Abstract
Objective
To determine whether end-tidal carbon dioxide (ETCO2)-guided chest compression delivery improves survival over standard cardiopulmonary resuscitation (CPR) after prolonged asphyxial arrest.
Design
Preclinical randomized controlled study.
Setting
University animal research laboratory.
Subjects
1–2-week-old swine.
Interventions
After undergoing a 20-minute asphyxial arrest, animals received either standard or ETCO2-guided CPR. In the standard group, chest compression delivery was optimized by video and verbal feedback to maintain the rate, depth, and release within published guidelines. In the ETCO2-guided group, chest compression rate and depth were adjusted to obtain a maximal ETCO2 level without other feedback. CPR included 10 minutes of basic life support followed by advanced life support for 10 minutes or until return of spontaneous circulation (ROSC).
Measurements and Main Results
Mean ETCO2 at 10 minutes of CPR was 34 ± 8 torr in the ETCO2 group (n=14) and 19 ± 9 torr in the standard group (n=14; p=0.0001). The ROSC rate was 7/14 (50%) in the ETCO2 group and 2/14 (14%) in the standard group (p=0.04). The chest compression rate averaged 143 ± 10/minute in the ETCO2 group and 102 ± 2/minute in the standard group (p<0.0001). Neither asphyxia-related hypercarbia nor epinephrine administration confounded the use of ETCO2-guided chest compression delivery. The response of the relaxation arterial pressure and cerebral perfusion pressure to the initial epinephrine administration was greater in the ETCO2 group than in the standard group (p=0.01 and p=0.03, respectively). The prevalence of resuscitation-related injuries was similar between groups.
Conclusions
ETCO2-guided chest compression delivery is an effective resuscitation method that improves early survival after prolonged asphyxial arrest in this neonatal piglet model. Optimizing ETCO2 levels during CPR required that chest compression delivery rate exceed current guidelines. The use of physiologic feedback during CPR has the potential to provide optimized and individualized resuscitative efforts.
Keywords: asphyxia, heart arrest, cardiopulmonary resuscitation, capnography, feedback, pediatrics, neonatal
INTRODUCTION
Survival to discharge from out-of-hospital cardiac arrest in infants and children remains low (<10%), despite guidelines that emphasize chest compression delivery (1–5). After a prolonged cardiac arrest, resuscitation efforts must be maximally effective to achieve return of spontaneous circulation (ROSC) and minimize neurologic injury (1, 2, 6). Resuscitation methods that incorporate physiologic feedback have the potential to individualize and optimize chest compression delivery, increase the effectiveness of resuscitative efforts, and improve outcome. A previously described resuscitation method that incorporates physiologic feedback is hemodynamic-directed cardiopulmonary resuscitation (CPR), which uses arterial pressure monitoring. Hemodynamic-directed CPR has been used to guide chest compression delivery (increased rate and force) and epinephrine administration frequency in adults (7) and to improve outcome in animal models (8–10).
A novel physiologic parameter for guiding resuscitation efforts utilizes end-tidal carbon dioxide (ETCO2) as an indicator of chest compression efficacy (11). The basis for this method is that the ETCO2 level during CPR represents the movement of blood containing carbon dioxide to the lungs, and thereby becomes a surrogate for resuscitation-generated systemic blood flow (cardiac output). This relationship of the ETCO2 level with systemic blood flow is strong because during CPR, the ETCO2 level becomes less dependent on CO2 production and minute ventilation and more dependent on cardiac output (12–24). An advantage to using ETCO2 level as physiologic feedback during CPR is that it can be measured both easily and continuously. ETCO2 physiologic feedback also has the potential to be used with the currently recommended quality feedback about chest compression rate, depth, and release, and with the promising physiologic feedback method of hemodynamic-directed CPR.
The 2015 American Heart Association (AHA) resuscitation guidelines suggested the use of ETCO2 during adult CPR as a prognostication tool either immediately after intubation or after 20 minutes of resuscitation, but did not provide instruction for using ETCO2 to direct resuscitation efforts (25). The pediatric 2015 AHA guidelines reported a lack of evidence about the use of ETCO2 to direct chest compression delivery and were unable to make a recommendation (26). The 2013 AHA consensus regarding CPR quality and resuscitation outcome improvement suggested the development of “a more reliable, inexpensive, noninvasive physiological monitor that will increase our ability to optimize CPR for individual victims of cardiac arrest” and recommended that future research “determine optimal titration of hemodynamic and ETCO2 monitoring during human CPR” (27).
In our previous study that used a brief (90-second) fibrillatory cardiac arrest, the use of ETCO2 feedback to guide chest compression delivery was equivalent to using quality feedback about compression rate and depth (11). In the current study, we used a more prolonged (20-minute) asphyxial cardiac arrest to determine if ETCO2-guided chest compression delivery can improve survival compared to standard CPR guided by rate and depth feedback. We also investigated concerns that the hypercarbia from asphyxia or the administration of epinephrine during advanced life support (ALS) might confound the use of ETCO2-guided compression delivery. We hypothesized that if extreme hypercarbia and epinephrine administration do not interfere, then the use of ETCO2-guided chest compression delivery after a prolonged cardiac arrest would optimize resuscitative efforts and improve survival over standard CPR.
MATERIALS AND METHODS
All studies were carried out with approval from the Animal Care and Use Committee of Johns Hopkins University in accordance with institutional guidelines. Twenty-eight male domestic swine (7–14 days old, 3–4 kg) received anesthesia induction and maintenance with 2% isoflurane, 70% nitrous oxide, and 30% oxygen. A 4.0 cuffed tracheal tube was secured via tracheostomy. A femoral artery catheter was advanced to the mid-aorta for hemodynamic and blood gas determinations. A pacing wire placed through one femoral vein was advanced into the right ventricle as indicated by ventricular irritation on electrocardiogram. A catheter advanced through the other femoral vein to a mid-thoracic position was used to measure central venous pressure (CVP) and to administer fluid and epinephrine. A sagittal sinus catheter was placed through a burr hole at the bregma to measure intracranial pressure (ICP) and venous blood gases. Finally, the anteroposterior chest diameter was measured before arrest (and after CPR) to determine compression-induced deformity. After the surgery, isoflurane was decreased and fentanyl (10 mcg/kg) and vecuronium (0.3 mg/kg) administered. A heating pad was adjusted to maintain rectal temperature at 38–39°C. Pressure-controlled ventilation was adjusted to maintain a PaCO2 of 35–45 torr at a rate of 20 breaths per minute, and FiO2 was decreased from 30% to 21%. Vital signs, including ETCO2, mean arterial blood pressure (MAP), mean CVP, arterial relaxation pressure (aka diastolic blood pressure (DBP)), mean ICP, heart rate, and rectal temperature, were recorded before protocol initiation (baseline) and at 30-second intervals during CPR. Hemodynamic values measured during the interval of ALS after ROSC were removed from the values on the graphs displayed as ALS.
