Abstract
Background & Aims
Gastroparesis is a complication of diabetes with few treatment options. Relamorelin (also called RM-131) is a selective, prokinetic agonist of ghrelin. We aimed to evaluate efficacy of relamorelin on symptoms and gastric emptying (GE) in a 12-week, phase 2B study of diabetic patients with moderate to severe gastroparesis symptoms (DG).
Methods
We performed a study of 393 patients with DG (37.7% male; 9.9% with type 1 diabetes; median age, 58.2 years [range 20–76]; median body mass index, 31.4 kg/m2 [range, 18.2–60.1]; HbA1c level, 7.6%, [range, 5.2–11.0]). All participants had 13C-spirulina GE breath test T1/2 values of 79 min or more (with 89.8% delayed relative to 90th %ile of normal, 85.75 minutes), recent vomiting, and gastroparesis cardinal symptom index-daily diary scores of 2.6 or more. Patients were randomly assigned to groups given placebo (n=104) or relamorelin (10 μg [n=98], 30 μg [n=109], or 100 μg [n=82] twice daily) for 12 weeks, following a 2-week, single-blind, placebo run-in period. Patient-reported outcomes were determined from DG Symptom Severity daily e-diaries, in which patients recorded vomiting frequency and symptom scores (nausea, abdominal pain, postprandial fullness, and bloating) on a 0–10 scale. Endpoints were change from baseline in vomiting frequency, composite DG Symptom Severity score, GE, and safety. We performed longitudinal, mixed-effects model analysis using repeated measures, with baseline and baseline-by-week interaction values as covariates.
Results
Patients given relamorelin had a 75% reduction in vomiting frequency compared to baseline, but this difference was not significant compared with the placebo group. All 4 symptoms of DG (composite or individual symptoms) were significantly reduced over the 12-week study period in all 3 relamorelin dose groups, compared to the placebo group (all P<.05, based on longitudinal analysis over 12 weeks). Relamorelin significantly accelerated GE from baseline compared with placebo (by 12%, P<.05 for the 10 and 30 μg groups; P=.051 for the 100 μg group). Dose-related worsening of glycemic control was noted in 14.5% of patients who received relamorelin; some required insulin or other diabetes drug dosage adjustments.
Conclusions
In a phase 2B randomized trial of patients with moderate to severe DG, relamorelin significantly reduced core symptoms of DG and overall composite score compared to placebo, accelerated GE, and was generally safe and well tolerated.
Keywords: nausea, vomiting, fullness, bloating
INTRODUCTION
Gastroparesis is defined as delayed gastric emptying with associated symptoms in the absence of mechanical obstruction.1 The cardinal symptoms are upper abdominal pain, postprandial fullness, bloating, early satiety, nausea and vomiting. Weight loss, malnutrition, dehydration, electrolyte imbalance, bezoar formation, and aspiration pneumonia may occur in advanced cases.2,3
Gastroparesis is a clinically important complication of diabetes mellitus. Maleki et al. documented upper gastrointestinal symptoms such as nausea and vomiting in ~15% of patients with type 1 or 2 diabetes mellitus in a population-based questionnaire study in southeastern Minnesota.4 Similarly, epidemiological studies conducted in Australia5 showed that diabetes mellitus is associated with an increased prevalence of upper and lower gastrointestinal symptoms, including vomiting (OR, 2.51; 95% CI, 1.12–5.66), which were linked to poor glycemic control, but not to duration of diabetes or type of treatment. In contrast, based on the medical records of people in the same community (Olmsted County, MN), the population-based incidence of definite gastroparesis (based on objective measurement of gastric emptying by scintigraphy using a 320 kcal, 30% fat solid-liquid meal) ranged from 6.3 to 17.2 cases per 100,000 person-years when adjusted for age and sex.6 In that analysis, the prevalence of gastroparesis was estimated to be 5% among patients with type 1 diabetes mellitus, 1% among patients with type 2 diabetes mellitus, and 0.2% in non-diabetic controls.7
Besides suffering from similar gastrointestinal symptoms and other associated complications as described above, patients with established diabetic gastroparesis also face challenges in managing diabetic glycemic control. Gastroparesis has a significant impact on patients’ perceived ability to self-manage and control their diabetes.8 Delayed gastric transit and vomiting can exacerbate efforts to match caloric intake with insulin or other therapies for diabetes, frequently leading to increased hypo- and hyperglycemic excursions as a consequence. Many patients with diabetic gastroparesis, particularly patients with underlying type 1 diabetes mellitus or insulin-treated type 2 diabetes mellitus, are less motivated to achieve HbA1c target levels if the occurrence of hypoglycemia is a concern. Thus, overall glycemic control is suboptimal and further hyperglycemic complications can continue to confound the management of patients with diabetic gastroparesis who have long-standing type 1 diabetes mellitus or type 2 diabetes mellitus. Indeed, it has also been shown that, among 78 patients with type 1 diabetes mellitus followed for 20 years, delayed gastric emptying was associated with early and long-term hyperglycemia.9 Moreover, gastric emptying can impact glycemic control; for example, in patients with type 2 diabetes not on insulin, delayed gastric emptying in the absence of symptoms may potentially be advantageous in relation to postprandial glycemic control,10 and this is the basis for treatment with amylin analogs or GLP-1 receptor agonists in patients with type 2 diabetes.
Unfortunately, there are few approved or efficacious treatment options for diabetic gastroparesis. The 5-HT4 receptor agonist, cisapride, has been withdrawn from the prescription markets in most countries. In a prior systematic analysis of prokinetics in gastroparesis, erythromycin was the most efficacious in stimulating gastric emptying, while both erythromycin and domperidone were noted to improve overall symptoms of gastroparesis.11 However, this analysis did not consider the tachyphylaxis associated with longer term use of motilin receptor agonists, including erythromycin.12 Tachyphylaxis resulted in failed therapy or drop-outs beyond 4 weeks of treatment with erythromycin.13 Domperidone, a dopamine receptor antagonist, is not approved by the Food and Drug Administration (FDA) for treatment of gastroparesis. Another dopamine receptor antagonist, metoclopramide, is currently the only prokinetic approved by FDA for diabetic gastroparesis. Metoclopramide targets both D1 and D2 dopamine receptors with a peripheral gastrointestinal prokinetic effect (predominantly in the stomach) and central antiemetic action in the chemoreceptor trigger zone on the floor of the 4th ventricle.14 Metoclopramide, administered in a nasal spray, decreased symptoms of gastroparesis in women (not in men) with diabetic gastroparesis.15 The side effects of metoclopramide include akathisia, restlessness, insomnia and agitation (usually occuring 1–2 weeks into therapy), depression, tardive dyskinesia (FDA boxed warning) and prolongation of the corrected QT interval.16 The FDA warning also advises against prescribing metoclopramide for longer than 3 months, unless therapeutic benefits outweigh the potential risks.
