Abstract
This report establishes a novel application of a commercially available porcine urinary bladder extracellular matrix, MatriStem (ACell, Inc., Columbia, MD), in the salvage of partial loss of the skin paddle of a fibula osteoseptocutaneous free flap that was utilized for mandibular reconstruction.
Keywords: urinary bladder extracellular matrix, MatriStem, fibula free flap
Since the first report in the literature by Taylor et al 1 in 1975, and its subsequent adaptation for mandibular reconstruction by Hidalgo 2 in 1989, the fibula free flap has become a mainstay in mandibular reconstruction. The ability of this flap to provide bone, muscle, and skin for reconstruction of composite defects of the mandible coupled with its relative consistent anatomy, long vascular pedicle, and length of available bone has solidified the fibula free flap as a mainstay in mandibular reconstruction. 3 The flap's dual periosteal blood supply provided by the peroneal artery and the endosteal blood supply that is based on nutrient arteries grants reconstructive surgeons the ability to transfer up to 25 cm of fibula bone as well as the ability to incorporate multiple osteotomies, making the fibula an ideal choice for the reconstruction of long span mandibular defects. 3 For example, Rodriguez et al reported on the reconstruction of a 21-cm near total mandibular defect with a single fibula free flap. 4 Subsequently, Winters et al reported on a case of total mandibular reconstruction using bilateral fibula free flaps. 5
Even though the osseous portion of the fibula free flap is well defined and has been pushed to new reconstructive limits, the inclusion of a reliable skin paddle until recently was considered the most challenging aspect of the fibula free flap harvest. 3 Chen et al first reported in 1983 on the inclusion of a skin paddle with the harvest of the fibula bone. 6 Wei et al would go on to report in 1986 a detail description of the location of the septocutaneous vessels to increase the reliability of fibula free flap skin paddle. 7 Subsequent reports, such as the method described by Yu and colleagues that emphasizes the use of common anatomic landmarks, have allowed for an increase in the reliability of the fibula free flap skin paddle. 8 Nonetheless, what truly causes either partial or complete loss of the fibula skin paddle is dependent on a multitude of factors, such as iatrogenic vessel damage during flap elevation; poor perforator positioning resulting in kinking and/or compression; and variations in perforator anatomy (septocutaneous vs. musculocutaneous). 3 While there exists a significant body of literature devoted to increasing the reliability of the fibula skin paddle, not much is known on how to salvage partial or complete loss of the skin paddle in the presence of a viable fibula bone flap. 3 8 Therefore, in this technical note, we describe the application of a decellularized porcine urinary bladder matrix (UBM), MatriStem Surgical Matrix Thick (ACell, Inc., Columbia, MD), which consists of the lamina propria and epithelial basement membrane of a porcine bladder in the salvage of the skin paddle of a fibula osteoseptocutaneous free flap. Our rational for the use of this bioengineered construct was to optimize and/or accelerate the wound healing process toward a more natural native tissue in avoidance of scar tissue formation.
Report of a Case
A 63-year-old woman was referred for evaluation of right posterior mandibular pain and a chronically draining orocutaneous fistula of the right neck. The patient was diagnosed with medication-related osteonecrosis of the right mandible 2 years following extraction of tooth no. 31. This diagnosis was attributed to a 3-year history of zoledronic acid (Zometa; Novartis Pharma AG, Basel, Switzerland) use for metastatic breast cancer. Patient had progression of mandibular osteonecrosis in the setting of long-term antimicrobial therapy. Patient's medical history was significant for hypertension and metastatic breast cancer. She was diagnosed with breast cancer 16 years prior, and underwent breast conserving surgery with combination chemotherapy consisting of cyclophosphamide, methotrexate, and 5-fluorouracil. At the time of initial presentation, the patient was undergoing treatment with Docetaxel (Taxotere). Patient was a never smoker, and the remainder of her social history was noncontributory.
