Summary
Objectives
Specific changes in the functional connectivity of brain networks occur in patients with epilepsy. Yet whether such changes reflect a stable disease effect or one that is a function of active seizure burden remains unclear. Here we longitudinally assessed the connectivity of canonical cognitive functional networks in patients with intractable temporal lobe epilepsy (TLE), both before and after undergoing epilepsy surgery and achieving seizure freedom.
Methods
Seventeen patients with intractable TLE who underwent epilepsy surgery with Engel Class I outcome and seventeen matched healthy controls took part in the study. The functional connectivity of a set of cognitive functional networks derived from typical cognitive tasks was assessed in patients, preoperatively and postoperatively, as well as in controls, using stringent methods of artifact reduction.
Results
Preoperatively, functional networks in TLE patients differed significantly from healthy controls, with differences that largely, but not exclusively, involved the default mode and temporal/auditory subnetworks. However, undergoing epilepsy surgery and achieving seizure freedom did not lead to significant changes in network connectivity, with postoperative functional network abnormalities closely mirroring the preoperative state.
Significance
This result argues for a stable chronic effect of the disease on brain connectivity, with changes that are largely “burned in” by the time a patient with intractable TLE undergoes epilepsy surgery, which typically occurs years after the initial diagnosis. The result has potential implications for the treatment of intractable epilepsy, suggesting that delaying surgical intervention that may achieve seizure freedom may lead to functional network changes that are no longer reversible by the time of epilepsy surgery.
Keywords: TLE, epilepsy surgery, fcMRI, functional network, longitudinal
Introduction
Epilepsy has been associated with specific changes in the resting-state connectivity of human brain functional networks1, primarily but not exclusively involving the default mode network2. It is unclear whether these changes reflect a chronic effect of the disease on brain connectivity or whether they are an index of acute seizure burden3; 4. Epilepsy surgery is a potential therapeutic option for patients with medically intractable epilepsy that in many patients can drastically reduce seizure burden5; 6. In addition, while several studies have examined preoperative functional connectivity as a predictor of surgical outcome7–9, or for presurgical localization of the seizure focus3; 10; 11, the effect of surgery on the resting-state connectivity of brain networks in epilepsy has been infrequently studied12–15 (with a notable case study showing significant normalization of resting-state brain networks after corpus callosotomy in a pediatric patient with severe epileptic encephalopathy14). To assess whether a significant reduction in seizure burden affects the atypical functional connectivity of brains of patients with epilepsy, we studied the preoperative and postoperative brain connectivity of patients with TLE who underwent epilepsy surgery with Engel Class I outcome (i.e. seizure-free after surgery)16, specifically focusing on cognitive functional networks validated in the healthy adult human population17 and employing recently validated stringent methods of artifact removal for fMRI time-series18.
Methods
Participants
Patients were recruited from the Washington University Adult Epilepsy Center at the Washington University School of Medicine and screened for inclusion based on a history of unilateral intractable temporal lobe epilepsy confirmed via video-EEG and epilepsy surgery with Engel Class I outcome16. Typical ictal clinical signs included arrested activity, automatisms, and decreased awareness or responsiveness19. Only participants who underwent temporal lobe epilepsy surgery (either selective amygdalo-hippocampectomy or temporal lobectomy) were included in the study. Of 21 participants initially enrolled in the study, 17 had Engel Class I outcome and were included in the final analysis (Supporting Table 1). A group of healthy participants (n = 17) individually matched to patients in terms of age (+/− 2 years), sex and handedness were studied under identical imaging conditions. Healthy participants and preoperative TLE patients completed their first MRI scan between 2009 and 2013. In patients, a second brain MRI was acquired postoperatively under identical imaging conditions between 2012 and 2014. Criteria for exclusion included age <18 years, clinical or electrographic evidence of bitemporal or extratemporal seizures, developmental anomalies, cortical malformations or other focal lesions on structural MRI, contraindication to MRI, including suspected pregnancy, history of substance or alcohol abuse, and non-proficiency in the English language. All participants provided informed written consent as required by the Washington University Institutional Review Board.
Surgery and Pathologic Findings
Patients with medically intractable TLE were deemed to be candidates for surgery based on video-EEG and neuroradiologic findings. Surgery was performed in standard fashion: patients underwent either selective amygdalo-hippocampectomy (5 left, 4 right), with the superior colliculus marking the approximate plane of the posterior resection, or a more extensive anterior temporal lobectomy (5 left, 3 right). The extent of the medial resection was similar in both groups, though the right temporal group had a more extensive lateral resection. Pathologic findings reflected a typical cross-section of patients with temporal lobe seizures, with 14 patients showing evidence of mesial temporal sclerosis, three showing malformations of cortical development, and one showing a cavernous angioma (Supporting Table 1).
Image Preprocessing and Artifact Removal
MRI scans were performed at the Center for Clinical Imaging and Research (CCIR) at Washington University in a 3T Siemens Trio MRI Scanner (Erlangen, Germany). A high-resolution (0.42 × 0.42 × 0.9 mm) T1-weighted MPRAGE (TI 800 ms, TE 3.29 ms) structural scan was obtained in each subject for the purpose of anatomic segmentation, registration of functional images, and atlas transformation. Two BOLD resting state functional scans (echoplanar, TR 2200 ms, TE 27 ms, flip angle 90 deg, 4×4×4 mm voxels) were also acquired for each subject. Each functional run consisted of 164 whole-brain volumes (approximately 6 minutes in duration). Study participants were asked to relax while visually fixating on a cross hair centered on the screen.
