Abstract
Background & Aims
Diarrhea associated with inflammatory bowel diseases (IBD) has been associated with increased levels of inflammatory cytokines, including tumor necrosis factor (TNF). The intestinal mucosa of patients with IBD has reduced expression of solute carrier family 26 member 3 (SLC26A3, also called DRA). We investigated whether TNF directly affects expression of DRA in human intestinal epithelial cells (IECs) and in intestines of mice, and studied the mechanisms of these effects.
Methods
We performed quantitative reverse transcription PCR, immunofluorescence, and immunoblot analyses of Caco-2, HT-29, and T-84 cells human IECs cultured in 2 or 3 dimensions with or without TNF (50 ng/ml for 6–24 hrs). We purified nuclear extracts and quantified NF-κB activation and DNA binding. We isolated intestinal crypts from C57/BL6 mice, cultured enteroids, incubated these with TNF (50 ng/ml, 24 hrs), and quantified mRNAs. DRA-mediated exchange of Cl− for HCO3− was measured by uptake of 125I. Expression of the NFKB inhibitor alpha (NFKBIA, also called IKBA) was knocked down in Caco-2 or HT-29 cells with small interfering RNAs. Activation of NF-κB in response to TNF was measured by luciferase reporter assays; binding of the NF-κB subunit p65 in cells was analyzed in chromatin precipitation assays. DRA promoter activity was measured in a luciferase reporter assay. C57BL/6J mice were injected with TNF (5 μg/mouse for 3–6 hrs) or vehicle (control); intestines were collected and analyzed by immunofluorescence, or RNA and protein were collected from the mucosa.
Results
Incubation of IECs with TNF reduced expression of DRA. Knockdown of IKBA in IECs led to nuclear translocation of the NF-κB subunit p65 and reduced levels of DRA mRNA and protein. Expression of a transgene encoding p65 or p50 in IECs led to significant reductions in the promoter activity of DRA and its expression. In chromatin precipitation assays, p65 bound directly to the promoter of DRA, at the regions of −935 of −629 and −375 to −84. Injection of mice with TNF or incubation of crypt-derived organoids with TNF reduced their expression of DRA mRNA and protein.
Conclusions
In human IECs and intestinal tissues from mice, we found TNF to activate NF-κB, which reduced expression of the Cl− to HCO3− exchanger DRA (SLC26A3), via direct binding to the promoter of DRA. This pathway is an important therapeutic target for IBD-associated diarrhea.
Keywords: ion transporter, intestinal inflammation, mouse model, NaCl absorption
Introduction
Diarrhea is one of the most debilitating symptoms of inflammatory bowel diseases (IBD). Diarrhea results from either increased secretion, decreased absorption, or both of water and electrolytes across intestinal epithelium. Increasing evidence shows that impairment of NaCl absorption appears to be the predominant mechanism underlying IBD-associated diarrhea. 1 Electroneutral NaCl absorption in the human ileum and colon involves coupling of luminal Na+/H+ exchange (NHE) and Cl−/OH−(HCO3−) exchange activities 2. Little is known about the molecular mechanisms underlying the modulation of Cl− absorption in intestinal inflammation. Two members of the multifunctional anion exchanger SLC26 gene family, SLC26A3 or DRA (Down Regulated in Adenoma) and SLC26A6 or PAT-1 (putative anion transporter-1) are involved in mediating luminal Cl−/HCO3− (OH−) exchange in the human intestine 3. Substantial evidence indicates that DRA couples to NHE3 to mediate electroneutral NaCl absorption in the intestine 3,4. Loss of function mutations in the DRA gene have been shown to cause congenial chloride diarrhea (CLD) 5. Further, recent studies showed that DRA−/− but not PAT1−/− knockout mice exhibit profound diarrheal phenotype with serum electrolyte imbalances similar to the CLD 6 indicating that DRA but not PAT1 plays the key role in intestinal chloride and fluid absorption. Previous studies from our lab and others have shown that DRA function or expression was significantly reduced in models of intestinal infection and/or inflammation associated with diarrhea. For example, enteropathogenic E coli infection of intestinal epithelial cells 7 caused a significant reduction in DRA levels on luminal plasma membrane concomitant with a decrease in its function. Furthermore, DRA expression was remarkably suppressed in animal models of infection by C. rodentium, the murine counterpart of EPEC, explaining the associated diarrhea 8. DRA expression was also significantly decreased in IL-10 knockout and DSS models of colitis in mice 9,10 and in patients with ulcerative colitis 11. However, the exact mechanisms involved in the reduction of DRA in intestinal inflammation and role of cytokines in the process are not fully understood. A recent study by Xiao et al. showed decreased expression of DRA in TNF-α over expressing (TNF+/ΔARE) transgenic mice12 suggesting a role for TNF in mediating the decrease in DRA. However, TNF+/ΔARE transgenic mice also displayed high levels of IL-1β and IFN-γ in both ileum and colon. Whether TNF directly influences DRA expression remains elusive, and the potential involvement of NF-κB pathways in this effect has not been investigated.
TNF exerts its effects mainly via activation of NFκB pathway. NF-κB/Rel protein family includes p50 and p65 subunits. In the inactive stage, p65/p50 subunits (NF-κB complex) are sequestered in the cytoplasm through interaction with inhibitory protein IκBα. Upon activation, NF-κB complex translocates to the nucleus where it regulates the expression of target genes13. Indeed, activation of the NFκB pathway by external stimuli such as proinflammatory cytokines or infectious agents plays a critical role in regulating the expression of a variety of genes linked to immune response and inflammation 14. Therefore, the present studies were undertaken to test the hypothesis that TNF decreases DRA expression via NF-κB mediated pathway in intestinal inflammation.
Our results demonstrated that TNF decreased DRA expression in both in vitro and in vivo models. This repression was mediated via eliciting NF-κB pathway and involved direct binding of p65 to the promoter region of the DRA gene. Our findings, for the first time, established that DRA is a direct target of NF-κB. These data provide mechanistic insights into the observed repression of electrolyte absorption and diarrhea associated with IBD.
Materials and Methods
Materials
Caco-2, T84 and HT-29 cells were obtained from American Type Cell Collection (ATCC, Manassas, VA). The Human TNF was obtained from Sigma Aldrich (St.Louis, MO) and murine TNF was procured from Peprotech (Rocky Hill, NJ). RNeasy kits for RNA extraction were obtained from Qiagen (Valencia, CA) and real-time quantitative RT-PCR (qRT-PCR) kits were from Stratagene (La Jolla, CA). Ready-made SDS-PAGE gels were procured from Bio-Rad (Hercules, CA). DRA antibodies were raised against the C-terminal amino acid sequence INTNGGLRNRVYEPVETKF of DRA (Accession number: BC025671) as previously described 15.
We had validated DRA antibodies utilizing DRA knockout 4, keratin-8 knockout 16 and C. rodentium-infected mice 17, where DRA expression is markedly reduced or is absent, as well as in Caco2 cells where DRA expression was knocked down by shRNA.