Experimental Protocol
A timeline for the experimental protocol is provided in Figure 1. We produced a 20-minute asphyxial-ventricular fibrillation arrest by clamping the tracheostomy tube for 14 minutes and then inducing 6 minutes of fibrillation by applying 50 mA of alternating current via the pacing wire. This procedure was similar to the model used to study hemodynamic-directed CPR (8). We included the period of fibrillation to prevent ROSC during the 10-minute basic life support (BLS) interval. This guaranteed 10-minute interval without ROSC allowed hemodynamic comparison between study groups during BLS. We chose to induce fibrillation at 14 minutes because this duration allowed the asphyxia to proceed to pulselessness (which usually requires 8–12 minutes) and was not so long that the hypoxemia produced a low amplitude fibrillation that was difficult to detect. After the 20 minutes of arrest, BLS was begun. Ventilation was resumed with an FiO2 of 1.0, the pre-arrest rate of 20 breaths/minute, and the pre-arrest inspiratory pressures. The heating pad was adjusted to maintain rectal temperature at 38–39°C during CPR. In both groups, resuscitators performed chest compressions with a two-thumb, encircling technique at a compression rate that varied by study group (see below). The resuscitators providing chest compression delivery switched every 2 minutes.
Figure 1.
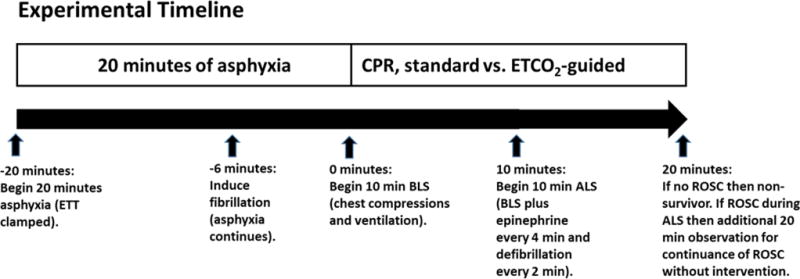
Experimental timeline indicating events during asphyxia and resuscitation in minutes.
Standard group
In this group, a marker was placed across the animal at 2/3 the anteroposterior diameter for chest compression depth (see Figure 1 in Hamrick et al. (11)). The targets for chest compression delivery throughout BLS and ALS were a rate of 100/minute, a depth of 1/3 the anteroposterior diameter, and full release between compressions. Real-time video and verbal feedback about the rate, depth, and release were provided. The resuscitators were blinded to the ETCO2 value.
ETCO2 group
In this group, resuscitators adjusted the chest compression rate, force, depth, and thumb positions throughout BLS and ALS to maximize the level of ETCO2 produced. ETCO2 level was the only feedback available.
Both groups
After the 10-minute interval of BLS, ALS was begun. Resuscitators administered 30 joules of biphasic defibrillation during compressor changes at 0, 2, 4, 6, and 8 minutes of ALS. Additionally, 300 mcg of epinephrine was administered into the mid-thoracic venous catheter at 0, 4, and 8 minutes of ALS. ALS was terminated when ROSC occurred or after 10 minutes (20 minutes of CPR total). Survival was defined as continued ROSC (sustained arterial pulsatility) for 20 minutes without further intervention. Non-survival was no ROSC after 10 minutes of ALS (20 minutes of CPR) or ROSC that was not sustained for 20 minutes. No additional compressions, medications, or defibrillations were provided after ROSC occurred. Arterial and venous blood gases were examined at baseline, 10 minutes of asphyxia, 8 minutes of BLS, and 20 minutes of ALS for non-survivors or 20 minutes of ROSC for survivors. After 20 minutes of ROSC, survivors were euthanized per Animal Care and Use Committee guidelines. We performed autopsies on all animals to determine resuscitation-related injuries (superficial liver injury, hemoperitoneum, hemothorax, hemopericardium, atelectasis, or superficial cardiac injury).
Statistical Analysis
Descriptive data were used to characterize baseline cardiorespiratory variables. All values are expressed as mean ± SD. Systemic perfusion pressure (SPP) was calculated as MAP – mean CVP. Cerebral perfusion pressure (CPP) was calculated as MAP – mean ICP. Differences between groups in resuscitation and injury statistics were compared with an unpaired, 2-tailed t test for continuous variables, and X2 or Fisher’s exact test for categorical variables, as appropriate. p-values <0.05 were considered statistically significant. Repeated-measures analysis of variance (ANOVA) was used to investigate the effect of time and group. All data were analyzed in Stata (Version 14; Statacorp LP, College Station, TX).
RESULTS
Baseline blood gas and hemodynamic results were similar between study groups (Table 1). PaCO2 at 10 minutes of asphyxia averaged 122 and 124 torr in the ETCO2 and standard groups, respectively. ETCO2 at the start of chest compressions exceeded the limit of detection (>99 torr) in both groups. At 30 seconds of chest compressions, ETCO2 averaged 64 and 65 torr, and at 1 minute it was 32 ± 10 and 25 ± 8 torr (p=0.046) in the ETCO2 and standard groups, respectively (Fig. 2A). ETCO2 throughout CPR was >30 torr in the ETCO2 group but fell to 11.5 torr in the standard group. The PaCO2 at 8 minutes of BLS was 54 ± 20 in the ETCO2 group and 42 ± 30 torr (p=0.23) in the standard group. The ETCO2 was higher in the ETCO2 group than in the standard group during both BLS and ALS (Table 2). ETCO2 did not change with epinephrine administration at 10, 14, or 18 minutes of CPR in either study group nor when analyzed for survival. ETCO2 level during BLS was higher in survivors (Table 3).
Table 1.
