Abstract
Immunotherapy induces durable responses in a subset of patients with cancer. High TMB may be a response biomarker for PD-1/PD-L1 blockade in tumors such as melanoma and non-small cell lung cancer (NSCLC). Our aim was to examine the relationship between TMB and outcome in diverse cancers treated with various immunotherapies. We reviewed data on 1,638 patients who had undergone comprehensive genomic profiling and had TMB assessment. Immunotherapy-treated patients (N = 151) were analyzed for response rate (RR), progression-free and overall survival (PFS, OS). Higher TMB was independently associated with better outcome parameters (multivariable analysis). The RR for patients with high (≥ 20 mutations/mb) vs. low to intermediate TMB was 22/38 (58%) vs. 23/113 (20%) (P = 0.0001); median PFS, 12.8 vs. 3.3 months (P = <0.0001); median OS, not reached vs. 16.3 months (P = 0.0036). Results were similar when anti-PD-1/PD-L1 monotherapy was analyzed (N = 102 patients), with a linear correlation between higher TMB and favorable outcome parameters; the median TMB for responders vs. non-responders treated with anti-PD-1/PD-L1 monotherapy was 18.0 vs. 5.0 mutations/mb (P < 0.0001). Interestingly, anti-CTLA4/anti-PD-1/PD-L1 combinations vs. anti-PD-1/PD-L1 monotherapy was selected as a factor independent of TMB for predicting better RR (77% vs. 21%) (P = 0.004) and PFS (P = 0.024). Higher TMB predicts favorable outcome to PD-1/PD-L1 blockade across diverse tumors. Benefit from dual checkpoint blockade did not show a similarly strong dependence on TMB.
INTRODUCTION
Immunotherapeutics, including high dose interleukin-2 (IL2) and antibodies that block programmed death receptor-1 (PD-1)/programmed death-ligand 1 (PD-L1) and cytotoxic T-lymphocyte associated protein-4 (CTLA4) can induce durable responses across numerous types of solid tumors (1–7) and hematologic malignancies (8,9). However, the majority of unselected patients will not respond to immunotherapy, even among those with responsive tumor types. For example, response rates to single-agent PD-1/PD-L1 inhibition in patients with melanoma, non-small cell lung cancer (NSCLC), and renal cell carcinoma (RCC) are 40% (1,10), 25% (2,3), and 19% (4), respectively.
There is an unmet need for biomarkers that will identify patients more likely to respond to PD-1/PD-L1 blockade as well as other immunotherapeutics (11). The use of tumor PD-L1 expression as a biomarker has been studied extensively. In general, across all tumor types, anti-PD-1/PD-L1 therapy results in response rates of 0–17% in patients with PD-L1-negative tumors, whereas, in those with tumors that express PD-L1, response rates range from 36–100% (12). However, widespread use and standardization of PD-L1 as a biomarker has been limited by the different detection methods used in practice (immunohistochemistry (IHC), flow cytometry, versus mRNA expression) (9). In addition, there is no standard definition as to what level of PD-L1 expression defines positivity (13). Furthermore, many tumors not only express PD-L1 on malignant cells, but also on the non-malignant cells with in the tumor microenvirnoment (14). Finally, PD-L1 expression is only applicable to patients treated with PD-1/PD-L1 blockade and not other types of immunotherapy.
Cancers are caused by the accumulation of somatic mutations that can result in the expression of neoantigens (15). Neoantigens occasionally elicit successful T-cell-dependent immune responses against tumors by activating CD8+ cytotoxic T-cells (CTLs). Primed CTLs can recognize target antigen that is peptide bound to major histocompatibility complex class I (MHC I) and presented on tumor cells, and hence initiate tumor cell lysis(16).
The most robust responses to PD-1/PD-L1 blockade have been seen in melanoma and NSCLC, which are both tumors with a high tumor mutational burden (TMB) (17). Higher non-synonymous mutational burden in NSCLC, assessed by whole exome sequencing (WES), is associated with an improved overall response rate (RR), durable clinical benefit, and progression-free survival (PFS) in patients treated with anti-PD-1/PD-L1 therapy (18). Despite the proven utility of WES in measuring TMB and predicting response to PD-1/PD-L1 blockade, it has many limitations. WES is expensive, time consuming, and labor intensive, and, therefore, difficult to incorporate into clinical practice (19).
Hybrid capture-based next generation sequencing (NGS) permits simultaneous identification of all classes of DNA alterations (base substitutions, indels, gene rearrangements and copy number changes) and TMB from a single specimen (20–25). TMB, measured by hybrid based NGS, has been shown to correlate with response to PD-1/PD-L1 blockade in patients with melanoma (19,26), NSCLC (26,27), and urothelial carcinoma (28,29). Patients with colorectal cancer and mismatch repair gene anomalies (which are generally associated with high TMB) also commonly respond to PD-1/PD-L1 blockade(30). However, it is unknown whether TMB serves as a useful biomarker for predicting response to other forms of immunotherapy and to PD-1/PD-L1 blockade in other tumor histologies(31–33). We hypothesized that TMB, measured by hybrid capture-based NGS, would prove clinically useful in predicting response to immunotherapy across a wide array of tumor histologies.
MATERIALS and METHODS
Patient selection
We reviewed the charts of 1,638 cancer patients who had undergone hybrid capture based NGS (Foundation Medicine (Cambridge MA) at UC San Diego Moores Cancer Center (October 2012 until August 2016). Only patients treated with immunotherapy were further analyzed. Immunotherapy agents included anti-PD-1/PD-L1, anti-CTLA4, combination anti-CTLA4/anti-PD-1/PD-L1, high dose IL2, and other agents (see Table 1). This study was performed and consents were obtained in accordance with UCSD Institutional Review Board guidelines for data analysis (NCT02478931) and for any investigational treatments.
Table 1.
Patient demographics by TMB low or intermediate vs. high (N = 151)*
| Variable | Group | Number for all patients N = 151 (%) |
TMB low to intermediate N = 113 (%) |
TMB high N = 38 (%) |
P-value1 |
|---|---|---|---|---|---|
| Age | <60 years | 78 (52%) | 67 (59%) | 11 (29%) | 0.0014 |
| ≥60 years | 73 (48%) | 46 (41%) | 27 (71%) | ||
| Sex | Men | 93 (62%) | 64 (56%) | 29 (76%) | 0.0349 |
| Women | 58 (38%) | 49 (44%) | 9 (24%) | ||
| Ethnicity | Caucasian | 111 (74%) | 77 (68%) | 34 (89%) | 0.0104 |
| Hispanic | 18 (12%) | 18 (16%) | 0 (0%) | 0.0070 | |
| Asian | 9 (6%) | 7 (6%) | 2 (5%) | 1.0000 | |
| African American | 9 (6%) | 8 (7%) | 1 (3%) | 0.4505 | |
| Other | 4 (3%) | 3 (3%) | 1 (3%) | 1.0000 | |
| Tumor type | Melanoma | 52 (34%) | 34 (30%) | 18 (47%) | 0.0748 |
| NSCLC2 | 36 (24%) | 33 (29%) | 3 (8%) | 0.0077 | |
| Other tumors3 | 63 (42%) | 46 (41%) | 17 (45%) | 0.7060 | |
| Type of Immunotherapy | Anti-PD-1/PD-L1 alone | 102 (68%) | 80 (71%) | 22 (58%) | 0.1631 |
| Anti-CTLA4 alone | 15 (10%) | 10 (9%) | 5 (13%) | 0.5308 | |
| High dose IL2 | 9 (6%) | 7 (6%) | 2 (5%) | 1.0000 | |
| Anti-CTLA4/Anti-PD-1/PD-L1 | 17 (11%) | 11 (10%) | 6 (16%) | 0.3732 | |
| Other immunotherapy4 | 8 (5%) | 5 (3%) | 3 (8%) | 0.4157 | |
| Response | CR/PR | 45 (30%) | 23 (20%) | 22 (58%) | 0.0001 (OR = 5.38, 95% CI 2.44–11.58)5 |
| SD or PD | 106 (70%) | 90 (80%) | 16 (42%) | 0.0001 (OR = 0.19, 95% CI 0.09–0.41) 5 | |
| PFS | Median (months) | 4.6 | 3.3 (2.98) | 12.8 (0.34) | <0.0001 (HR = 0.34, 95% CI 0.23–0.50) 5 |
| OS | Median (months) | 25.4 | 16.3 (3.03) | Not reached (median f/u of 10.5 mos) | 0.0036 (HR = 0.33, 95% CI 0.19–0.58) 5 |
See Supplemental Table 2 for patient demographics by TMB low vs. intermediate to high.
