Abstract
Objectives
Bisphosphonates (BP) are the first-line treatment for preventing fragility fractures. However, concern regarding their efficacy is growing because bisphosphonate is associated with over-suppression of remodelling and accumulation of microcracks. While dual-energy X-ray absorptiometry (DXA) scanning may show a gain in bone density, the impact of this class of drug on mechanical properties remains unclear. We therefore sought to quantify the mechanical strength of bone treated with BP (oral alendronate), and correlate data with the microarchitecture and density of microcracks in comparison with untreated controls.
Methods
Trabecular bone from hip fracture patients treated with BP (n = 10) was compared with naïve fractured (n = 14) and non-fractured controls (n = 6). Trabecular cores were synchrotron scanned and micro-CT scanned for microstructural analysis, including quantification of bone volume fraction, microarchitecture and microcracks. The specimens were then mechanically tested in compression.
Results
BP bone was 28% lower in strength than untreated hip fracture bone, and 48% lower in strength than non-fractured control bone (4.6 MPa vs 6.4 MPa vs 8.9 MPa). BP-treated bone had 24% more microcracks than naïve fractured bone and 51% more than non-fractured control (8.12/cm2 vs 6.55/cm2 vs 5.25/cm2). BP and naïve fracture bone exhibited similar trabecular microarchitecture, with significantly lower bone volume fraction and connectivity than non-fractured controls.
Conclusion
BP therapy had no detectable mechanical benefit in the specimens examined. Instead, its use was associated with substantially reduced bone strength. This low strength may be due to the greater accumulation of microcracks and a lack of any discernible improvement in bone volume or microarchitecture. This preliminary study suggests that the clinical impact of BP-induced microcrack accumulation may be significant.
Cite this article: A. Jin, J. Cobb, U. Hansen, R. Bhattacharya, C. Reinhard, N. Vo, R. Atwood, J. Li, A. Karunaratne, C. Wiles, R. Abel. The effect of long-term bisphosphonate therapy on trabecular bone strength and microcrack density. Bone Joint Res 2017;6:602–609. DOI: 10.1302/2046-3758.610.BJR-2016-0321.R1.
Keywords: Ageing, Biomechanics, Osteoporosis, Antiresorptives
Article Focus
Bisphosphonate suppresses osteoclastic activity to preserve bone structure.
Therapy may block remodelling, causing the accumulation of microcracks.
We examined the effect of bisphosphonate on trabecular microarchitecture, microdamage and compressive strength.
Key Messages
Bisphosphonate-treated bone was lower in compressive strength than untreated controls, which may be due to the greater accumulation of microcracks.
The clinical impact of induced microcrack accumulation may affect fragility.
Strengths and Limitations
Bisphosphonate-treated patients may exhibit less bone strength because of disease (selection bias) rather than BP treatment.
However, BP and untreated fracture controls possess the same microarchitecture, i.e. the groups are comparable in health.
This is the first study to employ state-of-the-art synchrotron micro-CT to visualise microcracks in humans.
Introduction
Bone fragility, or osteoporosis, is a major disease affecting 200 million people worldwide and contributing to 8.9 million fractures annually.1,2 This process is characterised by a reduction in bone mass, resulting from a reduction in the number, thickness, and connectivity of trabeculae, which can be documented and monitored by dual energy X-ray absorptiometry (DXA)3 or quantitative computed tomography (QCT)4 imaging. These imaging modalities quantify the amount of bone mineral present per unit area or volume and correlate reasonably with fracture risk.3,5 The bisphosphonate (BP) family of drugs has, for some years, been the first-line treatment option for prevention of fragility fractures.6,7 BP is said to improve bone strength by reducing bone remodelling8 and increasing DXA-based measures of bone mineral density (BMD).9 This increase in BMD is thought to be the result of microarchitectural changes caused by BP,10 but the mechanical consequences of these changes may not be entirely benign. Two clinical conditions are now linked to BP use: atypical femoral fractures where the femur breaks without warning,11 and osteonecrosis of the jaw, which causes poor bone healing following dental work.12 However, to our knowledge, no studies have reported the impact of BP treatment on the mechanical properties of human bone.