Baseline Characteristics of Subjects
| Variable | ETCO2 Group | Standard Group | p |
|---|---|---|---|
| Weight (kg) | 3.51 ± 0.6 | 3.57 ± 0.5 | 0.78 |
| ETCO2 (torr) | 45 ± 4.4 | 46 ± 3.1 | 0.56 |
| DBP (mmHg) | 64 ± 11 | 66 ± 13 | 0.59 |
| MAP (mmHg) | 78 ± 12 | 79 ± 15 | 0.87 |
| CVP (mmHg) | 12 ± 2.0 | 11 ± 1.5 | 0.4 |
| SPP (mmHg) | 66 ± 12 | 68 ± 15 | 0.60 |
| ICP (mmHg) | 14 ± 3.0 | 13 ± 1.5 | 0.47 |
| CPP (mmHg) | 64 ± 12 | 66 ± 16 | 0.78 |
| HR (beats/min) | 206 ± 37 | 225 ± 43 | 0.21 |
| pHa | 7.38 ± 0 | 7.37 ± 0 | 0.67 |
| pHv | 7.35 ± 0 | 7.32 ± 0 | 0.07 |
| PaCO2 (torr) | 39 ± 2.9 | 40 ± 4.4 | 0.74 |
| PvCO2 (torr) | 45 ± 2.0 | 45 ± 4.3 | 0.69 |
| PaO2 (torr) | 86 ± 13 | 90 ± 30 | 0.68 |
| PvO2 (torr) | 40 ± 5.7 | 46 ± 16 | 0.22 |
| BEa | −1.6 ± 2.1 | −0.7 ± 4.9 | 0.51 |
| BEv | −0.9 ± 2.7 | −2.3 ± 3.3 | 0.26 |
| SaO2 (%) | 97 ± 2.3 | 95 ± 3.6 | 0.11 |
| SvO2 (%) | 62 ± 15 | 60 ± 16 | 0.73 |
| Hb (g/dL) | 7.6 ± 1.7 | 8.4 ± 1.4 | 0.19 |
| Temperature (°C) | 38.5 ± 0.5 | 38.6 ± 0.7 | 0.65 |
Data are collected prior to asphyxia, separated by study group, and shown as mean ± SD.
BEa = base excess arterial; BEv = base excess venous; CPP = cerebral perfusion pressure; CVP = central venous pressure; DBP = diastolic blood pressure; ETCO2 = end-tidal carbon dioxide; Hb = hemoglobin; HR = heart rate; ICP = intracranial pressure; MAP = mean arterial blood pressure; SPP = systemic perfusion pressure; pHa = arterial pH; pHv = venous pH; SaO2 = arterial oxygen saturation; SvO2 = venous oxygen saturation.
Figure 2.
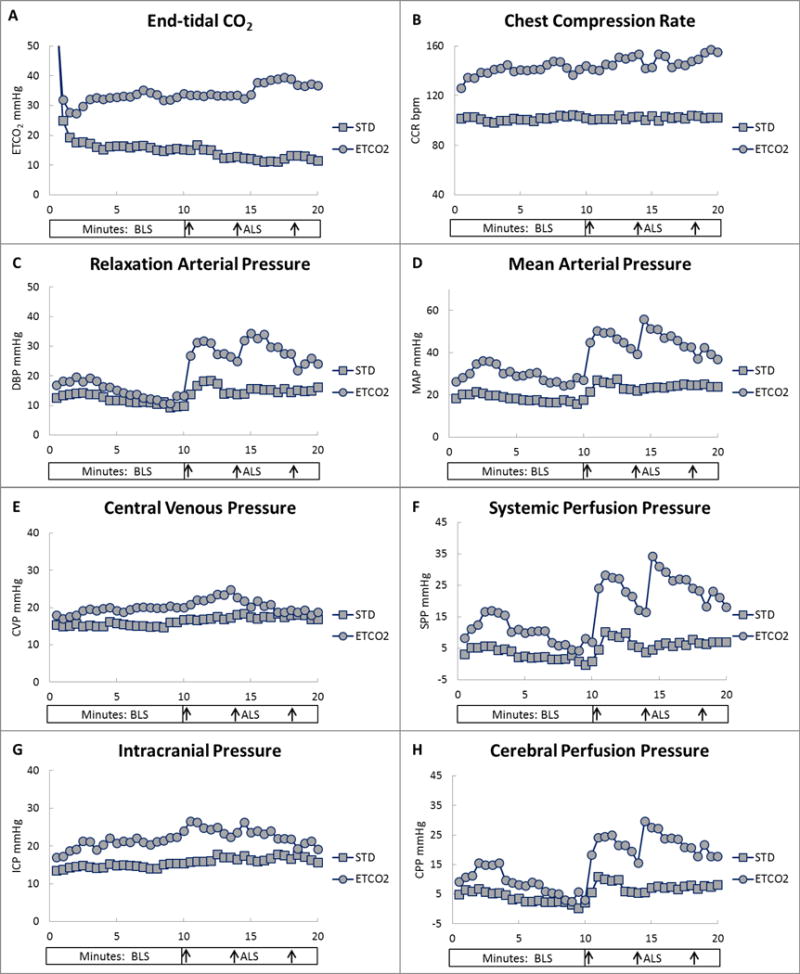
End-tidal CO2 (ETCO2), chest compression rate (CCR), and hemodynamic variables by study group (standard CPR [STD] or ETCO2-guided CPR [ETCO2]). A, ETCO2. B, CCR. C, Relaxation arterial (diastolic blood) pressure (DBP). D, Mean arterial pressure (MAP). E, Central venous pressure (CVP). F, Systemic perfusion pressure (SPP). G, Intracranial pressure (ICP). H, Cerebral perfusion pressure (CPP). Each data point represents the mean value at 30-second intervals. Y-axis values are in mmHg or beats per minute (bpm). X-axis values are in minutes of basic life support (BLS) and advanced life support (ALS). X-axis arrows indicate timing of epinephrine administration at 10, 14, and 18 minutes of CPR.
Table 2.
Hemodynamic Results During Basic and Advanced Life Support by Study Group
| Parameter | BLS (0–10 min)
|
ALS (10–20 min)
|
||||
|---|---|---|---|---|---|---|
| ETCO2 (n=14) | Standard (n=14) | p | ETCO2 (n=14) | Standard (n=14) | p | |
|
|
|
|||||
| ETCO2 (torr) | 34.4 ± 7.5 | 20.7 ± 8.7 | 0.0001 | 34.3 ± 9.5 | 16.2 ± 12 | 0.0001 |
| Comp/min | 140 ± 12 | 102 ± 3 | <0.0001 | 147 ± 19 | 102 ± 2 | <0.0001 |
| DBP (mmHg) | 14.5 ± 9.1 | 11.9 ± 5.5 | 0.37 | 30.7 ± 14 | 18.4 ± 9.9 | 0.01 |
| MAP (mmHg) | 29.5 ± 18 | 18.3 ± 6.5 | 0.04 | 47.4 ± 19 | 28.2 ± 12 | 0.004 |
| CVP (mmHg) | 19.4 ± 6.1 | 15.4 ± 2.1 | 0.02 | 20.5 ± 5.5 | 17.2 ± 2.1 | 0.04 |
| SPP (mmHg) | 10.6 ± 16 | 3.2 ± 1.8 | 0.07 | 27.6 ± 17 | 11.1 ± 13 | 0.004 |
| ICP (mmHg) | 20.7 ± 4.1 | 14.6 ± 1.8 | 0.0000 | 24.1 ± 5.0 | 16.7 ± 2.3 | <0.0001 |
| CPP (mmHg) | 8.8 ± 16 | 3.7 ± 6.9 | 0.29 | 23.6 ± 17 | 11.6 ± 12 | 0.04 |
Data represent the cumulative 30-second values during the 10 minutes of basic life support or the 10 minutes of advanced life support and are shown as mean ± SD.
