Abstract
Background
Despite extensive research regarding risk factors for adverse events after total joint arthroplasty (TJA), there are few publications describing the timing at which such adverse events occur.
Questions/purposes
(1) On which postoperative day do certain adverse events occur? (2) What adverse events occur earlier after TKA than after THA? (3) For each adverse event, what proportion occurred after hospital discharge?
Methods
We screened the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) to identify all patients undergoing primary THA and primary TKA between 2005 and 2013, resulting in a study population of 124,657 patients evaluated as part of this retrospective database analysis. For each of eight different adverse events, the median postoperative day of diagnosis, interquartile range for day of diagnosis, and middle 80% for day of diagnosis were determined. Multivariate Cox proportional hazards modeling was used to test whether there is a difference of timing for each adverse event as stratified by TKA or THA. The proportion of adverse events occurring after versus before discharge was also calculated.
Results
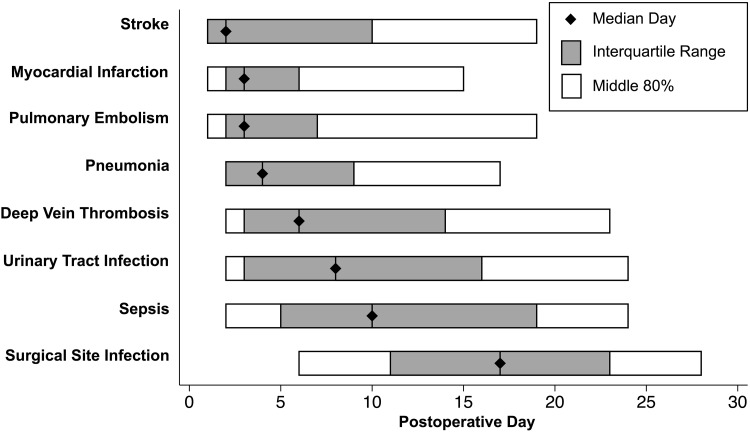
The median day of diagnosis (and interquartile range; middle 80%) for stroke was 2 (1–10; 1–19), myocardial infarction 3 (2–6; 1–15), pulmonary embolism 3 (2–7; 1–19), pneumonia 4 (2–9; 2–17), deep vein thrombosis 6 (3–14; 2–23), urinary tract infection 8 (3–16; 2–24), sepsis 10 (5–19; 2–24), and surgical site infection 17 (11–23; 6–28). For the later occurring adverse events (surgical site infection, sepsis), the rate of occurrence remained high at the end of the 30-day postoperative period. Timing was earlier in patients undergoing TKA for pulmonary embolism (day 3 [interquartile range 2–6] versus 5 [3–17], p < 0.001) and deep vein thrombosis (day 5 [2–11] versus 13 [6–22], p < 0.001). The proportion of events occurring after discharge for myocardial infarction was 97 of 283 (34%), stroke 42 of 118 (36%), pulmonary embolism 223 of 625 (36%), pneumonia 171 of 426 (40%), deep vein thrombosis 576 of 956 (60%), urinary tract infection 958 of 1406 (68%), sepsis 284 of 416 (68%), and surgical site infection 1147 of 1212 (95%).
Conclusions
As lengths of hospital stay after TJA continue to decrease, our findings suggest that caution is in order because several acute and immediately life-threatening findings, including myocardial infarction and pulmonary embolism, might occur after discharge. Furthermore, the timing of surgical site infection and sepsis suggests that even the 30-day followup afforded by the ACS-NSQIP may not be sufficient to study the latest occurring adverse events. Additionally, both pulmonary embolism and deep vein thrombosis tend to occur earlier after TKA than THA, and this should guide clinical surveillance efforts in patients undergoing those procedures. These findings also indicate that inpatient-only databases (such as the Nationwide Inpatient Sample) may fail to capture a very large proportion of postoperative adverse events, weakening the conclusions of many published studies using those databases.
Level of Evidence
Level III, therapeutic study.
