Abstract
Apolipoprotein A-IV (apoA-IV) is a satiation factor that acts in the hypothalamus, however, the intracellular mechanisms responsible for this action are still largely unknown. Here we report that apoA-IV treatment elicited a rapid activation of the phosphatidylinositol-3-kinase (PI3K) signaling pathway in cultured primary hypothalamic neurons, and this effect was significantly attenuated by pretreatment with LY294002, an inhibitor of the PI3K pathway. To determine if the activation of PI3K is required for apoA-IV’s inhibitory effect on food intake, apoA-IV was administered intracerebroventricularly. We found that apoA-IV significantly reduced food intake and activated PI3K signaling in the hypothalamus, and these effects were abolished by icv pre-treatment with LY294002. To identify the distinct brain sites where apoA-IV exerts its anorectic action, apoA-IV was administered into the ventromedial hypothalamus (VMH) through implanted bilateral cannula. At a low dose (0.5 μg), apoA-IV significantly inhibited food intake and activated PI3K signaling pathway in the VMH of lean rats, but not in high-fat diet-induced obese (DIO) rats. These results collectively demonstrate a critical role of the PI3K/Akt pathway in apoA-IV’s anorectic action in lean rats and suggest a defective PI3K pathway in the VMH is responsible for the impaired apoA-IV’s anorectic action in the DIO animals.
Keywords: Apolipoprotein, PI3K/Akt signaling, hypothalamus, food intake, obesity
Introduction
Apolipoprotein A-IV (apoA-IV) is a satiation factor [1,2]. Previously we reported that intracerebroventricular (icv) administration of apoA-IV significantly decreases food intake, whereas icv infusion of apoA-IV antiserum stimulates feeding, implying that endogenous apoA-IV tonically inhibits food intake [1,2]. However, the intracellular mechanisms mediating apoA-IV’s anorectic function are largely unknown.
Phosphatidylinositol 3-kinases (PI3Ks) are heterodimeric complexes composed of regulatory and catalytic subunits that control a broad spectrum of cellular functions including survival, growth and metabolism [3]. These functions are related to the ability of PI3K to activate Akt (also known as protein kinase B), a serine/threonine kinase that mediates the downstream effects of PI3K. PI3K signaling pathway in the hypothalamus has been implicated in the regulation of energy homeostasis [4]. For example, leptin and insulin activate hypothalamic PI3K pathway, which is required for them to suppress food intake [5,6]. Therefore, PI3K signaling in the hypothalamic neurons may be a common pathway that integrates metabolic cues to provide a coordinated control of energy homeostasis.
In the present study, we sought to determine whether the PI3K/Akt signaling pathway was modulated by apoA-IV in cultured primary hypothalamic neurons, and whether the activation of PI3K/Akt was required for the anorectic effect induced by icv administered apoA-IV in rats. Since it was unclear at which brain site(s) apoA-IV exerts its effect on feeding, we also examined apoA-IV’s anorectic effect in lean rats with bilateral cannula implanted in the ventromedial hypothalamus (VMH), and further determined if apoA-IV’s actions on feeding and PI3K signaling pathway were impaired in the VMH of high-fat diet-induced obese (DIO) rats.
2. Materials and methods
2.1. Animals
Pregnant Long-Evans female and adult male rats were purchased from Harlan (Indianapolis, IN) and individually housed in a temperature-controlled vivarium on a 12/12-h light/dark cycle. Standard rodent diet and water were provided ad libitum except where noted. All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Cincinnati.
2.2. Materials
The selective inhibitor of the PI3K signaling pathway, LY294002, and rabbit monoclonal antibodies, anti-pAKt-Ser473 (Cat. No. 4060) and anti-Akt (Cat. No. 9272) were purchased from Cell Signaling Technologies (Beverly, MA). Recombinant rat apoA-IV was described previously [7,8]. Cell culture medium was from Thermo Fisher Scientific (Waltham, MA). All other chemicals were purchased from Sigma (St. Louis, MO). Two pelleted semi-purified (AIN-93M), nutritionally complete experimental diets prepared by Dyets (Bethlehem, PA) were used. The high fat diet (HFD) contained 20 g fat/100 g diet by weight and the low-fat diet (LFD) contained 4 g fat/100 g diet by weight, as we reported before [9].
