Abstract
Background
Pelvic reconstruction after periacetabular tumor resection is technically difficult and characterized by a high complication rate. Although endoprosthetic replacement can result in immediate postoperative functional recovery, biologic reconstructions with autograft may provide an enhanced prognosis in patients with long-term survival; however, little has been published regarding this approach. We therefore wished to evaluate whether whole-bulk femoral head autograft that is not contaminated by tumor can be used to reconstruct segmental bone defects after intraarticular resection of periacetabular tumors.
Questions/purposes
In a pilot study, we evaluated (1) local tumor control, (2) complications, and (3) postoperative function as measured by the Musculoskeletal Tumor Society score.
Methods
Between 2009 and 2015, we treated 13 patients with periacetabular malignant or aggressive benign tumors with en bloc resection, bulk femoral head autograft, and cemented THA (with or without a titanium acetabular reconstruction cup), and all were included for analysis here. During that time, the general indications for this approach were (1) patients anticipated to have a good oncologic prognosis and adequate surgical margins to allow this approach, (2) patients whose pelvic bone defects did not exceed two types (Types I + II or Types II + III as defined by Enneking and Dunham), and (3) patients whose medical insurance would not cover what otherwise might have been a pelvic tumor prosthesis. During this period, another 91 patients were treated with pelvic prosthetic replacement, which was our preferred approach. Median followup in this study was 36 months (range, 24–99 months among surviving patients; one patient died 8 months after surgery); no patients were lost to followup. Bone defects were Types II + III in five patients, and Types I + II in eight. After intraarticular resection, ipsilateral femoral head autograft combined with THA was used to reconstruct the segmental bone defect of the acetabulum. In patients with Types I + II resections, the connection between the sacrum and the acetabulum was reestablished with a fibular autograft or a titanium cage filled with dried bone-allograft particles which was enhanced by using a pedicle screw and rod system. Functional evaluation was done in 11 patients who remained alive and maintained the femoral head autograft at final followup; one other patient received secondary resection involving removal of the femoral head autograft and internal fixation, and was excluded from functional evaluation. Endpoints were assessed by chart review.
Results
Two patients experienced local tumor recurrence. Finally, eight patients did not show signs of the disease, one patient died of disease for local and distant tumor relapse, and four patients survived, but still had the disease. Three of these four patients had distant metastases without local recurrence and one had local control after secondary resection but still experienced system relapse. We observed the following complications: hematoma (one patient; treated surgically with hematoma clearance), delayed wound healing (one patient; treated by débridement), deep vein thrombosis (one patient), and hip dislocation (one patient; treated with open reduction). The median 1993 Musculoskeletal Tumor Society score was 83% (25 of 30 points; range, 19–29 points), and all patients were community ambulators; one used a cane, three used a walker, and nine did not use any assistive devices.
Conclusions
In this small series at short-term followup, we found that reconstruction of segmental bone defects after intraarticular resection of periacetabular tumors with femoral head autograft does not appear to impede local tumor control; complications were in the range of what might be expected in a series of large pelvic reconstructions, and postoperative function was generally good.
Level of Evidence
Level IV, therapeutic study.
Introduction
Pelvic reconstruction after periacetabular tumor resection is technically difficult and associated with high complication rates and functional limitations. Commonly used methods include hip transposition, arthrodesis, autograft with recycled tumor-bearing bone, allograft-prosthetic composite, and custom-made or modular pelvic prostheses [26]. Pseudarthrosis and arthrodesis elicit stable long-term effects but result in problems with hip function and leg length discrepancy [24, 25]. Autograft with recycled tumor-bearing bone and allograft is associated with complications, including infection, nonunion, and fracture [5, 16]. Pelvic endoprosthetic replacement is generally the preferred approach when it is possible to be performed, but it requires adequate bone stock in the ilium for prosthesis fixation [4] and is associated with a high risk of infection and mechanical failure [3, 15].
Autograft is another option for pelvic reconstruction after tumor resection. Vascularized or nonvascularized fibular and iliac autografts have been used to maintain the continuity of the pelvic ring after the ilium is resected [1, 21, 22]. However, structural autograft is seldom used in reconstruction after periacetabular tumor resection. Biau et al. [2] reported on 13 patients with tumor resection in the acetabulum and pubic region. They procured and implanted the proximal part of the medial portion of the ipsilateral femur for pelvic reconstruction. Laffosse et al. [19] used the entire proximal femur autograft, and six of the 10 included patients had periacetabular tumors involving the ilium. The harvested femoral autograft was fixed to the pelvis with screws and one or more osteosynthesis plates. A new acetabulum was prepared in the remaining trochanteric process in the usual manner using all-polyethylene cups. Their reconstruction yielded satisfactory mechanical and functional effects, and no fracture or nonunion of autograft was observed. However, this method results in sacrifice of the proximal femur. Bulk femoral head autograft has been used in other pelvic reconstructions [18, 27], and therefore, it seemed reasonable to explore its use in tumor situations. However, no studies of which we are aware have focused on this reconstructive method after periacetabular tumor resection.
In a pilot study, we therefore evaluated (1) local tumor control, (2) complications, and (3) postoperative function as measured by the Musculoskeletal Tumor Society (MSTS) score.
