Abstract
Abnormal long-range temporal correlation (LRTC) in EEG oscillation has been observed in several brain pathologies and mental disorders. This study examined the relationship between the LRTC of broadband EEG oscillation and depression following cerebral infarction with different hemispheric lesions to provide a novel insight into such depressive disorders. Resting EEGs of 16 channels in 18 depressed (9 left and 9 right lesions) and 21 non-depressed (11 left and 10 right lesions) subjects following cerebral infarction and 19 healthy control subjects were analysed by means of detrended fluctuation analysis, a quantitative measurement of LRTC. The difference among groups and the correlation between the severity of depression and LRTC in EEG oscillation were investigated by statistical analysis. The results showed that LRTC of broadband EEG oscillations in depressive subjects was still preserved but attenuated in right hemispheric lesion subjects especially in left pre-frontal and right inferior frontal and posterior temporal regions. Moreover, an association between the severity of psychiatric symptoms and the attenuation of the LRTC was found in frontal, central and temporal regions for stroke subjects with right lesions. A high discriminating ability of the LRTC in the frontal and central regions to distinguish depressive from non-depressive subjects suggested potential feasibility for LRTC as an assessment indicator for depression following right hemispheric cerebral infarction. Different performance of temporal correlation in depressed subjects following the two hemispheric lesions implied complex association between depression and stroke lesion location.
Keywords: Depression, Hemispheric cerebral infarction, EEG oscillation, Long-range temporal correlation (LRTC), Detrended fluctuation analysis (DFA)
Introduction
Neural oscillations are known to show great variability and apparently random changes over time and brain conditions (Dimitriadis et al. 2015; Fiol-Veny et al. 2015; Florin and Baillet 2015; Fields and Glazebrook 2017; Qu et al. 2014). Several studies have proven that neuronal oscillations in the human brain possess long-range temporal correlations (LRTC) in frequency, amplitude, duration and recurrence (Linkenkaer-Hansen et al. 2005, 2007; Nikulin and Brismar 2004, 2005). The presence of LRTC is thought to be beneficial for a reliable transfer of information in neuronal networks (Beggs and Plenz 2003; Stam et al. 2004). The analysis of LRTC, which provides a quantitative index to dynamical oscillation of different time scales, has been applied to study electroencephalographic (EEG) oscillations of the human brain under different physiological and pathological states (Bornas et al. 2013, 2015; Lee et al. 2007; Montez et al. 2009; Nikulin et al. 2012; Pu et al. 2016; Takahashi et al. 2009).
Abnormal LRTC in EEG oscillation has been observed in several brain pathologies and mental disorders, including anaesthesia (Li et al. 2008), Alzheimer’s disease (Montez et al. 2009), schizophrenia (Nikulin et al. 2012) and attention deficit hyperactivity disorder (ADHD) (Smit et al. 2011). Furthermore, the scaling analysis was supposed to have diagnostic potential in some psychiatric disorders (Linkenkaer-Hansen et al. 2005; Smit et al. 2011). Studies have also demonstrated aberrant temporal dynamics in patients with depressive disorder (Linkenkaer-Hansen et al. 2005; Lee et al. 2007; Leistedt et al. 2007a, b). Linkenkaer-Hansen et al. (2005) found a breakdown of LRTC in the theta-frequency band with time scales ranging from 5 to 100 s in patients with major depressive disorder (MDD) compared to healthy controls. Lee et al. (2007) found a more persistent tendency of broadband (0.6–46 Hz) LRTC over 1-s scales in MDD patients than healthy controls in all brain regions. Correlation between the LRTC and the severity of depressed patients was also proved in the above studies. Additionally, negative emotion regulation strategies were observed to be associated with the LRTC of both broad- and theta-band oscillations in another study, which suggested that aberrant temporal of dynamics may exist before depression manifestation (Bornas et al. 2013, 2015).
Despite the studies on the LRTC of neuronal oscillations for different frequency and time scales in patients with depressive disorders, none of the above focused on depression following cerebral infarction. Cerebral infarction is a major cause of adult-onset disability and dependency worldwide, and neuropsychiatric disorders are often associated with it. Depressive disorder, which refers to emotional disorders with main symptoms of dropped interest and depression, is one of the most common emotional disorders afflicting stroke sufferers, which impedes the rehabilitation and recovery process, jeopardizes quality of life and severely increases mortality (Berg et al. 2003; Gaete and Bogousslavsky 2008; De Man-Van Ginkel et al. 2013). Its pathophysiological mechanism has not yet been fully elucidated and is assumed to be a multifactorial process involving biological, behavioural and social factors (Chemerinski et al. 2001; de Coster et al. 2005; Singh et al. 2000; Pedroso et al. 2015; Zhang et al. 2015). Among them, biological and psychological perspectives were two main viewpoints. The lesion location hypothesis was one of the major biological theories on the post-stroke pathophysiology of depression (Bogousslavsky 2003; Sinyor et al. 1986; Zhang et al. 2012). Some studies have proposed that there is a higher risk of depression when the lesion is in the left hemisphere (Robinson 2003, 2006), but other studies were not able to replicate the finding (House et al. 1990; Herrmann et al. 1995; Berg et al. 2001). At present, the relationship between lesion location and depression is not yet well understood (Carson et al. 2000; Bhogal et al. 2004).
Given that both neuronal oscillation and EEG analysis have been used to investigate neurophysiological changes in depression, we proposed to investigate the LRTC of EEG oscillation in depressive subjects after cerebral infarction. In this study, the resting EEG oscillation of depressed and non-depressed subjects with unilateral hemispheric basal ganglia infarction and healthy controls was evaluated using Detrended Fluctuation Analysis (DFA), a nonlinear analysis technique that permits the detection of the LRTC in seemingly non-stationary time series through the value of scaling exponents (Peng et al. 1995). Statistical analysis was conducted to find the difference among different groups and correlation between the severity of depression and EEG oscillation, hoping to provide a novel insight into depression following cerebral infarction.
