Abstract
Human induced-pluripotent stem cells (iPSCs) are a promising resource for propagation of myogenic progenitors. Our group recently reported a unique protocol for the derivation of myogenic progenitors directly (without genetic modification) from human pluripotent cells using free-floating spherical culture. Here we expand our previous efforts and attempt to determine how differentiation duration, culture surface coatings, and nutrient supplements in the medium influence progenitor differentiation and formation of skeletal myotubes containing sarcomeric structures. A long differentiation period (over 6 weeks) promoted the differentiation of iPSC-derived myogenic progenitors and subsequent myotube formation. These iPSC-derived myotubes contained representative sarcomeric structures, consisting of organized myosin and actin filaments, and could spontaneously contract. We also found that a bioengineering approach using three-dimensional (3D) artificial muscle constructs could facilitate the formation of elongated myotubes. Lastly, we determined how culture surface coating matrices and different supplements would influence terminal differentiation. While both Matrigel and laminin coatings showed comparable effects on muscle differentiation, B27 serum-free supplement in the differentiation medium significantly enhanced myogenesis compared to horse serum. Our findings support the possibility to create an in vitro model of contractile sarcomeric myofibrils for disease modeling and drug screening to study neuromuscular diseases.
Keywords: Skeletal muscle, human pluripotent stem cells, induced pluripotent stem cells, muscle differentiation, sarcomere, neuromuscular diseases
1. Introduction
Pluripotent stem cells, such as embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), have received much attention because of their potential use for cell-based therapies and in vitro modeling (Hosoyama et al., 2012; Rinaldi and Perlingeiro, 2014; Roca et al., 2015). Human iPSCs, established from somatic cells, represent a valuable source of tissue for generating human myogenic progenitors (Tedesco and Cossu, 2012). Furthermore, these progenitors are able to form myotubes in culture, which can provide a useful platform for understanding normal muscle development and disease mechanisms in vitro (Hosoyama et al., 2012; Rinaldi and Perlingeiro, 2014; Roca et al., 2015).
In recent years, several protocols have been reported to propagate human myogenic progenitors from pluripotent cell sources and to differentiate these progenitors into the skeletal muscle cell lineage as myoblasts or myotubes (Zhu et al., 2014). While many protocols require cell sorting and/or rely on exogenous expression of myogenic genes such as PAX3, PAX7, and MYOD (Abujarour et al., 2014; Darabi et al., 2012; Maffioletti et al., 2015; Skoglund et al., 2014; Tanaka et al., 2013), more recent advances have been made with the application of small molecules and growth factors to directly promote myogenic differentiation from human iPSCs (Barberi et al., 2007; Borchin et al., 2013; Caron et al., 2016; Chal et al., 2016; Chal et al., 2015; Choi et al., 2016; Hosoyama et al., 2014; Hwang et al., 2013; Shelton et al., 2014; Xu et al., 2013). Our group recently reported a unique method for the derivation of myogenic progenitors from human pluripotent cells using free-floating spherical culture (Hosoyama et al., 2014). Human ESC and iPSC colonies were expanded in medium supplemented with high concentrations of fibroblast growth factor-2 (FGF-2) and epidermal growth factor (EGF). The cells then formed sphere aggregates named EZ spheres. We could confirm Pax7-positive (Pax7+) myogenic progenitors (approximately 40–60% of total cells) in EZ spheres, and myosin heavy chain-positive (MHC+) myotubes were identified following sphere dissociation and 2 weeks of terminal differentiation (Hosoyama et al., 2014). Importantly, our culture protocol is applicable to create myogenic progenitors and myotubes from human iPSCs generated from both healthy donors and patients with neuromuscular disorders (Hosoyama et al., 2014).
In the present study, we expand our previous efforts and attempt to create mature skeletal myotubes that contain organized sarcomeric structures from iPSCs. Sarcomere formation is critical for morphologically modeling the functional units of muscle contraction (Alter, 2004). The rationale of the present study is based on the previous observations using satellite cells and primary myoblasts, which showed that differentiation duration, culture surface coatings, and nutrient supplements in the medium can significantly influence muscle differentiation (Grefte et al., 2012; Hartley and Yablonka-Reuveni, 1990; Lawson and Purslow, 2000; Molnar et al., 2007). Here we determined the time course of muscle differentiation and sarcomere formation in EZ sphere-derived myogenic progenitors. We also used a bioengineering approach and tested three-dimensional (3D) cultures to create elongated and matured myotubes. Further, we evaluated the effects of different extracellular matrix coatings and serum-free or serum supplements for myotube differentiation.
2. Materials and Methods
2.1. Human induced pluripotent stem cells
A human iPSC line (IMR90) was used in this study and maintained following feeder-independent protocols (Ludwig et al., 2006). This iPSC line was obtained from WiCell (Madison, WI, USA) and was originally created from human fibroblasts transduced with lentivirus to overexpress OCT4, NANOG, SOX2, and LIN28 (Yu et al., 2007). iPSC colonies were cultured in mTeSR1 medium on a 6-well plate coated with Matrigel (BD Bioscience; San Jose, CA), and passaged using Versene (Life Technologies, Grans Island, NY, USA).
2.2. Differentiation of iPSCs to myotubes
Human iPSC-derived myogenic progenitors and myotubes were prepared using our protocol as recently described (Fig. 1) (Hosoyama et al., 2014). Briefly, iPSC colonies were lifted by 0.1% collagenase (Life Technologies) and transferred into expansion medium [Stemline medium (S-3194, Sigma-Aldrich, St. Louis, MO, USA) supplemented with 100 ng/ml recombinant human FGF-2 (WiCell), 100 ng/ml human EGF (Millipore, Billerica, MA, USA), 5 ng/ml heparin sulfate (Sigma-Aldrich), and penicillin/streptomycin/amphotericin B (PSA, 1%v/v; Life Technologies)]. After 1 week, the colonies formed spherical aggregates (EZ spheres). The spheres were passaged every week by mechanical chopping using a McIlwain tissue chopper (Mickle Laboratory Engineering, Surrey, UK).
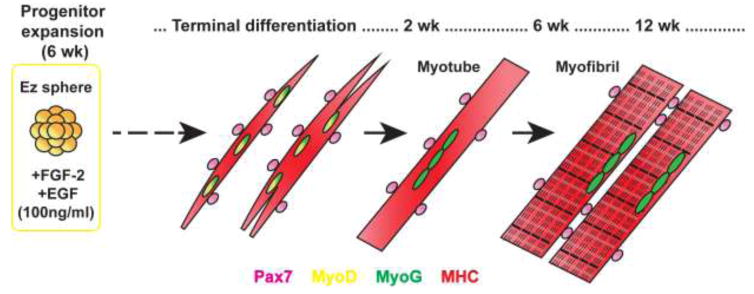
Figure 1. Muscle differentiation of iPSC-derived myogenic progenitors.
Human iPSCs were maintained as spherical aggregates (termed as EZ spheres) in suspension medium containing high concentration (100 ng/ml) of FGF-2 and EGF for 6 weeks. Myogenic progenitors in EZ spheres were plated on coverslips and terminally differentiated for 2–12 weeks. In this study, we determined the time course of muscle differentiation and sarcomere formation in EZ sphere-derived myogenic progenitors. Further, we evaluated the effects of different extracellular matrix coatings and serum-free or serum supplements for myotube differentiation. The stage of muscle cells was determined by the expression of Pax7 (myogenic progenitors), MyoD (myoblasts), Myogenin (MyoG; committed myocytes), and myosin heavy chain (MHC).
After 6 weeks, EZ spheres were dissociated using trypsin (TrypLE, Life Technologies), plated on coverslips coated with poly-L-ornithine (0.1 mg/ml) and laminin (50 μg/ml) (both Sigma-Aldrich) (Hosoyama et al., 2014). Alternatively, in order to determine the effect of Matrigel on myogenic differentiation, coverslips were coated with Matrigel (83.3 μg/ml; Corning Incorporated, Corning NY, USA) instead of poly-L-ornithine and laminin. Unless otherwise specified, the plated cells were then maintained in terminal differentiation medium [Dulbecco’s Modified Eagle’s Medium (DMEM, Sigma-Aldrich) containing 2% B27 supplement (Life Technologies) and 1% PSA] for differentiation into myotubes. To evaluate the effect of horse serum on myogenic differentiation, 2% horse serum (Life Technologies) was used instead of B27 supplement.