Relamorelin, a pentapeptide ghrelin receptor agonist, has potent prokinetic effects. In nonclinical studies, the effects of relamorelin on gastric emptying were 15- to 130-fold more potent than natural ghrelin.17 Relamorelin, administered at a dose of 100 μg s.c., accelerated gastric half-emptying time of solids in type 1 and type 2 diabetes mellitus with prior documentation of delayed gastric emptying,18,19 and, at a single dose of 30 μg, it increased frequency of distal antral motor contractions without inhibiting gastric accommodation or inducing satiation.20 In a large phase 2A, randomized, 4-week, controlled trial in patients with diabetic gastroparesis, relamorelin, at a dose of 10 μg b.i.d., significantly accelerated gastric emptying and decreased vomiting by ~60%; in the subgroup of patients with vomiting during the 1-week, run-in period, it decreased other symptoms such as nausea, abdominal pain, bloating and early satiety.21 Thus, the range of efficacy of relamorelin spans from 10 μg to 100 μg, which was the dose range selected for this study.
The aims of the current study were to evaluate the safety and efficacy of relamorelin on disease symptoms and gastric emptying across a 10-fold dose range (10 to 100 μg, b.i.d.) over 12 weeks in diabetic patients with moderate to severe symptoms of gastroparesis with evidence of vomiting at baseline.
METHODS
Study Design
We conducted a 12-week, randomized, double-blind, placebo-controlled, parallel-group study with a 2-week, single-blind, placebo run-in (Figure 1A). The study was conducted at clinical sites in the United States, Israel and Europe. The allocation ratio across treatment groups is shown in Figure 1B.
Figure 1.
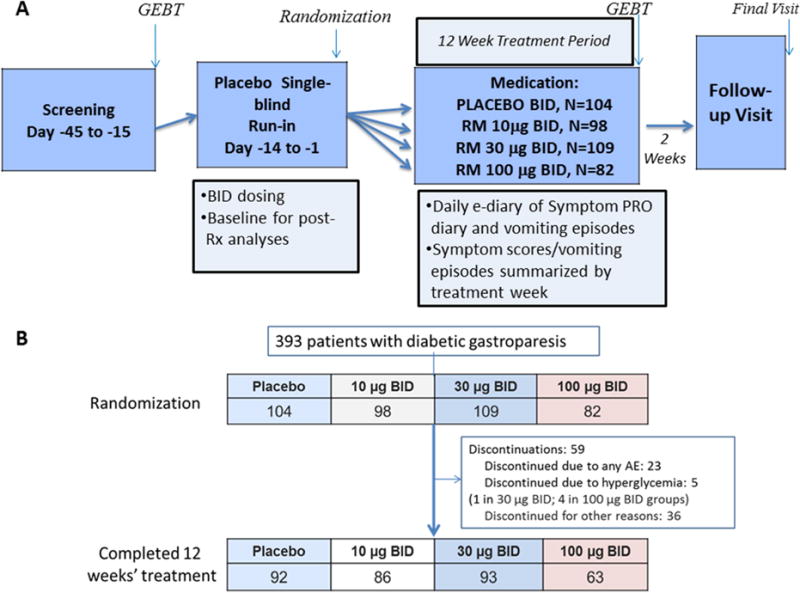
A. Experimental design
PRO=Patient reported outcome diary; GEBT=gastric emptying breath test; Sx=symptom; Rx=treatment; QD=once daily; BID=twice daily
B. CONSORT flow chart
Patients
Eligible patients with diabetic gastroparesis had type 1 or type 2 diabetes mellitus with HbA1c ≤11%, ≥3 months history of ongoing gastroparesis symptoms, a history of vomiting with ≥2 episodes during the 2 weeks prior to screening and at least one episode of vomiting reported during the single-blind placebo run-in period, 13C-spirulina gastric emptying breath test (GEBT) T1/2 values ≥79 minutes (which was the 75th percentile of GE T1/2 of healthy volunteers in prior validation studies), and a Gastroparesis Cardinal Symptom Index-Daily Diary (GCSI-DD) score ≥2.6 based on the recorded symptoms during the 2-week baseline period. The 75th percentile was selected to exclude patients with accelerated gastric emptying who may also present with similar upper gastrointestinal symptoms, as seen in patients with gastroparesis. Thus, to define an accurate diabetic gastroparesis population to treat safely with a prokinetic agent in this trial, patients who had accelerated GE T1/2 were excluded. The plan for enrollment [NCT02357420] required at least 50% of patients with GE T1/2 ≥97 minutes (i.e., the 95th percentile of normal range based on 38 healthy volunteers.22 The percentage of patients with delayed GE T1/2 (>85.75min, that is the 90th percentile of normal controls22 in each treatment groups was estimated and reported (Table 1). Patients on renal dialysis were excluded, though no specific cut-offs of serum creatinine or creatinine clearance were used for eligibility. In prior pharmacokinetic studies, it was estimated that only 8% of relamorelin is excreted in urine, and pharmacokinetics of relamorelin appears similar in healthy volunteers and patients with diabetes mellitus.
Table 1.
Baseline demographics and data on gastric emptying and smptoms at baseline (data show % or mean values)
| Placebo | 10 μg BID | 30 μg BID | 100 μg BID | P value | |
|---|---|---|---|---|---|
|
| |||||
| Number of Patients | 104 | 98 | 109 | 82 | |
|
| |||||
| Age (years) | 55.7 | 59.3 | 56.0 | 57.1 | ns |
|
| |||||
| Female % | 61.5 | 60.2 | 59.6 | 69.5 | ns |
|
| |||||
| Race (% Caucasian) | 81% | 81% | 80% | 75% | ns |
|
| |||||
| Diabetes Mellitus | |||||
| Type 1 (%) | 13% | 6% | 9% | 12% | |
| Type 2 (%) | 87% | 94% | 91% | 88% | ns |
| Diabetes duration (years) | 12.7 | 13.0 | 14.0 | 14.9 | ns |
| Current insulin use (%) | 43% | 31% | 37% | 46% | ns |
| Baseline HbA1c (%) | 7.8 | 7.4 | 7.7 | 8.1 | ns |
|
| |||||
| Body Mass Index (kg/m2) | 33.2 | 31.7 | 32.5 | 32.2 | ns |
|
| |||||
| GEBT T1/2 (min) | 127 | 127 | 129 | 134 | ns |
|
| |||||
| % (n) with delayed GE T1/2 (>85.8 min) | 88.5% (92) |
88.8% (87) |
89.9% (98) |
96.3% (79) |
ns |
|
| |||||
| Baseline Symptoms | |||||
| Weekly vomiting episodes | 5.9 | 7.9 | 6.2 | 5.4 | ns |
| 4-symptom composite score | 21.4 | 21.6 | 20.9 | 21.9 | ns |
Symptoms were rated by the patients using the choices: none (0), very mild (1), mild (2), moderate (3), severe (4), and very severe (5). The GCSI-DD total score equals the sum of the nausea/vomiting, bloating, and fullness/early satiety subscales divided by 3.23
The exclusion criteria were upper gastrointestinal obstruction, prior gastric surgery, HbA1c >11.0%, use of opiates, and use of metoclopramide, domperidone, erythromycin or antiemetics ≥2 weeks prior to randomization and throughout the study.
