Abstract
Background
Monitoring cancer risk among HIV-infected people in the modern antiretroviral therapy (ART) era is critical given their elevated risk for many cancers and prolonged survival with immunosuppression, ART exposure, and aging. Our study described cancer risk in HIV-infected people in the United States relative to the general population.
Methods
Utilizing data from linked population-based HIV and cancer registries (nine areas; 1996–2012), we calculated standardized incidence ratios (SIRs). We tested SIR differences by AIDS status and over time using Poisson regression.
Findings
Among 448,258 HIV-infected people, risk was elevated (p<0·0001) for cancer overall (SIR 1·69; 95%CI: 1·67–1·72), AIDS-defining cancers (Kaposi sarcoma [498; 478–519], non-Hodgkin lymphoma [11·5; 11·1–11·9], and cervix [3·24; 2·94–3·56]), most other virus-related cancers (e.g., anus [19·1; 18·1–20·0], liver [3·21; 3·02–3·41], and Hodgkin lymphoma [7·70; 7·20–8·23]), and some virus-unrelated cancers (e.g., lung [1·97; 1·89–2·05]), but not for other common cancers. Risk for several cancers was higher after AIDS onset and declined across calendar periods. After multivariable adjustment, SIRs decreased significantly across 1996–2012 for six cancers (Kaposi sarcoma, two non-Hodgkin lymphoma subtypes, anus, liver, and lung) but remained elevated in the latest period. SIRs did not increase over time for any cancer.
Interpretation
Risks for several virus-related cancers and lung cancer declined among HIV-infected people, likely reflecting ART expansion since 1996. Despite declines, risk for many cancers remain elevated in the modern treatment era.
Funding
National Cancer Institute.
Introduction
HIV-infected people have an elevated risk for many cancers, largely due to HIV-related immunosuppression, which impairs control of oncogenic viral infections.1–3 A high prevalence of these infections and other cancer risk factors (e.g., smoking and alcohol use) contributes to the elevated risk.1–4 Kaposi sarcoma (KS), some non-Hodgkin lymphoma (NHL) subtypes, and cervical cancer are caused by viruses (KS-associated herpesvirus, Epstein-Barr virus [EBV], and human papillomavirus [HPV], respectively) and are among conditions that can mark the onset of acquired immunodeficiency syndrome (AIDS).3 HIV-infected people have elevated risk for these AIDS-defining cancers (ADCs) and other virus-related non-ADCs (VRNADCs), but not for most virus-unrelated non-ADCs (VUNADCs).1–3
After the introduction of effective antiretroviral therapy (ART) in 1996, risk for AIDS and death declined dramatically in HIV-infected people.3 KS and NHL incidence has also declined in the ART era but remains highly elevated in HIV-infected people compared to the general population; trends for other cancers are less clear.3,5–12 Recent and comprehensive population-based data on cancer risk for HIV-infected people are limited.6,8–10,12
Risk for some cancer types may continue to decline as ART regimens improve, treatment is initiated at earlier stages of HIV disease, and access to ART widens.13 However, treatment may not fully reverse the impact of early immune suppression, and immune dysfunction and chronic inflammation can persist among persons on ART.2 HIV-infected people, including those who have not developed AIDS, may therefore still be at elevated risk of developing cancer. Further, many cancer types have latency periods of decades, and the modern ART era is only twenty years old; it is thus possible that elevated risk for some cancers will emerge over time. Finally, with prolonged survival the HIV population is aging, and the impact of HIV-related immunosuppression in an aging population is unclear.10,14
For these reasons, continued monitoring of cancer risk in this high-risk population is vital. In the present study, we describe the spectrum of cancer risk among HIV-infected people in the United States (US) during the modern ART era, using linked data from multiple population-based HIV and cancer registries.
Methods
Study design, participants, and data sources
The HIV/AIDS Cancer Match (HACM) Study uses linked data collected by US HIV and cancer registries (https://hivmatch.cancer.gov/).15 The study was approved by institutional review boards at participating HIV and cancer registries, as required, and received exemption from review at the National Institutes of Health. Because the study uses data collected for public health surveillance, consent of participants was not required.
The present analysis evaluated a cohort of HIV-infected people identified in HIV registries from Colorado (1996–2007), Connecticut (2005–2010), Georgia (2004–2012), Maryland (2008–2012), Michigan (1996–2010), New Jersey (1996–2012), New York (2001–2012), Puerto Rico (2003–2012), and Texas (1999–2009). For each registry, follow-up for each cohort member started three months after the latest of the beginning of systematic name-based state HIV registration, HIV report date (or AIDS diagnosis, if this was earlier), start of cancer registration, or January 1, 1996, and ended at the earliest of death, end of cancer registry coverage, or December 31, 2012. The first three months of follow-up were excluded to remove prevalent cancers, i.e., cancer cases that prompted HIV testing and reporting.
Procedures
Cancer diagnoses were identified through linkage with the corresponding cancer registries (see Appendix Table 1 for coding scheme [pp 2–3]). We evaluated individual cancer types and several broad categories, including all cancers, ADCs (KS; AIDS-defining NHLs: diffuse large B-cell lymphoma [DLBCL], Burkitt lymphoma, unspecified NHL, and central nervous system [CNS] NHL; and cervical cancer), and non-ADCs (NADCs). NADCs were sub-classified into VRNADCs (cancers of the anus, vagina, vulva, penis, and selected oral cavity/pharynx sites [caused by HPV]; liver cancer [hepatitis B and C viruses]; Hodgkin lymphoma [EBV]; and Merkel cell carcinoma [Merkel cell polyomavirus]) and VUNADCs (remaining NADCs).
Statistical analysis
We used standardized incidence ratios (SIRs) to measure cancer risk in HIV-infected people relative to the US general population. SIRs were calculated by dividing the observed number of cases in HIV-infected people by the expected number, estimated by applying general population cancer incidence rates to person-time in the HIV population based on sex, age, race/ethnicity, calendar year and registry. For KS and CNS NHL (for which contemporaneous general population rates largely reflect HIV-related cases), expected rates were based on data collected by the Surveillance, Epidemiology, and End Results (SEER) cancer registries before the AIDS epidemic (1973–1979).15 To calculate SIRs, all cancers were counted (not just first cancers), including multiple cancers of the same type.
In preliminary analyses, we observed that the SIR for our miscellaneous cancers category was significantly elevated. We therefore reviewed this miscellaneous category and pulled out additional cancer types with at least 10 cases for separate assessment (cancers of the extrahepatic bile duct, nasal cavity, accessory sinuses, scrotum, conjunctiva, and thymus, Merkel cell carcinoma, appendageal carcinoma of the skin, sarcomas of the skin, polycythemia vera, essential thrombocythemia, and myelodysplastic syndrome). These cancers are no longer included in the miscellaneous category but are instead included separately in the tables.