Keywords: Urinary Tract Infection, Deep Vein Thrombosis, Total Joint Arthroplasty, Periprosthetic Joint Infection, Nationwide Inpatient Sample
Introduction
Long lists of risk factors have been identified for each of the common adverse events after total joint arthroplasty (TJA), including, for example, venous thromboembolism [16, 17, 21, 24, 31, 36], surgical site infection [4, 7, 10, 30], and myocardial infarction [1, 13, 15, 18, 35]. Although risk factors for postoperative adverse events are critical to understand, an important dimension that has received far less attention is the timing of adverse events. In terms of existing research, Parvizi and colleagues described a group of 1,636 patients undergoing either primary THA or THA and found that 90% of major complications occurred within 4 days of the index surgery [23]. Pulido et al. examined 9245 patients undergoing primary hip or knee arthroplasty from a local data set with 12 months of followup and found that 27% of periprosthetic joint infections were diagnosed within the first 30 days after arthroplasty [28]. Finally, Mantilla and colleagues examined 10,244 patients undergoing primary THA or TKA and discovered that the median times to myocardial infarction, pulmonary embolism, and death after the index procedures were 1, 4, and 9 days, respectively [16]. Although the aforementioned recent studies have been helpful in providing valuable information regarding the timing of complications after TJA, the results are limited by relatively small numbers, local cohorts, or cursory analyses affecting the power, generalizability, and breadth of the conclusions.
A better understanding of the timing of adverse events after TJA would allow surgeons to lower the thresholds for postoperative diagnostic testing during the time periods of greatest risk. Additionally, such information has the potential to guide research in terms of what duration postoperatively patients need to be followed to capture the occurrence of specific adverse events. Perhaps most importantly, the length of hospital stay after TJA has been decreasing with much recent research focused on the safety of rapid recovery protocols and outpatient procedures in both THA and TKA [11, 14, 20]. However, the timing of adverse events is an important consideration in the safety of these protocols because acute and life-threatening complications, including myocardial infraction and pulmonary embolism, may be more quickly diagnosed and effectively treated if the patient has yet to be discharged.
Therefore, we asked: (1) On which postoperative day do certain adverse events occur? (2) What adverse events occur earlier after TKA than after THA? (3) For each adverse event, what proportion occurred after hospital discharge?
Materials and Methods
A retrospective comparative study was conducted using the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database [12, 19, 32, 33]. The ACS-NSQIP is a surgical registry in which patients undergoing major surgical procedures at several hundred institutions nationwide are identified preoperatively and then followed postoperatively for the development of postoperative adverse events. Highly trained surgical clinical reviewers review inpatient and outpatient records at the end of a 30-day postoperative followup period for the occurrence of any of these events. In cases in which occurrence is unclear, ACS-NSQIP reviewers will contact either the patients or the providers to clarify. The program has undergone regular rigorous review and has been demonstrated to have a high degree of reliability [33]. It has now been used extensively in orthopaedic and particularly in TJA research [2, 3, 8, 19].
After obtaining approval from our institutional review board, the 2005 to 2013 ACS-NSQIP database was accessed. Patients undergoing either primary THA or primary TKA were identified using Current Procedural Terminology codes (27130 for THA, 27447 for TKA). In total, 146,553 cases were initially identified. However, cases were excluded if there was any indication that they may not be a pure, simple, elective primary TJA (for example, coding included hardware removal, major ligament reconstruction, acute trauma, additional unrelated procedures, preoperative infection, etc). Hence, 124,657 of the 146,553 patients (85.1%) undergoing TJA met inclusion criteria (Table 1). Of these, 48,076 (38.6%) underwent THA and 76,581 (61.4%) underwent TKA. The following adverse events were analyzed: urinary tract infection, surgical site infection, deep vein thrombosis, pulmonary embolism, pneumonia, sepsis, myocardial infarction, and stroke (Table 2).
Table 1.
Patient population
| Population characteristics | Number | Percent |
|---|---|---|
| Total | 124,657 | 100 |
| Age | ||
| 18–59 years | 31,997 | 26 |
| 60–69 years | 43,627 | 35 |
| 70–79 years | 34,642 | 28 |
| ≥ 80 years | 14,391 | 12 |
| Sex | ||
| Male | 49,559 | 40 |
| Female | 75,098 | 60 |
| Body mass index | ||
| ≤ 24.9 kg/m2 | 18,433 | 15 |
| 25.0–29.9 kg/m2 | 38,142 | 31 |
| 30.0–34.9 kg/m2 | 33,445 | 27 |
| ≥ 35.0 kg/m2 | 34,637 | 28 |
| Functional status | ||
| Independent | 121,696 | 98 |
| Dependent | 2961 | 2 |
| Diabetes | 17,484 | 14 |
| Dyspnea on exertion | 8304 | 7 |
| Hypertension | 78,851 | 63 |
| COPD | 4688 | 4 |
| Current smoker | 67,712 | 10 |
| Procedure | ||
| THA | 48,076 | 36 |
| TKA | 76,581 | 61 |
| Anesthesia | ||
| Regional | 56,658 | 46 |
| General | 67,712 | 54 |
COPD = chronic obstructive pulmonary disease.