Medication
Relamorelin is a novel synthetic pentapeptide amide with potent ghrelin-receptor agonist activity and with similar characteristics to native ghrelin, but with enhanced potency, plasma stability, and circulating half-life. Pharmacokinetic studies in humans have shown a terminal half-life of ~4.5 hours, though, at highest doses, the terminal half-life was 19.4 hours.21 Toxicologic studies with relamorelin provide >750-fold safety margins compared with dose exposures reported in clinical trials. In prior pharmacokinetic studies, it was estimated that only 8% of relamorelin is excreted in urine, and pharmacokinetics of relamorelin appears similar in healthy volunteers and patients with diabetes mellitus.
Randomization
Patients were randomized to twice daily (b.i.d.) subcutaneous (s.c.) injections of placebo or 1 of 3 doses of relamorelin (10 μg, 30 μg, or 100 μg); interventions had similar appearance. Study drug was recommended to be administered approximately 30 minutes before breakfast and dinner, or during typical breakfast and dinner meal times (e.g., 6–9 AM and 5–8 PM) if a patient did not regularly eat breakfast or dinner. The planned sample sizes were 105 patients each in the placebo and relamorelin 10 μg and 30 μg groups, and 80 patients in the relamorelin 100 μg group (Figure 1). Randomization was concealed. A computer generated randomization schedule was produced and study medication was provided to each clinical trial site for patients according to the central randomization.
Patient Reported Outcome (PRO) Measurements
Key endpoints were reported using the Diabetic Gastroparesis Symptom Severity Diary (DGSSD), a daily e-diary designed to collect patient-reported vomiting frequency and symptoms of nausea, abdominal pain, early satiety, postprandial fullness, bloating and vomiting severity using a 0–10 scale. Qualitative research has been conducted in 41 patients with diabetic gastroparesis to validate the DGSSD-PRO.24 The results showed validity of a 6-item instrument addressing severity of nausea, vomiting, abdominal pain, early satiety, and bloating, as well as frequency of vomiting. Measurement properties were generally strong for weekly averages of daily item and composite scores, with intra-class correlation coefficients for individual items ranging from 0.79 to 0.97, and Cronbach’s alpha for various 3- and 4-item composites ranging from 0.85 to 0.93. Whereas measurement properties of the vomiting frequency were highly skewed, three effect size methods demonstrated the responsiveness of the item level and composite measures.
Within this study, we also used the data to validate the symptom-based patient reported outcome (PRO), and we collected information to appraise the context as well as content validity of the DGSSD by gathering data using the GCSI Daily Diary (GCSI-DD),25 by Global Assessment of Symptoms (“Overall, how would you rate your symptoms in the past 7 days?”), and using the Patient Assessment of Upper Gastrointestinal Symptom Severity Index (PAGI-SYM).26
Gastric Emptying
Gastric emptying was determined via GEBT during baseline (screening) and at 12 weeks using an FDA-approved method (https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm441370.htm). GEBT T1/2 is a continuous measurement representing the calculated time to empty from the stomach into the small intestine half of a 13C-labeled, 230 kcal test meal [27 grams of rehydrated, pasteurized scrambled egg mix containing a dose of 43 mg of 13C (provided by approximately 100 mg of 13C-Spirulina), 6 saltine crackers, 180 mL water, and consisting of 29.9% kcal as carbohydrates, 25.5% as protein, and 44.6% as fat].
GEBT T1/2 was calculated from values of 13CO2-enriched exhaled breath collected for up to 240 minutes after ingestion of the test meal, which is the last time point used in the studies for GEBT validation.22 In previous validation studies in 38 healthy volunteers, the mean GEBT T1/2 was 67.8 minutes, the 75th percentile was 79 minutes, and the 90th percentile (used to define upper limit of normal in the current study) was 85.75 minutes.22
Endpoints
All participants and study personnel (care providers and those assessing outcomes) were blinded to treatment assignment throughout the study. The prespecified primary endpoint was vomiting frequency. Vomiting frequency was selected for the primary endpoint as a result of the observations in a phase 2A study with relamorelin, which showed significant benefit in patients with vomiting severity rated >0 at baseline.21 The change from baseline through week 12 was also analyzed using a 4-symptom composite of DGSSD symptoms based on weekly normalized scores for nausea, abdominal pain, postprandial fullness and bloating.
Other secondary and exploratory endpoints were vomiting severity, gastric emptying T1/2 measured by GEBT, individual symptoms, GCSI-DD score, and safety.
Safety was evaluated by vital signs, adverse events, and laboratory measured parameters including blood glucose and HbA1c levels, which were monitored throughout the 12-week treatment period. In addition, there was a 30-day, follow-up period after stopping study treatments to assess safety in accordance with clinical trial practice. Blood glucose monitoring was not routine in most patients, but patients were advised to monitor their blood glucose more frequently if they or the study centers detected significant increases in blood glucose compared to the individual’s norms.
Statistical Power
With 105 patients randomized to the placebo and to the two lower relamorelin dose groups, this study was expected to have approximately 90% or greater power for the comparison of relamorelin treatment with placebo. Furthermore, assuming an approximately similar effect size for the relamorelin high dose group as for the two lower relamorelin dose groups, and with ~80 patients randomized to the 100 μg b.i.d. group, the comparison of the 100 μg b.i.d. dose group with placebo would have greater than 80% power. The highest dose of relamorelin, 100 μg b.i.d., was included to determine whether it provides significant advantage over lower doses in this dose-ranging phase 2B trial, or could be excluded in the phase 3 trials.
Statistical Analysis
Statistical analysis to assess treatment effects at each treatment week was based on a longitudinal, mixed-effects model with repeated measures, with baseline and baseline-by-week interaction values as covariates and unstructured variance-covariance matrix. The longitudinal analysis cumulated over the 12 weeks the severity of symptoms of nausea, abdominal pain, postprandial fullness, and bloating using a 0–10 scale or the mean score for all those symptoms for the composite symptom score. The unit for this analysis were arbitrary area under the curve (AUC) units expressed with a positive number that described the area of reduction in symptom scores below the baseline values for each individual symptom and the four-symptom composite score. Thus, the increase in AUC (higher postive values) reflects reductions of symptom scores over the 12 weeks of treatment in this clinical trial.
All authors had access to the study data and reviewed and approved the final manuscript.
RESULTS
Baseline Demographics
A total of 393 patients with diabetic gastroparesis [male 37.7%; median age 58.2 years (range: 20–76); median BMI 31.4 kg/m2 (range 18.2–60.1); median HbA1c 7.6% (range 5.2–11.0); those with type 1 diabetes mellitus 9.9%] were randomized at clinical sites in the United States, Israel and Europe. The study start date was January 2015 and the completion date was July 2016 after all patient visits were completed, in accordance with the experimental design (Figure 1A, NCT #02357420). Table 1 compares the baseline data for the 4 treatment groups; there were no differences in the baseline parameters (demographics, type of diabetes, treatment of diabetes, delay in gastric emptying, and symptoms). Figure 1B shows the CONSORT flow chart describing trial evolution.
Symptom Endpoints
1° Endpoint: Change from Baseline to Week 12 in Weekly Vomiting Episodes
Table 2 shows there was no significant difference in the treatment groups for the change from baseline to week 12 in weekly vomiting episodes. The 10, 30 and 100 μg relamorelin treatments induced approximately 75% reduction in vomiting episodes, with a remarkable placebo response reduction of ~70%.
