Abstract
Aims
Advanced stage of oral squamous cell carcinoma (OSCC) exhibits different properties compared with the early stage for example an invasion ability. The present study investigated a differential gene expression of surgical margin between advanced and early stage of OSCC.
Methods
Gene Expression Omnibus dataset (GSE31056) was downloaded and re-analyzed. Surgical margin samples were categorized into 2 groups; early stage and late stage. Differential gene expression analysis was performed. Dysregulated genes were further analyzed for gene ontology, enriched pathway, and disease association using a network-based analysis tools.
Results
Eighty-five dysregulated genes were identified in margin of late stage OSCC. Metabolic process and biological regulation were the main gene ontology of dysregulated genes. Genes involved in Jak-STAT signaling pathway were upregulated in late stage of surgical margin samples. In addition, seven upregulated genes in late stage group, namely CEBPB, S1PR1, IL6, CEBPD, CHI3L1, PTX3, and SOCS3, were categorized in acute phase reaction and inflammation categories of disease association analysis.
Conclusion
The differential expressed genes in surgical margin of late stage OSCC could be further employed to understand cancer's behavior and to identify target pathway to prevent OSCC invasion.
Keywords: Interleukin-6, Oral squamous cell carcinoma, Stage, Expression profiling, Inflammation
1. Introduction
Oral squamous cell carcinoma (OSCC) is one of the common oral cancers.1, 2 Prevalence of OSCC is range from 60% to 100% of all oral cancers and is high in Asian country due to predisposing risk factors for example betel nut chewing.1, 2, 3 A lymph node metastasis, tumor volume, and tumor invasiveness are factors related to poor prognosis.4, 5 Various pathways were dysregulated in OSCC compared to the normal tissues including metabolic pathway, extracellular matrix-receptor interaction, focal adhesion, cytokine-cytokine receptor interaction, and cell cycle progression.6
Surgical resection is one of the treatment options of OSCC. Margin assessment is indeed an essential prognostic factor. Gross inspection and frozen section evaluation have been employed to identify surgical margin.7 Margin dysplasia is related to the lower survival rate.8 However, studies illustrated that surgical margin of the lesion which appears normal in histological observation exhibited a genetic alteration. These molecular changes could lead to the recurrence of OSCC after resection.9 In this respect, the positive staining of p53 was observed in basal and parabasal cells in the surgical margin.10 Previous work also reported that the expression of MMP1, COL4A1, P4HA2, and THBS2 in surgical margin was associated with OSCC recurrence.9
Gene expression in the margin has been shown to correlate with the aggressiveness of OSCC, for example the VEGF overexpression.11 This gene were highly expressed in tumor margin compared with OSCC and in stage 3–4 compared with stage 1–2.11 These results suggest that the differential expression of gene expression in the tumor margin at different cancer stage may participate in OSCC progression.11 The present study aimed to investigate a differential gene expression of surgical margin between advanced and early stage of OSCC.
2. Methods
2.1. Dataset processing
Public available microarray expression profiling of OSCC was identified from Gene Expression Omnibus (GEO) database. GEO dataset, GSE31056, was downloaded.9 Probe IDs that did not match official gene symbol or matched with multiple official gene symbol were excluded from the analysis. The included criteria for GSE samples were (1) surgical margin; (2) known disease stage. Samples from tumors and normal tissues were excluded. The included GSE samples were categorized into 2 groups. First group was the surgical margin samples of OSCC stage 1 and 2. The second group was the surgical margin samples of OSCC stage 3 and 4.
2.2. Bioinformatic analysis
Processed dataset was uploaded to NetworkAnalyst, a network-based analysis of gene expression data.12, 13, 14 Mean intensity was calculated and variance data filtering was set at 15%. Limma statistical method was employed. Differentially expressed genes, which exhibited adjusted p-value <0.05 and log 2 fold change >1, were included for further analysis. Heatmap visualization of differentially expressed genes was performed using NetworkAnalyst. Gene ontology, KEGG enriched pathway, pathway commons, disease association analysis were performed using WebGestalt.15, 16 The analyses were performed using hypergeometric methods with significance level set at p < 0.05.
