Abstract
Objectives
The pharmacokinetics of infliximab are highly variable in children with Crohn's disease (CD), and a one-size-fits-all approach to dosing is inadequate. Model-based drug dosing can help individualize dosing strategies. We evaluated the predictive performance and clinical utility of a published population pharmacokinetic model of infliximab in children with CD.
Methods
Within a cohort of 34 children with CD who had infliximab trough concentrations measured, the pharmacokinetics of each patient was estimated in NONMEM® using a published population pharmacokinetic model. Infliximab concentrations were then predicted based on each patient's dosing history and compared to actual measured concentrations (n=59). In addition, doses 5-10 mg/kg and dosing intervals every 4-8 weeks were simulated in each patient to examine dose-trough relationships.
Results
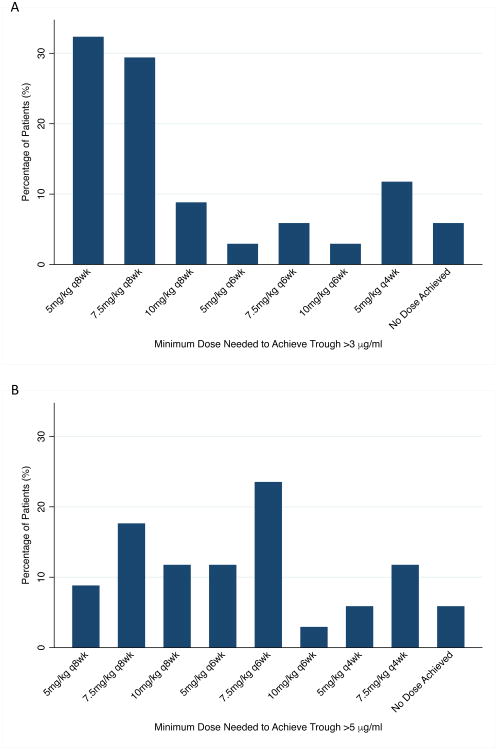
Predicted concentrations were within ±1.0 μg/ml of actual measured concentrations for 88% of measurements. The median prediction error (i.e. measure of bias) was -0.15 μg/ml (95%CI: -0.37 to -0.05 μg/ml) and absolute prediction error (i.e. measure of precision) was 0.26 μg/ml (95%CI: 0.15 to 0.40 μg/ml). At standard maintenance dosing of 5 mg/kg every 8 weeks, a trough >3 μg/ml was predicted to be achieved in 32% of patients. To achieve a trough >3 μg/ml, a dosing interval ≤ every 6 weeks was predicted to be required in 29% of patients.
Conclusions
A published infliximab population pharmacokinetic model demonstrated accurate predictive performance in a pediatric CD population. Individualized infliximab dosing strategies in children with CD will be critical to consistently achieve trough concentrations associated with optimal outcomes.
Keywords: Infliximab, Pharmacokinetics, Children, Crohn's Disease, inflammatory bowel disease, Modeling, Monte Carlo Simulation
Introduction
Infliximab is the most commonly used first-line biologic agent for the treatment of moderate to severe Crohn's disease in children. The current standard infliximab maintenance dosing in children of 5 mg/kg every 8 weeks is based on the original randomized clinical trials demonstrating efficacy in adults and children with Crohn's disease.(1,2) However, the pharmacokinetics of infliximab in children with Crohn's disease is highly variable, and a one-size-fits-all approach to dosing will not result in similar exposures across patients.(3) This variation in infliximab exposure is clinically relevant. There is increasing evidence in adults and children that adequate infliximab exposure is critical when treating Crohn's disease, and trough concentrations <3 μg/ml are associated with treatment failure and worse outcomes.(4–10)
To better understand infliximab dose-exposure relationships, we recently conducted a pharmacokinetic modeling and simulation analysis in children with Crohn's disease and demonstrated that at the standard infliximab dosing of 5 mg/kg every 8 weeks more than 60% of children were predicted to have a trough concentration <3 μg/ml.(11) Higher infliximab doses and/or shorter dosing intervals were predicted to be needed to consistently achieve a trough concentration >3 μg/ml during maintenance dosing. Similarly, a prospective observational study found that 44% (n=10/23) of children with Crohn's disease had a trough concentration <3 μg/ml when receiving standard infliximab maintenance dosing of 5 mg/kg every 8 weeks.(12) Together, these studies highlight the need for more individualized dosing strategies of infliximab in Crohn's disease to ensure adequate exposure and account for the pharmacokinetic variation between patients.