ALS = advanced life support; BLS = basic life support; Comp/min = compressions per minute; CPP = cerebral perfusion pressure; CVP = central venous pressure; DBP = diastolic blood pressure; ETCO2 = end-tidal carbon dioxide; ICP = intracranial pressure; MAP = mean arterial blood pressure; SPP = systemic perfusion pressure.
Table 3.
Hemodynamic Results During Basic and Advanced Life Support by Survival Group
| Parameter | BLS (0–10 min)
|
ALS (10–20 min)
|
||||
|---|---|---|---|---|---|---|
| Survivors (n=9) |
Non-survivors (n=19) |
p | Survivors (n=9) |
Non-survivors (n=19) |
p | |
|
|
|
|||||
| ETCO2 (torr) | 35.1 ± 7.1 | 24 ± 10 | 0.007 | 32.5 ± 8.5 | 21.8 ± 14 | 0.06 |
| Comp/min | 130 ± 19 | 117 ± 22 | 0.15 | 126 ± 22 | 120 ± 25 | 0.59 |
| DBP (mmHg) | 16.9 ± 6.4 | 11.4 ± 7.4 | 0.06 | 34.6 ± 11 | 19.7 ± 12 | 0.004 |
| MAP (mmHg) | 28.0 ± 7.6 | 22.0 ± 16 | 0.31 | 49.1 ± 12 | 32.5 ± 18 | 0.02 |
| CVP (mmHg) | 19.1 ± 7 | 16.6 ± 3.5 | 0.21 | 20.3 ± 6.1 | 18.2 ± 3.4 | 0.25 |
| SPP (mmHg) | 9.4 ± 3.7 | 5.7± 3.1 | 0.24 | 29.3 ± 13 | 14.6 ± 17 | 0.02 |
| ICP (mmHg) | 19.8 ± 3.7 | 16.7 ± 4.4 | 0.09 | 23.5 ± 5 | 18.9 ± 5 | 0.03 |
| CPP (mmHg) | 8.2 ± 9.3 | 5.3 ± 14 | 0.57 | 26.1 ± 12 | 13.6 ± 16 | 0.04 |
Data represent the cumulative 30-second values during the 10 minutes of basic life support or the 10 minutes of advanced life support and are shown as mean ± SD. ALS = advanced life support; BLS = basic life support; Comp/min = compressions per minute; CPP = cerebral perfusion pressure; CVP = central venous pressure; DBP = diastolic blood pressure; ETCO2 = end-tidal carbon dioxide; ICP = intracranial pressure; MAP = mean arterial blood pressure; SPP = systemic perfusion pressure.
The ROSC rate was greater in the ETCO2 group (50% vs 14%, p=0.04; Table 4). Two of the seven non-survivors in the ETCO2 group had ROSC less than the required 20 minutes (8 and 16 minutes), none of the twelve non-survivors in the standard group had ROSC of any duration. There were no differences between groups in defibrillation attempts, time to defibrillation, or epinephrine doses. Figure 3A shows the ETCO2 level during CPR by survival and illustrates that survivors had consistently higher ETCO2 levels after 1 minute of CPR. Figure 3A1 shows the ETCO2 level during CPR by group and survival. In the ETCO2 group, both survivors and non-survivors maintained ETCO2 during CPR in the upper 20s to 30 torr, whereas the ETCO2 in the standard group showed the expected relationship with survival (high 20s in survivors, low 10s in non-survivors).
Table 4.
Outcomes, Blood Gas, and Cardiopulmonary Resuscitation-related Injuries by Study Group
| Variable | ETCO2 Group | Standard Group | p |
|---|---|---|---|
| Outcome results | |||
| Successful ROSC, n (%) | 7/14 (50) | 2/14 (14) | 0.04 |
| Time to ROSC (survivors), min | 15.2 ± 3.9 | 15.6 ± 6.2 | 0.9 |
| Successfully defibrillated, n (%) | 9/14 (64) | 5/14 (36) | 0.26 |
| Time to defibrillation, min | 14.6 ± 3.0 | 14.0 ± 5.3 | 0.8 |
| Defibrillation attempts | 3.6 ± 1.5 | 4.2 ± 1.3 | 0.29 |
| Doses of epinephrine required | 2.3 ± 0.8 | 2.7 ± 0.7 | 0.16 |
| 8-minute CPR arterial blood gas results | |||
| pH | 6.98 ± 0.1 | 7.02 ± 0.2 | 0.77 |
| PaCO2 | 54 ± 20 | 42 ± 29 | 0.12 |
| PaO2 | 132 ± 77 | 120 ± 67 | 0.33 |
| BE | −18 ± 3.4 | −20 ± 3.6 | 0.09 |
| Autopsy results | |||
| Atelectasis, n (%) | 10/13 (77) | 8/12 (66) | 0.67 |
| Liver laceration, n (%) | 0/13 (0) | 0/12 (0) | N/A |
| Epicardial hemorrhages, n (%) | 10/13 (77) | 4/12 (33) | 0.05 |
| Hemothorax, n (%) | 3/13 (23) | 0/12 (0) | 0.12 |
Data are shown as mean ± SD unless otherwise specified. Percentages are in parentheses. BE = base excess; N/A = not applicable; PaCO2 = arterial partial pressure of carbon dioxide; PaO2 = arterial partial pressure of oxygen; ROSC = return of spontaneous circulation.
Figure 3.