Calculated using Fisher’s exact test and log-rank (Mantel-Cox) test where appropriate.
Histologies included: adenocarcinoma (N=30) and squamous cell carcinoma (N=6 )
Tumors included: Adrenal carcinoma (n=1), appendix adenocarcinoma (n=1), basal cell carcinoma (n=2), bladder transitional cell carcinoma (n=4), breast cancer (n=3), cervical cancer (n=2), colon adenocarcinoma (n=5), cutaneous squamous cell carcinoma (n=8), hepatocellular carcinoma (n=3), head and neck (n=13), Merkel cell carcinoma (n=2), ovarian carcinoma (n=2), pleural mesothelioma (n=1), prostate cancer (n=1), renal cell carcinoma (n=6), sarcoma (n=3), thyroid cancer (n=3), unknown primary squamous cell carcinoma (n=2), and urethral squamous cell carcinoma (n=1)
Other immunotherapy: OX40 (n=3), anti-CD73 (n=1), Talimogene laherparepvec (n=2), OX40+anti-PD-L1 (n=1), and IDO+anti-PD-1 (n=1)
Odds Ratio (OR) >1.0 implies higher chance of response; Hazard Ratio (HR) <1.0 implies less chance of progression or death; OR and HR refer to TMB high versus TMB low to intermediate
Abbreviations: CI = confidence interval; CR = complete response; CTLA4 = cytotoxic T-lymphocyte associated protein 4; HR = hazard ratio; IL2 = interleukin 2; NSCLC = non-small cell lung cancer; OR = odds ratio; OS = overall survival; PD-1 = programmed death receptor-1; PD-L1 programmed death receptor-ligand 1; PD = progressive disease: PFS = progression free survival; PR = partial response; SD = stable disease; TMB = tumor mutational burden
Next Generation Sequencing and Assessment of Tumor Mutational Burden
Formalin-fixed paraffin embedded tumor samples were submitted for NGS to Foundation Medicine (clinical laboratory improvement amendments (CLIA)-certified lab). The FoundationOne assay was used (hybrid-capture-based NGS; 182, 236 or 315 genes, depending on the time period) (http://www.foundationone.com/). The methods have been previously described (20). Average sequencing depth of coverage was greater than 250×, with >100× at >99% of exons. For TMB, the number of somatic mutations detected on NGS (interrogating 1.2 mb of the genome) are quantified and that value extrapolated to the whole exome using a validated algorithm (19,28). Alterations likely or known to be bona fide oncogenic drivers and germline polymorphisms are excluded. TMB was measured in mutations per megabase (mb). TMB levels were divided into three groups based off the Foundation Medicine official reports: low (1–5 mutations/mb), intermediate (6–19 mutations/mb), and high (≥ 20 mutations/mb), which in a large cohort approximately divided ~50% of patients to low TMB, ~40% intermediate TMB, and 10% high TMB (34). 100 non-synonymous mutations per exome was used previously as a threshold in other papers. Our threshold of 20 coding mutations per megabase is roughly equivalent to 400 non-synonymous mutations per exome (20 coding mutations/MB * 30 MB / exome * 2/3 non-synonymous/coding).
For outcome analyses, comparisons were made between both low to intermediate vs. high and low vs. intermediate to high TMB. In addition, the linearity of TMB across all levels was assessed.
Statistical Analysis and Outcome Evaluation
The Fisher’s exact test was used to assess categorical variables. P values ≤ 0.05 were considered significant. Responses were assessed based on physician notation; physicians used RECIST criteria. PFS and OS were calculated by the method of Kaplan and Meier (P values by log-rank (Mantel-Cox) test). Linear regressions were performed using the least squares method. Patients who died early were considered evaluable (as progressive disease). For patients who received multiple immunotherapy regimens, the treatment with the longest PFS was chosen for analysis. (However, a second analysis that included all treatments given to all patients was also performed). OS was defined as the time from initiation of the immunotherapy with longest PFS until patient death. Patients were considered inevaluable for inclusion in the survival analysis if they were lost to follow up before their first restaging. Patients were censored at date of last follow up for PFS and OS, if they had not progressed or died, respectively. Statistical analyses were carried out by SK using Graph-Pad Prism version 7.0 (San Diego, CA, USA) and IBM SPSS Statistics version 24.
RESULTS
Patient characteristics
Overall, 151 patients treated with various immunotherapies were evaluable for outcome (Supplemental Figure 1). Median age was 59 years (range, 19 to 88 years). The most common tumor types were melanoma and NSCLC (N = 52 and 36 patients, respectively). Sixty-three patients had 19 other tumor types (Tables 1 and 2). All patients had locally advanced or metastatic disease. Thirty-seven patients received multiple lines of immunotherapy (range 2–5) (Supplemental Table 1). The outcome data is compiled for the immunotherapy with best PFS (see Methods) unless otherwise stated. The most common treatment evaluated was anti-PD-1/PD-L1 monotherapy (N = 102, anti-PD1 = 99 and anti-PD-L1 = 3).
Table 2.
Univariate and multivariate analysis of factors affecting outcome for all patients treated with immunotherapy agents (TMB low or intermediate vs. high) (N = 151)*
| Variable | Group (N) | PR/CR N (%) | OR (95% CI)1 | P –value univariate (PR/CR)2 | P-value multivariate (PR/CR) | Median PFS (mos)3 | HR (95% CI) (PFS)1 | P-value univariate (PFS)4 | P-value multivariate (PFS) | Median OS (mos)3 | HR (95% CI) (OS)1 | P-value univariate (OS)4 | P-value multivariate (OS) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | ≤60 years (N = 78) | 20 (26%) | 0.66 (0.32–1.33) | 0.2873 | 4.0 | 1.12 (0.77–1.63) | 0.5396 | 28.4 | 1.03 (0.61–1.72) | 0.9165 | |||
| >60 years (N = 73) | 25 (34%) | 1.51 (0.75–3.14) | 5.7 | 0.89 (0.61–1.29) | 25.4 | 0.97 (0.58–1.63) | |||||||
| Gender | Men (N = 93) | 33 (36%) | 2.11 (0.97–4.57) | 0.0675 | 0.235 | 5.8 | 0.70 (0.47–1.03) | 0.0572 | 0.362 | 28.4 | 0.88 ().51–1.50) | 0.6204 | |