Patients and Methods
A retrospective study was performed after approval was obtained from the institutional review board of our hospital. The medical history and followup information of patients manifesting pelvic bone neoplasm and receiving surgical treatment at our institute were collected and reviewed. The following inclusion criteria were considered: (1) patients who underwent an intraarticular acetabular resection and had a preserved uninvolved ipsilateral femoral head for later reconstruction; (2) patients who had a complete or partial acetabular segmental defect after tumor resection; (3) patients who underwent reconstruction of the acetabulum with an ipsilateral femoral head autograft; and (4) patients who underwent THA for hip function repair. The following exclusion criteria were considered: (1) patients with preserved continuity of the acetabulum; (2) patients who received a chip bone autograft; and (3) patients who underwent other reconstructions such as a hemipelvic endoprosthesis, allograft, arthrodesis, or hip transposition.
Between 2009 and 2015, we treated 13 patients with periacetabular malignant or aggressive benign tumors with en bloc resection and bulk femoral head autograft, and all were included for analysis here (Table 1). During that time, the general indications for this approach were (1) patients anticipated to have a good oncologic prognosis and adequate surgical margins to allow this approach, (2) patients whose pelvic bone defects did not exceed two types (Types I + II or II + III) as defined by Enneking and Dunham (Fig. 1) [8], and (3) patients whose medical insurance would not cover what otherwise might have been a pelvic tumor prosthesis. During this period, another 91 patients were treated with pelvic prosthetic replacement, which was our preferred approach. Median followup in this study was 36 months (range, 24–99 months among surviving patients; one patient died at 8 months). No patients were lost to followup. Bone defects were Types II + III in five patients, and Types I + II in eight.
Table 1.
Patient characteristics and outcomes
| Patient number | Sex | Age (years) | Diagnosis | Resection type | Surgical margin | Blood loss (mL) | Operation time (minutes) | Instruments for acetabular reconstruction | Followup time (months) | Local recurrence | Survival | Complications | MSTS score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 39 | Chordoma | II + III | Adequate | 3100 | 540 | CS | 25 | None | NED | None | 19 |
| 2 | Male | 29 | Chondrosarcoma | II + III | Inadequate | 6300 | 380 | CS | 41 | None | NED | Hematoma | 27 |
| 3 | Female | 21 | Giant cell tumor | II + III | Adequate | 400 | 260 | CS | 99 | None | NED | None | 28 |
| 4 | Male | 16 | Chondroblastoma | II + III | Inadequate | 1000 | 330 | CS | 46 | None | NED | None | 29 |
| 5 | Male | 40 | Epithelioid hemangioendothelioma | II + III | Adequate | 2900 | 190 | CS | 47 | None | NED | None | 29 |
| 6 | Female | 65 | Solitary fibrous tumor | I + II | Adequate | 1000 | 360 | CS; TC; PSRS | 24 | None | AWD | Superficial wound problem/DVT | 21 |
| 7 | Female | 25 | Ewing’s sarcoma | I + II | Adequate | 1400 | 300 | CS; TC; PSRS | 26 | None | NED | None | 22 |
| 8 | Male | 27 | Chondrosarcoma | I + II | Adequate | 3000 | 600 | CS; TC; PSRS | 28 | None | AWD | Dislocation | 22 |
| 9 | Male | 45 | Angiosarcoma | I + II | Adequate | 500 | 190 | CS; PSRS | 24 | None | NED | None | 24 |
| 10 | Female | 30 | Dedifferentiated chondrosarcoma | I + II | Adequate | 1800 | 420 | CS; PSRS | 36 | None | NED | None | 25 |
| 11 | Male | 28 | Alveolar soft part sarcoma | I + II | Adequate | 3900 | 420 | CS; TC; PSRS | 27 | None | AWD | None | 28 |
| 12 | Female | 38 | Chondrosarcoma | I + II | Inadequate | 3800 | 400 | CS; PSRS | 43 | Yes | AWD | None | None |
| 13 | Female | 57 | Dedifferentiated chondrosarcoma | I + II | Inadequate | 6300 | 510 | CS; TC; PSRS | 8 | Yes | DOD | None | None |
MSTS = Musculoskeletal Tumor Society; NED = no evidence of disease; AWD = alive with disease; DOD = died of disease; DVT = deep vein thrombosis; CS = cancellous screws; TC = titanium cage; PSRS = pedicle screw and rod system.
Fig. 1.