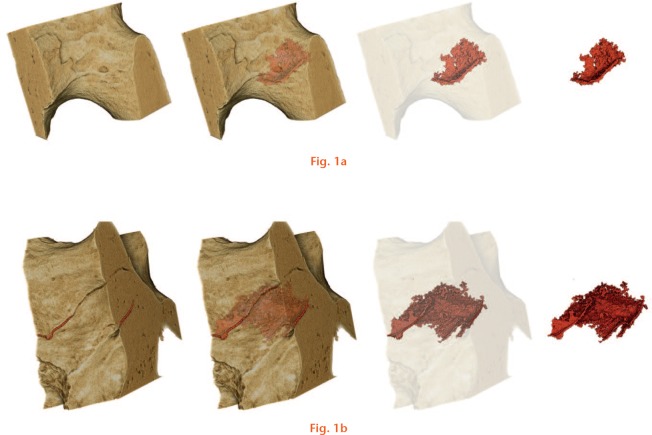
Suppression of remodelling by BP may cause microcracks to accumulate in bone.13 Microcracks are a normal feature of bone (Fig. 1 and Supplementary movie 1); they are a consequence of stress, and act as a stimulus for bone remodelling, but cannot be detected by DXA or QCT. A substantial increase in microcrack density as a result of BP treatment has been reported in both canine and human studies,14,15 but the relationship between microcrack density and mechanical properties has only been demonstrated in animal studies.10,16 These found that a reduction in fracture resistance was inversely related to microcrack density, which increased with duration of use17 and dose of BP treatment.18 This important relationship between the microarchitecture, the density of microcracks, and the mechanical properties of bone has not been established in humans for normal osteoporotic or BP-treated bone.
Synchrotron micro-CT images of trabecular bone and microcracks. Rendered scan data depicting microcracks within a) a trabeculum, and b) a node where several elements join together. Bone is shown in light brown and the microcracks in dark red. The computer models were created with colour, light and perspective using the software VG Studio Max.
Our aim was therefore threefold: first, to determine whether BP (oral alendronate 70 mg, taken weekly) therapy was associated with any microstructural changes that are not visible on conventional bone quality metrics in the femoral head and neck, an area of bone that is highly clinically relevant; second, to measure the mechanical properties of human bone from the BP-treated group and control groups; and third, to relate the microstructural features to these mechanical properties.
Patients and Methods
Imperial College Tissue Bank (R13004) approved collection and research of human tissue from Charing Cross Hospital and St Mary’s Hospital (London, United Kingdom) between May 2014 and September 2014. A consecutive series of femoral heads was collected from 25 patients with an intracapsular fracture after written informed consent was provided. In total, 24 specimens were included in the study and one was excluded due to avascular necrosis. Patients were divided into those who had taken BP and those who had not (Table I). Patient history of metabolic bone disease and treatment was collected from patient records. Ten fracture patients (61 to 94 years) had been prescribed BP (oral alendronate 70 mg, weekly) for between one and nine years. The mean duration of treatment was 3.1 years (sd 2.6). A total of 14 hip fracture patients (74 to 95 years) had not received any treatment for metabolic bone disease. These were then compared with six matched cadaveric femoral heads from ‘healthy’ ageing people who had no history of hip disease or bone metabolic medication (73 to 84 years). The mean age of the three groups was not significantly different (one-way analysis of variance F = 1.529, p = 0.223); BP-treated patients tended to be younger (78 years, sd 8) than both untreated fracture (83 years, sd 8) and non-fractured controls (79 years, sd 4). The gender ratio was skewed towards females, probably because osteoporosis affects more women than men.
Table I.