| Women (N = 58) | 12 (21%) | 0.47 (0.22–1.03) | 3.6 | 1.44 (0.97–2.12) | 16.3 | 1.14 (0.67–1.95) | |||||||
| Ethnicity | Caucasian (N = 111) | 38 (34%) | 2.45 (0.99–6.29) | 0.0685 | 0.604 | 5.9 | 0.58 (0.36–0.92) | 0.0066 | 0.983 | 28.4 | 0.65 (0.35–1.20) | 0.1192 | |
| Hispanic (N = 18) | 3 (17%) | 0.43 (0.13–1.57) | 0.2745 | 2.6 | 1.38 (0.73–2.61) | 0.2543 | 15.6 | 1.46 (0.64–3.30) | 0.2927 | ||||
| Asian (N = 9) | 1 (11%) | 0.28 (0.02–1.94) | 0.2808 | 2.0 | 2.61 (0.86–7.90) | 0.0063 | 0.083 | Not reached (median f/u 3.4 mos) | 1.84 (0.49–7.01) | 0.2265 | |||
| African American (N = 9) | 3 (33%) | 1.19 (0.31–4.45) | 1.0000 | 3.9 | 1.30 (0.55–3.07) | 0.5002 | Not reached (median f/u 6.5 mos) | 1.26 (0.35–4.60) | 0.6916 | ||||
| Other (N = 4) | 0 (9%) | 0 (0–2.40) | 0.3181 | 4.1 | 1.54 (0.45–5.23) | 0.3902 | 38.33 | 1.02 (0.25–4.27) | 0.9730 | ||||
| Tumor Type | Melanoma (N = 52) | 26 (50%) | 3.68 (1.71–7.82) | 0.0007 | 0.562 | 9.3 | 0.36 (0.25–0.51) | <0.0001 | 0.035 | Not reached (median f/u of 15.6 mos) | 0.26 (0.16–0.43) | <0.0001 | 0.006 |
| NSCLC (N = 36) | 7 (19%) | 0.49 (0.19–1.23) | 0.1460 | 2.8 | 2.00 (1.21–3.27) | 0.0007 | 0.992 | 8.0 | 1.90 (0.97–3.72) | 0.0213 | 0.794 | ||
| Other tumors5 (N = 63) | 12 (19%) | 0.39 (0.18–5.51) | 0.0187 | 0.301 | 2.9 | 1.67 (1.12–2.50) | 0.0056 | 0.992 | 11.2 | 2.13 (1.23–3.69) | 0.0022 | 0.794 | |
| TMB | Low to Intermediate (N = 113) | 23 (20%) | 0.19 (0.09–0.41) | 0.0001 | <0.001 | 3.3 | 2.98 (2.02–4.41) | <0.0001 | <0.001 | 16.3 | 3.03 (1.72–5.33) | 0.0036 | 0.016 |
| High (N = 38) | 22 (58%) | 5.38 (2.44–11.58) | 12.8 | 0.34 (0.23–0.50) | Not reached (median f/u of 10.5 mos) | 0.33 (0.19–0.58) | |||||||
| Type of immunotherapy | Anti-PD-1/PD-L1 monotherapy (N = 102) | 21 (21%) | 0.27 (0.13–0.58) | 0.0006 | 0.743 | 3.3 | 2.41 (1.65–3.50) | <0.0001 | 0.588 | 15.7 | 2.74 (1.63–4.59) | 0.0005 | 0.820 |
| Anti-CTLA4 alone (N = 15) | 2 (13%) | 0.33 (0.07–1.40) | 0.2329 | 4.7 | 1.08 (0.58–2.01) | 0.7957 | 28.4 | 0.87 (0.39–1.95) | 0.7529 | ||||
| High dose IL2 (N = 9) | 5 (56%) | 3.19 (0.88–10.72) | 0.1270 | 37.0 | 0.40 (0.23–0.71) | 0.0146 | 0.070 | Not reached (median f/u of 34.6 mos) | 0.37 (0.17–0.80) | 0.0614 | 0.284 | ||
| Anti-CTLA4/Anti-PD-1/PD-L1 (N = 17) | 13 (77%) | 10.36 (3.05–30.18) | <0.0001 | 0.004 | Not reached (median f/u 9.2 mos) | 0.27 (0.16–0.44) | 0.0006 | 0.024 | Not reached (median f/u of 16.3 mos) | 0.20 (0.10–0.40) | 0.0107 | 0.172 | |
| Other immunotherapy6 (N = 8) | 4 (50%) | 2.49 (0.69–8.82) | 0.2392 | 8.9 | 0.77 (0.32–1.87) | 0.6044 | Not reached (median f/u of 5.5 mos) | 0.81 (0.23–2.92) | 0.7694 |
All univariate P values of ≤0.1 were included in the multivariate analysis. For a similar analysis by TMB low vs. intermediate to high, see Supplemental Table 4.
Odds Ratio (OR) >1.0 implies higher chance of response; Hazard Ratio (HR) <1.0 implies less chance of progression or death
Calculated using Fisher’s exact test
All medians for PFS and OS calculated by Kaplan Meier
Calculated using log-rank (Mantel-Cox) test
Other tumors: Adrenal carcinoma (n=1), appendix adenocarcinoma (n=1), basal cell carcinoma (n=2), bladder transitional cell carcinoma (n=4), breast cancer (n=3), cervical cancer (n=2), colon adenocarcinoma (n=5), cutaneous squamous cell carcinoma (n=8), hepatocellular carcinoma (n=3), head and neck (n=13), Merkel cell carcinoma (n=2), ovarian carcinoma (n=2), pleural mesothelioma (n=1), prostate cancer (n=1), renal cell carcinoma (n=6), sarcoma (n=3), thyroid cancer (n=3), unknown primary squamous cell carcinoma (n=2), and urethral squamous cell carcinoma (n=1)
Other immunotherapy: OX40 (n=3), anti-CD73 (n=1), Talimogene laherparepvec (n=2), OX40+anti-PD-L1 (n=1), and IDO+anti-PD-1/PD-L1 (n=1)
Abbreviations: CI = confidence interval; CR = complete response; CTLA4 = cytotoxic T-lymphocyte associated protein 4; HR = hazard ratio; IL2 = interleukin 2; OR = odds ratio; OS = overall survival; PD-1 = programmed death receptor-1; PD-L1 programmed death receptor-ligand 1; PD = progressive disease: PFS = progression free survival; PR = partial response; TMB = tumor mutational burden
Of the 151 patients, 65 (43%) had low TMB (1–5 mutations/mb); 48 (32%), intermediate (6–19 mutations/mb); and 38 (25%), high TMB (≥ 20 mutations/mb). The median time from biopsy for NGS/TMB to immunotherapy initiation was 8.0, 9.2, and 6.4 months for tumors with low, intermediate, and high TMB (P = 0.2208). The median TMB was 6 mutations/mb (range, 1 to 347). The median TMB for patients with melanoma (N = 52) was 10.5 (range, 1 to 133); for NSCLC (N = 36 ), 5 (range, 1 to 57); and for tumors other than melanoma or NSCLC (N = 63), median TMB was 6 (range, 1 to 347).
Amongst the 151 patients, the number who attained CR/PR was 45 (30 %); median PFS, 4.6 months; median OS, 25.4 months (Table 1)
Outcome by TMB
When TMB was dichotomized by high vs. low to intermediate, age ≥ 60 (P = 0.0014), male sex (P = 0.0349), and Caucasian ethnicity (P = 0.0104) were all associated with a high TMB while age < 60 (p = 0.0014), female sex (P = 0.0349), Hispanic ethnicity (P = 0.0070), and NSCLC histology (P =0.0077) were associated with a low to intermediate TMB (Table 1). CR/PR rates were 22/38 (58%) vs. 23/113 (20%) (P = 0.0001); median PFS, 12.8 vs. 3.3 months (P = <0.0001); median OS, 16.3 months vs. not reached (P = 0.0036). Supplemental Table 2 shows similar results when TMB was dichotomized by low versus intermediate and high (except that age and sex are no longer significantly associated with TMB stratification).
The median TMB was 19 vs. 5 mutations/mb for responders vs. non-responders for all 151 patients (P = <0.0001) (Figure 1); it was 32 versus 6 mutations/mb for the 63 patients that did not include melanoma and NSCLC (P = 0.0001), and it was 16 vs. 5 mutations/mb for the 88 melanoma and NSCLC patients (p<0.0003) (Supplemental Table 3).
Figure 1. Forest plots comparing TMB for patients treated with immunotherapy agents: responders vs. non-responders.
