Abstract
Background
Concerns exist that clozapine is underutilized in the management of treatment-resistant schizophrenia. Although a 2015 change in the US Food and Drug Administration (FDA) monitoring recommendations lowered the threshold of the absolute neutrophil count for treatment interruption from 1,500/μL to 1,000/μL and removed white blood cell count thresholds from the monitoring algorithm, the implications of this policy change on clozapine interruptions remain unknown.
Methods
We analyzed outpatient prescribing records for antipsychotic medications in the Veterans Integrated Service Network 7 (VISN 7) database between 1999 and 2012 to assess the potential impact of the recent changes in FDA neutropenia monitoring recommendations on clozapine treatment discontinuation. We evaluated results of complete blood count monitoring to compare percentages of patients who developed or would have developed ≥ 1 hematologic event under the previous and current FDA guidelines in the first year following initiation of clozapine.
Results
From a cohort of 14,620 patients with schizophrenia (ICD-9-295.x), 246 patients received clozapine treatment (1.7%). No agranulocytosis was observed during the study period. Under the former recommendations, 5 patients in the clozapine initiation cohort (n=160, 3.1%; 95% CI, 0.43–5.83) qualified for treatment interruption during the first year of clozapine treatment, while only 1 patient (0.6%) qualified under the current recommendations. Under the former recommendations, hematologic events occurred at a similar rate for individuals taking and not taking clozapine.
Conclusions
While clozapine remains an underused medication, the new FDA monitoring guidelines are likely to substantially reduce the percentage of patients who meet criteria for clozapine-associated hematologic events requiring treatment interruption. This decrease may reduce the clinical burden of managing patients on clozapine and therefore increase the number of individuals treated with this uniquely effective medication. However, prospective studies of individuals treated under the new guidelines are needed to fully assess safety of the FDA’s change.
Schizophrenia is a significant mental disorder affecting approximately 1% of the adult population and is a major cause of disability in the United States and worldwide.1 Within the Veterans Affairs (VA) population, rates of diagnosis are higher than that of the general population.2 Antipsychotics are the cornerstone of treatment for schizophrenia; however, as much as 30% of patients receive little benefit from standard antipsychotic medications.3–5 Patients who do not respond well to multiple trials of antipsychotic medications are considered treatment-resistant cases and have greater disability.6
Clozapine remains the single medication approved by the US Food and Drug Administration (FDA) for treatment-resistant schizophrenia. Clinical trials have demonstrated clozapine’s superior efficacy in reducing psychotic symptoms in treatment-resistant schizophrenia; it may be the only medication that improves both positive and negative symptoms.7,8 Patients treated with clozapine have also been shown to have lower rates of suicide attempts, psychiatric hospitalizations, antipsychotic nonadherence, and use of an additional antipsychotic as compared with patients treated with other antipsychotics.9,10 However, a recent meta-analysis11 has generated some debate regarding clozapine’s superiority over other second-generation antipsychotics for treatment-resistant schizophrenia.12
Despite clozapine’s indication for treatment-refractory patients with schizophrenia, only a minority of such patients receive this medication. Although as much as 30% of individuals with schizophrenia are considered treatment-resistant, clozapine accounts for less than 6% of antipsychotics prescribed to people with schizophrenia in the United States.3,13 Concerns about tolerability and side-effect complications are barriers to treatment.14 Specifically, treatment with clozapine poses an increased risk of agranulocytosis and requires monitoring through regular blood draws examining white cell counts.15 The complexity of this monitoring system differs across countries and health systems16 and can be arduous, presenting further barriers to treatment.
To help address these barriers, the FDA instituted in 2015 several changes to the guidelines regarding clozapine treatment and monitoring. The new recommendations seek to increase clozapine treatment continuity without endangering patients’ health. Among other changes, the FDA reduced the threshold for interruption of clozapine treatment for absolute neutrophil counts (ANC) from 1,500/μL to 1,000/μL and removed total white blood cell count (WBC) thresholds from the monitoring algorithm (Table 1).16 These changes substantially reduced the complexity of the clozapine monitoring algorithm. The intent of the new policy is to reduce barriers to initiation and continuity of clozapine treatment.
Table 1.