Table 2.
Adverse event rates (N = 124,657)
| Adverse event | Number | Percent | 95% CI |
|---|---|---|---|
| Urinary tract infection | 1406 | 1.13 | 1.07–1.19 |
| Surgical site infection | 1212 | 0.97 | 0.92–1.03 |
| Deep vein thrombosis | 956 | 0.77 | 0.72–0.82 |
| Pulmonary embolism | 625 | 0.50 | 0.46–0.54 |
| Pneumonia | 426 | 0.34 | 0.31–0.37 |
| Sepsis | 416 | 0.33 | 0.30–0.37 |
| Myocardial infarction | 283 | 0.23 | 0.20–0.25 |
| Stroke | 118 | 0.09 | 0.08–0.11 |
CI = confidence interval.
First, for each adverse event, the following was determined: the rate of occurrence in the first 30 postoperative days, the median postoperative day of diagnosis, and associated interquartile range and middle 80%. Second, differences in timing for each adverse event as stratified by TKA or THA were analyzed. This relied on multivariate Cox proportional hazards modeling to test for demographic, comorbidity, and procedural associations with early versus late occurrence during the 30-day postoperative interval. Each multivariate model included the following predictor variables: age, sex, body mass index, functional status (independent versus dependent), performance of TKA versus THA, use of general versus regional anesthesia, current smoking status, and the presence of diabetes, dyspnea on exertion, hypertension, and chronic obstructive pulmonary disease. Independent functional status is defined as the ability to perform all activities of daily living without the assistance of another person in the 30 days before surgery. Dependent functional status is defined as some or total assistance in completing some or all activities of daily living in the 30 days before surgery. Finally, the proportion of adverse events occurring before versus after discharge were calculated. The level of significance was set at p < 0.05.
Results
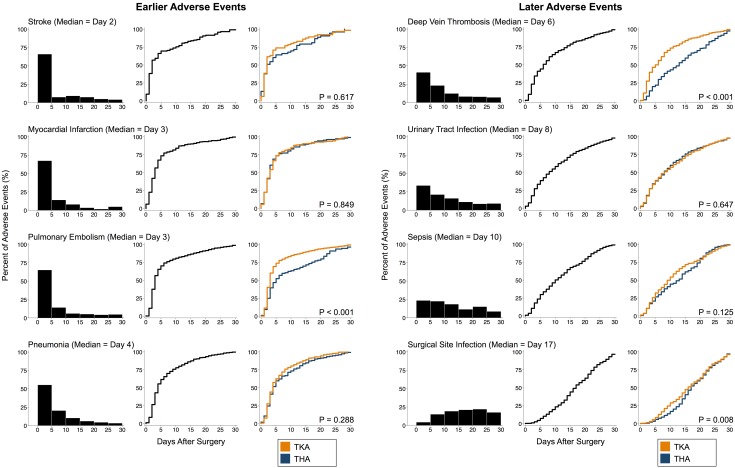
The median day of diagnosis (and interquartile range; middle 80%; Fig. 1) for stroke was 2 (1–10; 1–19), myocardial infarction 3 (2–6; 1–15), pulmonary embolism 3 (2–7; 1–19), pneumonia 4 (2–9; 2–17), deep vein thrombosis 6 (3–14; 2–23), urinary tract infection 8 (3–16; 2–24), sepsis 10 (5–19; 2–24), and surgical site infection 17 (11–23; 6–28). For the earliest occurring adverse events (stroke, myocardial infarction), the rate of occurrence had nearly plateaued by the end of the 30-day postoperative period. In contrast, for the latest occurring adverse events (eg, surgical site infection, sepsis), the rate of occurrence remained high at the end of the 30-day postoperative period (Fig. 2).
Fig. 1.
This figure displays the summary statistics for the timing of adverse events. The median day of diagnosis (and interquartile range; middle 80%) for stroke was 2 (1–10; 1–19), myocardial infarction 3 (2–6; 1–15), pulmonary embolism 3 (2–7; 1–19), pneumonia 4 (2–9; 2–17), deep vein thrombosis 6 (3–14; 2–23), urinary tract infection 8 (3–16; 2–24), sepsis 10 (5–19; 2–24), and surgical site infection 17 (11–23; 6–28).