Age and gender distribution of patients
| Group | Mean age, yrs (sd) | Female, n (%) | Male, n (%) | Total, n |
|---|---|---|---|---|
| Non-fracture control | 78.8 (4.0) | 4 (66.7) | 2 (33.3) | 6 |
| Fracture control | 82.9 (7.8) | 8 (57.1) | 6 (42.9) | 14 |
| Bisphosphonate therapy | 77.3 (8.0) | 7 (70.0) | 3 (30.0) | 10 |
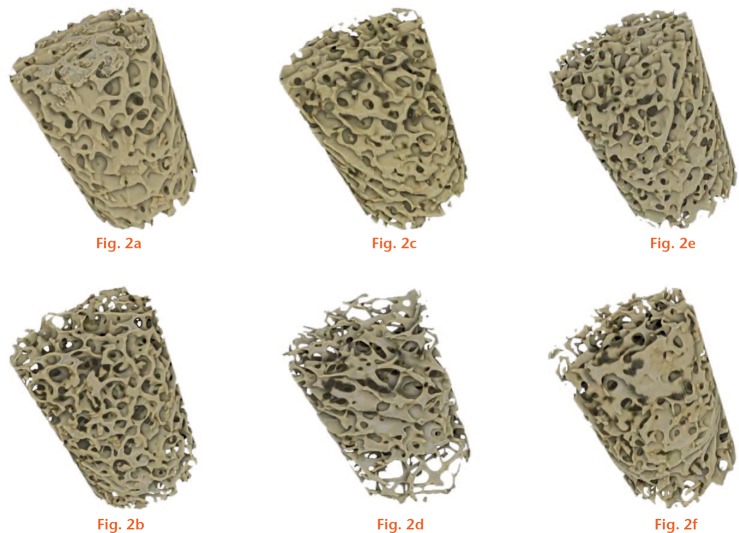
Two cores of trabecular bone (10 mm height × 7 mm diameter, an approximate ratio of 3:2) were prepared from the primary compressive trabecular arcade in each of the 30 femoral heads (Fig. 2 and Supplementary movie 2). In order to minimise damage caused by the preparation process, a low-speed (500 rpm) bench pillar drill (Jet JDP-15B, Jet Tools, La Vergne, Tennessee) was used under irrigation with a diamond drill bit (DK Holdings Ltd, Staplehurst, United Kingdom). Cores were collected from the trabecular chiasma and the region directly inferior. Cores were micro-CT scanned using a Nikon X-Tek HMXST-225 system, and reconstructed using CTPro 2.0 (Nikon Metrology UK, Tring, United Kingdom). The settings were: tungsten target; X-Ray beam 180 keV and 200 µA, voxel size 30 µm; 3124 projections; 360o rotation.
Rendered micro-CT images of trabecular bone cores. The top and bottom rows represent cores with the highest and lowest bone volume fraction (BVF) for a) and b) non-fractured; c) and d) untreated fracture patients; and e) and f) BP-treated fracture patients.
Trabecular microarchitecture was quantified using the BoneJ plugin for ImageJ v1.49 (an open source software), which has been validated for measuring bone.19 Scans were thresholded into binary images for microarchitectural analysis using the optimise threshold plugin. Bone volume fraction (BVF), connectivity density and trabecular thickness were measured. BVF is the ratio of the number of voxels that represent bone relative to the number that represent the core. Connectivity density was calculated by first determining the Euler characteristic, which can be used to provide an estimate of connectivity, and then dividing by the volume of the core. Trabecular thickness was calculated by fitting spheres within the trabeculae and measuring the diameter of the maximum fitted sphere.