Patient subsequently underwent right segmental mandibulectomy with condylar disarticulation with immediate reconstruction utilizing a two-segment fibula osteoseptocutaneous free flap. The skin paddle of the fibula free flap was utilized for the reconstruction of the intraoral soft-tissue defect ( Fig. 1a ) with primary closure of the right neck fistulectomy wound. Upon inset and microvascular anastomosis of the fibula free flap, appropriate patency of arterial anastomosis was confirmed with a triphasic Doppler signal throughout the vascular pedicle. Strip test confirmed appropriate venous outflow. No Doppler signal was noted along the surface of the skin of the skin paddle; however, bleeding was noted along edges of skin paddle. Kinking and compression of the perforator were rule out. A decision was made at this time to observe the flap postoperatively. The patient was subsequently returned to the operating room 12 hours postoperatively due to clinical signs of congestion of the skin paddle. Upon exploration of the flap and neck wound, congealed blood was identified in the neck that was consistent with a hematoma. No obvious bleeding vessel was appreciated, and the neck wound was irrigated with copious amounts of normal saline. Both arterial and venous anastomoses were confirmed to be patent. Indocyanine green–based fluorescence angiography (SPY Imaging; LifeCell Corp, Branchburg, NJ) confirmed perfusion of the skin paddle of the flap.
Fig. 1.
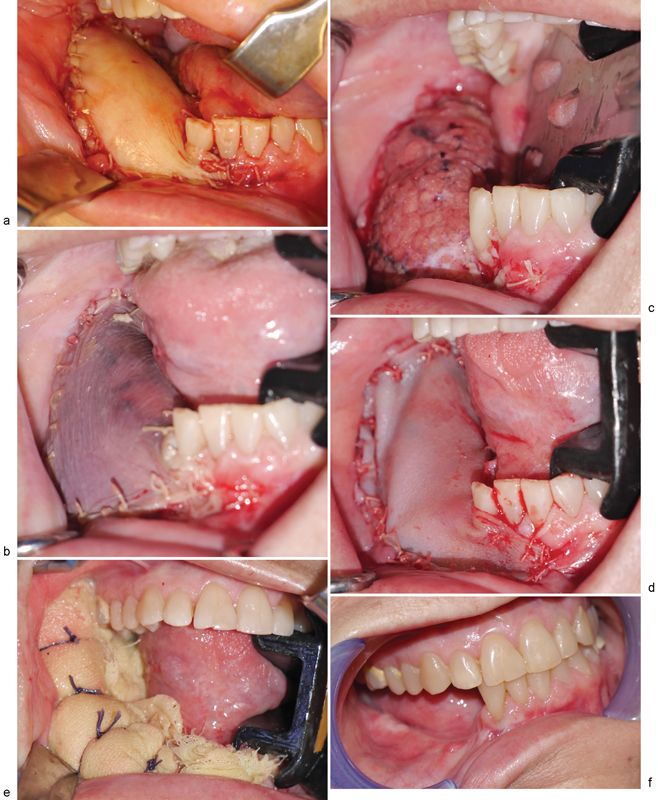
Clinical photographs. ( a ) Fibula free flap skin paddle immediately after inset and microvascular anastomosis. ( b ) Congested skin paddle on postoperative day 7. ( c ) Remaining portion of skin paddle following debridement of clinically apparent congested tissues. ( d ) Urinary bladder matrix (UBM) construct sutured in place. ( e ) Xeroform bolster in place to maintain intimate contact between UBM construct and underlying soft tissues. ( f ) 4.5 months following application of UBM, note the minimal scar tissue and maintenance of vestibule.
On postoperative day 7, the skin paddle of the fibula free flap appeared bluish/dusky ( Fig. 1b ) with brisk bleeding of dark blood on pinprick. There was complete absence of Doppler signal from skin paddle. There was no change in skin turgor. Clinical assessment of the flap confirmed worsening congestion of the skin paddle. Patient was taken to the operating room on this day for debridement of the skin paddle. After removing only the skin portion of the skin paddle, the underlying adipose tissue was confirmed to be viable ( Fig. 1c ); therefore, no further debridement of tissue from the skin paddle was performed. An eight-layer, vacuum-pressed UBM device, MatriStem Surgical Matrix Thick (ACell, Inc.), was rehydrated in room temperature normal saline (0.9%) as described in the manufacturer's instructions for use. The construct was next secured to the soft-tissue defect bed using an absorbable suture with the epithelial basement membrane surface away from the wound bed ( Fig. 1d ). A Xeroform (Tyco Healthcare, Mansfield, MA) bolster ( Fig. 1e ) was placed over the UBM construct, and the bolster was removed 7 days later. Tooth no. 26 was eventually extracted due to sensitivity of the tooth in the presence of significant tooth mobility. The patient was followed up closely following discharge from the hospital, and at 4.5 months postoperatively the patient appeared to have healthy soft tissues ( Fig. 1f ) without exposure of any hardware, native mandible, or fibula bone. The patient would go on to receive dental endosseous implants within the fibula free flap 6.5 months postoperatively ( Fig. 2 ).