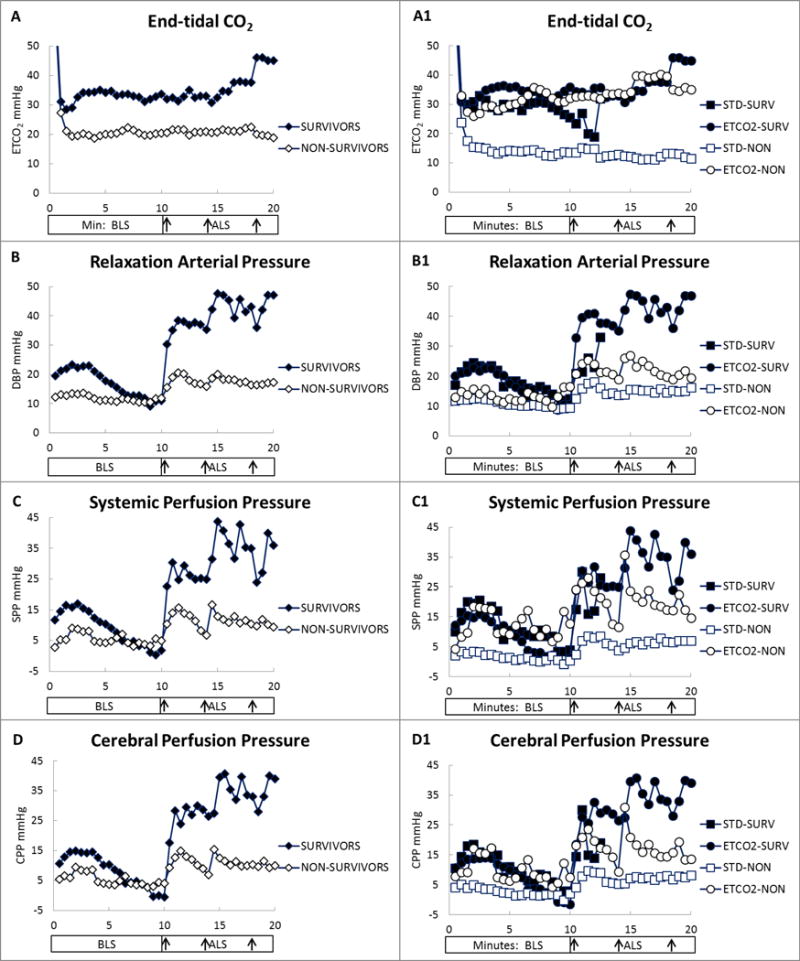
End-tidal CO2 (ETCO2) and hemodynamic variables by survival (STD-SURV = standard group survivors, ETCO2-SURV = ETCO2 group survivors, STD-NON = standard group non-survivors, ETCO2-NON = ETCO2 group non-survivors). A, ETCO2. A1, ETCO2 by study group and survival. B, Relaxation arterial (diastolic blood) pressure (DBP). B1, Relaxation arterial pressure by study group and survival. C, Systemic perfusion pressure (SPP). C1, Systemic perfusion pressure by study group and survival. D, Cerebral perfusion pressure (CPP). D1, Cerebral perfusion pressure by study group and survival. Each data point represents the mean value at 30-second intervals. Y-axis values are in mmHg. X-axis values are in minutes of basic life support (BLS) and advanced life support (ALS). X-axis arrows indicate timing of epinephrine administration at 10, 14, and 18 minutes of CPR.
The chest compression rate over the 20 minutes of CPR was 102 ± 2/minute (range, 84–114) in the standard group and 143 ± 10/minute (range, 72–182) in the ETCO2 group (p<0.0001) (Table 2, Fig. 2B). Compression rate did not differ by survival (Table 3; p=0.14).
The DBP (an indicator of coronary perfusion during CPR) did not differ between study groups during BLS, but it was higher in the ETCO2 group during ALS (Table 2, Fig. 2C). The ETCO2 group exhibited a greater increase in DBP than did the standard group 1 minute after the first epinephrine administration (DBP: 20 ± 16 vs 7 ± 7 mmHg, p=0.01). Survivors exhibited higher DBP early in BLS (0–8 minutes; 18.5 ± 7 vs 11.6 ± 7 mmHg, p=0.02), but not during late BLS (8–10 minutes, 10.7 vs 10.7; Fig. 3B). During ALS, DBP was higher in survivors than in non-survivors (Fig. 3B). Figure 3B1 shows that the DBP during BLS was similar for survival between study groups, unlike the ETCO2 level in Figure 3A1. The first epinephrine administration produced a greater increase in DBP in survivors than in non-survivors (25 ± 17 vs 8 ± 7 mmHg, p=0.001). ANOVA of DBP by group and survival showed a significant difference during ALS (p=0.01), with DBP in ETCO2-guided survivors higher than that in standard group non-survivors (p=0.001) and ETCO2 group non-survivors (p=0.04; Fig. 3B1).
MAP, CVP, and ICP (but not SPP or CPP) were higher in the ETCO2 group than in the standard group during BLS (Table 2, Fig. 2D–H), but all five variables were higher in the ETCO2 group during ALS. After the first epinephrine administration, we observed greater increases in MAP (24 ± 18 vs 9 ± 11 mmHg, p=0.02), SPP (23 ± 18 vs 9 ± 12 mmHg, p=0.01), and CPP (21 ± 16 vs 9 ± 11 mmHg, p=0.03), but not CVP or ICP, in the ETCO2 group. MAP, CPP, ICP, and SPP were higher in survivors than in non-survivors during ALS (Table 3, Fig. 3C and 3D). After the first epinephrine administration, MAP increased more in survivors than in non-survivors (33 ± 18 vs 9 ± 8 mmHg, p=0.0001) as did SPP (30 ± 21 vs 9 ± 9 mmHg, p=0.0005), ICP (4 ± 7 vs 0.3 ± 3 mmHg, p=0.03), and CPP (29 ± 18 vs 9 ± 8 mmHg, p=0.0003). ANOVA of SPP by group and survival demonstrated a significant difference during ALS (p=0.02), with SPP in the ETCO2 group survivors and nonsurvivors being greater than that of the standard group non-survivors (p=.006 & p=.031, respectively; Fig. 3C1).
Autopsy results revealed that the chest deformity (change in pre-arrest to post-resuscitation anteroposterior chest diameter) was less in the ETCO2 group than in the standard group (1.1 ± 0.7 vs 1.8 ± 1.0 cm, p=0.05). This difference may be due to restoration of chest shape by ventilation during the period of ROSC, which occurred more often in the ETCO2 group. Epicardial hemorrhages, which were superficial and along the distribution of the right coronary artery, were greater in the ETCO2 group (77% vs 33%). These superficial hemorrhages were more common in survivors than in non-survivors in both groups (78% vs 37%). If related to resuscitative efforts, their presence did not appear to interfere with the rate of ROSC. We also noted a nonsignificant increase in hemothorax in the ETCO2-guided group. These hemothoraces appeared to be caused by the fibrillation wire perforating the vasculature during BLS and were corrected by withdrawing the wire before CPR in subsequent experiments. Other injuries did not differ between study groups (Table 4).
DISCUSSION
This proof-of-concept study showed that ETCO2-guided chest compression can improve survival compared to standard CPR for cardiac arrest. By providing real-time, physiologic feedback, ETCO2 monitoring enabled rescuers to adjust chest compression delivery to optimize the patient’s response. In this study, resuscitators adjusted chest compression factors such as hand position, rate, depth, force, and release in response to ETCO2 level. The sustained improvement in the ETCO2 level is believed to correspond to improved pulmonary blood flow and systemic cardiac output.