2.3. Immunocytochemistry
Immunocytochemistry was performed as described previously (Hosoyama et al., 2014). Briefly, cells were fixed with 4% paraformaldehyde (PFA), permeabilized in 0.2% Triton-X 100, and blocked with 5% normal donkey serum in phosphate-buffered saline (PBS). The fixed cells were stained with primary antibodies against Pax3, Pax7, MyoD, or Myogenin (MyoG). For labeling myosin heavy chain (MHC), MYH isoforms, and titin, cells were fixed in ice-cold methanol. To identify co-localization of multiple markers for myogenic cells, rabbit polyclonal anti-MyoG antibody was co-labeled with mouse monoclonal antibodies against Pax7. After incubating with primary antibody overnight, the cells were stained with secondary antibody conjugated to Alexa Fluor 488 or Cy3 (anti-IgG, 1:1,000, Jackson Immunotech Research Laboratories, West Grove, PA, USA). To identify the expression of Pax3, Pax7, and MyoG in myotubes, anti-MHC antibodies directly conjugated with Alexa Fluor 488 or Alexa Fluor 660 were additionally used. The details of the primary antibodies used in this study are described in Table 1. Hoechst 33258 (0.5 μg/ml in PBS, Sigma-Aldrich) was used to label cell nuclei. The fluorescence images were captured at 20x objective using a Nikon Eclipse 80i fluorescence microscope with a DS-QilMC CCD camera (Nikon, Tokyo, Japan) or Leica TCS SP8 confocal microscope (Leica, St. Gallen, Switzerland). For each staining, we prepared technical replicates (2–3 coverslips), and the positive cells were counted from 6 randomly selected fields per coverslip. We repeated three independent experiments for cell counting.
Table 1.
Primary antibodies used for muscle cell identification.
| Antibody | Clone | Company | Dilution |
|---|---|---|---|
| Pax7 | Pax7 | DSHB (Iowa City, IA, USA) | 1:40 |
| Pax3 | Pax3 | DSHB (Iowa City, IA, USA) | 1:40 |
| MyoD | 5.8A | Vector laboratories (Burlingame, CA, USA) | 1:40 |
| MyoG (mouse monoclonal) | F5D | DSHB | 1:40 |
| MyoG (rabbit polyclonal) | M-225 | Santa Cruz (Dallas, TX, USA) | 1:400 |
| MHC | MF20 | DSHB | 1:40 |
| MHC (Alexa Fluor 480 or Alexa Fluor 660 conjugated) | MF20 | eBioscience (San Diego, CA, USA) | 1:500 |
| MYH3 (embryonic MHC) | F1.652 | DSHB | 1:40 |
| MYH8 (fetal/perinatal MHC) | N3.36 | DSHB | 1:40 |
| MYH2 (adult MHC) | A4.74 | DSHB | 1:40 |
| Titin | 9D10 | DSHB | 1:40 |
| Laminin | L9393 | Sigma-Aldrich | 1:100 |
MyoG, Myogenin; DSHB, Developmental Study Hybridoma Bank.
2.4. Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted and purified from cell lysates using an RNeasy kit (Qiagen, Germantown, MD, USA). RT-PCR was run using RT reaction mix and PCR master mix (Promega, Madison, WI, USA) in a thermal cycler (Eppendorf, Hamburg, Germany). The same primers for PAX3, PAX7, MYF5, MYOD, MYOGENIN, or β-ACTIN (loading control) were used as described in our recent study (Hosoyama et al., 2014). We newly designed the primers for myosin heavy chain 3 (MYH3; forward 5′-GTGGTGGACTCAAAGGAAGAA-3′, reverse 5′-AGAAGAGGCCTGAGTAGGTATAG-3′), myosin heavy chain 8 (MYH8; forward 5′-GGAGAAGAGTGAGCTGAAGATG-3′, reverse 5′-TGGCATCCGTCTCGTATTTG-3′), myosin heavy chain 2 (MYH2; 5′-CTGCTGAAGGAGAGGGAGCT-3′, 5′-TGATTAGCTGGTCACACCTT-3′) (Shi et al., 2004), which can identify embryonic, fetal/perinatal, and adult forms of MHC, respectively (Osterlund et al., 2012). The gel images were captured by a UVP BioSpectrum Imaging System (UVP, Upland, CA, USA).
2.5. Electron microscopy
The differentiated myotubes or 3D muscle constructs were fixed in 2% PFA and 2.5% glutaraldehyde in 0.1 M cacodylate buffer for 1 hour, and stored in 0.1 M cacodylate buffer overnight at 4°C. The cells were then post-fixed in 1% osmium tetroxide for 1 hour. After multiple rinses with 0.1 M cacodylate buffer and deionized water, fixed cells were dehydrated through an ethanol gradient, embedded in Durcapan (Sigma-Aldrich), sectioned at 60 nm thickness, and stained with lead citrate and uranium acetate. A Philips CM120 transmission electron microscope (Eimdhoven, The Netherlands) was used for captured electron microscopic images.
2.6. Skeletal muscle video capture
Plated cells from dissociated EZ spheres were monitored every 3 days under microscope until cells started contraction. The contraction of myotubes was captured using a 20x objective on a Nikon Eclipse TS 100 inverted microscope with a QImaging camera (Surrey, BC, Canada). Two hundred static frames were captured using 100 millisecond exposure time and 1 millisecond interval. Video was recorded as AVI files by converting sequence images with Q capture Pro Software (QImaging). Contractions were quantified manually by counting the number of contractions within the time period of the movie clip, and using the length of the movie clip to calculate how many beats per minute. Two different counts were obtained. One count was a total of individual myotube contractions occurring within the field of view. Another count was for coordinated contractions involving an area that appeared to consist of multiple merged myotubes.
2.7. Preparation of three-dimensional (3D) muscle constructs
3D muscle constructs were prepared with sphere-derived myogenic progenitors by the modified protocol as outlined in a previous paper (van der Schaft et al., 2013). Briefly, +/− 4 mm Velcro strips (also known as hook-and-loop fasteners or touch fasteners, only using the loop side; Velcro USA, Manchester, NH, USA) were cut to resemble an irregular pentagon with two parallel sides (house-like or home plate shaped). Two Velcro pentagons were cemented opposite one another into wells of 6-well culture plates at a space of 10 mm between points (see Fig. 6A for example). EZ spheres were trypsinized as described above, and the dissociated cells (1,250,000 cells per construct) were carefully suspended in a Matrigel/collagen matrix on ice. The matrix (150 μl per construct) consisted of 1.6 mg/ml collagen (rat tail collagen type I, BD Bioscience), 1.8 μg/ml Matrigel and 0.5 M sodium hydroxide (5 μl). The cell-suspended matrix was gently mixed with the terminal differentiation medium (see above; 100 μl per construct) and then strung between Velcro cutouts. After 2–3 weeks, the cell/matrix formed a small fibrous structure. The muscle constructs were maintained in a terminal differentiation medium for 6–12 weeks (van der Schaft et al., 2013).
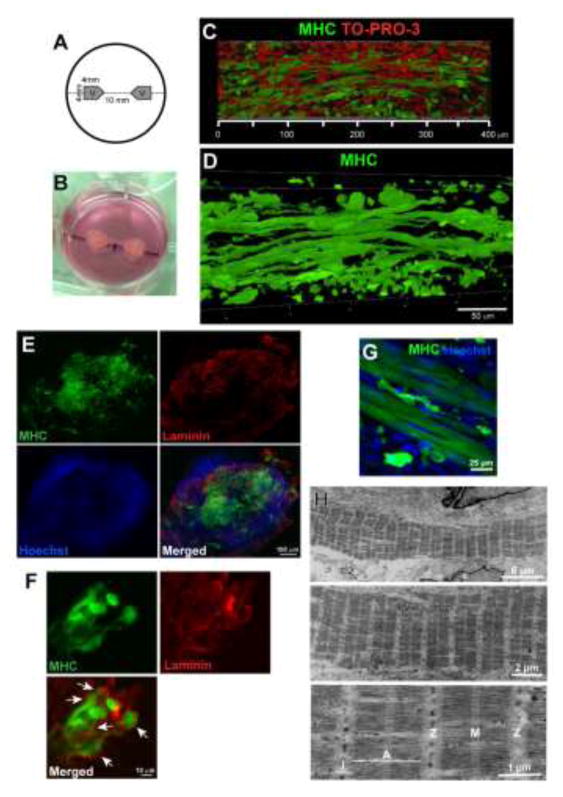
Figure 6. Human pluripotent stem cells can be derived into elongated myotubes in 3D culture.