2.3. Primary hypothalamic neuron cultures and treatments
Primary neuronal cells were prepared from rat embryos on the 18th day of gestation and cultured in a serum-free neurobasal medium supplemented with B-27, as we reported previously [10]. After cultured for 6 days, the primary hypothalamic cells were treated with apoA-IV (100 nM) for 0, 5, 15, 30, or 60 min. The cells were then washed in ice-cold PBS, the lysates were prepared, and pAkt and total Akt levels were measured by immunoblot analyses [10]. To determine the dose-effect relationship, the cultured neuronal cells were treated with different doses of apoA-IV (10, 100 or 1000 nM) for 15 min. The treated cells were then harvested for immunoblot analyses.
2.4. Pre-treatment with PI3K inhibitor in cultured neuronal cells
The cultured primary hypothalamic cells were pretreated with the PI3K inhibitor LY294002 (10 μM) or vehicle (dimethylsulfoxide, DMSO) [11]. Thirty minutes later, the cells were treated with apoA-IV (100 nM) or vehicle (PBS) for an additional 15 min and then the cells were harvested for immunoblot analyses.
2.5. Surgical procedure for intracerebroventricular (icv) cannula implantation
After anesthetized, each rat was stereotaxically implanted with a guide cannula (22-gauge; Plastics One, Roanoke, VA) into the 3rd-cerebral ventricle, as we reported previously [12].
2.6. Icv delivery of drugs and food intake
The rats with icv cannula were fasted for 4-h. Ninety min before lights off, LY294002 (1 nmol, 1μl) or vehicle (10% DMSO in artificial cerebrospinal fluid, aCSF, 1 μl) was injected into the 3rd ventricle [13]. We selected this dose of LY294002 because it had no effect on food intake when administered alone, but significantly attenuated the decrease of food intake induced by leptin [6]. Sixty min later, the rats received an icv injection of apoA-IV (4 μg, 1 μl) [14] or vehicle (aCSF, 1 μl). At the onset of the dark cycle, rats were presented with preweighed food, and cumulative food intake was measured at 1, 2, 4, and 24 h post-food presentation. Body weight was recorded 24 h post-food presentation.
2.7. Hypothalamic tissue collection
Seven days after the feeding test described above, the same rats were used to examine the activation of PI3K signaling. The LY294002 or DMSO was icv injected followed by apoA-IV or aCSF administration, as described above. Thirty min after the 2nd injection, rats were sacrificed, and the brains were quickly frozen. Coronal hypothalamus brain sections were cut using a cryostat, and the tissues of hypothalamus and cortex (for control) were micropunched for immunoblot analysis, as we reported previously [15].
2.8. Surgical procedure for intra-VMH bilateral cannula implantation
Rats were anesthetized and a bilateral guide-cannula (22-gauge, Plastics One) was positioned with its tip 2.0 mm above the center of the VMH with the following coordinates: anteroposterior from bregma −2.5 mm, mediolateral ± 0.7 mm, and dorsoventral −7.8 mm), based on the atlas of Paxinos and Watson [16] and a previous report [17]. Correct bilateral VMH cannula placement was confirmed histologically post-mortem by the injection of methylene blue (0.1 μl) at the end of experiments [18].
2.9. Intra-VMH delivery of drugs and food intake
After recovery from surgery, the rats were fasted for 4 h before dark. Thirty min before dark cycle onset, three doses of apoA-IV (0.125, 0. 25, or 0.5 μg in 0.2 μl) or vehicle (aCSF, 0.2 μl) were administered into each side of the VMH. Therefore, the doses of apoA-IV were 0.25, 0.5 and 1.0 μg per rat. We used a 28-gauge bilateral infusion cannula, which was connected through a polyethylene tube to a 5-μl microsyringe. A volume of 0.2 μl was infused with a 5-μl Hamilton syringe attached to a Motorized Integrated Stereotaxic Injector (iSi) system (Stoelting Co.) into one side of the VMH over 1 min and the infusion cannula was kept in place for an additional 2 min to minimize backflow of the injectant [13]. Food was returned at the onset of the dark cycle, and intake was measured at 1, 2, 4, and 24 h post food presentation.
