Rosenberg S, Nicholas P, Restifo N. Hematopoietic stem cells promote the expansion and function of adoptively transferred anti-tumor CD8 T-cells. J Clin Invest. 2007;117:492–501. doi: 10.1172/JCI30414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankelevich M, Goodell M, Kaplan J. Efficacy of delayed administration of post-chemo-therapy granulocyte colony-stimulating factor: Evidence from murine studies of bone marrow cell kinetics. Exp Hematol. 2008;36:9–16. doi: 10.1016/j.exphem.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Wheeler C, Zeltzer P, Ying H, Finger D, Lee P, Yong W, Incardona F, Thompson R, Ridinger M, et al. Vaccination of malignant glioma patients with peptide-pulsed dendritic cells elicits systemic cytotoxicity and intracranial T-cell infiltration. Cancer Res. 2001;1:842–847. [PubMed] [Google Scholar]