(A) Diagram of 3D muscle construct setup. (B) Representative 3D construct. (C and D) 3D rendering images of MHC+ myotubes grown in the constructs. (E) Immunostaining of laminin and MHC in the transverse section of the muscle construct. (F) Laminin-positive basal lamina determined the boundaries of the individual fibers (white arrows). (G) Striated patterns were visible in the differentiated myotubes. After 12 weeks of differentiation, iPSC-derived myotubes were labeled with MHC+ in the 3D muscle construct. (H) Ultrastructures of myotubes differentiated in the 3D muscle construct for 6 weeks. Specific sarcomare structures (I-band, A-band, M-line, and Z-lines) were labeled.
2.8. Immunohistochemistry for 3D muscle constructs
The muscle construct was collected, transferred into a microcentrifuge tube, and fixed with 4% PFA for 20 minutes. For whole mount immunohistochemistry, the fixed construct was washed in PBS twice and then placed in a blocking solution (5% NGS, 0.1% Triton-X 100 in PBS) at room temperature for 2 hours. The construct was incubated with anti-MHC antibodies conjugated with Alexa Fluor 488 (1:40, clone MF20, Affymetrix Inc., Santa Clara, CA, USA) at 4°C overnight. After washing with PBS, TO-PRO-3 Iodide (1μM in PBS, Life Technologies) or Hoechst 33258 (0.5 μg/ml in PBS, Sigma-Aldrich) was applied to identify cell nuclei. The stained construct was transferred to a glass slide and mounted under a coverslip in Fluoromount-G (SouthernBiotech, Birmingham, AL, USA). For preparing transverse sections, the fixed construct was washed in PBS twice, transversely chopped by a McIlwain tissue chopper at 150 μm thickness, and expanded on the slide glass. Double labeling of MHC and laminin was then performed using these sections as described in Section 2.3, to identify the laminar layers of individual myotubes. The fluorescence signals were detected by Leica TCS SP8 confocal microscope and Nikon Eclipse 80i fluorescence microscope.
2.9. Statistical Analysis
The analyses of the cell counting were from triplicated experiments and presented as means ± SEM. Unpaired two-tailed Student’s t-test analyses were performed using the GraphPad Prism software (La Jolla, CA) to compare two groups: 2 weeks vs 6 weeks, B27 vs horse serum, and laminin vs Matrigel. Differences were considered significant when p<0.05.
3. Results
3.1. Long-term culture supports the differentiation of myogenic progenitors into myotubes
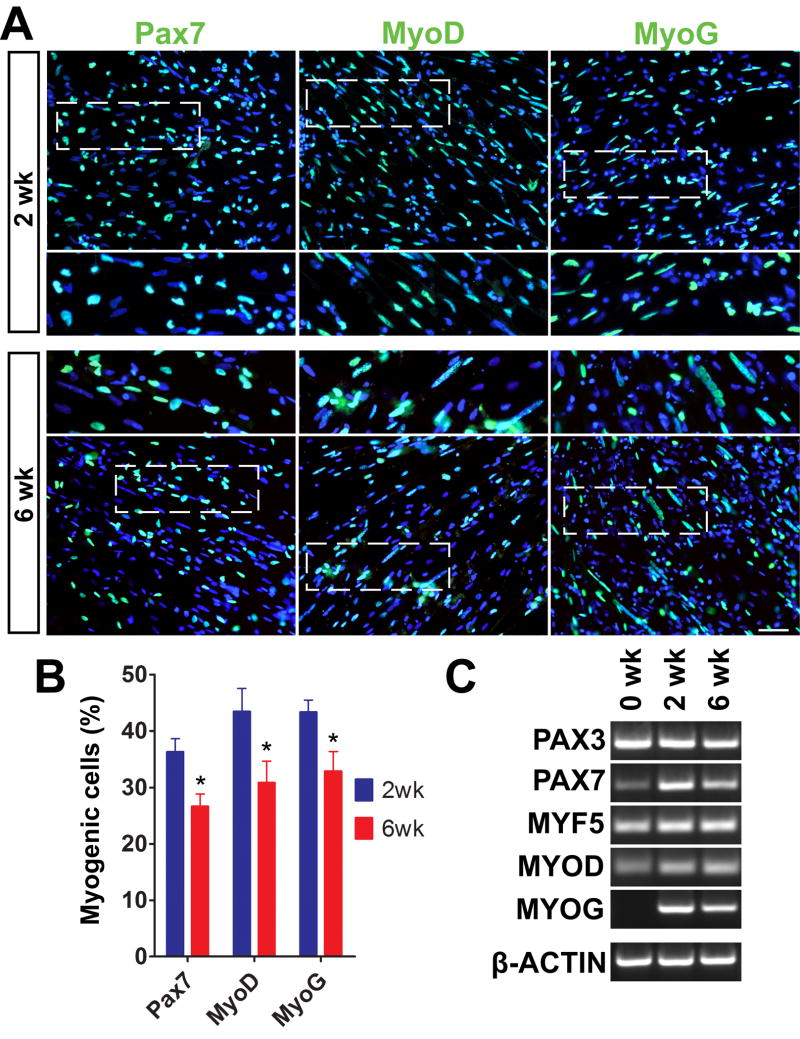
We had previously reported that myogenic progenitors cultured as EZ spheres can successfully form MHC+ myotubes when dissociated, plated down, and cultured in terminal differentiation medium for 2 weeks (Hosoyama et al., 2014). In the present study, we first determined how a longer duration of culture would influence the differentiation of myogenic progenitors. iPSC-derived EZ spheres were dissociated using trypsin, plated on coverslips, and maintained in the terminal differentiation medium for either 2 or 6 weeks. The cells were fixed and immunostained for Pax7 (muscle progenitors), MyoD (myoblasts), and Myogenin (MyoG; committed myocytes) (Fig. 2A). We observed a considerable number of Pax7+ (36.3 ± 2.2%), MyoD+ (43.4 ± 4.0%), and MyoG+ (43.3 ± 2.1%) cells following 2 weeks of terminal differentiation (Fig. 2B). After 6 weeks, the percentages of Pax7+ (26.6 ± 2.1%), MyoD+ (30.8 ± 3.7%), and MyoG+ (32.8 ± 3.4%) cells were significantly decreased compared to 2 weeks (p<0.05; Fig. 2B). Notably, whereas Pax7+ cells were single-nucleated without fusion, MyoD+ and MyoG+ nuclei were elongated and lined up (Fig. 2A, insets). Such nuclear morphology was similarly observed in human primary myoblasts (Serena et al., 2016). Further, we performed RT-PCR to determine relative mRNA levels of myogenic genes in the differentiated cells (Fig. 2C). Pax7, MyoD, and MyoG mRNAs were increased after 2 weeks of terminal differentiation. The expression of these mRNAs was then decreased at 6 weeks of differentiation, supporting our immunocytochemical observations (Fig. 2A and B). On the other hand, Pax3 mRNA was consistently detected during terminal differentiation (Fig. 2C). We also performed immunocytochemistry using anti-Pax3 antibodies and found that Pax3+ cells were identified in the differentiated cells at both 2 and 6 weeks (Supplementary Fig. 1).
Figure 2. Longer differentiation allows the generation of multinucleated myotubes in iPSC-derived myogenic progenitors.
(A) Representative images of immunocytochemistry labeled myogenic cell markers. The plated cells lined up as nascent myotubes after 2 weeks of terminal differentiation and then formed multinucleated myotubes at 6 weeks. Scale bar = 50 μm. (B) Approximately 30–40% of total nuclei expressed Pax7+, MyoD+ and MyoG+ at 2 weeks, but these numbers were significantly reduced at 6 weeks (*p<0.05 vs. 2 weeks) toward myotube maturation. (C) Muscle gene expression on RT-PCR was characterized from mRNA isolation of 0, 2, and 6 weeks differentiated cells.