To develop individualized infliximab dosing strategies in patients, utilization of therapeutic drug monitoring (TDM) and population pharmacokinetic models will be essential.(13) TDM has already been successfully implemented in the clinical care of patients with inflammatory bowel disease and is a component of some treatment guidelines.(14–17) In addition, several commercial assays that measure serum infliximab concentrations are readily available. Similarly, a population pharmacokinetic model for infliximab in Crohn's disease patients was previously developed using data from 112 children in the REACH trial and 580 adults in the ACCENT I trial.(3) In this pharmacokinetic model, weight, serum albumin, antibodies to infliximab, and concomitant immunomodulation therapy all significantly impacted infliximab clearance, and consideration of these clinical characteristics may help optimize a patient's individual dose need. However, before such model-based dosing can be applied to aid in therapeutic decision making, ‘validation’ of the underlying population pharmacokinetic model is critical (i.e., can the model accurately and precisely predict drug concentrations in the patient population it will be applied?). The primary aim of the current study was to evaluate the predictive performance and clinical utility of a previously published infliximab population pharmacokinetic model within a cohort of 34 children with Crohn's disease receiving infliximab. The secondary aim of the analysis was to examine the relationship between dosing strategy and trough concentration achievement in each patient to shed further light on dose needs in this population.
Methods
Patient Cohort
To evaluate the predictive performance of the infliximab population pharmacokinetic model, data from a previously collected prospective cohort of children <18 years with Crohn's disease receiving infliximab maintenance treatment were examined.(12) Patients in the cohort were enrolled consecutively at two academic hospitals and one district hospital in the Netherlands over a one-year period. All patients had previously responded to an induction regimen with 5 mg/kg infliximab at week 0, 2, and 6 followed by infusions every 8 weeks (q8w). There were no exclusion criteria. The original study was approved by the institutional review boards at all sites.
In each patient, infliximab trough concentrations were measured prior to two consecutive infliximab infusions. Additional laboratory data collected included antibodies to infliximab (ATI), C-reactive protein (CRP), and serum albumin (ALB). Clinical data collected included weight (WT), age, sex, and concomitant immunomodulation therapy (IMM). Infliximab serum concentrations and ATIs were determined by Sanquin Diagnostics Services (Amsterdam, the Netherlands) using a well-established ELISA-based test.(18) This method did not allow detection of ATIs in the presence of infliximab. The lower limit of quantification of infliximab was 0.002 μg/ml.
Model Evaluation
A published infliximab population pharmacokinetic model developed from 112 children in the REACH trial and 580 adults in the ACCENT I trial was implemented in the non-linear mixed effects modeling software NONMEM 7.2 (ICON Development Solutions, Ellicott City, MD).(3) Briefly, a two-compartment model with first-order elimination was used to describe infliximab pharmacokinetics. Clearance (CL) was predicted according to the following equation:
where IMM = 1 in patients receiving concomitant immunomodulation therapy and 0 in patients not receiving concomitant immunomodulation therapy; ATI = 1 in presence of antibodies to infliximab and 0 in patients without presence of antibodies to infliximab. Sex had no impact on clearance in the published model. Central and peripheral volumes of distribution (Vc and Vp) were predicted by weight as given by the equations:
Inter-compartmental clearance (Q) was constant (2.26 ml/kg/day). After accounting for known predictors, the remaining variation between children was described by an exponential error model for CL (% coefficient of variation [% CV] 30.7%),Vc (12.6%) and Vp (% CV 55.3%). Residual variability was captured using a combined proportional (%CV 29.2%) and additive error model (standard deviation [SD] ± 0.371 μg/ml).
For each patient, predicted infliximab concentrations were simulated using the population pharmacokinetic model and dosing history. Predictions were calculated in two ways: 1) using a patient's clinical characteristics only (i.e. population prediction) and 2) using a patient's clinical characteristics & infliximab concentrations (i.e.,individual Bayesian prediction). Concentrations were simulated only at times for which a patient had infliximab concentrations measured. The predicted concentrations were then compared to the corresponding observed infliximab concentrations.
The bias and precision of the model were assessed by calculating the median prediction error and median absolute prediction error according to the following formulas(19):
The 95% confidence interval of the median prediction error and median absolute prediction error were estimated using a bootstrap re-sampling procedure of 1000 replication datasets. The percentage of predicted concentrations within ± 1 μg/ml of the observed concentration was also calculated.
Lastly, to evaluate the model's hypothetical application in the clinical setting, the ability of the model to predict a patient's second infliximab concentration (measured after the next dose) using only the first concentration was calculated in the same manner as described above but limiting the dataset to only include the patient's first infliximab concentration when estimating a patient's individual Bayesian prediction.