2D cell culture and treatments
Cells were grown at 37°C in an atmosphere of 5% CO2 /95% Air in T-75 flask. T84 Cells were maintained in DMEM/F12 with high glucose, 5% fetal bovine serum and HT-29 Cells were maintained in McCoy’s media with 10% fetal bovine serum and 20 mM HEPES, 100 IU/ml penicillin, 100 μg/ml streptomycin and 2 μg/ml gentamicin. Studies were performed in fully differentiated monolayers grown (12–14 days post-plating) on 12-well Transwell inserts between passages 25–45. Monolayers were treated with TNF (50 ng/ml) for 6–24 h in culture medium supplemented with 0.2% BSA.
3D-Cell culture
To culture Caco2 cells in 3-dimensional system, 6-well plates were pre-coated with 80 μl of matrigel (BD biosciences). Caco2 cells were then plated at a density of 25000 cells/well in 6-well plates and allowed to solidify for 10 min at 37°C, then overlayed with 2 ml of Eagle’s medium (EMEM) supplemented with 20% FBS and penicillin–gentamicin (100 IU/ml and 100 μg/ml, respectively) mixed with Hepes. Cells were subsequently maintained for 12 days at 37°C in 5% CO2 for differentiation and formation of cysts. For TNF treatment, 3D Caco2 cysts were treated with 50 ng/ml TNF for 24h and then processed for RNA, protein extraction and immunostaining.
Mouse intestinal organoid generation
Mouse intestinal crypts were isolated and enteroids were cultured as previously described 18. Briefly, small intestine was dissected and opened longitudinally and cut into small (~1 cm) pieces after cleaning with ice-cold PBS (penicillin, 100 I.U./ml/streptomycin, 100 μg/ml). The tissues were rocked in PBS with 2 mM EDTA for 30 min at 4°C and then switched to PBS with 54.9 mM d -sorbitol and 43.4 mM sucrose. The tissues were then vortexed for 1–2 min and filtered through a 70 μm sterile cell strainer. The crypts were collected by centrifugation at 150g for 10 min at 4°C. Approximately 500 crypts were s uspended in 50 μL growth factor reduced phenol-free Matrigel (BD Biosciences, San Jose, CA). Next, a 50 μL droplet of Matrigel/crypt mix was placed in the center of the well of a 12-well plate. After 30 min of polymerization, 650 μl of mini gut medium was overlaid 18 (Mini gut medium: advanced DMEM/F12 supplemented with HEPES, L-glutamine, N2 and B27) was added to the culture, along with R-Spondin, Noggin, and EGF. The medium was changed every 2–3 days. For passage, enteroids were removed from Matrigel and broken up mechanically by passing through a syringe and needle (27G, BD Biosciences), then transferred to fresh matrigel. The passage was performed every 7–10 days with a 1:4 split ratio. Each experiment was repeated thrice. Each condition was examined in triplicate.
RNA Extraction and mRNA expression
RNA was isolated from Caco-2(3D), HT-29, mouse intestinal tissues or enteroids using RNeasy Mini Kit (Qiagen) according to manufacturer’s instructions. An equal amount of RNA for each sample was reverse-transcribed and amplified in one-step reaction using Brilliant SYBR Green QRT-PCR master mix kit (Stratagene, La Jolla, CA) using Mx 3000 (Stratagene). The gene specific primers for human or mouse DRA, NHE2, PAT1 and GAPDH have been described previously17.The gene specific primers for human ZO-1 were as follows: sense primer 5′-CGGTCCTCTGAGCCTGTAAG -3′, antisense primer 5′-GGATCTACATGCGACGACAA -3′ The ΔCt-DRA, ΔCt-NHE2, ΔCt-PAT1, ΔCt-ZO-1 and ΔCt-GAPDH represented the difference between the threshold cycle of amplification of DRA, NHE2, PAT1, Z0-1 and GAPDH.
Cl−/HCO3− exchange activity
DRA-mediated Cl−/HCO3− exchange activity was measured as DIDS-sensitive 125I− uptake in the base-loaded cells as described previously by us7, 15.
Preparation of nuclear extracts and measurement of p65
Nuclear extracts from control and TNF treated HT-29 or Caco-2 cells were prepared using the nuclear extraction kit from Thermo Scientific (Waltham, MA) following the manufacturer’s protocol. NF-κB activation was assessed by measuring levels of nuclear p65 by immunoblotting with anti-p65 antibody (Abcam).
NF-κB activity
NF-κB activation in response to TNF incubation was measured by NF-κB-Luciferase reporter assay as described earlier 19.
Western blotting
After treatment, IECs were washed twice with ice-cold 1X PBS and lysed as described previously 20. Lysates were run on an 7.5% gel and then transferred onto nitrocellulose membrane. Immunoblotting was carried out with DRA antibody as previously described 20. Bands were visualized with enhanced chemiluminescence (ECL) detection reagents.
siRNA silencing
Expression of IκBα was selectively silenced utilizing predesigned siRNAs (Cell signaling). Scrambled siRNA was used as a non-targeting control. Caco-2 or HT-29 cells were transiently transfected with 100 pmol of siRNA duplexes for 48 h using Lipofectamine 2000 (Invitrogen, Grand Island, NY). Silencing was validated by real-time PCR and Western blotting utilizing IκBα specific primers and specific antibodies (Abcam, Cambridge, MA).
Assessment of promoter activity
IECs were transfected with human DRA promoter (p-1183/+114) fragment cloned upstream of the luciferase reporter gene in pGL2-Basic and β-galactosidase expression vector by electroporation using Amaxa Nucleofactor System as described previously 21, 22. Activities of firefly luciferase and β-galactosidase were measured according to the manufacturer’s instructions (Promega, Madison,WI). DRA promoter activity was expressed in terms of relative luciferase activity normalized to β-galactosidase activity.
ChIP assays
ChIP assay was performed utilizing the commercially available ChIP One-Day Kit essentially according to the manufacturer’s instructions (Qiagen, Germantown, MD). Briefly, cells over expressing p65 or vehicle transfected cells were fixed in 1% (vol/vol) formaldehyde and chromatin was sonicated; then immunoprecipitated with 5 μg of specific antibodies against NF-κB p65 subunit (overnight) at room temperature (Abcam). Normal rabbit IgG was used as a control (Abcam). After reverse cross-linking and DNA extraction, immunoprecipitated chromatin was used as the template for real-time quantitative PCR utilizing primers within the spanning regions flanking nucleotides −1183/+114 of hDRA.
In vivo studies
In vivo studies performed in C57BL/6J mice were approved by the Institutional Animal Care Committee of University of Illinois at Chicago and Jesse Brown Veterans Affairs Medical Center. Mice were injected i.p. with either 5 μg TNF in 200 μl of PBS or sterile PBS with 0.2% BSA as vehicle for 3 and 6h. Intestines were resected and mucosa was scraped for RNA and protein extraction. A section (~2 cm) of different regions of the intestine (ileum and colon) was immediately snap frozen in optimal cutting temperature (OCT) embedding medium for immunofluorescence studies.
Statistical analysis
The data presented are mean ± SEM of 3–5 independent experiments. Values are means ± SE. One-way ANOVA (analysis of variance) with Tukey’s test or Student’s t-test was utilized for statistical analysis. P ≤ 0.05 was considered statistically significant.