To assess the association of cancer risk with advancing immunosuppression, we calculated SIRs separately for person-time with AIDS and without AIDS (i.e., HIV-only). The AIDS onset month was considered the end of the HIV-only period, so that some ADCs were counted as occurring in people with HIV-only. We compared SIRs for the HIV-only and AIDS periods using Poisson regression. These models yielded SIR ratios adjusted for sex/HIV-risk group (men-who-have-sex-with-men [MSM], other male, female), attained age (<30, 30–39, 40–49, 50–59, 60+ years), race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic/Latino), calendar year (modeled as one continuous variable, except for KS, DLBCL and CNS NHL which were modeled as separate segments as informed by Joinpoint analysis, see below), registry, and early vs. late attained follow-up duration (<10 vs. 10+ years after the later of HIV report or AIDS diagnosis).
We considered calendar trends in cancer risk to reflect increasing use of ART at the population level. To screen for time trends, we first calculated SIRs for four calendar periods (1996–1999, 2000–2004, 2005–2008, 2009–2012) and tested for a trend in SIRs across the periods using unadjusted Poisson models. For selected cancers for which the SIR was elevated overall and the trend across periods was significant, we further evaluated trends across individual calendar years. We first used Joinpoint (version 4.3.1, National Cancer Institute) to identify significant changes in SIR trends over calendar time, allowing up to four calendar segments. Incorporating the interval parameterization identified in Joinpoint, we then used Poisson regression to characterize calendar trends adjusted for sex/HIV-risk group, attained age, race/ethnicity, registry, and attained follow-up duration.
If individuals move out of cancer registry areas (i.e., out-migration), SIRs will be underestimated, especially with extended time after HIV registration. In a sensitivity analysis to address this possible bias, we recalculated SIRs after decreasing the expected cancer counts by 27% for the late follow-up period defined above (see Appendix Methods [p 1]). Participating registries provided data for varying calendar intervals, which might affect overall calendar trends, so in another sensitivity analysis we recalculated the trends excluding one registry at a time.
All statistical analyses, except the Joinpoint analysis, were performed using SAS (version 9.3, SAS Institute, Cary, NC). We present 95% confidence intervals (95%CIs), but because we made multiple comparisons, we used a conservative two-sided p-value of 0·001 to determine significance.
Role of the Funding Source
The National Cancer Institute reviewed and approved final submission but had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the aggregate data in the study and had final responsibility for the decision to submit for publication.
Results
A total of 448,258 HIV-infected people contributed 3,090,033 person-years of follow-up (Table 1). Seventy-one percent of the person-time was contributed by males, 38% by subjects aged 40–49 years old, 47% by non-Hispanic blacks, 33% by MSM, 64% by subjects after an AIDS diagnosis, and 85% by subjects followed within 10 years of HIV report/AIDS diagnosis. Across calendar periods during 1996–2012, the contribution by females and older age groups increased, while the contribution from non-Hispanic whites and injection drug users decreased. The proportion of follow-up time 10+ years after HIV report/AIDS diagnosis increased from 0·4% (1996–1999) to 24·3% (2009–2012).
Table 1.
Demographic characteristics of person-time contributed by HIV-infected people in the United States according to calendar year period (1996–2012)
| Characteristic | Person-years (%)*
|
||||
|---|---|---|---|---|---|
| All | 1996–1999 | 2000–2004 | 2005–2008 | 2009–2012 | |
| Total | 3,090,033 (100) | 155,342 (100) | 756,530 (100) | 1,071,041 (100) | 1,107,119 (100) |
| Sex | |||||
| Male | 2,196,707 (71·1) | 114,047 (73·4) | 546,223 (72·2) | 763,422 (71·3) | 773,015 (69·8) |
| Female | 893,325 (28·9) | 41,295 (26·6) | 210,307 (27·8) | 307,619 (28·7) | 334,104 (30·2) |
| Attained age group, years | |||||
| <30 | 279,425 (9·0) | 18,337 (11·8) | 66,529 (8·8) | 94,737 (8·9) | 99,822 (9·0) |
| 30–39 | 706,853 (22·9) | 64,699 (41·6) | 226,440 (29·9) | 232,781 (21·7) | 182,933 (16·5) |
| 40–49 | 1,175,561 (38·0) | 54,550 (35·1) | 301,772 (39·9) | 428,241 (40·0) | 390,998 (35·3) |
| 50–59 | 697,455 (22·6) | 14,223 (9·2) | 127,919 (16·9) | 242,397 (22·6) | 312,917 (28·3) |
| 60+ | 230,739 (7·5) | 3,533 (2·3) | 33,871 (4·5) | 72,885 (6·8) | 120,449 (10·9) |
| Race/ethnicity | |||||
| Non-Hispanic white | 765,400 (24·8) | 54,793 (35·3) | 210,162 (27·8) | 269,384 (25·2) | 231,061 (20·9) |
| Non-Hispanic black | 1,460,565 (47·3) | 74,115 (47·7) | 333,717 (44·1) | 487,712 (45·5) | 565,022 (51·0) |
| Hispanic/Latino | 864,067 (28·0) | 26,435 (17·0) | 212,652 (28·1) | 313,944 (29·3) | 311,036 (28·1) |
| HIV-risk group | |||||
| MSM | 1,006,462 (32·6) | 52,624 (33·9) | 244,707 (32·3) | 355,841 (33·2) | 353,289 (31·9) |
| Male IDU | 455,865 (14·8) | 30,403 (19·6) | 129,171 (17·1) | 153,977 (14·4) | 142,315 (12·9) |
| MSM/IDU | 141,466 (4·6) | 10,436 (6·7) | 41,367 (5·5) | 49,761 (4·7) | 39,901 (3·6) |
| Male other/unknown | 592,915 (19·2) | 20,584 (13·3) | 130,978 (17·3) | 203,843 (19·0) | 237,510 (21·5) |
| Female IDU | 230,618 (7·5) | 17,363 (11·2) | 65,706 (8·7) | 76,567 (7·2) | 70,981 (6·4) |
| Female other/unknown | 662,708 (21·4) | 23,932 (15·4) | 144,601 (19·1) | 231,052 (21·6) | 263,123 (23·8) |
| AIDS onset | |||||
| HIV-only | 1,114,805 (36·1) | 66,588 (42·9) | 235,367 (31·1) | 384,110 (35·9) | 428,741 (38·7) |
| AIDS | 1,975,228 (63·9) | 88,755 (57·1) | 521,164 (68·9) | 686,931 (64·1) | 678,378 (61·3) |
| Time since HIV report/AIDS diagnosis, years | |||||
| <10 | 2,622,217 (84·9) | 154,787 (99·6) | 722,117 (95·5) | 907,374 (84·7) | 837,939 (75·7) |
| 10+ | 467,816 (15·1) | 555 (0·4) | 34,414 (4·6) | 163,666 (15·3) | 269,181 (24·3) |
Abbreviations: AIDS, acquired immunodeficiency syndrome; IDU, injection drug users; MSM, men who have sex with men.
Person-years are rounded to the nearest whole number so subtotals across categories may not add up to the total.