BOLD data were pre-processed using stringent artifact removal criteria according to recently published methods18. The steps included, in order: 1) slice time correction (to realign slices in time to the beginning of each volume’s acquisition), 2) rigid-body realignment to correct for movement within and across functional runs, 3) within-run intensity normalization, 4) atlas transformation to Talairach 3 mm × 3 mm standard space20, 5) censoring/scrubbing of timepoints with a framewise displacement greater than 0.2 mm, 6) de-meaning and de-trending across each functional run, 7) regression of nuisance covariates and their first-time derivatives, including movement (translation in three dimensions and rotation in three dimensions), global signal, white matter signal, and ventricular signal across runs, 8) interpolation of censored/excluded timepoints with generated spectral data matching the retained timepoints, 9) bandpass filtering (0.009 – 0.08 Hz), and 10) spatial smoothing (6-mm full width-half maximum). Timepoints that survived artifact removal were then used to compute connectivity measures for each participant.
Cognitive Functional Networks
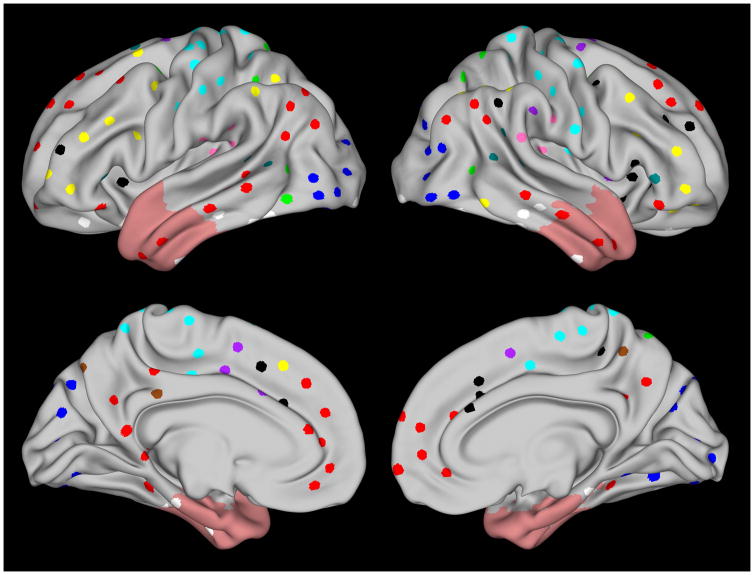
The goal of the study was to assess whether epilepsy surgery and seizure-freedom affect the connectivity of networks of regions that form connections based on involvement in specific cognitive functions, rather than purely on the basis of anatomical location. To that end we used 264 regions of interest (ROIs) obtained from the Petersen and Schlaggar laboratories at Washington University and based on groups of brain regions that co-activate during performance of common types of cognitive tasks17. The ROIs were distributed across 14 functional networks, spanning broad cognitive domains and functions, including attention, memory, cognitive control, salience, default mode, vision, etc. (Figure 1; see also Supporting Table 2). Functional connectivity between ROIs was computed as the Fisher z-transformed Pearson’s r along the temporal dimension. Functional connectivity within and between functional subnetworks was computed as the mean functional connectivity between ROIs within and between the respective subnetworks. Node-node connectivity changes were corrected for multiple comparison via false discovery rate.
Figure 1.
Regions of interest studied are shown projected onto the Human Connectome Project 440-subject (R440) group-average mid-thickness surface for each hemisphere39; 40. Functional subnetworks are color-coded and include sensory/somatomotor (cyan), visual (blue), auditory (pink), default mode (red), cingulo-opercular task control (purple), fronto-parietal task control (yellow), salience (black), memory retrieval (brown), ventral attention (teal), dorsal attention (green) and uncertain classification (white). Light red shading shows the extent of the mask used to exclude nodes within or immediately near surgically resected areas across patients.
Statistical Analysis
For ROI pair analyses statistical models were designed to test the effect of disease on the dependent variable of functional connectivity strength between ROIs in a given pair (i.e. the Fisher’s z-transformed correlation between the two ROI time-series). Contributions from individual ROIs were averaged within each functional network. The first ANOVA focused on the effect of TLE on the functional connectivity of networks in patients compared to healthy controls: the model thus included fixed independent factors of group (healthy control vs. TLE preoperative) and functional network (coded as two separate factors to account for their interaction) and a random independent factor of participant. The second ANOVA focused on the effect of surgery and seizure freedom, replacing the group factor with a factor of preoperative/postoperative status, but was otherwise identical to the first ANOVA. Post-hoc t-tests (unpaired for healthy vs. preoperative TLE, paired for preoperative vs. postoperative TLE), corrected for multiple comparisons via false discovery rate, were conducted for significant effects revealed by the ANOVAs.
Results
Controls were individually matched to patients with regards to age, sex (8 males in each group) and handedness (2 left-handers in each group). There was no significant difference in age between controls (mean age = 41.3 years, range: 21–58 years) and patients (mean age between preoperative and postoperative scans = 43.2 years, range: 22–63 years; Welch t-test: t = 0.44, d.f. 29.9, p = 0.66). In patients the mean number of months between the preoperative scan and surgery was 8.5 (S.D. 7.1, range 0–27), while the mean number of months between surgery and the postoperative scan was 23.5 (S.D. 12.5, range 5–45).