The higher ETCO2 level and ROSC rate in the ETCO2-guided group were due primarily to increases in chest compression rate and force in response to the ongoing feedback. The observers thought that chest compression release was good and that neither group exhibited excessive leaning. In one of the ETCO2-guided experiments, the ETCO2 level improved noticeably when the compressor’s thumbs were moved to the left of midline, but no change in ETCO2 was noticed with hand position changes in the other 13 experiments of that group. Because a pediatric accelerometer did not work well on the small chest of the animals, objective data for depth and force are not available. The increases in MAP, mean CVP, and mean ICP seen in the ETCO2-guided group probably represent an increase in force by the resuscitators. The increased force did not cause significant chest compression deformity or injuries detected by autopsy. The chest compression rate was considerably increased by the resuscitators in the ETCO2-guided group to maximize ETCO2 levels (126/minute at 30 seconds to 157/minute at 20 minutes; Fig. 2B). The benefit of the fast compression rate may be specific for our model and relate to the very compliant chest wall and a cardiac-pump type mechanism of blood flow (28). Additional research is needed to determine if the use of compression rates above the guideline recommendations (100–120 per minute) are effective and safe before this method is considered for clinical use.
In our previous study, ETCO2-guided CPR was comparable to standard CPR after a short-duration arrest interval (11). In that study, the group guided by ETCO2 alone saw a 70% rate of ROSC, whereas the standard CPR group, which received verbal and visual feedback about CPR quality saw a 65% rate of ROSC. In the current study, we tested whether ETCO2-guided chest compression delivery would improve the rate of ROSC when survival from standard CPR was likely to be very low. Therefore, we used a prolonged, 20-minute asphyxia-ventricular fibrillation arrest model that produces an ROSC rate of only 15% with standard CPR. The 50% rate of ROSC with ETCO2-guided chest compression indicates that this method has the potential to improve resuscitative efforts after prolonged arrest or during prolonged resuscitation.
An interesting finding is that survivors and non-survivors in the ETCO2 group had similar ETCO2 levels (approximately 30 mmHg) throughout CPR. Low levels of ETCO2 during CPR have been associated with low-quality CPR, a decreased rate of ROSC, and futility while high levels of ETCO2 have been associated with survival (18, 25, 29). Our results indicate that attempting to raise ETCO2 levels during CPR can result in increased ETCO2 levels that do not indicate likelihood of ROSC. Figure 3B1 shows that the relationship between the relaxation arterial pressure (a surrogate for myocardial perfusion (10, 30–32)) and survival is present in both study groups during BLS. In contrast, the ETCO2, SPP, and CPP levels during BLS in the non-survivors of the ETCO2-guided group overlap the survivors of both groups (Fig. 3A1, 3C1, and 3D1). The ETCO2 levels produced by ETCO2-guided chest compression delivery appear to correspond better to systemic perfusion than myocardial perfusion. Despite this apparent disconnect between ETCO2 and DBP, the ETCO2-guided group was more likely to experience ROSC. The increased response in MAP and DBP in the ETCO2 group to early epinephrine administration suggests that improved systemic perfusion better maintains vascular responsiveness during prolonged CPR. A better understanding of the relationship between DBP and ETCO2 during prolonged CPR is needed to determine how to maximize both when ROSC is difficult to achieve.
One concern of using ETCO2-guided chest compression for pediatric arrest has been that significant hypercarbia caused by prolonged asphyxia might cofound implementation of ETCO2 guidance. The ETCO2 in the standard and ETCO2 groups fell to 25 and 32 mmHg, respectively, at 1 minute of CPR, a range that was useful and persisted during the remainder of resuscitation. Others have also reported a similar rapid decrease in ETCO2 after asphyxial arrest (33, 34). Another potential confounder of ETCO2-guided CPR is that epinephrine administration might affect pulmonary blood flow and ETCO2 levels. Others have reported that ETCO2 levels could be temporarily increased or decreased after epinephrine administration (35–42). However, we did not observe fluctuations in ETCO2 in either study group after epinephrine administration at 10, 14, or 18 minutes of CPR (Fig. 2A). The fact that ETCO2 levels did not decrease as the effect of epinephrine on DBP and SPP (Fig. 2C and 2F) wore off at 12 and 16 minutes of CPR may indicate that ETCO2 cannot be used to guide early epinephrine administration and that invasive monitoring would be most useful for this role. We will need additional studies to determine if the lack of ETCO2 responsiveness to epinephrine administration is due to the long arrest interval used in this model. In our previous study that used a short arrest interval, most animals achieved ROSC with the first epinephrine dose, making it difficult to know if the increased ETCO2 level was from epinephrine, ROSC, or both (11).
CPR guided by physiologic feedback has the potential to improve cardiac and neurologic outcomes when resuscitation efforts become prolonged. Extracorporeal CPR (eCPR) technology is improving, but rates of neurologic injury and failure to wean from support after recovery remain high. Hemodynamic-directed CPR has shown great promise for improving survival and neurologic outcome in preclinical models when an arterial line is present to guide epinephrine administration (8–10, 32). Near-infrared spectroscopy measurements and amplitude analysis of ventricular fibrillation are other physiologic responses that have the potential to guide resuscitation efforts. Resuscitation guidelines offer evidence-based recommendations for a common initial resuscitation approach and for CPR quality. The use of physiologic feedback offers the potential to maximize those recommendations, individualize efforts, and, when needed, modify resuscitative efforts beyond current guidelines.
Several limitations of this study deserve attention. First, rate of ROSC is a limited outcome measure for resuscitation studies. Longer-term outcomes and neurologic status are much more useful and will need to be addressed in future studies. With the current prolonged arrest interval, long-term survival will likely require intensive care, and neurologic outcomes may be very poor. Second, we did not have an accelerometer to measure compression depth, force, or release. Chest compression rate was the only objective measure we had of alterations to CPR delivery. It is very likely that force, and possibly depth or release, were different between groups and contributed to our findings. When a neonatal accelerometer becomes commercially available, it would be very useful to add it to our studies. Third, it is unclear whether an asphyxial arrest model in healthy animals is applicable to neonatal, infant, or pediatric arrests. This model might correlate with apnea that leads to arrest in cases of sudden infant death syndrome, choking, suffocation, or drowning. However, the study does not address the usefulness of an ETCO2-guided resuscitation method when lung disease leads to respiratory and then cardiac arrest. Therefore, we cannot generalize its applicability to all pediatric cardiopulmonary arrests. Fourth, baseline ventilation parameters (a breath rate of 20 per minute) and mechanical ventilation were used during CPR which may limit the clinical applicability of this study. Current recommendations are for reduced breath rates of 10–12 per minute to prevent over-ventilation during CPR. Mechanical ventilation was continued, rather than switching to manual ventilation, to minimize the number of variables under investigator control in this necessarily unblinded study. Pressure controlled ventilation is commonly used in children and its use during experimental CPR can result in reduced tidal volumes and require an increased breath rate. Table 4 shows that the blood gas mean values at eight minutes of BLS are appropriate (PaCO2 54 and 42 mmHg and PaO2 132 and 120 mmHg) and that using mechanical ventilation and a breath rate of 20 per minute did not produce over-ventilation. Fifth, the lack of blinding of resuscitators may provide bias in the study. The standard group was observed for performance of the recommended chest compression rate, depth, release, and hand position (see diagrams and description in reference 11) and was blinded to ETCO2 level, whereas the experimental group was instructed to improve ETCO2 levels by adjusting these variables. Thus, improving resuscitator performance in the ETCO2 group was part of the study design. Nevertheless, study outcomes were objectively measured (rate of ROSC, hemodynamic variables, and presence of injuries).