There were 21,294 incident cancers diagnosed during 1996–2012 (incidence: 689 per 100,000 person-years), of which 6,384 (30·0%) were ADCs, 14,344 (67·4%) were NADCs, and 566 (2·6%) were poorly specified (Table 2). Among NADCs, 4,144 (28·9%) were VRNADCs and 10,200 (71·1%) were VUNADCs. Overall, cancer risk was 69% higher among HIV-infected people than in the general population (SIR 1·69; 95%CI: 1·67–1·72). Risk was elevated for ADCs (SIR 14·0; 95%CI: 13·6–14·3), as well for NADCs (1·21; 1·19–1·23), driven by the elevation for VRNADCs (5·39; 5·23–5·55); risk for VUNADCs was actually slightly decreased (0·92; 0·90–0·94).
Table 2.
Standardized incidence ratios for cancer in HIV-infected people, 1996–2012
| Cancer types | Observed cases | SIR (95% CI)* |
|---|---|---|
| All cancers | 21,294 | 1·69 (1·67, 1·72) |
| ADCs | 6,384 | 14·0 (13·6, 14·3) |
| KS | 2,269 | 498 (478, 519) |
| AIDS-defining NHLs | 3,687 | 11·5 (11·1, 11·9) |
| DLBCL | 2,242 | 10·3 (9·89, 10·7) |
| Burkitt lymphoma | 435 | 20·2 (18·4, 22·2) |
| Unspecified NHL | 1,010 | 12·4 (11·7, 13·2) |
| CNS NHL† | 528 | 153 (140, 167) |
| Cervix | 428 | 3·24 (2·94, 3·56) |
| NADCs | 14,344 | 1·21 (1·19, 1·23) |
| VRNADCs | 4,144 | 5·39 (5·23, 5·55) |
| HPV-related oral cavity/pharynx | 297 | 1·64 (1·46, 1·84) |
| Anus | 1,568 | 19·1 (18·1, 20·0) |
| Liver | 1,104 | 3·21 (3·02, 3·41) |
| Merkel cell carcinoma | 10 | 2·58 (1·24, 4·74) |
| Vagina | 25 | 3·55 (2·30, 5·24) |
| Vulva | 151 | 9·35 (7·91, 11·0) |
| Penis | 114 | 5·33 (4·39, 6·40) |
| Hodgkin lymphoma | 875 | 7·70 (7·20, 8·23) |
| VUNADCs | 10,200 | 0·92 (0·90, 0·94) |
| Lip | 20 | 2·35 (1·43, 3·62) |
| Salivary gland | 33 | 0·89 (0·62, 1·26) |
| Nasopharynx | 31 | 1·20 (0·82, 1·71) |
| Non-HPV oral cavity/pharynx | 343 | 2·20 (1·98, 2·45) |
| Esophagus | 190 | 1·23 (1·06, 1·42) |
| Stomach | 185 | 0·74 (0·64, 0·86) |
| Small Intestine | 54 | 0·71 (0·53, 0·93) |
| Colon | 477 | 0·61 (0·56, 0·67) |
| Rectum/rectosigmoid junction | 272 | 0·69 (0·61, 0·77) |
| Intrahepatic bile duct | 21 | 1·21 (0·75, 1·85) |
| Gallbladder | 36 | 1·34 (0·94, 1·85) |
| Extrahepatic bile duct | 20 | 1·04 (0·64, 1·61) |
| Pancreas | 307 | 1·13 (1·01, 1·26) |
| Nasal cavity | 25 | 2·66 (1·72, 3·93) |
| Accessory sinuses | 17 | 1·32 (0·77, 2·12) |
| Larynx | 347 | 2·11 (1·89, 2·34) |
| Lung | 2,475 | 1·97 (1·89, 2·05) |
| Bones and joints | 15 | 0·62 (0·35, 1·03) |
| Soft tissue including heart | 99 | 1·02 (0·83, 1·24) |
| Melanoma | 213 | 0·86 (0·75, 0·98) |
| Appendageal carcinoma of the skin | 14 | 1·68 (0·92, 2·81) |
| Sarcomas of the skin | 16 | 0·79 (0·45, 1·28) |
| Female breast | 688 | 0·63 (0·58, 0·68) |
| Uterus | 83 | 0·43 (0·34, 0·53) |
| Ovary | 60 | 0·69 (0·52, 0·88) |
| Prostate | 1,522 | 0·48 (0·46, 0·51) |
| Testis | 88 | 0·86 (0·69, 1·06) |
| Scrotum | 20 | 6·84 (4·18, 10·6) |
| Urinary bladder | 171 | 0·88 (0·75, 1·02) |
| Kidney/renal pelvis | 360 | 0·74 (0·66, 0·82) |
| Conjunctiva | 21 | 5·56 (3·44, 8·50) |
| Brain‡ | 83 | 0·57 (0·45, 0·70) |
| Thyroid | 164 | 0·50 (0·42, 0·58) |
| Thymus | 13 | 0·89 (0·47, 1·52) |
| Non-AIDS-defining NHLs | 510 | 1·32 (1·20, 1·44) |
| Myeloma | 206 | 0·89 (0·78, 1·02) |
| Myeloid and monocytic leukemias | 165 | 1·18 (1·00, 1·37) |
| Polycythemia vera | 79 | 2·26 (1·79, 2·81) |
| Essential thrombocythemia | 28 | 1·02 (0·68, 1·47) |
| Myelodysplastic syndrome | 104 | 2·04 (1·66, 2·47) |
| Mesothelioma | 17 | 1·15 (0·67, 1·84) |
| Miscellaneous | 608 | 1·77 (1·63, 1·92) |
| Poorly specified histology at any site | 566 | 2·35 (2·16, 2·55) |
Abbreviations: ADCs, AIDS-defining cancers; AIDS, acquired immunodeficiency syndrome; CI, confidence interval; CNS, central nervous system; DLBCL, diffuse large B-cell lymphoma; HPV, human papillomavirus; KS, Kaposi sarcoma; NADCs, non-AIDS-defining cancers; NHL, non-Hodgkin lymphoma; SIR, standardized incidence ratio; VRNADCs, virus-related non-AIDS-defining cancers; VUNADCs, virus-unrelated non-AIDS-defining cancers.
Underlining indicates that the SIR is significant with p<0·0001.
CNS NHL is defined based on site rather than histology, so this category overlaps with other AIDS-defining NHL subtypes, and the total of the subcategories is greater than for AIDS-defining NHL overall.
This category does not include CNS NHL.
