Summary
Objective
Low-cost evidence-based tools are needed to facilitate the early identification of patients with possible psychogenic nonepileptic seizures (PNES). Prior to accurate diagnosis, patients with PNES do not receive interventions that address the cause of their seizures and therefore incur high medical costs and disability due to an uncontrolled seizure disorder. Both seizures and comorbidities may contribute to this high cost.
Methods
Based on data from 1,365 adult patients with video-electroencephalography confirmed diagnoses from a single center, we used logistic and Poisson regression to compare the total number of comorbidities, number of medications and presence of specific comorbidities in five mutually exclusive groups of diagnoses: epileptic seizures (ES) only, PNES only, mixed PNES and ES, physiologic nonepileptic seizure-like events, and inconclusive monitoring. To determine the diagnostic utility of comorbid diagnoses and medication history to differentiate PNES only from ES only, we used multivariate logistic regression, controlling for sex and age, trained using a retrospective database and validated using a prospective database.
Results
Our model differentiated PNES only from ES only with a prospective accuracy of 78% (95% CI 72–84%) and AUC of 79%. With a few exceptions, the number of comorbidities and medications was more predictive than a specific comorbidity. Comorbidities associated with PNES were asthma, chronic pain and migraines (p<0.01). Comorbidities associated with ES were diabetes mellitus and non-metastatic neoplasm (p<0.01). The population-level analysis suggested that patients with mixed PNES plus ES may be a distinct population from patients with either condition alone.
Significance
An accurate patient-reported past medical history and medication history can be useful when screening for possible PNES. Our prospectively validated and objective score may assist in the interpretation of the medication and medical history in the context of the seizure description and history.
Keywords: screening, psychogenic nonepileptic attack disorder, machine learning, asthma, migraines
1. Introduction
Psychogenic nonepileptic seizures (PNES) appear behaviorally similar to epileptic seizures (ES) but are not caused by the abnormally synchronous neural activity of epilepsy 1. A recent study of time from first seizure to diagnosis of PNES at our center revealed an average of 8.4 years2, but this delay is exponentially distributed, varies substantially across centers and has been reported to be as short at 18 months in some pediatric populations3; 4. During this diagnostic delay, the direct and indirect cost of psychogenic seizures is similar to medication resistant epileptic seizures: 21,000 euros/22,500 US dollars annually 5; 6. This high cost is due to both the impact of seizures and multiple comorbid conditions that limit quality of life 7–9.
Early differentiation of PNES from ES was associated with improved long-term seizure outcomes and reduced cost 10–14. Early diagnosis is hindered by witnesses’ and patients’ descriptions of seizure behavior that often are inaccurate and rarely are sufficient for definitive diagnosis 15; 16. Moreover, a normal interictal electroencephalograph (EEG) does not exclude the diagnosis of epilepsy 17; 18. Therefore, there is a need to establish other objective, evidence-based methods to diagnose PNES using information obtained early in the patient’s disease course 19. The standard of care to document PNES is simultaneous video-EEG observation of a typical event 20.
Most frequently, PNES are a sign of conversion disorder where patients express and cope with psychological stressors through physical symptoms 1; 14; 21. Patients with PNES report more positive symptoms on review of systems questionnaires 22 and frequently are diagnosed with other medical comorbidities associated with psychiatric disorders including asthma 23, chronic pain 9, and fibromyalgia 9. These comorbidities and other medically unexplained symptoms have an impact upon patients’ quality of life 24–26.
In this manuscript, we evaluate if the patient-reported comorbidities can differentiate between patients with PNES and ES. We also evaluate if the number and type of medications prescribed can further differentiate the populations by providing information regarding comorbidity severity. This work also will help establish the frequency of comorbidities in a large unselected population of patients with medication resistant seizures.
2. Methods
Our patient population comprises all patients admitted to the UCLA adult video-electroencephalography (vEEG) monitoring unit from January 2006 to November 2016. Diagnosis was expert clinical opinion based on the available clinical history, physical exam, vEEG, structural MRI, FDG-PET, MEG and SPECT. We placed the patients in five mutually exclusive categories: psychogenic nonepileptic seizures (PNES), epileptic seizures (ES), mixed nonepileptic and epileptic seizures, physiologic nonepileptic seizure-like episodes (PSLE), and inconclusive monitoring. These categories are heterogeneous populations with many important subtypes, but the description of subtypes within PNES and ES is outside our scope with one exception: temporal lobe epilepsy (TLE). In previous literature mood disorders were more common in patients with TLE, therefore we compared TLE to other ES as well as the other five diagnostic categories. We will specify mixed seizures when referring to patients with both PNES and ES. We kept mixed seizures and PNES separate because patients with mixed seizures would benefit from anti-seizure medication (ASM) treatment where PNES alone would not, and there is insufficient evidence to suggest that the mechanism and risk factors for PNES are the same in mixed and PNES alone 7.
Inconclusive monitoring occurred when patients did not experience sufficient characteristic events during vEEG monitoring to yield a definitive diagnosis for all reported types of the patient’s seizures. While patients with inconclusive monitoring represent a mixture of the other groups, we do not know the relative composition of the other groups within the inconclusive group. Inclusion of these patients allowed us to state that we included all patients in our analysis, thereby reducing the potential for selection bias, and improves our ability to control for confounding variables while otherwise not affecting the results or conclusions.