CONCLUSIONS
ETCO2-guided chest compression delivery has the potential to improve neonatal/infant outcomes from prolonged cardiac arrest over standard CPR. Concerns that interference from hypercarbia or epinephrine administration might interfere with the use of ETCO2-guidance were unsupported. Additional study is needed to determine the benefit on long-term outcomes of ETCO2-guided chest compression delivery and how it can be used to individualize and optimize resuscitation by manipulating parameters within and beyond published guidelines.
Acknowledgments
Sources of Funding: Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development award R21HD072845, by the National Institute of Neurological Disorders and Stroke awards K08NS080984 and R01NS060703, and by the National Center for Research Resources of the National Institutes of Health Roadmap for Medical Research grant UL1RR025005. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Copyright form disclosure:
Dr. Lee’s institution received funding from the NIH; she received support for article research from the NIH; and she received funding from Medtronic. Dr. Koehler received support for article research from the NIH. Dr. Hunt received support for article research from the NIH; her institution received grant funding from Laerdal Foundation for Acute Care Medicine and from Hartwell Foundation; and she received funding from Zoll Medical Corporation (consulting, patents where Zoll Medical now has a non-exclusive license for two of the devices). Dr. Shaffner’s institution received funding from the National Institute of Child Health and Human Development and the National Institute of Neurological Disorders and Stroke; he received funding from Wolters Kluwer; and he received support for article research from the NIH.
The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Atkins DL, Everson-Stewart S, Sears GK, et al. Epidemiology and outcomes from out-of-hospital cardiac arrest in children: the Resuscitation Outcomes Consortium Epistry-Cardiac Arrest. Circulation. 2009;119:1484–1491. doi: 10.1161/CIRCULATIONAHA.108.802678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berg MD, Schexnayder SM, Chameides L, et al. Part 13: pediatric basic life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S862–S875. doi: 10.1161/CIRCULATIONAHA.110.971085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kleinman ME, Chameides L, Schexnayder SM, et al. Pediatric advanced life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Pediatrics. 2010;126:e1361–e1399. doi: 10.1542/peds.2010-2972D. [DOI] [PubMed] [Google Scholar]
- 4.Girotra S, Spertus JA, Li Y, et al. Survival trends in pediatric in-hospital cardiac arrests: an analysis from Get With the Guidelines-Resuscitation. Circ Cardiovasc Qual Outcomes. 2013;6:42–49. doi: 10.1161/CIRCOUTCOMES.112.967968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fink EL, Prince DK, Kaltman JR, et al. Unchanged pediatric out-of-hospital cardiac arrest incidence and survival rates with regional variation in North America. Resuscitation. 2016;107:121–128. doi: 10.1016/j.resuscitation.2016.07.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuisma M, Suominen P, Korpela R. Paediatric out-of-hospital cardiac arrests–epidemiology and outcome. Resuscitation. 1995;30:141–150. doi: 10.1016/0300-9572(95)00888-z. [DOI] [PubMed] [Google Scholar]
- 7.Manning JE. Feasibility of blind aortic catheter placement in the prehospital environment to guide resuscitation in cardiac arrest. J Trauma Acute Care Surg. 2013;75:S173–7. doi: 10.1097/TA.0b013e318299d9ee. [DOI] [PubMed] [Google Scholar]
- 8.Sutton RM, Friess SH, Bhalala U, et al. Hemodynamic directed CPR improves short-term survival from asphyxia-associated cardiac arrest. Resuscitation. 2013;84:696–701. doi: 10.1016/j.resuscitation.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friess SH, Sutton RM, French B, et al. Hemodynamic directed CPR improves cerebral perfusion pressure and brain tissue oxygenation. Resuscitation. 2014;85:1298–1303. doi: 10.1016/j.resuscitation.2014.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naim MY, Sutton RM, Friess SH, et al. Blood pressure- and coronary perfusion pressure-targeted cardiopulmonary resuscitation improves 24-hour survival from ventricular fibrillation cardiac arrest. Crit Care Med. 2016;44:e1111–e1117. doi: 10.1097/CCM.0000000000001859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamrick JL, Hamrick JT, Lee JK, et al. Efficacy of chest compressions directed by end-tidal CO2 feedback in a pediatric resuscitation model of basic life support. J Am Heart Assoc. 2014;3:e000450. doi: 10.1161/JAHA.113.000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanders AB, Atlas M, Ewy GA, et al. Expired PCO2 as an index of coronary perfusion pressure. Am J Emerg Med. 1985;3:147–149. doi: 10.1016/0735-6757(85)90039-7. [DOI] [PubMed] [Google Scholar]
- 13.Weil MH, Bisera J, Trevino RP, et al. Cardiac output and end-tidal carbon dioxide. Crit Care Med. 1985;13:907–909. doi: 10.1097/00003246-198511000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Garnett AR, Ornato JP, Gonzalez ER, et al. End-tidal carbon dioxide monitoring during cardiopulmonary resuscitation. JAMA. 1987;257:512–515. [PubMed] [Google Scholar]
- 15.Falk JL, Rackow EC, Weil MH. End-tidal carbon dioxide concentration during cardiopulmonary resuscitation. N Engl J Med. 1988;318:607–611. doi: 10.1056/NEJM198803103181005. [DOI] [PubMed] [Google Scholar]
- 16.Gazmuri RJ, von Planta M, Weil MH, et al. Arterial PCO2 as an indicator of systemic perfusion during cardiopulmonary resuscitation. Crit Care Med. 1989;17:237–240. doi: 10.1097/00003246-198903000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Ornato JP, Levine RL, Young DS, et al. The effect of applied chest compression force on systemic arterial pressure and end-tidal carbon dioxide concentration during CPR in human beings. Ann Emerg Med. 1989;18:732–737. doi: 10.1016/s0196-0644(89)80005-8. [DOI] [PubMed] [Google Scholar]
- 18.Sanders AB, Kern KB, Otto CW, et al. End-tidal carbon dioxide monitoring during cardiopulmonary resuscitation. A prognostic indicator for survival JAMA. 1989;262:1347–1351. [PubMed] [Google Scholar]