Materials and methods
Participants
18 depressed post-infarction (DPI) (9 with left hemispheric lesions and 9 with right); 21 non-depressed post-infarction (NDPI) (11 with left and 10 with right) and 19 age-matched healthy control (HC) subjects were included for this study. All participants were recruited from the Rehabilitation Medical Department of Tianjin Union Medical Centre and were informed of the aims and protocols of the experiments before the EEG recordings. The study was approved by the Human Experimental Ethics committee at the Tianjin Union Medical Centre. All participants were right-handed and free of psychotropic drugs for at least one week before the study. For the cerebral infarction patients, the inclusion criteria were as follows: ① First onset middle cerebral arterial (MCA) ischaemic stroke with damaged lesion of basal ganglia in the unilateral hemisphere confirmed by brain magnetic resonance imaging (MRI). ② Ability to complete the necessary investigations and questionnaires. ③ Chronic phase with more than 1 month and less than 12 months after the onset of stroke. Exclusion criteria for all the participants were as follows: ① Diagnosis of anxiety, schizophrenia or other psychotic disorders, psychopathic personality disorder or alcohol/drug abuse of dependence; ② History of seizures, brain surgery, organic brain disease or organic affective disturbance; ③ cognitive dysfunction with Montreal Cognitive Assessment (MoCA) >25; ④ EEG abnormalities consistent with encephalitis, or use of medications that could confound EEG assessment, such as benzodiazepines, tricyclic antidepressants, or neuroleptic medications. The clinical diagnosis of depression subtypes was established prospectively using the Diagnostic and Statistical Manual, Fourth Edition (DSM-IV) criteria for major depression (American Psychiatric Association 1994). The severity of depression was assessed by the 24-item Hamilton Depression Rating Scale (HDRS) (Hamilton 1960), which was administered by the same trained psychiatric interviewer.
Experimental conditions and EEG recordings
Resting-state EEGs were collected in the study. All subjects were instructed to close their eyes and maintain consciousness during the recording. They were also asked to avoid blinking and unnecessary movements. The acquisition room remained quiet with dim light and was free from electromagnetic interference. Eight minutes of EEGs were collected with a NicoletOne digital video electroencephalograph using 16 Ag/AgCl scalp electrodes (Fp1, Fp2, F3, F4, F7, F8, C3, C4, T3, T4, P3, P4, O1, O2, T5 and T6) placed according to the international 10–20 electrode position system (American Electroencephalographic Society 1994). The impedance of all electrodes was below 10 kΩ. The EEG data were recorded in the 0.1–70 Hz frequency range and sampled at 250 Hz, and linked earlobes were chosen to be the reference electrodes.
Data analysis
The collected 8 min EEG data were preprocessed before long-range temporal correlation analysis. The original EEG data were visually inspected, and segments containing artefacts were rejected. After that, the raw EEG was band-pass filtered with finite impulse response filtering (3rd order, Hamming window) in the 0.5–30 Hz range for broadband oscillations. This range was chosen because approximately 98% of spectral power lies within these limits (Fingelkurts and Fingelkurts 2015). Finally, 200 s of consecutive filtered EEG data were selected for the LRTC analysis. The length 200 s EEG data was chosen to accommodate subjects who got the shortest length after removing artefacts.
The preprocessed EEG data were segmented into 20 s with a 5 s overlap. We could obtain 13 segments of EEG data after that to do the LRTC analysis. The following detrended fluctuation analysis (DFA) method was used to quantify the LRTC (Peng et al. 1995; Karkare et al. 2009). The LRTC of one subject was described by the mean value of the DFA parameters of the 13 segments.
We assumed time series x(i), i = {1,2,…,N} represent the EEG signals of one channel, the DFA was calculated using the following algorithm.
First, cumulative sum sequences y(m) minus the mean value were constructed according to formula (1), where <x> is the average of time series x(i). Second, the integrated sequences y(m) were divided into nonoverlapping windows of size n which corresponded to time scales t of length n/250 s. In this study, values of t were varied from 0.2 to 10 s with a sampling interval of 0.1 s. Linear fitting sequences were obtained with least squares for every subsequence. The root mean square of the time series detrended fluctuation F(n) was calculated by formula (2). The procedure of calculating F(n) was repeated for different time scales t. Finally, n and F(n) were plotted in a double logarithmic coordinate system. If the time series x(i) had the property of self-similarity, the graph would display a linear scaling region. The slope of the least-squares line in this graph is called the scaling exponent, which is the result of DFA and quantifies the LRTC. All routines above were implemented in MATLAB (MathWorks, Inc).
| 1 |
| 2 |
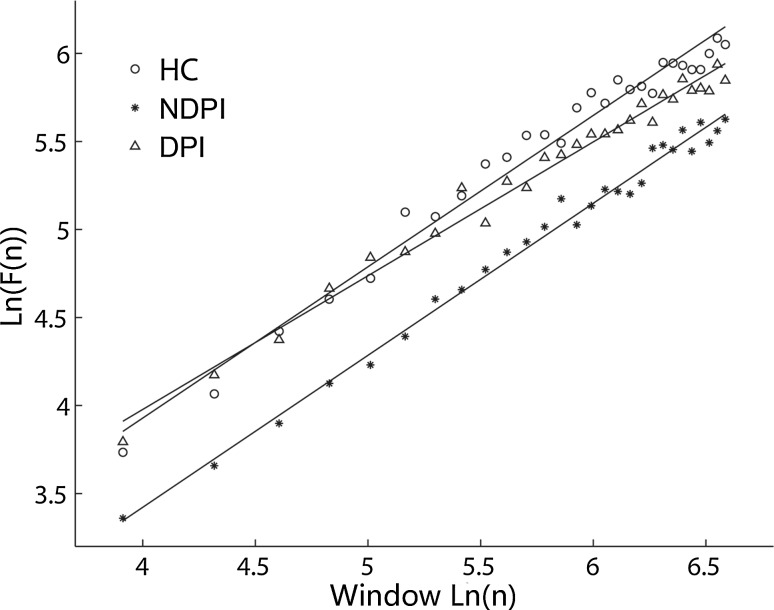
The scaling exponent provided a quantitative measure for the LRTC of EEG signals. The scaling exponent in the 0.5–1 range indicates the presence of persistent temporal correlations. A larger DFA exponent in the interval of 0.5–1.0 indicates a greater persistence of temporal correlations (stronger autocorrelations). According to the above calculation method, the linear scaling region with the slope of 0.5–1 was found in the time range of 0.2–3 s, which means the broadband EEG oscillation showed LRTC in the range of 0.2–3 s. Figure 1 shows three examples of the linear relationship in a double logarithmic plot between the magnitude of fluctuation F(n) and n for the FP1 channel in three subjects from DPI, NDPI and HC groups separately. Scaling exponents were calculated for broadband oscillations in each channel and data segment.