Dosing Strategy and Trough Concentration Achievement
Following model evaluation, the relationship between trough concentration and dosing strategy was examined. The individual Bayesian pharmacokinetic parameter estimates for each patient were used to calculate steady-state trough concentrations after different infliximab maintenance dosing strategies. Doses of 5, 7.5, and 10 mg/kg at dosing intervals of every 4, 6, and 8 weeks were examined. Predicted infliximab trough concentrations achieved in each patient were then summarized. In addition, the percentage of children that achieved a trough concentration >3 μg/ml and >5 μg/ml were calculated for each infliximab dosing strategy. Lastly, the minimum dosing strategy for each patient that resulted in a trough concentration >3 μg/ml and >5 μg/ml was assessed. To account for both dose amount and dosing interval, dosing strategies were converted to mg/kg/week when evaluating the minimum dosing strategy. Target trough concentrations of > 3 μg/ml and > 5 μg/ml were chosen based on previous reports noting an association with treatment response.(4–10) Statistical analyses of the data and figure productions were performed using STATA 13 (StataCorp LP, College Station, TX).
Results
Patient Cohort
Overall, data from a total of 34 children with Crohn's disease were available for analysis. Demographic and clinical characteristics of the children are shown in Table 1. The median (IQR) infliximab dose was 5.0 (5.0-5.0) mg/kg and median (IQR) dosing interval was 7.9 (7.0-8.0) weeks. Three (9%) patients received a dose of 10 mg/kg, and 7 (21%) patients had a dosing interval <7 weeks. Two trough concentrations were measured in 25 (74%) children, and one trough concentration was measured in 9 (26%) children.
Table 1. Patient Characteristics (n = 34).
| Median or No. | IQR | Min, Max | |
|---|---|---|---|
| Age, years | 14.9 | 13.2 – 15.9 | 5, 17.9 |
| Weight, kg | 53 | 48 – 62 | 22, 120 |
| Female, n (%) | 13 (38%) | - | - |
| Time since diagnosis, years | 1.9 | 1.1 – 2.9 | 0.3, 7.6 |
| Time since start of IFX, wk | 29.7 | 13.9 – 56.1 | 2.0 - 174 |
| Albumin, g/dL | 4.3 | 4.0 – 4.5 | 3.4, 4.8 |
| CRP, mg/L | 2.4 | 0.9 – 4.8 | 0.2, 18.4 |
| Concomitant immunomodulation, n (%) | 15 (44%) | - | - |
| Detectable ATI, n (%) | 4 (12%) | - | - |
IQR, interquartile range; IFX, infliximab; CRP, c-reactive protein; ATI, antibodies to infliximab; Concomitant immunomodulation refers to purine-analogue or methotrexate.
Model Evaluation
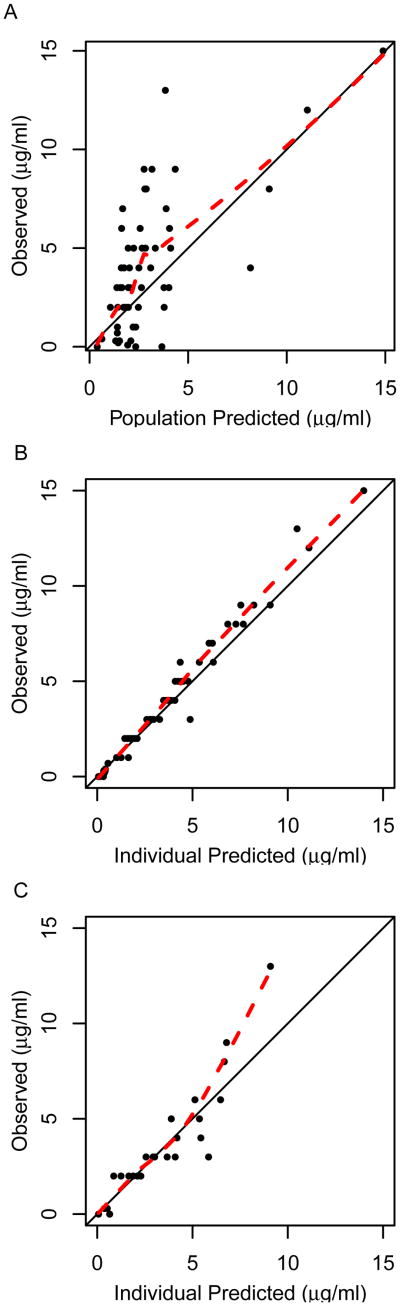
When using only a patient's clinical characteristics to predict infliximab concentration (i.e. population prediction), the pharmacokinetic model predicted the observed infliximab concentrations with low precision and accuracy (Figure 1A; Table 2). For the population predictions, the median prediction error (i.e. measure of bias) was -0.57 μg/ml (95% CI: -1.4 to 0.03 μg/ml) and the median absolute prediction error (i.e. measure of precision) was 1.3 μg/ml (95% CI: 1.0 to 1.8 μg/ml). Only 36% of population predictions were within ± 1 μg/ml of the observed concentration.
Figure 1.
Observed vs. predicted infliximab trough concentrations in a cohort of children with Crohn's disease applying a published pharmacokinetic model. A) Population predicted concentrations when using only clinical characteristics (i.e. weight, serum albumin, concomitant immunomodulation therapy, and presence of antibodies to infliximab; n=34 patients). B) Individual predicted concentrations when using clinical characteristics and all infliximab concentration data (n=34 patients). C) Individual predicted second trough concentration when using clinical characteristics and only the first trough concentration data (n=25 patients). Dashed line indicates loess smooth.