Comparison of FDA Changes to CBC Guidelinesa for Interruption of Clozapine Treatment
| Guidelines | WBCs [μL] | ANCs [μL] |
|---|---|---|
| Previous | <3,000 | <1,500 |
| Current | Not monitored | <1,000 |
Abbreviations: ANC=absolute neutrophil count, CBC=complete blood count, FDA=US Food and Drug Administration, WBC=white blood cell count.
Little is known about the potential effects of the new FDA guidelines on clinical practice. Specifically, it is unknown how the new thresholds for defining hematologic events are likely to affect the number of patients who will no longer meet the hematologic threshold for treatment interruption. The VA offers a unique opportunity to examine this question, as it generates data on clozapine monitoring for patients with schizophrenia from 1999 forward. We examined these data to assess the effects the new guidelines would have in the VA schizophrenia population and on the detection of hematologic events within that population during the first year of clozapine treatment.
METHODS
This retrospective cohort study evaluated the effects of the 2015 revisions to the FDA complete blood count (CBC) monitoring recommendations for clozapine treatment for individuals with schizophrenia. Within the VA health care system, we compared patients’ qualifying hematologic events under the original and revised guidelines. The study was approved and exempted from our respective Institutional Review Board Committees.
Data Source
The Veterans Integrated Service Network (VISN) Southeast Network is VISN 7, encompassing the VA medical centers of Alabama, Georgia, and South Carolina. The VISN 7 Corporate Database consists of computerized clinical and administrative data downloaded from each VA medical facility within VISN 7. The data are housed on a server in a format that permits extraction of data for research and management purposes.
Cohort Construction
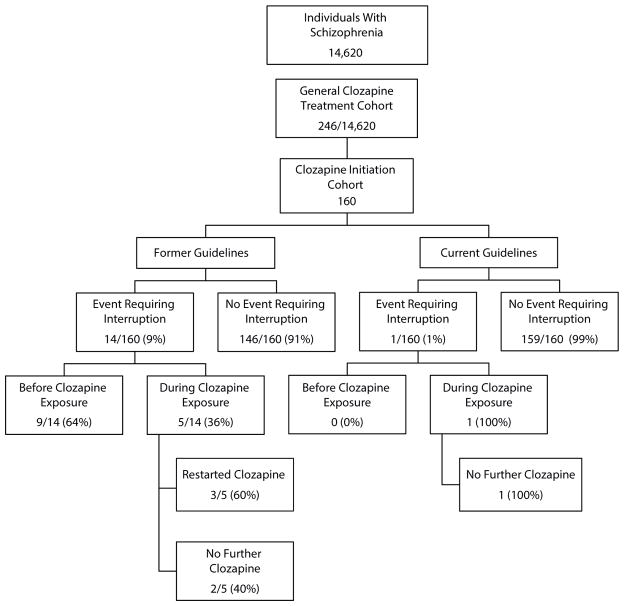
We analyzed the outpatient prescribing records for antipsychotics in VISN 7 between 1999 and 2012. The subjects were all patients treated with a diagnosis of schizophrenia. For purposes of this study, the diagnosis of schizophrenia was defined by at least 2 visits in a VA mental health clinic for which a mental health clinician (psychiatrist, psychologist, or nurse practitioner) conferred an encounter diagnosis of schizophrenia (ICD-9–295.xx).
In our analysis, we included 2 cohorts, a general clozapine treatment cohort and a clozapine initiation cohort. The general clozapine treatment cohort included all individuals with a diagnosis of schizophrenia in the VISN 7 network who received a clozapine prescription during the study period. The clozapine initiation cohort included those individuals who had new treatment episodes of clozapine. For the initiation cohort, we monitored events that occurred while patients were taking clozapine within the first year of clozapine initiation. We focused on new treatment episodes and the first year of exposure because this period is believed to be the high-risk period for neutropenia and agranulocytosis.17
New treatment episodes included only individuals who had 5 years of observed service use without clozapine before their first clozapine prescription and who provided at least 1 year of follow-up from their first prescription. The treatment episodes were considered continuous if the patients refilled their clozapine prescription within 30 days of the termination of their days of supply. Follow-up was censored at 1 year after clozapine initiation or loss of VA eligibility, whichever occurred first.