Results
TNF decreases DRA expression and function in IECs
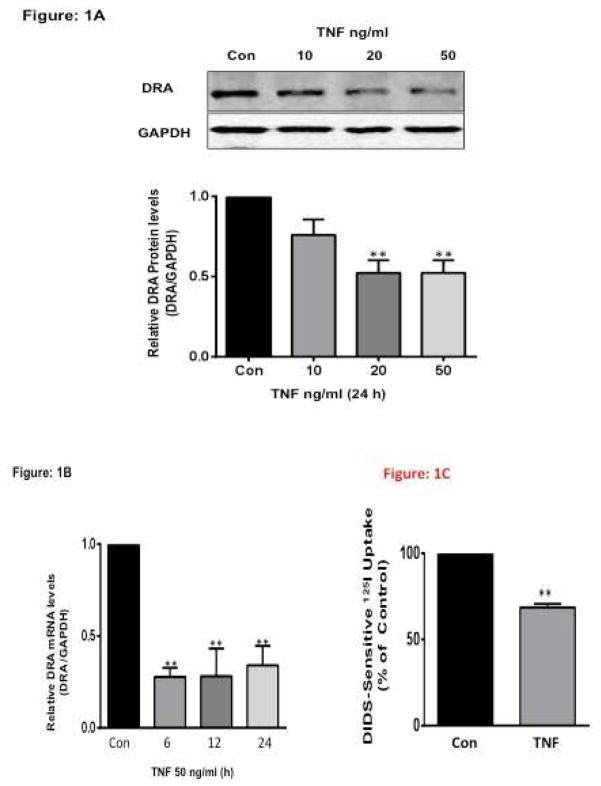
TNF is a well-known pro inflammatory cytokine that is elevated in patients with inflammatory bowel disease (IBD) 23. Initial experiments examined whether TNF modulates DRA expression in HT29 cells. Treatment of HT-29 cells with 10, 20 or 50 ng/ml TNF 24 for 24h significantly decreased DRA protein expression by ~50% as compared to untreated cells (Figure 1A). Consistent with protein, TNF (50 ng/ml) significantly decreased DRA mRNA levels at 6, 12 and 24h, (Figure 1B) indicating that TNF decreases DRA mRNA expression as early as 6h and the decrease remained persistent for 24h. To determine the effects of TNF on Cl−/HCO3− exchange activity, T-84 cells were treated with TNF (50 ng/ml) for 24 h. TNF significantly decreased the DIDS-sensitive 125I− uptake by ~30% compared to control (Figure 1C).
Figure 1. TNF decreases DRA expression in HT-29 Cells.
HT-29 cells grown on Transwell inserts for 10–12 days were treated with different concentrations of TNF (in culture medium supplemented with 0.2% BSA) for 24h or with TNF (10–50 ng/L), (A). Protein lysates were prepared, run on 7.5% SDS-PAGE, transblotted, and Western blotting was performed utilizing anti-DRA antibody. Representative blot of 3 different experiments. Densitometric analysis shows relative expression of DRA normalized to GAPDH. **P < 0.001 vs. control. (B) Total RNA was extracted and quantitative real-time RT-PCR was performed utilizing SYBR green fluorescent dye. DRA mRNA levels were normalized to respective GAPDH mRNA (internal control) levels. Results are expressed as fold-changes in mRNA levels in treated cells compared with control. Values are means ± SEM from ≥3 different experiments performed in triplicate. **p<0.001 vs. control (C) TNF decreased apical Cl−/HCO3− exchange activity in T-84 cells. Cells were treated with TNF (50 ng/mL) for 24 h from basolateral sides. Cl−/HCO3− exchange activity was measured as DIDS-sensitive 125I− uptake for 5 min. Results are expressed as % of control and represent means ± SEM of 6 separate experiments performed in triplicate. **p<0.001 vs. control.
TNF decreases DRA expression in 3Dimensional culture of Caco-2 Cells
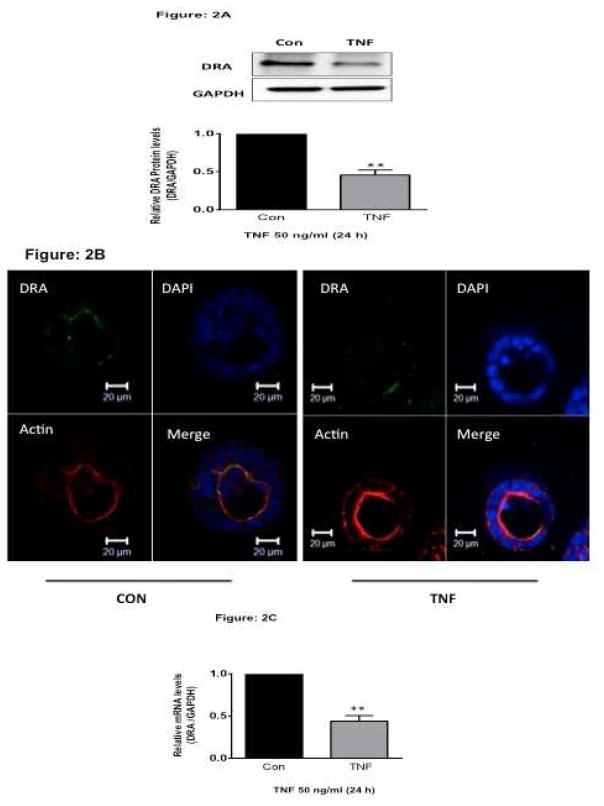
2-D cell culture systems have often been used to study function and regulation of ion transporters. One of the limitations in this model system is that the cells are forced to adapt to an artificial, flat and rigid surface and are known to have very low levels of TNF receptors. To further substantiate our results in a more physiological setting, we utilized the three-dimensional (3D) Caco-2 culture system. At 12–14 days post-plating, Caco-2 cells formed cysts containing a lumen in a matrigel-based 3D culture. To determine the effects of TNF on DRA expression, Caco-2 cells in 3D were treated with 50 ng/ml TNF for 6–24h from the basolateral side. Consistent with the data in HT-29 cells, TNF (50 ng/ml) also significantly decreased DRA protein expression in 3D Caco-2 cells as analyzed by Western blotting (Figure 2A) and confocal microscopy (Figure 2B). In addition, TNF treatment also showed significant decrease in DRA mRNA expression compared to controls (Figure 2C). These results further indicate that endogenous DRA is down regulated by TNF and that the effects are not cell line specific.
Figure 2. TNF decreases DRA expression in 3D-culture of Caco-2 cells.
Caco-2 cells grown in 3D culture system were treated with TNF (50 ng/ml, 24 h). (A). Protein lysates were prepared, run on 7.5% SDS-PAGE, transblotted, and Western blotting was performed utilizing anti-DRA antibody. Representative blot of 3 different experiments is presented. Densitometric analysis shows relative expression of DRA normalized to GAPDH. **P < 0.001 vs. control. (B). Untreated or TNF (50 ng/ml, 24 h) treated Caco-2 cells in 3D culture system were stained for DRA (green), Phalloidin (red) and DAPI (blue) and visualized by confocal microscopy. XY planar images and orthogonal XZ images were obtained with a Zeiss LSM 710 confocal microscope. (C). Total RNA was extracted and quantitative real-time RT-PCR was performed utilizing SYBR green fluorescent dye. Data represent the relative expression of DRA normalized to the respective GAPDH mRNA (internal control) levels. Results are expressed as fold-change in mRNA levels in treated cells compared with control. Data represent mean ± SEM of 4 separate experiments. ** p<0.001 compared with control.