Table 2.
Predictive performance of the infliximab population pharmacokinetic model in an external cohort of children with Crohn's disease.
| Predictions using Clinical Characteristicsa Only | Predictions using Clinical Characteristicsa & IFX Concentrations | |
|---|---|---|
| Prediction errorb (μg/mL) | ||
|
| ||
| Median | -0.57 | -0.15 |
| 95% confidence interval | -1.4 to -0.03 | -0.37 to -0.05 |
|
| ||
| Percentage prediction error (%) | ||
|
| ||
| Median (%) | -13.6% | -5.6% |
| 95% confidence interval | -34.5 to -0.8% | -8.7 to -2.3% |
|
| ||
| Absolute prediction errorc (μg/mL) | ||
|
| ||
| Median | 1.3 | 0.26 |
| 95% confidence interval | 1.0 to 1.8 | 0.15 to 0.40 |
|
| ||
| Absolute percentage prediction error (%) | ||
|
| ||
| Median | 49.3% | 10.7% |
| 95% confidence interval | 41.4 to 59.7% | 7.4 to 13.3% |
IFX, Infliximab
Clinical characteristics that predicted infliximab pharmacokinetics in the model were weight, serum albumin, concomitant immunomodulation therapy, and presence of antibodies to infliximab.
Prediction error is a measure of bias of the pharmacokinetic model.
Absolute prediction error is a measure of precision of the pharmacokinetic model.
When including a patient's trough concentration(s) to predict infliximab concentration (i.e. individual Bayesian prediction), the predictive performance of the pharmacokinetic model improved and was able to adequately predict the observed infliximab concentrations (Figure 1B; Table 2). For the individual Bayesian predictions, the median prediction error was -0.15 μg/ml (95% CI: -0.37 to -0.05 μg/ml) and the median absolute prediction error was 0.26 μg/ml (95% CI: 0.15 to 0.40 μg/ml). Individual predicted concentrations were within ± 1.0 μg/ml and ± 1.5 μg/ml of the observed concentrations for 88% and 95% of measurements.
The ability of the pharmacokinetic model to predict a patient's second trough concentration using only the first trough concentration was evaluated in 25 patients (Figure 1C). The median prediction error was 0.07 μg/ml (95% CI: -0.44 to 0.29 μg/ml) and the median absolute prediction error was 0.47 μg/ml (95% CI: 0.23 to 1.1 μg/ml). Predicted concentrations were within ± 1.0 μg/ml and ± 1.5 μg/ml of the observed concentrations for 68% and 88% of patients, respectively.
Dosing Strategy and Trough Concentration Achievement
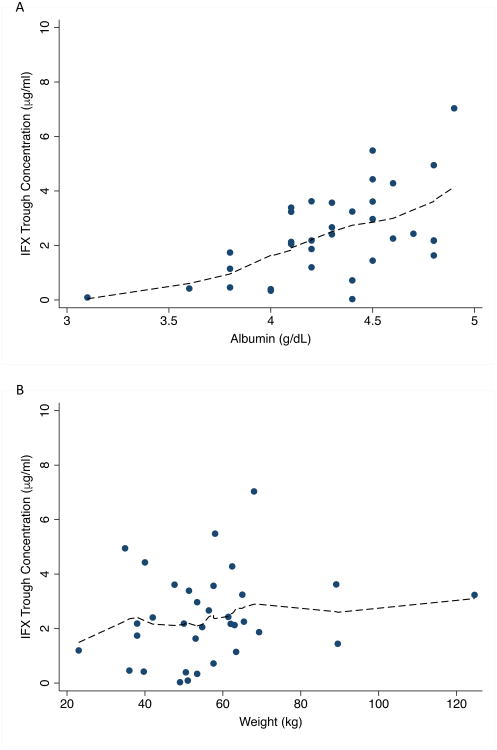
The predicted trough concentrations in each patient for various dosing strategies are shown in Table 3. At the standard infliximab maintenance dosing of 5 mg/kg every 8 weeks, the median (IQR) predicted trough concentration was 2.2 (1.2-3.4) μg/ml, and a trough concentration >3 μg/ml and >5 μg/ml was predicted to be achieved in 32% and 6% of patients, respectively. The trough concentration decreased as serum albumin decreased (Figure 2A), and no patient who had a serum albumin ≤4 g/dL (n=0/7) was predicted to achieve a trough concentration >3 μg/ml at the standard infliximab maintenance dosing of 5 mg/kg every 8 weeks. No trends in trough concentration achievement and patient weight (Figure 2B), sex (data not shown), or concomitant immunomodulatory status (data not shown) were seen.