Hematologic Events
For this analysis, neutropenic and leukopenic events were defined according to FDA guidelines for WBC values that required clozapine treatment interruption or discontinuation. Under former recommendations, ANCs less than 1,500/μL or WBCs less than 3,000/μL defined a hematologic event. Current recommendations dictate interruption or discontinuation of clozapine treatment only for events with ANCs less than 1,000/μL (Table 1). If an individual qualified on the basis of an ANC and a WBC result and those results occurred fewer than 5 days apart, the hematologic event was counted only once.
Monitored Characteristics
We recorded basic clinical and demographic characteristics including age, sex, race, and CBC results. For those individuals who had hematologic events that qualified for treatment interruption or termination, we compared current milligrams of clozapine, number of events, whether clozapine was restarted, and the number of days into titration when the event occurred. We evaluated the medical complexity of the patients as determined by greater than 1 clinical diagnosis of diabetes mellitus, hypertension, or hyperlipidemia.
Analysis Plan
For the general clozapine treatment cohort, we determined the proportion of patients with a schizophrenia diagnosis per year who received any clozapine treatment during the study period, total clozapine treatment exposure, and rate of agranulocytosis. All other analyses were conducted with the clozapine initiation cohort only.
Using the clozapine initiation cohort, we evaluated results of CBC monitoring to determine percentage of the cohort that developed ≥ 1 hematologic event under the former FDA guidelines as compared with those same individuals under the current guidelines. This comparison of the number of hematologic events that required treatment interruption allowed us to evaluate treatment continuity and discontinuation under the different guidelines. We calculated percentages of individuals with events and mean number of events. We assessed the clinical and demographic characteristics of those individuals with events. In a post hoc analysis, we examined hematologic events that occurred in the 5 years preceding patients’ clozapine treatment episode. These hematologic events would have qualified for clozapine interruption had they occurred during clozapine treatment.
RESULTS
General Clozapine Treatment Cohort
From a sample of 14,620 individuals with schizophrenia (ICD-9–295.x), 246 (1.7%) patients received any clozapine treatment between 1999 and 2012. Over the 13-year period, those 246 individuals contributed 509,562 days (1,396 years) of clozapine treatment exposure. The annualized rate of clozapine treatment trended downward during the study period from a rate of 2.3% (1999) to 1.1% (2012) with a mean of 1.3%. None of these individuals had reported agranulocytosis (ANC <500 μL) while treated with clozapine.
Clozapine Initiation Cohort
From the original population of 246 individuals, 160 individuals were eligible for the clozapine initiation cohort. These 160 patients represented only new clozapine treatment episodes. Under previous guidelines, 5 individuals (3.1%; 95% CI, 0.43–5.83) contributed 7 events within the first year of clozapine treatment that qualified for treatment interruption or termination. Under current guidelines, 1 person (0.6%) would have contributed 1 event that qualified as treatment interruption or discontinuation during the follow-up year. Mean number of events per person was 0.04 (95% CI, 0.00–0.08). Among those who had an event, the mean number of events per person was 1.4 (95% CI, 0.12–1.68).
Characteristics of patients with hematologic events
Table 2 characterizes the 5 patients who met criteria for clozapine treatment interruption or discontinuation under previous or current guidelines. All patients were male. The mean age was 45.8 years for patients who met criteria for a hematologic event under the previous guidelines. Three of the individuals were identified as white and 2 as black. One of the individuals was defined as medically complex. For individual patients, mean doses of clozapine during events ranged from 200 mg to 600 mg (overall mean = 375 mg). Maximum number of events for 1 individual was 3 (all others had only 1 event). Mean number of days into clozapine treatment before an event occurred was 146 (range, 34–335) days. Three (60.0%) of the patients were restarted on clozapine after their event. Under current guidelines, 1 patient would have met criteria for interruption of treatment. He was 52 years old, black, not medically complex, and receiving 600 mg of clozapine per day.
Table 2.