Fig. 2.
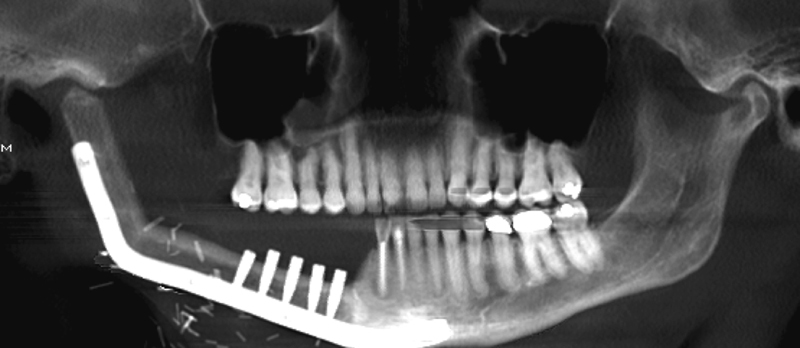
Orthopantomogram following placement of dental endosseous implants within distal fibula segment.
Technical Note
UBM is commercially available under the name MatriStem for reinforcement of soft tissue, where weakness exists in patients requiring gastroenterological, urological, and other reconstructive procedures. MatriStem devices consist of two to eight layers of vacuum-pressed UBM, which provides a range of strength and handling characteristics. UBM is also available under the name Cytal (ACell, Inc.) for general wound management indications, and both MatriStem and Cytal devices are provided in a sterile double peel-open packaging. 9 Based on the manufacturer's recommendations, UBM should be stored in a dry environment at a temperature ranging from 15 to 35°C while being protected from high humidity, freezing, or excessive heat. Prior to its use, the sheet devices should be rehydrated in room temperature normal (0.9%) saline as described in the appropriate instructions for use. The epithelial basement membrane should always face away from the defect, while the lamina propria should face the defect. To place the construct in the appropriate orientation, the indicator notch should always be in the right upper hand corner with the construct in a vertical orientation ( Fig. 3 ). In this orientation, the basement membrane side is facing outward, and the lamina propria side is facing inward. This is critical because in the senior author's (D.K.) experience, it is difficult with the naked eye to distinguish between the two surfaces (basement membrane and lamina propria) of the construct. As with an autogenous skin graft or bioengineered skin substitute, the construct should maintain close approximation to the tissue of the defect. As described in our case, this can be achieved with proper suturing technique and with a tissue bolster. While sensitivity or allergy to porcine materials is the only reported contraindication to the use of this product, there is a black box warning regarding its use in the presence of an active infection.
Fig. 3.

Photograph of appropriate orientation of construct with tab along the upper right hand corner when the construct is in the vertical position. Of note, the outward face represents the basement membrane, and the inward face represents the lamina propria.