2.1 Comorbidity and Medication Databases
Our population is split into two parts based on how their data was collected; retrospectively (January 2006–April 2015) and prospectively (May 2015–November 2016). Prior to May 2015, records from patients were acquired though retrospective chart review. In the interest of developing an early screening tool, if multiple notes were available, we used a single neurologist’s note from the earliest outpatient or inpatient clinical encounter that described of their seizures, comorbidities and medication history. Formal psychiatric assessment was not routine during either outpatient or inpatient encounters, therefore our data reflect patient-reported or neurologist-documented comorbidities. Patients admitted after April 2015 underwent standardized interview within 48 hours of vEEG admission by a trained non-neurologist (E.A.J., S.R.D., W.T.K., or M.A.). To simulate the data that would be available during a neurology or primary care outpatient visit, no information from the health record was used to supplement the patient-provided history except if the patient stated that they were unable to provide a full list of current or past medications. If retrospective patients were re-admitted during the prospective period (i.e. due to inconclusive initial monitoring), they were excluded from the prospective analysis, information from the standardized interview was not used, and their diagnosis was updated in the retrospective dataset. This reduces the frequency of inconclusive monitoring in the retrospective group and ensures that the comorbidity and medication information is blinded to vEEG results. Age was recorded as the age at the time of the clinical note or standardized interview.
For retrospective patients, the list of co-morbidities was based on past medical history, identifying history of present illness section of the clinical report and conditions specifically indicated by medications but were not otherwise discussed (for more details, see Supplemental Text). We recorded the total number of medical comorbidities, psychiatric comorbidities, as well as the presence of a targeted list of specific comorbidities (Table 1). These comorbidities were chosen based on hypothesized or established association with either epilepsy or PNES (see supplemental text). Formal psychiatric assessment was not routine, therefore psychiatric comorbidities reflect patient-reported and neurologist-documented diagnoses that may differ from formal psychiatric diagnoses.
Table 1.
| Comorbidity |
|---|
| Migraines |
| Asthma |
| Chronic Pain |
| Psychosomatic disorder |
| Depression |
| Anxiety |
| Developmental Delay |
| Neurodegenerative disease |
| Non-metastatic neoplasia |
| Metastatic neoplasia |
| Diabetes mellitus |
| Cardiovascular disease |
| Hypertension |
| Hypertensive encephalopathy |
| Atrial Fibrillation |
| Stroke |
| Transient Ischemic Attack |
| Hydrocephalus |
| Catastrophic Illness |
| Hypothyroidism |
| GERD/Ulcers |
| Neurofibromatosis Type 1 |
List of specifically targeted comorbidities studied. Abbreviation: Gastroesophageal reflux disease (GERD)
Each patient’s medication list was split into four mutually exclusive categories: anti-seizure medication (ASM), psychiatric medication, non-medical supplements and other medication. Medications prescribed as needed were included. If a psychotropic or anti-seizure medication was discussed specifically as being used to treat a non-psychiatric or non-seizure condition, the medication was counted based on its purpose instead of the medication class (e.g. duloxetine treating fibromyalgia and lamotrigine for bipolar disorder). For ASMs, we also recorded the number of ASMs previously tried and the number of ASMs stopped due to side effects or lack of efficacy in seizure control.
2.2 Statistical Modeling
We analyzed the relationship of co-morbidities and medications using both population-level descriptive statistics and individual-level predictive statistics. In descriptive statistics, we ask if the probability of a specific comorbidity or number of comorbidities was associated with seizure etiology on a population level; whereas in predictive statistics, we ask the reverse: if the seizure etiology of an individual patient can be predicted by the presence of a specific comorbidity or the number of comorbidities. Predictive statistics and individual comorbidities were modeled using logistic regression, whereas the number of comorbidities and medications was modeled using Poisson regression. We controlled for patient’s sex and age in all regressions. When analyzing specific comorbidities, we controlled for the total number of medical comorbidities to interpret if the contribution of each comorbidity was conditionally independent of the nonspecific increase in overall number of comorbidities. When analyzing the reason for ASM failure, we controlled for the number of failed ASMs because patients exposed to more ASMs had more opportunity for ASM failure. For predictive statistics, post-hoc tests of odds ratios were conducted on a model that included all studied variables. To create a succinct score and accurately estimate the intercept, a separate logistic regression as trained including only significant variables (p<0.05).
For predictive statistics, we trained our model on the patients with either PNES alone or ES alone in the retrospective dataset to so that we could assess our performance on the independently collected prospective data. Instead of positive and negative predictive values, we report the predictive value of PNES and ES that are defined similarly because our population lacks healthy negative controls. Statistically, the binary comparison of PNES versus ES is well posed and well-studied but there are not well-established, highly effective statistical methods to accomplish multinomial prediction between all five diagnostic classes. We report the predicted composition of PSLE, mixed PNES plus ES and patients with inconclusive monitoring based on the predictive algorithm for PNES versus ES. We combine the retrospective and prospective datasets for these diagnostic groups because they were not used in training.
For population level descriptive statistics, we combined the retrospective and prospective datasets because this practice results in the best linear and unbiased estimate of the effect of each studied factor 27. We recognize that the method of data collection differed between retrospective and prospective groups and have included details of the difference in each of our studied factors between the groups in Supplemental Tables 1 and 2.