Comparison of Hematologic Events for Individuals With Schizophrenia Requiring Interruption of Clozapine Treatment During First Year of Clozapine Titrationa
| Guidelines | Identity | Age | Sex | Race | Medically Complexb | WBC (ANC) Result, μL | Number of Days Into Titration | Dose During Event, mg | Number of Events | Clozapine Restarted After Event |
|---|---|---|---|---|---|---|---|---|---|---|
| Previous | A | 46 | Male | White | No | (1.44) | 87 | 500 | 1 | Yes |
| B | 30 | Male | Black | Yes | 2.6 | 34 | 300 | 1 | Yes | |
| C | 57 | Male | White | No | 2.9 | 144 | 250 | 3 | Yes | |
| C | 2.6 | 177 | 200 | |||||||
| C | 2.9 | 182 | 200 | |||||||
| D | 44 | Male | White | No | 2.6 | 61 | 350 | 1 | No | |
| E | 52 | Male | Black | No | 2.81 (0.83) | 335 | 600 | 1 | No | |
| Current | E | 52 | Male | Black | No | (0.83) | 335 | 600 | 1 | No |
Italics highlight the 1 patient who had multiple hematologic events.
Defined as greater than clinical condition (hypertension, diabetes mellitus, hyperlipidemia).
Abbreviations: ANC=absolute neutrophil count, WBC=white blood cell count.
Hematologic events before and during clozapine treatment
Figure 1 compares the rate of hematologic events before and during clozapine treatment for the previous and current guidelines. In the 5 years before clozapine initiation, there were 35 events that would have qualified for clozapine termination or interruption under the previous guidelines had the patients been taking clozapine during those events. Conversely, using current guidelines, no hematologic events occurred that would have required treatment interruption or termination during the preceding 5 years. Under previous guidelines, rate of hematologic events during and predating clozapine treatment was 0.6 events/100 persons/year.
Figure 1. Comparison of Annual Rates of Hematologic Eventsa for Individuals With Schizophrenia Requiring Treatment Interruption Before and During Clozapine Exposure.
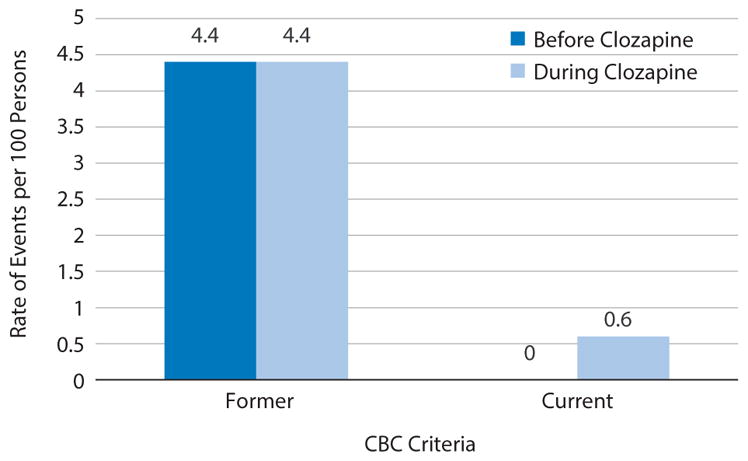
aDetermined by using US Food and Drug Administration complete blood count (CBC) criteria.
Figure 2 outlines and compares the distribution of individuals who required or would have required termination or interruption of the clozapine treatment under former and current guidelines based on their CBC values. Under previous guidelines, 9 individuals had hematologic events that would have qualified for termination or interruption of treatment had the individuals been on clozapine during the event. Under current guidelines, no individual had hematologic events predating clozapine that would have generated an interruption or termination of treatment or that would possibly have deterred a clinician from considering or starting clozapine.
Figure 2.
Comparison of Numbers of Individuals With Schizophrenia Requiring Clozapine Treatment Interruption Due to Hematologic Events Under Former and Current Guidelines From the US Food and Drug Administration
DISCUSSION
Consistent with prior research,17 we report that clozapine-induced agranulocytosis is a rare event. Further, clozapine-induced hematologic episodes that warrant discontinuation or interruption of treatment, though uncommon, were considerably more prevalent under the previous guidelines than they would be using the current FDA monitoring recommendations. These findings are consistent with the intent of the new FDA guidelines and have practical implications for the continuity of clozapine treatment in community practice.