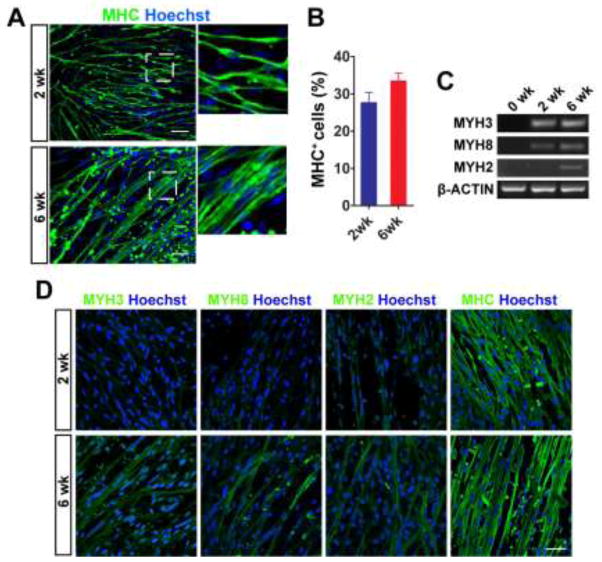
We next asked whether longer-term differentiation enhanced fusion of myoblasts and formation of multinucleated myotubes. The plated cells were maintained up to 6 weeks and immunolabeled with MHC to identify skeletal myotubes. MHC+ myotubes at 6 weeks seemed to be more integrated with adjacent cells and formed wider fibers, compared to the myotubes at 2 weeks (Fig. 3A). We also counted MHC+ cells and found that the number of MHC+ cells tended to be increased at 6 weeks of differentiation (32.7 ± 1.0% vs. 26.7 ± 2.8% at 2 weeks), although the difference was not significant (Fig. 3B). To identify the process of MHC maturation in culture, we performed RT-PCR using specific primers for embryonic (MYH3), fetal/perinatal (MYH8), and adult isoform of MHC (MYH2) (Fig. 3C). The expression of these MHC isoforms was not detectable in pre-differentiated cells (0 week). MYH3 gene expression was increased after 2 weeks of differentiation, and the level of mRNA was sustained at 6 weeks. Fetal/perinatal (MYH8) and adult MHC (MYH2) were detectable at 6 weeks, although their expression was quite faint. Next, we performed immunocytochemistry to test specific antibodies against MYH3 (clone F1.652), MYH8 (N3.36), and MYH2 (A4.74) for differentiated myotubes. MYH3, MYH8, and MYH2 proteins could be identified in the differentiated myotubes when using the specific antibody clone (Fig. 3D). The expression of MYH3, MYH8, and MYH2 proteins was weakly detectable in the culture of 2 weeks of differentiation and then much more obvious at 6 weeks. Our semi-quantitative analyses using RT-PCR and immunocytochemistry suggested that the levels of MYH mRNA and protein expression (specifi cally MYH3) were not fully correlated. Translation of the MYH mRNA might not actively begin until after 2 weeks of differentiation.
Figure 3. Longer differentiation supports more mature phenotype in myotubes.
(A) Representative image of MHC+ myotubes. From 2 to 6 weeks, single nucleated nascent myotubes migrated and fused to form larger multinucleated MHC+ myotubes. Scale bar = 50 μm. (B) The number of MHC+ cells tended to be increased at 6 weeks, although the difference did not reach statistically significance. (C) RT-PCR analysis of MYH genes in differentiated cells. Specific primers were used to detect the gene expression of embryonic (MYH3), fetal/perinatal (MYH8), and adult form of MHC (MYH2). (D) Immunocytochemistry of MYH3, MYH8, MYH2, and MHC (with MF20 antibody clone) in differentiated myotubes. Scale bar = 50 μm.
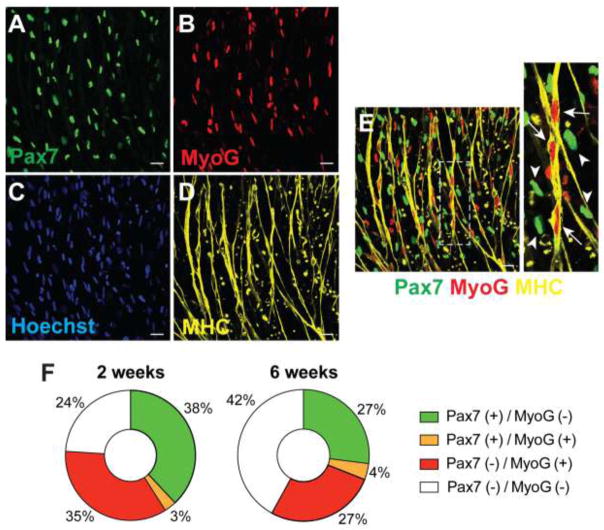
Immunostaining for an individual muscle cell marker could identify a specific population of differentiated cells expressing Pax7, MyoD, MyoG, or MHC. To further characterize the stage of muscle cells following differentiation, we performed triple staining with Pax7, MyoG, and MHC and determined how these markers were overlapped (Fig. 4). Most of the nuclei in MHC+ myotubes were elongated with Pax7−/MyoG+ (Fig. 4E, arrows). In contrast, Pax7+/MyoG− cells had single and oval-shaped nuclei and were not overlapped with myotubes (Fig. 4E, arrow heads). Approximately 38% of total cells were Pax7+/MyoG− in the differentiated cells at 2 weeks, while 35% of total cells were Pax7−/MyoG+ (Fig. 4F). Although both Pax7+/MyoG− and Pax7−/MyoG+ cells were decreased to 27% at 6 weeks of differentiation, Pax7+ cells were still identified even after the longer-term differentiation up to 12 weeks (data not shown).
Figure 4. Co-localization of myogenic proteins in iPSC-derived myotubes.
(A–E) Multiple staining with Pax7 (A), MyoG (B), Hoechst (C), and MHC (D) to identify how these markers were overlapped. (E) Merged images from A, B, and D. Most of the nuclei in MHC+ myotubes were elongated with Pax7−/MyoG+ (arrows), while Pax7+/MyoG− cells were not overlapped with myotubes (arrow heads). Scale bar = 25 μm. (F) The percentage of Pax7 and MyoG expressing cells in differentiated cells.
3.2. Long-term differentiation can give rise to striated and spontaneously contracting myotubes
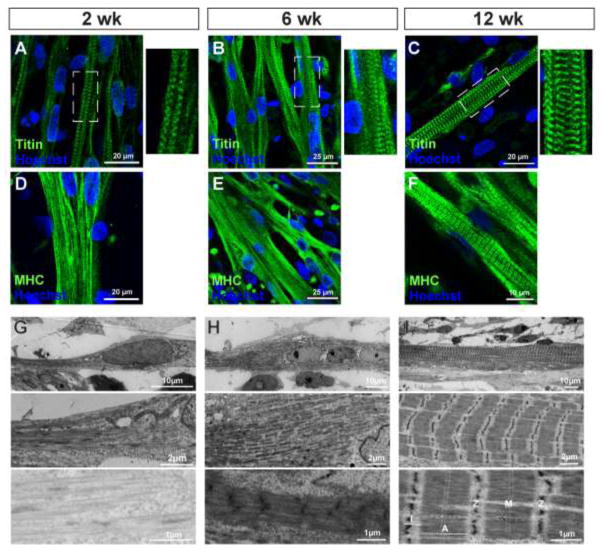
We analyzed the process of sarcomere formation in the differentiated myotubes. We first performed immunostaining with titin, a sarcomeric component protein that spans the half-sarcomere and links the Z-disk and M-line (Lin et al., 2017). This protein has been used to identify the level of muscle maturation in iPSC-derived muscle cells (Chal et al., 2016; Choi et al., 2016). Titin-positive striated patterns were already identified at 2 weeks of terminal differentiation (Fig. 5A). These patterns were clearly detectable in the myotubes at 6 and 12 weeks (Fig. 5B and C). When using MHC staining for the same cell preparations, the striations were less apparent at 2 and 6 weeks (Fig. 5D and E) but became more obvious at 12 weeks (Fig. 5F).
Figure 5. Sarcomere formation in iPSC-derived myotubes.
(A–C) Immunolabeling with a sarcomeric filament titin. Titin staining revealed that striated patterns were already identified at 2 weeks of terminal differentiation (A). These patterns were clearly visible in the myotubes at 6 (B) and 12 weeks (C). (D–F) MHC staining in the same cell preparations used for titin labeling. (G–I) Ultrastructures of iPSC-derived myofibrils. After 2 weeks of differentiation, spindle-shaped myotubes contained un-defined filaments with no sarcomere structure (G). After 6 weeks of differentiation, thick filaments assembled with a nascent Z line but no M-line (H). After 12 weeks of differentiation, mature sarcomeres were observed assembled into myofibrils (I). Morphological hallmarks, including I-band of actin filaments and A-band with distinct M-line across myosin filaments, were clearly visible. Sarcomere Z lines appeared to be reasonably aligned and gave rise to a striated pattern.
We next characterized the ultrastructural morphology of the differentiated myotubes using electron microscopy. Striated patterns were not obvious in the cells at 2 weeks of differentiation (Fig. 5G), although spindle-shaped myotubes were identified following fluorescent immunocytochemistry (Fig. 3A). Specific features of sarcomeric organization could be identified in the myotubes after 6 weeks of differentiation. However, these features seemed to be in the process of maturation. Nascent Z lines were clearly observed in the myotubes; however, thick myosin and thin actin filaments still could not be distinguished (Fig. 5H). At 12 weeks of terminal differentiation, the myotubes contained well-organized sarcomeric structures located between Z lines and consistent with myosin and actin organization (Fig. 5H) (Alter, 2004). These myofibrils had specific structures, such as A bands consisting of myosin filaments, I bands representing actin filaments, and Z lines. Further, M lines, a hallmark of sarcomeric maturation (Yang et al., 2014), were identified across A bands (Fig. 5I).