TNF decreases DRA expression in crypt-derived Ileal enteroids
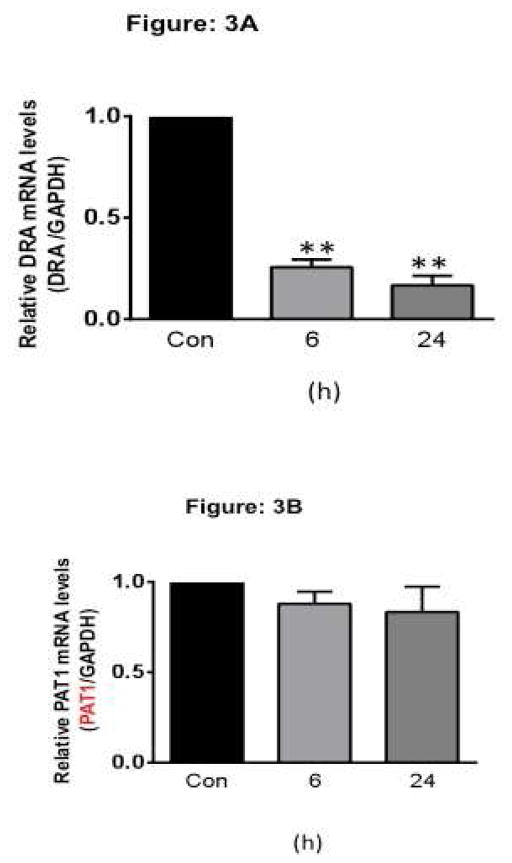
Enteroids cultured from crypts represent an excellent ex vivo model as they recapitulate the multiple cell types of normal intestinal epithelium, in vivo tissue architecture with self-renewing capacity 25. To further validate our results in a complex physiological setting, we utilized the ex-vivo mouse crypt derived ileal enteroid culture systems. To determine the effects of TNF on DRA expression, ileal enteroids were treated with 50 ng/ml TNF for 6h or 24h from the basolateral side. Consistent with the results observed in HT-29 and 3D Caco-2 cells, TNF treatment showed significant alterations in mouse DRA expression as compared to controls in both 6h and 24h time point (Figure 3A). However, TNF did not alter the expression levels of another apical anion exchanger, mouse PAT-1 (SLC26A6) (Figure 3B), indicating the specificity of the effects.
Figure 3. TNF decreases mouse DRA but not mouse PAT-1 mRNA expression in crypt derived mouse ileal enteroids.
Crypt derived mouse Ileal enteroids grown on matrigel were treated for 6 or 24 h with TNF (50 ng/ml). Total RNA was extracted and quantitative real-time RT-PCR was performed utilizing SYBR green fluorescent dye. Data represent the relative expression of DRA (A) or PAT1 (B) normalized to the respective GAPDH mRNA (internal control) levels. Results are expressed as fold-change in mRNA levels in treated cells compared with control. Values are means ± SEM from ≥3 different experiments performed in triplicate. **p < 0.001 vs. control
TNF decreases DRA Promoter Activity
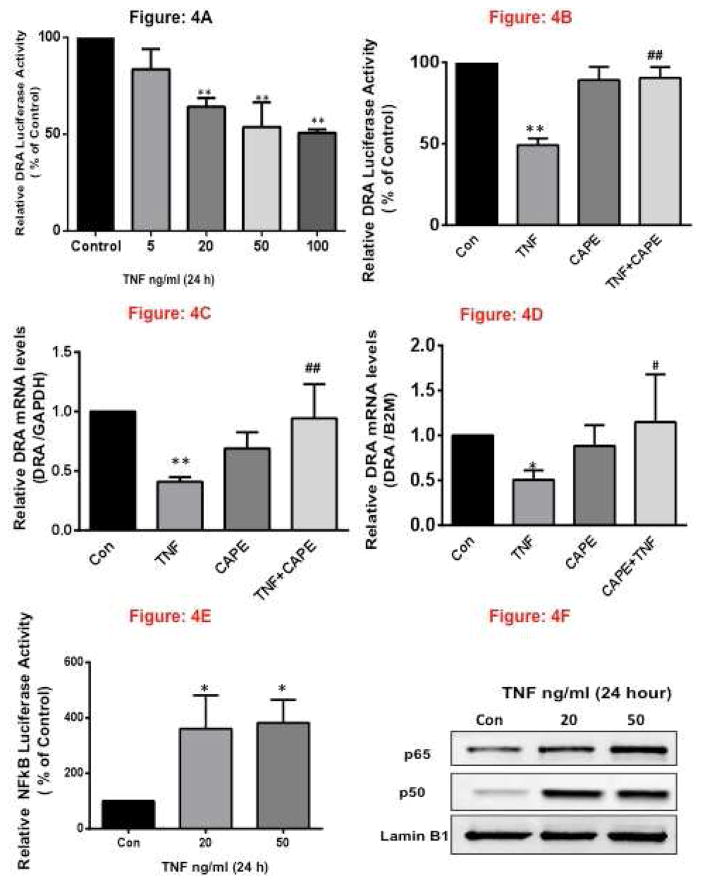
To elucidate the molecular mechanisms underlying decreased DRA expression by TNF, we next examined whether DRA promoter activity was altered. The promoter activity was assessed by transient cotransfection of DRA promoter construct in HT-29 cells along with the β-galactosidase mammalian expression vector as an internal control to adjust for transfection efficiency. Results demonstrated that TNF significantly inhibited DRA promoter activity at 20–100 ng/ml. Time-course studies demonstrated a decrease in DRA promoter activity at 6, 12, and 24h time points following TNF treatments (Figure 4A) & (Supplementary Figure 1A, 1B). These results suggest that inhibition of DRA by TNF occurs specifically via suppression of DRA promoter as early as 6h.
Figure 4. TNF Decreases DRA promoter activity via NF-κ B pathway.