The most common individual cancer types were AIDS-defining NHLs (3,687; 17·3%), lung cancer (2,475; 11·6%), KS (2,269; 10·7%), anal cancer (1,568; 7·4%), prostate cancer (1,522; 7·1%), liver cancer (1,104; 5·2%), and Hodgkin lymphoma (875; 4·1%; Table 2). Risk was significantly elevated for almost all virus-related cancers: KS (SIR 498; 95%CI: 478–519), AIDS-defining NHLs (11·5; 11·1–11·9), cancers of the cervix (3·24; 2·94–3·56), HPV-related oral cavity/pharynx (1·64; 1·46–1·84), anus (19·1; 18·1–20·0), liver (3·21; 3·02–3·41), vagina (3·55; 2·30–5·24), vulva (9·35; 7·91–11·0), and penis (5·33; 4·39–6·40), and Hodgkin lymphoma (7·70; 7·20–8·23); the exception was Merkel cell carcinoma (SIR 2·58; 1·24–4·74, p=0·0133). Risk was significantly elevated for each AIDS-defining NHL subtype: DLBCL (SIR 10·3; 9·89–10·7), Burkitt lymphoma (20·2; 18·4–22·2), unspecified NHL (12·4; 11·7–13·2), and CNS NHL (153; 140–167). Risks were also significantly elevated for cancers of the (non-HPV) oral cavity/pharynx (SIR 2·20; 1·98–2·45), nasal cavity (2·66; 1·72–3·93), larynx (2·11; 1·89–2·34), lung (1·97; 1·89–2·05), scrotum (6·84; 4·18–10·6), and conjunctiva (5·56; 3·44–8·50), as well as non-AIDS-defining NHLs (1·32; 1·20–1·44), polycythemia vera (2·26; 1·79–2·81), myelodysplastic syndrome (2·04; 1·66–2·47), and miscellaneous cancers (1·77; 1·63–1·92). In contrast, risks were significantly decreased for cancers of the stomach (SIR 0·74; 95%CI: 0·64–0·86), colon (0·61; 0·56–0·67), rectum/rectosigmoid junction (0·69; 0·61–0·77), female breast (0·63; 0·58–0·68), uterus (0·43; 0·34–0·53), prostate (0·48; 0·46–0·51), kidney/renal pelvis (0·74; 0·66–0·82), brain (0·57; 0·45–0·70), and thyroid (0·50; 0·42–0·58). Correcting SIRs for potential outmigration did not affect the estimates appreciably (Appendix Table 2 [p 4]).
Compared with people with HIV-only, people with AIDS had significantly higher SIRs for ADCs, VRNADCs, and VUNADCs considered as grouped outcomes (Table 3). This pattern was also present for each individual ADC and VRNADC, except liver and vaginal cancers, and Merkel cell carcinoma. Risks were also higher for people with AIDS than those with HIV-only for the following VUNADCs: cancers of the (non-HPV) oral cavity/pharynx, esophagus, and lung, non-AIDS-defining NHLs, myeloid and monocytic leukemias, and myelodysplastic syndrome.
Table 3.
Standardized incidence ratios for cancer in HIV-infected people by AIDS status
| Cancer types | SIR (95% CI) | Adjusted SIR ratio, AIDS vs HIV-only* |
||
|---|---|---|---|---|
|
| ||||
| HIV-only | AIDS | Ratio (95% CI) | P value | |
| All cancers | 1·24 (1·20, 1·28) | 1·88 (1·85, 1·91) | 1·83 (1·77, 1·89) | <0·0001 |
| ADCs | 6·99 (6·58, 7·43) | 17·3 (16·9, 17·8) | 3·15 (2·94, 3·37) | <0·0001 |
| KS | 277 (249, 307) | 586 (560, 613) | 3·21 (2·86, 3·60) | <0·0001 |
| AIDS-defining NHLs | 5·90 (5·43, 6·40) | 14·0 (13·5, 14·5) | 3·12 (2·85, 3·42) | <0·0001 |
| DLBCL | 5·05 (4·53, 5·62) | 12·6 (12·1, 13·2) | 3·32 (2·94, 3·73) | <0·0001 |
| Burkitt lymphoma | 17·5 (14·5, 20·9) | 21·5 (19·2, 24·0) | 1·63 (1·31, 2·03) | <0·0001 |
| Unspecified NHL | 4·92 (4·09, 5·87) | 15·7 (14·7, 16·8) | 4·27 (3·52, 5·17) | <0·0001 |
| CNS NHL | 30·4 (20·8, 43·0) | 207 (189, 225) | 10·1 (7·02, 14·5) | <0·0001 |
| Cervix | 2·04 (1·66, 2·48) | 3·94 (3·53, 4·39) | 2·20 (1·74, 2·76) | <0·0001 |
| NADCs | 0·99 (0·96, 1·02) | 1·30 (1·27, 1·32) | 1·45 (1·39, 1·51) | <0·0001 |
| VRNADCs | 3·25 (3·02, 3·50) | 6·27 (6·06, 6·49) | 2·21 (2·03, 2·40) | <0·0001 |
| HPV-related oral cavity/pharynx | 1·15 (0·88, 1·48) | 1·84 (1·61, 2·09) | 1·76 (1·32, 2·36) | 0·0001 |
| Anus | 7·41 (6·39, 8·56) | 24·2 (22·9, 25·5) | 3·49 (2·98, 4·08) | <0·0001 |
| Liver | 2·81 (2·48, 3·17) | 3·36 (3·14, 3·59) | 1·25 (1·08, 1·44) | 0·0029 |
| Merkel cell carcinoma | 1·84 (0·22, 6·63) | 2·87 (1·24, 5·65) | 1·56 (0·33, 7·35) | 0·5734† |
| Vagina | 0·84 (0·10, 3·02) | 4·95 (3·14, 7·43) | 6·76 (1·58, 28·9) | 0·0100 |
| Vulva | 4·00 (2·54, 6·00) | 12·3 (10·3, 14·6) | 3·22 (2·04, 5·08) | <0·0001 |
| Penis | 1·94 (0·97, 3·48) | 6·54 (5·34, 7·93) | 3·67 (1·94, 6·92) | <0·0001 |
| Hodgkin lymphoma | 4·64 (4·00, 5·35) | 9·42 (8·72, 10·1) | 2·12 (1·80, 2·51) | <0·0001 |
| VUNADCs | 0·84 (0·81, 0·87) | 0·95 (0·93, 0·97) | 1·25 (1·20, 1·31) | <0·0001 |
| Lip | 0·76 (0·09, 2·75) | 3·05 (1·81, 4·83) | 4·30 (0·96, 19·3) | 0·0568 |
| Salivary gland | 0·52 (0·19, 1·14) | 1·06 (0·70, 1·55) | 2·27 (0·92, 5·58) | 0·0745 |
| Nasopharynx | 1·29 (0·62, 2·38) | 1·17 (0·72, 1·78) | 0·90 (0·43, 1·92) | 0·7903† |
| Non-HPV oral cavity/pharynx | 1·35 (1·03, 1·74) | 2·54 (2·26, 2·86) | 2·20 (1·65, 2·92) | <0·0001 |
| Esophagus | 0·79 (0·55, 1·10) | 1·40 (1·19, 1·64) | 2·20 (1·51, 3·21) | <0·0001 |
| Stomach | 0·69 (0·51, 0·92) | 0·76 (0·64, 0·90) | 1·18 (0·84, 1·66) | 0·3275 |
| Small Intestine | 0·83 (0·50, 1·30) | 0·66 (0·46, 0·92) | 0·85 (0·48, 1·53) | 0·5931 |
| Colon | 0·68 (0·58, 0·80) | 0·58 (0·52, 0·65) | 0·95 (0·78, 1·16) | 0·5943 |