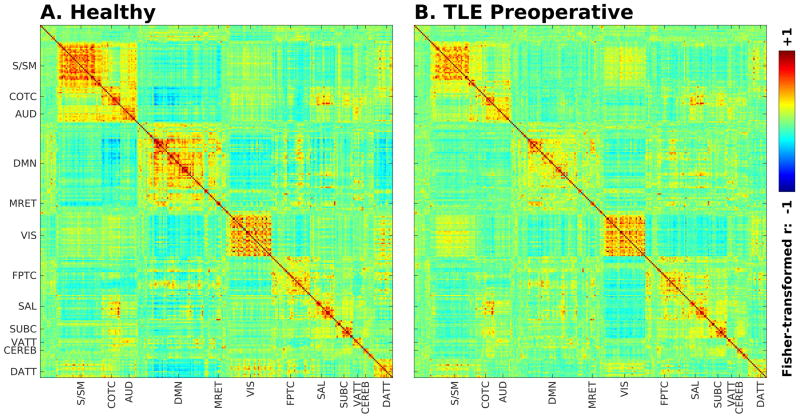
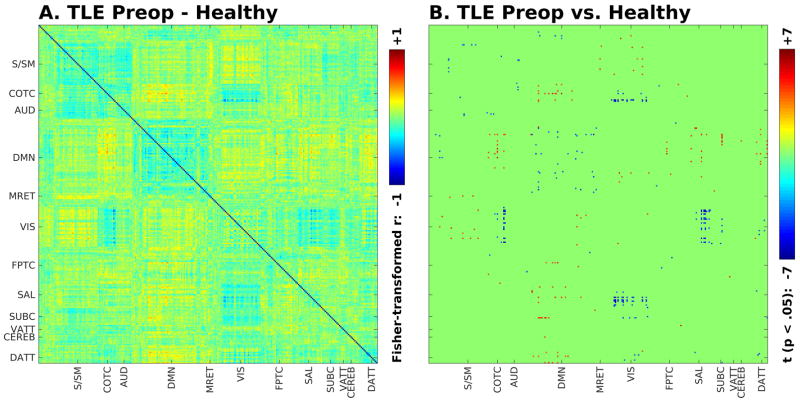
Replicating prior studies1, functional connectivity within and between networks differed in specific ways between preoperative TLE patients and controls, as shown in Figures 2 and 3. The strongest differences involved decreased within-network connectivity in the default mode network, and decreased coupling (whether in the form of positive or negative connectivity) between specific networks, including between default mode and cingulo-opercular task control and salience networks, and between cingulo-opercular task control and salience networks and visual networks (Figure 3). An ANOVA testing the effect of disease on the average connectivity between functional networks in controls and TLE patients showed a significant effect of disease (F = 6.9, d.f. 1, p < .05), as well as highly significant effects of network (F = 52, d.f. 11, p < 1 × 10−15), interaction between networks (F = 70, d.f. 55, p < 1 × 10−11), and interaction between network and disease (F = 2.5, d.f. 11, p < .005). The greatest amount of variance in the model (43%) was explained by the interaction between networks, suggesting that most of the variance was accounted for by the differences in functional connectivity between networks.
Figure 2.
Seed-seed group functional connectivity matrices for healthy controls (A) and preoperative TLE patients (B). Connectivity strength is displayed as Fisher-transformed Pearson’s r (with color scale arbitrarily bounded between −1 and +1). Axis labels denote approximate distribution of functional cognitive subnetworks (S/SM – sensory/somato-motor, COTC – cingulo-opercular task control, AUD – auditory, DMN – default mode, MRET – memory retrieval, VIS – visual, FPTC – fronto-parietal task control, SAL – salience, SUBC – subcortical, VATT – ventral attention, CEREB – cerebellar, DATT – dorsal attention).
Figure 3.
Seed-seed mean group connectivity difference (A) and t-value for seed-seed connections significantly different between preoperative TLE patients compared to controls (unpaired t-test, p < .05, corrected for multiple comparisons) (B).