Fig. 1.
An example of the linear relationship in a double logarithmic plot in the range of 0.2–3 s between the magnitude of fluctuation F(n) and n for the FP1 channel in three subjects from depression post-infarction (DPI) and non-depression post-infarction (NDPI) with right hemispheric lesion and healthy control (HC). The scaling exponents of the three subjects were 0.760 (DPI), 0.863 (NDPI) and 0.860 (HC)
Statistical analysis
Statistical analysis of clinical characteristic and scaling exponents was performed using the IBM SPSS statistics 19.0 software program. We described the demographic and clinical characteristics in DPI, NDPI and HC subjects using analysis of variance (ANOVA) (Gutiérrez and Ramírez-Moreno 2016) and an independent samples t test.
For the broadband LRTC of different subjects, multiple analysis of variance (MANOVA) was first used to evaluate the difference in scaling exponents among the five groups (DPI subjects with left-hemisphere lesions, DPI subjects with right-hemisphere lesions, NDPI subjects with left-hemisphere lesions, NDPI subjects with right-hemisphere lesions and HC subjects). Post hoc analysis using the Bonferroni test was used to compare the between-differences of HC, DPI and NDPI subjects with left- and right-hemisphere lesions separately. To explore the influences of unilateral stroke damage on the LRTC of broadband EEG oscillation without the effect of depressive disorder, we also compared the between-differences of HC, NDPI subjects with left- and right-hemisphere lesions. Then, Pearson linear correlation analysis between scaling exponents and HDRS scores was used to examine the relationship between EEG oscillation and depressive symptoms. Because of small numbers of depressive subjects for each lesion side, we performed the correlation analysis in infarction subjects for each lesion. The correlation coefficients and significance level were corrected for multiple comparisons using the Benjamini and Hochberg False Discovery Rate (BH FDR). Finally, receiver operating characteristic (ROC) curves were used to estimate the discriminating ability of scaling exponents to distinguish DPI subjects from NDPI subjects through quantifying the balance between sensitivity/specificity. The mean area under curves (AUCs) of ROC near the upper-left corner is close to 1, indicating better diagnostic capabilities.
Results
Demographic information and descriptive data
Relevant demographic (age and gender) and clinical (HDRS, lesion side and time post-infarction) characteristics are shown in Table 1. According to the ANOVAs, no statistically significant difference (p > 0.05) was found for gender and age among DPI, NDPI and HC subjects. There was also no significant difference in post-stroke time among DPI and NDPI patients with different lesion sides. The HDRS scores showed a significant difference among DPI, NDPI and HC groups (p < 0.001). The post hoc test with least significant difference (LSD) indicated that the HDRS score of DPI subjects was higher than that of the HC and NDPI (p < 0.001). The HDRS was also higher in NDPI subjects than the HC (p < 0.01). However, we noted that compared with the HC group, the main cause of elevated scores in the NDPI group was the diminished capacity of daily living activities instead of a depressed mood. The HDRS score and 7 items of sub-scores showed no significant difference between the left and right lesion sides in DPI subjects. According to the percentage of sub-scores to the whole HDRS, we could see that the common depressive symptoms for DPI were retardation, desperation, anxiety/somatization and sleep with no difference between right and left lesion sides.
Table 1.
Demographic (age and gender) and clinical (HDRS, lesion sides and time post-infarction) characteristics in depression post-infarction (DPI), non-depression post-infarction (NDPI) and healthy control (HC) subjects
| Variables | DPI (N = 18) | NDPI (N = 21) | HC (N = 19) | F | df | p | ||