Our understanding of the mechanism of clozapine-induced agranulocytosis remains unclear, though some research suggests a genetic vulnerability.18–20 However, the FDA’s decision to change the criteria for interruption and termination of clozapine treatment is consistent with the known clinical and basic science knowledge of immunology. The neutrophil’s role in phagocytic defense of the host is generally sufficient at counts above 1,000/μL. Lower counts are associated with increased susceptibility to infection, and counts below 500/μL place the host at risk for opportunistic infections. A neutrophil count of less than 200/μL provides no inflammatory defense.21
None of the individuals in our study population had an episode of agranulocytosis while taking clozapine. Previous research17 indicates that approximately 0.8% develop agranulocytosis in the first year of treatment. Our lower rate of agranulocytosis (0%) may be due to sample size or clinical characteristics, such as the rate of clozapine dose titration. While agranulocytosis in clozapine has never been linked to dose titration, dose–titration-related adverse events are seen in other medications,22 and the current recommended clozapine titrations are less aggressive than those used in older studies.7,23
Clozapine is associated with mild to moderate neutropenia, which is far more common and typically transient and benign.15 However, due to concerns that neutropenia could develop into agranulocytosis, occurrence of these events may lead to clozapine discontinuation.24 Approximately 3% of patients may develop severe neutropenia in the first year of clozapine treatment.22 Under the former guidelines, 3.1% of this study cohort had an event requiring treatment interruption or discontinuation. Given that some of these individuals were restarted on clozapine, their clozapine-induced hematologic events may have been transient.25 If this assumption is accurate, it would mean that the FDA’s new guidelines will reduce the frequency of unnecessary clozapine discontinuations.
In the clozapine treatment cohort, 14 individuals had a total of 42 events that would have qualified for clozapine termination under previous guidelines. However, most of these events (35 or 83.3%) occurred in the 5 years preceding any clozapine exposure. Under current guidelines, no events occurred outside of a clozapine treatment episode that would have generated an interruption or discontinuation or that could have deterred clinicians from even considering or starting clozapine treatment. This finding suggests that current guidelines are more specific in identifying clozapine-induced neutropenia and may allow more patients to start and continue clozapine as clinically appropriate.
When comparing the rate of hematologic events per year, individuals currently receiving clozapine treatment had similar rates of events before clozapine treatment (0.6 events/100 persons/year) as they had during treatment (0.6 events/100 persons/year). This result is supported by findings in the literature.24 Before clozapine initiation, these individuals were most likely treated with another antipsychotic. A recent article comparing individuals on clozapine to other second-generation antipsychotics found no statistical difference in the rate of neutropenia.24
Clozapine has a unique and highly regulated monitoring system developed out of concerns for its risk of agranulocytosis.16 A review26 of medications associated with agranulocytosis revealed 125 drugs with definite or probable causality to agranulocytosis. While some of these drugs have recommended blood monitoring, none have required monitoring systems similar to clozapine’s. The FDA’s change to the clozapine monitoring system places clozapine closer in line with other pharmacologic agents that have been associated with agranulocytosis.
While comparing clozapine with other drugs was not the primary goal of this study, these results further illustrate the very low prescribing of clozapine as a treatment for schizophrenia. The mean annual rate of 1.3% in a VA sample of schizophrenia is surprisingly low given expected rates of treatment-resistance of around 30%.3,13 Low rates of clozapine prescribing have been described previously in the VA, public, and private sectors.13,27–30 One international comparison suggests that lower rates in the United States may in part be due to more stringent monitoring guidelines in this country.30
Previous work demonstrating lower rates of clozapine in the VA system, as compared with private insurance, has been accounted for by prescriber reluctance to start clozapine in a population with high levels of disability and social isolation, which could increase nonadherence with blood monitoring.27 Developing “Clozapine Clinics,” similar to Warfarin Clinics, which would include support staff, such as phlebotomy, in addition to psychiatrists, could alleviate some of the administrative burden of clozapine treatment and potentially improve compliance.31 The VA system, as an integrated medical center, represents an opportunity to pioneer such a solution.