Fig. 2.
This figure displays the histograms and timing curves for the timing of adverse events. For each adverse event, the left most graph is the histogram depicting the percentage of the total number of adverse events that occur in each 5-day interval, the middle graph is the timing curve representing the cumulative percentage of adverse events that have occurred by day for all patients, and the right graph is the timing curves displaying the cumulative percentage of adverse events that have occurred by day stratified by TKA and THA. Probability values in the right-sided timing curves are results from the multivariate Cox proportional hazards models that compared timing between THA and TKA while controlling for all other factors. Timing did not differ between THA and TKA with three exceptions: for pulmonary embolism, timing was earlier for TKA (day 3 versus 5, p < 0.001); for deep vein thrombosis, timing was earlier for TKA (day 5 versus 13, p < 0.001); and for surgical site infection, timing was earlier for TKA (day 16 versus 17, p = 0.008).
Timing was earlier in patients undergoing TKA for pulmonary embolism (day 3 [interquartile rage 2–6] versus 5 [3–17], p < 0.001) and deep vein thrombosis (day 5 [2–11] versus 13 [6–22], p < 0.001) (Table 3). Some adverse events were associated with certain baseline characteristics. For myocardial infarction, earlier occurrence was associated with female sex (median of 2 versus 3 days, p < 0.001). For pulmonary embolism, earlier occurrence was also associated with independent functional status (3 versus 16 days, p = 0.004). For pneumonia, earlier occurrence was associated with) the use of regional anesthesia (3 versus 9 days, p = 0.001). For urinary tract infection, earlier occurrence was associated with obesity (7 versus 9 days, p < 0.004). There were no associations with the timing of stroke or sepsis.
Table 3.
Timing of adverse events by baseline characteristics*
| Adverse event and baseline characteristic | Median day without characteristic (and interquartile range) | Median day with characteristic (and interquartile range) | p value |
|---|---|---|---|
| Stroke | |||
| No associations | – | – | – |
| Myocardial infarction | |||
| Female | 3 (2–8) | 2 (1–4) | < 0.001 |
| Pulmonary embolism | |||
| Dependent status (versus independent) | 3 (2–7) | 16 (7–20) | 0.004 |
| TKA† | 5 (3–17) | 3 (2–6) | < 0.001 |
| Pneumonia | |||
| Dyspnea on exertion | 6 (3–12) | 5 (3–11) | 0.002 |
| General anesthesia (versus regional) | 3 (2–8) | 9 (3–15) | 0.001 |
| Deep vein thrombosis | |||
| TKA† | 13 (6–22) | 5 (2–11) | < 0.001 |
| Urinary tract infection | |||
| Obese (BMI ≥ 30 kg/m2) | 9 (4–17) | 7 (3–15) | 0.004 |
| Sepsis | |||
| No associations | – | – | – |
| Surgical site infection | |||
| Obese (BMI ≥ 30 kg/m2) | 15 (9–22) | 17 (12–23) | < 0.001 |
| TKA† | 17 (13–23) | 16 (10–23) | 0.008 |
* Only statistically significant associations are shown; †in comparison with THA; BMI = body mass index.
The proportion of events occurring after discharge for myocardial infarction was 97 of 283 (34%), stroke 42 of 118 (36%), pulmonary embolism 223 of 625 (36%), pneumonia 171 of 426 (40%), deep vein thrombosis 576 of 956 (60%), urinary tract infection 958 of 1406 (68%), sepsis 284 of 416 (68%), and surgical site infection 1147 of 1212 (95%).
Discussion
Although risk factors for postoperative adverse events are critical to understand, an important dimension that has received less attention is the timing of when the most common and most severe adverse events actually occur in the days and weeks after elective arthroplasty. A better understanding of the timing of adverse events after TJA would allow surgeons to lower the thresholds for postoperative diagnostic testing during the time periods of greatest risk. Additionally, such information has the potential to guide research in terms of for what duration postoperatively patients need to be followed to capture the occurrence of specific adverse events. Finally, the trend toward rapid recovery protocols and outpatient joint arthroplasty could impact the location of the patient during acute and life-threatening complications, including myocardial infarction and pulmonary embolism [11, 14, 20]. Knowing when these events occur could help to guide timing of discharge. Therefore, we aimed to characterize the timing of eight different postoperative adverse events after THA and TKA, to compare the timing between procedures, and to identify what proportion of adverse events occur after discharge.