| Rectum/rectosigmoid junction | 0·60 (0·46, 0·75) | 0·72 (0·63, 0·83) | 1·40 (1·02, 1·92) | 0·0360‡ |
| Intrahepatic bile duct | 1·20 (0·44, 2·61) | 1·21 (0·68, 2·00) | 1·01 (0·39, 2·61) | 0·9800† |
| Gallbladder | 1·03 (0·44, 2·02) | 1·47 (0·97, 2·12) | 1·39 (0·61, 3·18) | 0·4367 |
| Extrahepatic bile duct | 0·54 (0·11, 1·59) | 1·24 (0·72, 1·99) | 2·29 (0·67, 7·80) | 0·1865† |
| Pancreas | 0·98 (0·77, 1·22) | 1·19 (1·04, 1·36) | 1·26 (0·96, 1·65) | 0·0953 |
| Nasal cavity | 1·41 (0·38, 3·60) | 3·21 (1·98, 4·90) | 2·61 (0·87, 7·80) | 0·0861 |
| Accessory sinuses | 1·58 (0·58, 3·44) | 1·22 (0·61, 2·18) | 0·77 (0·28, 2·08) | 0·6057† |
| Larynx | 1·71 (1·35, 2·13) | 2·26 (2·00, 2·54) | 1·51 (1·17, 1·96) | 0·0019 |
| Lung | 1·57 (1·45, 1·71) | 2·13 (2·03, 2·22) | 1·51 (1·37, 1·66) | <0·0001 |
| Bones and joints | 0·82 (0·33, 1·68) | 0·52 (0·22, 1·02) | 0·73 (0·25, 2·10) | 0·5603 |
| Soft tissue including heart | 0·77 (0·50, 1·14) | 1·14 (0·90, 1·44) | 1·70 (1·06, 2·73) | 0·0272 |
| Melanoma | 0·87 (0·68, 1·09) | 0·85 (0·72, 1·01) | 1·07 (0·80, 1·44) | 0·6575 |
| Appendageal carcinoma of the skin | 0·00 (0·00, 1·42) | 2·44 (1·33, 4·09) | ||
| Sarcomas of the skin | 0·56 (0·15, 1·42) | 0·92 (0·48, 1·61) | 1·66 (0·54, 5·14) | 0·3808† |
| Female breast | 0·67 (0·59, 0·76) | 0·60 (0·55, 0·66) | 0·95 (0·81, 1·11) | 0·4958 |
| Uterus | 0·38 (0·25, 0·57) | 0·45 (0·34, 0·58) | 1·27 (0·78, 2·06) | 0·3316 |
| Ovary | 0·59 (0·35, 0·93) | 0·74 (0·53, 1·00) | 1·35 (0·77, 2·38) | 0·2956 |
| Prostate | 0·55 (0·50, 0·60) | 0·46 (0·43, 0·49) | 0·91 (0·81, 1·02) | 0·1131 |
| Testis | 0·88 (0·61, 1·22) | 0·85 (0·64, 1·12) | 0·99 (0·63, 1·54) | 0·9529 |
| Scrotum | 5·86 (1·90, 13·7) | 7·24 (4·05, 11·9) | 1·23 (0·45, 3·40) | 0·6833† |
| Urinary bladder | 0·81 (0·59, 1·09) | 0·90 (0·75, 1·08) | 1·24 (0·87, 1·78) | 0·2353 |
| Kidney/renal pelvis | 0·80 (0·67, 0·96) | 0·71 (0·62, 0·80) | 0·90 (0·71, 1·13) | 0·3576 |
| Conjunctiva | 1·88 (0·23, 6·79) | 7·00 (4·22, 10·9) | 3·72 (0·87, 16·0) | 0·0770† |
| Brain§ | 0·48 (0·30, 0·72) | 0·61 (0·46, 0·78) | 1·32 (0·80, 2·19) | 0·2735 |
| Thyroid | 0·55 (0·42, 0·70) | 0·47 (0·38, 0·57) | 0·85 (0·61, 1·19) | 0·3540 |
| Thymus | 0·42 (0·05, 1·53) | 1·11 (0·55, 1·99) | 2·62 (0·58, 11·8) | 0·2098† |
| Non-AIDS-defining NHLs | 1·14 (0·95, 1·35) | 1·39 (1·26, 1·54) | 1·44 (1·18, 1·77) | 0.0004 |
| Myeloma | 1·08 (0·84, 1·36) | 0·82 (0·69, 0·97) | 0·83 (0·61, 1·12) | 0·2173 |
| Myeloid and monocytic leukemias | 0·78 (0·54, 1·08) | 1·37 (1·14, 1·62) | 1·97 (1·34, 2·89) | 0.0006 |
| Polycythemia vera | 1·97 (1·22, 3·02) | 2·38 (1·81, 3·08) | 1·40 (0·83, 2·35) | 0·2114 |
| Essential thrombocythemia | 0·55 (0·18, 1·29) | 1·24 (0·79, 1·86) | 2·24 (0·85, 5·88) | 0·1029† |
| Myelodysplastic syndrome | 0·86 (0·46, 1·48) | 2·53 (2·03, 3·10) | 3·51 (1·94, 6·34) | <0·0001 |
| Mesothelioma | 0·70 (0·14, 2·04) | 1·33 (0·73, 2·23) | 1·91 (0·55, 6·64) | 0·3097† |
| Miscellaneous | 1·53 (1·30, 1·79) | 1·87 (1·70, 2·06) | 1·32 (1·09, 1·59) | 0·0038 |
| Poorly specified histology at any site | 1·49 (1·22, 1·81) | 2·69 (2·45, 2·95) | 2·21 (1·77, 2·75) | <0·0001 |
Abbreviations: ADCs, AIDS-defining cancers; AIDS, acquired immunodeficiency syndrome; CI, confidence interval; CNS, central nervous system; DLBCL, diffuse large B-cell lymphoma; HPV, human papillomavirus; KS, Kaposi sarcoma; NADCs, non-AIDS-defining cancers; NHL, non-Hodgkin lymphoma; SIR, standardized incidence ratio; VRNADCs, virus-related non-AIDS-defining cancers; VUNADCs, virus-unrelated non-AIDS-defining cancers.
Unless otherwise indicated, SIR ratios are from models adjusted for sex/HIV-risk group (men who have sex with men, other male, female), attained age (<30, 30–39, 40–49, 50–59, 60+ years), race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic/Latino), calendar year (modeled as one continuous variable, except for KS, DLBCL and CNS NHL which were modeled as separate segments as informed by Joinpoint analysis, see Methods), registry, and attained follow-up duration (<10 vs. 10+ years after the later of HIV report or AIDS diagnosis).
SIR ratio (95% CI) and p value from unadjusted model, as adjusted model did not converge.
SIR ratio (95% CI) and p value for rectal cancer only, as adjusted model for rectosigmoid junction cancer did not converge.
This category does not include CNS NHL.
SIRs did not increase across calendar periods for any cancer (Appendix Table 3 [pp 5–6]). Moreover, SIRs decreased significantly for many cancer types, including some for which risk was elevated overall (grouped ADCs, KS, each AIDS-defining NHL, grouped VRNADCs, anus, liver, lung, non-AIDS-defining NHLs, and miscellaneous), grouped VUNADCs and myeloma; borderline decreasing trends were also observed for cervical cancer and Hodgkin lymphoma. Despite these decreases, SIRs remained elevated, with 95%CIs excluding 1·00, for several cancer types in the most recent period (2009–2012, Appendix Table 3 [pp 5–6]).