HT-29 cells were transiently transfected with human DRA promoter construct (p-1183/+114) along with the mammalian expression vector for β-galactosidase (pCMV-βgal). 24h post-transfection, HT-29 cells were treated with different doses of TNF ranging from 5–100 ng/ml for 24h (A) and with and without CAPE (B). Promoter activity was measured by luciferase assays. Values were normalized to β-galactosidase activity to correct for transfection efficiency. (C) HT-29 cells grown on Transwell inserts for 10–12 days were treated for 24h with TNF (50 ng/ml) and with or without, NF-κB inhibitor, CAPE (25 μM) (D) Crypt derived mouse Ileal enteroids grown on matrigel were treated for 24 h with TNF (50 ng/ml) with and without CAPE. Total RNA was extracted and quantitative real-time RT-PCR was performed utilizing SYBR green fluorescent dye. Data represent the relative expression of DRA normalized to the respective GAPDH or B2M mRNA (internal control) levels. Results are expressed as fold change in mRNA levels in treated cells compared with control. (E) HT-29 cells were transiently transfected with NFκB promoter luciferase reporter construct along with the mammalian expression vector for β-galactosidase (pCMV-β-gal). 24h post-transfection, HT-29 cells were treated with 20 or 50 ng/ml of TNF for 24h. Promoter activity was measured by luciferase assays. Values were normalized to β-galactosidase activity to correct for transfection efficiency, (F) HT-29 cells grown on Transwell inserts for 10–12 days were treated for 24h with 20 or 50 ng/ml TNF. Nuclear lysates were prepared, run on 10% SDS-PAGE, transblotted, and Western blotting was performed utilizing anti-p65 or p50 subunit antibody. Representative blot of 3 different experiments is shown. Results represent mean ± SEM of 3–4 separate experiments performed in triplicate and expressed as % of control comparing transfected cells treated with TNF to untreated cells (control). **p<0.001 compared with control, *p<0.05, compared with control. ##p<0.001 compared with TNF, #p<0.05, compared with TNF.
TNF effects on DRA expression are NF-κB dependent
Previous studies have shown that TNF activates NF-κB dependent signaling pathways 26, 27. We, therefore, examined the role of NF-κB pathway in TNF-induced downregulation of DRA. The specific NF-κB inhibitor, CAPE (caffeic acid phenethyl ester, 25 μM) blocked the inhibitory effects of TNF on DRA promoter activity (Figure 4B) and DRA mRNA levels in HT-29 cells (Figure 4C) and in mice ileal enteroids (Figure 4D) suggesting the involvement of NFkB on DRA expression. Further we examined the NF-κB reporter activity in response to TNF treatment in HT-29 cells by luciferase assays. Interestingly, TNF significantly upregulated NF-κB luciferase activity normalized to B-galactosidase (Figure 4E). In addition, TNF (50 ng/ml, 24h) increased nuclear localization of NF-κB subunit p65 and p50 in HT-29 cells (Figure 4F) indicative of its activation.
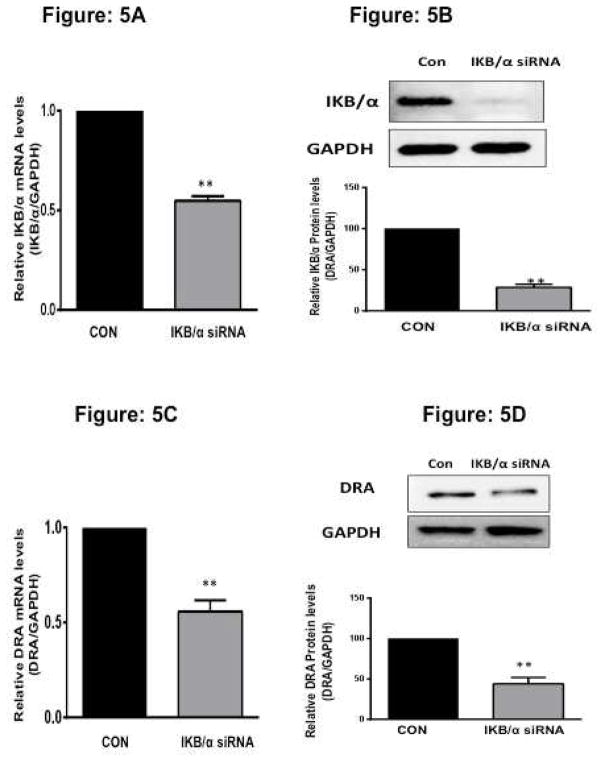
siRNA knockdown of IκBα decreases DRA expression
In inactivated state, NF-κB is located in the cytosol with the inhibitory protein IκBα. TNF activates the enzyme IκB kinase (IKK) and in turn phosphorylates the IκBα protein. This results in ubiquitination and dissociation of IκBα from NF-κB, activating NF-κB to translocate to the nucleus. 13 To directly test the role of NF-κB in modulating DRA expression, we utilized predesigned siRNAs to silence IkBα expression in Caco-2 cells. After 48h of treatment with 100 nM siRNA duplexes, selective downregulation of IκBα mRNA and protein expression (Figure 5A and 5B) (normalized to GAPDH as the internal control) was achieved compared with scrambled siRNA controls. IκBα knock down also resulted in translocation of p65 subunit to nucleus (Supplementary Figure 2) The levels of p65 and p50 mRNA remained unaltered with IκBα silencing (data not shown) indicating specificity of inhibition. Interestingly, DRA mRNA levels were decreased (60%) with IκBα silencing compared to scrambled siRNA control. Consistent with mRNA levels (Figure 5C), silencing of IκBα also decreased DRA protein expression in HT-29 cells (Figure 5D).
Figure 5. siRNA knockdown of IkBα decreases DRA expression.
Caco-2 cells were transfected with scrambled or IκB/α specific small interfering RNA (siRNA) for 48h. siRNA-treated knockdown of IκB/α was confirmed by mRNA (A) and immunoblotting (B) utilizing primers specific for IκB/α and anti-IκB/α antibody. (C) Total RNA was extracted and quantitative real-time RT-PCR for DRA was performed utilizing SYBR green fluorescent dye. Data represent the relative expression of DRA normalized to the respective GAPDH mRNA (internal control) levels. Results are expressed as fold-change in mRNA levels in treated cells compared with control. Values represent means ± SEM 3 separate experiments. **p < 0.001 compared with control. (D) Protein lysates were prepared, run on 7.5 % SDS-PAGE, transblotted, and Western blotting was performed utilizing anti-DRA antibody. Representative blot of 3 different experiments is shown. Densitometric analysis shows relative expression of DRA normalized to GAPDH. **p < 0.001 vs. control.
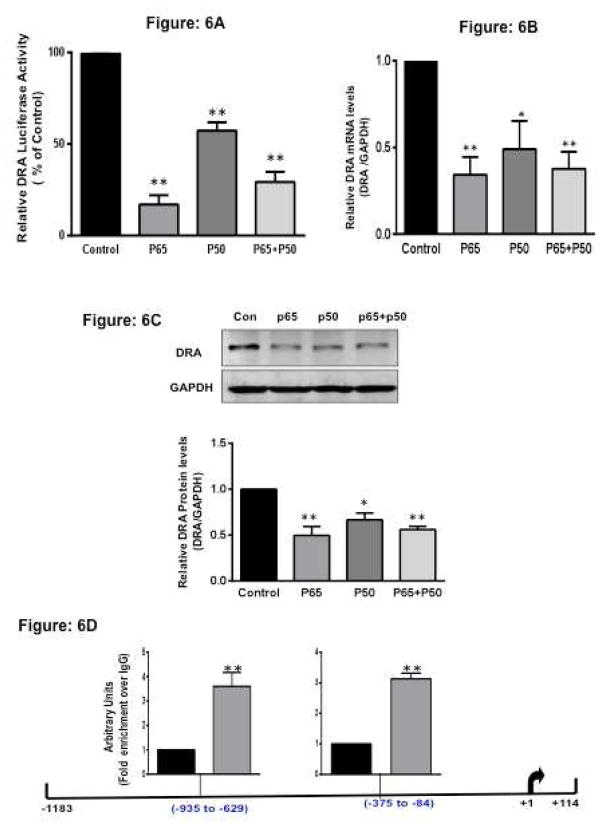
Overexpression of p50 and p65 attenuated DRA promoter activity in IECs
To examine whether DRA promoter activity is affected by NF-κB, mammalian expression vectors for p65 and/or p50 subunits were overexpressed in Caco-2 cells along with DRA promoter. Ectopic expression of either p65 or p50 and the combination (Supplementary Figure 3) significantly decreased DRA promoter activity in Caco-2 cells (Figure 6A, Supplementary figures 4A and 4B) Consistent with the promoter activity, DRA mRNA expression was significantly decreased in response to the over expression of p65 and p50 subunits by ~60% and ~40% (Figure 6B), respectively. Also, DRA protein expression was decreased by p65 (~ 60%) and by p50 (~ 40%) (Figure 6C). Similar effects were observed in T-84 cells in response to overexpression of the NF-κB subunits (Supplementary Figure 4C)
Figure 6. Ectopic expression of NFκB subunit (p65 and p50) inhibits DRA expression.