Limitations of the present study are those of the ACS-NSQIP. First, the ACS-NSQIP only follows patients for 30 days; hence, the occurrence of adverse events beyond the 30-day postoperative period could not be investigated. This does not present a problem for the earlier occurring adverse events; however, it would have been helpful to see the timing curves extend out further for the later occurring adverse events, most notably surgical site infection, sepsis, and urinary tract infection. Second, the ACS-NSQIP does not collect data on orthopaedic-specific adverse events such as dislocation, loosening, and catastrophic failure; instead, the program focuses on short-term, general medical/surgical adverse events. Additionally, the ACS-NSQIP does not collect data on patient-reported outcomes, so the timing of improvement in such outcomes (such as pain scores and Knee Society Score) could not be characterized. ACS-NSQIP, like all national data sets, must be used with caution with respect to the accuracy of the coding. However, the use of highly trained clinical reviewers for data abstraction combined with regular quality reviews has been shown to be effective in producing reliable data [33]. Finally, being retrospective in nature and largely focused on the timing of adverse events, these data cannot support any specific clinical algorithm for the diagnosis or treatment of adverse events.
The major implications of the present study are to urge caution in the adoption of decreasing lengths of hospital stay after TJA. Rapid recovery protocols and outpatient procedures have been receiving more attention in part as a result of improvements in minimally invasive techniques, changing reimbursements, and rising patient expectations. However, the median time of acute and life-threatening adverse events, including myocardial infarction and pulmonary embolism, is on the third day. Patients discharged rapidly are at risk of delayed diagnosis and treatment of these conditions in comparison with those who are still hospitalized. Additionally, the results of the current study direct clinical practice in that surgeons should have the lowest thresholds for ordering postoperative tests during the time periods of greatest risk. For example, a postoperative stroke is far more likely to occur during the several days after surgery than during postoperative days 15 to 30. Hence, the threshold for ordering head CT and/or neurology consult should be much lower during these early days. Similarly, pretest probabilities should be altered accordingly with an increase in the pretest probability for each adverse event during the time period identified here to be of greatest risk. Finally, the findings regarding the latest occurring adverse events (sepsis and surgical site infection) indicate that, although the ACS-NSQIP is routinely used to study adverse events [8, 27, 34], it may not be best to study the latest occurring events because it does not account for occurrences after 30 days.
It was interesting that thromboembolic events (deep vein thrombosis and pulmonary embolism) tended to occur substantially earlier after TKA than THA. This has been demonstrated on one other occasion with respect to pulmonary embolism (but not deep vein thrombosis) [22] and is confirmed here. The cause for this difference is not known. We speculate that it could be related to differences in patient positioning or the use of a tourniquet intraoperatively with knee flexion during portions of the case or the use of a tourniquet predisposing to venous compression and thrombosis. That is, as a result of increased venous compression during TKA compared with THA, a higher proportion of venous thromboembolic events may be initiated intraoperatively and thus be seen earlier during the postoperative course. A second difference in timing that was noted was the substantially later occurrence of pulmonary embolism in patients with dependent functional status. To our knowledge, this has not been previously reported. We speculate that this difference may be related to the fact that for many patients, thrombosis may initiate intraoperatively; however, patients with dependent functional status likely spend increased postoperative time immobilized, predisposing them to postoperative initiation of venous thrombosis. As a result, a greater proportion of pulmonary emboli in those with functional dependence may be observed later in the postoperative course. Finally, the reader might note that we did observe a difference in 1 day in the timing of surgical site infection between THA and TKA; although statistically significant, this difference was not thought to be clinically significant.
Databases that only capture inpatient adverse events may inadequately reflect actual occurrence, particular for deep vein thrombosis, urinary tract infection, surgical site infection, and sepsis because more than half of these events occurred after discharge. This is a particularly important consideration given the increase in the use of inpatient-only data collection programs to study the occurrence of adverse events [2, 3, 25, 27, 29]. An example of such a program is the Nationwide Inpatient Sample (NIS), which samples hospitals across the country and captures data regarding procedures and adverse events during the inpatient stay. Hence, databases such as NIS fail to capture more than half of these adverse events. Nevertheless, these adverse events remain commonly investigated using inpatient-only data [5–7, 9, 26, 27, 29].