3. Results
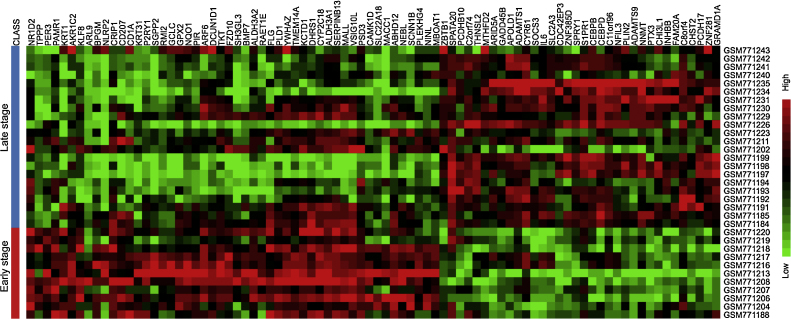
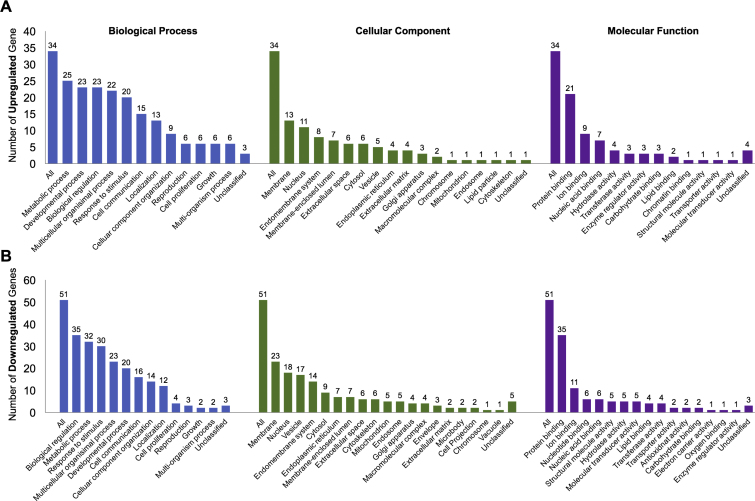
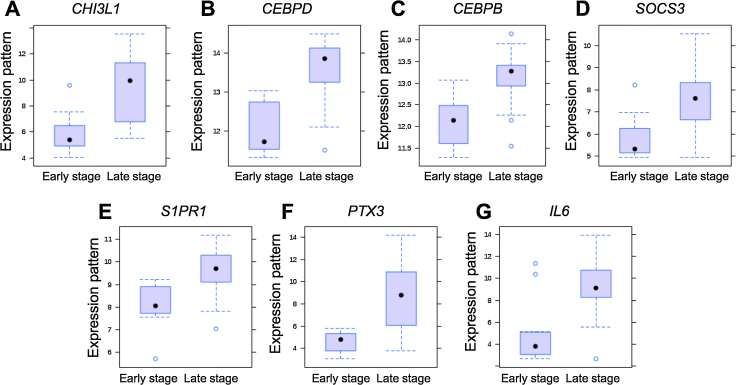
Dataset contained 11 samples for group 1 (margin of early stage OSCC) and 22 samples for group 2 (margin of late stage OSCC). Total of 85 dysregulated genes were identified, composing of 34 upregulated and 51 downregulated genes in margin of late stage OSCC (Fig. 1). Top 10 differentially expressed genes were shown in Table 1. Gene ontology analysis of biological process demonstrated that majority of upregulated genes were related to metabolic process while the downregulated genes were involved in biological regulation (Fig. 2). Main dysregulated genes were categorized on membrane and protein binding group in cellular component and molecular function categories (Fig. 2). KEGG pathway enrichment analysis demonstrated that the upregulated genes were clustered in Jak-STAT signaling pathway (Table 2). Corresponding to Pathway Commons analysis, IL6-mediated signaling events and Interleukin-6 signaling categories were observed for the upregulated genes (Table 3). While the downregulated genes were clustered in metabolic pathway in both KEGG and Pathway Commons analysis. Using disease association analysis, seven upregulated genes were categorized in acute phase reaction and inflammation (Table 4). These genes were CEBPB, S1PR1, IL6, CEBPD, CHI3L1, PTX3, and SOCS3. Among these, the upregulation of IL6, CHI3L1, and PTX3 was higher than 3.5 folds (Fig. 3).
Fig. 1.
Heatmap demonstrated the differentially expressed genes between surgical margins of early and late stage OSCC.
Table 1.