(A). Caco-2 cells were transiently transfected with DRA promoter construct with and without p65 or p50 subunit expression vector or together along with the mammalian expression vector for β-galactosidase (pCMV-β-gal). 24h post-transfection, promoter activity was measured by luciferase assay. Values were normalized to β-galactosidase activity. (B) Caco-2 cells were transiently transfected with and without p65 or p50 subunit expression vector or both together. Total RNA was extracted and quantitative real-time RT-PCR was performed utilizing SYBR green fluorescent dye. Data represent the relative expression of DRA normalized to the respective GAPDH mRNA (internal control) levels. (C) Caco-2 cells were transiently transfected with and without p65 or p50 subunit expression vector or both. Protein lysates were prepared, run on 7.5% SDS-PAGE, transblotted, and Western blotting was performed utilizing anti-DRA antibody. Representative blot of 3 different experiments is shown. Densitometric analysis shows relative expression of DRA normalized to GAPDH. (D) Identification of the NF-κB responsive region in DRA promoter. Caco-2 cells transiently transfected with and without p65 subunit expression vector were fixed in formaldehyde, chromatin was sonicated, and immunoprecipitated with specific antibody against p65. After reverse cross-linking and DNA extraction, immunoprecipitated DNA was used as the template for real-time quantitative PCR utilizing primers spanning nucleotides −1183/+114 of DRA. Enrichment of the p65 was observed at −375/−84 bp and −935/−629 bp regions of DRA promoter. Values are means ± SEM from 3 or more different experiments. *p < 0.05, **p < 0.001 vs. control.
Identification of the NF-κB responsive region in the DRA promoter
Since ectopic expression of p65 inhibited DRA expression, we next examined the direct binding of p65 with DRA promoter region by ChIP assays. Chromatin from control cells and cells overexpressing p65 was immunoprecipitated with p65 antibody. Enrichment of DRA promoter with the immunoprecipitates of p65 was assessed by real-time PCR utilizing specific primers flanking different regions of DRA promoter (−1183/+114). In cells overexpressing p65, there was 3.5 fold increase in association of p65 with −935 to −629 bp and 3 fold increase with −375 to −84 bp regions in DRA promoter as compared to control cells (Figure 6D). However region between 1183 to −1028 on DRA gene showed no enrichment with p65 immunoprecipitate, indicating that the results are specific. These data for the first time suggested that NF-κB directly modulates transcription of the DRA gene in the human intestinal epithelial cells.
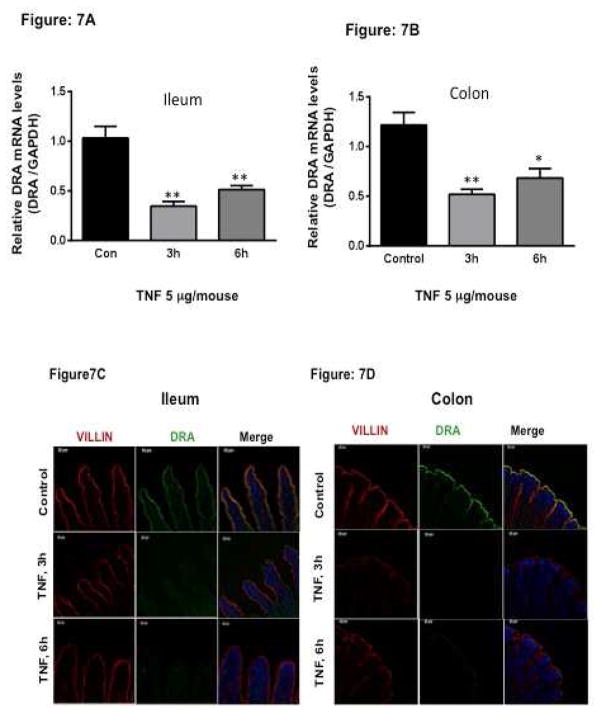
In vivo effects of TNF on DRA expression in C57BL/6 mice
Our in vitro studies in 3D Caco-2, HT-29 cells and mouse enteroids clearly demonstrated that treatment with TNF significantly decreased DRA expression. Therefore, to further investigate the effect of TNF in a complex physiological setting, we next examined the effects of TNF on DRA expression in an in vivo mouse model. TNF (5 μg/mouse in 200 μl PBS) or vehicle alone was injected to C57BL/6 mice 28. As shown in Figure 7A and Figure 7B, DRA mRNA levels significantly decreased in response to TNF in ileum and colon, however, TNF did not change mRNA levels of PAT1 (data not shown). Further, TNF effects on DRA protein expression were examined by immunofluorescence staining of ileal and colonic mucosal sections. As shown in Figure 7C, and Figure 7D, TNF treatment resulted in marked loss of DRA staining at 3h and 6h time point as compared to control.
Figure 7. In vivo effects of TNF on DRA expression in C57BL/6 mice.
TNF (5 μg/mouse in 200 μl PBS) or vehicle alone was injected i.p. to C57BL/6 mice. After 3 or 6 h, mice were sacrificed, intestines were removed and mucosa scraped from ileal and colonic regions for RNA isolation. Mouse DRA mRNA was measured by real-time PCR using gene specific primers. Relative DRA mRNA expression in ileal (A) and colonic mucosa (B) of mice treated with vehicle or TNF. Values are means ± SEM from 5 mice. **p < 0.001, *p < 0.05 vs. control. TNF decreases DRA immunostaining in Ileum (C) and colon (D). Green: DRA; Red: Villin; Blue: Nuclei.