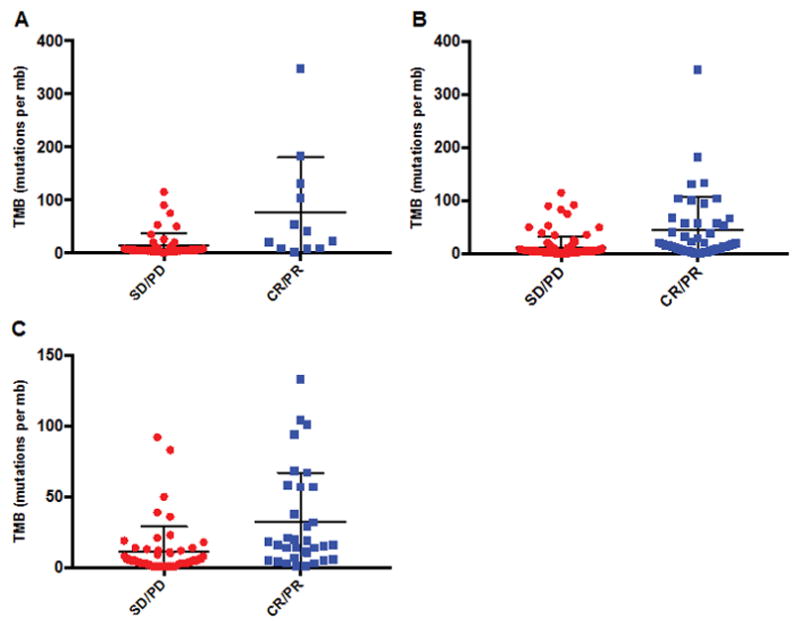
The mean with standard deviation is represented.
Panel A: Patients with all tumors excluding melanoma and NSCLC (N = 63) (P<0.0001).
Panel B: Patients with all tumors including melanoma and NSCLC (N = 151) (P = 0.0001).
Panel C: Patients with melanoma or NSCLC (N = 88) (P = 0.0003).
Abbreviations: CR = complete response, mb = megabase, NSCLC = non small cell lung cancer; PD = progressive disease, PR = partial response, SD = stable disease, TMB = tumor mutational burden
Multivariate Analysis of Factors Predicting Outcome after Immunotherapy
All tumor types considered together
The key independent factors affecting outcome in multivariate analysis of all 151 patients included having a high vs. low to intermediate TMB (CR/PR rate = 58% versus 20%) (P <0.001), and receiving combination therapy with anti-CTLA4/anti-PD-1/PD-L1 vs. anti-PD-1/PD-L1 alone (CR/PR rate = 77% versus 21%) (P = 0.004) (Table 2). Independent factors correlating with longer PFS included having melanoma (P = 0.035), combination therapy with anti-CTLA4/anti-PD-1/PD-L1 vs. anti-PD-1/PD-L1 alone (P= 0.024), and TMB high versus low to intermediate (p<0.001). It is interesting that the combined anti-CTLA4/anti-PD-1/PD-L1 remained an independent factor predicting outcome despite the fact that all but one patient receiving the combination had melanoma, (Note that 16 of 52 patients with melanoma received the combination immunotherapy regimen). Independent predictors of longer OS included having melanoma (p = 0.006) and TMB high versus low to intermediate (p = 0.016). Identical independent factors were selected for predicting outcome when TMB was dichotomized by low versus intermediate plus high (Supplemental Table 4).
Tumor types other than melanoma and NSCLC
For 63 patients with tumor types other than melanoma and NSCLC, only TMB (high vs. low to intermediate) (Table 3) was selected for independently predicting RR (CR/PR rate = 47% versus 9%; P = 0.005) and PFS (median PFS 10 vs. 2.1 months; p = 0.0007) (but not OS (P = 0.1847)). Similar results were seen when TMB was dichotomized by intermediate to high versus low (Supplemental Table 5).
Table 3.
Univariate and multivariate analysis of factors affecting outcome for patients with all tumor types excluding melanoma and NSCLC treated with immunotherapy agents (TMB low or intermediate vs. high) (N = 63)*
| Variable | Group (N) | PR/CR N (%) | OR (95% CI)1 | P–value univariate (PR/CR)2 | P-value multivariate (PR/CR) | Median PFS (mos)3 | HR (95% CI) (PFS)1 | P-value univariate (PFS)4 | p-value multivariate (PFS) | Median OS (mos)3 | HR (95% CI) (OS)1 | P-value univariate (OS)4 | P-value multivariate (OS) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | ≤60 years (N = 33) | 4 (12%) | 0.38 (0.12–1.35) | 0.2017 | 3.4 | 1.44 (0.82–2.54) | 0.2033 | 11.1 | 1.25 (0.60–2.65) | 0.5449 | |||
| >60 years (N = 30) | 8 (27%) | 2.64 (0.74–8.55) | 2.7 | 0.69 (0.39–1.22) | 11.2 | 0.80 (0.39–1.66) | |||||||
| Gender | Men (N = 41) | 11 (27%) | 7.7 (1.11–86.57) | 0.0433 | 0.219 | 2.9 | 0.70 (0.38–1.29) | 0.2128 | 11.1 | 1.23 (0.56–2.69) | 0.6085 | ||
| Women (N = 22) | 1 (5%) | 0.13 (0.01–0.90) | 3.5 | 1.43 (0.78–2.63) | Not reached (median f/u of 5.4 mos) | 0.81 (0.37–1.77) | |||||||
| Ethnicity | Caucasian (N = 40) | 10 (25%) | 3.50 (0.77–17.00) | 0.1830 | 0.254 | 3.6 | 0.64 (0.35–1.18) | 0.1179 | 0.499 | 11.2 | 0.63 (0.29–1.38) | 0.2047 | |
| Hispanic (N = 7) | 0 (0%) | 0 (0–2.58) | 0.3292 | 1.9 | 1.38 (0.52–3.65) | 0.4515 | 3.1 | 2.82 (0.76–10.44) | 0.0168 | 0.053 | |||
| Asian (N = 7) | 1 (14%) | 0.68 (0.05–5.40) | 1.0000 | 2.0 | 1.62 (0.57–4.56) | 0.2597 | Not reached (median f/u of 3.7 mos) | 1.15 (0.32–4.07) | 0.8179 | ||||
| African American (N = 6) | 1 (17%) | 0.70 (0.06–5.51) | 1.0000 | 5.0 | 1.047 (0.41–2.69) | 0.9212 | Not reached (median f/u of 6.0 mos) | 0.80 (0.22–2.97) | 0.7625 | ||||
| Other (N = 3) | 0 (0%) | 0 (0–4.99) | 1.0000 | 3.6 | 1.66 (0.38–7.21) | 0.3849 | Not reached (median f/u of 8.6 mos) | 0.72 (0.13–4.05) | 0.7491 | ||||
| TMB | Low to Intermediate (N = 46) | 4 (9%) | 0.11 (0.03–0.44) | 0.0016 | 0.006 | 2.1 | 3.31 (1.86–5.91) | 0.0007 | 0.003 | 11.1 | 1.88 (0.84–4.22) | 0.1847 | 0.362 |
| High (N = 17) | 8 (47%) | 9.33 (2.28–31.23) | 10.0 | 0.30 (0.17–0.54) | 11.2 | 0.53 (0.24–1.20) | |||||||
| Type of immunotherapy | Anti-PD-1/PD-L1 monotherapy (N = 55) | 9 (16%) | 0.33 (0.06–1.43) | 0.1700 | 0.120 | 2.9 | 0.97 (0.38–2.48) | 0.9477 | 11.2 | 1.23 (0.46–3.28) | 0.6633 | ||
| Other immunotherapy5 (N = 8) | 3 (38%) | 3.07 (0.70–15.99) | 2.6 | 1.03 (0.40–2.63) | 25.4 | 0.81 (0.31–2.17) |
All univariate P values of ≤0.2 were included in the multivariate analysis. For a similar analysis by TMB low vs. intermediate to high, see Supplemental Table 5. For an analysis of melanoma and NSCLC on their own, see Supplemental Tables 20–23. Tumors included: Adrenal carcinoma (n=1), appendix adenocarcinoma (n=1), basal cell carcinoma (n=2), bladder transitional cell carcinoma (n=4), breast cancer (n=3), cervical cancer (n=2), colon adenocarcinoma (n=5), cutaneous squamous cell carcinoma (n=8), hepatocellular carcinoma (n=3), head and neck (n=13), Merkel cell carcinoma (n=2), ovarian carcinoma (n=2), pleural mesothelioma (n=1), prostate cancer (n=1), renal cell carcinoma (n=6), sarcoma (n=3), thyroid cancer (n=3), unknown primary squamous cell carcinoma (n=2), and urethral squamous cell carcinoma (n=1)
Odds Ratio (OR) >1.0 implies higher chance of response; Hazard Ratio (HR) <1.0 implies less chance of progression or death
Calculated using Fisher’s exact test
All medians for PFS and OS calculated by Kaplan Meier
Calculated using log-rank (Mantel-Cox) test
Other immunotherapy: OX40 (n=2), anti-CD73 (n=1), anti-CTLA4 (n=2), OX40+anti-PD-L1 (n=1), anti-CTLA4/anti-PD-1/PD-L1 (n=1), and IDO+anti-PD-1/PD-L1 (n=1)
Abbreviations: CI = confidence interval; CR = complete response; CTLA4 = cytotoxic T-lymphocyte associated protein 4; HR = hazard ratio; IL2 = interleukin 2; OR = odds ratio; OS = overall survival; PD-1 = programmed death receptor-1; PD-L1 programmed death receptor-ligand 1; PD = progressive disease: PFS = progression free survival; PR = partial response; TMB = tumor mutational burden
Melanoma and NSCLC analysis
Supplemental Tables 6 and 7 show that TMB, dichotomized either as high vs. low to intermediate or as intermediate to high vs. low, was also an independent predictor of outcome (RR and PFS) when only the 88 patients with melanoma and NSCLC were included. Treatment with combined anti-CTLA4/anti-PD1/PD-L1 also predicted significantly better outcomes (RR and PFS) (p values ranged from 0.042 to 0.003). For OS, the only factor that showed a trend to predict a better outcome was TMB high versus low to intermediate (p = 0.055).