Table 2.
Change from baseline to week 12 in DGSSD (4-symptom composite scorea) and GEBT
| Parameter | Placebo BID (n=88) | RM 10 μg BID (n=86) | RM 30 μg BID (n=91) | RM 100 μg BID (n=63) |
|---|---|---|---|---|
| Weekly Vomiting Episodes | ||||
| Placebo | 10 μg | 30 μg | 100 μg | |
| N=85 | N=81 | N=86 | N=66 | |
| Baseline, mean ± SD | 5.7 ±6.0 | 7.7 ±17.2 | 6.9 ±10.3 | 4.8 ±5.2 |
| Week 12, mean ± SD | 2.8 ±5.9 | 3.9 ±17.1 | 3.1 ±9.1 | 3.8 ±13.7 |
| Change from baseline, mean ± SD | −2.9 ±5.8 | −3.7 ±12.5 | −3.8 ±7.6 | −1.1 ±13.5 |
| Percent change from baselinea | −70.5% | −74.9% | −75.8% | −73.4 |
| p-valueb (difference from placebo) | 0.36 | 0.25 | 0.59 | |
| DGSSD 4 symptom composite scorec | ||||
| Baseline, mean ± SD | 22.7 ± 7.3 | 22.7 ± 7.9 | 22.4 ± 6.7 | 24.0 ± 7.5 |
| Week 12, mean ± SD | 17.1 ± 8.8 | 14.0 ± 10.3 | 13.8 ± 9.6 | 14.2 ± 9.5 |
| Change from baseline, mean ± SD | −5.6 ± 8.8 | −8.7 ± 9.0 | −8.7 ± 9.0 | −9.7 ± 8.8 |
| Change from baselined | −6.07 | −7.91 | −8.41 | −8.64 |
| LS mean difference vs. placebo | −1.85 | −2.34 | −2.57 | |
| p-value (difference from placebo)e | 0.134 | 0.053 | 0.052 | |
| GEBT (T½ in min) | ||||
| Baseline, mean ± SD | 127.1 ± 36.5 | 126.8 ± 37.6 | 128.6 ± 35.9 | 133.6 ± 35.4 |
| Week 12, mean ± SD | 126.3 ± 39.8 | 112.8 ± 43.5 | 115.8 ± 45.7 | 118.0 ± 49.5 |
| Change from baseline, mean ± SD | −0.0 ± 38.5 | −12.7 ± 38.1 | −12.8 ± 36.5 | −13.6 ± 40.5 |
| Change from baseline | −0.43 | −13.36 | −12.55 | −12.47 |
| LS mean difference vs. placebo | −12.93 | −12.12 | −12.04 | |
| 95% CI of difference | −24.04; −1.82 | −22.92; −1.33 | −24.11; 0.04 | |
| p-value (difference from placebo)b | 0.023 | 0.028 | 0.051 | |
BID, twice daily; CI = confidence interval; DGSSD, Diabetic Gastroparesis Symptom Severity Diary; GEBT, gastric emptying breath test; LS, least squares; RM, relamorelin; SD, standard deviation
Analysis was done on log-transformed change-from-baseline of weekly least square means for vomiting data, and reported as percent change from baseline.
Two-sided p-value from longitudinal, mixed-effects model with repeated measures, including fixed effects for treatment, week, treatment-by-week interaction, as well as baseline and baseline-by-week interaction values as the covariates with unstructured variance-covariance correlation matrix being common to all subjects for the repeated measures over treatment weeks
Nausea, post-prandial fullness, abdominal pain, bloating; composite score in total numeric points;
Analysis done on change-from-baseline data for sum of weekly averages of 4 individual symptom scores (nausea, abdominal pain, post-prandial fullness, and bloating);
Composite Endpoints
Table 2 shows the change from baseline to week 12 in the DGSSD 4-symptom composite score, and Table 3 shows the longitudinal analysis over 12 weeks of the composite and individual symptom scores. While the change from baseline to week 12 shows borderline effects in the 30 and 100 μg treatment groups, the longitudinal analysis (Table 3) shows highly significant effects with all three relamorein treatment groups compared to placebo for the DGSSD 4-symptom composite score. Improvements were also evident for each of the individual symptoms comprising the composite score, namely, nausea, postprandial fullness, abdominal pain and bloating (Figures 2A and 2B). Finally, the magnitude of response and separation of the relamorelin treatment arms from placebo are apparent after 4 weeks of dosing and remain consistent relative to placebo between 4 and 12 weeks (Figure 2A).
Table 3.
Composite and individual symptom scores over 12 weeks: longitudinal analysis of reduction from baseline in arbitrary AUC (area under the curve relative to baseline expressed as positive values) units. (Pbo = placebo)
| Symptom | Mean + SD | Placebo, N=88 | 10 μg RM, N=86 | 30 μg RM, N=91 | 100 μg RM, N=63 |
|---|---|---|---|---|---|
| 4-Symptom Composite | 318.1 ±546.9 | 501.0 ±402.4 | 544.0 ±577.5 | 554.3 ±541.4 | |
| LS Mean Δ from Pbo | 183.51 | 231.77 | 209.48 | ||
| 95% CI of difference | 24.11; 342.92 | 74.58; 388.96 | 35.68; 383.27 | ||
| P-value | 0.02 | <0.01 | <0.02 | ||
| Nausea | 94.9 ±161.2 | 128.0 ±145.6 | 149.9 ±157.8 | 152.0 ±157.6 | |
| LS Mean Δ from Pbo | 34.32 | 57.01 | 50.38 | ||
| 95% CI of difference | −9.19; 77.83 | 14.11; 99.92 | 2.98; 97.77 | ||
| P-value | 0.12 | <0.01 | 0.04 | ||
| Post-prandial Fullness | 67.3 ±139.9 | 118.2 ±142.0 | 124.1 ±150.3 | 126.7 ±143.9 | |
| LS Mean Δ from Pbo | 53.72 | 57.28 | 54.44 | ||
| 95% CI of difference | 11.64 ; 95.80 | 15.81; 98.75 | 8.60; 100.29 | ||
| P-value | <0.01 | <0.01 | 0.02 | ||
| Abdominal Pain | 81.7 ±148.3 | 127.4 ±141.1 | 133.3 ±151.9 | 145.2 ±140.1 | |
| LS Mean Δ from Pbo | 43.09 | 54.42 | 56.47 | ||
| 95% CI of difference | 1.18 ; 84.99 | 13.09; 95.74 | 10.79; 102.15 | ||
| P-value | 0.04 | <0.01 | 0.02 | ||
| Bloating | 74.2 ±148.4 | 127.4 ±139.0 | 136.6 ±150.0 | 130.3 ± 142.5 | |
| LS Mean Δ from Pbo | 52.18 | 63.21 | 48.39 | ||
| 95% CI of difference | 10.51; 93.86 | 22.12; 104.31 | 2.94; 93.84 | ||
| P-value | <0.01 | <0.01 | 0.04 |
Figure 2.