In conclusion, the findings of this manuscript emphasize caution in adopting ever increasing shortening lengths of hospital stay after TJA because the occurrence of life-threatening and treatable complications will more often occur in the outpatient setting, potentially leading to delayed diagnosis and management. Additionally, these data can be applied immediately in the guidance of postoperative diagnostic testing when there is suspicion for one of these eight events. Specifically, thresholds for diagnostic testing should be lowered and pretest probabilities should be raised for each adverse event during the time periods identified here to be of greatest risk. Moving forward, future research may focus on reasons for the earlier occurrence of thromboembolism after TKA in comparison with THA as well as on implications of the use of data sets that do not collect postdischarge information.
Footnotes
One of the authors (DDB) reports the following financial activities outside the submitted work: current grant with the Cervical Spine Research Society (Rosemont, IL, USA). One of the authors (BRL) reports the following financial activities outside the submitted work: current consultancy with Zimmer (Newington, CT, USA), Link (Rockaway, NJ, USA), McGraw-Hill (New York, NY, USA), Janssen (Titusville, NJ, USA), Krames (Waterbury, CT, USA), Human Kinetics (Champaign, IL, USA), and Slack Inc (Thorofare, NJ, USA). One of the authors (JNG) reports the following financial activities outside the submitted work: current consultancy with Bioventus (Durham, NC, USA), ISTO Technologies (St Louis, MO, USA), Medtronic (Minneapolis, MN, USA), Stryker (Mahwah, NJ, USA), Novella Clinical (Morrisville, NC, USA), Andante Medical Devices (White Plains, NY, USA), and Vertex (Minneapolis, MN, USA); ongoing expert testimony with legal case reviews; and a current grant with the Orthopaedic Trauma Association (Rosemont, IL, USA).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his institution approved or waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the Rush University Medical Center, Chicago, IL, USA; and the Yale School of Medicine, New Haven, CT, USA.
A comment to this article is available at https://doi.org/10.1007/s11999-017-5261-5.
References
- 1.Belmont PJ, Jr, Goodman GP, Kusnezov NA, Magee C, Bader JO, Waterman BR, Schoenfeld AJ. Postoperative myocardial infarction and cardiac arrest following primary total knee and hip arthroplasty: rates, risk factors, and time of occurrence. J Bone Joint Surg Am. 2014;96:2025–2031. doi: 10.2106/JBJS.N.00153. [DOI] [PubMed] [Google Scholar]
- 2.Bohl DD, Basques BA, Golinvaux NS, Baumgaertner MR, Grauer JN. Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472:1672–1680. doi: 10.1007/s11999-014-3559-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bohl DD, Russo GS, Basques BA, Golinvaux NS, Fu MC, Long WD, 3rd, Grauer JN. Variations in data collection methods between national databases affect study results: a comparison of the Nationwide Inpatient Sample and National Surgical Quality Improvement Program databases for lumbar spine fusion procedures. J Bone Joint Surg Am. 2014;96:e193. doi: 10.2106/JBJS.M.01490. [DOI] [PubMed] [Google Scholar]
- 4.Bohl DD, Shen MR, Kayupov E, Della Valle CJ. Hypoalbuminemia independently predicts surgical site infection, pneumonia, length of stay, and readmission after total joint arthroplasty. J Arthroplasty. 2016;31:15–21. doi: 10.1016/j.arth.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 5.Browne JA, Novicoff WM, D’Apuzzo MR. Medicaid payer status is associated with in-hospital morbidity and resource utilization following primary total joint arthroplasty. J Bone Joint Surg Am. 2014;96:e180. doi: 10.2106/JBJS.N.00133. [DOI] [PubMed] [Google Scholar]
- 6.Cavanaugh PK, Chen AF, Rasouli MR, Post ZD, Orozco FR, Ong AC. Total joint arthroplasty in transplant recipients: in-hospital adverse outcomes. J Arthroplasty. 2015;30:840–845. doi: 10.1016/j.arth.2014.11.037. [DOI] [PubMed] [Google Scholar]