We performed separate population-level analyses that split patients with temporal lobe epilepsy (TLE) from other epilepsies to test the hypothesis that patients with TLE experience more depression, anxiety and other psychiatric symptoms 28.
All patients consented for the use of their records in research, and the UCLA Institutional Review Board approved this study. This work is consistent with Declaration of Helsinki. De-identified raw data, code and online predictive score for this study is available at http://www.brainmapping.org/MarkCohen/research.html.
3. Results
Demographic differences between diagnostic groups are summarized in Table 2. No significant demographic differences existed between the retrospective and prospective datasets within each diagnostic group (p<0.05, Supplemental Table 1). Inconclusive monitoring occurred more often in the prospective dataset (26% vs 10%, Fisher exact test, p<0.01). Standardized interviews were performed with 83% (246/296) of patients admitted during the prospective period. Patients were not interviewed due to declining the interview, inability of the patient or family to communicate with the interviewer (i.e. language barrier and declined translation services), or no trained interviewers were available during admission.
Table 2.
| Diagnostic Group | Number | Age Min (95%CI) Max |
Sex %F |
Delay Min (95%CI) Max |
|---|---|---|---|---|
| PNES + ES | 51 | 13 (33–43) 79 | 59 | 1d (13–23) 54 |
| PNES | 331 | 13 (37–41) 88 | 71 | 1d (7–10) 71 |
| PSLE | 37 | 13 (43–58) 95 | 65 | 1d (4–16) 82 |
| Inconclusive | 199 | 10 (36–41) 84 | 59 | 1d (10–14) 53 |
| Epilepsy | 747 | 6 (34–37) 86 | 51 | 1d (15–18) 61 |
| TLE | 297 | 11 (35–39) 73 | 54 | 1w (15–19) 59 |
|
| ||||
| All | 1365 | 6 (36–39) 95 | 58 | 0 (13–15) 82 |
Demographic and delay from first seizure to neurology presentation information for each diagnostic category. Abbreviations: confidence interval (CI), percent female (%F), day (d), week (w).
3.1. Individual-Level Prediction
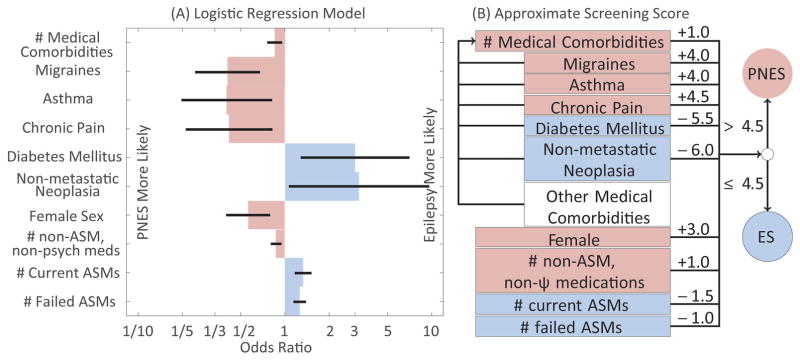
A summary of the significant factors that contributed to the individual level model is summarized in Figure 1. The performance of our model on the prospective dataset is summarized in Figure 2. The specific non-metastatic neoplasias observed are listed in Supplemental Table 3. Of the 26 non-metastatic neoplasias in the retrospective dataset (odds in logistic regression 3.2, p=0.04), 11 were intracranial and 16 were extracranial. Of the intracranial neoplasias, 4 and 6 were in patients with PNES and ES, respectively (odds 0.74, p=0.73). Of the extracranial neoplasias, 2 and 12 were in patients with PNES and ES, respectively (odds 3.0, p=0.16). Female sex was positively associated with PNES (odds 1.77, 95% CI 1.24–2.51, p=0.001), whereas age had no significant linear effect (log odds 0.99 per year, p=0.59). The overall model fit the data better than an intercept only model (intercept deviance 1202, residual deviance 910, df=26, p<10−46). Exact model statistics are available in Supplemental Table 4. The performance of a leave-one-out cross validation model using the retrospective data is summarized in Supplemental Figure 1.
Figure 1.
Individual-level predictive model trained on the retrospective dataset. Blue reflects factors that increase the likelihood of ES, whereas pink reflects factors that increase the likelihood of PNES. A) Odds ratios of all significant factors (Wald test, p<0.05). Error bars reflect standard error. B) Translation of log-odds ratios to a rounded score for implementation. Medical comorbidities (clear) other than the 5 listed contribute to the total number of comorbidities, but do not otherwise contribute to our 10-item score. Abbreviations: anti-seizure medication (ASM), and psychiatric (ψ).