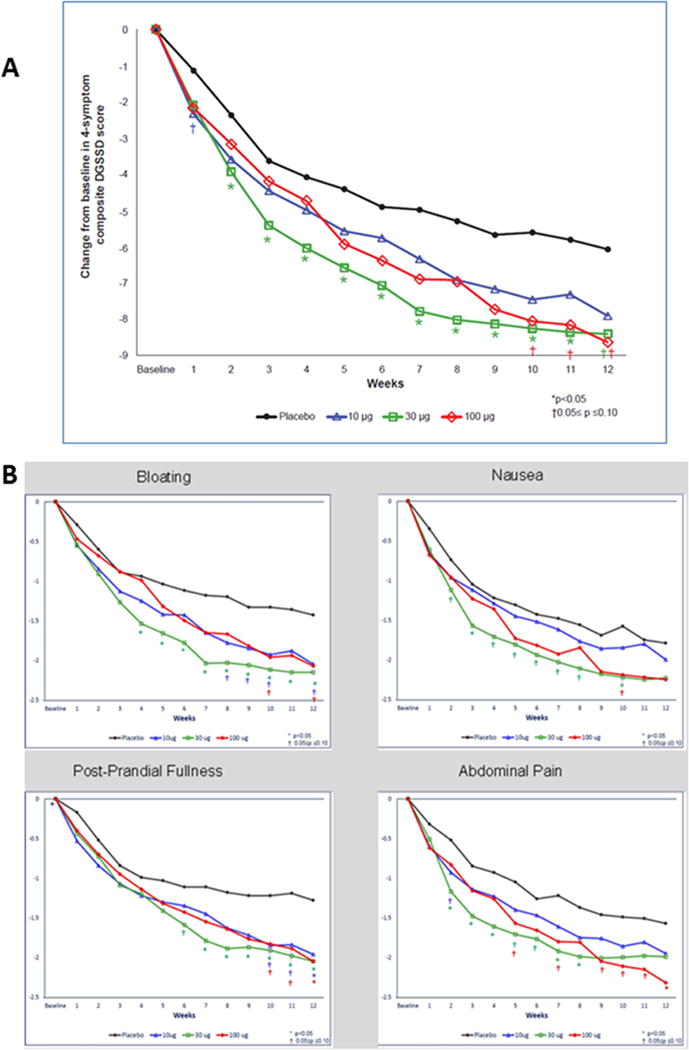
A. Change from baseline through week 12 in DGSSD [4-symptom (nausea, postprandial fullness, abdominal pain, bloating) composite score in total numeric points; full analysis set]. Note that the * and † symbols reflect differences relative to placebo treatment.
B. Change from baseline through week 12 for each individual symptom. Note that the * and † symbols reflect differences relative to placebo treatment.
Other Symptom-Related Observations
For all doses, there were also improvements in GCSI-DD at week 12 relative to baseline (Supplementary Figure 1), changes from baseline over 12 weeks for the Global Assessment of Symptoms [“Overall, how would you rate your symptoms in the past 7 days?” (Supplementary Table 1)] and PAGI-SYM improvements (Supplementary Figure 1) at week 12.
Gastric Emptying
Relamorelin accelerated gastric emptying T1/2 at all 3 doses compared to placebo (Table 2), with approximately 10% acceleration from baseline (shortened T1/2 by 12 to 13 minutes overall) for the relamorelin treatment groups and <1% change in the placebo group (Supplementary Figure 2). The difference in gastric emptying with relamorelin compared to placebo was significant (p<0.05) for the fully powered 10 and 30 μg relamorelin dose arms.
Safety, Completion and Adverse Events
Relamorelin was safe and well tolerated with high compliance (98.8% based on syringe/kit reconciliations during the double-blind period) and study completion rates (85% overall: 88.5, 87.8, 85.3, and 76.8% for placebo, and 10, 30 and 100 μg relamorelin, respectively). Subcutaneous injections were well tolerated with no clinically important injection site reactions. Adverse events reported are shown in Table 4.
Table 4.
Treatment emergent (TE) serious adverse effects (SAEs), discontinuations and AEs of special interest
| Placebo | 10 μg BID | 30 μg BID | 100 μg BID | |
|---|---|---|---|---|
|
| ||||
| Number of Patients | 104 | 98 | 109 | 82 |
|
| ||||
| Completed (%) | 92 (86%) | 86 (88%) | 93 (85%) | 63 (77%) |
|
| ||||
| TE SAEs | 8 (7.7%) | 7 (7.1%) | 10 (9.2%) | 6 (7.3%) |
|
| ||||
| Reasons for Discontinuation | ||||
|
| ||||
| Discontinued (%) | 15 (14%) | 13 (13%) | 17 (16%) | 19 (23%) |
|
| ||||
| TEAEs (%) | 3 (2.9%) | 3 (3.1%) | 8 (7.3%) | 9 (11.0%) |
| Withdrew Consent | 4 (3.8%) | 8 (8.2%) | 7 (6.4%) | 6 (7.3%) |
| Lost to Follow-Up | 3 (2.9%) | 2 (2.0%) | 2 (1.8%) | 3 (3.7%) |
| Other | 4 (3.8%) | 1 (1.2%) | ||
|
| ||||
| TEAEs of Special Interest | ||||
|
| ||||
| Hyperglycemia AEs | 3 (2.9%) | 8 (8.2%) | 17 (15.6%) | 17 (20.7%) |
| DKA (serious AE) | 0 (0.0%) | 1 (1.0%) | 1 (0.9%) | 1 (1.2%) |
| LFT AEs | 1 (1.0%) | 2 (2.0%) | 4 (3.7%) | 2 (2.4%) |
| GI Disorders | 13 (12.5%) | 6 (6.1%) | 18 (16.5%) | 13 (15.9%) |
| Diarrhea | 0 (0.0%) | 4 (4.1%) | 7 (7.3%) | 6 (6.4%) |
Worsening of glycemic control early after treatment initiation was noted in some patients. A total of 45 hyperglycemia events were reported during the 12 weeks of treatment or during the 30-day safety follow-up period. More hyperglycemia events were observed with relamorelin treatment compared with placebo (3 on placebo, and 8 on 10 μg, 17 on 30 μg, and 17 on 100 μg relamorelin). Many hyperglycemia events represented random glucose laboratory abnormalities, with some patients withdrawing without having undergone adjustments in insulin or oral hypoglycemic drug dose, or other attempts to manage hyperglycemia.
As with random and postprandial blood glucose values, there were increases in HbA1c values noted during the first 8 weeks of treatment (the median increase compared to baseline in the relamorelin treatment groups was +0.20%, +0.60%, and +0.35% for the 10, 30 and 100 μg doses respectively); no further rise in group median HbA1c was observed over the last 4 weeks of treatment at any dose level of relamorelin compared to placebo.
Serious adverse events associated with hyperglycemia in relamorelin-treated patients were 3 events of diabetic ketoacidosis (one of which was observed at follow-up at 12 days after relamorelin 10 μg, b.i.d. had been stopped) and 2 hyperglycemia events leading to hospitalizations. All 5 serious adverse events were associated with concurrent factors including infections (2 urinary tract infections and 1 pneumonia). Lapses in antidiabetic treatment (insulin or metformin) also coincided with 4 of these 5 events.