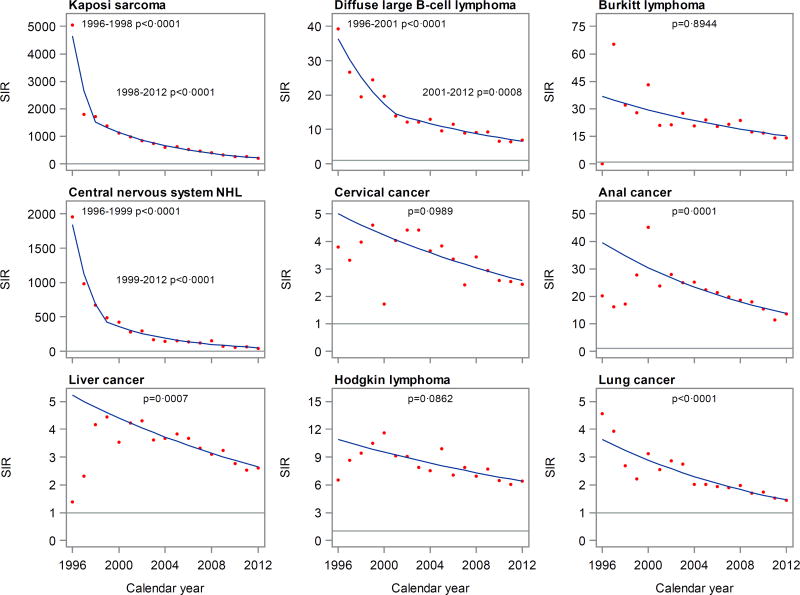
For most cancers selected for detailed calendar trend analysis, SIRs appeared to decrease steadily over time (Figure 1). For KS, DLBCL, and CNS NHL, moderating changes in slope were identified, i.e., steep early declines became attenuated after 1998, 2001, and 1999, respectively. For anal cancer, liver cancer, and Hodgkin lymphoma, there was a suggestive increase in the earliest years, but Joinpoint analysis identified only a single decreasing trend for these cancers across all of 1996–2012. After multivariable adjustment, SIR trends significantly declined for KS, DLBCL, CNS NHL, and cancers of the anus, liver, and lung. Trends were not significant for Burkitt lymphoma, cervical cancer, or Hodgkin lymphoma. Exclusion of one registry at a time did not change the trends appreciably (not shown).
Figure 1. Calendar trends in standardized incidence ratios for selected cancers in HIV-infected people in the United States.
Abbreviations: NHL, non-Hodgkin lymphoma; SIR, standardized incidence ratio.
Dots depict observed SIRs and lines depict fitted crude trends characterized by Joinpoint. Horizontal lines indicate a SIR of 1.
P values are tests of trend from Poisson models adjusted for sex/HIV-risk group (men who have sex with men, other male, female), attained age (<30, 30–39, 40–49, 50–59, 60+ years), race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic/Latino), registry, and attained follow-up duration (<10 vs. 10+ years after the later of HIV report or AIDS diagnosis).
Discussion
During 1996–2012, HIV-infected people in the US, including those who had not yet developed AIDS, had elevated risk for many cancer types, especially those of viral cause. There was a decline in risk during this period for several virus-related cancers and lung cancer, presumably resulting, at least in part, from improving efficacy, earlier use, and wider access to ART over time.12,13 Although risk remained elevated for several cancers even in the most recent years, reassuringly we did not observe increasing trends in relative risk for any cancer. The elevated risk for many cancers, especially after AIDS onset, highlights the continuing contribution of immunosuppression to cancer risk in this population.
A decline in risk for ADCs, especially KS and NHL, has been well-established in the ART era in the US and other developed countries.3,5,6,8,10–12 Decreasing trends for KS, and NHL (mainly DLBCL and CNS NHL) have been steep, 3,5,6,8,10–12,15–17 but we observe here that they have moderated in more recent years. The trend for Burkitt lymphoma has been less clear,15–17 and in the present study, after accounting for demographic changes, the decline during 1996–2012 was not significant (Figure 1). Furthermore, our finding that the difference in risk between people with HIV-only and AIDS was much smaller for Burkitt lymphoma than for KS and other AIDS-defining NHLs confirms a previous analysis from the HACM Study.17 These observations for Burkitt lymphoma, along with evidence that EBV is less frequently detected in AIDS-related Burkitt lymphoma tumor cells than in other NHL subtypes,16 point to a somewhat complex etiologic relationship with immunosuppression.18
Some recent data suggest that cervical cancer risk among HIV-infected women may also be decreasing.3,8,10 After multivariable adjustment, we did not find a significant trend, although SIRs decreased over time (Figure 1). We also found that cervical cancer risk was most highly elevated among women with a prior AIDS diagnosis, consistent with an etiologic role for long-term immunosuppression. An AIDS diagnosis could also be a marker for lack of appropriate medical care and inadequate cervical cancer screening.19
The elevated risks for VRNADCs, particularly after an AIDS diagnosis, highlight the biological relevance of immunosuppression for these cancers as well. In previous studies,1–3,5–7,9,10,12,20–22 risk for each VRNADC was also found to be elevated, and risk for Hodgkin lymphoma and some HPV-related cancers was found to increase in relation to AIDS onset. Consistent with some recent studies we observed decreasing trends in risk relative to the general population for anal and liver cancers,9,12 but other recent studies observed null or increasing trends.5–7,9,10,12 These discrepancies among studies may partly be explained by differences in the included calendar years, as our plots suggest an increasing trend for these cancers in the earliest years, followed by a more recent decline.
Risks were also elevated compared with the general population for lung cancer and several other VUNADCs. Among HIV-infected people in our study, lung cancer was second only to AIDS-defining NHLs in incidence. The elevated risk of lung cancer has been previously documented, and is partly, though not entirely, explained by a high prevalence of smoking among HIV-infected people.1–4,23 The more pronounced risk for lung cancer among people with AIDS and the decline in SIRs over time (which is consistent with other studies5,9,10 and tracks with widened access to effective ART) both support a contribution from immunosuppression.23 HIV-related chronic pulmonary inflammation, abnormal immune activation, and repeated lung infections may also play a role.20,23,24 Smoking likely contributes to the elevated risk we observed for cancers of the oral cavity/pharynx, nasal cavity, and larynx.25 We also found increased risks for scrotal cancer, conjunctival cancer, polycythemia vera, and myelodysplastic syndrome. It is possible that scrotal cancer, like other anogenital cancers, could be caused by HPV, as suggested by reported detection of this virus in some tumors.26 An elevated risk of conjunctival cancer has been observed previously, especially among HIV-infected people in Africa, although an infectious agent has not been clearly identified.27,28 Risks for polycythemia vera and myelodysplastic syndrome are increased among immunosuppressed transplant recipients,29 although they do not have known viral causes.