- 7.Crowe B, Payne A, Evangelista PJ, Stachel A, Phillips MS, Slover JD, Inneh IA, Iorio R, Bosco JA. Risk factors for infection following total knee arthroplasty: a series of 3836 cases from one institution. J Arthroplasty. 2015;30:2275–2278. doi: 10.1016/j.arth.2015.06.058. [DOI] [PubMed] [Google Scholar]
- 8.Duchman KR, Gao Y, Pugely AJ, Martin CT, Noiseux NO, Callaghan JJ. The effect of smoking on short-term complications following total hip and knee arthroplasty. J Bone Joint Surg Am. 2015;97:1049–1058. doi: 10.2106/JBJS.N.01016. [DOI] [PubMed] [Google Scholar]
- 9.Enayatollahi MA, Novack TA, Maltenfort MG, Tabatabaee RM, Chen AF, Parvizi J. In-hospital morbidity and mortality following total joint arthroplasty in patients with hemoglobinopathies. J Arthroplasty. 2015;30:1308–1312. doi: 10.1016/j.arth.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 10.Florschutz AV, Fagan RP, Matar WY, Sawyer RG, Berrios-Torres SI. Surgical site infection risk factors and risk stratification. J Am Acad Orthop Surg. 2015;23(Suppl):S8–s11. doi: 10.5435/JAAOS-D-14-00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goyal N, Chen AF, Padgett SE, Tan TL, Kheir MM, Hopper RH Jr, Hamilton WG, Hozack WJ. Otto Aufranc Award: A multicenter, randomized study of outpatient versus inpatient total hip arthroplasty. Clin Orthop Relat Res. 2016 Jun 10. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 12.Khuri SF. The NSQIP: a new frontier in surgery. Surgery. 2005;138:837–843. doi: 10.1016/j.surg.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 13.Kumar A, Tsai WC, Tan TS, Chiu LT, Kung PT, Lo CK, Ku MC. Risk of post-TKA acute myocardial infarction in patients with a history of myocardial infarction or coronary stent. Clin Orthop Relat Res. 2016;474:479–486. doi: 10.1007/s11999-015-4616-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lovecchio F, Alvi H, Sahota S, Beal M, Manning D. Is outpatient arthroplasty as safe as fast-track inpatient arthroplasty? A propensity score matched analysis. J Arthroplasty. 2016;31:197–201. doi: 10.1016/j.arth.2016.05.037. [DOI] [PubMed] [Google Scholar]
- 15.Lu N, Misra D, Neogi T, Choi HK, Zhang Y. Total joint arthroplasty and the risk of myocardial infarction: a general population, propensity score-matched cohort study. Arthritis Rheumatol (Hoboken NJ). 2015;67:2771–2779. doi: 10.1002/art.39246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mantilla CB, Horlocker TT, Schroeder DR, Berry DJ, Brown DL. Frequency of myocardial infarction, pulmonary embolism, deep venous thrombosis, and death following primary hip or knee arthroplasty. Anesthesiology. 2002;96:1140–1146. doi: 10.1097/00000542-200205000-00017. [DOI] [PubMed] [Google Scholar]
- 17.Mantilla CB, Horlocker TT, Schroeder DR, Berry DJ, Brown DL. Risk factors for clinically relevant pulmonary embolism and deep venous thrombosis in patients undergoing primary hip or knee arthroplasty. Anesthesiology. 2003;99:552–560; discussion 555A. [DOI] [PubMed]
- 18.Menendez ME, Memtsoudis SG, Opperer M, Boettner F, Gonzalez Della Valle A. A nationwide analysis of risk factors for in-hospital myocardial infarction after total joint arthroplasty. Int Orthop. 2015;39:777–786. [DOI] [PubMed]
- 19.Molina CS, Thakore RV, Blumer A, Obremskey WT, Sethi MK. Use of the National Surgical Quality Improvement Program in orthopaedic surgery. Clin Orthop Relat Res. 2015;473:1574–1581. doi: 10.1007/s11999-014-3597-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otero JE, Gholson JJ, Pugely AJ, Gao Y, Bedard NA, Callaghan JJ. Length of hospitalization after joint arthroplasty: does early discharge affect complications and readmission rates? J Arthroplasty. 2016;31:2714–2725. doi: 10.1016/j.arth.