A third core was cut from each of a subset of 18 of the femoral heads (six randomly selected from each of the three treatment and control groups) directly superior to the chiasma. The specimens were synchrotron micro-CT scanned at Diamond Light Source Ltd (Didcot, United Kingdom) using Beamline I12. The settings were: sample-to-detector distance 1000 mm, scan time five minutes, X-Ray beam 55 keV, 1800 projections; exposure time 1.3 seconds; 180o rotation; ring energy 3.1281 J; ring current 301.5 mA; and Wiggler readback 4.2 T. A volume of interest 2.76 mm in height and 3.28 mm in diameter was scanned at the centre of the core in order to avoid the region at the edge of the sample which was damaged during preparation. The total volume was 23.31 mm3 and the voxel size was 1.3 µm. Tomographic images were reconstructed using DAWN (Diamond Light Source, Didcot, United Kingdom),20 which uses filter back-projection and ring artefact removal.21
Synchrotron scans were used to calculate microcrack density. Each scan contained 2200 slices and was subdivided into 11 subsets consisting of 200 slices. The number of microcracks per mm2 was counted in 11 slices selected at random, one from each subset. A cumulative frequency plot of microcrack density revealed that increasing the number of slices beyond 11 per block did not produce any observable change in mean microcrack density. A single value of crack density was calculated from each sample for use in statistical analysis.
After scanning, cores were tested to failure in compression using an Instron 5565 machine (Instron Engineering Corporation, Norwood, Massachusetts). Apparent compressive strength was calculated as the maximum load divided by the cross-sectional area. The maximum loads that the samples withstood during the compression test were also dependent on the amount of tissue, porosity and size of the sample. Therefore, to investigate other effects of BP on bone strength, isolated from that of porosity, the strength divided by BVF (which is inversely equal to the porosity) was also calculated, i.e. tissue strength.
Parametric descriptive statistics and tests were calculated using SPSS (IBM, Armonk, New York) with one-way ANOVA with Tukey’s post hoc test.
Results
Apparent strength of BP-treated bone was 29% less in compression than the untreated fracture cores, which in turn were 27% less than the non-fractured controls (Fig. 3a). BP-treated bone tissue strength was 28% less than the untreated fracture bone, and 38% less than the non-fractured control bone, which were not statistically significantly different from each other (Fig. 3b).
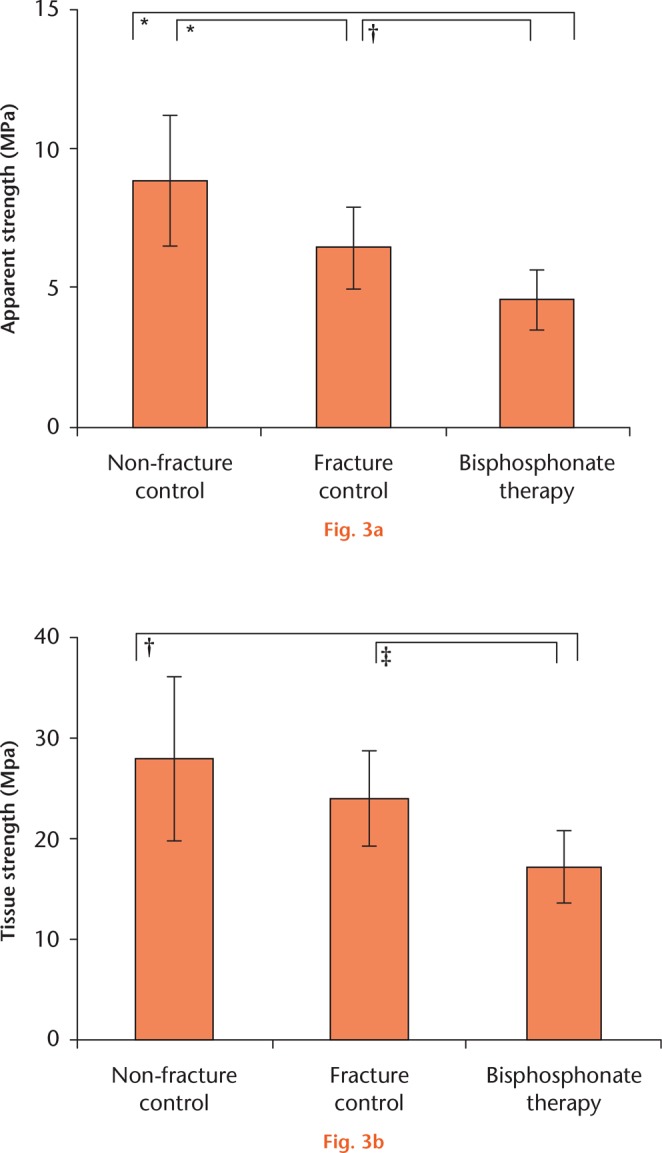
Compressive strength of BP-treated bone cores in comparison with controls. Trabecular cores were compared using one-way ANOVA with Tukey’s post hoc test. a) F = 27.3, p < 0.001; b) F = 6.59, p = 0.003. Symbols denote significant pairwise difference at *p < 0.001, †p < 0.01, and ‡p < 0.05.