Top 10 differentially expressed genes.
| Gene symbol | Gene name | Entrez ID | Log 2FC | Adjusted p value |
|---|---|---|---|---|
| TPPP | Tubulin polymerization promoting protein | 11076 | 2.0765 | 1.40e−03 |
| PER3 | Period homolog 3 (Drosophila) | 8863 | 1.0577 | 1.96e−03 |
| KRT31 | Keratin 31 | 3881 | 4.0921 | 2.51e−03 |
| CEBPD | CCAAT/enhancer binding protein (C/EBP), delta | 1052 | −1.5553 | 3.59e−03 |
| ZNF281 | Zinc finger protein 281 | 23528 | −1.0125 | 3.59e−03 |
| PAMR1 | Peptidase domain containing associated with muscle regeneration 1 | 25891 | 1.7659 | 7.32e−03 |
| P2RY1 | Purinergic receptor P2Y, G-protein coupled, 1 | 5028 | 1.7692 | 8.72e−03 |
| NQO1 | NAD(P)H dehydrogenase, quinone 1 | 1728 | 2.0162 | 1.26e−02 |
| APOLD1 | Apolipoprotein L domain containing 1 | 81575 | −1.9391 | 1.26e−02 |
| ALDH3A2 | Aldehyde dehydrogenase 3 family, member A2 | 224 | 1.084 | 1.26e−02 |
Fig. 2.
Gene ontology analysis of the differentially expressed genes between surgical margins of early and late stage OSCC: (A) upregulated genes and (B) downregulated genes.
Table 2.
KEGG pathway enrichment analysis.
| Pathway name | Number of genes | Adjusted p value |
|---|---|---|
| Downregulated genes | ||
| Metabolism of xenobiotics by cytochrome P450 | 4 | 0.0004 |
| Glutathione metabolism | 3 | 0.0018 |
| Glycosis/gluconeogenesis | 3 | 0.0024 |
| Drug metabolism-cytochrome P450 | 3 | 0.0024 |
| Endocytosis | 4 | 0.0038 |
| Histidine metabolism | 2 | 0.0048 |
| Metabolic pathways | 8 | 0.0105 |
| Glycerolipid metabolism | 2 | 0.0105 |
| Arachinodic acid metabolism | 2 | 0.0129 |
| Glycerophospholipid metabolism | 2 | 0.0208 |
| Fc gamma R-mediated phagocytosis | 2 | 0.0255 |
| Upregulated genes | ||
| Jak-STAT signaling pathway | 3 | 0.0066 |
Table 3.
Top 10 pathway commons analysis.
| Pathway name | Number of genes | Adjusted p value |
|---|---|---|
| Downregulated genes | ||
| Glutathione conjugation | 2 | 0.0408 |
| Upregulated genes | ||
| IL6-mediated signaling events | 4 | 0.0000851 |
| AP-1 transcription factor network | 7 | 0.0017 |
| Integrin-linked kinase signaling | 7 | 0.0017 |
| Interleukin-6 signaling | 2 | 0.0017 |
| Nectin adhesion pathway | 8 | 0.0022 |
| EGF receptor (ErbB1) signaling pathway | 8 | 0.0022 |
| VEGF and VEGFR signaling network | 8 | 0.0022 |
| PDGFR-beta signaling pathway | 8 | 0.0022 |
| Alpha9 beta1 integrin signaling events | 8 | 0.0022 |
| Arf6 trafficking events | 8 | 0.0022 |
Table 4.
Disease association analysis for upregulated genes.
| Pathway name | Gene symbol | Adjusted p value |
|---|---|---|
| Acute phase reaction | CEBPB, IL6, CEBPD, PTX3, SOCS3 | 1.58e−08 |
| Inflammation | CEBPB, S1PR1, IL6, CEBPD, CHI3L1, PTX3, SOCS3 | 5.92e−06 |
Fig. 3.
Expression pattern of upregulated genes involved in acute phase reaction and inflammation categories in disease association analysis.
4. Discussion
It should be noted that the present study identified the dysregulated genes using bioinformatics re-analysis of public available expression microarray database without the validation information of tissue expression. Hence, the interpretation and further applications should be done with caution. The validation of the dysregulated genes in the tissue sections either by polymerase chain reaction or immunocytochemistry staining is indeed necessitated. However, previous report of this dataset has been validated.9 In addition, it has been demonstrated some dysregulated genes identified in the present study in the tissue samples. NAD(P)H dehydrogenase, quinone 1 (NQO1) mRNA expression was significantly higher in the margin of late stage OSCC than those of early stage samples. Correspondingly, previous work demonstrated that NQO1 was expressed in head and neck squamous cell carcinoma and their margins.17 Though, the normal margin exhibited lower NQO1 protein expression than those of cancer tissues. Further, KRT31 was upregulated in metastatic head and neck carcinoma, while less expression was noted in normal and N0/N1 lesion.18 These evidences confirmed the expression of dysregulated genes in cancer and its margin.