Discussion
As free flaps continue to play a key role in the reconstruction of soft-tissue defects of the oral cavity and the jaws, it is imperative to identify flap salvage options that spare the patient an additional donor site and a lengthy operative intervention. A myriad of options are available to the reconstructive surgeon for the management of wounds within the oral cavity after partial flap failure with subsequent debridement of a segment of the flap, such as local flaps, autogenous skin grafts, biologic and synthetic skin substitutes, or tissue closure by secondary intention. In this case, we utilized a commercially available acellular porcine UBM construct. Our interest in this product stemmed from its reported effectiveness in the reconstruction of complex wounds in both animal models and human case reports/case series. 9 10 11 12 13 14 15 For instance, Rommer et al reported on the use of sheets of UBM for the reconstruction of recalcitrant nonhealing radiation wounds of the abdomen, thigh, and sacrum. 12 Afaneh et al reported that when used for reinforcement of esophagojejunal anastomoses, UBM decreased the incidence of esophagojejunal anastomotic leaks following total gastrectomy for malignancy. 13 Kruper et al also reported on the use of the same UBM constructs to salvage failed local and regional flaps in the reconstruction of cutaneous head and neck defects. 14
The extracellular matrix (ECM) is the secreted product of cells that populate a given tissue that contains a myriad of structural and functional proteins, such as type I collagen, type IV collagen, fibronectin, laminin, glycosaminoglycans, and other biological molecules. 16 17 18 Interest in the utilization of the ECM in wound healing relates to the unique tissue-specific arrangement of these aforementioned structural and functional proteins in providing structural support/tensile strength to the healing wound, while maintaining attachment sites for cell surface receptors and serving as a reservoir for signaling factors that modulate cell migration, cell proliferation, and angiogenesis. 15 16
ECM-based biomaterials have been shown in animal models to facilitate granulation, epithelialization, and cell growth to enhance wound closure. 12 15 16 UBM constructs are prepared via the decellularization of the porcine urinary bladder to leave behind only ECM, which primarily involves soaking the tissue in a middle acid and salt solutions with the intended goal of retaining the architecture and composition of the native tissue. 19
The UBM construct described in our case is composed of an immunologically inert decellularized epithelial basement membrane and lamina propria derived from the porcine urinary bladder that are jointly referred to as UBM. The preparation of this biologic construct involves the following processes: decellularization, removal of the submucosal and muscular layers, and subsequent electron beam radiation sterilization. 10 This process results in an acellular ECM that retains its complex native composition and structure. 17 18 This material is also nonchemically cross-linked and completely resorbable. 10 The degradation of the UBM is facilitated by the usual body proteases. 9 The upper surface of the construct corresponds to the basement membrane, which serves as a barrier between the epithelium and the normal tissue. 18 The inner surface of the construct represents the lamina propria, which is a more open structure that facilitates the incorporation of native tissues from within the bed of the defect. 18 20 As previously described, this surface of the construct should intimately contact the surface of the defect.
While the exact mechanism of UBM as a mechanical substrate for regenerative medicine is not fully understood, what is known is that ECM-based constructs appear to guide the wound healing process by providing a complex molecular architecture and the body responds to the presence of UBM by modulating the immune response. 21 Currently, the in vivo role of this property of the UBM is not fully understood.
Conclusion
In this report we described the novel application of a commercially available ECM UBM (ACell, Inc.), in the salvage of partial loss of the skin paddle of a fibula osteoseptocutaneous free flap that was utilized for mandibular reconstruction. The UBM construct provides an off-the-shelf reconstructive option for wounds that would have been classically managed with autogenous tissues, such as full-thickness or split-thickness skin grafts.
References
- 1.Taylor G I, Miller G D, Ham F J. The free vascularized bone graft. A clinical extension of microvascular techniques. Plast Reconstr Surg. 1975;55(05):533–544. doi: 10.1097/00006534-197505000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Hidalgo D A. Fibula free flap: a new method of mandible reconstruction. Plast Reconstr Surg. 1989;84(01):71–79. [PubMed] [Google Scholar]