DISCUSSION
This dose-ranging, phase 2B trial of the effects of relamorelin over a 10-fold dose range in patients with diabetic gastroparesis and vomiting has demonstrated that this ghrelin receptor agonist is efficacious in the improvement of individual and composite symptom scores. Although there was no significant treatment effect on the number of vomiting episodes per week, which was the prespecified primary endpoint, it is relevant to note that there was an average 75% reduction in vomiting episodes with relamorelin treatment, and there was an unusually high (70%) placebo response. The reason for this high placebo response rate is unclear, though it questions the use of number of vomiting episodes as a primary endpoint in studies of diabetic gastroparesis. There was a very wide range in the number of vomiting episodes in all groups, both at baseline and during the treatment phases, especially evident by the large standard deviation in the number of vomiting episodes in the 10 and 100 μg, b.i.d. dose groups. In addition, among the primary symptoms of gastroparesis evaluated in DGSSD, vomiting frequency proved to be the least valid patient reported outcome on formal psychometric evaluation.24
Relamorelin demonstrated substantial efficacy at all 3 doses tested, with reductions in critical diabetic gastroparesis symptoms of nausea, postprandial fullness, abdominal pain and bloating, measured as a key composite endpoint. These are very relevant symptoms of patients with diabetic gastroparesis, as documented in prior studies including the large database from the NIH Gastroparesis Consortium.27 Therefore, this study shows that relamorelin relieves relevant symptoms, as was also documented in evaluation of individual symptoms that overlap with another PRO, the GCSI-DD.25 Improvements in diabetic gastroparesis symptoms were apparent for all individual symptoms and became greater or, at least, remained stable by 12 weeks of treatment. In general, the magnitude of the effect of relamorelin over the effect of placebo represented decreases by approximately 33 to 50% from the baseline symptom scores (least square means, data in Table 3 and Supplementary Figure 1) for all symptoms other than vomiting. We excluded patients on renal dialysis and therefore our study results cannot be generalized to patients with diabetic gastroparesis who are receiving dialysis treatment.
Relamorelin demonstrated prokinetic pharmacodynamic properties, with acceleration of slow gastric emptying which is characteristic of gastroparesis. This effect was quantified by the decrease in GEBT T½, with results similar to findings evident after 4 weeks of relamorelin treatment in a prior diabetic gastroparesis clinical trial.21 The difference of >10% over placebo needs to be considered in relation to the relatively low calorie content of the test meal (230 kcal). The robust effects of relamorelin on some diabetic gastroparesis symptoms (particularly nausea, postprandial fullness, bloating and abdominal pain) are consistent with the prokinetic action of relamorelin on gastric emptying. There was no correlation of acceleration of gastric emptying and diabetic gastroparesis symptom improvements across this moderate to severe diabetic gastroparesis population. This finding is consistent with analyses of other studies that used diverse methods for assessing gastric emptying and symptoms, and a wide range of pharmacological treatments.28 However,there is documented association of delayed gastric emptying and symptoms,29–31 and other studies using effective prokinetic agents (e.g., cisapride) have demonstrated a positive correlation between gastric emptying and symptom improvement.32,33 Overall, individual patients may demonstrate meaningful symptomatic improvements, in part, due to shortened gastric emptying time resulting from the prokinetic effects of relamorelin. Vomiting and related symptoms, in contrast, could arguably respond more to an antiemetic approach, especially if they are incessant and refractory in nature.
The current trial also showed that relamorelin was efficacious in the relief of individual symptoms such as nausea, fullness, bloating and abdominal pain as measured by composite scores made up of these 4 symptoms and other patient global-response outcomes including the GCSI-DD, Global Assessment of Symptoms (“Overall, how would you rate your symptoms in the past 7 days?”), and PAGI-SYM. These improvements consolidate the observations with the DGSSD.
A second important result from this study is that relamorelin appears to be safe and well tolerated in patients with diabetic gastroparesis, with no tolerability problems with subcutaneous injections. The results also suggest that there is no dose response relationship between the three doses tested and that future trials of relamorelin might not need to include the 100 μg b.i.d. dose.
Important adverse effects were the increased frequency of hyperglycemic episodes and the propensity to experience diarrhea observed with relamorelin compared to placebo. Given the normal small intestinal absorption in patients with diabetes, it follows that enhanced emptying of nutrients from the stomach could contribute to hyperglycemia, if there is no adjustment in the dose or timing of treatment for hyperglycemia. Indeed, in type 1 diabetes mellitus, pharmacologically-mediated acceleration of gastric emptying increases postprandial levels of glucose assessed accurately with continuous glucose monitoring.34 Considering the difficulty of achieving glycemic control in this diabetic gastroparesis population, the number of reported episodes of hyperglycemia was not unexpected. Optimized glycemic management should be considered in future clinical trials.
It is theoretically conceivable that the ghrelin receptor agonist might have other diverse endocrine effects (e.g., stimulation of pituitary growth hormone release, contributing to glucose counterregulatory effects, impairment of insulin sensitivity, or an insulinostatic effect), which may contribute to the hyperglycemia. Relamorelin stimulated pituitary growth hormone release only modestly and attenuated rapidly over time, with measured growth hormone levels returning to normal after 2 weeks of treatment in prior studies.17,18,21 The insulinostatic effect of ghrelin has been demonstrated predominantly in pancreatic islet beta cell preparations from rodents and in fasting human volunteers or in conditions that mimic ghrelin levels observed in starvation35–37 or result from effects of ghrelin produced by pancreatic islet cells. However, the doses used in rats to reduce insulin are extremely high (e.g. 10 nmol/L) relative to the dose of synthetic human ghrelin administered systemically in patients to induce physiological increases in growth hormone levels, which is 0.33 micrograms/kg.38 This is equivalent to 7 nmol ghrelin, or 0.167 nmol/L assuming a volume of distribution over the entire body of 42 L. Likewise, the effect of ghrelin infusion in healthy fasting humans to suppress insulin release may be overridden by hyperglycemia in the setting of diabetic gastroparesis.36,37 Therefore, it is deemed highly unlikely that the administration of the pentapeptide ghrelin receptor agonist, relamorelin, had an inhibitory effect on insulin production to induce hyperglycemia.
There was a higher prevalence of diarrhea with relamorelin (4–7.5% more in the relamorelin treatment groups compared to placebo), but there were no drop-outs due to diarrhea in the trial. This finding of mild diarrhea is not surprising, given prior observations of the presence of ghrelin receptors in the enteric neural control of the colon,39 as well as the relief of constipation and acceleration of colonic transit with relamorelin in patients with chronic constipation.40
While the first goal of our study was not met, in that there was no significant treatment effect compared to placebo on vomiting frequency, this is counter-balanced by the effects of relamorelin on individual symptoms and composite symptom scores, which had the highest level of validation in a study of the psychometric performance of the patient reported outcomes in the DGSSD instrument.24 It is also worth noting that vomiting frequency had the most highly skewed measurement properties in the psychometric validation study24 and, therefore, the greatest potential for error in the estimate of treatment effects of relamorelin compared to placebo.