2.10. Chronic HFD feeding in rats
Rats were randomly divided into 3 groups. Groups 1 and 2 received ad libitum access to the HFD and the LFD for 10 wks, respectively. Because HF and LF rats consumed different amounts of energy each day, a control group (pair-fed HF group, PHF), was included. The PHF rats were provided the HFD each day, but in an amount limited to the average daily caloric consumption of the rats fed the LFD ad libitum, as we reported previously [19]. By the end of experiment, blood samples were taken from the tail vein, and plasma leptin and insulin were measured with ELISA kits (Millipore, Billerica MA).
After been maintained on the different diets for 10 wk, each rat was implanted a bilateral intra-VMH cannula as described above. After recovery from the surgery, 4-h fasted rats received intra-VMH injection of apoA-IV (0.25 μg/side) or aCSF (0.2 μl/side) before the dark cycle. Food was returned at the onset of the dark cycle, and intake was measured at 1, 2, 4, and 24 h post-food presentation.
Seven days after the feeding test as described above, the same rats were used to examine the activation of PI3K pathway. Four h fasted rats received intra-VMH administration of apoA-IV (0.25 μg/side) before the dark cycle. Thirty min later, the rats were sacrificed and the VMH were micropunched for immunoblot analyses [15].
2.11. Immunoblot analyses for pAkt/Akt measurement
The cultured neuronal cells, rat hypothalamus, cortex or VMH tissues were homogenized, and the supernatant proteins (10 μg protein) were prepared for immunoblot analyses, as we have done previously [10]. The dilution of both pAkt and Akt antibodies were 1:1,000. The amount of pAkt was normalized to total Akt protein level at each individual sample and was expressed as a ratio [10].
2.12. Statistical analyses
The data are presented as mean ± standard error (SEM). Data were analyzed using GraphPad Prism to evaluate normal distribution and variations within and among groups. In vitro experiments were carried out in triplicate and performed on 3–4 separate occasions. In in vivo studies, differences among the groups were determined using one-way or two-way repeated measures ANOVA analyses followed by Student-Newman-Keuls test for comparison between treatments. P values less than 0.05 were considered statistically significant.
3. Results
3.1. ApoA-IV time-dependently increased PI3K activation in cultured hypothalamic neurons
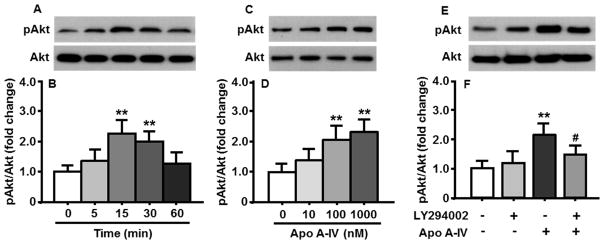
As depicted in Fig. 1A and 1B, apoA-IV (100 nM) treatment significantly increased the ratio of phosphorylated Akt (pAkt) to total Akt in the cultured neuronal cells at both 15 and 30 min, compared to that in prior to apoA-IV treatment (0 min). The pAkt level induced by apoA-IV was significantly reduced at 60 min.
Fig. 1.
ApoA-IV time- (A and B) and dose-dependently (C and D) increased the activation of PI3K/Akt signaling pathway in cultured primary hypothalamic neuronal cells. ApoA-IV-induced Akt phosphorylation was significantly attenuated by LY294002 (E and F). A, C and E are representative immunoblots of pAkt and total Akt; and B, D, F are quantitative analysis of data. Mean ± SEM; n = 3. **P <0.01, vs. 0 min or vehicle-treated cells. #P <0.05, vs. apoA-IV-treated neuronal cells.
3.2. ApoA-IV dose-dependently activated PI3K/Akt pathway in cultured neurons
Because the highest level of pAkt induced by ApoA-IV was observed at 15 min, the cultured neuronal cells were incubated with different concentrations of apoA-IV (10, 100 and 1000 nM) for 15 min. We found that apoA-IV at doses ≥ 100 nM significantly increased the pAkt levels in those cells (Fig. 1C and 1D).
3.3. Pretreatment of PI3K inhibitor attenuated apoA-IV effect on PI3K/Akt pathway
If apoA-IV-induced phosphorylation of Akt is mediated through the PI3K signaling pathway, this effect should be blocked/attenuated by pharmacological inhibition of the PI3K signaling pathway. Consistent with this hypothesis, LY294002 (10 μM) significantly attenuated the effect of apoA-IV on the phosphorylation of Akt in the cultured primary cells (Fig. 1E and 1F).