Similarly as seen in primary myotube cultures (Liao et al., 2008; Manabe et al., 2012), spontaneous contractions were observed in our human iPSC-derived myotubes (Hosoyama et al., 2014). In this study, we determined how the occurrence of spontaneous contractions was changed in iPSC-derived myotubes following long-term culture. The differentiating myotubes were regularly monitored under light microscopy every 2–3 days immediately after feeding. We could identify the initiation of spontaneous contractions at approximately 5 weeks of differentiation (Supplementary online video 1A). Frequent contractions were observed in the culture between 6 weeks (Supplementary online video 1B) and 13 weeks. Sporadic contractions were still identified in some areas of the plated cells after 14 weeks of differentiation (Supplementary online video 1C), but their occurrence and frequency were gradually attenuated.
We manually counted the number of contractions within the time period of the movie clips. Two different types of contraction were evaluated: 1) the sum of individual contractions from single (non-fused) myotubes, and 2) coordinated contractions observed in an area consisting of multiple fused myotubes. The individual myotube contractions were the first to appear around 4 weeks then peaked at 13 weeks (135 contractions/min). The individual contractions were then decreased dramatically at 14 weeks (3 contractions/min) and diminished at week 15, as the myotube cultures matured and became more synchronized. The coordinated contractions were first confirmed at 6 weeks (5 contractions/min) and maximized at 13 weeks (45 contractions/min).
3.3. 3D culture supports muscle differentiation to form elongated myotubes
While we have commonly used two-dimensional culture to differentiate muscle progenitors into myotubes, we hypothesized that 3D culture platforms would further facilitate terminal differentiation and formation by more closely representing the in vivo environment. To test this idea, 3D muscle constructs were prepared with sphere-derived myogenic progenitors cultured on Velcro anchors (van der Schaft et al., 2013). The dissociated cells from EZ spheres were mixed with collagen/Matrigel and plated between two small pentagonal cutouts of Velcro on a 6-well-cuture plate (Fig. 6A). These Velcro pieces acted as anchoring points for the fibrous structure formed by the cell/matrix mixture after 12 weeks (Fig. 6B). Additional analyses by confocal microscopy indicated that these elongated myotubes had over a 400 μm length (Fig. 6C and D; Supplementary online video 2). Next, we determined whether the myotubes had their own basal lamina. We prepared transverse sections of the 3D constructs and immunolabeled with laminin and MHC. We found that the expression of laminin was associated with the area with MHC+ myotubes (Fig. 6E). High-magnification imaging revealed that laminin-positive basal lamina surrounded individual fibers (Fig. 6F). We also analyzed sarcomere formation in the myotubes found in the 3D constructs. Whole-mount immunohistochemistry revealed that the constructs contained well-striated MHC+ myotubes at 12 weeks of differentiation (Fig. 6G). Ultrastructural hallmarks of sarcomere organization were identified in the myotubes at 6 weeks of differentiation (Fig. 6H), although these features were still immature. Striated patterns were more visible and aligned, when compared to the myotubes differentiated on the coverslip at 6 weeks (Fig. 5H).
3.4. Matrigel coating shows comparable effects on myogenic differentiation to laminin coating
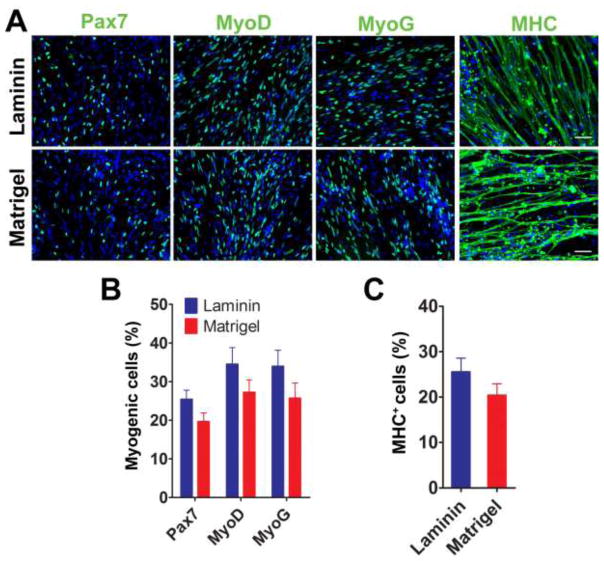
Basement membrane matrix has been used to promote cell differentiation in vitro (Kleinman et al., 1987). We hypothesized that different coatings of extracellular matrix could improve the efficiency and speed of muscle differentiation and maturation of human iPSC-derived myogenic progenitors. While our group has primarily used laminin-coated coverslips to plate myogenic progenitors for terminal differentiation (Hosoyama et al., 2014), other studies have claimed that Matrigel coating promoted myogenic differentiation of satellite cells, primary myoblasts, and human iPSC-derived myogenic progenitors (Chal et al., 2015; Grefte et al., 2012; Hartley and Yablonka-Reuveni, 1990; Shelton et al., 2014). We hypothesized that Matrigel would improve the efficiency of muscle differentiation and maturation of iPSC-derived myogenic progenitors. To test this hypothesis, EZ spheres were dissociated and plated on coverslips pre-coated with either laminin or Matrigel. After 2 weeks of terminal differentiation, we found a significant number of Pax7+, MyoD+, and MyoG+ nuclei, and MHC+ myotubes on both laminin- and Matrigel-coated coverslips (Fig. 7A). The number of Pax7+ nuclei tended to be higher when using laminin (25.4 ± 2.2%) compared to Matrigel (19.6 ± 2.2%). The numbers of MyoD+ (34.5 ± 4.3% with laminin vs. 27.2 ± 3.2% with Matrigel) and MyoG+ (34.0 ± 4.1% vs. 25.7 ± 3.9%) nuclei also showed a similar trend but were not statistically significant (Fig. 7B). There was no significant difference in the number of MHC+ myotubes between the coatings (25.5 ± 3.0% with laminin vs. 20.3 ± 2.5% with Matrigel; Fig. 7C). Similar to the cells plated on laminin, we were also able to maintain the cells plated on Matrigel for up to 12 weeks and observe sarcomeric striations in the MHC+ myotubes (data not shown).
Figure 7. Laminin coating induces similar number of myotubes compared to Matrigel coating.
(A) Representative images of immunostaining at 2 weeks differentiation demonstrated that both laminin and Matrigel coating resulted in myotube formation. Pax7+, MyoD+, MyoG+, and MHC+ cells were identified from each coating. Scale bar = 50 μm. There was no significant difference in the number of myogenic cells (B) and MHC+ myotubes (C) between laminin and Matrigel coating.
3.5. Serum-free B27 supplement enhances myogenic differentiation compared with horse serum
We have used serum-free supplement (2% B27 supplement) for inducing terminal differentiation of myogenic progenitors to form myotubes. In contrast, one previous study reported that horse serum could enhance myotube fusion better than a serum-free supplement (Lawson and Purslow, 2000). To compare the effects of B27 supplement and horse serum on myotube formation, EZ spheres were dissociated, plated on laminin-coated coverslips, and maintained in the medium supplemented with either B27 supplement or horse serum (Fig. 8A). After 2 weeks of terminal differentiation, more Pax7+ and MyoG+ nuclei were identified in the B27-supplemented culture compared to horse serum (23.2 ± 2.7% vs. 8.8 ± 3.2% in Pax7+; 42.3 ± 2.8% vs. 13.5 ± 1.9% in MyoG+; Fig. 8B). Further, there were more MHC+ cells present in the cultures supplemented with B27 compared to horse serum (35.4 ± 3.6% vs. 6.3 ± 0.5%; p<0.0001; Fig. 8C). Together, we concluded that serum-free B27 supplement sufficiently enhanced myogenic differentiation in human iPSC-derived myogenic progenitors.
Figure 8. Serum-free supplement (B27) drives more myotube formation than horse serum (HS) supplement.
(A) Immunolabeling of myogenic markers revealed that myotubes could be formed using either serum-free supplement or horse serum. Scale bar = 50 μm. (B) At 2 weeks of differentiation, Pax7+ progenitors and MyoG+ myocytes were significantly more in B27 than horse serum. *p<0.05. (C) B27 showed a significantly higher number of MHC+ cells compared to horse serum. ***p<0.0001.