The microarchitecture of non-fractured bone differed significantly from the two fracture groups: healthy bone had significantly more bone per unit volume, with greater trabecular connectivity, while the two fracture groups could not be distinguished by any microarchitectural metric (Fig. 4).
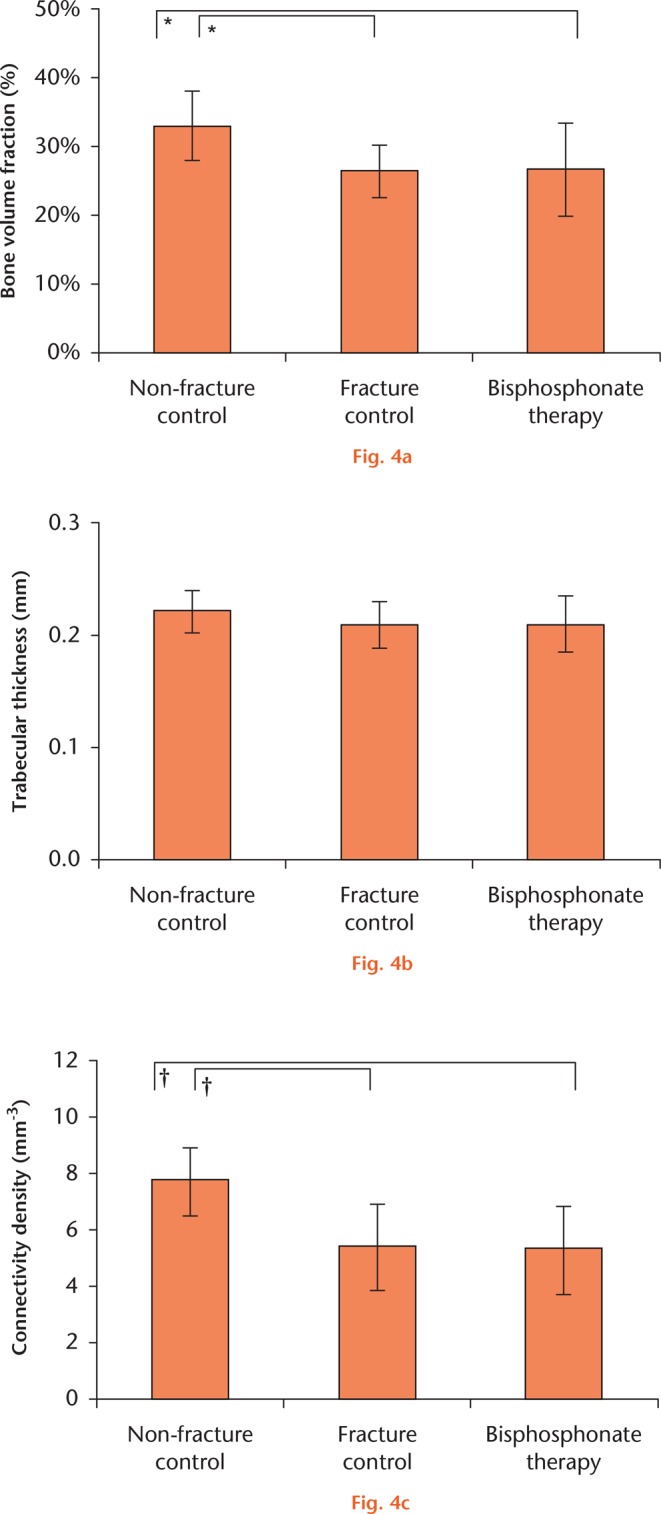
Microarchitecture of BP-treated bone cores in comparison with controls. Trabecular cores were compared using one-way ANOVA with Tukey’s post hoc test. a) F = 5.839, p = 0.005; b) F = 11.96, p < 0.001; c) F = 1.291, p = 0.285. Symbols denote significant pairwise difference at *p < 0.01 and †p < 0.001.