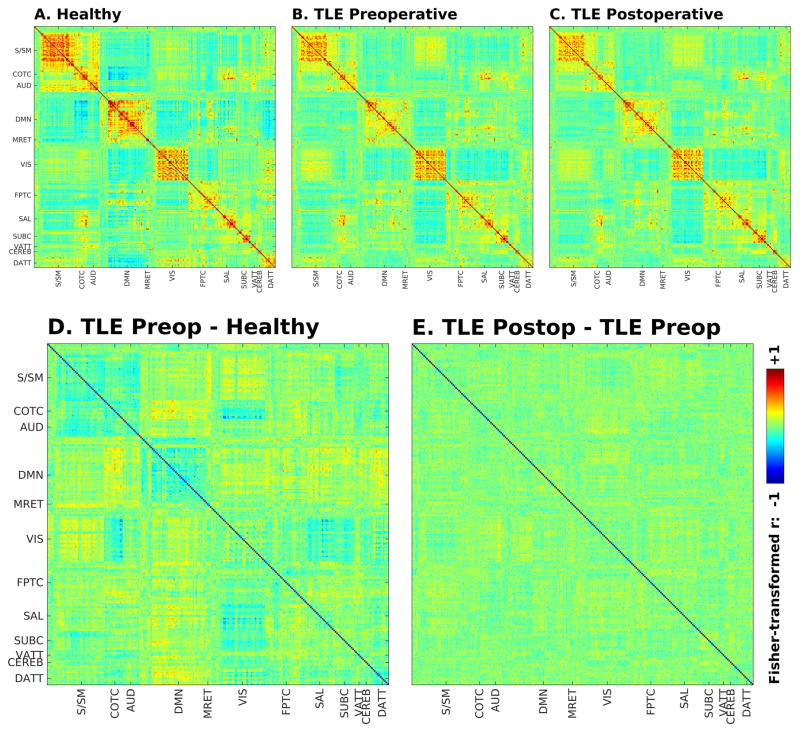
Importantly, functional connectivity was, however, largely unaffected by surgery and the resultant seizure freedom, as shown in Figure 4. To eliminate the direct effect of surgery targeting specific nodes in the subnetworks studied, the analysis comparing patients preoperatively to postoperatively was conducted involving only nodes that were not within or immediately adjacent to the resection region across all patients (or within its contralateral equivalent - see red-shaded region in Figure 1). This approach effectively removed 29 nodes within the bilateral temporal lobes, with the same nodes removed for each participant’s data in this analysis. Statistically, an ANOVA assessing effects on functional connectivity in TLE patients before and after surgery did not show a significant effect of operative status (preoperative vs. postoperative). There were, however, significant effects of network (F = 47, d.f. 11, p < 1 × 10−15) and interaction between networks (F = 62, d.f. 55, p < 1 × 10−15). Note the similarity between preoperative and postoperative TLE patient correlograms (Figure 4). No functional connectivity differences survived multiple-comparison correction when comparing patients preoperatively to postoperatively. Finally, adding terms of age, disease duration, seizure frequency, time interval between preoperative and postoperative scans, time interval between preoperative scan and surgery, or time interval between surgery and postoperative scan to the statistical models did not yield additional significant effects.
Figure 4.
Seed-seed group functional connectivity matrices for healthy controls (A), preoperative TLE patients (B) and postoperative TLE patients (C) in non-surgical nodes. Difference between connectivity matrices for TLE preoperative patients and controls (D) and between TLE postoperative patients and preoperative patients (E) in non-surgical nodes.
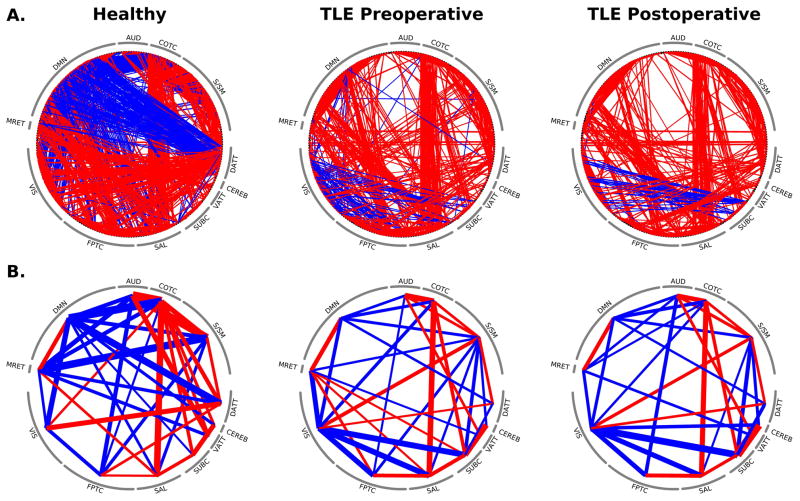
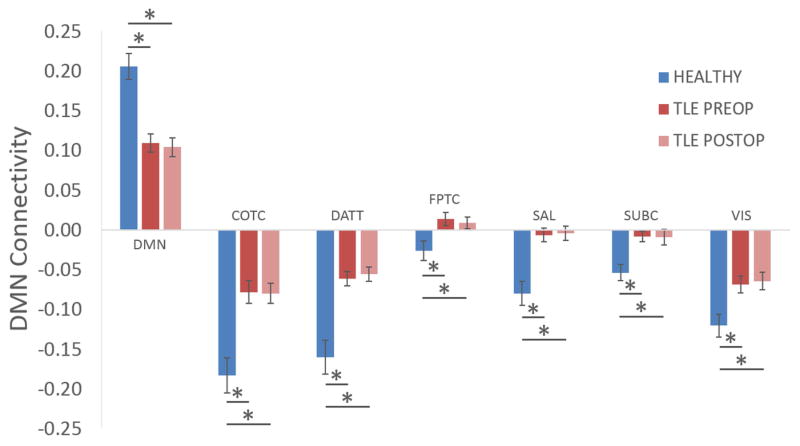
To emphasize the differences in functional connectivity across subnetworks, circular plots of the connectivity for controls, preoperative TLE and postoperative TLE patients are shown in Figure 5. One of the most striking findings is the decreased connectivity between the default mode network and the dorsal attention network as a result of the disease. These networks exhibit a strong anti-correlation in controls but appear to be minimally connected in patients, both pre- and postoperatively. Some cross-network connections, on the other hand, were unaffected both across controls and patients and within patients in equal fashion: the connectivity between cingulo-opercular task control (COTC) and salience networks, for instance, was strongly positive and minimally changed across the groups and across operative periods, suggesting that not all connectivity patterns were different between healthy controls and TLE patients. Focusing specifically on the default mode network, which has often been cited as being adversely affected by TLE2, the preoperative connectivity of specific brain networks with the default mode network in patients with TLE differed significantly from healthy controls; however achieving seizure freedom after temporal lobe surgery did not significantly change or normalize these altered connections (Figure 6).