4. Discussion
Human iPSC-derived myogenic progenitors are attractive tools in muscle biology, because these cells allow us to analyze myotube formation, sarcomeric assembly, and functional contraction in vitro. In the present study, we tested different culture conditions to refine our differentiation protocol for myotube formation and maturation in iPSC-derived myogenic progenitors and myoblasts. It was recently demonstrated that human myogenic progenitors prepared by inducible expression of PAX7 transcription factors could be differentiated into mature myotubes with physiological and ultrastructural features (Skoglund et al., 2014). Here we confirmed the presence of contractile sarcomeric myofibrils in iPSC-derived myogenic progenitors directly prepared under defined culture condition without exogenous genetic modification. In this study, we also focused on the time course change of myotube maturation and sarcomere formation following terminal differentiation of iPSC-derived myogenic progenitors. While several other studies have already confirmed the existence of sarcomere structures in iPSC-derived myocytes (Choi et al., 2016; Skoglund et al., 2014), the process of sarcomere formation throughout the differentiation period has not been fully analyzed.
The duration of the terminal differentiation step significantly influenced myogenic differentiation in iPSC-derived progenitors. Our previous results demonstrated that iPSC-derived myogenic progenitors could consistently differentiate into MHC+ myotubes after being plated on laminin-coated coverslips and maintained for 2 weeks in terminal differentiation medium containing B27 serum-free supplement (Hosoyama et al., 2014). Without genetic modification, our model demonstrated the maturation process of myotube formation. In this study, we found that longer-term differentiation of over 6 weeks significantly reduced the number of immature Pax7+ myogenic progenitors and MyoD+/MyoG+ myoblasts but increased the number of multi-nucleated myotubes with more integrated and wider fiber morphology, compared to the myotubes at 2 weeks. Myogenic cells gradually develop with a typical transition from randomly organized myofilament networks to striated myofibril patterns (Osterlund et al., 2012). In our study, electron microscopy revealed that at least 6 weeks were required for the myotubes to exhibit the ultrastructural features of sarcomere and myofibril assembly. This indicates that a specific period (at least 6 weeks) is required for myotube formation in iPSC-derived myocytes. As an additional note, our titin staining showed striated patterns in the myotubes at 2 weeks. In contrast, the level of sarcomere formation was not obvious in these myotubes when analyzing by electron microscopy. Although this immunostaining has been used to characterize maturity in iPSC-derived myotubes (Chal et al., 2016; Choi et al., 2016), our results suggest that titin organization may precede actin and myosin organization into sarcomere structures and that titin staining alone may not be a sufficient definition of myotube maturation.
Further, the triple staining of Pax7, MyoG, and MHC proteins revealed that many nuclei in MHC+ myotubes still retained MyoG expression even after 6 weeks of differentiation. Although specific sarcomeric structures were observed in differentiated myotubes in the present study using electron microscopy, completely organized structures characterized in fully matured muscle fibers could not be identified. RT-PCR and immunocytochemistry for different MHC forms indicated that embryonic (MYH3) and fetal/perinatal MHC (MYH8) are still detectable even after 6 weeks of differentiation. Together, the present results suggest that culture conditions need to be further improved to achieve fully matured myotubes like the myofibrils in adult muscles in vivo. While our protocol requires over 12 weeks (6 weeks of sphere and over 6 weeks of terminal differentiation) for myofibril maturation, other protocols have proposed different durations of myotube derivation. A recent publication reported that myotubes with sarcomeres could be identified after 30 days when using a differentiation method that involves a GSK-3β inhibitor (CHIR99021) and a γ-secretase inhibitor (DAPT) (Choi et al., 2016). Another study reported that robust myogenesis could be identified within 1 month by the dual modulation of Wnt and bone morphogenetic protein (BMP) pathways (Chal et al., 2016; Chal et al., 2015). To optimize the best culture condition for myotube maturation with well-organized myofibrils, it would be worth comparing the myotubes prepared by these differentiation protocols with regards to the level of myotube maturation and sarcomeric organization. Additionally, most Pax7+ nuclei were not located in myotubes, but these positive cells were distributed adjacent to myotubes. These Pax7+ cells may represent satellite-like cells that were identified in the recent method of myogenic differentiation of human iPSC (Chal et al., 2016; Chal et al., 2015). In this study, we also found that Pax3+ cells remained even after 6 weeks of differentiation. This did not seem to be fully reflective of the progression of myogenic differentiation in vivo, but one possible explanation is that these Pax3+ cells may represent non-myogenic cells like neuronal progenitors and other mesodermal progenitors (Hosoyama et al., 2014). Further studies are necessary to answer what type of cells still express Pax7 and/or Pax3, and whether Pax7+ cells represent satellite cells or myoblasts and exhibit regenerative properties.
In this study, we confirmed that myogenic progenitors from our EZ sphere culture could form differentiated, elongated, and multinucleated myotubes in 3D muscle-like constructs prepared between Velcro anchors. Our results support an idea that 3D muscle constructs are potentially applicable to create functional skeletal muscle in vitro and to evaluate anatomical and physiological properties of differentiated myotubes (Chal et al., 2016). Although the sphere culture protocol is efficient for deriving a relatively high number of myogenic progenitors, not all of the resulting cells differentiate into myoblasts/myotubes (Hosoyama et al., 2014). Further studies are necessary to improve the efficiency of progenitor derivation from iPSCs; for example, improvements can be made by preparing the muscle constructs with pure populations of myogenic progenitors. It is anticipated that with increased myotube differentiation efficiency, 3D constructs will demonstrate detectable contractile force with stimulation (Madden et al., 2015).
We also tested the effects of an alternate coating and culture supplement on myogenic differentiation and myotube formation. Both Matrigel and laminin showed comparable effects on muscle differentiation in the present study. These coatings have been known to function as chemical cues supporting muscle development. Matrigel was originally extracted from the Engelbreth-Holm-Swarm mouse sarcoma and contains Collagen IV, laminin, proteoglycan and other undefined extracellular matrix components (Hughes et al., 2010). Laminin is a fibrous protein present in the basal lamina of the epithelia and has been shown to increase adhesion, migration (Pawlikowski et al., 2009), formation, and proliferation of skeletal myocytes (Foster et al., 1987; Kuhl et al., 1982; von der Mark and Ocalan, 1989). Interestingly, laminin is also a major component of basal lamina at the neuromuscular junction. This specificity of laminin expression at the neuromuscular junction may explain why laminin coating showed trends toward better efficiency of myogenic differentiation from myogenic progenitors compared to Matrigel. It has not been fully determined how these extracellular substrates enhance muscle differentiation in culture. However, one previous study showed that in porcine muscle stem cells, Matrigel or laminin significantly increased the expression of myogenic genes and enhanced myogenesis by activating intracellular signaling such as integrin and Wnt signaling pathways (Wilschut et al., 2010). Although we only compared two coatings in this study, other types of extracellular matrix-derived coatings might provide greater effects on differentiation suitable for specific cell lineage. For instance, vitronectin could potentially enhance myotube formation. When mouse myoblast C2C12 cells were maintained on a vitronectin basement, myotube formation was initiated earlier at 3 days with vitronectin compared to 7 days with laminin (Molnar et al., 2007). Exploring additional matrices will be important for improving efficiency of myogenic progenitors and well-differentiated myotubes.
Our study also showed that B27 serum-free supplement enhanced myogenesis compared to horse serum. Our findings agree with a previous study demonstrating that a serum-free medium (Ultroser-G supplemented IGF-I) could enhance differentiation in C2C12 murine myoblasts when compared to horse serum (Gawlitta et al., 2008). Another study showed that a stronger active tension was generated in differentiated C2C12 myoblasts cultured under serum-free condition (AIM-V and Ultroser-G) compared to horse serum (Fujita et al., 2010). However, there is still contradiction over which nutrient supplement, either serum or serum-free supplement, is preferable for muscle differentiation and maintenance of myotubes. Myoblasts derived from different species may show different responses to serum or serum-free supplement. Horse serum promotes a higher level of myotube differentiation in rat L6 and chicken myoblasts, whereas the serum-free condition enhanced myotube formation in mouse C2C12 myoblasts (Lawson and Purslow, 2000). In another study, chicken myoblasts formed more myotubes in the culture medium with horse serum compared to the culture medium with a serum-free supplement AIM-V (Lawson and Purslow, 2000). The use of serum-free supplements such as N2, B27, ITS (insulin, transferrin, selenium), knockout serum replacement (Barberi et al., 2007; Borchin et al., 2013; Chal et al., 2016; Hosoyama et al., 2014; Shelton et al., 2014) or serum supplements such as 10% fetal bovine serum, 2–5% horse serum can drive skeletal myocyte differentiation (Caron et al., 2016; Hwang et al., 2013; Xu et al., 2013) in the transgene-free protocols. A well-defined serum-free culture condition would be an ideal for future medical application and quality control due to lot-to-lot variability of serum product. Optimizing culture conditions is important for developing a suitable environment for terminal differentiation of human myogenic progenitors and myoblasts.