Table 3.
Predicted infliximab trough concentrations at steady-state in children with Crohn's Disease by dosing strategy (n=34).
| Infliximab Maintenance Dosing Regimen | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 5 mg/kg | 7.5 mg/kg | 10 mg/kg | |||||||
| q 8 wk | q 6 wk | q 4 wk | q 8 wk | q 6 wk | q 4 wk | q 8 wk | q 6 wk | q 4 wk | |
| Trough, ug/ml median (IQR) | 2.2 (1.2-3.4) | 4.8 (3.0-7.1) | 11.5 (8.4-15.6) | 3.3 (1.8-5.1) | 7.2 (4.5-10.6) | 17.3 (12.7-23.4) | 4.4 (2.4-6.8) | 9.5 (6.0-14.2) | 23.0 (16.9-31.2) |
| % >3 ug/ml | 32 | 74 | 94 | 62 | 79 | 94 | 71 | 82 | 94 |
| % >5 ug/ml | 6 | 47 | 82 | 26 | 74 | 94 | 38 | 76 | 94 |
Dosing interval expressed in weeks (wk). Trough concentrations were predicted at the end of the dosing interval for a given strategy.
Figure 2.
Predicted infliximab trough concentration by (A) serum albumin and (B) weight at a dose of 5 mg/kg every 8 weeks in a cohort of children with Crohn's disease (n=34 patients). Dashed line indicates loess smooth. IFX, infliximab.
The minimum dosing strategy needed in each patient to achieve a trough >3 μg/ml and >5 μg/ml is shown in Figure 3. To achieve a trough >3 μg/ml and >5 μg/ml, a dosing interval ≤ every 6 weeks was predicted to be required in 29% and 62% of patients, respectively. Two patients did not achieve the target trough at even the highest dose examined (10 mg/kg every 4 weeks). Both patients had detectable antibodies to infliximab. In the two other patients with detectable antibodies to infliximab, the minimum dosing strategy needed to achieve a trough >3 μg/ml was 10 mg/kg every 6 weeks and 5 mg/kg every 4 weeks.
Figure 3.
Minimum infliximab dose predicted to be needed for each patient to achieve a trough concentration (A) >3 μg/ml or (B) >5 μg/ml in a cohort of children with Crohn's disease (n=34 patients). For each patient, all dosing strategies were examined and the minimum dose that resulted in a trough concentration >3 μg/ml and >5 μg/ml was selected. Two patients did not achieve the target infliximab trough concentration at even the highest dose examined 10 mg/kg every 4 weeks. Both patients had detectable antibodies for infliximab.
Discussion
In this study we demonstrate the predictive performance of a previously published population pharmacokinetic model for infliximab in a prospectively followed cohort of children with Crohn's disease. Overall, there was a high-degree of agreement between model predicted and observed infliximab concentrations, as indicated by the low prediction errors. Using only the clinical characteristics of a patient and one infliximab drug concentration, the model could predict the subsequent infliximab concentration of a patient within ± 1.0 μg/ml and ± 1.5 μg/ml of the observed concentration for 68% and 88% of patients, respectively. This study represents the first published data to support the clinical application of a model-based approach to aid in the optimization of infliximab dosing in children with Crohn's disease.
Population pharmacokinetic models offer a powerful tool to help individualize dosing based on a patient's unique pharmacokinetics.(13) The approach integrates patient specific predictors of a drug's pharmacokinetics (i.e. weight, serum albumin, concomitant immunomodulator therapy, infliximab antibody status for infliximab), dose history, and measured drug concentrations within a Bayesian framework to calculate an individualized dose most likely to achieve a defined exposure target for a patient.(20–22) Such model-based dosing has previously been shown to improve therapeutic target achievement, reduce hospital stay, reduce drug toxicity, and improve outcome when applied to other drugs.(23–25)
Similarly, there is great opportunity for model-based dosing to help advance therapeutic decision making for infliximab in patients with Crohn's disease. The pharmacokinetics of infliximab are highly variable in this population and consequently dose requirements will vary between patients.(3,11) In the cohort of children examined in the current study, the predicted infliximab maintenance dosing strategy needed to achieve a trough concentration >3 μg/ml was quite heterogeneous and ranged from the standard dosing of 5 mg/kg every 8 weeks up to 5 mg/kg every 4 weeks (Figure 3A). Our cohort was homogenous in terms of serum albumin (only 1 patient had a serum albumin < 3.5), and serum albumin is the most influential predictor of infliximab clearance and trough achievement.(11) Even greater heterogeneity in dose needs will likely be present in a larger, more diverse population. In addition, we demonstrated the potential challenges of achieving trough concentrations >3 μg/ml with even the most aggressive dosing (i.e. 10 mg/kg every 4 weeks) in children who have developed antibodies to infliximab. Taken together, more robust, model-based clinical dosing support tools are needed to help guide clinicians with dose individualization.