Figure 5.
Circular network plots for node-node (A) and subnetwork-subnetwork (B) connections for non-surgical nodes in healthy controls and preoperative and postoperative TLE patients. Line thickness indicates suprathreshold positive (red) and negative (blue) connectivity strength. Nodes are ordered in terms of cognitive subnetworks matching prior figures.
Figure 6.
Connectivity within the default mode network (DMN) and between the DMN and other networks was significantly different between healthy controls and preoperative TLE patients, but undergoing temporal lobe surgery and achieving seizure freedom had no significant effect on this difference. Asterisk denotes significant difference (p < .05) on Welch t-test after correction for multiple comparisons.
Discussion
Temporal lobe epilepsy exerts a significant effect on specific cognitive networks, including but not limited to the default mode network and other subnetworks in the temporal lobe (e.g. language, memory and auditory networks). Our findings replicate those of prior studies in our preoperative patient cohort1. Surprisingly, however, epilepsy surgery, which targets significant portions of the medial (and at times lateral) temporal lobe, and the resulting seizure freedom, failed to significantly affect the abnormal connectivity of TLE patients postoperatively. If the connectivity of these networks is changed by the disease, why does it not revert when the disease is controlled? One possibility is that functional network abnormalities (assessed in our sample in a range of months to years after the surgery) at least partially reflect the inherent stability of functional networks in individuals across the lifespan. In other words, functional network connections in healthy individuals as detected by functional MRI may reflect stable relationships between cognitive networks formed over many years. Abnormalities in these connections may in turn reflect a time-averaged or “burnt in” effect on connectivity resulting from years of disease. Another possibility is that the intrinsic etiology of epileptic seizures may induce functional network changes during the process of epileptogenesis, with network changes primarily reflecting the initial insult that caused the seizures, rather than the effect of chronic, ongoing epilepsy. The possible causes of network changes in our cohort in fact parallel those of mesial temporal sclerosis itself, which may be caused by the effects of chronic ongoing seizures, or by damage from an acute insult, such as encephalitis or febrile seizures.21 Such an important distinction could only be reliably settled through prospective trials early in the course of temporal lobe epilepsy.22 Regardless of the etiology of the abnormal connections the achievement of seizure freedom, while life-altering to patients who reach it, appears to be insufficient to normalize the abnormal connections, at least within the follow up time of our study. It is possible that future studies examining changes over longer time periods after seizure freedom will detect a significant shift towards normalization of network connections.14 Furthermore, while the time to postoperative scan in our study had a somewhat wide range, this factor did not significantly affect the statistical model when explicitly tested. Larger data sets in future studies may capture such an effect, which may be complex and non-linear, e.g. with short-term changes that are reversed in the longer term.
A second finding of this study was the observation that the typical effect of epilepsy on the connections between cognitive subnetworks was deleterious or disruptive. Whether the original connection was positive (coupled) or negative (anti-coupled) TLE appeared to reduce the coupling in many, though not all, instances. In a few instances connections were more strongly coupled (e.g. the visual network with the sensory/somatomotor network) or anti-coupled (e.g. the visual network with the salience, cingulo-opercular task control or subcortical networks) in TLE patients compared to controls. This results replicates and expands prior findings of generally diminished connectivity in patients with epilepsy,23; 24 with the exception of relative increases in connectivity in areas with physiologic links to the pathophysiologic process3. Typically, prior studies have focused on regions defined by anatomic location, while our approach used regions of interest derived from well-defined functional networks involved in the performance of a range of specific cognitive tasks. As a rule, TLE thus disrupts coupling between cognitive functional networks, intuitively explained by neuronal loss or dysfunction from the underlying pathophysiology of chronic epilepsy. However, neuronal loss or dysfunction seems a less probable cause of strengthened network coupling. Instead, increased coupling/anti-coupling compared to controls may reflect abnormal functional connections that underlie the primary pathophysiologic process in focal epilepsy, such as an abnormally facilitated pathway of propagation for focal epileptic seizures. Multiple extratemporal brain regions have in fact been implicated metabolically in typical patterns of propagation in temporal lobe seizures, including, for instance, the somatosensory region around the central sulcus, which shows hyperperfusion during the ictal period.25–27 At the structural level some of the same regions show cortical thinning in patients with TLE with a very similar extratemporal distribution28. Extending beyond temporal lobe epilepsy, Xiao and colleagues demonstrated abnormally increased functional connectivity between primary motor cortex and IFG, parietal lobe, and supramarginal gyrus in patients with rolandic epilepsy, arguing that increased connectivity changes may capture the disease process in focal epilepsy in general.29
A key caveat for this study is that tests of preoperative and postoperative cognitive function were not formally compared to the disease-related functional network reorganization and its lack of a change after epilepsy surgery. First, while neuropsychological batteries for epilepsy surgery planning share key features across institutions, there is a relative lack of standardizations in terms of which tests are performed, even within institution, in part motivated by a need to tailoring testing to the individual patient being assessed30. Furthermore, neuropsychological testing done for the purpose of epilepsy presurgical planning often utilizes tests of cognitive function that are not well matched to the type of tests used to delineate cognitive functional networks in the neuroimaging literature31, i.e. routine clinical neuropsychological tests would not necessarily track the functional networks explored in this study. Therefore, as a rule, future studies pairing cognitive tests well matched to the functional networks explored may be able to capture more nuanced changes resulting from epilepsy surgery, and specifically tie a neuroimaging correlate to a cognitive change.