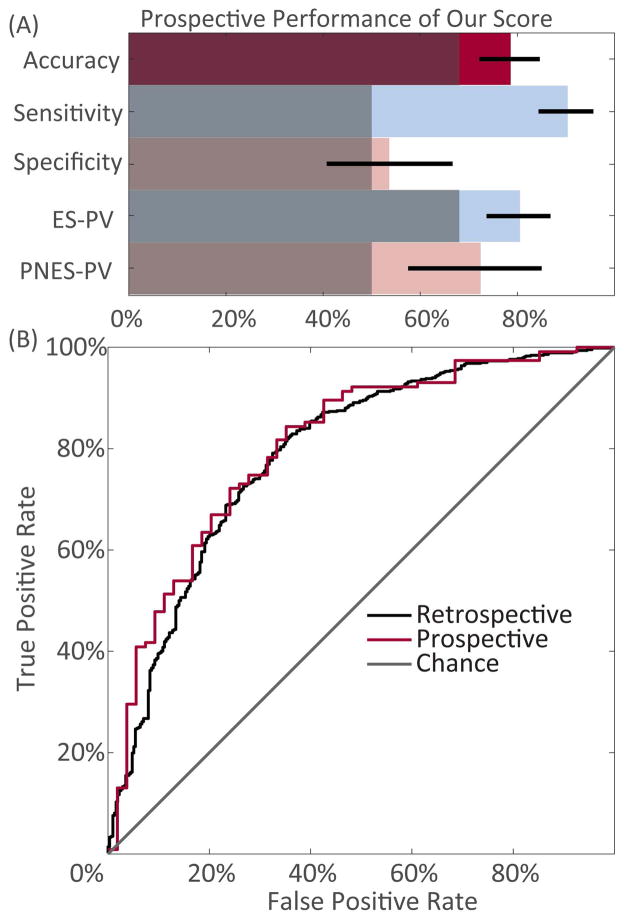
Figure 2.
Performance statistics of the predictive model when applied to the prospective dataset. A) Binary summary statistics of our model compared to uninformed classification methods (gray). Uninformed classification was done by either naïvely assuming that all patients have epilepsy or simple guessing with a 50% chance of guessing PNES. Exact numbers displayed in Supplemental Table 3. B) Receiver-operating curve of score from our predictive model applied to retrospective or prospective datasets, compared to chance.
Combining inconclusive patients from the retrospective and prospective groups, this model predicts that 61% (95% CI 53–70%) would go on to be diagnosed with epilepsy. Similarly, when applied to the patients with mixed PNES plus ES, the model predicted 80% had ES alone (95% CI 68–92%).
3.2. Population-Level Statistics
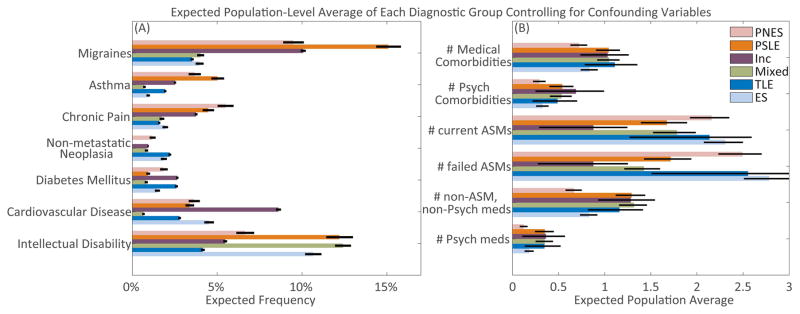
The average within diagnostic class for all factors that differed significantly between PNES and ES after controlling for confounding variables are summarized in Figure 3 (Wald tests, p<0.05). Exact model statistics including overall model fits are summarized in Supplemental Table 5. Analogous figures based on analyzing the data from the retrospective and prospective datasets separately and without controlling for other factors are displayed in Supplemental Table 2.
Figure 3.
Summary statistics of studied factors with significant differences (p<0.05) observed between patients with PNES as compared to ES controlling for age, sex, number of medical comorbidities and number of failed ASMs. Error bars reflect standard error of the model-based prediction for a single patient. A) Comorbidities. B) Count variables.
All specifically noted pairwise differences below control for the non-specific effect of the total number of comorbidities and, when appropriate, the total number of ASMs failed and are significant with 95% or greater confidence. See Supplemental Table 5 for a full summary of all differences.
The total number of medical comorbidities was lower in ES than in PNES, PSLE, mixed and inconclusive (Poisson lambda difference>0.22, standard error (SE) <0.18, Wald p<0.03). Migraines were less common in ES and TLE than in PNES, inconclusive and PSLE (log odds difference>3.3, SE<0.50, p<0.001). Asthma was more common in PNES and PSLE than ES (log odds difference>5.0, SE 1.0, p<0.03). Chronic pain was less common in ES than in PNES (log odds difference 5.1, SE 0.72, p=0.04). There were no significant differences in non-metastatic neoplasia or diabetes mellitus between any populations (p>0.25). Cardiovascular disease was most common in inconclusive patients compared to patients with TLE, mixed and PSLE (log odds difference>0.90, SE 0.57, p<0.03). Intellectual disability was less common in TLE than in other epilepsies (log odds difference=1.01, SE 0.52, p=0.02).
Prior to controlling for age, sex and the number of medical comorbidities, both anxiety and depression were more common in PNES than ES (anxiety: 8.5% ES vs 16.5% PNES, Fisher exact p<10−25; depression 16.8% ES vs 28.0% PNES, Fisher exact p<10−24). After controlling for the confounders, anxiety was more common in PNES than ES (log odds difference 0.69, SE 0.23, p=0.046) and depression tended to be more common in PNES than ES, but was not statistically significant (log odds difference 0.55, SE 0.19, p=0.052). Anxiety was more common in the prospective database than the retrospective database (ES: 6.8% retrospective vs 17.8% prospective, Fisher p=0.003; PNES 12.6% retrospective vs 26.4% prospective, Fisher p=0.051). Patients with ES and TLE had fewer psychiatric comorbidities than PNES, PSLE or inconclusive (Poisson lambda difference 0.49, SE 0.11, p=0.003). There were no significant psychiatric differences between ES and TLE (p>0.43).