A challenge to date with model-based dosing is that most pharmacokinetic modeling software, such as NONMEM®, is positioned better for research applications than clinical use. Several more user-friendly pharmacokinetic software programs are available, which have focused on antibiotic dosing.(26) Explorations are underway to develop a model-based clinical dosing support tool for infliximab that highlights user experience and the potential for integration within the electronic medical record. The ability to develop and deliver a model-based dosing approach within the clinical workflow will be important for clinical adoption.
Previous retrospective and prospective studies, including randomized controlled trials, have shown the potential value of TDM to support infliximab therapeutic decision making in adults with inflammatory bowel disease.(14–16,27) Our study further highlights the value of TDM in the context of infliximab dose individualization. With the availability of just one infliximab concentration, the precision of the model improved by 60% compared to using clinical characteristics alone to predict a patient's dose-exposure relationship. Utilization of TDM will be an important clinical piece in individualizing the dosing strategy and ensuring adequate infliximab exposure within a model-based framework.
A current challenge in the application of TDM in the clinical setting is the delayed turnaround time in measuring infliximab concentrations. The current commercially available assays are often send out laboratories (e.g. PROMETHEUS® Anser™ IFX) and can take several days to come back. As a result, the infliximab concentration is not available to guide dosing decisions when drawn on the same day as the infusion. An advantage of model-based dosing is that the approach is robust in terms of the timing of when concentrations are measured and is not constrained to utilizing only a trough concentration. Therefore, the pharmacokinetics of a patient may be estimated using an infliximab concentration measured several days to even weeks before the infusion. This would allow for the result to come back, and the individualized dose to then be calculated prior to the infusion. Nonetheless, even this approach is logistically cumbersome and not ideal from a patient convenience standpoint. In addition, further prospective validation of this approach would be necessary. The recent development of a bedside point-of-care infliximab assay is promising and would alleviate many of these challenges.(28).
In our current study, we selected a trough concentration target >3 μg/ml along with a more aggressive target > 5 μg/ml based on current evidence noting an association with treatment response.(4–10) However, the precise trough concentration to target in children with Crohn's disease is still not clear. The model-based approach is agnostic to the specific trough concentration target chosen, and the clinician has the ability to adjust the target concentration. This flexibility will also allow for ongoing individualization as more specific trough concentration targets are established in patient subtypes. In addition, as new and innovative pharmacodynamic biomarkers of response are developed in inflammatory bowel disease,(29) our work serves as a framework to incorporate such pharmacodynamic biomarkers. The model-based approach can be continuously updated to reflect the current treatment goals and further advance individualized dosing strategies.
A limitation of the current study is the relatively small sample size of the cohort of children with Crohn's disease available for validation. The true variation and distribution of potential children with Crohn's disease was likely not captured. Nonetheless, external validation is the most rigorous method for testing a developed model.(30) Future real-world applications of the population pharmacokinetic model are necessary to assess the robustness of predicted infliximab concentrations in diverse inflammatory bowel disease populations. For example, there appears to be a slight trend at higher concentrations for the model to under predict, and the ability of the model to predict concentrations in patients with very low (or high) infliximab clearance warrants further evaluation. In addition, since the pharmacokinetics of infliximab in children with Crohn's disease is unique and complex, performance of the model in adult Crohn's disease patients and ulcerative colitis patients, in general, is yet to be determined but underway.
In conclusion, our study further supports the clinical rationale to individualize infliximab dosing in patients with Crohn's disease. A one-size-fits-all infliximab dosing strategy results in sub-therapeutic infliximab trough levels in the majority of Crohn's disease patients. Individualization of infliximab dosing will be critical to consistently achieve trough concentrations associated with optimal outcomes. The application of a population pharmacokinetic model to aid in the optimization of infliximab dosing in children with Crohn's disease was also supported. Incorporation of such a model based approach into a robust clinical dosing support tool can help guide clinicians with dose individualization.
What is Known.
Infliximab pharmacokinetics are highly variable in children with Crohn's disease (CD), and a one-size-fits-all dosing will lead to large differences in drug exposure.
Population pharmacokinetic models and therapeutic drug monitoring can guide individualized dosing strategies to ensure adequate drug exposure.
What's New Here.
We evaluated the predictive performance of a previously published population pharmacokinetic model in 34 CD children receiving infliximab who had drug concentrations measured.
The pharmacokinetic model predicted infliximab trough concentrations within ±1.0 μg/ml of actual infliximab trough concentrations for 88% of measurements.
Standard infliximab 5mg/kg every 8 weeks dosing predicted a trough concentration >3 μg/ml in only 32% of patients — necessitating increased dosing or frequency in the majority of patients to achieve adequate drug exposure.