In conclusion, we identified skeletal myotubes from iPSC-derived myogenic progenitors following terminal differentiation (over 6 weeks) and optimized culture conditions. These results support the ability of our culture protocol to generate mature and functional skeletal myocytes using iPSC-derived spheres for in vitro muscle modeling. Importantly, as patient-specific iPSC lines become available, our protocol can be used to produce mature myotubes created from those cell lines. These myocytes could be applied for use in drug screening, and modeling of neuromuscular diseases such as muscular dystrophy and Pompe disease (glycogen storage disease type II) where muscle function is impaired due to muscle pathology. Further, creating 3D models of muscle tissue may offer a potential advantage for studying neuromuscular disease mechanisms and testing potential treatments.
Supplementary Material
Pax3 proteins were clearly detectable in the differentiated cells at 2 weeks (left). Even after 6 weeks, Pax3+ cells were identified (white arrows in the right image). Notably, these positive cells were not overlapped with myotubes. Scale bar = 50μm.
Sequential videos of spontaneous contraction of iPSC-derived myotubes. (A) The first slight contraction was observed at 5 weeks of differentiation. (B) More dramatic contraction was captured at 6 weeks. (C) Spontaneous contractions gradually attenuated by 14 weeks of differentiation.
3D image of human myogenic progenitor-derived myotubes. Long-cylinder myotubes were labeled with MHC+ staining (green) showing the muscle alignment.
Highlights.
Long-term culture promotes the differentiation of iPSC-derived myogenic progenitors.
Longer differentiation can give rise to striated myotubes.
3D culture promotes muscle differentiation to form elongated myotubes.
Serum-free B27 supplement enhances myogenic differentiation compared to horse serum.
Acknowledgments
The authors would like to thank Ben August (Electron Microscope Facility, UW School of Medicine and Public Health) for his kindly expert assistance in electron microscope technique. We thank Christina Lewis (University of Wisconsin, Madison) for the help in preparing this manuscript. The antibodies for Pax3, Pax7, MYOGENIN (F5D), titin (9D10), and myosin heavy chain (MF20, F1.652, N3.36, A4.74) were obtained from the DSHB developed under the NICHD and maintained by the University of Iowa. The first author (S.J.) would like to thank the financial support from the Royal Thai Government Scholarship. This work was supported by grants from the ALS Association (15-IIP-201, M.S.), NIH/NINDS (R01NS091540, M.S.) and the University of Wisconsin Foundation (M.S).
Footnotes
Competing interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abujarour R, Bennett M, Valamehr B, Lee TT, Robinson M, Robbins D, Le T, Lai K, Flynn P. Myogenic differentiation of muscular dystrophy-specific induced pluripotent stem cells for use in drug discovery. Stem cells translational medicine. 2014;3:149–160. doi: 10.5966/sctm.2013-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter JM. Science of Flexibility. 3. Human Kinetics; Illinois: 2004. pp. 19–25. [Google Scholar]
- Barberi T, Bradbury M, Dincer Z, Panagiotakos G, Socci ND, Studer L. Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nat Med. 2007;13:642–648. doi: 10.1038/nm1533. [DOI] [PubMed] [Google Scholar]
- Borchin B, Chen J, Barberi T. Derivation and FACS-Mediated Purification of PAX3+/PAX7+ Skeletal Muscle Precursors from Human Pluripotent Stem Cells. Stem Cell Reports. 2013;1:620–631. doi: 10.1016/j.stemcr.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron L, Kher D, Lee KL, McKernan R, Dumevska B, Hidalgo A, Li J, Yang H, Main H, Ferri G, Petek LM, Poellinger L, Miller DG, Gabellini D, Schmidt U. A Human Pluripotent Stem Cell Model of Facioscapulohumeral Muscular Dystrophy-Affected Skeletal Muscles. Stem Cells Transl Med. 2016;5:1145–1161. doi: 10.5966/sctm.2015-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chal J, Al Tanoury Z, Hestin M, Gobert B, Aivio S, Hick A, Cherrier T, Nesmith AP, Parker KK, Pourquie O. Generation of human muscle fibers and satellite-like cells from human pluripotent stem cells in vitro. Nat Protoc. 2016;11:1833–1850. doi: 10.1038/nprot.2016.110. [DOI] [PubMed] [Google Scholar]
- Chal J, Oginuma M, Al Tanoury Z, Gobert B, Sumara O, Hick A, Bousson F, Zidouni Y, Mursch C, Moncuquet P, Tassy O, Vincent S, Miyanari A, Bera A, Garnier JM, Guevara G, Hestin M, Kennedy L, Hayashi S, Drayton B, Cherrier T, Gayraud-Morel B, Gussoni E, Relaix F, Tajbakhsh S, Pourquie O. Differentiation of pluripotent stem cells to muscle fiber to model Duchenne muscular dystrophy. Nat Biotechnol. 2015;33:962–969. doi: 10.1038/nbt.3297. [DOI] [PubMed] [Google Scholar]
- Choi IY, Lim H, Estrellas K, Mula J, Cohen TV, Zhang Y, Donnelly CJ, Richard JP, Kim YJ, Kim H, Kazuki Y, Oshimura M, Li HL, Hotta A, Rothstein J, Maragakis N, Wagner KR, Lee G. Concordant but Varied Phenotypes among Duchenne Muscular Dystrophy Patient-Specific Myoblasts Derived using a Human iPSC-Based Model. Cell Rep. 2016;15:2301–2312. doi: 10.1016/j.celrep.2016.05.016. [DOI] [PubMed] [Google Scholar]
- Darabi R, Arpke RW, Irion S, Dimos JT, Grskovic M, Kyba M, Perlingeiro RC. Human ES- and iPS-derived myogenic progenitors restore DYSTROPHIN and improve contractility upon transplantation in dystrophic mice. Cell Stem Cell. 2012;10:610–619. doi: 10.1016/j.stem.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster RF, Thompson JM, Kaufman SJ. A laminin substrate promotes myogenesis in rat skeletal muscle cultures: analysis of replication and development using antidesmin and anti-BrdUrd monoclonal antibodies. Developmental biology. 1987;122:11–20. doi: 10.1016/0012-1606(87)90327-7. [DOI] [PubMed] [Google Scholar]
- Fujita H, Endo A, Shimizu K, Nagamori E. Evaluation of serum-free differentiation conditions for C2C12 myoblast cells assessed as to active tension generation capability. Biotechnology and bioengineering. 2010;107:894–901. doi: 10.1002/bit.22865. [DOI] [PubMed] [Google Scholar]
- Gawlitta D, Boonen KJ, Oomens CW, Baaijens FP, Bouten CV. The influence of serum-free culture conditions on skeletal muscle differentiation in a tissue-engineered model. Tissue engineering Part A. 2008;14:161–171. doi: 10.1089/ten.a.2007.0095. [DOI] [PubMed] [Google Scholar]
- Grefte S, Vullinghs S, Kuijpers-Jagtman AM, Torensma R, Von den Hoff JW. Matrigel, but not collagen I, maintains the differentiation capacity of muscle derived cells in vitro. Biomedical materials. 2012;7:055004. doi: 10.1088/1748-6041/7/5/055004. [DOI] [PubMed] [Google Scholar]
- Hartley RS, Yablonka-Reuveni Z. Long-term maintenance of primary myogenic cultures on a reconstituted basement membrane. In vitro cellular & developmental biology: journal of the Tissue Culture Association. 1990;26:955–961. doi: 10.1007/BF02624469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoyama T, McGivern JV, Van Dyke JM, Ebert AD, Suzuki M. Derivation of myogenic progenitors directly from human pluripotent stem cells using a sphere-based culture. Stem Cells Transl Med. 2014;3:564–574. doi: 10.5966/sctm.2013-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoyama T, Van Dyke J, Suzuki M. Applications of skeletal muscle progenitor cells for neuromuscular diseases. Am J Stem Cells. 2012;1:253–263. [PMC free article] [PubMed] [Google Scholar]
- Hughes CS, Postovit LM, Lajoie GA. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics. 2010;10:1886–1890. doi: 10.1002/pmic.200900758. [DOI] [PubMed] [Google Scholar]