3.4. Inhibition of PI3K signaling pathway reduced the potency of apoA-IV anorectic action
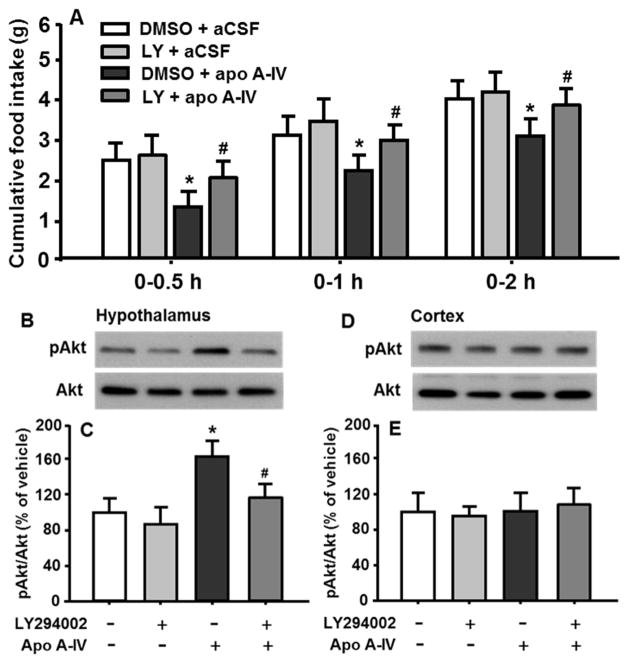
Consistent with our previous report [14], apoA-IV at a dose of 4 μg significantly reduced food intake at 0.5, 1 and 2 h, compared with vehicle. In vivo pretreatment with LY294002 significantly attenuated the potency of satiation induced by apoA-IV (Fig. 2A). No significant differences in food intake and body weight of rats over 4 h after apoA-IV or aCSF injection was observed (data not shown).
Fig. 2.
ApoA-IV-induced anorexigenic effect (A) and the activation of PI3K/Akt pathway were significantly attenuated in the hypothalamus (B and C). As a control, no significant difference in pAkt levels were found in the cortex after apoA-IV or LY294002 treatment (D and E). Mean ± SEM; n = 6–8, *P <0.05, vs. vehicle-control. #P <0.05, vs. apoA-IV-treated rats.
3.5. The effect of apoA-IV on PI3K/Akt pathway was inhibited by LY294002
To verify the influence of PI3K inhibitor on in vivo activation of PI3K pathway induced by apoA-IV, 4 h fasted rats received icv administration of LY294002 or vehicle, followed by apoA-IV or aCSF icv injection, as described above. The hypothalamus and cortex (for control) were micropunched for immunoblot analyses. As depicted in Fig. 2B and 2C, apoA-IV significantly increased pAkt level, compared to vehicle. Pretreatment with LY294002 attenuated apoA-IV-induced Akt phosphorylation by 36% (P < 0.05), whereas LY294002 administration alone did not affect the level of pAkt significantly. The ratio of pAkt/Akt in the cortex were comparable among all four groups (Fig. 2D and 2E).
3.6. Intra-VMH injection of apoA-IV dose-dependently suppressed food intake
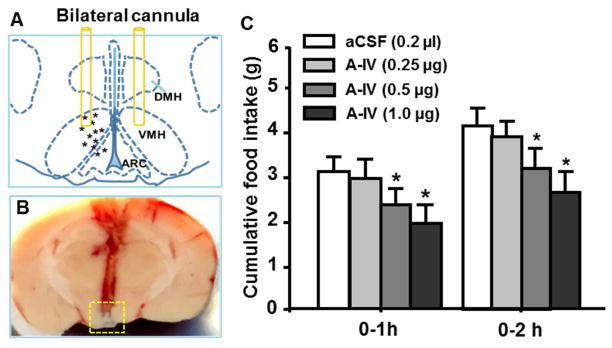
While icv injection of apoA-IV significantly reduces food intake, it is unclear at which brain site(s) apoA-IV exerts its anorectic action. To determine if the VMH was the key site for apoA-IV’s anorectic effect, lean rats with bilateral intra-VMH cannula was used. No significant difference in food intake was found between the vehicle and apoA-IV at a dose of 0.25 μg (0.125 μg per side of the VMH) at any time point. However, the higher dose of apoA-IV (0.5 and 1.0 μg) significantly decreased food intake at both 1 and 2 h (Fig. 3C). Therefore, the minimal effective dose of apoA-IV (0.5 μg/rat) was selected to determine the changes in response to apoA-IV treatment in HFD-fed rats below.