Discussion
DRA functions as the key mediator of Cl−/HCO3− exchange in electroneutral NaCl absorption process. Previous studies have demonstrated that DRA expression was decreased in experimental models of inflammation as well as in patients with ulcerative colitis 9,11. Also, DRA KO mice have been shown to be more susceptible to DSS-induced colitis 29. Whether this decrease in DRA expression under inflammatory conditions is a cause or effect of inflammation is not very clear. In this regard, our previous studies and those of others have shown that proinflammatory cytokines such as IL-1β and IFN-γ directly reduce DRA expression. For example, IFN-γ downregulated DRA expression via transcriptional mechanisms involving JAK/STAT1 pathway 22. Another study showed that TNF overexpressing mouse (with elevated cytokine levels including TNF, IL-1β and IFN-γ) caused a decrease in DRA expression in the ileum and colon 12, although the underlying mechanisms were not investigated. Our current studies utilizing a number of state-of-the-art in vitro, ex vivo and in vivo approaches showed that TNF decreases DRA expression via activation of NF-κB pathway and by direct binding of p65 to −935 to −629 bp and −375 to −84 bp regions of DRA promoter to suppress DRA gene expression. These results provide novel evidence supporting an important role of NF-κB as a major pathway underlying the direct inhibition of DRA expression in intestinal inflammation.
We have recently shown that Caco-2 cells grown as 3D-cysts represent a more suitable model to examine responsiveness of ion transporters to pathophysiological stimuli because of its higher expression of critical signaling components including TNFR2 30. Our current data demonstrated that TNF treatment of Caco-2 3D-cysts significantly downregulated DRA expression. In addition, HT-29 cells representing a colonic crypt cell line also showed a similar decrease in DRA expression in response to TNF indicating that the effects were not cell line specific. Since both Caco-2 and HT-29 cells represent cancer cell lines, we further utilized enteroids, the recently developed ex-vivo 3-D culture system that are expected to recapitulate the multiple cell types of normal intestinal epithelium and overcome recognized limitations of cancer cell lines.25 TNF also significantly decreased DRA mRNA expression in crypt derived mouse ileal enteroids. In contrast, under the same conditions, mRNA expression of other ion transporters, such as PAT-1, was not changed, indicating that the effects were specific to DRA. Since the effects of TNF in IECs vary depending on the experimental models and the doses used in the study, we further examined if the effects of TNF could be validated in an in vivo mouse model. Similar to our in vitro results, mice injected with TNF also showed a significant decrease in DRA expression in both ileum and colon.
We further showed that TNF effects on DRA expression occurred at the transcriptional level as TNF decreased DRA promoter activity. These inhibitory effects of TNF appear to be predominantly mediated via transcriptional mechanisms; however, the possibility of contribution of changes in DRA mRNA stability also cannot be ruled out. TNF exerts its effects mainly via activation of NF-κB, JNK, and p38-MAPK signaling pathways 31. The decrease in DRA mRNA expression by TNF was blocked in the presence of specific NF-κB inhibitor CAPE. NF-κB /Rel protein family consists of five members that include p50 and p65. These proteins form homo- and hetero-dimers, with p50/p65 heterodimers being the predominant forms in the NF complex. NF-κB activation plays an important role in inducing the expression of genes involved in inflammation of the gastrointestinal tract. 32, 33, 34, 35 For example, NF-κB is known to affect the levels of tight junction proteins by mediating their internalization in intestinal epithelial cells 36. Furthermore, ZO-1 expression was affected by activation of NF-κB by pro-inflammatory cytokines such as TNF and IL-1β. Intestinal galactose transporter expression was shown to be reduced in response to IL-1β where the effects are driven by activated NF-κB. In addition, CFTR expression was found to be suppressed by the NF-κB inflammatory signaling in lung epithelial (H441) and engineered (H57) cell lines 37. Our current data, for the first time, showed that the ectopic expression of NF-κB subunits decreased DRA promoter activity. Activation of NF-κB pathway achieved by siRNA knockdown of IKB-α (which increased nuclear translocation of p65) resulted in decreased DRA mRNA and protein expression. ChIP assays showed the direct binding of p65 to the DRA promoter at −935 to −629 bp and −375 to −84 bp regions.
In summary, our findings for the first time demonstrate downregulation of DRA expression by TNF via transcriptional mechanisms involving NF-κB activation. Since DRA is one of the key transporters involved in coupled electroneutral NaCl absorption in the intestine, TNF-mediated activation of NF-κB may represent a major pathway underlying the inhibition of DRA expression in intestinal inflammation. Thus, reduction of epithelial NF-κB activation in IBD could attenuate the decrease in DRA expression and function resulting in reduced diarrheal episodes via attenuating loss of Cl− ions.
Supplementary Material
Acknowledgments
Supported by Department of Veterans Affairs Merit Award # BX002011 (P. K. Dudeja), Merit Award # BX000152 (W. A. Alrefai), Merit Award BX002867(S. Saksena), and VA Senior Research Career Scientist Award (P. K. Dudeja), and Research Career Scientist Award (W.A. Alrefai), and the NIDDK grants DK54016, DK81858 and DK92441 (P.K. Dudeja), DK 98170 (R. K. Gill) and DK 71596, DK 109709 (W. A. Alrefai).
Footnotes
Conflict of Interests: The authors have no conflicts of interest to declare
Author contributions: AK, IC, TG, AA, HC, ANA: performed experiments; AK, AB, RKG, WAA, PKD: analyzed and interpreted data; AK: drafted manuscript, prepared figures; AB, SS, RKG, WAA, PKD: edited and revised the manuscript; AB, WAA, PKD: approved the final version of the manuscript; AB, RKG, PKD: responsible for conception and design of the study
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Priyamvada S, Gomes R, Gill RK, et al. Mechanisms Underlying Dysregulation of Electrolyte Absorption in Inflammatory Bowel Disease–Associated Diarrhea. Inflammatory bowel diseases. 2015;21:2926–2935. doi: 10.1097/MIB.0000000000000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dudeja PK, Baldwin ML, Harig JM, et al. Mechanisms of Na+ transport in human distal colonic apical membrane vesicles. Biochim Biophys Acta. 1994;1193:67–76. doi: 10.1016/0005-2736(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 3.Kato ARM. Regulation of Electrneutral NaCl by the Small Intestine. Annu Rev Physiol. 2010 doi: 10.1146/annurev-physiol-012110-142244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker NM, Simpson JE, Brazill JM, et al. Role of down-regulated in adenoma anion exchanger in HCO3− secretion across murine duodenum. Gastroenterology. 2009;136:893–901. doi: 10.1053/j.gastro.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makela S, Kere J, Holmberg C, et al. SLC26A3 mutations in congenital chloride diarrhea. Hum Mutat. 2002;20:425–38. doi: 10.1002/humu.10139. [DOI] [PubMed] [Google Scholar]