Patients with ES or TLE took more ASMs than patients with PNES, PSLE and inconclusive (Poisson lambda difference>0.25, SE 0.06, p<0.002), and patients with PSLE took fewer ASMs than patients with PNES and inconclusive (Poisson lambda difference>0.71, SE 0.23, p<0.0001). All diagnostic groups significantly differed in the number of ASM failures (Poisson lambda difference>0.49, SE<0.23, p<0.01) except there was no significant difference between mixed or ES and TLE (p>0.12). The order of average ASM failures from most to least is ES, TLE, mixed, inconclusive, PNES then PSLE. The quality of the data regarding the reason for failure was poor with patients frequently not remembering why each medication had failed. With the reported data, patients with ES had more ASM failures due to efficacy than PNES or PSLE (Poisson lambda difference>1.3, SE<0.5, p<0.01). Patients with ES also had more ASM failures due to lack of side effects than PNES (Poisson lambda difference=0.26, SE 0.13, p=0.04).
Patients with ES and TLE took fewer other medications or psychiatric medications than all other groups, and patients with TLE took fewer other medications or psychiatric medications than patients with other epilepsies (Poisson lambda difference>0.64, SE<0.22, p<0.02). Patients with inconclusive monitoring took more non-medical supplements than patients in all other groups (Poisson lambda difference>0.46, SE<0.2, p<0.003).
4. Discussion
By understanding the context of comorbidities in which seizures present, we differentiated patients with PNES and ES. While the associations we observed were expected, our model translates this knowledge into an accurate, prospectively validated score using evidence-based relative weights from a large, unselected patient population. This low cost, objective score based on only 10 questions routinely asked in outpatient interviews had the potential to assist in early identification of patients with PNES without requiring additional testing or increasing the time needed to interview a patient (Figure 1 and online calculator linked on brainmapping.org/MarkCohen/research.html).
Our predictive performance was achieved through an impressive sensitivity but mediocre specificity. The difference between sensitivity and specificity may reflect the heterogeneity of the population of patients with PNES 29–31. Contrary to our hypothesis that patients with PNES have more comorbidities, a substantial subpopulation of patients with PNES do not have more medical comorbidities than patients with ES. This is consistent with our recent publication that the many patients with PNES did not have a broadly positive review-of-systems 22.
Assessment of other aspects of the patient’s history, like ictal behavior and risk factors of sexual abuse and traumatic brain injury, may raise the specificity by identifying characteristics of patients with PNES that do not have more medical comorbidities. Therefore, our score should be one part of a detailed patient assessment by a health care provider, and not a replacement for the clinical interview and diagnostic testing. Consistent with the recommendations of LaFrance and colleagues 20, historical factors only raise or lower the suspicion for PNES while vEEG remains necessary to characterize the seizures definitively.
The strongest difference between patients with PNES and ES was seen in the ASM history. Patients with ES were taking, and had previously taken more ASMs. This suggests that when seizures were epileptic, providers and patients attempted to control seizures with ASMs longer prior to referral, even though current international league against epilepsy (ILAE) guidelines recommend referral after failure of 2 ASMs 32. Accordingly, more ASM trials in PNES was associated with longer delay to diagnosis 2. These data suggest that a substantial proportion of patients with medication refractory seizures of both epileptic and psychogenic types are not being referred in a timely fashion.
Patients with PNES were more medically complex than patients with ES, as evidenced by more medical comorbidities and medications to treat these conditions. In addition to more review-of-systems complaints 22, our results suggest that patients with PNES have more symptoms attributed to clinical conditions requiring medication. The absolute number of conditions and medications provided more information than the identity of the specific conditions with a few key exceptions: asthma, chronic pain, migraines, diabetes mellitus and non-metastatic cancer.
The three specific conditions that were associated positively with PNES represent well-established risk factors. Asthma and migraines both are episodic conditions where the severity of attacks are difficult to quantify except by patient report 23; 33; 34. Similarly, the severity of chronic pain is based patients’ experience of pain and is difficult to correlate with objective measures 9. Because these conditions are based upon patient-experienced symptoms that are difficult to measure objectively, elements of these conditions may be understood as separate manifestations of conversion disorder.
The positive association of diabetes mellitus with ES has been discussed recently and may reflect immune abnormalities, microvascular lesions, local brain damage, metabolic factors and gene associations 35. The positive association of non-metastatic neoplasm with ES included both by intracranial and extracranial masses. Based on post-hoc analysis, this effect may be driven more by extracranial masses than intracranial masses, but there was insufficient power to separate these effects reliably. Intracranial masses can lead to tumor-related epilepsies 36. In addition, there are numerous paraneoplastic epilepsies primarily associated with breast cancer, germ-line tumors, and other tumors we observed in our patients 37. We did not identify any paraneoplastic antibodies in any of our patients. Both positive associations of diabetes mellitus and non-metastatic neoplasia were only significant in the individual-level model and not the population-level models (p=0.16), suggesting that it only provided diagnostic information after also controlling for medication history and the presence of migraines, asthma and chronic pain.