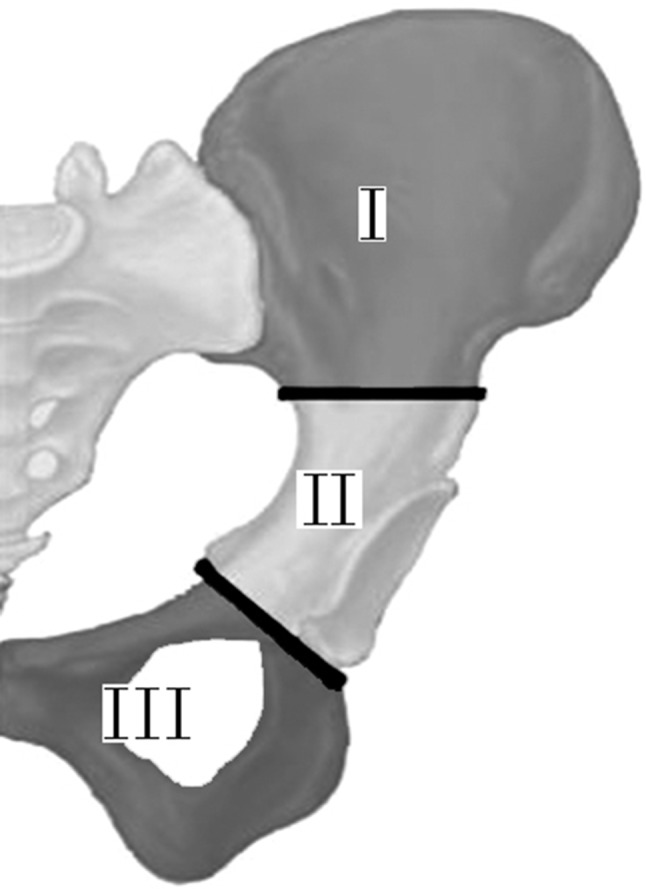
The types of pelvic resections, as described by Enneking and Dunham [8], are shown.
Of these patients, six were men and seven were women, with a mean age of 33 years (range, 16–65 years). Their pathologic diagnoses included five chondrosarcomas, one Ewing’s sarcoma, one angiosarcoma, one epithelioid hemangioendothelioma, one giant cell tumor, one chondroblastoma, and three metastatic bone tumors that originated from chordoma, alveolar soft part sarcoma, and solitary fibrous tumor. The preoperative MR images of the pelvises of the patients were evaluated thoroughly to ascertain that the tumor did not extend into the hip. En bloc tumor resections were performed in all of the patients. The resected gross specimens were inspected thoroughly during surgery by surgeons. This femoral head autograft reconstruction was used only in patients without obvious tumor violation and there was anticipation of obtaining satisfied surgical margins. Postoperatively, the final surgical margins were ascertained by an experienced pathologist (KS). According to the classification of Fuchs et al. [10], nine patients were characterized as having adequate margins (including wide and marginal resections), and four had inadequate margins on pathological evaluation (including intralesional resections).
Tumor resection and pelvic bone reconstruction were performed by two teams of surgeons, separately. The reconstruction group was responsible for placement of spinal instruments. The procedure begins by placing the patients in a lateral position, and using an approach that had elements of the ilioinguinal, posterior iliac, and Smith-Petersen approach. Briefly, the dissection began at the posterior superior iliac spine, was carried along the iliac crest to the anterior superior iliac spine, and then directed medially over the inguinal ligament to the pubic symphysis. Then, an anterior (Smith-Petersen) approach was performed to create a T-shaped field. The tumor was resected routinely with an attempt to achieve adequate surgical margins. The ipsilateral femoral head and neck, whose surface cartilage was removed from the subchondral bone, were procured and custom-shaped for autograft. Pelvic reconstructions were divided in two categories (Fig. 2) according to different combinations of pelvic resection type. In five patients with Types II + III resections (acetabulum, pubis, and ischium), autografts were fixed to the residual acetabulum or the osteotomy site of the ilium with at least two cancellous compression screws, and the puboischium bone was not subjected to reconstruction [6]. In eight patients with Types I + II resections (acetabulum and ilium), the femoral head was fixed to the residual acetabulum or the osteotomy site of the pubis and ischium. For overall consideration of the scale of bone defects, desire of the patient, and convenience of manipulation, different choices of reestablishing the connection between the sacrum and the new acetabulum were made. A nonvascularized fibular autograft was used in one patient (Patient 10, Fig. 3), an autologous iliac crest bone graft in two patients (Patients 9 and 12), and a titanium cage filled with dried bone allograft particles in five patients (Fig. 4). Continuity of the pelvic ring and stability among the acetabulum, sacral, and lower lumbar vertebrae were enhanced by using a pedicle screw and rod system. Usually, the pedicle screws were inserted in L5 and S1 in the proximal part, while the other two pedicle screws were fixed to the pubis and ischium, respectively. The connections between screws were linked by titanium rods after compressed fixation. This autograft construct then was reamed in the usual way to form a new acetabulum, and reamings were packed in the remaining minor defects. Afterward, a cemented THA routinely was performed. In patients who had a large acetabular defect before placement of the autograft, and especially in patients with complete acetabular resection, a titanium acetabular reconstruction cup was considered with the goal of reducing the risk of mechanical failure. A constrained liner and a 28-mm diameter femoral head were used to avoid hip dislocation. The remaining abductor muscles were reattached to the ilium or autograft bony structures, and sutured with abdominal wall muscles to retain muscle tone, reduce hip dislocation, and improve postoperative gait. The mean operative time was 377 minutes (range, 190–600 minutes), and the average blood loss during surgery was 2723 mL (range, 400–6300 mL).