Treatment with anti-PD1/PD-L1 monotherapy and outcome by TMB
All tumor types considered together
For the 102 patients treated with single-agent anti-PD-1/PD-L1 antibodies, high TMB correlated with better outcomes as compared to low to intermediate TMB (CR/PR rate = 46% vs. 14%; p = 0.0025) (PFS = 10 vs. 2.2 months; P = 0.0005) (OS = 11.1 months vs. not reached, P = 0.0557) (Supplemental Table 8; Figure 2B and 2E). Similar results were obtained when TMB was dichotomized at intermediate to high versus low (Supplemental Table 9: P = 0.0002, P<0.0001 and P = 0.0103, respectively) (Supplemental Figure 2B and 2E).
Figure 2. Kaplan Meier curves for PFS and OS (for patients treated with anti-PD-1/PD-L1 monotherapy).
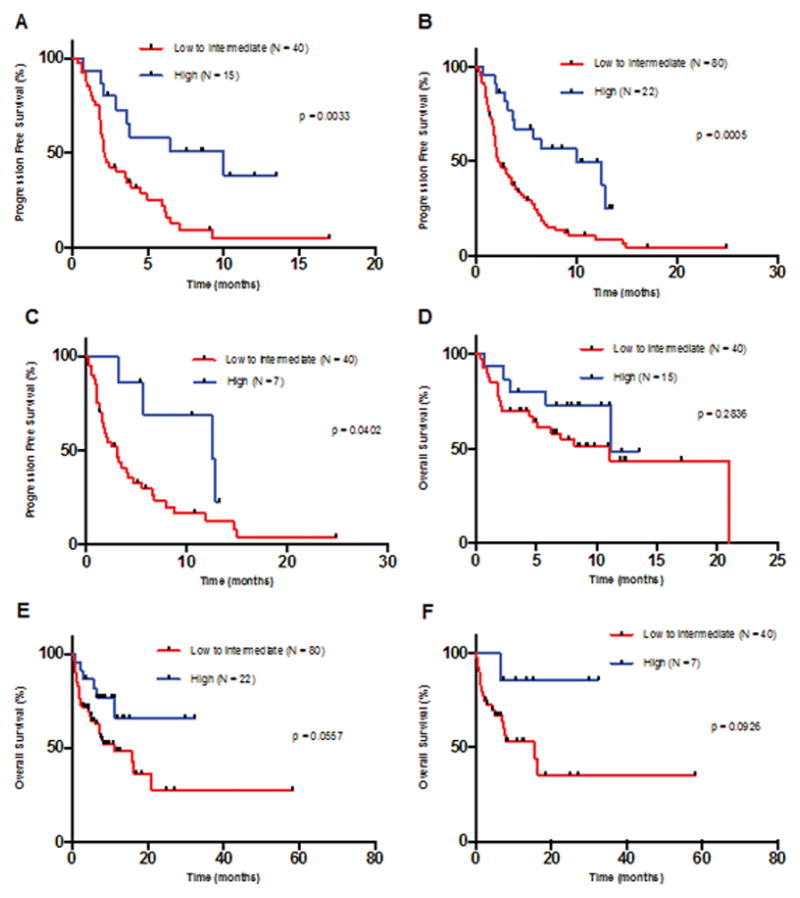
Tick marks represent patients at the time of censoring, and P values were calculated using log-rank (Mantel-Cox) test. For a similar analysis by TMB low vs. intermediate to high, see Supplemental Figure 2.
Panel A: PFS for patients with all tumor types excluding melanoma and NSCLC – TMB low to intermediate vs. high [P = 0.0033, HR = 0.35 (95% CI 0.19 to 0.64)]. For TMB low to intermediate, N = 40 with 35 events. For TMB high, N = 15 with 8 events.
Panel B: PFS for patients with all tumor types including melanoma and NSCLC – TMB low to intermediate vs. high [P = 0.0005, HR = 0.36 (95% CI 0.23 to 0.58)]. For TMB low to intermediate, N = 80, with 66 events. For TMB high, N = 22 with 12 events.
Panel C: PFS for patients with melanoma or NSCLC – TMB low to intermediate vs. high [P = 0.0402, HR = 0.36 (95% CI 0.17 to 0.77)]. For TMB low to intermediate, N = 40 with 31 events. For TMB high, N = 7 with 4 events.
Panel D: OS for patients with all tumor types excluding melanoma and NSCLC – TMB low to intermediate vs. high for all tumor types excluding melanoma and NSCLC [P = 0.2836, HR = 0.59 (95% CI 0.25 to 1.40]. For TMB low to intermediate, N = 40 with 20 events. For TMB high, N = 15 with 5 events.
Panel E: OS for patients with all tumor types including melanoma and NSCLC – TMB low to intermediate vs. high [P = 0.0557, HR = 0.44 (95% CI 0.23 to 0.87)]. For TMB low to intermediate, N = 80 with 36 events. For TMB high, N = 22 with 6 events.
Panel F: OS for patients with melanoma or NSCLC – TMB low to intermediate vs. high [P = 0.0926, HR = 0.21 (95% CI 0.07 to 0.63)]. For TMB low to intermediate, N = 40 with 16 events. For TMB high, N = 7 with 1 events.
Abbreviations: CI = confidence interval; HR = hazard ratio; NSCLC = non-small cell lung cancer; OS = overall survival; PD-1 = programmed death receptor-1; PD-L1 = programmed death receptor-ligand 1; PFS = progression free survival; TMB = tumor mutational burden
For anti-PD-1/PD-L1 monotherapy, the response rate was 4% (2/46) for low TMB, 26% (9/34) for intermediate TMB, and 45% (10/22) for high TMB. For patients with very high TMB (which we designate as >50 mutations/mb) the response rate was 67% (8/12). Furthermore, as demonstrated in Supplemental Tables 10, Supplemental Table 11, and Figure 3, as the cutoff used to dichotomize TMB between low and high increases, the outcome improves in a linear fashion, favoring the TMB high group. This can be seen both for the OR for response (Figure 3A), the HR for PFS (Figure 3B), and the HR for OS (Figure 3C).
Figure 3. Linear correlation1 between TMB cutoff for OR2 for CR/PR rates and HR2 for PFS, and OS depending on TMB for patients treated with anti-PD-1/PD-L1 monotherapy (N = 102).