Acknowledgments
Conflict of Interest and Source of Funding: KTP has received research support from AbbVie, Janssen, and Takeda, outside the submitted work. MAB has received research support from Janssen Biologics, outside the submitted work. AK has received research support from Janssen Biologics, outside the submitted work. AF is supported in part by the National Institutes of Health (K23 HD079557) for this work. KP is supported in part by the National Institutes of Health (K08 DK094868) for this work.
Footnotes
Author contributions: AF: designed the study, performed analyses, interpreted the data, and drafted the manuscript.
KTP: designed the study, interpreted the data, and drafted the manuscript.
DRH: designed the study, made substantial contributions to the acquisition and interpretation of data, and revised the manuscript critically for intellectual content.
TLP: made substantial contributions to the interpretation of data and revised the manuscript critically for intellectual content.
TGM, TZH, MAB, AK: made substantial contributions to the acquisition of data and revised the manuscript critically for intellectual content.
All authors approved the final version of the manuscript.
References
- 1.Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002 May 4;359(9317):1541–9. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 2.Hyams J, Crandall W, Kugathasan S, et al. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn's disease in children. Gastroenterology. 2007 Mar;132(3):863–873. 1166. doi: 10.1053/j.gastro.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Fasanmade AA, Adedokun OJ, Blank M, Zhou H, Davis HM. Pharmacokinetic properties of infliximab in children and adults with Crohn's disease: a retrospective analysis of data from 2 phase III clinical trials. Clin Ther. 2011 Jul;33(7):946–64. doi: 10.1016/j.clinthera.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Singh N, Rosenthal CJ, Melmed GY, et al. Early infliximab trough levels are associated with persistent remission in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2014 Oct;20(10):1708–13. doi: 10.1097/MIB.0000000000000137. [DOI] [PubMed] [Google Scholar]
- 5.Bortlik M, Duricova D, Malickova K, et al. Infliximab trough levels may predict sustained response to infliximab in patients with Crohn's disease. J Crohns Colitis. 2013 Oct;7(9):736–43. doi: 10.1016/j.crohns.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 6.Vande Casteele N, Khanna R, Levesque BG, et al. The relationship between infliximab concentrations, antibodies to infliximab and disease activity in Crohn's disease. Gut. 2015 Oct;64(10):1539–45. doi: 10.1136/gutjnl-2014-307883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki Y, Matsui T, Ito H, et al. Circulating Interleukin 6 and Albumin, and Infliximab Levels Are Good Predictors of Recovering Efficacy After Dose Escalation Infliximab Therapy in Patients with Loss of Response to Treatment for Crohn's Disease: A Prospective Clinical Trial. Inflamm Bowel Dis. 2015 Sep;21(9):2114–22. doi: 10.1097/MIB.0000000000000475. [DOI] [PubMed] [Google Scholar]
- 8.Cornillie F, Hanauer SB, Diamond RH, et al. Postinduction serum infliximab trough level and decrease of C-reactive protein level are associated with durable sustained response to infliximab: a retrospective analysis of the ACCENT I trial. Gut. 2014 Nov;63(11):1721–7. doi: 10.1136/gutjnl-2012-304094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levesque BG, Greenberg GR, Zou G, et al. A prospective cohort study to determine the relationship between serum infliximab concentration and efficacy in patients with luminal Crohn's disease. Aliment Pharmacol Ther. 2014 May;39(10):1126–35. doi: 10.1111/apt.12733. [DOI] [PubMed] [Google Scholar]
- 10.Ungar B, Levy I, Yavne Y, et al. Optimizing Anti-TNF-α Therapy: Serum Levels of Infliximab and Adalimumab Are Associated With Mucosal Healing in Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol. 2016;14(4):550–557. doi: 10.1016/j.cgh.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 11.Frymoyer A, Piester TL, Park KT. Infliximab Dosing Strategies and Predicted Trough Exposure in Children With Crohn Disease. J Pediatr Gastroenterol Nutr. 2016 May;62(5):723–7. doi: 10.1097/MPG.0000000000001123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoekman DR, Brandse JF, de Meij TG, et al. The association of infliximab trough levels with disease activity in pediatric inflammatory bowel disease. Scand J Gastroenterol. 2015 Sep;50(9):1110–7. doi: 10.3109/00365521.2015.1027264. [DOI] [PubMed] [Google Scholar]