We did not observe elevated risk for most VUNADCs, consistent with prior studies in the ART era.1–3,5,6,10,12,22 Indeed, risk was actually decreased compared with the general population for some VUNADCs. These deficits confirmed prior observations for breast and prostate cancers, and identified new deficits for other cancers with previously inconclusive results (e.g., uterine and colorectal cancers) or modest elevations (e.g., stomach and kidney/renal pelvis cancers).1–3,5,6,10,12,22 We considered that the deficits might reflect under-ascertainment of cancers, specifically due to out-migration (see Methods). However, these deficits persisted after we allowed for 27% out-migration 10+ years after HIV report/AIDS diagnosis, even though out-migration to such an extent seems unlikely. The deficits may have biological explanations (e.g., hormonal and/or metabolic abnormalities),1 which could be evaluated in future studies.
Limitations of our study include the lack of individual-level data on ART use and HIV disease markers (i.e., CD4 count and HIV viral load). Instead, we used calendar year as a population-level measure of ART use (with more recent calendar years associated with more effective ART, wider use, and earlier initiation), and AIDS onset as an indicator of ever having had advanced immune suppression. Changes in the prevalence of oncogenic viral infections and other cancer risk factors (e.g., smoking and alcohol use) over time may have potentially influenced our results, but the lack of data on these factors precluded us from evaluating them as confounders for the patterns we observed. Finally, we assessed a large number of cancers, so some findings could be due to chance. However, we used a stringent threshold (p<0·001) to identify cancers for which risk differed significantly from the general population, and to test differences in risk by AIDS status and over time.
A major strength of our study was its population-based design. The study covered eight states and Puerto Rico across a 17-year calendar period during the ART era. The study population comprised all people with known HIV infection living in these areas, including all HIV-risk groups and ages. The approximate similarity between the distribution of the demographic characteristics of our population and the US HIV population14 favors the representativeness of our sample and the generalizability of our results. Moreover, cancers were ascertained using linked cancer registry data, which have greater validity than other data sources.22 HACM is the largest study of cancer in HIV-infected people, which enabled the examination of a breadth of individual cancers, including NHL subtypes and rare cancers. Furthermore, since aging and other changes in the demographic characteristics of the HIV population could influence time trends in the SIRs, we adjusted the calendar trends using multivariable regression. Finally, in a sensitivity analysis, we observed similar trends when excluding one registry area at a time, which suggested that the observed overall trends were not disproportionally affected by one registry or the varying calendar intervals by registry.
Additional efforts aimed at cancer prevention and screening in HIV-infected people are warranted. Although SIRs did not increase for any cancer, and have declined over time for several cancers, SIRs were still elevated in HIV-infected people in recent years.3,8–10,12 Because the HIV population is aging and growing in size,3,14 the burden of cancer may increase even in the absence of increasing incidence rates.11 Early diagnosis of HIV-infection, prompt and sustained ART after diagnosis, and reduction of non-HIV cancer risk factors are crucial for cancer prevention.2,4,30 With further improvement of ART and expansion of ART use, reductions in the risk of KS, AIDS-defining NHLs, and potentially other cancers can be expected. Since current ART does not completely restore immunologic health,2 close monitoring of cancer risk factors and evaluation of symptoms possibly related to cancer is needed, even in virally suppressed patients. Efforts should aim to optimize smoking and alcohol cessation, and treatment of HCV and HBV infections.30 Screening for cervical cancer, and possibly for anal, liver and lung cancers, is appropriate for high risk populations.30
In conclusion, cancer risk has declined in HIV-infected people in the US, but remains elevated for a characteristic spectrum of cancers, notably for ADCs, many other virus-related cancers and lung cancer. Sustained and widened access to ART has likely contributed to declines in cancer risk, but improvements are needed to reduce the cancer burden further.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed, reviewed personal collections and examined reference lists to identify publications on cancer risk among HIV-infected people, compared to the general population or uninfected groups, that reported risk estimates overall and by AIDS onset, and recent trends covering the effective antiretroviral therapy (ART) era beginning in 1996. Previous evidence indicates that HIV-infected people, especially those with AIDS, have an elevated risk for many cancers, especially those of viral cause. Evidence also indicates that, after the introduction of ART in 1996, incidence declined for two AIDS-defining cancers, Kaposi sarcoma and non-Hodgkin lymphoma. However, trends for other cancers are less clear, and recent and comprehensive population-based data on cancer risk for HIV-infected people are limited.
Added value of this study
The HIV/AIDS Cancer Match (HACM) Study is the largest population-based study of cancer in HIV-infected people in the world. We used HACM Study data to assess cancer risk in a sample of 448,258 HIV-infected people in the United States during 1996–2012. We found that, compared with the general population, HIV-infected people (including those without AIDS) had elevated risk for a broad but characteristic spectrum of cancers, including AIDS-defining cancers, many other viral-related cancers, and lung cancer. While there was a decline over time in the risk for Kaposi sarcoma and non-Hodgkin lymphoma, and some non-AIDS defining cancers (anus, liver, and lung), risk remained quite elevated even in the most recent years.
Implications of all the available evidence
The declines in cancer risk likely reflect sustained and widened ART utilization. Despite these declines, cancer risk in HIV-infected people has remained elevated during the modern treatment era, indicating that continued cancer control efforts are warranted.
Acknowledgments
The authors gratefully acknowledge the support and assistance provided by individuals at the following HIV/AIDS and cancer registries: Colorado, Connecticut, Georgia, Maryland, Michigan, New Jersey, New York, Puerto Rico, and Texas. We also thank Timothy S. McNeel at Information Management Services for programming support.
This research was supported in part by the Intramural Research Program of the National Cancer Institute. RUHR and RD were supported by grant R01-CA165937 from the National Cancer Institute.
The following cancer registries were supported by the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute: Connecticut (HHSN261201300019) and New Jersey (HHSN261201300021I, N01-PC-2013-00021). The following cancer registries were supported by the National Program of Cancer Registries of the Centers for Disease Control and Prevention: Colorado (5U58DP003868-04), Georgia (5U58DP003875-01), Maryland (5NU58DP003919-05-00), Michigan (5U58DP003921-03), New Jersey (5U58/DP003931-02), and Texas (5U58DP000824-04). The New Jersey State Cancer Registry was also supported by the state of New Jersey, and the Maryland Cancer Registry was supported by the State of Maryland and the Maryland Cigarette Restitution Fund. The following HIV registries were supported by HIV Incidence and Case Surveillance Branch of the Centers for Disease Control and Prevention, National HIV Surveillance Systems: Colorado, Connecticut (5U62PS001005-05), Michigan (U62PS004011-02), and New Jersey (U62PS004001-2).
The views expressed in this paper are those of the authors and should not be interpreted to reflect the views or policies of the National Cancer Institute, HIV/AIDS or cancer registries, or their contractors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
All the authors declared no competing interests.
Author Contributions
RUHR and EAE wrote the first draft of the manuscript. RUHR conducted the statistical analysis. MSS and EAE are the HACM Study principal investigators; they acquired the data, obtained funding and supervised the study. All the authors contributed to the study design and the interpretation of the data, critically reviewed the manuscript for important intellectual content, and approved the final version of the article.