|---|---|---|---|---|---|---|---|---|
| Lesion side | Left (N = 9) | Right (N = 9) | Left (N = 11) | Right (N = 10) | – | |||
| Gender (female/male) | 5/4 | 4/5 | 4/7 | 4/6 | 11/8 | 0.381 | 4/53 | 0.821 |
| Age (M ± SD) | 61.4 ± 8.7 | 60.2 ± 2.3 | 61.1 ± 10.5 | 58.5 ± 7.2 | 55.2 ± 9.3 | 1.337 | 4/53 | 0.268 |
| TPI (M ± SD)(/mon) | 4.3 ± 4.1 | 7.06 ± 6.0 | 6.3 ± 4.6 | 3.0 ± 2.7 | – | 1.676 | 3/35 | 0.190 |
| HDRS (M ± SD) | 23.6 ± 5.1 | 20.0 ± 5.1 | 5.5 ± 1.6 | 6.6 ± 4.6 | 1.79 ± 2.46 | 78.844 | 4/53 | 0.000** |
| T | df | p | ||||||
| Anxiety/Somatization (M ± SD (PCT)) | 4.6 ± 2.1 (19.1%) | 3.4 ± 1.7 (16.9%) | – | – | – | −1.161 | 16 | 0.265 |
| Weight (M ± SD (PCT)) | 0.43 ± 0.79 (1.8%) | 0 ± 0 (0%) | – | – | – | −1.441 | 16 | 0.200 |
| Cognize (M ± SD (PCT)) | 1.6 ± 1.0 (6.6%) | 2.56 ± 1.59 (12.7%) | – | – | – | 1.435 | 16 | 0.173 |
| Diurnal variation (M ± SD (PCT)) | 0.7 ± 1.0 (2.9%) | 1.1 ± 0.78 (5.4%) | – | – | – | 0.917 | 16 | 0.375 |
| Retardation (M ± SD (PCT)) | 6.0 ± 2.6 (24.9%) | 4.9 ± 2.3 (24.3%) | – | – | – | −0.917 | 16 | 0.375 |
| Sleep (M ± SD (PCT)) | 4.4 ± 2.1 (18.3%) | 2.7 ± 2.1 (13.4%) | – | – | – | −1.639 | 16 | 0.124 |
| Desperation (M ± SD (PCT)) | 6.3 ± 1.5 (26.1%) | 5.4 ± 1.9 (26.7%) | – | – | – | −0.968 | 16 | 0.350 |
M Mean, SD standard deviation, mon Month, HDRS Hamilton depression rating scales, PCT the percentage of sub-scores to the whole HDRS, TPI time post-infarction
**p < 0.01
Difference of the LRTC in broadband EEG among different groups
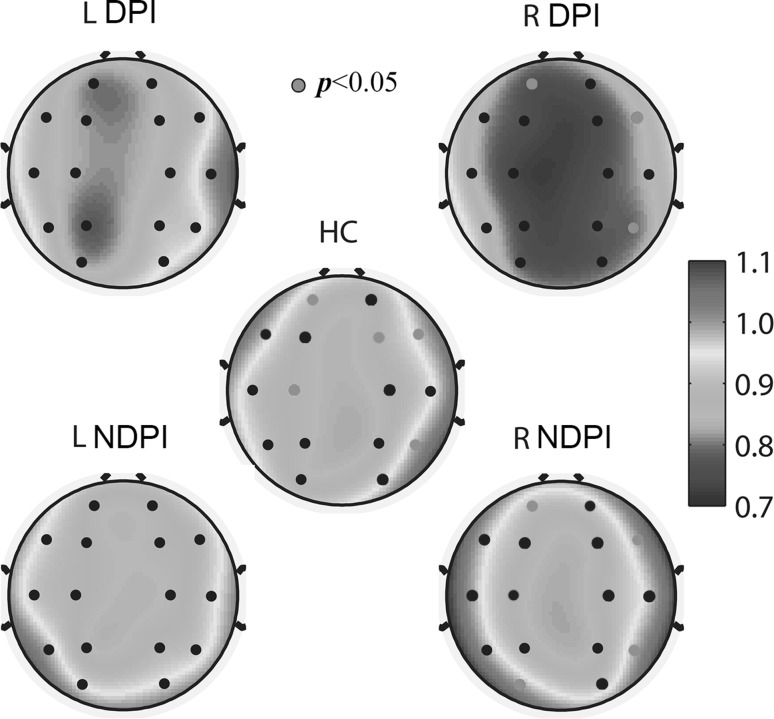
All scaling exponents of the three group subjects were in the interval of 0.5–1.0 in the range of 0.2–3 s, indicating persistent LRTC of EEG oscillation. The spatial distribution of the scaling exponents is presented in Fig. 2. According to the MANOVA analysis, significantly different scaling exponents of the five groups were found in regions FP1, FP2, F3, F4, C3, P4, O1, F7, F8 and T6 (p < 0.05). For DPI subjects with left lesion side, post hoc testing showed no significant difference with HC and NDPI subjects. For DPI subjects with right lesion side, LRTC was significantly attenuated (p < 0.05) in the pre-frontal and occipital of the left hemisphere (FP1 and O1) and inferior frontal and posterior temporal areas of the right hemisphere (F8 and T6) in contrast to NDPI subjects, and in the pre-frontal and central of the left hemisphere (FP1 and C3) and frontal and posterior temporal areas of the right hemisphere (F4, F8 and T6) in contrast to HC subjects. Table 2 shows the post hoc testing results for DPI subjects in a right lesion with NDPI and HC subjects separately. The most abnormal area for DPI subjects with a right lesion was the left pre-frontal (FP1), right inferior frontal (F8) and posterior temporal (T6), in which the scaling exponents were significantly different from both NDPI and HC subjects. Additionally, there was no significant difference among NDPI and HC subjects (p > 0.05), indicating no significant influences of unilateral stroke damage to the LRTC of broadband EEG oscillation without the effect of a depressive disorder.
Fig. 2.
Topographic plots of the scaling exponents of broadband oscillations in depression post-infarction (DPI) and non-depression post-infarction (NDPI) subjects with left and right hemispheric lesions and healthy control (HC) subjects. The pink colour of electrode locations of HC and NDPI with right hemispheric lesions represents p values of the t test compared to DPI less than 0.05. The pink colour of electrode locations of DPI indicates the channel in which both p values of the t-test with HC and NDPI were less than 0.05. (Color figure online)
Table 2.
Post hoc testing results of scaling exponents in the time range of 0.2–3 s in depression post-infarction (DPI) subjects with right-hemisphere lesion and non-depressed post-infarction (NDPI) subjects with right-hemisphere lesion and healthy controls (HC)