- 19.Isserles SA, Breen PH. Can changes in end-tidal PCO2 measure changes in cardiac output? Anesth Analg. 1991;73:808–814. doi: 10.1213/00000539-199112000-00023. [DOI] [PubMed] [Google Scholar]
- 20.Gazmuri RJ, Weil MH, Bisera J, et al. End-tidal carbon dioxide tension as a monitor of native blood flow during resuscitation by extracorporeal circulation. J Thorac Cardiovasc Surg. 1991;101:984–988. [PubMed] [Google Scholar]
- 21.Ward KR, Menegazzi JJ, Zelenak RR, et al. A comparison of chest compressions between mechanical and manual CPR by monitoring end-tidal PCO2 during human cardiac arrest. Ann Emerg Med. 1993;22:669–674. doi: 10.1016/s0196-0644(05)81845-1. [DOI] [PubMed] [Google Scholar]
- 22.Yannopoulos D, Aufderheide TP, McKnite S, et al. Hemodynamic and respiratory effects of negative tracheal pressure during CPR in pigs. Resuscitation. 2006;69:487–494. doi: 10.1016/j.resuscitation.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Sheak KR, Wiebe DJ, Leary M, et al. Quantitative relationship between end-tidal carbon dioxide and CPR quality during both in-hospital and out-of-hospital cardiac arrest. Resuscitation. 2015;89:149–154. doi: 10.1016/j.resuscitation.2015.01.026. [DOI] [PubMed] [Google Scholar]
- 24.Gudipati CV, Weil MH, Bisera J, et al. Expired carbon dioxide: a noninvasive monitor of cardiopulmonary resuscitation. Circulation. 1988;77:234–9. doi: 10.1161/01.cir.77.1.234. [DOI] [PubMed] [Google Scholar]
- 25.Link MS, Berkow LC, Kudenchuk PJ, et al. Part 7: Adult Advanced Cardiovascular Life Support: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132:S444–64. doi: 10.1161/CIR.0000000000000261. [DOI] [PubMed] [Google Scholar]
- 26.de Caen AR, Maconochie IK, Aickin R, et al. Part 6: Pediatric Basic Life Support and Pediatric Advanced Life Support: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2015;132:S177–203. doi: 10.1161/CIR.0000000000000275. [DOI] [PubMed] [Google Scholar]
- 27.Meaney PA, Bobrow BJ, Mancini ME, et al. Cardiopulmonary resuscitation quality: improving cardiac resuscitation outcomes both inside and outside the hospital: a consensus statement from the American Heart Association. Circulation. 2013;23:128, 417–35. doi: 10.1161/CIR.0b013e31829d8654. [DOI] [PubMed] [Google Scholar]
- 28.Babbs CF. New versus old theories of blood flow during CPR. Crit Care Med. 1980;8:191–195. doi: 10.1097/00003246-198003000-00026. [DOI] [PubMed] [Google Scholar]
- 29.Levine RL, Wayne MA, Miller CC. End-tidal carbon dioxide and outcome of out-of-hospital cardiac arrest. N Engl J Med. 1997;337:301–306. doi: 10.1056/NEJM199707313370503. [DOI] [PubMed] [Google Scholar]
- 30.Sanders AB, Ewy GA, Taft TV. Prognostic and therapeutic importance of the aortic diastolic pressure in resuscitation from cardiac arrest. Crit Care Med. 1984;12:871–873. doi: 10.1097/00003246-198410000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Sanders AB, Ogle M, Ewy GA. Coronary perfusion pressure during cardiopulmonary resuscitation. Am J Emerg Med. 1985;3:11–14. doi: 10.1016/0735-6757(85)90003-8. [DOI] [PubMed] [Google Scholar]
- 32.Morgan RW, French B, Kilbaugh TJ, et al. A quantitative comparison of physiologic indicators of cardiopulmonary resuscitation quality: diastolic blood pressure versus end-tidal carbon dioxide. Resuscitation. 2016;104:6–11. doi: 10.1016/j.resuscitation.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berg RA, Henry C, Otto CW, et al. Initial end-tidal CO2 is markedly elevated during cardiopulmonary resuscitation after asphyxial cardiac arrest. Pediatr Emerg Care. 1996;12:245–248. doi: 10.1097/00006565-199608000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Grmec S, Lah K, Tusek-Bunc K. Difference in end-tidal CO2 between asphyxia cardiac arrest and ventricular fibrillation/pulseless ventricular tachycardia cardiac arrest in prehospital setting. Crit Care. 2003;7:R139–R144. doi: 10.1186/cc2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gonzalez ER, Ornato JP, Garnett AR, et al. Dose-dependent vasopressor response to epinephrine during CPR in human beings. Ann Emerg Med. 1989;18:920–926. doi: 10.1016/s0196-0644(89)80453-6. [DOI] [PubMed] [Google Scholar]
- 36.Martin GB, Gentile NT, Paradis NA, et al. Effect of epinephrine on end-tidal carbon dioxide monitoring during CPR. Ann Emerg Med. 1990;19:396–398. doi: 10.1016/s0196-0644(05)82345-5. [DOI] [PubMed] [Google Scholar]
- 37.Tang W, Weil MH, Gazmuri RJ, et al. Pulmonary ventilation/perfusion defects induced by epinephrine during cardiopulmonary resuscitation. Circulation. 1991;84:2101–2107. doi: 10.1161/01.cir.84.5.2101. [DOI] [PubMed] [Google Scholar]
- 38.Callaham M, Barton C, Matthay M. Effect of epinephrine on the ability of end-tidal carbon dioxide readings to predict initial resuscitation from cardiac arrest. Crit Care Med. 1992;20:337–343. doi: 10.1097/00003246-199203000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Chase PB, Kern KB, Sanders AB, et al. Effects of graded doses of epinephrine on both noninvasive and invasive measures of myocardial perfusion and blood flow during cardiopulmonary resuscitation. Crit Care Med. 1993;21:413–419. doi: 10.1097/00003246-199303000-00020. [DOI] [PubMed] [Google Scholar]
- 40.Cantineau JP, Merckx P, Lambert Y, et al. Effect of epinephrine on end-tidal carbon dioxide pressure during prehospital cardiopulmonary resuscitation. Am J Emerg Med. 1994;12:267–270. doi: 10.1016/0735-6757(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 41.Rivers EP, Wortsman J, Rady MY, et al. The effect of the total cumulative epinephrine dose administered during human CPR on hemodynamic, oxygen transport, and utilization variables in the postresuscitation period. Chest. 1994;106:1499–1507. doi: 10.1378/chest.106.5.1499. [DOI] [PubMed] [Google Scholar]
- 42.Ryu SJ, Lee SJ, Park CH, et al. Arterial pressure, end-tidal carbon dioxide, and central venous oxygen saturation in reflecting compression depth. Acta Anaesthesiol Scand. 2016;60:1012–1023. doi: 10.1111/aas.12728. [DOI] [PubMed] [Google Scholar]