Fig. 3.
Intra-VMH administration of apoA-IV reduced food intake in lean rats. A, schematic drawing showing the placement of cannulas in the VMH of rats. B, representative photograph showing placement of bilateral cannula in the VMH. Tips of bilateral injectors in the VMH were indicated by blue ink within the yellow-dashed outline. C, intra-VMH injection of apoA-IV dose-dependently suppressed food intake of rats at 1 and 2 h after the injection, compared to vehicle (aCSF). VMH, ventromedial hypothalamus; ARC, arcuate nucleus of hypothalamus; DMH, dorsomedial nucleus of hypothalamus. Mean ± SEM; n = 6–7, *P <0.05, vs. vehicle-controls.
3.7. Chronic HFD consumption significantly diminished apoA-IV actions on feeding and the PI3K activation in the VMH
To determine the influence of diet-induced obesity on apoA-IV anorectic action, the rats were fed LFD or HFD for 10 wks. Consistent with our previous report [9], the HFD-fed rat gained significantly more body weight (P < 0.05), compared with the LFD-fed rats (578 ± 22.6 g vs. 517 ± 27.2 g). Importantly, the HFD-fed rats had 47% more body fat mass (P < 0.01) than LF-fed rats (72 ± 9.6 vs. 49 ± 11.3 g). Daily food intake in the HFD-fed rats on Wk-10 was significantly higher than that in LFD-fed group (455 ± 27.9 vs. 401 ± 25.6 KJ/day) (P < 0.05). Additionally, the HFD-fed rats were hyperleptinemia, compared to LFD-fed rats (plasma leptin level: 19.6 ± 3.2 vs. 14.2 ± 2.4 ng/ml) (P < 0.05). Although blood glucose levels were comparable between the HFD-fed and LFD-fed rats (116.2 ± 8.4 vs. 105.6 ± 7.9 mg/dl), the insulin levels in the plasma of HFD-fed rats were significantly higher (P < 0.05) than that in LFD-fed rats (2.5 ± 0.3 vs. 1.4 ± 0.2 ng/ml). However, these metabolic parameters described above are comparable between the LFD-fed and PHF-fed rats.
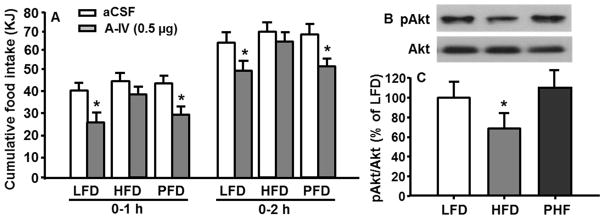
To determine whether apoA-IV-induced anorectic effect was diminished in the DIO rats, 4-h fasted rats received intra-VMH injection of apoA-IV (0.5 μg/rat, or 0.25 μg in 0.2 μl/side of VMH) or sCSF (0.2 μl/side). As depicted in Fig. 4A, apoA-IV significantly reduced food intake in LFD-fed rats (P < 0.05), but not in HFD-fed rats. ApoA-IV’s anorectic effect in the PHF-fed rats was comparable to that in LFD-fed rats.
Fig. 4.
HFD rats have diminished response to apoA-IV administered into the VMH in the reduction of food intake (A) and the activation of PI3K/Akt signaling pathway (B and C). Means ± SEM, n = 6–8, *P <0.05, vs. vehicle-treated rats at the same time point (A), or LFD-fed rats (C).
After recovery from the feeding test above, all rats received intra-VMH injection of apoA-IV (0.5 μg/rat). Thirty min later, the rats were sacrificed and the VMHs were then micropunched. The pAkt/Akt levels in the VMH were analyzed by immunoblot analysis. Compared with LFD-fed rats, HFD-fed rats had significantly reduced pAkt levels (Fig. 4B and 4C). The PHF-fed rats were still responsive to apoA-IV treatment and the ratio of pAkt/Akt was comparable to that in LFD rats.