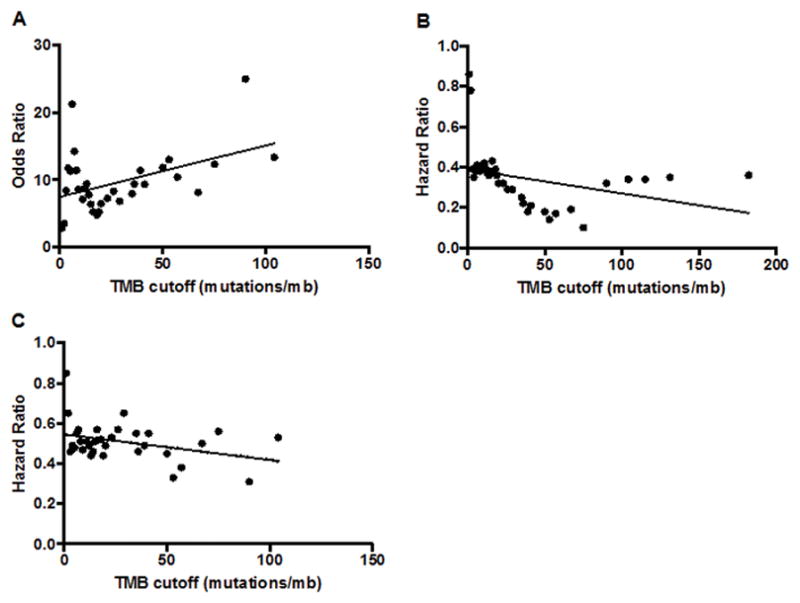
Panel A: OR for CR/PR rate depending on TMB cutoff (R2 = 0.1985, P = 0.0106, Y = 0.07617*X + 7.494).
Panel B: HR for PFS depending on TMB cutoff (R2 = 0.1246, P = 0.0487, Y = −0.001184*X + 0.3886).
Panel C: HR for OS depending on TMB cutoff (R2 = 0.1985, P = 0.0476, Y = −0.001275*X + 0.5462).
1Linear regression performed using the least squares method.
2Odds Ratio (OR) >1.0 implies higher chance of response. The OR was calculated by comparing RR above and below the cut-off for each value. Hazard Ratio (HR) <1.0 implies less chance of progression or death. The HR was evaluated by comparing OS above and below the cut-off for each value.
Abbreviations: CR = complete response, HR = hazard ratio; mb = megabase, NSCLC = non small cell lung cancer; OR = odds ratio; OS = overall survival; PD-1 = programmed death receptor-1; PD-L1 programmed death receptor-ligand 1; PFS = progression free survival; PD = progressive disease; PR = partial response; SD = stable disease; TMB = tumor mutational burden
Tumor types other than melanoma and NSCLC
When melanoma and NSCLC were excluded (55 patients analyzed; Supplemental Table 12; Figure 2A and 2D for PFS and OS), the CR/PR rate for TMB high versus low to intermediate was 40% vs. 8% (P = 0.0086); median PFS was 10 vs. 2.1 months (p = 0.0033), but median OS did not differ significantly. When comparing this same group of patients and separating them by TMB intermediate to high vs. low, the RR and PFS was 26% vs. 4% (P = 0.0620) and 6.2 versus 2.0 (p < 0.0001), respectively (Supplemental Figure 2A and 2D; Supplemental Table 13).
Melanoma and NSCLC analysis
Finally, when only melanoma and NSCLC were included, CR/PR rates, PFS and OS all showed either a strong trend or significantly better outcomes as TMB increased (Supplemental Tables 14 and 15 and Supplemental Figure 3). For instance, when TMB was dichotomized as intermediate to high vs. low (Supplemental Table 15), CR/PR rate was 44% versus 5% (P = 0.0023), PFS (median 5.7 versus 1.9 months) (P = 0.0023) and OS (median not reached versus 8.0 months) (P = 0.0791) (Figure 2C and 2F; Supplemental Figure 2C and 2F).
When analyzing the 102 patients treated with anti-PD1/PD-L1 monotherapy, including individuals with melanoma and NSCLC, the median TMB for responders vs. non-responders was 18.0 and 5.0 mutations/mb (P < 0.0001) (Supplemental Table 16). For the 55 patients with tumors other than melanoma and NSCLC, the median TMB for responders vs. non-responders was 53.0 vs. 5.5 mutations/mb (P < 0.0001). For 47 patients with melanoma and NSCLC, the median TMB for responders versus non-responders was 15.5 vs. 5 mutations/mb (P = 0.0005).
Treatment with a combination of anti-CTLA4 and antiPD1 therapy
Seventeen patients received combination therapy. All but one of these patients had melanoma. Thirteen (77%) achieved CR/PR. The median TMB for responders versus non-responders did not differ (P = 0.6535). Amongst the 17 patients, 6 had a high TMB and, of these, 5 (83%) responded; 11 had a low or intermediate TMB and of these, 8 (67%) responded (P = 1.0000).
Because of the relatively small number of patients in the above analysis which, per Methods, included only patients whose best PFS was on combination treatment, we repeated the analysis with all instances of combination treatment (N = 27) (Supplemental Table 17). There were 16 responders (59%). Median TMB for responders was 9.5 mutations/mb (range, 1–133); for non-responders, 6 (1–83) (P = 0.4061). Median PFS also did not differ by TMB (P = 0.3051).
Treatment with other modalities: anti-CTLA4 and IL-2
When considering therapy with best PFS in each patient, there were 15 patients treated with anti-CTLA4 monotherapy. Their CR/PR rate was 13% (2/15 patients) (The TMB of responders was 20 and 68 mutations/mb; median (range) TMB of non-responders was 8 mutations/mb (range, 2 to 92). We also assessed the total treatments with anti-CTLA 4 alone (N = 29) (Supplemental Table 17). There were six responders (21%). Median (range) TMB (mutations/mb) for responders versus non-responders was 20.5 (16–68) versus 8 (1–92) (P = 0.24). Median PFS for high versus low to intermediate TMB was 6.4 versus 2.7 months (HR = 0.38; 95% CI, 0.17–0.81) (P = 0.0144).
When considering therapy with best PFS, there were nine patients treated with high-dose IL-2. Their CR/PR rate was 56% (5/9 patients). TMB of responders was 1, 3, 4, 38 and 58 mutations/mb (median = 4); for non-responders, 1, 2, 4 and 9 mutations/mb (median = 3). We also assessed all treatments with high-dose IL2 (N = 22) (Supplemental Table 17). There were nine responders (41%). Median (range) TMB (mutations/mb) for responders versus non-responders was 16 (1–58) versus 5 (1–16) (P = 0.056). Median PFS for high versus low to intermediate TMB was 38.9 versus 4.2 months (P = 0.1; HR 0.24: 95% CI, 0.08–0.77).
DISCUSSION
To our knowledge, this is the first study evaluating the utility of TMB as a biomarker of response to immunotherapy in patients with diverse tumor histologies treated with various types of immunotherapy. Our results suggest that TMB, measured by hybrid capture-based NGS interrogating 1.2 mb of the genome, can predict better outcomes after anti-PD-1/PD-L1 immunotherapy in many tumor types, in addition to melanoma and NSCLC.
Although NGS technology is young, oncologists are beginning to effectively customize treatment for patients by matching targeted therapies with cognate alterations (35–37). NGS also has the ability to recognize alterations that can predict response to immunotherapy by identifying mutations in mismatch repair genes (21), microsatellite instability (MSI) (24,25,30,38,39), and PD-L1 amplification (40).
Supplemental Table 18 summarizes many of the published abstracts and manuscripts that have evaluated somatic mutational burden in cancer. Most of these studies are descriptive and do not correlate outcome after immunotherapy to TMB. Two published manuscripts (19,26) and one abstract (27) suggest that TMB measured by NGS predicts response to anti-PD-1/PD-L1 monotherapy in melanoma and NSCLC. In addition, patients with urothelial carcinoma, who responded to treatment with atezolizumab (anti-PD-L1), had a significantly increased TMB compared to non-responders (12.4 versus 6.4 mutations/mb, respectively). Finally, patients with colorectal cancer and mismatch repair defects (which are known to result in high TMB) also respond to PD-1/PD-L1 blockade(30).