More specifically, it is also possible that the range of cognitive function spanned by the functional networks studied here does not perfectly overlap and capture the cognitive changes that typically occur after epilepsy surgery. In TLE in particular, epilepsy surgery has a tendency to adversely affect memory, and to a lesser degree language, depending on the anatomic structures targeted. There exists, however, considerable variability even within the postoperative outcomes of neuropsychological measures alone, with the well-known finding of some patients experiencing paradoxical improvement in cognitive function32. In this study, the goal was to assess the resilience in the connectivity of nodes not directly resected by surgery. Therefore we excluded nodes in the temporal lobe directly resected in surgery, with an exclusion region that was purposefully bilateral to avoid confounding effects (Figure 1), resulting in several temporal nodes being excluded from the analysis. Memory in particular has notoriously been challenging to assess with fMRI, including in the case of epilepsy33; 34, due in part to technical limitations of low signal-to-noise in temporal regions near areas of air/parenchyma or bone/parenchyma interfaces. Therefore, not only is there heterogeneity within postoperative neuropsychological outcome, but fMRI may capture changes in brain regions that are 1) not typically resected by surgery or 2) not formally tested via routine neuropsychological batteries.
A related limitation lies in the strategy of combining patients with left and right TLE, which may reduce power to detect changes unique to those specific subsets of patients. This would be particularly important for the connectivity of brain regions with nodes found within the resected area and the contralateral region, since both were explicitly eliminated in the pre/post comparison to avoid left/right mismatch. However, since the main goal of this part of the study was to assess cognitive networks and nodes not directly targeted by surgery, the set of nodes we retained for the preoperative/postoperative comparison was largely outside of the temporal area typically resected in TLE surgery (i.e. only 29 nodes were removed from the original set of 264). Furthermore, the strategy that other groups, including our own, have used in the past of collapsing across left and right TLE into ipsilateral and contralateral hemisphere, would not be possible in this type of analysis as several of the nodes tested do not have a clear homologous counterpart in the contralateral hemisphere. In general, future studies with sufficient numbers of patients with left and right TLE would be able to address this issue directly by including TLE lateralization explicitly as a separate factor.
Despite these limitations, however, our study showed large and significant differences in primarily extra-temporal functional networks of TLE patients compared to healthy controls, and these differences were essentially unaffected by surgery. It is thus possible, if not probable, that these disease-related network abnormalities capture a form of cognitive dysfunction that is a manifestation of the chronic effect of the disease. Functional connectivity, while more limited in the assessment of certain brain regions due to technical limitations, may be able to assess neural correlates of cognitive function that are only partially assessed by routine neuropsychological tests. Future studies directly querying cognitive tasks matched to the functional networks studied here may be better poised to detect a relationship between specific cognitive measures and specific network changes. However, based on our findings here, we speculate that the main effect or central tendency is likely to be essentially unchanged between the preoperative and the postoperative state.
Another consideration is that both individual variability and disease-related variability in the spatial distribution of specific functional networks may lead to significant differences between epilepsy patients and healthy controls, which would be of crucial importance when directly comparing brain regions based on a standardized location. In other words, a plausible explanation for the significant differences in connectivity noted between the healthy and diseased state may be due to a disease-related reorganization of brain networks, or even merely because of individual variation, especially but not exclusively in terms spatial extent35. This is particularly relevant with regards to our conclusion that the changes in connectivity are “burnt in” after years of disease, given that reorganization of functional networks is likely to occur over longer time periods. In our study, since the nodes queried were selected a priori based on well-established cognitive functional networks, the disease-specific effects mentioned above would only be captured to the extent that the nodes reflect the typical connectivity patterns of functional networks in the healthy state. Canonical locations for typical cognitive network distribution may be altered by disease, leading to differences with the healthy state that are not mere changes in connectivity strength but rather reflect a re-distribution of regions involved in specific cognitive tasks. This has been observed across neurologic disease, such as with the abnormal connectivity and distribution of dorsal attention and somatosensory networks in stroke patients36 or the reorganized sensorimotor network in exhibited by amputees.37 Studies based on ROIs in predefined locations are thus invariably subject to the criticism that the predefined location may not reflect an individual’s or disease-related reorganization of cortical function. To the extent that an analysis which utilizes a priori fixed ROI locations fails to capture such a reorganization, it would be comparably unable to capture a normalization (or move towards normalization) of connectivity after surgery. A potential solution would thus lie in the ability of detecting and defining distinct functional networks at the level of the individual, which is a non-trivial but potentially feasible endeavor, especially with very recent techniques using rich multimodal data sets.38 Future studies that can reliably delineate functional network extent at the individual level may therefore be able to capture evidence of post-operative normalization of connectivity that a study using a priori ROIs would not detect.
To conclude, the key finding of our study was the stability of the effect of temporal lobe epilepsy on cognitive network connectivity before and after undergoing epilepsy surgery and achieving seizure freedom. This result not only suggests that the connectivity changes observed here and in the recent literature in epilepsy reflect the lifetime of the disease’s disruptive effects on functional networks, but also serves as a validation of the stability of the technique in terms of capturing physiologically meaningful changes over time. Furthermore, the life-altering event of achieving seizure freedom does not result in normalization of abnormal functional connections, at least in the approximately two-year period following surgery. Future longitudinal studies targeting the effects of epilepsy earlier in the history of the disease may shed further light on the pathophysiologic process as it first exerts its disruptive effect on the brain’s connectivity.