4. Discussion
Previously we have reported that icv administration of apoA-IV significantly suppresses food intake [1,2]. Since the apoA-IV does not cross the blood-brain barrier, and icv infusion of apoA-IV antiserum stimulates feeding [20], the anorectic action induced by apoA-IV seems likely to occur within the brain. Given that icv administered apoA-IV reduces food intake acutely, it is unlikely that meal termination is controlled by transcriptional events because they typically require more time. We therefore hypothesized that a signaling cascade might be responsible for apoA-IV’s anorectic effect.
PI3Ks are a family of enzymes involved in cellular functions such as cell growth, proliferation, differentiation, motility, and intracellular trafficking [3]. In numerous cell types, PI3K acts in a heterodimeric form consisting of one 85-kDa regulatory (p85) and one 110-kDa catalytic (p110) subunit. Activation of PI3K signaling pathway rapidly induces the phosphorylation of Akt via serine and threonine phosphorylation [21]. Recent studies suggest that hypothalamic PI3K signaling is important in the regulation of both energy balance and glucose metabolism [4].
To determine whether the PI3K signaling pathway mediates anorectic action of apoA-IV in the hypothalamus, we first determined if apoA-IV could affect the phosphorylation of Akt in cultured primary hypothalamic neurons. We found that apoA-IV time- and dose-dependently increased the activation of PI3K/Akt signaling pathway in the neuronal cells. If the phosphorylation of Akt were mediated through the PI3K signaling pathway, the increased levels of pAkt induced by apoA-IV should be attenuated by pharmacological inhibition of the PI3K signaling pathway. Consistent with this hypothesis, LY294002 was found to significantly reduce the phosphorylation of Akt induced by apoA-IV in cultured neuronal cells.
To determine whether apoA-IV-induced anorexia is affected by in vivo pretreatment with the PI3K inhibitor, LY294002 was infused into the 3rd ventricle prior to apoA-IV administration. We found that apoA-IV induced the reduction of on food intake and the phosphorylation of Akt were significantly attenuated by LY294002. These effects seemed tissue-specific because no changes in pAkt levels were found in the cortex. These results support our hypothesis that apoA-IV engages the PI3K signaling pathway and its downstream effector mechanisms to activate hypothalamic neuronal cells and exert its anorectic effect.
While the molecular mechanism of how apoA-IV increases the activation of PI3K signaling pathway remains to be investigated, our results extend the integrative role of PI3K/Akt in the regulation of food intake in the hypothalamus. Previous studies have demonstrated that systemic administration of leptin in rats activates hypothalamic PI3K/Akt pathway and icv infusion of LY294002 blocks leptin-induced anorexia [6,22]. Insulin acts on the brain to reduce food intake and this anorectic effect is completely abolished by the pre-treatment of the inhibitor of PI3K [5,22]. Thus, the hypothalamic PI3K/Akt signaling pathway might be a common pathway that integrates metabolic cues to provide a coordinated control of energy homeostasis.
Obesity has become a serious health problem in the United States. One well-established risk factor predisposing to obesity is the amount of fat in the diet. Rodent DIO models represent the condition occurring in most obese humans. The DIO rats had significantly increased body weight, which is associated with increased calorie intake. Recent data suggest that obese animals have blunted satiety, raising the possibility that defective signaling of apoA-IV in the hypothalamus. Our present study demonstrated that PI3K/Akt signaling played an important role in transducing apoA-IV anorectic action in lean rats. We then asked if the PI3K signaling is impaired in the VMH of rats after chronic HFD consumption. The reasons we focus on the VMH are because it is a brain site intimately involved in the integration of signals for energy homeostasis [2,10], and because apoA-IV is highly expressed in the VMH [20].
To test this possibility, apoA-IV was first injected into the VMH of lean rats through an implanted bilateral cannula. We found that apoA-IV at doses ≥ 0.5 μg/rat significantly reduced food intake in those lean rats. However, when an effective dose of apoA-IV (0.5 μg) was administered into the VMH of the DIO rats, no significant reduction of food intake was found, indicating the impaired response to apoA-IV in those DIO rats. Additionally, the exogenous apoA-IV treatment increased pAkt levels in the VMH of LFD rats, but not in DIO rats, suggesting a defective PI3K pathway in the VMH of rats is responsible to the impairment of apoA-IV anorectic action after chronic HFD feeding. Interestingly, the PHF-fed rats were still responsive to apoA-IV treatment and the ratio of pAkt/Akt was comparable to that in LFD rats, these results indicate that it is the extra calories consumed, but not the HFD itself, which was responsible for the alteration of apo A-IV’s anorectic action.