One limitation of this study was the finding that the primary endpoint of vomiting frequency was not significantly impacted by the relamorelin treatment relative to placebo. Nonetheless, the significant effects on the composite endpoint, as well as on individual symptoms of gastroparesis measured with a validated patient reported outcome instrument suggest the medication provides clinically relevant benefit. In addition, there was significant effect on gastric emptying T1/2 and GCSI-DD scores.
The study was strengthened by its generalizability, having enrolled a DG patient population with typical symptoms of diabetic gastroparesis with a predominance of type 2 diabetes and consistency of baseline demographics and other measurements across the 4 treatment groups.
In conclusion, the prokinetic ghrelin receptor agonist, relamorelin, demonstrated substantially improved core diabetic gastroparesis symptoms individually and using a composite total score, and it was generally safe and well tolerated. Relamorelin also demonstrated efficacy and safety that should be further assessed in pivotal hase 3 trials; the results showed no additional benefit in patient reported outcomes with the highest dose of relamorelin, 100 μg b.i.d., suggesting that it could be excluded in the phase 3 trials. The infrequent occurrence of postprandial hyperglycemia, that may result from the acceleration of gastric emptying,and enhanced nutritional intake achievable with relamorelin, can be monitored and managed with additional attention to glycemic control in future trials.
Supplementary Material
Acknowledgments
The authors acknowledge the significant contributions of the co-investigators and study coordinators at the study sites. We thank Mrs. Cindy Stanislav for excellent secretarial support.
Funding: This study was supported by Motus.
Dr. Camilleri is supported by grant PO1-DK68055 from the National Institutes of Health (diabetic gastroenteropathy studies).
Dr. McCallum is supported by grant U01-DK073975 from the National Institutes of Health (Gastroparesis Consortium studies).
Dr. Tack is supported by a Methusalem grant from Leuven University.
Abbreviations
- AUC
area under the curve
- DGSSD
Diabetic Gastroparesis Symptom Severity Diary
- GCSI-DD
Gastroparesis Cardinal Symptom Index-Daily Diary
- GEBT
gastric emptying breath test
- PAGI-SYM
Patient Assessment of Upper Gastrointestinal Symptom Severity Index
- PRO
patient reported outcome
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: Ms. Spence is a consultant with Motus. Drs. Gottesdiener and Fiedorek are employees and have equity interest in Motus, a prior subsidiary of Rhythm Pharmaceuticals. The other authors have no conflicts of interest.
Authors’ contributions:
Michael Camilleri: Senior author of manuscript; project concept and design
Richard W. McCallum: Reviewed data; participated in writing and editing of the manuscript
Jan Tack: Study investigator; reviewed data and participated in writing and editing of the manuscript
Sharon C. Spence: Joint author with Dr. Fiedorek on clinical protocol; operational study oversight; participated in writing and editing of the manuscript
Keith Gottesdiener: Sponsor employee; reviewed final data and edited the manuscript
Fred T. Fiedorek: Sponsor employee and clinical lead for study design and conduct; reviewed final data and participated with senior author in manuscript preparation and co-authorship
ClinicalTrials.gov Identifier: NCT02357420
References
- 1.Camilleri M, Parkman HP, Shafi MA, et al. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108:18–37. doi: 10.1038/ajg.2012.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abell TL, Bernstein RK, Cutts T, et al. Treatment of gastroparesis: a multidisciplinary clinical review. Neurogastroenterol Motil. 2006;18:263–283. doi: 10.1111/j.1365-2982.2006.00760.x. [DOI] [PubMed] [Google Scholar]
- 3.Lacy BE, Weiser K. Gastric motility, gastroparesis, and gastric stimulation. Surg Clin NA. 2005;85:967–987. doi: 10.1016/j.suc.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Maleki D, Locke GR, 3rd, Camilleri M, et al. Gastrointestinal tract symptoms among persons with diabetes mellitus in the community. Arch Intern Med. 2000;160:2808–2816. doi: 10.1001/archinte.160.18.2808. [DOI] [PubMed] [Google Scholar]
- 5.Bytzer P, Talley NJ, Leemon M, et al. Prevalence of gastrointestinal symptoms associated with diabetes mellitus: a population-based survey of 15,000 adults. Arch Intern Med. 2001;161:1989–1996. doi: 10.1001/archinte.161.16.1989. [DOI] [PubMed] [Google Scholar]
- 6.Jung HK, Choung RS, Locke GR, 3rd, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136:1225–1233. doi: 10.1053/j.gastro.2008.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choung RS, Locke GR, 3rd, Schleck CD, et al. Risk of gastroparesis in subjects with type 1 and 2 diabetes in the general population. Am J Gastroenterol. 2012;107:82–88. doi: 10.1038/ajg.2011.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Homko C, Siraj ES, Parkman HP. The impact of gastroparesis on diabetes control: Patient perceptions. J Diabetes Complications. 2016;30:826–829. doi: 10.1016/j.jdiacomp.2016.03.025. [DOI] [PubMed] [Google Scholar]
- 9.Bharucha AE, Batey-Schaefer B, Cleary PA, et al. Delayed gastric emptying is associated with early and long-term hyperglycemia in type 1 diabetes mellitus. Gastroenterology. 2015;149:330–339. doi: 10.1053/j.gastro.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phillips LK, Deane AM, Jones KL, et al. Gastric emptying and glycaemia in health and diabetes mellitus. Nat Rev Endocrinol. 2015;11:112–128. doi: 10.1038/nrendo.2014.202. [DOI] [PubMed] [Google Scholar]
- 11.Sturm A, Holtmann G, Goebell H, et al. Prokinetics in patients with gastroparesis: a systematic analysis. Digestion. 1999;60:422–427. doi: 10.1159/000007687. [DOI] [PubMed] [Google Scholar]
- 12.Thielemans L, Depoortere I, Perret J, et al. Desensitization of the human motilin receptor by motilides. J Pharmacol Exp Ther. 2005;313:1397–1405. doi: 10.1124/jpet.104.081497. [DOI] [PubMed] [Google Scholar]
- 13.Richards RD, Davenport K, McCallum RW. The treatment of idiopathic and diabetic gastroparesis with acute intravenous and chronic oral erythromycin. Am J Gastroenterol. 1993;88:203–207. [PubMed] [Google Scholar]
- 14.Pasricha PJ, Pehlivanov N, Sugumar A, et al. Drug insight: from disturbed motility to disordered movement–a review of the clinical benefits and medicolegal risks of metoclopramide. Nat Clin Pract Gastroenterol Hepatol. 2006;3:138–148. doi: 10.1038/ncpgasthep0442. [DOI] [PubMed] [Google Scholar]
- 15.Parkman HP, Carlson MR, Gonyer D. Metoclopramide nasal spray reduces symptoms of gastroparesis in women, but not men, with diabetes: results of a phase 2B randomized study. Clin Gastroenterol Hepatol. 2015;13:1256–1263. doi: 10.1016/j.cgh.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 16.Sarganas G, Garbe E, Klimpel A, et al. Epidemiology of symptomatic drug-induced long QT syndrome and Torsade de Pointes in Germany. Europace. 2014;16:101–108. doi: 10.1093/europace/eut214. [DOI] [PubMed] [Google Scholar]