- 13.Mould DR, D'Haens G, Upton RN. Clinical Decision Support Tools: The Evolution of a Revolution. Clin Pharmacol Ther. 2016 Apr;99(4):405–18. doi: 10.1002/cpt.334. [DOI] [PubMed] [Google Scholar]
- 14.Warman A, Straathof JWA, Derijks LJJ. Therapeutic drug monitoring of infliximab in inflammatory bowel disease patients in a teaching hospital setting: results of a prospective cohort study. Eur J Gastroenterol Hepatol. 2015 Mar;27(3):242–8. doi: 10.1097/MEG.0000000000000279. [DOI] [PubMed] [Google Scholar]
- 15.Steenholdt C, Brynskov J, Thomsen OØ, et al. Individualized Therapy Is a Long-Term Cost-Effective Method Compared to Dose Intensification in Crohn's Disease Patients Failing Infliximab. Dig Dis Sci. 2015;60(9):2762–70. doi: 10.1007/s10620-015-3581-4. [DOI] [PubMed] [Google Scholar]
- 16.Vande Casteele N, Ferrante M, Van Assche G, et al. Trough Concentrations of Infliximab Guide Dosing for Patients With Inflammatory Bowel Disease. Gastroenterology. 2015;148(7):1320–9. doi: 10.1053/j.gastro.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 17.Ding NS, Hart A, De Cruz P. Systematic review: predicting and optimising response to anti-TNF therapy in Crohn's disease - algorithm for practical management. Aliment Pharmacol Ther. 2016 Jan;43(1):30–51. doi: 10.1111/apt.13445. [DOI] [PubMed] [Google Scholar]
- 18.Wolbink GJ, Vis M, Lems W, et al. Development of antiinfliximab antibodies and relationship to clinical response in patients with rheumatoid arthritis. Arthritis Rheum. 2006 Mar;54(3):711–5. doi: 10.1002/art.21671. [DOI] [PubMed] [Google Scholar]
- 19.Sheiner LB, Beal SL. Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm. 1981 Aug;9(4):503–12. doi: 10.1007/BF01060893. [DOI] [PubMed] [Google Scholar]
- 20.Sheiner LB, Beal S, Rosenberg B, Marathe VV. Forecasting individual pharmacokinetics. Clin Pharmacol Ther. 1979 Sep;26(3):294–305. doi: 10.1002/cpt1979263294. [DOI] [PubMed] [Google Scholar]
- 21.Sheiner LB, Rosenberg B, Melmon KL. Modelling of individual pharmacokinetics for computer-aided drug dosage. Comput Biomed Res. 1972 Oct;5(5):411–59. doi: 10.1016/0010-4809(72)90051-1. [DOI] [PubMed] [Google Scholar]
- 22.Sheiner LB, Beal SL. Bayesian individualization of pharmacokinetics: Simple implementation and comparison with non-Bayesian methods. J Pharm Sci. 1982 Dec 1;71(12):1344–8. doi: 10.1002/jps.2600711209. [DOI] [PubMed] [Google Scholar]
- 23.Evans WE, Relling MV, Rodman JH, Crom WR, Boyett JM, Pui CH. Conventional compared with individualized chemotherapy for childhood acute lymphoblastic leukemia. N Engl J Med. 1998 Feb 19;338(8):499–505. doi: 10.1056/NEJM199802193380803. [DOI] [PubMed] [Google Scholar]
- 24.Tobler A, Mühlebach S. Intravenous phenytoin: a retrospective analysis of Bayesian forecasting versus conventional dosing in patients. Int J Clin Pharm. 2013 Oct;35(5):790–7. doi: 10.1007/s11096-013-9809-5. [DOI] [PubMed] [Google Scholar]
- 25.van Lent-Evers NA, Mathôt RA, Geus WP, van Hout BA, Vinks AA. Impact of goal-oriented and model-based clinical pharmacokinetic dosing of aminoglycosides on clinical outcome: a cost-effectiveness analysis. Ther Drug Monit. 1999 Feb;21(1):63–73. doi: 10.1097/00007691-199902000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Roberts JA, Abdul-Aziz MH, Lipman J, et al. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis. 2014 Jun;14(6):498–509. doi: 10.1016/S1473-3099(14)70036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Billiet T, Cleynen I, Ballet V, et al. Prognostic factors for long-term infliximab treatment in Crohn's disease patients: a 20-year single centre experience. Aliment Pharmacol Ther. 2016 Oct;44(7):673–83. doi: 10.1111/apt.13754. [DOI] [PubMed] [Google Scholar]
- 28.Afonso J, Lopes S, Gonçalves R, et al. Proactive therapeutic drug monitoring of infliximab: a comparative study of a new point-of-care quantitative test with two established ELISA assays. Aliment Pharmacol Ther. 2016;44(7):684–92. doi: 10.1111/apt.13757. [DOI] [PubMed] [Google Scholar]
- 29.Heier CR, Fiorillo AA, Chaisson E, et al. Identification of Pathway-Specific Serum Biomarkers of Response to Glucocorticoid and Infliximab Treatment in Children with Inflammatory Bowel Disease. Clin Transl Gastroenterol. 2016;7(9):e192. doi: 10.1038/ctg.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sherwin CMT, Kiang TKL, Spigarelli MG, Ensom MHH. Fundamentals of population pharmacokinetic modelling: validation methods. Clin Pharmacokinet. 2012 Sep 1;51(9):573–90. doi: 10.1007/BF03261932. [DOI] [PubMed] [Google Scholar]