The present study demonstrated that surgical margin of late stage OSCC expressed higher genes in Jak-STAT signaling pathway, namely IL6, SOCS3, and SPRY1. Corresponding with previous study, a meta-analysis of expression profiling of OSCC showed the upregulation of Jak-STAT pathway compared to the normal tissues.6 STAT3 dysregulation involved in carcinogenesis, treatment resistance and immune escape of head and neck squamous cell carcinoma (HNSCC).19, 20 Thus, several studies targeted this pathway as a candidate treatment approach. It has been shown that Jak kinase inhibition resulted in the reduction of HNSCC cell proliferation in vitro and tumor growth in vivo.21 In addition, an active compound of Magnolia officinalis, Honokiol, induced OSCC apoptosis via the suppression of Jak-STAT, Akt and Erk pathway.22 Together, the overexpression of Jak-STAT signaling pathway in margin of late stage OSCC may link to the tumor aggressiveness.
IL6 acts as both pro- and anti-inflammatory cytokine. Serum and saliva IL6 protein levels were significantly increased in OSCC patients compared to the healthy control.23, 24 IL6 promoted migration of OSCC cells in vitro.22 Further, IL6 expression correlated with pattern of invasion, vascular invasion and pathological nodal status.25 IL6 overexpression led to the decrease of disease free survival rate in OSCC patients.25 It has been shown that SOCS3 polymorphisms was linked with the risk of head and neck squamous cell carcinoma.26 Upregulation of SOCS3 was observed in OSCC compared with the control.27 SPRY1 is a modulator of a receptor tyrosine kinase signaling. Though, role in OSCC has not yet been reported. Among these three genes, IL6 fold change was the highest (4.32 folds). Thus, IL6 expression could be used as a marker to identify normal tumor margin.
After performing disease association analysis, the acute phase reaction and inflammation categories were identified for the upregulated genes. Genes that related to these two categories were CEBPB, S1PR1, IL6, CEBPD, CHI3L1, PTX3, and SOCS3. Beside the influence of IL6 and SOCS3 in OSCC described above, PTX3 is another molecule which has been investigated the participation in OSCC. PTX3 has been proposed as a maker for cancer-related inflammation. PTX3 was induced in fibroblast after co-culture with OSCC cell line via IL1 signaling.28 However, an influence of CEBPB, S1PR1, CEBPD, and CHI3L1 in OSCC has not yet been identified. It has been shown that CHI3L1 overexpression was associated with poor prognosis of thyroid carcinoma.29 CHI3L1 may contribute to breast cancer growth and metastasis as it induced the expression of CCL2, CXCL2, and MMP9.30 CEBPB and CEBPD were shown to be involved in the chemoresistance in other cancer cell types.31, 32 S1PR1 expression correlated with poor prognosis in urothelial carcinoma.33
Inflammation induced/mediated carcinogenesis in OSCC has been widely investigated.34 Anti-inflammatory medication are considered to be combined with conventional OSCC treatment.34 However, the inflammation rather promotes cancer progression than induced cancer formation.35 Corresponding with the present study, the upregulation of inflammation related genes at tumor margin of late stage OSCC may participate to recurrence and progression of the disease.
Conflict of interest
The authors have none to declare.
Acknowledgement
The present study was supported by the Dental Research Project Funding, Faculty of Dentistry. Authors thank Miss. Nonrut Prompianpong and Miss. Suchaya Peachpaibul for GSE dataset preparation.