| Channel | Mean (±SD) | Sig | |||
|---|---|---|---|---|---|
| DPI | NDPI | HC | DPI Versus NDPI | DPI Versus HC | |
| FP1 | 0.763 ± 0.076 | 0.910 ± 0.105 | 0.904 ± 0.112 | 0.038* | 0.010* |
| FP2 | 0.792 ± 0.100 | 0.922 ± 0.088 | 0.868 ± 0.078 | 0.062 | 0.259 |
| F3 | 0.764 ± 0.112 | 0.883 ± 0.107 | 0.867 ± 0.085 | 0.054 | 0.075 |
| F4 | 0.753 ± 0.089 | 0.866 ± 0.096 | 0.883 ± 0.117 | 0.281 | 0.017* |
| C3 | 0.740 ± 0.103 | 0.870 ± 0.086 | 0.868 ± 0.135 | 0.218 | 0.037* |
| C4 | 0.780 ± 0.077 | 0.890 ± 0.092 | 0.867 ± 0.137 | 0.641 | 0.206 |
| P3 | 0.757 ± 0.103 | 0.882 ± 0.153 | 0.856 ± 0.166 | 0.244 | 0.658 |
| P4 | 0.781 ± 0.067 | 0.858 ± 0.110 | 0.859 ± 0.124 | 1.000 | 0.133 |
| O1 | 0.786 ± 0.112 | 0.995 ± 0.249 | 0.861 ± 0.139 | 0.029* | 1.000 |
| O2 | 0.780 ± 0.123 | 0.916 ± 0.130 | 0.939 ± 0.197 | 1.000 | 0.162 |
| F7 | 0.826 ± 0.099 | 0.973 ± 0.131 | 0.971 ± 0.126 | 0.206 | 0.056 |
| F8 | 0.839 ± 0.135 | 0.993 ± 0.080 | 0.966 ± 0.096 | 0.016* | 0.008* |
| T3 | 0.856 ± 0.127 | 0.991 ± 0.132 | 0.911 ± 0.113 | 0.261 | 1.000 |
| T4 | 0.848 ± 0.106 | 0.970 ± 0.129 | 0.940 ± 0.129 | 0.455 | 0.476 |
| T5 | 0.873 ± 0.107 | 0.987 ± 0.174 | 0.907 ± 0.143 | 0.645 | 1.000 |
| T6 | 0.791 ± 0.144 | 0.979 ± 0.115 | 0.980 ± 0.161 | 0.039* | 0.004* |
The italics represent significant difference between DPI and NDPI or HC subjects
* p < 0.05
Correlation of LRTC with severity of depressive symptoms
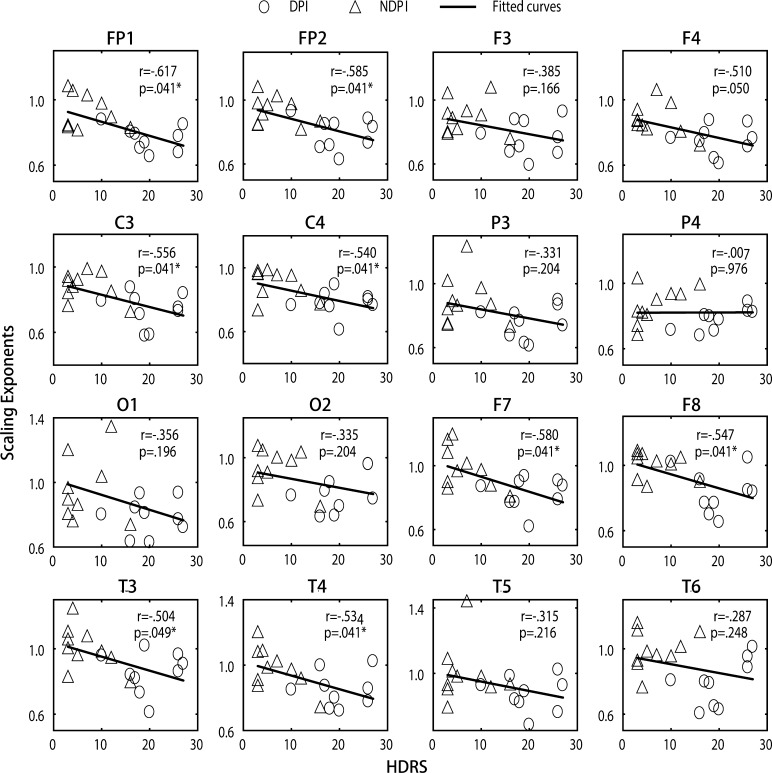
Linear correlation between scaling exponents and HDRS scores was analysed in stroke subjects with the right lesion side to find the relationship between LRTC abnormality and depressive severity. The result showed that the scaling exponents in the frontal (FP1, FP2, F7 and F8), central (C3 and C4) and temporal (T3 and T4) areas significantly declined with increasing HDRS scores (p < 0.05) (shown in Fig. 3).
Fig. 3.
Scatter diagram of the scaling exponents and HDRS scores in stroke subjects with right hemispheric lesions. r represents the Pearson correlation coefficient, and p represents the significance value
Discriminating ability of the LRTC for depressive disorders with right hemispheric lesion
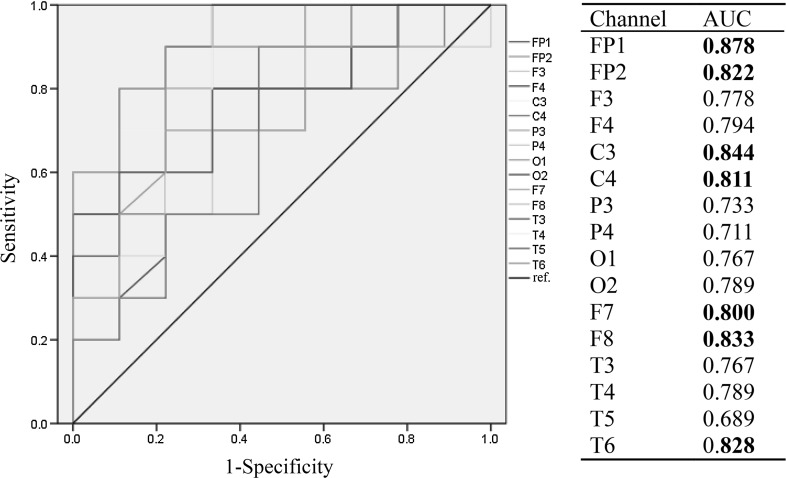
The ROC curves estimated the capability of scaling exponents of different regions to distinguish DPI subjects from NDPI subjects with right stroke lesions. Figure 4 displays the ROC curves and AUCs of scaling exponents for 16 channels. AUCs in the frontal (FP1, FP2, F7 and F8), central (C3 and C4) and right posterior temporal (T6) were up to 0.8, indicating a high diagnostic ability of scaling exponents in these areas to depressive disorders.
Fig. 4.
ROC curves of scaling exponents for 16 channels in cerebral infarction subjects with right hemispheric lesions. Boldface indicates that the area under the curve (AUC) was greater than 0.8
Discussion and conclusion
In this study, spontaneous broadband (0.5–30 Hz) EEG oscillation in DPI, NDPI and HC subjects was analysed by means of DFA, a quantitative measurement of the LRTC, to find the relationship between EEG oscillation and DPI with different lesion sides. The main finding of our study was that broadband EEG oscillations in DPI, NDPI and HC subjects all exhibit LRTC in the range of 0.2–3 s. Lesion lateralization of stroke had little effect on the LRTC of EEG oscillation. However, the persistence of LRTC was attenuated in most of the brain regions, especially the frontal and temporal areas for DPI subjects with the right lesion side but not with the left. Moreover, we observed an association between the severity of psychiatric symptoms and attenuation of LRTC in the frontal, central and temporal regions for stroke subjects with right lesions. The relatively high discriminating ability of scaling exponents in the frontal and central regions distinguishing DPI subjects from NDPI subjects, which suggested potential feasibility for LRTC as assessment indicators of depression with a right lesion stroke.