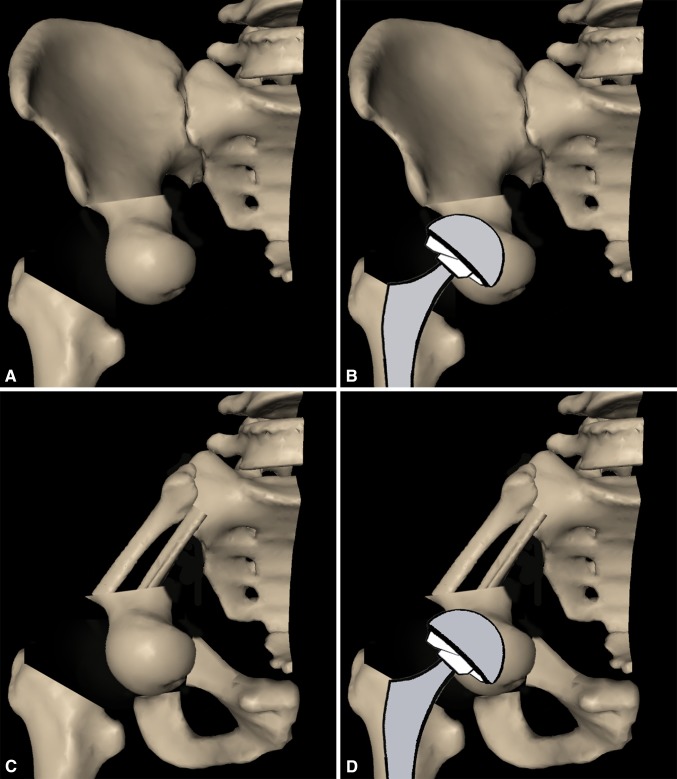
Fig. 2A–D.
A pelvic reconstruction with an ipsilateral femoral head autograft is presented. (A) The ipsilateral femoral head is harvested and fixed to the residual ilium after Types II + III pelvic resection. (B) A cemented THA is performed to maintain joint function. (C) After a Types I + II pelvic resection, the acetabulum and continuity of the pelvic ring are reestablished by the ipsilateral femoral head combined with fibular autografts. (D) The hip is replaced by a cemented endoprosthesis.
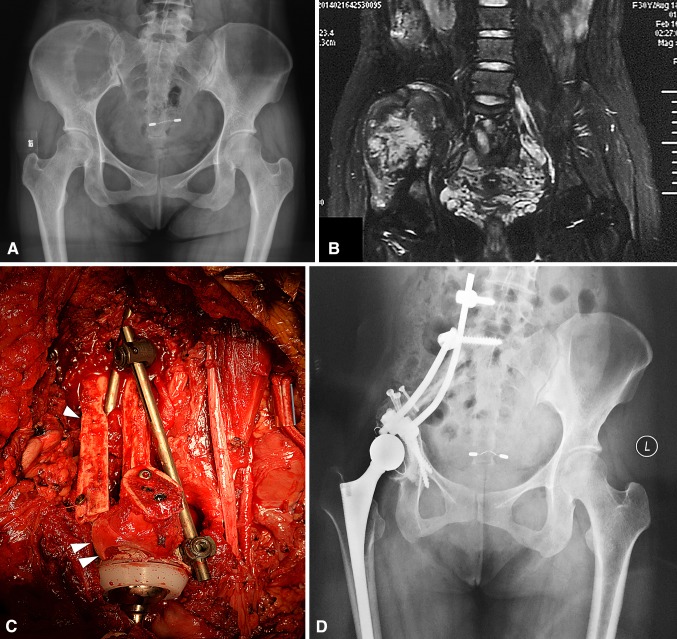
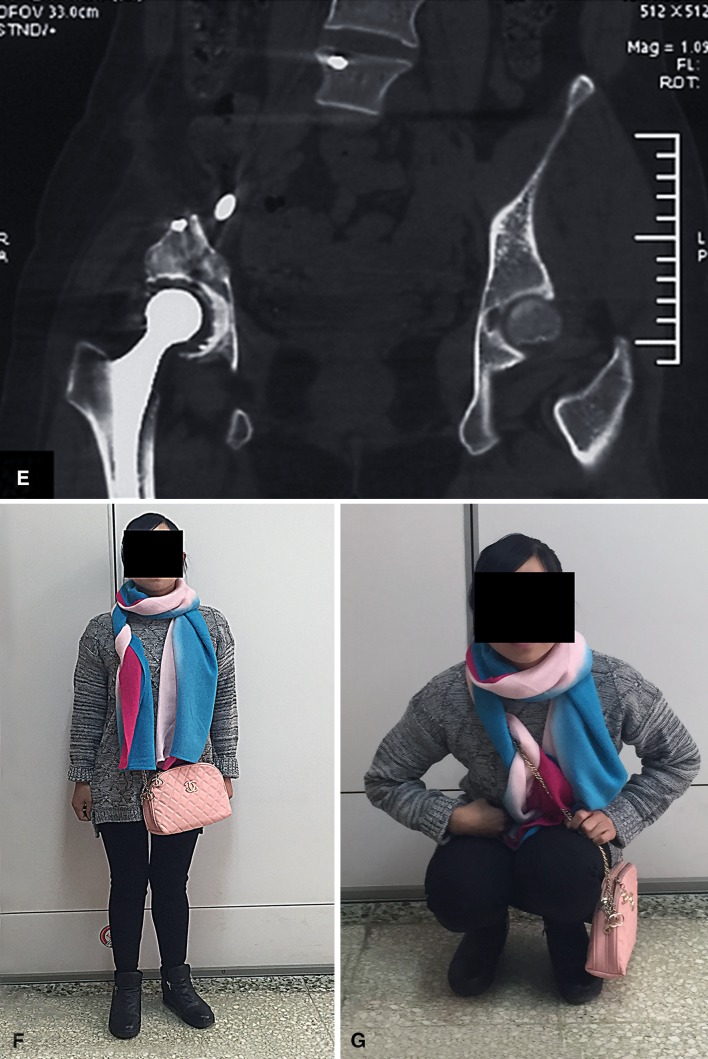
Fig. 3A–H.