- 6.Schweinfest CW, Spyropoulos DD, Henderson KW, et al. slc26a3 (dra)-deficient mice display chloride-losing diarrhea, enhanced colonic proliferation, and distinct up-regulation of ion transporters in the colon. J Biol Chem. 2006;281:37962–71. doi: 10.1074/jbc.M607527200. [DOI] [PubMed] [Google Scholar]
- 7.Gill RK, Borthakur A, Hodges K, et al. Mechanism underlying inhibition of intestinal apical Cl/OH exchange following infection with enteropathogenic E. coli. J Clin Invest. 2007;117:428–37. doi: 10.1172/JCI29625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borenshtein D, Schlieper KA, Rickman BH, et al. Decreased expression of colonic Slc26a3 and carbonic anhydrase iv as a cause of fatal infectious diarrhea in mice. Infect Immun. 2009;77:3639–50. doi: 10.1128/IAI.00225-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuhn R, Lohler J, Rennick D, et al. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–74. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 10.Singh V, Kumar A, Raheja G, et al. Lactobacillus acidophilus attenuates downregulation of DRA function and expression in inflammatory models. Am J Physiol Gastrointest Liver Physiol. 2014;307:G623–31. doi: 10.1152/ajpgi.00104.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang H, Jiang W, Furth EE, et al. Intestinal inflammation reduces expression of DRA, a transporter responsible for congenital chloride diarrhea. Am J Physiol. 1998;275:G1445–53. doi: 10.1152/ajpgi.1998.275.6.G1445. [DOI] [PubMed] [Google Scholar]
- 12.Xiao F, Juric M, Li J, et al. Loss of downregulated in adenoma (DRA) impairs mucosal HCO3(−) secretion in murine ileocolonic inflammation. Inflamm Bowel Dis. 2012;18:101–11. doi: 10.1002/ibd.21744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54. e42. doi: 10.1053/j.gastro.2011.10.001. quiz e30. [DOI] [PubMed] [Google Scholar]
- 15.Kumar A, Hecht C, Priyamvada S, et al. Probiotic Bifidobacterium species stimulate human SLC26A3 gene function and expression in in vitro and in vivo models. Gastroenterology (Abstract) 2012;142:S–542. [Google Scholar]
- 16.Asghar MN, Priyamvada S, Nystrom JH, et al. Keratin 8 knockdown leads to loss of the chloride transporter DRA in the colon. Am J Physiol Gastrointest Liver Physiol. 2016;310:G1147–54. doi: 10.1152/ajpgi.00354.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar A, Anbazhagan AN, Coffing H, et al. Lactobacillus acidophilus counteracts inhibition of NHE3 and DRA expression and alleviates diarrheal phenotype in mice infected with Citrobacter rodentium. Am J Physiol Gastrointest Liver Physiol. 2016;311:G817–G826. doi: 10.1152/ajpgi.00173.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang YG, Wu S, Xia Y, et al. Salmonella-infected crypt-derived intestinal organoid culture system for host-bacterial interactions. Physiol Rep. 2014:2. doi: 10.14814/phy2.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watson WH, Burke TJ, Doll MA, et al. S-Adenosylhomocysteine Inhibits NF-κB-Mediated Gene Expression in Hepatocytes and Confers Sensitivity to TNF Cytotoxicity. Alcoholism: Clinical and Experimental Research. 2014;38:889–896. doi: 10.1111/acer.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar A, Anbazhagan AN, Coffing H, et al. Lactobacillus acidophilus counteracts inhibition of NHE3 and DRA expression and alleviates diarrheal phenotype in mice infected with Citrobacter rodentium. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2016;311:G817–G826. doi: 10.1152/ajpgi.00173.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alrefai WA, Wen X, Jiang W, et al. Molecular cloning and promoter analysis of downregulated in adenoma (DRA) Am J Physiol Gastrointest Liver Physiol. 2007;293:G923–34. doi: 10.1152/ajpgi.00029.2007. [DOI] [PubMed] [Google Scholar]
- 22.Saksena S, Singla A, Goyal S, et al. Mechanisms of transcriptional modulation of the human anion exchanger SLC26A3 gene expression by IFN-{gamma} Am J Physiol Gastrointest Liver Physiol. 2010;298:G159–66. doi: 10.1152/ajpgi.00374.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1756–1767. e1. doi: 10.1053/j.gastro.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poritz LS, Garver KI, Tilberg AF, et al. Tumor necrosis factor alpha disrupts tight junction assembly. J Surg Res. 2004;116:14–8. doi: 10.1016/s0022-4804(03)00311-1. [DOI] [PubMed] [Google Scholar]
- 25.Lukovac S, Roeselers G. The Impact of Food Bioactives on Health. Springer; 2015. Intestinal crypt organoids as experimental models; pp. 245–253. [PubMed] [Google Scholar]
- 26.Resta-Lenert S, Barrett KE. Probiotics and commensals reverse TNF-alpha- and IFN-gamma-induced dysfunction in human intestinal epithelial cells. Gastroenterology. 2006;130:731–46. doi: 10.1053/j.gastro.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 27.Tao Y, Drabik KA, Waypa TS, et al. Soluble factors from Lactobacillus GG activate MAPKs and induce cytoprotective heat shock proteins in intestinal epithelial cells. Am J Physiol Cell Physiol. 2006;290:C1018–30. doi: 10.1152/ajpcell.00131.2005. [DOI] [PubMed] [Google Scholar]
- 28.Clayburgh DR, Musch MW, Leitges M, et al. Coordinated epithelial NHE3 inhibition and barrier dysfunction are required for TNF-mediated diarrhea in vivo. J Clin Invest. 2006;116:2682–94. doi: 10.1172/JCI29218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao F, Yu Q, Li J, et al. Slc26a3 deficiency is associated with loss of colonic HCO3 (−) secretion, absence of a firm mucus layer and barrier impairment in mice. Acta Physiol (Oxf) 2014;211:161–75. doi: 10.1111/apha.12220. [DOI] [PubMed] [Google Scholar]
- 30.Chatterjee I, Kumar A, Gill RK, et al. Mo1765 3D Cell Culture of CaCo2: A Better Model to Study the Functionality and Regulation of Intestinal Ion Transporters. Gastroenterology. 2014;5:S–654. [Google Scholar]
- 31.Amin MR, Orenuga T, Tyagi S, et al. Tumor necrosis factor-alpha represses the expression of NHE2 through NF-kappaB activation in intestinal epithelial cell model, C2BBe1. Inflamm Bowel Dis. 2011;17:720–31. doi: 10.1002/ibd.21419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ellis R, Goodlad J, Limb G, et al. Activation of nuclear factor kappa B in Crohn’s disease. Inflammation research. 1998;47:440–445. doi: 10.1007/s000110050358. [DOI] [PubMed] [Google Scholar]
- 33.Schmid R, Adler G, Liptay S. Activation of NFκB in inflammatory bowel disease. Gut. 1998;43:586–586. doi: 10.1136/gut.43.4.586c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghosh S, Hayden MS. New regulators of NF-κB in inflammation. Nature Reviews Immunology. 2008;8:837–848. doi: 10.1038/nri2423. [DOI] [PubMed] [Google Scholar]
- 35.Baeuerle PA, Baichwal VR. NF-kB as a frequent target for immunosuppressive and anti-inflammatory molecules. Advances in immunology. 1997;65:111–138. [PubMed] [Google Scholar]
- 36.Al-Sadi R, Boivin M, Ma T. Mechanism of cytokine modulation of epithelial tight junction barrier. Frontiers in bioscience: a journal and virtual library. 2009;14:2765. doi: 10.2741/3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hunter MJ, Treharne KJ, Winter AK, et al. Expression of wild-type CFTR suppresses NF-κB-driven inflammatory signalling. PLoS One. 2010;5:e11598. doi: 10.1371/journal.pone.0011598. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.