- 17.Van der Ploeg L, Laken H, Sharma S, et al. Preclinical gastrointestinal prokinetic efficacy and endocrine effects of the ghrelin mimetic RM-131. Life Sci. 2014;109:20–29. doi: 10.1016/j.lfs.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Shin A, Camilleri M, Busciglio I, et al. Randomized controlled phase Ib study of ghrelin agonist, RM-131, in type 2 diabetic women with delayed gastric emptying: pharmacokinetics and pharmacodynamics. Diab Care. 2013;36:41–48. doi: 10.2337/dc12-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shin A, Camilleri M, Busciglio I, et al. The ghrelin agonist RM-131 accelerates gastric emptying of solids and reduces symptoms in patients with type 1 diabetes mellitus. Clin Gastroenterol Hepatol. 2013;11:1453–1459. doi: 10.1016/j.cgh.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson AD, Camilleri M, Acosta A, et al. Effects of ghrelin receptor agonist, relamorelin, on gastric motor functions and satiation in healthy volunteers. Neurogastroenterol Motil. 2016;28:1705–1713. doi: 10.1111/nmo.12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lembo A, Camilleri M, McCallum R, et al. Relamorelin reduces vomiting frequency and severity and accelerates gastric emptying in adults with diabetic gastroparesis. Gastroenterology. 2016;151:87–96. doi: 10.1053/j.gastro.2016.03.038. [DOI] [PubMed] [Google Scholar]
- 22.Szarka LA, Camilleri M, Vella A, et al. A stable isotope breath test with a standard meal for abnormal gastric emptying of solids in the clinic and in research. Clin Gastroenterol Hepatol. 2008;6:635–643. doi: 10.1016/j.cgh.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Revicki DA, Camilleri M, Kuo B, et al. Development and content validity of a gastroparesis cardinal symptom index daily diary. Aliment Pharmacol Ther. 2009;30:670–680. doi: 10.1111/j.1365-2036.2009.04078.x. [DOI] [PubMed] [Google Scholar]
- 24.Fehnel S, Nelson L, DiBennedetti D, et al. Development and psychometric evaluation of the Diabetic Gastroparesis Symptom Severity Diary. Gastroenterology. 2017;152:S517. doi: 10.2147/CEG.S184016. (abstract). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Revicki DA, Camilleri M, Kuo B, et al. Evaluating symptom outcomes in gastroparesis clinical trials: validity and responsiveness of the Gastroparesis Cardinal Symptom Index-Daily Diary (GCSI-DD) Neurogastroenterol Motil. 2012;24:456–463. doi: 10.1111/j.1365-2982.2012.01879.x. [DOI] [PubMed] [Google Scholar]
- 26.Rentz AM, Kahrilas P, Stanghellini V, et al. Development and psychometric evaluation of the patient assessment of upper gastrointestinal symptom severity index (PAGI-SYM) in patients with upper gastrointestinal disorders. Qual Life Res. 2004;13:1737–1749. doi: 10.1007/s11136-004-9567-x. [DOI] [PubMed] [Google Scholar]
- 27.Parkman HP, Yates K, Hasler WL, et al. National Institute of Diabetes and Digestive and Kidney Diseases Gastroparesis Clinical Research Consortium. Similarities and differences between diabetic and idiopathic gastroparesis. Clin Gastroenterol Hepatol. 2011;9:1056–1064. doi: 10.1016/j.cgh.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janssen P, Harris MS, Jones M, et al. The relation between symptom improvement and gastric emptying in the treatment of diabetic and idiopathic gastroparesis. Am J Gastroenterol. 2013;108:1382–1391. doi: 10.1038/ajg.2013.118. [DOI] [PubMed] [Google Scholar]
- 29.Khayyam U, Sachdeva P, Gomez J, et al. Assessment of symptoms during gastric emptying scintigraphy to correlate symptoms to delayed gastric emptying. Neurogastroenterol Motil. 2010;22:539–545. doi: 10.1111/j.1365-2982.2009.01454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parkman HP, Hallinan EK, Hasler WL, et al. Early satiety and postprandial fullness in gastroparesis correlate with gastroparesis severity, gastric emptying, and water load testing. Neurogastroenterol Motil. 2017 Apr;29(4) doi: 10.1111/nmo.12981. Epub 2016 Oct 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Halawi H, Zinsmeister AR, Acosta A, et al. Relationship between gastric emptying or gastric accommodation and postprandial symptoms observed after ingestion of a maximum volume of a nutrient drink in 285 participants. Gastroenterology. 2017 abstract, in press. [Google Scholar]
- 32.Jian R, Ducrot F, Piedeloup C, et al. Measurement of gastric emptying in dyspeptic patients: effect of a new gastrokinetic agent (cisapride) Gut. 1985;26:352–358. doi: 10.1136/gut.26.4.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Corinaldesi R, Stanghellini V, Raiti C, et al. Effect of chronic administration of cisapride on gastric emptying of a solid meal and on dyspeptic symptoms in patients with idiopathic gastroparesis. Gut. 1987;28:300–305. doi: 10.1136/gut.28.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parthasarathy G, Kudva YC, Low PA, et al. Relationship between gastric emptying and diurnal glycemic control in type 1 diabetes mellitus: a randomized trial. J Clin Endocrinol Metab. 2017;102:398–406. doi: 10.1210/jc.2016-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dezaki K, Hosoda H, Kakei M, et al. Endogenous ghrelin in pancreatic islets restricts insulin release by attenuating Ca2+ signaling in β-cells: implication in the glycemic control in rodents. Diabetes. 2004;53:3142–3151. doi: 10.2337/diabetes.53.12.3142. [DOI] [PubMed] [Google Scholar]
- 36.Tong J, Davis HW, Gastaldelli A, et al. Ghrelin impairs prandial glucose tolerance and insulin secretion in healthy humans despite increasing GLP-1. J Clin Endocrinol Metab. 2016;101:2405–2414. doi: 10.1210/jc.2015-4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tong J, Prigeon RL, Davis HW, et al. Physiologic concentrations of exogenously infused ghrelin reduces insulin secretion without affecting insulin sensitivity in healthy humans. J Clin Endocrinol Metab. 2013;98:2536–2543. doi: 10.1210/jc.2012-4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cremonini F, Camilleri M, Vazquez Roque M, et al. Obesity does not increase effects of synthetic ghrelin on human gastric motor functions. Gastroenterology. 2006;131:1431–1439. doi: 10.1053/j.gastro.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 39.Kitazawa T, Nakamura T, Saeki A, et al. Molecular identification of ghrelin receptor (GHS-R1a) and its functional role in the gastrointestinal tract of the guinea-pig. Peptides. 2011;32:1876–1886. doi: 10.1016/j.peptides.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 40.Acosta A, Camilleri M, Kolar G, et al. Relamorelin relieves constipation and accelerates colonic transit in a phase 2, placebo-controlled, randomized trial. Clin Gastroenterol Hepatol. 2015;13:2312–2319. doi: 10.1016/j.cgh.2015.04.184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