A female patient (Patient 10) with pelvic dedifferentiated chondrosarcoma underwent femoral head autograft reconstruction. (A) Her preoperative plain radiograph and (B) T2-weighted MR image show involvement of the ilium and superior part of the acetabulum. (C) An intraoperative view shows the pelvic ring is reconstructed with a double nonvascularized fibular autograft (one arrow) and femoral head autograft (two arrows) enhanced by cancellous compression screws and a spinal pedicle screw-rod system. (D) A postoperative plain radiograph and (E) CT scan obtained at the 24-month followup show good local tumor control with no signs of mechanical failure of the endoprosthesis or internal fixation. (F) This patient could stand and (G) squat freely without aid at her 24-month followup.
Fig. 4.
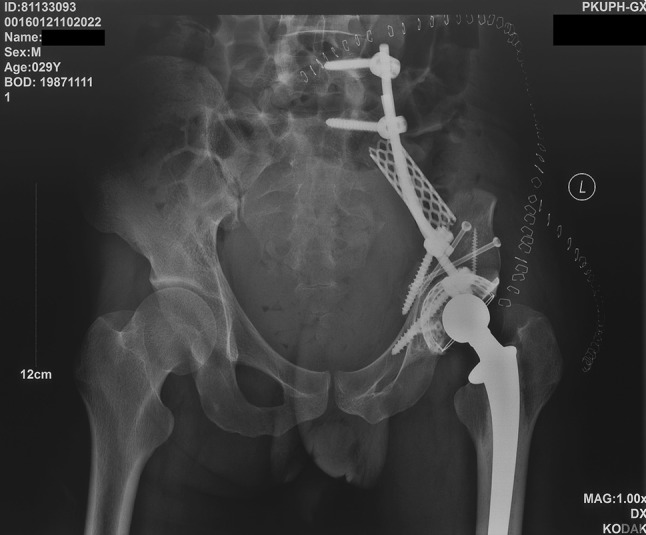
A patient’s (Patient 11) postoperative plain radiograph shows that the connection between the sacrum and the new acetabulum was reestablished by a titanium cage combined with a pedicle screw and rod system.
Postoperatively, two closed-suction drains were maintained until the volume of drainage was less than 30 mL per day. Intravenous antibiotic treatment typically was maintained for 1 week after surgery. The affected hip was immobilized with a brace for 4 to 6 weeks, and patients were asked to remain nonweightbearing, but they were encouraged to move the other limb during the early postoperative period. Partial weightbearing using crutches or a walker was started 6 weeks postoperatively; full weightbearing was not allowed until bone union was seen on plain radiographs and CT scans. All of the patients were followed up clinically and radiologically every 3 months for the first 2 years, every 6 months between 2 and 5 years, and yearly thereafter.
Functional evaluation was done in 11 patients who remained alive and maintained the femoral head autograft at final followup. One other patient received a secondary resection involving removal of the femoral head autograft and internal fixation, and was excluded from functional evaluation. Endpoints were assessed by chart review, including oncologic outcomes (local recurrence, distant metastasis, and death). Chart review was performed by a physician (HQ) who was not involved in the care of these patients. Perioperative and prosthetic-related complications were recorded. Autograft integration in the bone was evaluated based on the criteria described by Nigro and Grace [20]. The length of the legs and functional outcomes, which included pain, function, emotional acceptance, support, walking ability, and gait, were assessed during the final followup with the 1993 MSTS system [7] for the lower extremity.
Results
Local tumor recurrence occurred in two patients (at 5 and 14 months after surgery, in patients with dedifferentiated chondrosarcoma and classic chondrosarcoma, respectively) whose margins were inadequate at the time of surgery. The tumors recurred adjacent to the sacroiliac joint, rather than the femoral head autografts. One of these patients underwent secondary resection of the recurrent tumor, the femoral head autograft and internal fixation were removed, and the reconstruction was changed to a pelvic endoprosthesis. Finally, this patient was alive with local tumor control but still experienced system relapse. The other patient declined further treatment and died of metastasis at 8 months postoperatively. At latest followup, one patient died of the disease owing to local relapse and metastases, four patients were alive with disease with distant metastases, and eight patients had no evidence of disease. The following complications were observed: hematoma (one patient; treated surgically with hematoma clearance), delayed wound healing (one patient; treated with débridement), deep vein thrombosis (one patient), and hip dislocation (one patient; treated with open reduction). No patients experienced bone nonunion, fracture, or mechanical failure of internal fixation or endoprostheses. All autografts completely integrated in bone.
The median MSTS 93 score was 83% (25 of 30 points; range, 19–29 points). The median MSTS score of the patients with Types II + III resections was 88% (26 of 30 points; range, 19–29 points) and the median MSTS score of patients with Types I + II resections was 79% (24 of 30 points; range, 19–28 points). All patients were community ambulators; one used a cane, three used a walker, and nine did not use any assistive devices. The median limb length discrepancy of the 11 hips was 1.5 cm (range, 1–2.5 cm).