All scaling exponents of participants were found in the interval of 0.5–1.0, which demonstrated an LRTC of broadband EEG oscillation regardless of their neurologic and psychiatric condition. The values of the scaling exponents agreed with the previous studies showing an existence of LRTC in neuronal oscillations recorded with EEG/MEG (Linkenkaer-Hansen et al. 2001, 2005; Lee et al. 2007; Nikulin et al. 2012; Nikulin and Brismar 2004, 2005). Neither left nor right lesion sides subjects without depression showed difference from healthy controls, indicating that the infarct lesion side had little influence on the LRTC of EEG oscillation. This may be because EEG oscillation tends to be normal along with recovery in the chronic phase of a stroke (Juhász et al. 1997).
The persistence of the LRTC was attenuated in depressive patients with a right lesion location in most brain regions and was associated with the severity of psychiatric symptoms in right lesion stroke subjects, especially some emotion-related areas, such as frontal and temporal (Bhogal et al. 2004; Robinson and Coyle 1980; Yuvaraj and Murugappan 2016), but not in the left lesion. There is evidence that the right hemisphere is selectively involved in processing negative emotions, pessimistic thoughts and unconstructive thinking styles, all of which comprise the cognitive phenomenology of depression and in turn contribute to elevated anxiety and stress (Hecht 2010; Robinson and Starkstein 1989; Spalletta et al. 2007; Yuvaraj and Murugappan 2016). Investigators have recognized that emotional indifference was frequently the consequence of right frontal brain lesions (Denny-Brown et al. 1952; Bowers et al. 1991), and right hemisphere damage may influence the presentation of depressive disorders after stroke by disrupting emotion processing mechanisms (Paradiso et al. 2008). Some biochemical and behavioural responses related to depression were observed in experimental animal models following right-side infarcts but not left-side (Robinson and Coyle 1980). The LRTC described the dynamics of neuronal activations over long stretches of time. The attenuation of scaling exponents in depressive patients with right lesions indicates that the neuronal events in time are more loosely relating to each other than in infarction patients with no depressive and healthy controls. Thus, the attenuation is most likely due to the increased variability in neuronal activity (Nikulin et al. 2012). Because depression can be conceptualized as a syndrome of cortical-subcortical dysrhythmia with prefrontal or limbic abnormal activity marked by persistent resonance of several oscillations (Fingelkurts and Fingelkurts 2015), we hypothesize that the weaker temporal correlations might thus correspond to the basal ganglia infarction in the right side, which is related to emotion processing in depressive subjects.
Lesion location was one of the major biological theories on the pathophysiology of post-stroke depression (Pedroso et al. 2015; Zhang et al. 2012; Robinson 2003, 2006; Robinson and Coyle 1980), despite the inconsistency in association between depression and the location of brain lesions as a result of a stroke. Stroke lateralization was not related to depressive occurrence and symptoms in the present study, but a definite difference of the LRTC in broadband EEG oscillation was found among depressive patients with different lesion sides, which implied that the generation of depressive disorders with different hemisphere damage may be different and supports the notion that the association between depression following cerebral infarction and lesion location may be more complex than previously suggested (Sinyor et al. 1986; Bhogal et al. 2004; Wang et al. 2017).
In conclusion, this study found attenuated long-range temporal correlations of broadband EEG oscillation in depressive patients with right hemispheric cerebral infarction, which provided a novel insight into depression following stroke and may develop a new means to evaluate it. There are some limitations of the study. Although definite abnormality was found in depressive patients with right hemispheric lesions, more samples would be needed to obtain more reliable results regarding the abnormal brain regions and association with depressive symptoms. Because the EEG oscillatory pattern may be considered as a spatiotemporally organized superposition of multiple EEG oscillations in many frequency bands, different timescales and narrow frequency bands, including delta, theta, alpha and beta, will also be explored in future work to comprehensively prospect abnormal changes in depressive subjects following cerebral infarction.
Acknowledgement
This work was supported by Tianjin Municipal Commission of Health and Family Planning, Tianjin, China (14KG107, 2012KZ044).
Compliance with ethical standards
Conflict of interest
None of the authors have potential conflicts of interest to be disclosed.
Contributor Information
Chunfang Wang, Phone: +86-022-27557451, Email: chfwang@tju.edu.cn.
Jingang Du, Phone: +86-022-27557056, Email: rmyy126du@sina.com.