In addition to these pertinent associated factors, our population level results confirm previous demonstrations that patient-reported psychiatric disease was more common in PNES than ES, whereas the individual level predictive model suggests that the knowledge of psychiatric comorbidities did not add information that was not already accounted for based on medical comorbidities and medication history (p=0.35). While we observed more psychiatric disease in patients with PNES than ES on a population level (p<10−4), the presence of psychiatric comorbidities may have influenced ASM prescription patterns by raising the clinician’s suspicion for PNES, thereby reducing the number of ASMs failed prior to referral and the number of concurrent ASMs tried. Additionally, depression, anxiety and other psychiatric comorbidities are associated with more medical comorbidities 38. Lastly, we emphasize that our database includes only patient-reported or neurologist-documented psychiatric disease and not information from formal psychiatric assessment. This mirrors the quality of psychiatric diagnostic information available at an initial outpatient neurology or primary care visit.
The population-level statistics complement the individual-level predictions by providing information about the other patient populations. While patients with PSLE may appear similar to PNES in some aspects, patients with PSLE were easier to identify because they were tried on fewer ASMs prior to referral, and more frequently report neurodegenerative disease and migraines. This is consistent with the most common types of PSLE: complex migraines or dementia-related behavioral or confusion episodes.
The population with mixed ES plus PNES presents a difficult diagnostic challenge because they differed significantly from patients with both PNES alone and ES alone. This suggests that patients with mixed ES plus PNES should be viewed a separate group, and that stratifying patients based on high or low risk for PNES alone may not rule out mixed disease. The result that our model predicted that 80% of mixed patients had ES alone suggests that comorbidities and medications are a poor indicator of PNES in patients with known ES. Additional studies that separately discuss patients with mixed ES plus PNES are needed to better understand this patient population 7.
Unexpectedly, patients for whom monitoring is inconclusive were distinct from the other populations. Likely because of consistent differences from both patients with PNES and ES, our predicted result that 62% of patients would go on to be diagnosed with ES further confirms that inconclusive monitoring was non-diagnostic, and that seizures need to be observed to reach a definitive diagnosis 20.
Lastly, our results did not reproduce the effect that patients with TLE have more comorbid depression, anxiety or other psychiatric comorbidities than other patients with epilepsy (p>0.16), but the burden of depression, anxiety and psychiatric comorbidities was high in both populations. This lack of difference may be due to our reliance on patient-reported psychiatric comorbidities as compared to formal psychiatric assessments.
The patient population referred for VEEG monitoring at UCLA was similar to other datasets from epilepsy referral centers but did not reflect the population seen in emergency rooms and primary care practices. The delay to diagnosis in our dataset was substantially longer than the population that participated in the PNES treatment trials and in recent studies of pediatric PNES3; 4. Consequentially, our population may represent diagnostically challenging patients with PNES that may differ from other patients with PNES. In specific, our patients with PNES may have been tried on more ASMs due to the longer delay to diagnosis.
Outside of epilepsy referral centers, the relative prevalence of PNES is lower 39; 40. This difference reduces the PNES predictive value while increasing the ES predictive value. We highlight that at our center, and in emergency rooms and primary care practices, a formal psychiatric assessment was not obtained routinely, therefore our results may differ from previous literature that included this formal assessment.
There was a difference in method of data collection between our retrospective group and our prospective group. We view this as a strength of our work because predictive decisions were based upon physician-recorded retrospective record review that, conventionally, one would use to develop predictive algorithms. Prospective data was based on direct, standardized patient interview, which mirrors real-world application of our results.
5. Conclusions
A provider can begin to assess the possibility of PNES by discussing comorbid conditions and medication history with the patient and caregivers. Unlike seizure semiology that can be limited by ictal amnesia and healthcare literacy, patients and caregivers frequently can provide this history reliably. Our prospectively validated, evidence-based score illustrated in Figure 1B and available online could be used as an easy-to-use objective measure within a complete assessment of a patient to determine the likelihood of PNES and if the patient should be referred to vEEG for definitive diagnosis. However, this score has limited specificity and should be interpreted in the appropriate clinical context. We emphasize the ILAE guideline that irrespective of hypothesized seizure etiology any patient who has failed appropriate doses of two appropriately chosen ASMs should be referred for evaluation at an epilepsy center 2; 32.
Supplementary Material
Supplemental Figure 1. Summary statistics of all studied factors observed between patients with PNES as compared to ES, controlling for age, sex, number of comorbidities, and number of failed anti-seizure medications. Error bars reflect standard error. A) Comorbidities. B) Count variables.
Key Points.
Reliable early screening tools for psychogenic nonepileptic seizures (PNES) may reduce the time to diagnosis.
Medical comorbidities and anti-seizure medication history can identify patients at risk for psychogenic seizures.
Patients with PNES plus epilepsy have a different comorbidity and medication history than those with either epilepsy or PNES.
Acknowledgments
The authors thank Kirk Shattuck, Marc Nuwer, and Edward P. Lau for organization support, access to the data, and technical support. This work was supported by the UCLA-California Institute of Technology Medical Scientist Training Program (NIH T32 GM08042), the Neuroimaging Training Program (NIH T90 DA022768, R90 DA022768 & R90 DA023422 to MSC), the William M. Keck Foundation, research grants to JE (NS03310 & NS080181), and the UCLA Departments of Psychiatry & Biobehavioral Sciences and Biomathematics.