Discussion
Although biologically based reconstructions such as allografts have potential advantages in patients undergoing pelvic tumor resections whose oncologic prognosis is good, autograft pelvic reconstruction after periacetabular tumor resection has rarely been reported [2, 19]. Endoprosthetic replacement after tumor resection in the pelvis is associated with a high risk of complications and functional restrictions [3, 15, 17, 26]. In 2009, we therefore began to use the ipsilateral femoral head as an autograft to support a cemented THA for acetabular reconstruction after tumor resection in a way analogous to patients undergoing THA for developmental hip dysplasia [18, 27], although even during this time our preferred approach was endoprosthetic replacement. We generally used the autograft reported here for the more-challenging reconstructions. In this small study, we evaluated this approach in terms of tumor control, complications, and function at short term.
This study has some limitations. First, the number of included patients is limited and the followup period is short; future studies will need to evaluate whether the results remain durable with time, and whether they are generalizable in larger patient groups. Second, because we did not compare the approach described in this study with another approach, it is difficult to know whether it is superior or inferior to alternatives; however, in general, the patients in this series underwent more-difficult reconstructions. During the same time, we performed another 91 resections and reconstructions for pelvic tumors using endoprostheses, and that approach generally has shorter operative time and less blood loss [12, 29]. Third, this autograft can be used only in limited situations; it is not suitable for reconstruction of large pelvic bone defects after Types I + II + III resections. Fourth, selection bias existed in this study. Patients expected to have adequate surgical margins and better prognosis were candidates for this biological reconstruction, which might have had a positive effect on the results. However, approximately one-third of the patients in our study had intralesional resections and a poor prognosis, which would tend to suggest that this limitation was not severe. The prognosis of malignant tumors of the pelvis is influenced mainly by its anatomic site and biological behavior. On the contrary, the inclusion of patients without medical insurance might have a negative effect. Care of these patients could be more complicated and they might have a poor health status.
In this small series, two of 13 patients experienced local recurrences, and five of 13 had metastatic disease. In addition, four patients had contaminated margins at the time of postoperative pathological examination. In a systemic review [26], the local recurrence rates reported in numerous studies of pelvic bone tumor resections and reconstructions ranged from 20% to 30%. The risk of local recurrence after resection of pelvic tumors is greatly influenced by the ability to achieve adequate surgical margins [24, 28]. Han et al. [13] reported that the surgical margin correlated with local recurrence and it was identified as an independent prognostic factor in surgical treatment of pelvic sarcoma. In our study, two patients experienced local recurrence and had inadequate surgical margins. The result of local tumor control in our series is comparable to results in other reports [24, 26]. Although no local relapse occurred from the femoral head autograft, it is important to verify before surgery that the joint is free from tumor by using appropriate imaging tests so that the graft does not result in contamination of the reconstruction with tumor. In addition, the femoral head autograft should be changed to an endoprosthetic replacement or hip transposition if intraarticular contamination occurs.
Three patients in our series experienced four complications, and but there were no infections, nonunions, or early mechanical failures. While allograft-based approaches appear similar in some ways to the approach we used, allograft-based procedures seem more likely to be associated with serious complications, including a risk of failure as high as 41% [23]. Recycled tumor-bearing bones likewise are associated with complications. For instance, Jeon et al. [16] reported three infections, two fractures, and three hips with aseptic loosening in 14 patients who received pasteurized autograft-total hip prosthesis composites for periacetabular tumors. Pelvic endoprostheses provide immediate reconstruction and result in limited mechanical complications on a short-term scale. However, they are expensive, associated with a high infection risk, and nonbiologic with a high mechanical failure rate on a long-term scale. Although pelvic autograft reconstruction might have some advantages when compared with other methods, complications are still encountered. Hillmann et al. [14] retrospectively reviewed 110 patients who underwent surgical removal of pelvic tumors and reported that infection was more frequent after endoprosthetic reconstruction (six of 16 patients) and massive allograft reconstruction (five of 13 patients) than after autograft reconstruction (one of 12 patients). Laffosse et al. [19] examined 10 patients who had periacetabular tumors and received segmental ipsilateral femur autografts. They observed major complications, including three hip dislocations and one deep infection, but they did not find any graft fracture or nonunion. In another study [2] involving 13 patients with ipsilateral femur reconstruction of the acetabulum, two patients underwent revision surgery for mechanical failure and infection after 49 months of followup. Ipsilateral proximal femur autograft is used for reconstruction after a Type II resection and combined Types II and III resections. However, the stability created by a construct in a Type II resection combined with a Type I resection is likely insufficient. In our study, the resected ipsilateral femoral head was used as an acetabular autograft, an approach we believe is advantageous for several reasons. First, the cancellous bone of an autologous femoral head, which exhibits excellent biocompatibility, is suitable for bone union. Second, pelvic reconstruction with autograft instead of a tumor megaprosthesis could result in a lower deep infection rate, especially in patients with Types II + III resection who need only limited internal fixation instruments. Third, the stability of the hip after arthroplasty is maintained to some extent with an intact greater trochanter and reattachment of residual abductor muscles to autograft bony structures. Fourth, this method can be used for reconstruction after Types I and II resections. However, as mentioned above, this autograft approach took more surgical time and resulted in more blood loss compared with hemipelvic endoprosthetic replacement. Aortic balloon occlusion or other blood vessel control methods usually were considered in this series. Even so, two of our patients still had more than 6000 mL of blood loss. Both of these patients experienced an unexpected common iliac vein injury (which was repaired) during tumor resection which resulted in hemorrhage.