This study has several limitations. First, data are limited to individuals in the VA and to the period between 1999 and 2012; therefore, the population is middle-aged and largely male. Because the risks of clozapine-associated neutropenia and agranulocytosis may increase with age32 and may be higher in women than men,17 results might vary across patient populations. While we have no reason to believe that the rate of neutropenia or agranulocytosis would be different after 2012, further study of this phenomenon is warranted, and caution should be exercised in extending our findings outside of VA populations. Second, the uncommonness of hematologic, especially agranulocytic, events limits the precision of our analysis. Third, we did not account for the occurrence of benign ethnic neutropenia (BEN),33 but we expect that this makes our analysis more conservative, since the new guidelines allow for lower thresholds for patients with BEN.16 Fourth, individuals currently on clozapine treatment have regularly scheduled CBC monitoring. This monitoring creates a bias favoring detection of hematologic events during clozapine treatment episodes. Therefore, our comparisons with non-clozapine treatment periods should be viewed as exploratory. Finally, given that this study simulates the new guidelines for individuals who actually received treatment under the previous guidelines, our study was unable to assess the potential hematologic and other medical outcomes had clozapine treatment been continued, as the new guidelines would dictate. Therefore, we recommend prospective studies of the safety of the new monitoring system to help address this point.
The goal of the FDA’s changes to the clozapine monitoring system was to reduce complexity and improve access to a potentially life-altering treatment for patients with schizophrenia. Our findings demonstrate that clozapine-induced hematologic events are rare using either current or former guidelines. Clinicians should find these results reassuring. Moreover, the new monitoring guidelines are likely to substantially reduce the percentage of patients who meet criteria for clozapine-associated hematologic events requiring clinical intervention. This decrease may reduce the clinical burden of managing patients on this uniquely effective medication and increase its use in practice. It is important to note that along with the changing of the WBC criteria, the FDA also created a single monitoring system. This implementation has posed substantial administrative disruptions and obstacles for patients, providers, and health care systems. However, we expect that in the long term, these changes will reduce administrative barriers to clozapine treatment for patients and providers and may increase the use of clozapine in this population.
Finally, our analyses demonstrate the hypothetical implications of the FDA’s new monitoring guidelines on the frequency of clozapine treatment interruptions and that the new guidelines most likely promote longer periods of treatment continuity. This change by the FDA is consistent with basic science literature and moves clozapine treatment guidelines more in line with medications with similar hematologic concerns. However, our analysis is unable to assess the safety of these changes. Further, prospective studies with individuals treated under the new guidelines are necessary.
Clinical Points.
Despite the high prevalence of treatment-resistant schizophrenia, clozapine remains an underutilized treatment for patients with treatment-resistant schizophrenia. The recent update to US Food and Drug Administration (FDA) clozapine blood monitoring guidelines attempts to address this gap. However, its effect remains unknown.
The FDA’s update to clozapine monitoring guidelines substantially reduced the complexity of the clozapine monitoring system and reduces the frequency of interruption of clozapine treatment for schizophrenic patients.
The reduction in complexity and stringency of the clozapine monitoring guidelines should help clinicians and patients feel more comfortable with initiating and continuing this important treatment. However, close monitoring should be continued to fully assess the safety of these changes.
Acknowledgments
Funding/support: Funding was provided for Dr Sultan through a NIH T32 research training grant and to Dr Duncan by the Veterans Affairs Merit Review Program.
Role of the sponsor: The sponsors had no role in the study design, data collection, analysis, interpretation, or writing of this article.
Infrastructure support was provided by the Research and Development, Rehabilitation Research and Development, and Mental Health Service Lines of the Atlanta Veterans Affairs Medical Center; the Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine; and the New York State Psychiatric Institute and Columbia University College of Physicians and Surgeons. Dr Duncan is a full-time attending psychiatrist in the Mental Health Service Line at the Atlanta Veterans Affairs Medical Center, Decatur, Georgia.
Footnotes
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Veterans Affairs.
Potential conflicts of interest: Dr Correll has been a consultant and/or advisor to or has received honoraria from Alkermes, Allergan, Bristol-Myers Squibb, Forum, Gerson Lehrman Group, IntraCellular, Janssen/J&J, LB Pharma, Lundbeck, Medavante, Medscape, Neurocrine, Otsuka, Pfizer, ProPhase, Sunovion, Supernus, Takeda, and Teva; has provided expert testimony for Bristol-Myers Squibb, Janssen, and Otsuka; has served on a Data Safety Monitoring Board for Lundbeck and Pfizer; and received grant support from Takeda. Dr Duncan has received research support from Auspex. Drs Sultan and Olfson report no conflicts to disclose.