Supplementary Material
Key Point Box.
Cognitive functional networks in patients with TLE exhibit abnormal connectivity prior to epilepsy surgery compared to healthy controls.
Achieving seizure freedom after epilepsy surgery does not significantly change the abnormal connectivity of cognitive functional networks.
The abnormal connections between and within cognitive networks in TLE likely reflect a chronic, “burned in” effect of the disease.
Acknowledgments
This work was supported by the National Institute of Neurological Disorders and Stroke (1K23NS085028 to L.M.), the National Center for Advancing Translational Sciences (UL1TR000448, sub award KL2TR000450 to L.M.), and the Institute of Clinical and Translational Sciences at Washington University (UL1RR024992 to R.H.). Data for visualization of brain surfaces were provided in part by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University.
Footnotes
Disclosure of Conflicts of Interest
None of the authors have any conflict of interest to disclose.
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Cataldi M, Avoli M, de Villers-Sidani E. Resting state networks in temporal lobe epilepsy. Epilepsia. 2013;54:2048–2059. doi: 10.1111/epi.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liao W, Zhang Z, Pan Z, et al. Default mode network abnormalities in mesial temporal lobe epilepsy: a study combining fMRI and DTI. Hum Brain Mapp. 2011;32:883–895. doi: 10.1002/hbm.21076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maccotta L, He BJ, Snyder AZ, et al. Impaired and facilitated functional networks in temporal lobe epilepsy. Neuroimage Clin. 2013;2:862–872. doi: 10.1016/j.nicl.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Douw L, DeSalvo MN, Tanaka N, et al. Dissociated multimodal hubs and seizures in temporal lobe epilepsy. Ann Clin Transl Neurol. 2015;2:338–352. doi: 10.1002/acn3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tellez-Zenteno JF, Dhar R, Wiebe S. Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain. 2005;128:1188–1198. doi: 10.1093/brain/awh449. [DOI] [PubMed] [Google Scholar]
- 6.McIntosh AM, Averill CA, Kalnins RM, et al. Long-term seizure outcome and risk factors for recurrence after extratemporal epilepsy surgery. Epilepsia. 2012;53:970–978. doi: 10.1111/j.1528-1167.2012.03430.x. [DOI] [PubMed] [Google Scholar]
- 7.Osipowicz K, Sperling MR, Sharan AD, et al. Functional MRI, resting state fMRI, and DTI for predicting verbal fluency outcome following resective surgery for temporal lobe epilepsy. J Neurosurg. 2016;124:929–937. doi: 10.3171/2014.9.JNS131422. [DOI] [PubMed] [Google Scholar]
- 8.Doucet GE, Rider R, Taylor N, et al. Presurgery resting-state local graph-theory measures predict neurocognitive outcomes after brain surgery in temporal lobe epilepsy. Epilepsia. 2015;56:517–526. doi: 10.1111/epi.12936. [DOI] [PubMed] [Google Scholar]
- 9.Negishi M, Martuzzi R, Novotny EJ, et al. Functional MRI connectivity as a predictor of the surgical outcome of epilepsy. Epilepsia. 2011;52:1733–1740. doi: 10.1111/j.1528-1167.2011.03191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weaver KE, Chaovalitwongse WA, Novotny EJ, et al. Local functional connectivity as a pre-surgical tool for seizure focus identification in non-lesion, focal epilepsy. Front Neurol. 2013;4:43. doi: 10.3389/fneur.2013.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgan VL, Sonmezturk HH, Gore JC, et al. Lateralization of temporal lobe epilepsy using resting functional magnetic resonance imaging connectivity of hippocampal networks. Epilepsia. 2012;53:1628–1635. doi: 10.1111/j.1528-1167.2012.03590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ibrahim GM, Morgan BR, Smith ML, et al. Thalamocortical connectivity is enhanced following functional hemispherotomy for intractable lateralized epilepsy. Epilepsy Behav. 2015;51:281–285. doi: 10.1016/j.yebeh.2015.07.039. [DOI] [PubMed] [Google Scholar]
- 13.McCormick C, Quraan M, Cohn M, et al. Default mode network connectivity indicates episodic memory capacity in mesial temporal lobe epilepsy. Epilepsia. 2013;54:809–818. doi: 10.1111/epi.12098. [DOI] [PubMed] [Google Scholar]
- 14.Pizoli CE, Shah MN, Snyder AZ, et al. Resting-state activity in development and maintenance of normal brain function. Proc Natl Acad Sci U S A. 2011;108:11638–11643. doi: 10.1073/pnas.1109144108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnston JM, Vaishnavi SN, Smyth MD, et al. Loss of resting interhemispheric functional connectivity after complete section of the corpus callosum. J Neurosci. 2008;28:6453–6458. doi: 10.1523/JNEUROSCI.0573-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engel J, Jr, Van Ness PC, Rasmussen TB, et al. Outcome with respect to epileptic seizures. In: Engel J Jr, editor. Surgical treatment of the epilepsies. Raven Press, Ltd; New York: 1993. pp. 609–621. [Google Scholar]