MSTS scores were generally high in our small group. In patients with Type II or Types II + III pelvic resections, acceptable function of the lower limb usually is provided by applying pelvic endoprosthetic reconstruction to maintain an intact ilium. Bus et al. [3] reported on pedestal cup endoprosthetic reconstructions after periacetabular tumor resections and found a mean MSTS score for 24 patients (51%) of 21 of 30 points (70%; range, 30%–93%) after a median followup of 39 months. Falkinstein et al. [9] used endoprosthetic reconstruction of Type II pelvic resection and reported a mean MSTS functional outcome score of 20 (range, 11–27) for 10 survivors. They also observed that nine of the 10 patients were able to walk. However, function is relatively poor when periacetabular tumors affect the ilium. In one study [17] on endoprosthetic reconstruction after pelvic tumor resection, the mean MSTS 93 score for patients with iliosacral joint involvement was 39%, which is lower than that without iliosacral joint involvement (58%). Hip transposition is another option with few complications, but this procedure can result in leg-length discrepancy [11]. In the current study, most patients who received Types II + III and Types I + II resections could walk without assistive devices. However, the interference with gait is inevitable owing to loss of the gluteus and its bony attachment in patients with Types I + II resections.
In this small series at short-term followup, we found that reconstruction of segmental bone defects after intraarticular resection of periacetabular tumors with femoral head autograft does not seem to impede local tumor control; complications were in the range of what might be expected in a series of large pelvic reconstructions, and postoperative function was generally good. Future studies might be performed with larger numbers of patients with longer-term followup, to ascertain whether the apparent advantages of this biological reconstruction endure with time in a consistent way.
Acknowledgments
We thank Huayi Qu MD (Musculoskeletal Tumor Center, Peking University People’s Hospital) for help with chart review and Kunkun Sun MD (Department of Pathology, Peking University People’s Hospital) for pathology evaluations.
Footnotes
Each author certifies that neither he, nor any member of his immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Akiyama T, Clark JC, Miki Y, Choong PF. The non-vascularised fibular graft: a simple and successful method of reconstruction of the pelvic ring after internal hemipelvectomy. J Bone Joint Surg Br. 2010;92:999–1005. doi: 10.1302/0301-620X.92B7.23497. [DOI] [PubMed] [Google Scholar]
- 2.Biau DJ, Thevenin F, Dumaine V, Babinet A, Tomeno B, Anract P. Ipsilateral femoral autograft reconstruction after resection of a pelvic tumor. J Bone Joint Surg Am. 2009;91:142–151. doi: 10.2106/JBJS.G.01061. [DOI] [PubMed] [Google Scholar]
- 3.Bus MP, Boerhout EJ, Bramer JA, Dijkstra PD. Clinical outcome of pedestal cup endoprosthetic reconstruction after resection of a peri-acetabular tumour. Bone Joint J. 2014;96:1706–1712. doi: 10.1302/0301-620X.96B12.34622. [DOI] [PubMed] [Google Scholar]
- 4.Bus MP, Szafranski A, Sellevold S, Goryn T, Jutte PC, Bramer JA, Fiocco M, Streitburger A, Kotrych D, van de Sande MA, Dijkstra PD. LUMiC® endoprosthetic reconstruction after periacetabular tumor resection: short-term results. Clin Orthop Relat Res. 2017;475:686–695. doi: 10.1007/s11999-016-4805-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campanacci D, Chacon S, Mondanelli N, Beltrami G, Scoccianti G, Caff G, Frenos F, Capanna R. Pelvic massive allograft reconstruction after bone tumour resection. Int Orthop. 2012;36:2529–2536. doi: 10.1007/s00264-012-1677-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dominkus M, Darwish E, Funovics P. Reconstruction of the pelvis after resection of malignant bone tumours in children and adolescents. Recent Results Cancer Res. 2009;179:85–111. doi: 10.1007/978-3-540-77960-5_8. [DOI] [PubMed] [Google Scholar]
- 7.Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–246. [PubMed] [Google Scholar]
- 8.Enneking WF, Dunham WK. Resection and reconstruction for primary neoplasms involving the innominate bone. J Bone Joint Surg Am. 1978;60:731–746. doi: 10.2106/00004623-197860060-00002. [DOI] [PubMed] [Google Scholar]
- 9.Falkinstein Y, Ahlmann ER, Menendez LR. Reconstruction of type II pelvic resection with a new peri-acetabular reconstruction endoprosthesis. J Bone Joint Surg Br. 2008;90:371–376. doi: 10.1302/0301-620X.90B3.20144. [DOI] [PubMed] [Google Scholar]