Footnotes
7. Conflicts & Ethical Publication
Drs. Engel, Stern and Kerr have clinical responsibilities that include the diagnosis and treatment of patients with epilepsy and nonepileptic seizures. The remaining authors have no declared conflicts of interest. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Dickinson P, Looper KJ. Psychogenic nonepileptic seizures: a current overview. Epilepsia. 2012;53:1679–1689. doi: 10.1111/j.1528-1167.2012.03606.x. [DOI] [PubMed] [Google Scholar]
- 2.Kerr WT, Janio EA, Le JM, et al. Diagnostic delay in psychogenic seizures and the association with anti-seizure medication trials. Seizure. 2016 doi: 10.1016/j.seizure.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valente KD, Alessi R, Vincentiis S, et al. Risk Factors for Diagnostic Delay in Psychogenic Nonepileptic Seizures Among Children and Adolescents. Pediatr Neurol. 2017;67:71–77. doi: 10.1016/j.pediatrneurol.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 4.LaFrance WC, Jr, Baird GL, Barry JJ, et al. Multicenter pilot treatment trial for psychogenic nonepileptic seizures: a randomized clinical trial. JAMA Psychiatry. 2014;71:997–1005. doi: 10.1001/jamapsychiatry.2014.817. [DOI] [PubMed] [Google Scholar]
- 5.Begley CE, Famulari M, Annegers JF, et al. The cost of epilepsy in the United States: an estimate from population-based clinical and survey data. Epilepsia. 2000;41:342–351. doi: 10.1111/j.1528-1157.2000.tb00166.x. [DOI] [PubMed] [Google Scholar]
- 6.Magee JA, Burke T, Delanty N, et al. The economic cost of nonepileptic attack disorder in Ireland. Epilepsy Behav. 2014;33:45–48. doi: 10.1016/j.yebeh.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Baroni G, Piccinini V, Martins WA, et al. Variables associated with co-existing epileptic and psychogenic nonepileptic seizures: a systematic review. Seizure. 2016;37:35–40. doi: 10.1016/j.seizure.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Dixit R, Popeschu A, Bagic A, et al. Medical comorbidities in patients with psychogenic nonepileptic spells (PNES) referred for video-EEG monitoring. Epilepsy & Behavior. 2013;28:137–140. doi: 10.1016/j.yebeh.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Benbadis SR. A spell in the epilepsy clinic and a history of “chronic pain” or “fibromyalgia” independently predict a diagnosis of psychogenic seizures. Epilepsy Behav. 2005;6:264–265. doi: 10.1016/j.yebeh.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Razvi S, Mulhern S, Duncan R. Newly diagnosed psychogenic nonepileptic seizures: health care demand prior to and following diagnosis at a first seizure clinic. Epilepsy Behav. 2012;23:7–9. doi: 10.1016/j.yebeh.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Walczak TS, Papacostas S, Williams DT, et al. Outcome after diagnosis of psychogenic nonepileptic seizures. Epilepsia. 1995;36:1131–1137. doi: 10.1111/j.1528-1157.1995.tb00472.x. [DOI] [PubMed] [Google Scholar]
- 12.Jones SG, TJOB, Adams SJ, et al. Clinical characteristics and outcome in patients with psychogenic nonepileptic seizures. Psychosom Med. 2010;72:487–497. doi: 10.1097/PSY.0b013e3181d96550. [DOI] [PubMed] [Google Scholar]
- 13.LaFrance WC, Jr, Benbadis SR. Avoiding the costs of unrecognized psychological nonepileptic seizures. Neurology. 2006;66:1620–1621. doi: 10.1212/01.wnl.0000224953.94807.be. [DOI] [PubMed] [Google Scholar]
- 14.Perez DL, LaFrance WC. Nonepileptic seizures: an updated review. CNS Spectr. 2016;21:239–246. doi: 10.1017/S109285291600002X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Syed TU, LaFrance WC, Jr, Kahriman ES, et al. Can semiology predict psychogenic nonepileptic seizures? A prospective study. Ann Neurol. 2011;69:997–1004. doi: 10.1002/ana.22345. [DOI] [PubMed] [Google Scholar]
- 16.Syed TU, Arozullah AM, Suciu GP, et al. Do observer and self-reports of ictal eye closure predict psychogenic nonepileptic seizures? Epilepsia. 2008;49:898–904. doi: 10.1111/j.1528-1167.2007.01456.x. [DOI] [PubMed] [Google Scholar]
- 17.Kerr WT, Anderson A, Lau EP, et al. Automated diagnosis of epilepsy using EEG power spectrum. Epilepsia. 2012;53:e189–192. doi: 10.1111/j.1528-1167.2012.03653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilbert DL, Sethuraman G, Kotagal U, Buncher R. Meta-analysis of EEG test performance shows wide variation among studies. Neurology. 2003;60:564–570. doi: 10.1212/01.wnl.0000044152.79316.27. [DOI] [PubMed] [Google Scholar]
- 19.Syed TU, Arozullah AM, Loparo KL, et al. A self-administered screening instrument for psychogenic nonepileptic seizures. Neurology. 2009;72:1646–1652. doi: 10.1212/WNL.0b013e3181a55ef7. [DOI] [PubMed] [Google Scholar]