Herein, we confirm the correlation between TMB and outcome for patients with NSCLC and melanoma, and suggest that this correlation holds true in other tumor histologies (Tables 1–3, Figure 1). Patients with a high TMB had significantly higher response rates, and longer PFS and OS than those with a lower TMB, and the correlation between TMB and outcome was linear for patients treated with PD-1/PD-L1 monotherapy blockade (Figure 3). The association between higher TMB and better response rates and PFS remained significant when we excluded melanoma and NSCLC patients; however, OS did not (though the smaller number of patients may have precluded finding significance).
Patients with rare tumors generally have limited treatment options (41). Utilizing TMB as a biomarker may help select such patients for immunotherapy. For example, in our study, patients with cervical high-grade neuroendocrine carcinoma, metastatic basal cell carcinoma(42), and undifferentiated pleomorphic sarcoma, all of whom had failed multiple prior treatments and had intermediate to high TMB, responded to PD-1/PD-L1 blockade (Supplemental Table 19). Prospective basket trials evaluating patients with uncommon tumors harboring high TMB are needed.
Not surprisingly, TMB is not a perfect predictor of response to anti-PD-1/PD-L1 therapy. In our study, 2 of 46 patients (4.3%) with a low TMB responded to PD-1/PD-L1 blockade while 12 of 22 patients (54.5%) with a high TMB did not achieve an objective response. Of the two patients with a low TMB who responded, one patient had squamous cell NSCLC (TMB = 5 mutations/mb (the cutoff for intermediate TMB is ≥ 6 mutations/mb)). The other patient had Merkel cell carcinoma (TMB = 1 mutation/mb). Virus-associated Merkel cell carcinomas are known to carry a low mutational burden (43–45); however, these tumors are responsive to PD-1/PD-L1 blockade (46). Viral disease, which may up-regulate specific genes such as APOBEC (responsible for mRNA editing)(47), could create immunogenic neoantigens(48). Further, other biological mechanisms (e.g. PDL1 amplification) in addition to TMB contribute to immunotherapy response.
In seventeen of our patients, anti-PD-1/PD-L1 combined with anti-CTLA4 was the immunotherapy with the best PFS; all but one had melanoma. In these patients, combination therapy was a significant predictor of response and PFS, independent of TMB (multivariate analysis). We also evaluated all treatments with combination therapy (N = 27). Median TMB for responders did not differ from that in non-responders (P = 0.4061), and outcome data remained unrelated to TMB. Our analysis suggests that combinations of anti-PD-1/PD-L1/CTLA4-blocking antibodies can induce responses regardless of the TMB level. This observation is supported by prior studies reporting that combined ipilimumab and nivolumab produced similar response rates in PD-L1-expressing and non-expressing tumors,(49) which is relevant because increased PD-L1 expression correlates with higher TMB(50). The number of patients treated with combination therapy was, however, small in our study, and the implications of TMB level for combination therapy requires validation in larger cohorts.
We used the immunotherapy treatment with best PFS in each patient to assess outcome. However, because anti-CTLA4 or high-dose IL2 were therefore chosen for assessment in only a few patients, we also evaluated all treatments in all patients with these agents. Higher TMB showed a significant correlation or a strong trend to associate with better outcomes (anti-CTLA4 monotherapy (N = 29 treatments)) (high-dose IL2 (N = 22 treatments)). These results are consistent with those previously reported for ipilumumab in melanoma(32,33).
Our study has several limitations. First, it is retrospective. Further, only 151 patients could be analyzed for immunotherapy response. Second, the number of patients for any given malignancy (other than melanoma and NSCLC) and immunotherapy agent (other than anti-PD-1/PD-L1) were low. For this reason, we also assessed the total number of treatments given, which confirmed our observations. Third, cancers are not static, and can acquire mutations as they evolve. NGS is often performed on old biopsy specimens, and samples tested may therefore not accurately reflect the current mutational burden of a tumor. In our study, the median time to treatment with immunotherapy from biopsy was similar among TMB groups (median 8.0, 9.2, and 6.4 months for TMB low, intermediate, and high, respectively (P = 0.2208)). Even so, it would be ideal to have TMB assessment on tissue obtained immediately prior to therapy.
In conclusion, our study suggests that, across tumor diagnoses, cancers with a higher TMB, measured by comprehensive genomic profiling, have a higher likelihood of immunotherapy response, especially with PD-1/PD-L1 blockade. Similar findings were demonstrated with single agent anti-CTL4 or high-dose IL2, albeit in small numbers of patients. Outcome after anti-PD-1/PD-L1/anti-CTLA4 combinations appeared to be independent of TMB. Our observations should be validated in prospective cohorts, and clinical trials should incorporate TMB as a biomarker for assigning patients to single-agent immunotherapies such as checkpoint inhibitors. Larger studies are also needed to confirm if dual checkpoint inhibition is less reliant on higher TMB for response.
Supplementary Material
Acknowledgments
Funding: Funded in part by National Cancer Institute grant P30 CA016672 (R. Kurzrock) and the Joan and Irwin Jacobs Fund philanthropic fund (R. Kurzrock).
References
- 1.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–30. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 2.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. N Engl J Med. 2015;373:1627–39. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–28. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 4.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373:1803–13. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atkins MB, Kunkel L, Sznol M, Rosenberg SA. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am. 2000;6(Suppl 1):S11–4. [PubMed] [Google Scholar]
- 7.Fisher RI, Rosenberg SA, Fyfe G. Long-term survival update for high-dose recombinant interleukin-2 in patients with renal cell carcinoma. Cancer J Sci Am. 2000;6(Suppl 1):S55–7. [PubMed] [Google Scholar]
- 8.Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 Blockade with Nivolumab in Relapsed or Refractory Hodgkin’s Lymphoma. N Engl J Med. 2015;372:311–9. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodman A, Patel SP, Kurzrock R. PD-1-PD-L1 immune-checkpoint blockade in B-cell lymphomas. Nat Rev Clin Oncol [Internet] 2016 doi: 10.1038/nrclinonc.2016.168. [cited 2016 Nov 2];advance online publication. Available from: http://www.nature.com/nrclinonc/journal/vaop/ncurrent/abs/nrclinonc.2016.168.html. [DOI] [PubMed]
- 10.Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–84. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 11.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16:275–87. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel SP, Kurzrock R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol Cancer Ther. 2015;14:847–56. doi: 10.1158/1535-7163.MCT-14-0983. [DOI] [PubMed] [Google Scholar]
- 13.Grigg C, Rizvi NA. PD-L1 biomarker testing for non-small cell lung cancer: truth or fiction? J Immunother Cancer [Internet] 2016 doi: 10.1186/s40425-016-0153-x. [cited 2016 Nov 14];4. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4986262/ [DOI] [PMC free article] [PubMed]
- 14.Santarpia M, Karachaliou N. Tumor immune microenvironment characterization and response to anti-PD-1 therapy. Cancer Biol Med. 2015;12:74–8. doi: 10.7497/j.issn.2095-3941.2015.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gubin MM, Artyomov MN, Mardis ER, Schreiber RD. Tumor neoantigens: building a framework for personalized cancer immunotherapy. J Clin Invest. 2015;125:3413–21. doi: 10.1172/JCI80008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–42. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SAJR, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–21. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science. 2015;348:124–8. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson DB, Frampton GM, Rioth MJ, Yusko E, Xu Y, Guo X, et al. Targeted Next Generation Sequencing Identifies Markers of Response to PD-1 Blockade. Cancer Immunol Res [Internet] 2016 doi: 10.1158/2326-6066.CIR-16-0143. [cited 2016 Oct 20]; Available from: http://cancerimmunolres.aacrjournals.org/content/early/2016/10/05/2326-6066.CIR-16-0143. [DOI] [PMC free article] [PubMed]
- 20.Frampton GM, Fichtenholtz A, Otto GA, Wang K, Downing SR, He J, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol. 2013;31:1023–31. doi: 10.1038/nbt.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chalmers ZR, Huang FW, Gay LM, Ali SM, Chmielecki J, Ross JS, et al. Analysis of tumor mutation burden (TMB) in >51,000 clinical cancer patients to identify novel non-coding PMS2 promoter mutations associated with increased TMB [Abstract] J Clin Oncol [Internet] [cited 2016 Jul 6]; Available from: http://meetinglibrary.asco.org/content/167383-176.