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 21.Parvizi J, Huang R, Raphael IJ, Arnold WV, Rothman RH. Symptomatic pulmonary embolus after joint arthroplasty: stratification of risk factors. Clin Orthop Relat Res. 2014;472:903–912. doi: 10.1007/s11999-013-3358-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parvizi J, Huang R, Raphael IJ, Maltenfort MG, Arnold WV, Rothman RH. Timing of symptomatic pulmonary embolism with warfarin following arthroplasty. J Arthroplasty. 2015;30:1050–1053. doi: 10.1016/j.arth.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Parvizi J, Mui A, Purtill JJ, Sharkey PF, Hozack WJ, Rothman RH. Total joint arthroplasty: when do fatal or near-fatal complications occur? J Bone Joint Surg Am. 2007;89:27–32. doi: 10.2106/JBJS.E.01443. [DOI] [PubMed] [Google Scholar]
- 24.Pedersen AB, Sorensen HT, Mehnert F, Overgaard S, Johnsen SP. Risk factors for venous thromboembolism in patients undergoing total hip replacement and receiving routine thromboprophylaxis. J Bone Joint Surg Am. 2010;92:2156–2164. doi: 10.2106/JBJS.I.00882. [DOI] [PubMed] [Google Scholar]
- 25.Ponnusamy KE, Jain A, Thakkar SC, Sterling RS, Skolasky RL, Khanuja HS. Inpatient mortality and morbidity for dialysis-dependent patients undergoing primary total hip or knee arthroplasty. J Bone Joint Surg Am. 2015;97:1326–1332. doi: 10.2106/JBJS.N.01301. [DOI] [PubMed] [Google Scholar]
- 26.Poultsides LA, Ma Y, Della Valle AG, Chiu YL, Sculco TP, Memtsoudis SG. In-hospital surgical site infections after primary hip and knee arthroplasty–incidence and risk factors. J Arthroplasty. 2013;28:385–389. doi: 10.1016/j.arth.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 27.Pugely AJ, Martin CT, Harwood J, Ong KL, Bozic KJ, Callaghan JJ. Database and registry research in orthopaedic surgery: Part I: claims-based data. J Bone Joint Surg Am. 2015;97:1278–1287. doi: 10.2106/JBJS.N.01260. [DOI] [PubMed] [Google Scholar]
- 28.Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466:1710–1715. doi: 10.1007/s11999-008-0209-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rasouli MR, Maltenfort MG, Purtill JJ, Hozack WJ, Parvizi J. Has the rate of in-hospital infections after total joint arthroplasty decreased? Clin Orthop Relat Res. 2013;471:3102–3111. doi: 10.1007/s11999-013-2949-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rasouli MR, Restrepo C, Maltenfort MG, Purtill JJ, Parvizi J. Risk factors for surgical site infection following total joint arthroplasty. J Bone Joint Surg Am. 2014;96:e158. doi: 10.2106/JBJS.M.01363. [DOI] [PubMed] [Google Scholar]
- 31.Schiff RL, Kahn SR, Shrier I, Strulovitch C, Hammouda W, Cohen E, Zukor D. Identifying orthopedic patients at high risk for venous thromboembolism despite thromboprophylaxis. Chest. 2005;128:3364–3371. doi: 10.1378/chest.128.5.3364. [DOI] [PubMed] [Google Scholar]
- 32.Schilling PL, Hallstrom BR, Birkmeyer JD, Carpenter JE. Prioritizing perioperative quality improvement in orthopaedic surgery. J Bone Joint Surg Am. 2010;92:1884–1889. doi: 10.2106/JBJS.I.00735. [DOI] [PubMed] [Google Scholar]
- 33.Shiloach M, Frencher SK, Jr, Steeger JE, Rowell KS, Bartzokis K, Tomeh MG, Richards KE, Ko CY, Hall BL. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210:6–16. doi: 10.1016/j.jamcollsurg.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 34.Suleiman LI, Ortega G, Ong’uti SK, Gonzalez DO, Tran DD, Onyike A, Turner PL, Fullum TM. Does BMI affect perioperative complications following total knee and hip arthroplasty? J Surg Res. 2012;174:7–11. doi: 10.1016/j.jss.2011.05.057. [DOI] [PubMed] [Google Scholar]
- 35.Tabatabaee RM, Rasouli MR, Rezapoor M, Maltenfort MG, Ong AC, Parvizi J. Coronary revascularization and adverse events in joint arthroplasty. J Surg Res. 2015;198:135–142. doi: 10.1016/j.jss.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 36.White RH, Gettner S, Newman JM, Trauner KB, Romano PS. Predictors of rehospitalization for symptomatic venous thromboembolism after total hip arthroplasty. N Engl J Med. 2000;343:1758–1764. doi: 10.1056/NEJM200012143432403. [DOI] [PubMed] [Google Scholar]