- Hwang Y, Suk S, Lin S, Tierney M, Du B, Seo T, Mitchell A, Sacco A, Varghese S. Directed in vitro myogenesis of human embryonic stem cells and their in vivo engraftment. PLoS One. 2013;8:e72023. doi: 10.1371/journal.pone.0072023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman HK, Luckenbill-Edds L, Cannon FW, Sephel GC. Use of extracellular matrix components for cell culture. Analytical biochemistry. 1987;166:1–13. doi: 10.1016/0003-2697(87)90538-0. [DOI] [PubMed] [Google Scholar]
- Kuhl U, Timpl R, von der Mark K. Synthesis of type IV collagen and laminin in cultures of skeletal muscle cells and their assembly on the surface of myotubes. Developmental biology. 1982;93:344–354. doi: 10.1016/0012-1606(82)90122-1. [DOI] [PubMed] [Google Scholar]
- Lawson MA, Purslow PP. Differentiation of myoblasts in serum-free media: effects of modified media are cell line-specific. Cells, tissues, organs. 2000;167:130–137. doi: 10.1159/000016776. [DOI] [PubMed] [Google Scholar]
- Liao IC, Liu JB, Bursac N, Leong KW. Effect of Electromechanical Stimulation on the Maturation of Myotubes on Aligned Electrospun Fibers. Cellular and molecular bioengineering. 2008;1:133–145. doi: 10.1007/s12195-008-0021-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin BL, Song T, Sadayappan S. Myofilaments: Movers and Rulers of the Sarcomere. Compr Physiol. 2017;7:675–692. doi: 10.1002/cphy.c160026. [DOI] [PubMed] [Google Scholar]
- Ludwig TE, Bergendahl V, Levenstein ME, Yu J, Probasco MD, Thomson JA. Feeder-independent culture of human embryonic stem cells. Nat Methods. 2006;3:637–646. doi: 10.1038/nmeth902. [DOI] [PubMed] [Google Scholar]
- Madden L, Juhas M, Kraus WE, Truskey GA, Bursac N. Bioengineered human myobundles mimic clinical responses of skeletal muscle to drugs. Elife. 2015;4:e04885. doi: 10.7554/eLife.04885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffioletti SM, Gerli MF, Ragazzi M, Dastidar S, Benedetti S, Loperfido M, VandenDriessche T, Chuah MK, Tedesco FS. Efficient derivation and inducible differentiation of expandable skeletal myogenic cells from human ES and patient-specific iPS cells. Nat Protoc. 2015;10:941–958. doi: 10.1038/nprot.2015.057. [DOI] [PubMed] [Google Scholar]
- Manabe Y, Miyatake S, Takagi M, Nakamura M, Okeda A, Nakano T, Hirshman MF, Goodyear LJ, Fujii NL. Characterization of an acute muscle contraction model using cultured C2C12 myotubes. PloS one. 2012;7:e52592. doi: 10.1371/journal.pone.0052592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar P, Wang W, Natarajan A, Rumsey JW, Hickman JJ. Photolithographic patterning of C2C12 myotubes using vitronectin as growth substrate in serum-free medium. Biotechnology progress. 2007;23:265–268. doi: 10.1021/bp060302q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund C, Lindstrom M, Thornell LE, Eriksson PO. Remarkable heterogeneity in myosin heavy-chain composition of the human young masseter compared with young biceps brachii. Histochem Cell Biol. 2012;138:669–682. doi: 10.1007/s00418-012-0985-5. [DOI] [PubMed] [Google Scholar]
- Pawlikowski B, Lee L, Zuo J, Kramer RH. Analysis of human muscle stem cells reveals a differentiation-resistant progenitor cell population expressing Pax7 capable of self-renewal. Developmental dynamics: an official publication of the American Association of Anatomists. 2009;238:138–149. doi: 10.1002/dvdy.21833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi F, Perlingeiro RC. Stem cells for skeletal muscle regeneration: therapeutic potential and roadblocks. Transl Res. 2014;163:409–417. doi: 10.1016/j.trsl.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca I, Requena J, Edel MJ, Alvarez-Palomo AB. Myogenic Precursors from iPS Cells for Skeletal Muscle Cell Replacement Therapy. J Clin Med. 2015;4:243–259. doi: 10.3390/jcm4020243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serena E, Zatti S, Zoso A, Lo Verso F, Tedesco FS, Cossu G, Elvassore N. Skeletal Muscle Differentiation on a Chip Shows Human Donor Mesoangioblasts’ Efficiency in Restoring Dystrophin in a Duchenne Muscular Dystrophy Model. Stem cells translational medicine. 2016;5:1676–1683. doi: 10.5966/sctm.2015-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton M, Metz J, Liu J, Carpenedo RL, Demers SP, Stanford WL, Skerjanc IS. Derivation and expansion of PAX7-positive muscle progenitors from human and mouse embryonic stem cells. Stem Cell Reports. 2014;3:516–529. doi: 10.1016/j.stemcr.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi D, Reinecke H, Murry CE, Torok-Storb B. Myogenic fusion of human bone marrow stromal cells, but not hematopoietic cells. Blood. 2004;104:290–294. doi: 10.1182/blood-2003-03-0688. [DOI] [PubMed] [Google Scholar]
- Skoglund G, Laine J, Darabi R, Fournier E, Perlingeiro R, Tabti N. Physiological and ultrastructural features of human induced pluripotent and embryonic stem cell-derived skeletal myocytes in vitro. Proc Natl Acad Sci U S A. 2014;111:8275–8280. doi: 10.1073/pnas.1322258111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A, Woltjen K, Miyake K, Hotta A, Ikeya M, Yamamoto T, Nishino T, Shoji E, Sehara-Fujisawa A, Manabe Y, Fujii N, Hanaoka K, Era T, Yamashita S, Isobe K, Kimura E, Sakurai H. Efficient and reproducible myogenic differentiation from human iPS cells: prospects for modeling Miyoshi Myopathy in vitro. PloS one. 2013;8:e61540. doi: 10.1371/journal.pone.0061540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedesco FS, Cossu G. Stem cell therapies for muscle disorders. Curr Opin Neurol. 2012;25:597–603. doi: 10.1097/WCO.0b013e328357f288. [DOI] [PubMed] [Google Scholar]
- van der Schaft DW, van Spreeuwel AC, Boonen KJ, Langelaan ML, Bouten CV, Baaijens FP. Engineering skeletal muscle tissues from murine myoblast progenitor cells and application of electrical stimulation. Journal of visualized experiments: JoVE. 2013:e4267. doi: 10.3791/4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Mark K, Ocalan M. Antagonistic effects of laminin and fibronectin on the expression of the myogenic phenotype. Differentiation; research in biological diversity. 1989;40:150–157. doi: 10.1111/j.1432-0436.1989.tb00823.x. [DOI] [PubMed] [Google Scholar]
- Wilschut KJ, Haagsman HP, Roelen BA. Extracellular matrix components direct porcine muscle stem cell behavior. Experimental cell research. 2010;316:341–352. doi: 10.1016/j.yexcr.2009.10.014. [DOI] [PubMed] [Google Scholar]
- Xu C, Tabebordbar M, Iovino S, Ciarlo C, Liu J, Castiglioni A, Price E, Liu M, Barton ER, Kahn CR, Wagers AJ, Zon LI. A zebrafish embryo culture system defines factors that promote vertebrate myogenesis across species. Cell. 2013;155:909–921. doi: 10.1016/j.cell.2013.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Pabon L, Murry CE. Engineering adolescence: maturation of human pluripotent stem cell-derived cardiomyocytes. Circulation research. 2014;114:511–523. doi: 10.1161/CIRCRESAHA.114.300558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zhu X, Fu L, Yi F, Liu GH, Ocampo A, Qu J, Izpisua Belmonte JC. Regeneration: making muscle from hPSCs. Cell research. 2014;24:1159–1161. doi: 10.1038/cr.2014.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pax3 proteins were clearly detectable in the differentiated cells at 2 weeks (left). Even after 6 weeks, Pax3+ cells were identified (white arrows in the right image). Notably, these positive cells were not overlapped with myotubes. Scale bar = 50μm.
Sequential videos of spontaneous contraction of iPSC-derived myotubes. (A) The first slight contraction was observed at 5 weeks of differentiation. (B) More dramatic contraction was captured at 6 weeks. (C) Spontaneous contractions gradually attenuated by 14 weeks of differentiation.
3D image of human myogenic progenitor-derived myotubes. Long-cylinder myotubes were labeled with MHC+ staining (green) showing the muscle alignment.