References
- 1.World Health Organization. The Global Burden of Disease. Geneva, Switzerland: WHO Press; 2008. [Google Scholar]
- 2.Chwastiak LA, Rosenheck RA, Desai R, et al. Association of psychiatric illness and all-cause mortality in the National Department of Veterans Affairs Health Care System. Psychosom Med. 2010;72(8):817–822. doi: 10.1097/PSY.0b013e3181eb33e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conley RR, Kelly DL. Management of treatment resistance in schizophrenia. Biol Psychiatry. 2001;50(11):898–911. doi: 10.1016/s0006-3223(01)01271-9. [DOI] [PubMed] [Google Scholar]
- 4.Duggan A, Warner J, Knapp M, et al. Modelling the impact of clozapine on suicide in patients with treatment-resistant schizophrenia in the UK. Br J Psychiatry. 2003;182:505–508. doi: 10.1192/bjp.182.6.505. [DOI] [PubMed] [Google Scholar]
- 5.Kelly DL, Kreyenbuhl J, Dixon L, et al. Clozapine underutilization and discontinuation in African Americans due to leucopenia. Schizophr Bull. 2007;33(5):1221–1224. doi: 10.1093/schbul/sbl068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies LM, Drummond MF. Assessment of costs and benefits of drug therapy for treatment-resistant schizophrenia in the United Kingdom. Br J Psychiatry. 1993;162:38–42. doi: 10.1192/bjp.162.1.38. [DOI] [PubMed] [Google Scholar]
- 7.Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789–796. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- 8.Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951–962. doi: 10.1016/S0140-6736(13)60733-3. [DOI] [PubMed] [Google Scholar]
- 9.Meltzer HY, Alphs L, Green AI, et al. International Suicide Prevention Trial Study Group. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT) Arch Gen Psychiatry. 2003;60(1):82–91. doi: 10.1001/archpsyc.60.1.82. [DOI] [PubMed] [Google Scholar]
- 10.Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of clozapine and standard antipsychotic treatment in adults with schizophrenia. Am J Psychiatry. 2016;173(2):166–173. doi: 10.1176/appi.ajp.2015.15030332. [DOI] [PubMed] [Google Scholar]
- 11.Samara MT, Dold M, Gianatsi M, et al. Efficacy, acceptability, and tolerability of antipsychotics in treatment-resistant schizophrenia: a network meta-analysis. JAMA Psychiatry. 2016;73(3):199–210. doi: 10.1001/jamapsychiatry.2015.2955. [DOI] [PubMed] [Google Scholar]
- 12.Kane JM, Correll CU. The role of clozapine in treatment-resistant schizophrenia. JAMA Psychiatry. 2016;73(3):187–188. doi: 10.1001/jamapsychiatry.2015.2966. [DOI] [PubMed] [Google Scholar]
- 13.Stroup TS, Gerhard T, Crystal S, et al. Geographic and clinical variation in clozapine use in the United States. Psychiatr Serv. 2014;65(2):186–192. doi: 10.1176/appi.ps.201300180. [DOI] [PubMed] [Google Scholar]
- 14.Gee S, Vergunst F, Howes O, et al. Practitioner attitudes to clozapine initiation. Acta Psychiatr Scand. 2014;130(1):16–24. doi: 10.1111/acps.12193. [DOI] [PubMed] [Google Scholar]
- 15.Nielsen J, Correll CU, Manu P, et al. Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided? J Clin Psychiatry. 2013;74(6):603–613. doi: 10.4088/JCP.12r08064. quiz 613. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen J, Young C, Ifteni P, et al. Worldwide differences in regulations of clozapine use. CNS Drugs. 2016;30(2):149–161. doi: 10.1007/s40263-016-0311-1. [DOI] [PubMed] [Google Scholar]
- 17.Alvir JM, Lieberman JA, Safferman AZ, et al. Clozapine-induced agranulocytosis: incidence and risk factors in the United States. N Engl J Med. 1993;329(3):162–167. doi: 10.1056/NEJM199307153290303. [DOI] [PubMed] [Google Scholar]