In terms of microcrack density, BP-treated bone was highly abnormal, with 24% more microcracks per unit area than the fracture bone, and 54% more than the healthy control bone (Fig. 5). The incidence of microcracks varied considerably, but the difference between hip fracture and non-fracture controls in terms of microcrack density failed to reach statistical significance. The density of microcracks in BP-treated bone was significantly greater than either control group (Fig. 5).
Fig. 5.
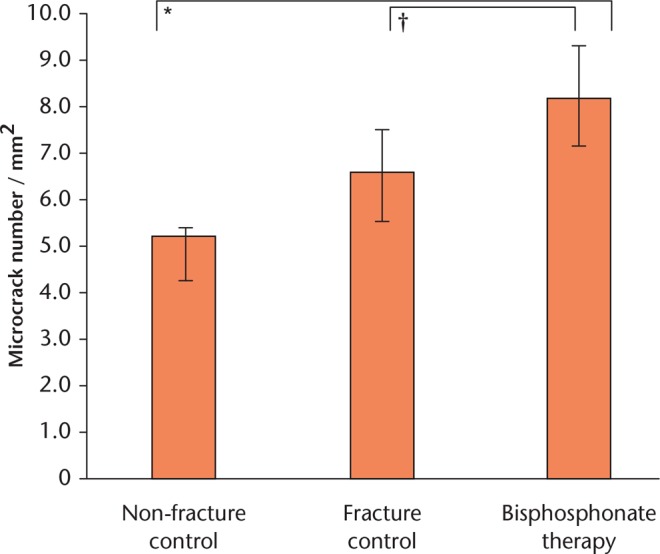
Microcrack density of BP-treated bone cores in comparison with controls. Trabecular cores were compared using one-way ANOVA with Tukey’s post hoc test: microcrack number/mm2 F = 13.754, p = 0.001. Symbols denote significant pairwise difference at *p < 0.001 and †p < 0.05.
Discussion
This is a small study of bone obtained following a hip fracture, so any results should be treated with caution: no observation can be made regarding bone healing, only regarding risk of fracture. However, this is the first study to link the microarchitecture, microcrack density, and mechanical strength of BP-treated bone from human patients. The most important finding is that, in a subgroup of patients who fractured after therapy, BP treatment was associated with a reduction in the compressive strength of bone, in comparison with both bone from untreated patients with hip fractures and bone from ‘healthy’ non-fractured controls. The reduced strength was associated with increased microdamage, mirroring findings from animal studies.10,16-18 This deleterious effect of accumulated microcracks on compressive strength in the BP-treated patients was not offset by any detectable improvements in bone volume or other microarchitectural measures.
BP therapy was associated with a reduction in the compressive strength of bone (Fig. 3). Bone from BP-treated fracture patients failed under just 4.6 MPa of compressive load (sd 1.1), while BP-naïve bone withstood 6.4 MPa (sd 1.5) or a 39% greater load. Non-fracture control bone was of course much stronger, withstanding 8.9 MPa (sd 2.3) or almost twice the load of BP-affected bone. When these results are corrected for bone volume fraction, any difference between the two samples of bone not exposed to BP failed to reach significance, suggesting that osteoporotic bone is fundamentally the same biomaterial as normal bone – there is just less of it. However, BP-exposed bone was much weaker: 40% weaker than untreated fracture bone, and 62% weaker than healthy ageing controls. This suggests that the impact of BP may not be entirely benign because the bone is lower in strength than expected for the volume.