In conclusion, our results identify an essential neural mechanism of apoA-IV’s action on food intake. We demonstrated that apoA-IV triggered the activation of the PI3K cascade in cultured primary hypothalamic neurons. In rats, the inhibition of the PI3K signaling cascade in the brain significantly decreased the potency of apoA-IV to reduce food intake. Furthermore, apoA-IV actions on feeding and the activation of PI3K/Akt signaling pathway were significantly impaired in the VMH of DIO rats. Thus, manipulations of the brain apoA-IV system, via the specific activation of the PI3K cascade within selected brain regions, e.g. the VMH, may provide novel therapeutic strategies for the prevention and treatment of obesity.
Highlights.
ApoA-IV increases the activation of PI3K/Akt signaling pathway in cultured primary hypothalamic neuronal cells.
Icv pretreatment of PI3K inhibitor significantly attenuates apoA-IV’s action on food intake.
ApoA-IV administered into ventromedial hypothalamus inhibits food intake and activates PI3K signaling pathway in lean, but not in DIO rats.
Acknowledgments
This work was supported by NIH grants NIH DK92779, DK95440 (to M.L).
Abbreviation
- ApoA-IV
apolipoprotein A-IV
- PI3K
Phosphatidylinositol-4,5-bisphosphate 3-kinase
- Akt
also known PKB, protein kinase B
- DMSO
dimethyl sulfoxide
- PHF
pair-fed HF
- aCSF
artificial cerebrospinal fluid
Footnotes
Author contributions
Conceived and designed the experiments: ML. Performed the experiments and datum analysis: LS and ML. Interpretation of data and revision of manuscript: ML, CCL, and LAW.
Conflicts of interest
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fujimoto K, Fukagawa K, Sakata T, Tso P. Suppression of food intake by apolipoprotein A-IV is mediated through the central nervous system in rats. J Clin Invest. 1993;91:1830–1833. doi: 10.1172/JCI116395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu M, Doi T, Shen L, Woods SC, Seeley RJ, Zheng S, Jackman A, Tso P. Intestinal satiety protein apolipoprotein AIV is synthesized and regulated in rat hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1382–R1387. doi: 10.1152/ajpregu.2001.280.5.R1382. [DOI] [PubMed] [Google Scholar]
- 3.Cantley LC. The phosphoinositide 3-kinase pathway. Science (80-) 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 4.Morton GJ, Gelling RW, Niswender KD, Morrison CD, Rhodes CJ, Schwartz MW. Leptin regulates insulin sensitivity via phosphatidylinositol-3-OH kinase signaling in mediobasal hypothalamic neurons. Cell Metab. 2005;2:411–420. doi: 10.1016/j.cmet.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Niswender KD, Morrison CD, Clegg DJ, Olson R, Baskin DG, Myers MG, Jr, Seeley RJ, Schwartz MW. Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus: a key mediator of insulin-induced anorexia. Diabetes. 2003;52:227–231. doi: 10.2337/diabetes.52.2.227. [DOI] [PubMed] [Google Scholar]
- 6.Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG, Jr, Schwartz MW. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature. 2001;413:794–795. doi: 10.1038/35101657. [DOI] [PubMed] [Google Scholar]
- 7.Lo CM, Xu M, Yang Q, Zheng S, Carey KM, Tubb MR, Davidson WS, Liu M, Woods SC, Tso P. Effect of intraperitoneal and intravenous administration of cholecystokinin-8 and apolipoprotein AIV on intestinal lymphatic CCK-8 and apo AIV concentration. Am J Physiol Regul Integr Comp Physiol. 2009;296:R43–R50. doi: 10.1152/ajpregu.90410.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu M, Maiorano N, Shen L, Pearson K, Tajima D, Zhang DM, Woods SC, Seeley RJ, Davidson WS, Tso P. Expression of biologically active rat apolipoprotein AIV in Escherichia coli. [accessed April 18, 2014];Physiol Behav. 2003 78:149–55. doi: 10.1016/s0031-9384(02)00959-9. http://www.ncbi.nlm.nih.gov/pubmed/12536022. [DOI] [PubMed] [Google Scholar]