- 22.Frampton GM, Fabrizio D, Chalmers ZR, Ross JS, Johnson DB, Lovly CM, et al. Assessment of tumor mutation burden from >60,000 clinical cancer patients using comprehensive genomic profiling. J Clin Oncol [Internet] 2016 [cited 2016 Nov 1];34. Available from: http://meetinglibrary.asco.org/content/167202-176.
- 23.Ross JS, Gay LM, Sheehan CE, Dalvi S, Voronel O, Elvin JA, et al. Biomarkers of immune checkpoint inhibitor response in metastatic breast cancer: PD-L1 protein expression, CD274 gene amplification, and total mutational burden. J Clin Oncol [Internet] 2016 [cited 2016 Nov 1];34. Available from: http://meetinglibrary.asco.org/content/163800-176.
- 24.Santin A, Moore KN, Gunderson C, Gowen K, Fabrizio D, Frampton GM, et al. Immunotherapy (IO) versus targeted therapy triage in endometrial adenocarcinoma (EA) by concurrent assessment of tumor mutation burden (TMB), microsatellite instability (MSI) status, and targetable genomic alterations (GA) J Clin Oncol [Internet] 2016 [cited 2016 Nov 1];34. Available from: http://meetinglibrary.asco.org/content/171461-176.
- 25.George TJ, Frampton GM, Sun J, Gowen K, Kennedy M, Greenbowe JR, et al. Tumor mutational burden as a potential biomarker for PD1/PD-L1 therapy in colorectal cancer. ASCO Meet Abstr. 2016;34:3587. [Google Scholar]
- 26.Campesato LF, Barroso-Sousa R, Jimenez L, Correa BR, Sabbaga J, Hoff PM, et al. Comprehensive cancer-gene panels can be used to estimate mutational load and predict clinical benefit to PD-1 blockade in clinical practice. Oncotarget. 2015;6:34221–7. doi: 10.18632/oncotarget.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kowanetz M, Zou W, Shames DS, Cummings C, Rizvi N, Spira AI, et al. Tumor mutation load assessed by FoundationOne (FM1) is associated with improved efficacy of atezolizumab (atezo) in patients with advanced NSCLC. Ann Oncol. 2016;27:77P. [Google Scholar]
- 28.Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet Lond Engl. 2016 doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenberg JE, Petrylak DP, Heijden MSVD, Necchi A, O’Donnell PH, Loriot Y, et al. PD-L1 expression, Cancer Genome Atlas (TCGA) subtype, and mutational load as independent predictors of response to atezolizumab (atezo) in metastatic urothelial carcinoma (mUC; IMvigor210) J Clin Oncol [Internet] 2016 [cited 2016 Nov 1];34. Available from: http://meetinglibrary.asco.org/content/165087-176.
- 30.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509–20. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, et al. Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell. 2017;168:542. doi: 10.1016/j.cell.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 32.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–99. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allen EMV, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350:207–11. doi: 10.1126/science.aad0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9:34. doi: 10.1186/s13073-017-0424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wheler J, Lee JJ, Kurzrock R. Unique Molecular Landscapes in Cancer: Implications for Individualized, Curated Drug Combinations. Cancer Res. 2014;74:7181–4. doi: 10.1158/0008-5472.CAN-14-2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwaederle M, Daniels GA, Piccioni DE, Fanta PT, Schwab RB, Shimabukuro KA, et al. On the Road to Precision Cancer Medicine: Analysis of Genomic Biomarker Actionability in 439 Patients. Mol Cancer Ther. 2015;14:1488–94. doi: 10.1158/1535-7163.MCT-14-1061. [DOI] [PubMed] [Google Scholar]
- 37.Schwaederle M, Parker BA, Schwab RB, Daniels GA, Piccioni DE, Kesari S, et al. Precision Oncology: The UC San Diego Moores Cancer Center PREDICT Experience. Mol Cancer Ther. 2016;15:743–52. doi: 10.1158/1535-7163.MCT-15-0795. [DOI] [PubMed] [Google Scholar]
- 38.Hall MJ, Gowen K, Sanford EM, Elvin JA, Ali SM, Kaczmar J, et al. Evaluation of microsatellite instability (MSI) status in 11,573 diverse solid tumors using comprehensive genomic profiling (CGP) J Clin Oncol [Internet] 2016 [cited 2016 Nov 1];34. Available from: http://meetinglibrary.asco.org/content/166691-176.
- 39.Khagi Y, Kurzrock R, Patel SP. Next generation predictive biomarkers for immune checkpoint inhibition. Cancer Metastasis Rev. 2016 doi: 10.1007/s10555-016-9652-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ikeda S, Goodman A, Cohen P, Jensen T, Ellison C, Frampton G, et al. Metastatic Basal Cell Carcinoma with Amplification of PD-L1: Exceptional Response to Anti-PD1 Therapy. Genomic Med. doi: 10.1038/npjgenmed.2016.37. In press; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dean A, Byrne A, Marinova M, Hayden I. Clinical Outcomes of Patients with Rare and Heavily Pretreated Solid Tumors Treated according to the Results of Tumor Molecular Profiling. BioMed Res Int. 2016;2016:e4627214. doi: 10.1155/2016/4627214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ikeda S, Goodman AM, Cohen PR, Jensen TJ, Ellison CK, Frampton G, et al. Metastatic basal cell carcinoma with amplification of PD-L1: exceptional response to anti-PD1 therapy. Npj Genomic Med. 2016;1:16037. doi: 10.1038/npjgenmed.2016.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goh G, Walradt T, Markarov V, Blom A, Riaz N, Doumani R, et al. Mutational landscape of MCPyV-positive and MCPyV-negative Merkel cell carcinomas with implications for immunotherapy. Oncotarget. 2015;7:3403–15. doi: 10.18632/oncotarget.6494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harms PW, Vats P, Verhaegen ME, Robinson DR, Wu Y-M, Dhanasekaran SM, et al. The Distinctive Mutational Spectra of Polyomavirus-Negative Merkel Cell Carcinoma. Cancer Res. 2015;75:3720–7. doi: 10.1158/0008-5472.CAN-15-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cohen PR, Tomson BN, Elkin SK, Marchlik E, Carter JL, Kurzrock R. Genomic portfolio of Merkel cell carcinoma as determined by comprehensive genomic profiling: implications for targeted therapeutics. Oncotarget. 2016;7:23454–67. doi: 10.18632/oncotarget.8032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar RR, Miller NJ, Annamalai L, et al. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N Engl J Med. 2016;374:2542–52. doi: 10.1056/NEJMoa1603702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boichard A, Tsigelny IF, Kurzrock R. High expression of PD-1 ligands is associated with kataegis mutational signature and APOBEC3 alterations. OncoImmunology. 2017;0:e1284719. doi: 10.1080/2162402X.2017.1284719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swanton C, McGranahan N, Starrett GJ, Harris RS. APOBEC Enzymes: Mutagenic Fuel for Cancer Evolution and Heterogeneity. Cancer Discov. 2015;5:704–12. doi: 10.1158/2159-8290.CD-15-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Madore J, Strbenac D, Vilain R, Menzies AM, Yang JYH, Thompson JF, et al. PD-L1 Negative Status is Associated with Lower Mutation Burden, Differential Expression of Immune-Related Genes, and Worse Survival in Stage III Melanoma. Clin Cancer Res. 2016;22:3915–23. doi: 10.1158/1078-0432.CCR-15-1714. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