References
- 1.Shiels MS, Cole SR, Kirk GD, Poole C. A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J Acquir Immune Defic Syndr. 2009;52(5):611–22. doi: 10.1097/QAI.0b013e3181b327ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dubrow R, Silverberg MJ, Park LS, Crothers K, Justice AC. HIV infection, aging, and immune function: implications for cancer risk and prevention. Curr Opin Oncol. 2012;24(5):506–16. doi: 10.1097/CCO.0b013e328355e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shiels MS, Engels EA. Evolving epidemiology of HIV-associated malignancies. Curr Opin HIV AIDS. 2017;12(1):6–11. doi: 10.1097/COH.0000000000000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park LS, Hernández-Ramírez RU, Silverberg MJ, Crothers K, Dubrow R. Prevalence of non-HIV cancer risk factors in persons living with HIV/AIDS: a meta-analysis. AIDS. 2016;30(2):273–91. doi: 10.1097/QAD.0000000000000922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silverberg MJ, Chao C, Leyden WA, et al. HIV infection and the risk of cancers with and without a known infectious cause. AIDS. 2009;23(17):2337–45. doi: 10.1097/QAD.0b013e3283319184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franceschi S, Lise M, Clifford GM, et al. Changing patterns of cancer incidence in the early- and late-HAART periods: the Swiss HIV Cohort Study. Br J Cancer. 2010;103(3):416–22. doi: 10.1038/sj.bjc.6605756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silverberg MJ, Lau B, Justice AC, et al. Risk of anal cancer in HIV-infected and HIV-uninfected individuals in North America. Clin Infect Dis. 2012;54(7):1026–34. doi: 10.1093/cid/cir1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hleyhel M, Belot A, Bouvier AM, et al. Risk of AIDS-defining cancers among HIV-1-infected patients in France between 1992 and 2009: results from the FHDH-ANRS CO4 cohort. Clin Infect Dis. 2013;57(11):1638–47. doi: 10.1093/cid/cit497. [DOI] [PubMed] [Google Scholar]
- 9.Hleyhel M Writing committee of the Cancer Risk Group of the French Hospital Database on HIV (FHDH-ANRS CO4) Risk of non-AIDS-defining cancers among HIV-1-infected individuals in France between 1997 and 2009: results from a French cohort. AIDS. 2014;28(14):2109–18. doi: 10.1097/QAD.0000000000000382. [DOI] [PubMed] [Google Scholar]
- 10.Robbins HA, Shiels MS, Pfeiffer RM, Engels EA. Epidemiologic contributions to recent cancer trends among HIV-infected people in the United States. AIDS. 2014;28(6):881–90. doi: 10.1097/QAD.0000000000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silverberg MJ, Lau B, Achenbach CJ, et al. Cumulative incidence of cancer among persons with HIV in North America: a cohort study. Ann Intern Med. 2015;163(7):507–18. doi: 10.7326/M14-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park LS, Tate JP, Sigel K, et al. Time trends in cancer incidence in persons living with HIV/AIDS in the antiretroviral therapy era: 1997–2012. AIDS. 2016;30(11):1795–806. doi: 10.1097/QAD.0000000000001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eholie SP, Badje A, Kouame GM, et al. Antiretroviral treatment regardless of CD4 count: the universal answer to a contextual question. AIDS Res Ther. 2016;13:27. doi: 10.1186/s12981-016-0111-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103(9):753–62. doi: 10.1093/jnci/djr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123(1):187–94. doi: 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- 16.Biggar RJ, Chaturvedi AK, Goedert JJ, Engels EA. AIDS-related cancer and severity of immunosuppression in persons with AIDS. J Natl Cancer Inst. 2007;99(12):962–72. doi: 10.1093/jnci/djm010. [DOI] [PubMed] [Google Scholar]
- 17.Gibson TM, Morton LM, Shiels MS, Clarke CA, Engels EA. Risk of non-Hodgkin lymphoma subtypes in HIV-infected people during the HAART era: a population-based study. AIDS. 2014;28(15):2313–8. doi: 10.1097/QAD.0000000000000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guech-Ongey M, Simard EP, Anderson WF, et al. AIDS-related Burkitt lymphoma in the United States: what do age and CD4 lymphocyte patterns tell us about etiology and/or biology? Blood. 2010;116(25):5600–4. doi: 10.1182/blood-2010-03-275917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chapman Lambert CL. Factors influencing cervical cancer screening in women infected with HIV: a review of the literature. J Assoc Nurses AIDS Care. 2013;24(3):189–97. doi: 10.1016/j.jana.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Engels EA. Non-AIDS-defining malignancies in HIV-infected persons: etiologic puzzles, epidemiologic perils, prevention opportunities. AIDS. 2009;23(8):875–85. doi: 10.1097/QAD.0b013e328329216a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaturvedi AK, Madeleine MM, Biggar RJ, Engels EA. Risk of human papillomavirus-associated cancers among persons with AIDS. J Natl Cancer Inst. 2009;101(16):1120–30. doi: 10.1093/jnci/djp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park LS, Tate JP, Rodriguez-Barradas MC, et al. Cancer incidence in HIVinfected versus uninfected veterans: comparison of cancer registry and ICD-9 code diagnoses. J AIDS Clin Res. 2014;5(7):1000318. doi: 10.4172/2155-6113.1000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirk GD, Merlo CA Lung HIVS. HIV infection in the etiology of lung cancer: confounding, causality, and consequences. Proc Am Thorac Soc. 2011;8(3):326–32. doi: 10.1513/pats.201009-061WR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sigel K, Wisnivesky J, Crothers K, et al. Immunological and infectious risk factors for lung cancer in US veterans with HIV: a longitudinal cohort study. Lancet HIV. 2017;4(2):e67–e73. doi: 10.1016/S2352-3018(16)30215-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gandini S, Botteri E, Iodice S, et al. Tobacco smoking and cancer: a metaanalysis. Int J Cancer. 2008;122(1):155–64. doi: 10.1002/ijc.23033. [DOI] [PubMed] [Google Scholar]
- 26.Vyas R, Zargar H, Trolio RD, Di Lorenzo G, Autorino R. Squamous cell carcinoma of the scrotum: a look beyond the chimneystacks. World J Clin Cases. 2014;2(11):654–60. doi: 10.12998/wjcc.v2.i11.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carreira H, Coutinho F, Carrilho C, Lunet N. HIV and HPV infections and ocular surface squamous neoplasia: systematic review and meta-analysis. Br J Cancer. 2013;109(7):1981–8. doi: 10.1038/bjc.2013.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng H, Taylor JL, Benos PV, et al. Human transcriptome subtraction by using short sequence tags to search for tumor viruses in conjunctival carcinoma. J Virol. 2007;81(20):11332–40. doi: 10.1128/JVI.00875-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morton LM, Gibson TM, Clarke CA, et al. Risk of myeloid neoplasms after solid organ transplantation. Leukemia. 2014;28(12):2317–23. doi: 10.1038/leu.2014.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goncalves PH, Montezuma-Rusca JM, Yarchoan R, Uldrick TS. Cancer prevention in HIV-infected populations. Semin Oncol. 2016;43(1):173–88. doi: 10.1053/j.seminoncol.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.