- 18.Athanasiou MC, Dettling M, Cascorbi I, et al. Candidate gene analysis identifies a polymorphism in HLA-DQB1 associated with clozapine-induced agranulocytosis. J Clin Psychiatry. 2011;72(4):458–463. doi: 10.4088/JCP.09m05527yel. [DOI] [PubMed] [Google Scholar]
- 19.Legge SE, Hamshere ML, Ripke S, et al. Genome-wide common and rare variant analysis provides novel insights into clozapine-associated neutropenia [published online ahead of print August 9, 2016] Mol Psychiatry. 2016 [Google Scholar]
- 20.Goldstein JI, Jarskog LF, Hilliard C, et al. Clozapine-induced agranulocytosis is associated with rare HLA-DQB1 and HLA-B alleles. Nat Commun. 2014;5:4757. doi: 10.1038/ncomms5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibson C, Berliner N. How we evaluate and treat neutropenia in adults. Blood. 2014;124(8):1251–1258. doi: 10.1182/blood-2014-02-482612. quiz 1378. [DOI] [PubMed] [Google Scholar]
- 22.Levi N, Bastuji-Garin S, Mockenhaupt M, et al. Medications as risk factors of Stevens-Johnson syndrome and toxic epidermal necrolysis in children: a pooled analysis. Pediatrics. 2009;123(2):e297–e304. doi: 10.1542/peds.2008-1923. [DOI] [PubMed] [Google Scholar]
- 23.Atkin K, Kendall F, Gould D, et al. Neutropenia and agranulocytosis in patients receiving clozapine in the UK and Ireland. Br J Psychiatry. 1996;169(4):483–488. doi: 10.1192/bjp.169.4.483. [DOI] [PubMed] [Google Scholar]
- 24.Rettenbacher MA, Hofer A, Kemmler G, et al. Neutropenia induced by second generation antipsychotics: a prospective investigation. Pharmacopsychiatry. 2010;43(2):41–44. doi: 10.1055/s-0030-1249071. [DOI] [PubMed] [Google Scholar]
- 25.Manu P, Sarpal D, Muir O, et al. When can patients with potentially life-threatening adverse effects be rechallenged with clozapine? a systematic review of the published literature. Schizophr Res. 2012;134(2–3):180–186. doi: 10.1016/j.schres.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andersohn F, Konzen C, Garbe E. Systematic review: agranulocytosis induced by nonchemotherapy drugs. Ann Intern Med. 2007;146(9):657–665. doi: 10.7326/0003-4819-146-9-200705010-00009. [DOI] [PubMed] [Google Scholar]
- 27.Leslie DL, Rosenheck RA. Benchmarking the quality of schizophrenia pharmacotherapy: a comparison of the Department of Veterans Affairs and the private sector. J Ment Health Policy Econ. 2003;6(3):113–121. [PubMed] [Google Scholar]
- 28.Olfson M, Gerhard T, Crystal S, et al. Clozapine for schizophrenia: state variation in evidence-based practice. Psychiatr Serv. 2016;67(2):152. doi: 10.1176/appi.ps.201500324. [DOI] [PubMed] [Google Scholar]
- 29.Leslie D, Rosenheck R. Tenth Annual Report on Schizophrenia Pharmacotherapy in VA. Northeast Program Evaluation Center. West Haven VAMC: 2009. [Google Scholar]
- 30.Conley RR, Kelly DL, Lambert TJ, et al. Comparison of clozapine use in Maryland and in Victoria, Australia. Psychiatr Serv. 2005;56(3):320–323. doi: 10.1176/appi.ps.56.3.320. [DOI] [PubMed] [Google Scholar]
- 31.Freudenreich O, Henderson DC, Sanders KM, et al. Training in a clozapine clinic for psychiatry residents: a plea and suggestions for implementation. Acad Psychiatry. 2013;37(1):27–30. doi: 10.1176/appi.ap.11090159. [DOI] [PubMed] [Google Scholar]
- 32.Munro J, O’Sullivan D, Andrews C, et al. Active monitoring of 12,760 clozapine recipients in the UK and Ireland: beyond pharmacovigilance. Br J Psychiatry. 1999;175:576–580. doi: 10.1192/bjp.175.6.576. [DOI] [PubMed] [Google Scholar]
- 33.Haddy TB, Rana SR, Castro O. Benign ethnic neutropenia: what is a normal absolute neutrophil count? J Lab Clin Med. 1999;133(1):15–22. doi: 10.1053/lc.1999.v133.a94931. [DOI] [PubMed] [Google Scholar]