References
- American Electroencephalographic Society Guideline thirteen: guidelines for standard electrode position nomenclature. J Clin Neurophysiol. 1994;11:111–113. doi: 10.1097/00004691-199401000-00014. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4 (DSM-IV) Washington: American Psychiatric Association; 1994. [Google Scholar]
- Beggs JM, Plenz D. Neuronal avalanches in neocortical circuits. J Neurosci. 2003;23:11167–11177. doi: 10.1523/JNEUROSCI.23-35-11167.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg A, Palomäki H, Lehtihalmes M, et al. Poststroke depression in acute phase after stroke. Cerebrovasc Dis. 2001;12:14–20. doi: 10.1159/000047675. [DOI] [PubMed] [Google Scholar]
- Berg A, Palomäki H, Lehtihalmes M, et al. Poststroke depression: an 18-month follow-up. Stroke. 2003;34:138–143. doi: 10.1161/01.STR.0000048149.84268.07. [DOI] [PubMed] [Google Scholar]
- Bhogal SK, Teasell R, Foley N, Speechley M. Lesion location and poststroke depression: systematic review of the methodological limitations in the literature. Stroke. 2004;35:794–802. doi: 10.1161/01.STR.0000117237.98749.26. [DOI] [PubMed] [Google Scholar]
- Bogousslavsky J. William Feinberg lecture 2002: emotions, mood, and behavior after stroke. Stroke. 2003;34:1046–1050. doi: 10.1161/01.STR.0000061887.33505.B9. [DOI] [PubMed] [Google Scholar]
- Bornas X, Noguera M, Balle M, et al. Long-range temporal correlations in resting EEG: its associations with depression-related emotion regulation strategies. J Psychophysiol. 2013;27:60–66. doi: 10.1027/0269-8803/a000087. [DOI] [Google Scholar]
- Bornas X, Fiol-Veny A, Balle M, et al. Long range temporal correlations in EEG oscillations of subclinically depressed individuals: their association with brooding and suppression. Cogn Neurodyn. 2015;9:53–62. doi: 10.1007/s11571-014-9313-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers D, Blonder LX, Feinberg T, Heilman KM. Differential impact of right and left hemisphere lesions on facial emotion and object imagery. Brain. 1991;114:2593–2609. doi: 10.1093/brain/114.6.2593. [DOI] [PubMed] [Google Scholar]
- Carson AJ, MacHale S, Allen K, et al. Depression after stroke and lesion location: a systematic review. Lancet. 2000;356:122–126. doi: 10.1016/S0140-6736(00)02448-X. [DOI] [PubMed] [Google Scholar]
- Chemerinski E, Robinson RG, Kosier JT. Improved recovery in activities of daily living associated with remission of poststroke depression. Stroke. 2001;32:113–117. doi: 10.1161/01.STR.32.1.113. [DOI] [PubMed] [Google Scholar]
- de Coster L, Leentjens AFG, Lodder J, Verhey FRJ. The sensitivity of somatic symptoms in post-stroke depression: a discriminant analytic approach. Int J Geriatr Psychiatry. 2005;20:358–362. doi: 10.1002/gps.1290. [DOI] [PubMed] [Google Scholar]
- De Man-Van Ginkel JM, Hafsteinsdóttir TB, Lindeman E, et al. In-hospital risk prediction for post-stroke depression: development and validation of the post-stroke depression prediction scale. Stroke. 2013;44:2441–2445. doi: 10.1161/STROKEAHA.111.000304. [DOI] [PubMed] [Google Scholar]
- Denny-brown D, Meyer JS, Horenstein S. The significance of perceptual rivalry resulting from parietal lesion. Brain. 1952;75:432–471. doi: 10.1093/brain/75.4.432. [DOI] [PubMed] [Google Scholar]
- Dimitriadis SI, Laskaris NA, Micheloyannis S. Transition dynamics of EEG-based network microstates during mental arithmetic and resting wakefulness reflects task-related modulations and developmental changes. Cogn Neurodyn. 2015;9:371–387. doi: 10.1007/s11571-015-9330-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields C, Glazebrook JF. Disrupted development and imbalanced function in the global neuronal workspace: a positive-feedback mechanism for the emergence of ASD in early infancy. Cogn Neurodyn. 2017;11:1–21. doi: 10.1007/s11571-016-9419-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingelkurts AA, Fingelkurts AA. Altered structure of dynamic electroencephalogram oscillatory pattern in major depression. Biol Psychiatry. 2015;77:1050–1060. doi: 10.1016/j.biopsych.2014.12.011. [DOI] [PubMed] [Google Scholar]
- Fiol-Veny A, Balle M, Bornas X. Long range temporal correlations in EEG and depression. Ann Depress Anxiety. 2015;2:1–5. doi: 10.1007/s11571-014-9313-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florin E, Baillet S. The brain’s resting-state activity is shaped by synchronized cross-frequency coupling of neural oscillations. Neuroimage. 2015;111:26–35. doi: 10.1016/j.neuroimage.2015.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaete JM, Bogousslavsky J. Post-stroke depression. Expert Rev Neurother. 2008;8:75–92. doi: 10.1586/14737175.8.1.75. [DOI] [PubMed] [Google Scholar]
- Gutiérrez D, Ramírez-Moreno MA. Assessing a learning process with functional ANOVA estimators of EEG power spectral densities. Cogn Neurodyn. 2016;10:175–183. doi: 10.1007/s11571-015-9368-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht D. Depression and the hyperactive right-hemisphere. Neurosci Res. 2010;68:77–87. doi: 10.1016/j.neures.2010.06.013. [DOI] [PubMed] [Google Scholar]
- Herrmann M, Bartels C, Schumacher M, Wallesch CW. Poststroke depression. Is there a pathoanatomic correlate for depression in the postacute stage of stroke? Stroke. 1995;26:850–856. doi: 10.1161/01.STR.26.5.850. [DOI] [PubMed] [Google Scholar]
- House A, Dennis M, Warlow C, et al. Mood disorders after stroke and their relation to lesion location. A CT scan study. Brain. 1990;113(Pt 4):1113–1129. doi: 10.1093/brain/113.4.1113. [DOI] [PubMed] [Google Scholar]
- Juhász C, Kamondi A, Szirmai I. Spectral EEG analysis following hemispheric stroke. Acta Neurol Scand. 1997;96:397–400. doi: 10.1111/j.1600-0404.1997.tb00305.x. [DOI] [PubMed] [Google Scholar]
- Karkare S, Saha G, Bhattacharya J. Investigating long-range correlation properties in EEG during complex cognitive tasks. Chaos Solitons Fractals. 2009;42:2067–2073. doi: 10.1016/j.chaos.2009.03.148. [DOI] [Google Scholar]
- Lee JS, Yang BH, Lee JH, et al. Detrended fluctuation analysis of resting EEG in depressed outpatients and healthy controls. Clin Neurophysiol. 2007;118:2489–2496. doi: 10.1016/j.clinph.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Leistedt S, Dumont M, Coumans N, et al. The modifications of the long-range temporal correlations of the sleep EEG due to major depressive episode disappear with the status of remission. Neuroscience. 2007;148:782–793. doi: 10.1016/j.neuroscience.2007.06.032. [DOI] [PubMed] [Google Scholar]