References
- 1.Al-Jaber A., Al-Nasser L., El-Metwally A. Epidemiology of oral cancer in Arab countries. Saudi Med J. 2016;37:249–255. doi: 10.15537/smj.2016.3.11388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maleki D., Ghojazadeh M., Mahmoudi S.S. Epidemiology of oral cancer in Iran: a systematic review. Asian Pac J Cancer Prev. 2015;16:5427–5432. doi: 10.7314/apjcp.2015.16.13.5427. [DOI] [PubMed] [Google Scholar]
- 3.Krishna Rao S.V., Mejia G., Roberts-Thomson K., Logan R. Epidemiology of oral cancer in Asia in the past decade – an update (2000–2012) Asian Pac J Cancer Prev. 2013;14:5567–5577. doi: 10.7314/apjcp.2013.14.10.5567. [DOI] [PubMed] [Google Scholar]
- 4.Lin C.S., de Oliveira Santos A.B., Silva E.L. Tumor volume as an independent predictive factor of worse survival in patients with oral cavity squamous cell carcinoma. Head Neck. 2017 doi: 10.1002/hed.24714. [DOI] [PubMed] [Google Scholar]
- 5.de Morais E.F., Mafra R.P., Gonzaga A.K., de Souza D.L., Pinto L.P., da Silveira E.J. Prognostic factors of oral squamous cell carcinoma in young patients: a systematic review. J Oral Maxillofac Surg. 2016 doi: 10.1016/j.joms.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 6.Osathanon T., Nowwarote N., Pavasant P. Expression and influence of Notch signaling in oral squamous cell carcinoma. J Oral Sci. 2016;58:283–294. doi: 10.2334/josnusd.15-0535. [DOI] [PubMed] [Google Scholar]
- 7.Mair M., Nair D., Nair S. Intraoperative gross examination vs frozen section for achievement of adequate margin in oral cancer surgery. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016 doi: 10.1016/j.oooo.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 8.Gokavarapu S., Parvataneni N., Pavagada S., Chandrasekhara Rao L.M., Raju K.V., Rao T.S. Mild to moderate dysplasia at surgical margin is a significant indicator of survival in patients with oral cancer. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123:330–337. doi: 10.1016/j.oooo.2016.10.028. [DOI] [PubMed] [Google Scholar]
- 9.Reis P.P., Waldron L., Perez-Ordonez B. A gene signature in histologically normal surgical margins is predictive of oral carcinoma recurrence. BMC Cancer. 2011;11:437. doi: 10.1186/1471-2407-11-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bilde A., von Buchwald C., Dabelsteen E., Therkildsen M.H., Dabelsteen S. Molecular markers in the surgical margin of oral carcinomas. J Oral Pathol Med. 2009;38:72–78. doi: 10.1111/j.1600-0714.2008.00715.x. [DOI] [PubMed] [Google Scholar]
- 11.Sales C.B., Buim M.E., de Souza R.O. Elevated VEGFA mRNA levels in oral squamous cell carcinomas and tumor margins: a preliminary study. J Oral Pathol Med. 2016;45:481–485. doi: 10.1111/jop.12398. [DOI] [PubMed] [Google Scholar]
- 12.Xia J., Gill E.E., Hancock R.E. NetworkAnalyst for statistical, visual and network-based meta-analysis of gene expression data. Nat Protoc. 2015;10:823–844. doi: 10.1038/nprot.2015.052. [DOI] [PubMed] [Google Scholar]
- 13.Xia J., Benner M.J., Hancock R.E. NetworkAnalyst – integrative approaches for protein-protein interaction network analysis and visual exploration. Nucleic Acids Res. 2014;42:W167–W174. doi: 10.1093/nar/gku443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia J., Lyle N.H., Mayer M.L., Pena O.M., Hancock R.E. INVEX – a web-based tool for integrative visualization of expression data. Bioinformatics. 2013;29:3232–3234. doi: 10.1093/bioinformatics/btt562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang B., Kirov S., Snoddy J. WebGestalt: an integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res. 2005;33:W741–W748. doi: 10.1093/nar/gki475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J., Duncan D., Shi Z., Zhang B. WEB-based GEne SeT AnaLysis Toolkit (WebGestalt): update 2013. Nucleic Acids Res. 2013;41:W77–W83. doi: 10.1093/nar/gkt439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li C., Wu H., Wang S., Zhu J. Expression and correlation of NRF2, KEAP1, NQO-1 and HO-1 in advanced squamous cell carcinoma of the larynx and their association with clinicopathologic features. Mol Med Rep. 2016;14:5171–5179. doi: 10.3892/mmr.2016.5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silveira N.J., Varuzza L., Machado-Lima A. Searching for molecular markers in head and neck squamous cell carcinomas (HNSCC) by statistical and bioinformatic analysis of larynx-derived SAGE libraries. BMC Med Genomics. 2008;1:56. doi: 10.1186/1755-8794-1-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geiger J.L., Grandis J.R., Bauman J.E. The STAT3 pathway as a therapeutic target in head and neck cancer: barriers and innovations. Oral Oncol. 2016;56:84–92. doi: 10.1016/j.oraloncology.2015.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagpal J.K., Mishra R., Das B.R. Activation of Stat-3 as one of the early events in tobacco chewing-mediated oral carcinogenesis. Cancer. 2002;94:2393–2400. doi: 10.1002/cncr.10499. [DOI] [PubMed] [Google Scholar]