- 9.Woods SC, Seeley RJ, Rushing PA, D’Alessio D, Tso P. A controlled high-fat diet induces an obese syndrome in rats. J Nutr. 2003;133:1081–1087. doi: 10.1093/jn/133.4.1081. [DOI] [PubMed] [Google Scholar]
- 10.Shen L, Tso P, Woods SC, Sakai RR, Davidson WS, Liu M. Hypothalamic Apolipoprotein A-IV Is Regulated by Leptin. Endocrinology. 2007;148:2681–2689. doi: 10.1210/en.2006-1596. [DOI] [PubMed] [Google Scholar]
- 11.Thon M, Hosoi T, Yoshii M, Ozawa K. Leptin induced GRP78 expression through the PI3K-mTOR pathway in neuronal cells. Sci Rep. 2014;4:7096. doi: 10.1038/srep07096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen L, Tso P, Woods SC, Clegg DJ, Barber KL, Carey K, Liu M. Brain apolipoprotein E: an important regulator of food intake in rats. Diabetes. 2008;57:2092–2098. doi: 10.2337/db08-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X, Garelick MG, Wang H, Li V, Athos J, Storm DR. PI3 kinase signaling is required for retrieval and extinction of contextual memory. Nat Neurosci. 2005;8:925–931. doi: 10.1038/nn1482. [DOI] [PubMed] [Google Scholar]
- 14.Gotoh K, Liu M, Benoit SC, Clegg DJ, Davidson WS, D’Alessio D, Seeley RJ, Tso P, Woods SC. Apolipoprotein A-IV interacts synergistically with melanocortins to reduce food intake. Am J Physiol Regul Integr Comp Physiol. 2006;290:R202–R207. doi: 10.1152/ajpregu.00502.2005. [DOI] [PubMed] [Google Scholar]
- 15.Shen L, Wang DQ, Lo CM, Tso P, Davidson WS, Woods SC, Liu M. Estradiol increases the anorectic effect of central apolipoprotein A-IV. Endocrinology. 2010;151:3163–3168. doi: 10.1210/en.2010-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paxinos G, Watson C. The rat brain in stereotaxic coordinates (the 4th version) Academic Press, Harcourt Brace & Company; New York: 1982. [Google Scholar]
- 17.Minokoshi Y, Haque MS, Shimazu T. Microinjection of leptin into the ventromedial hypothalamus increases glucose uptake in peripheral tissues in rats. [accessed April 12, 2014];Diabetes. 1999 48:287–91. doi: 10.2337/diabetes.48.2.287. http://www.ncbi.nlm.nih.gov/pubmed/10334303. [DOI] [PubMed] [Google Scholar]
- 18.He W, Li X, Adekunbi D, Liu Y, Long H, Wang L, Lyu Q, Kuang Y, O’Byrne KT. Hypothalamic effects of progesterone on regulation of the pulsatile and surge release of luteinising hormone in female rats. Sci Rep. 2017;7:8096. doi: 10.1038/s41598-017-08805-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu M, Shen L, Liu Y, Woods SC, Seeley RJ, D’Alessio D, Tso P. Obesity induced by a high-fat diet downregulates apolipoprotein A-IV gene expression in rat hypothalamus. Am J Physiol Endocrinol Metab. 2004;287:E366–E370. doi: 10.1152/ajpendo.00448.2003. [DOI] [PubMed] [Google Scholar]
- 20.Shen L, Pearson KJ, Xiong Y, Lo CM, Tso P, Woods SC, Davidson WS, Liu M. Characterization of apolipoprotein A-IV in brain areas involved in energy homeostasis. Physiol Behav. 2008;95:161–167. doi: 10.1016/j.physbeh.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saltiel AR, Pessin JE. Insulin signaling pathways in time and space. Trends Cell Biol. 2002;12:65–71. doi: 10.1016/s0962-8924(01)02207-3. [DOI] [PubMed] [Google Scholar]
- 22.Mirshamsi S, Laidlaw HA, Ning K, Anderson E, Burgess LA, Gray A, Sutherland C, Ashford ML. Leptin and insulin stimulation of signalling pathways in arcuate nucleus neurones: PI3K dependent actin reorganization and KATP channel activation. BMC Neurosci. 2004;5:54. doi: 10.1186/1471-2202-5-54. [DOI] [PMC free article] [PubMed] [Google Scholar]