- Leistedt S, Dumont M, Lanquart JP, et al. Characterization of the sleep EEG in acutely depressed men using detrended fluctuation analysis. Clin Neurophysiol. 2007;118:940–950. doi: 10.1016/j.clinph.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Li Y, Qiu J, Yan R, et al. Weakened long-range correlation of renal sympathetic nerve activity in Wistar rats after anaesthesia. Neurosci Lett. 2008;433:28–32. doi: 10.1016/j.neulet.2007.12.038. [DOI] [PubMed] [Google Scholar]
- Linkenkaer-Hansen K, Nikouline VV, Palva JM, et al. Long-range temporal correlations and scaling behavior in human brain oscillations. J Neurosci. 2001;21:1370–1377. doi: 10.1523/JNEUROSCI.21-04-01370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkenkaer-Hansen K, Monto S, Rytsälä H, et al. Breakdown of long-range temporal correlations in theta oscillations in patients with major depressive disorder. J Neurosci. 2005;25:10131–10137. doi: 10.1523/JNEUROSCI.3244-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkenkaer-Hansen K, Smit D, Barkil A, et al. Genetic contributions to long-range temporal correlations in ongoing oscillations. J Neurosci. 2007;27:13882–13889. doi: 10.1523/JNEUROSCI.3083-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montez T, Poil S-S, Jones BF, et al. Altered temporal correlations in parietal alpha and prefrontal theta oscillations in early-stage Alzheimer disease. Proc Natl Acad Sci USA. 2009;106:1614–1619. doi: 10.1073/pnas.0811699106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikulin VV, Brismar T. Long-range temporal correlations in alpha and beta oscillations: effect of arousal level and test-retest reliability. Clin Neurophysiol. 2004;115:1896–1908. doi: 10.1016/j.clinph.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Nikulin VV, Brismar T. Long-range temporal correlations in electroencephalographic oscillations: relation to topography, frequency band, age and gender. Neuroscience. 2005;130:549–558. doi: 10.1016/j.neuroscience.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Nikulin VV, Jönsson EG, Brismar T. Attenuation of long-range temporal correlations in the amplitude dynamics of alpha and beta neuronal oscillations in patients with schizophrenia. Neuroimage. 2012;61:162–169. doi: 10.1016/j.neuroimage.2012.03.008. [DOI] [PubMed] [Google Scholar]
- Paradiso S, Vaidya J, Tranel D. Nondysphoric depression following stroke. The Journal of neuropsychiatry and clinical neurosciences. 2008;20(1):52–61. doi: 10.1176/jnp.2008.20.1.52. [DOI] [PubMed] [Google Scholar]
- Pedroso VSP, de Souza LC, Brunoni AR, Teixeira AL. Post stroke depression: clinics, etiopathogenesis and therapeutics. Rev Psiquiatr Clin. 2015;42:18–24. doi: 10.1590/0101-60830000000041. [DOI] [Google Scholar]
- Peng CK, Havlin S, Stanley HE, Goldberger AL. Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos. 1995;5:82–87. doi: 10.1063/1.166141. [DOI] [PubMed] [Google Scholar]
- Pu J, Xu H, Wang Y, et al. Combined nonlinear metrics to evaluate spontaneous EEG recordings from chronic spinal cord injury in a rat model: a pilot study. Cogn Neurodyn. 2016;10:1–7. doi: 10.1007/s11571-016-9394-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu J, Wang R, Yan C, et al. Oscillations and synchrony in a cortical neural network. Cogn Neurodyn. 2014;8(2):157–166. doi: 10.1007/s11571-013-9268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson R. Poststroke depression: prevalence, diagnosis, treatment, and disease progression. Biol Psychiatry. 2003;54:376–387. doi: 10.1016/S0006-3223(03)00423-2. [DOI] [PubMed] [Google Scholar]
- Robinson RG. The clinical neuropsychiatry of stroke. New York: Cambridge University Press; 2006. [Google Scholar]
- Robinson RG, Coyle JT. The differential effect of right versus left hemispheric cerebral infarction on catecholamines and behavior in the rat. Brain Res. 1980;188:63–78. doi: 10.1016/0006-8993(80)90557-0. [DOI] [PubMed] [Google Scholar]
- Robinson RG, Starkstein SE. Mood disorders following stroke: new findings and future directions. J Geriatr Psychiatry. 1989;22:1–15. [PubMed] [Google Scholar]
- Singh A, Black SE, Herrmann N, et al. Functional and neuroanatomic correlations in poststroke depression: the Sunnybrook stroke study. Stroke. 2000;31:637–644. doi: 10.1161/01.STR.31.3.637. [DOI] [PubMed] [Google Scholar]
- Sinyor D, Jacques P, Kaloupek DG, et al. Poststroke depression and lesion location: an attempted replication. Brain. 1986;109:537–546. doi: 10.1093/brain/109.3.537. [DOI] [PubMed] [Google Scholar]
- Smit DJ, de Geus EJ, van de Nieuwenhuijzen ME, et al. Scale-free modulation of resting-state neuronal oscillations reflects prolonged brain maturation in humans. J Neurosci. 2011;31:13128–13136. doi: 10.1523/JNEUROSCI.1678-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalletta G, Serra L, Fadda L, et al. Unawareness of motor impairment and emotions in right hemispheric stroke: a preliminary investigation. Int J Geriatr Psychiatry. 2007;22:1241–1246. doi: 10.1002/gps.1822. [DOI] [PubMed] [Google Scholar]
- Stam CJ, de Bruin EA. Scale-free dynamics of global functional connectivity in the human brain. Hum Brain Mapp. 2004;22:97–109. doi: 10.1002/hbm.20016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Cho RY, Murata T, et al. Age-related variation in EEG complexity to photic stimulation: a multiscale entropy analysis. Clin Neurophysiol. 2009;120:476–483. doi: 10.1016/j.clinph.2008.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CF, Chen YY, Zhang Y, et al. Quantitative EEG abnormalities in major depressive disorder with basal ganglia stroke with lesions in different hemispheres. J Affect Disord. 2017;215:172–178. doi: 10.1016/j.jad.2017.02.030. [DOI] [PubMed] [Google Scholar]
- Yuvaraj R, Murugappan M. Hemispheric asymmetry non-linear analysis of EEG during emotional responses from idiopathic Parkinson’s disease patients. Cogn Neurodyn. 2016;10:225–234. doi: 10.1007/s11571-016-9375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Jing X, Zhao X, et al. Depression after stroke and lesion location: a systematic review. J Affect Disord. 2012;136:e83–e87. doi: 10.1016/j.jad.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang C, Sun C, et al (2015) Neural complexity in patients with poststroke depression: a resting EEG study. J Affect Disord 188:310–318. doi:10.1016/j.jad.2015.09.017 [DOI] [PubMed]