- 21.Sen M., Pollock N.I., Black J. JAK kinase inhibition abrogates STAT3 activation and head and neck squamous cell carcinoma tumor growth. Neoplasia. 2015;17:256–264. doi: 10.1016/j.neo.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang J.S., Yao C.J., Chuang S.E. Honokiol inhibits sphere formation and xenograft growth of oral cancer side population cells accompanied with JAK/STAT signaling pathway suppression and apoptosis induction. BMC Cancer. 2016;16:245. doi: 10.1186/s12885-016-2265-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dineshkumar T., Ashwini B.K., Rameshkumar A., Rajashree P., Ramya R., Rajkumar K. Salivary and serum interleukin-6 levels in oral premalignant disorders and squamous cell carcinoma: diagnostic value and clinicopathologic correlations. Asian Pac J Cancer Prev. 2016;17:4899–4906. doi: 10.22034/APJCP.2016.17.11.4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lotfi A., Shahidi N., Bayazian G. Serum level of interleukin-6 in patients with oral tongue squamous cell carcinoma. Iran J Otorhinolaryngol. 2015;27:207–211. [PMC free article] [PubMed] [Google Scholar]
- 25.Shinagawa K., Yanamoto S., Naruse T. Clinical roles of interleukin-6 and STAT3 in oral squamous cell carcinoma. Pathol Oncol Res. 2016 doi: 10.1007/s12253-016-0134-x. [DOI] [PubMed] [Google Scholar]
- 26.Hang D., Yin Y., Wang L. Effects of potentially functional polymorphisms in suppressor of cytokine signaling 3 (SOCS3) on the risk of head and neck squamous cancer. J Oral Pathol Med. 2016 doi: 10.1111/jop.12539. [DOI] [PubMed] [Google Scholar]
- 27.Kim K.Y., Zhang X., Cha I.H. Combined genomic expressions as a diagnostic factor for oral squamous cell carcinoma. Genomics. 2014;103:317–322. doi: 10.1016/j.ygeno.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 28.Hakelius M., Reyhani V., Rubin K., Gerdin B., Nowinski D. Normal oral keratinocytes and head and neck squamous carcinoma cells induce an innate response of fibroblasts. Anticancer Res. 2016;36:2131–2137. [PubMed] [Google Scholar]
- 29.Luo D., Chen H., Lu P. CHI3L1 overexpression is associated with metastasis and is an indicator of poor prognosis in papillary thyroid carcinoma. Cancer Biomark. 2016 doi: 10.3233/CBM-160255. [DOI] [PubMed] [Google Scholar]
- 30.Libreros S., Garcia-Areas R., Shibata Y., Carrio R., Torroella-Kouri M., Iragavarapu-Charyulu V. Induction of proinflammatory mediators by CHI3L1 is reduced by chitin treatment: decreased tumor metastasis in a breast cancer model. Int J Cancer. 2012;131:377–386. doi: 10.1002/ijc.26379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gardiner J.D., Abegglen L.M., Huang X. C/EBPbeta-1 promotes transformation and chemoresistance in Ewing sarcoma cells. Oncotarget. 2017 doi: 10.18632/oncotarget.14847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin S.R., Yeh H.C., Wang W.J. MiR-193b mediates CEBPD-induced cisplatin sensitization through targeting ETS1 and Cyclin D1 in human urothelial carcinoma cells. J Cell Biochem. 2016 doi: 10.1002/jcb.25818. [DOI] [PubMed] [Google Scholar]
- 33.Go H., Kim P.J., Jeon Y.K. Sphingosine-1-phosphate receptor 1 (S1PR1) expression in non-muscle invasive urothelial carcinoma: association with poor clinical outcome and potential therapeutic target. Eur J Cancer. 2015;51:1937–1945. doi: 10.1016/j.ejca.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 34.Sarode G.S., Sarode S.C., Patil A. Inflammation and oral cancer: an update review on targeted therapies. J Contemp Dent Pract. 2015;16:595–602. doi: 10.5005/jp-journals-10024-1727. [DOI] [PubMed] [Google Scholar]
- 35.Sun Y., Liu N., Guan X., Wu H., Sun Z., Zeng H. Immunosuppression induced by chronic inflammation and the progression to oral squamous cell carcinoma. Mediators Inflamm. 2016;2016:5715719. doi: 10.1155/2016/5715719. [DOI] [PMC free article] [PubMed] [Google Scholar]