- 20.LaFrance WC, Jr, Baker GA, Duncan R, et al. Minimum requirements for the diagnosis of psychogenic nonepileptic seizures: a staged approach: a report from the International League Against Epilepsy Nonepileptic Seizures Task Force. Epilepsia. 2013;54:2005–2018. doi: 10.1111/epi.12356. [DOI] [PubMed] [Google Scholar]
- 21.Prueter C, Schultz-Venrath U, Rimpau W. Dissociative and associated psychopathological symptoms in patients with epilepsy, pseudoseizures, and both seizure forms. Epilepsia. 2002;43:188–192. doi: 10.1046/j.1528-1157.2002.45900.x. [DOI] [PubMed] [Google Scholar]
- 22.Kerr WT, Janio EA, Braesch CT, et al. Diagnostic implications of review-of-systems questionnaires to differentiate epileptic seizures from psychogenic seizures. Epilepsy Behav. 2017;69:69–74. doi: 10.1016/j.yebeh.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Wet CJ, Mellers JDC, Gardner WN, et al. Pseudoseizures and asthma. Journal of Neurology Neurosurgery and Psychiatry. 2003;74:639–641. doi: 10.1136/jnnp.74.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sirven JI, Glosser DS. Psychogenic nonepileptic seizures: theoretic and clinical considerations. Neuropsychiatry Neuropsychol Behav Neurol. 1998;11:225–235. [PubMed] [Google Scholar]
- 25.Szaflarski JP, Hughes C, Szaflarski M, et al. Quality of life in psychogenic nonepileptic seizures. Epilepsia. 2003;44:236–242. doi: 10.1046/j.1528-1157.2003.35302.x. [DOI] [PubMed] [Google Scholar]
- 26.Szaflarski JP, Szaflarski M, Hughes C, et al. Psychopathology and quality of life: psychogenic non-epileptic seizures versus epilepsy. Med Sci Monit. 2003;9:CR113–118. [PubMed] [Google Scholar]
- 27.Miller ME, Hui SL, Tierney WM. Validation techniques for logistic regression models. Stat Med. 1991;10:1213–1226. doi: 10.1002/sim.4780100805. [DOI] [PubMed] [Google Scholar]
- 28.Kanner AM. Mood disorder and epilepsy: a neurobiologic perspective of their relationship. Dialogues Clin Neurosci. 2008;10:39–45. doi: 10.31887/DCNS.2008.10.1/amkanner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Groppel G, Kapitany T, Baumgartner C. Cluster analysis of clinical seizure semiology of psychogenic nonepileptic seizures. Epilepsia. 2000;41:610–614. doi: 10.1111/j.1528-1157.2000.tb00216.x. [DOI] [PubMed] [Google Scholar]
- 30.Szabo L, Siegler Z, Zubek L, et al. A detailed semiologic analysis of childhood psychogenic nonepileptic seizures. Epilepsia. 2012;53:565–570. doi: 10.1111/j.1528-1167.2012.03404.x. [DOI] [PubMed] [Google Scholar]
- 31.Cragar DE, Berry DT, Schmitt FA, et al. Cluster analysis of normal personality traits in patients with psychogenic nonepileptic seizures. Epilepsy Behav. 2005;6:593–600. doi: 10.1016/j.yebeh.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 32.Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069–1077. doi: 10.1111/j.1528-1167.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- 33.Rai D, Kerr MP, McManus S, et al. Epilepsy and psychiatric comorbidity: a nationally representative population-based study. Epilepsia. 2012;53:1095–1103. doi: 10.1111/j.1528-1167.2012.03500.x. [DOI] [PubMed] [Google Scholar]
- 34.Smitherman TA, Kolivas ED, Bailey JR. Panic disorder and migraine: comorbidity, mechanisms, and clinical implications. Headache. 2013;53:23–45. doi: 10.1111/head.12004. [DOI] [PubMed] [Google Scholar]
- 35.Yun C, Xuefeng W. Association between seizures and diabetes mellitus: a comprehensive review of literature. Curr Diabetes Rev. 2013;9:350–354. doi: 10.2174/15733998113099990060. [DOI] [PubMed] [Google Scholar]
- 36.Giulioni M, Marucci G, Martinoni M, et al. Epilepsy associated tumors: Review article. World J Clin Cases. 2014;2:623–641. doi: 10.12998/wjcc.v2.i11.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serafini A, Lukas RV, VanHaerents S, et al. Paraneoplastic epilepsy. Epilepsy Behav. 2016;61:51–58. doi: 10.1016/j.yebeh.2016.04.046. [DOI] [PubMed] [Google Scholar]
- 38.Kroenke K. Patients presenting with somatic complaints: epidemiology, psychiatric comorbidity and management. Int J Methods Psychiatr Res. 2003;12:34–43. doi: 10.1002/mpr.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lesser RP. Psychogenic seizures. Neurology. 1996;46:1499–1507. doi: 10.1212/wnl.46.6.1499. [DOI] [PubMed] [Google Scholar]
- 40.Gumnit RJ, Walczak TS National Association of Epilepsy C. Guidelines for essential services, personnel, and facilities in specialized epilepsy centers in the United States. Epilepsia. 2001;42:804–814. doi: 10.1046/j.1528-1157.2001.08701.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Summary statistics of all studied factors observed between patients with PNES as compared to ES, controlling for age, sex, number of comorbidities, and number of failed anti-seizure medications. Error bars reflect standard error. A) Comorbidities. B) Count variables.