- 3.Chen H H, Qiu S S, Lin C H, Kang C J, Liao C T. Fibula osteoseptocutaneous flap: advantages of beginning the harvesting from the posterior approach. Ann Plast Surg. 2015;77(06):635–639. doi: 10.1097/SAP.0000000000000638. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez E D, Bluebond-Langner R, Brazio P, Collins M. Near-total mandible reconstruction with a single fibula flap containing fibrous dysplasia in McCune Albright syndrome. J Craniofac Surg. 2007;18(06):1479–1482. doi: 10.1097/scs.0b013e3180a772ea. [DOI] [PubMed] [Google Scholar]
- 5.Winters R, Saad A, Beahm D D, Wise M W, St Hilaire H. Total autogenous mandibular reconstruction using virtual surgical planning. J Craniofac Surg. 2012;23(05):e405–e407. doi: 10.1097/SCS.0b013e31825bd302. [DOI] [PubMed] [Google Scholar]
- 6.Chen Z W, Yan W. The study and clinical application of the osteocutaneous flap of fibula. Microsurgery. 1983;4(01):11–16. doi: 10.1002/micr.1920040107. [DOI] [PubMed] [Google Scholar]
- 7.Wei F C, Chen H C, Chuang C C, Noordhoff M S. Fibular osteoseptocutaneous flap: anatomic study and clinical application. Plast Reconstr Surg. 1986;78(02):191–200. doi: 10.1097/00006534-198608000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Yu P, Chang E I, Hanasono M M. Design of a reliable skin paddle for the fibula osteocutaneous flap: perforator anatomy revisited. Plast Reconstr Surg. 2011;128(02):440–446. doi: 10.1097/PRS.0b013e31821e7058. [DOI] [PubMed] [Google Scholar]
- 9.Kimmel H, Rahn M, Gilbert T W. The clinical effectiveness in wound healing with extracellular matrix derived from porcine urinary bladder matrix: a case series on severe chronic wounds. J Am Col Certif Wound Spec. 2010;2(03):55–59. doi: 10.1016/j.jcws.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Remlinger N T, Gilbert T W, Yoshida M et al. Urinary bladder matrix promotes site appropriate tissue formation following right ventricle outflow tract repair. Organogenesis. 2013;9(03):149–160. doi: 10.4161/org.25394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eberli D, Atala A, Yoo J J. One and four layer acellular bladder matrix for fascial tissue reconstruction. J Mater Sci Mater Med. 2011;22(03):741–751. doi: 10.1007/s10856-011-4242-6. [DOI] [PubMed] [Google Scholar]
- 12.Rommer E A, Peric M, Wong A. Urinary bladder matrix for the treatment of recalcitrant nonhealing radiation wounds. Adv Skin Wound Care. 2013;26(10):450–455. doi: 10.1097/01.ASW.0000434617.57451.e6. [DOI] [PubMed] [Google Scholar]
- 13.Afaneh C, Abelson J, Schattner M et al. Esophageal reinforcement with an extracellular scaffold during total gastrectomy for gastric cancer. Ann Surg Oncol. 2015;22(04):1252–1257. doi: 10.1245/s10434-014-4125-4. [DOI] [PubMed] [Google Scholar]
- 14.Kruper G J, Vandegriend Z P, Lin H S, Zuliani G F. Salvage of failed local and regional flaps with porcine urinary bladder extracellular matrix aided tissue regeneration. Case Rep Otolaryngol. 2013;2013:917183. doi: 10.1155/2013/917183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eweida A M, Marei M K. Naturally occurring extracellular matrix scaffolds for dermal regeneration: do they really need cells? Biomed Res Int. 2015;2015:839694. doi: 10.1155/2015/839694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marçal H, Ahmed T, Badylak S F, Tottey S, Foster L J. A comprehensive protein expression profile of extracellular matrix biomaterial derived from porcine urinary bladder. Regen Med. 2012;7(02):159–166. doi: 10.2217/rme.12.6. [DOI] [PubMed] [Google Scholar]
- 17.Brown B, Lindberg K, Reing J, Stolz D B, Badylak S F. The basement membrane component of biologic scaffolds derived from extracellular matrix. Tissue Eng. 2006;12(03):519–526. doi: 10.1089/ten.2006.12.519. [DOI] [PubMed] [Google Scholar]
- 18.Balland O, Poinsard A S, Famose F et al. Use of a porcine urinary bladder acellular matrix for corneal reconstruction in dogs and cats. Vet Ophthalmol. 2015;19(06):454–463. doi: 10.1111/vop.12326. [DOI] [PubMed] [Google Scholar]
- 19.Brennan E P, Reing J, Chew D, Myers-Irvin J M, Young E J, Badylak S F. Antibacterial activity within degradation products of biological scaffolds composed of extracellular matrix. Tissue Eng. 2006;12(10):2949–2955. doi: 10.1089/ten.2006.12.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crapo P M, Gilbert T W, Badylak S F. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32(12):3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown B N, Londono R, Tottey S et al. Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater. 2012;8(03):978–987. doi: 10.1016/j.actbio.2011.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]