- 10.Fuchs B, Yaszemski MJ, Sim FH. Combined posterior pelvis and lumbar spine resection for sarcoma. Clin Orthop Relat Res. 2002;397:12–18. doi: 10.1097/00003086-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Gebert C, Wessling M, Hoffmann C, Roedl R, Winkelmann W, Gosheger G, Hardes J. Hip transposition as a limb salvage procedure following the resection of periacetabular tumors. J Surg Oncol. 2011;103:269–275. doi: 10.1002/jso.21820. [DOI] [PubMed] [Google Scholar]
- 12.Guo W, Li D, Tang X, Yang Y, Ji T. Reconstruction with modular hemipelvic prosthesies for periacetabular tumor. Clin Orthop Relat Res. 2007;461:180–188. doi: 10.1097/BLO.0b013e31806165d5. [DOI] [PubMed] [Google Scholar]
- 13.Han I, Lee YM, Cho HS, Oh JH, Lee SH, Kim HS. Outcome after surgical treatment of pelvic sarcomas. Clin Orthop Surg. 2010;2:160–166. doi: 10.4055/cios.2010.2.3.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hillmann A, Hoffmann C, Gosheger G, Rodl R, Winkelmann W, Ozaki T. Tumors of the pelvis: complications after reconstruction. Arch Orthop Trauma Surg. 2003;123:340–344. doi: 10.1007/s00402-003-0543-7. [DOI] [PubMed] [Google Scholar]
- 15.Jaiswal PK, Aston WJ, Grimer RJ, Abudu A, Carter S, Blunn G, Briggs TW, Cannon S. Peri-acetabular resection and endoprosthetic reconstruction for tumours of the acetabulum. J Bone Joint Surg Br. 2008;90:1222–1227. doi: 10.1302/0301-620X.90B9.20758. [DOI] [PubMed] [Google Scholar]
- 16.Jeon DG, Kim MS, Cho WH, Song WS, Lee SY. Reconstruction with pasteurized autograft-total hip prosthesis composite for periacetabular tumors. J Surg Oncol. 2007;96:493–502. doi: 10.1002/jso.20834. [DOI] [PubMed] [Google Scholar]
- 17.Ji T, Guo W, Yang RL, Tang XD, Wang YF. Modular hemipelvic endoprosthesis reconstruction: experience in 100 patients with mid-term follow-up results. Eur J Surg Oncol. 2013;39:53–60. doi: 10.1016/j.ejso.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Kim M, Kadowaki T. High long-term survival of bulk femoral head autograft for acetabular reconstruction in cementless THA for developmental hip dysplasia. Clin Orthop Relat Res. 2010;468:1611–1620. doi: 10.1007/s11999-010-1288-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laffosse JM, Pourcel A, Reina N, Tricoire JL, Bonnevialle P, Chiron P, Puget J. Primary tumor of the periacetabular region: resection and reconstruction using a segmental ipsilateral femur autograft. Orthop Traumatol Surg Res. 2012;98:309–318. doi: 10.1016/j.otsr.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Nigro N, Grace D. Radiographic evaluation of bone grafts. J Foot Ankle Surg. 1996;35:378–385. doi: 10.1016/S1067-2516(96)80055-X. [DOI] [PubMed] [Google Scholar]
- 21.Nishida J, Shiraishi H, Okada K, Ehara S, Shimamura T. Vascularized iliac bone graft for iliosacral bone defect after tumor excision. Clin Orthop Relat Res. 2006;447:145–151. doi: 10.1097/01.blo.0000203485.90711.1b. [DOI] [PubMed] [Google Scholar]
- 22.Ogura K, Sakuraba M, Miyamoto S, Fujiwara T, Chuman H, Kawai A. Pelvic ring reconstruction with a double-barreled free vascularized fibula graft after resection of malignant pelvic bone tumor. Arch Orthop Trauma Surg. 2015;135:619–625. doi: 10.1007/s00402-015-2197-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ozaki T, Hillmann A, Bettin D, Wuisman P, Winkelmann W. High complication rates with pelvic allografts: experience of 22 sarcoma resections. Acta Orthop Scand. 1996;67:333–338. doi: 10.3109/17453679609002326. [DOI] [PubMed] [Google Scholar]
- 24.Puri A, Pruthi M, Gulia A. Outcomes after limb sparing resection in primary malignant pelvic tumors. Eur J Surg Oncol. 2014;40:27–33. doi: 10.1016/j.ejso.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz AJ, Kiatisevi P, Eilber FC, Eilber FR, Eckardt JJ. The Friedman-Eilber resection arthroplasty of the pelvis. Clin Orthop Relat Res. 2009;467:2825–2830. doi: 10.1007/s11999-009-0844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shao QD, Yan X, Sun JY, Xu TM. Internal hemipelvectomy with reconstruction for primary pelvic neoplasm: a systematic review. ANZ J Surg. 2015;85:553–560. doi: 10.1111/ans.12895. [DOI] [PubMed] [Google Scholar]
- 27.Tsukada S, Wakui M. Bulk femoral head autograft without decortication in uncemented total hip arthroplasty: seven- to ten-year results. J Arthroplasty. 2012;27(437–444):e1. doi: 10.1016/j.arth.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Wirbel RJ, Schulte M, Maier B, Koschnik M, Mutschler WE. Chondrosarcoma of the pelvis: oncologic and functional outcome. Sarcoma. 2000;4:161–168. doi: 10.1155/2000/635246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zang J, Guo W, Yang Y, Xie L. Reconstruction of the hemipelvis with a modular prosthesis after resection of a primary malignant peri-acetabular tumour involving the sacroilliac joint. Bone Joint J. 2014;96:399–405. doi: 10.1302/0301-620X.96B3.32387. [DOI] [PubMed] [Google Scholar]