- 17.Power JD, Cohen AL, Nelson SM, et al. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Power JD, Mitra A, Laumann TO, et al. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wieser HG. Ictal manifestations of temporal lobe seizures. Adv Neurol. 1991;55:301–315. [PubMed] [Google Scholar]
- 20.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme Medical; New York: 1988. [Google Scholar]
- 21.Briellmann RS, Wellard RM, Jackson GD. Seizure-associated abnormalities in epilepsy: evidence from MR imaging. Epilepsia. 2005;46:760–766. doi: 10.1111/j.1528-1167.2005.47604.x. [DOI] [PubMed] [Google Scholar]
- 22.Bernhardt BC, Worsley KJ, Kim H, et al. Longitudinal and cross-sectional analysis of atrophy in pharmacoresistant temporal lobe epilepsy. Neurology. 2009;72:1747–1754. doi: 10.1212/01.wnl.0000345969.57574.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song M, Du H, Wu N, et al. Impaired resting-state functional integrations within default mode network of generalized tonic-clonic seizures epilepsy. PLoS One. 2011;6:e17294. doi: 10.1371/journal.pone.0017294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Voets NL, Beckmann CF, Cole DM, et al. Structural substrates for resting network disruption in temporal lobe epilepsy. Brain. 2012;135:2350–2357. doi: 10.1093/brain/aws137. [DOI] [PubMed] [Google Scholar]
- 25.Hogan RE, Kaiboriboon K, Bertrand ME, et al. Composite SISCOM perfusion patterns in right and left temporal seizures. Arch Neurol. 2006;63:1419–1426. doi: 10.1001/archneur.63.10.1419. [DOI] [PubMed] [Google Scholar]
- 26.Tae WS, Joo EY, Kim JH, et al. Cerebral perfusion changes in mesial temporal lobe epilepsy: SPM analysis of ictal and interictal SPECT. Neuroimage. 2005;24:101–110. doi: 10.1016/j.neuroimage.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Van Paesschen W, Dupont P, Van Driel G, et al. SPECT perfusion changes during complex partial seizures in patients with hippocampal sclerosis. Brain. 2003;126:1103–1111. doi: 10.1093/brain/awg108. [DOI] [PubMed] [Google Scholar]
- 28.Bernhardt BC, Bernasconi N, Concha L, et al. Cortical thickness analysis in temporal lobe epilepsy: reproducibility and relation to outcome. Neurology. 2010;74:1776–1784. doi: 10.1212/WNL.0b013e3181e0f80a. [DOI] [PubMed] [Google Scholar]
- 29.Xiao F, An D, Lei D, et al. Real-time effects of centrotemporal spikes on cognition in rolandic epilepsy: An EEG-fMRI study. Neurology. 2016;86:544–551. doi: 10.1212/WNL.0000000000002358. [DOI] [PubMed] [Google Scholar]
- 30.Vogt VL, Aikia M, Del Barrio A, et al. Current standards of neuropsychological assessment in epilepsy surgery centers across Europe. Epilepsia. 2017;58:343–355. doi: 10.1111/epi.13646. [DOI] [PubMed] [Google Scholar]
- 31.Baxendale S, Thompson P. Beyond localization: the role of traditional neuropsychological tests in an age of imaging. Epilepsia. 2010;51:2225–2230. doi: 10.1111/j.1528-1167.2010.02710.x. [DOI] [PubMed] [Google Scholar]
- 32.Sherman EM, Wiebe S, Fay-McClymont TB, et al. Neuropsychological outcomes after epilepsy surgery: systematic review and pooled estimates. Epilepsia. 2011;52:857–869. doi: 10.1111/j.1528-1167.2011.03022.x. [DOI] [PubMed] [Google Scholar]
- 33.Detre JA, Maccotta L, King D, et al. Functional MRI lateralization of memory in temporal lobe epilepsy. Neurology. 1998;50:926–932. doi: 10.1212/wnl.50.4.926. [DOI] [PubMed] [Google Scholar]
- 34.Jokeit H, Okujava M, Woermann FG. Memory fMRI lateralizes temporal lobe epilepsy. Neurology. 2001;57:1786–1793. doi: 10.1212/wnl.57.10.1786. [DOI] [PubMed] [Google Scholar]
- 35.Voets NL, Zamboni G, Stokes MG, et al. Aberrant functional connectivity in dissociable hippocampal networks is associated with deficits in memory. J Neurosci. 2014;34:4920–4928. doi: 10.1523/JNEUROSCI.4281-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baldassarre A, Ramsey L, Hacker CL, et al. Large-scale changes in network interactions as a physiological signature of spatial neglect. Brain. 2014;137:3267–3283. doi: 10.1093/brain/awu297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Makin TR, Filippini N, Duff EP, et al. Network-level reorganisation of functional connectivity following arm amputation. Neuroimage. 2015;114:217–225. doi: 10.1016/j.neuroimage.2015.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glasser MF, Coalson TS, Robinson EC, et al. A multi-modal parcellation of human cerebral cortex. Nature. 2016;536:171–178. doi: 10.1038/nature18933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Essen DC, Smith SM, Barch DM, et al. The WU-Minn Human Connectome Project: an overview. Neuroimage. 2013;80:62–79. doi: 10.1016/j.neuroimage.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Essen DC, Glasser MF, Dierker DL, et al. Parcellations and hemispheric asymmetries of human cerebral cortex analyzed on surface-based atlases. Cereb Cortex. 2012;22:2241–2262. doi: 10.1093/cercor/bhr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.