Regarding the individual microstructural variables that contribute towards mechanical strength in bone that has not been subjected to BP, BVF (the microscopic equivalent of bone density) is a major determinant of strength (Fig. 4), as is connectivity, which probably diminishes with bone loss. Healthy bone has more trabeculae that are more interlinked than in either fracture group, which were comparable on both these measures (Fig. 4). The trabeculae of BP-treated bone were only 4% narrower than in healthy bone, while those from untreated fracture bone were 6% narrower (211 µm, sd 25 vs 208 µm, sd 21 vs 221 µm, sd 19). This small difference in trabecular thickness, which may be a BP effect, did not reach statistical significance.
The synchrotron micro-CT images provide a new dimension of information (Fig. 1 and Supplementary movie 1): BP-treated bone had significantly more microcracks than either non-fractured or fractured control bone (Fig. 5). The microcrack density exhibited by the non-fracture and hip fracture controls were similar, while the BP fracture patients had 24% more microcracks per unit area. It is possible that some of the microcracks may have been created by the sample preparation process and synchrotron radiation (SR) scanning. However, in this comparative study any effect was presumably similar for all groups. To avoid scanning bone tissue damaged during the coring process, the SR micro-CT volume of interest was imaged at the centre of the bone core away from the cut edges (following).22 Several studies have reported that SR micro-CT is an accurate technique for quantifying bone microstructure in 3D.23-25 A comparison between 2D histology, 3D micro-CT and 3D SR micro-CT reported that the structural parameters were highly correlated (r2 = 0.84),26 hence it is unlikely that the preparation of SR micro-CT affected microstructure.
Perhaps BP-treated bone is associated with lower strength in compression than the fracture controls because the accumulated microcracks propagated and/or merged under mechanical loading, culminating in gross failure of the trabeculae. This proposal is not new. It was already proposed to explain the association between BP therapy and relatively rare ‘atypical’ femoral fractures (AFFs) of the subtrochanteric cortices.27 These data suggest that the same mechanism may also be relevant to typical hip fractures of the femoral neck or to other fragility fractures. If our findings from the femoral heads are representative of the proximal femur in general, they may have relevance to typical fractures of the femoral neck, suggesting that the neck might be more prone to a ‘typical’ fracture, despite any improvements in bone apparent density or mineral density.
The evidence presented here falls short of demonstrating a causal relationship between accumulated microcracks, reduced compressive strength, and increased fracture risk. There may well be residual confounding factors biasing the patient selection. Patients receiving BP may have had reduced bone strength to begin with because people more severely affected by osteoporosis tend to be prescribed BP. Osteoporosis is characterised by a loss of bone mass and deterioration of the trabecular microstructure, leading to increased fracture risk.28 The increased fracture risk is correlated with low BMD.29,30 We were not able to measure BMD directly in this study, but we did measure the BVF and characterise the microarchitecture of the bone specimens. The BVF and microarchitecture (trabecular thickness and connectivity) were similar, yet the BP-treated bone was significantly lower in strength when normalised for porosity (Fig. 3) than the bone in the untreated fracture group. As such, BP-treated bone did not appear to be lower in strength through any detectable difference in trabecular bone volume or microarchitecture associated with osteoporosis, so we have no direct evidence of residual confounding bias in our patient groups.
Instead, our data suggest that the relative low strength of BP-treated bone (in our subgroup of patients who had suffered a fracture during BP therapy) is due to a significantly higher density of microcracks than was found in either hip fracture or non-fracture controls. This interpretation supports, and is supported by, a recent nested case-control study of 2009 hip fracture patients and 10 045 controls.31 Overall, hip fracture risk did not differ between BP-treated patients and controls. However, the odds ratio favoured BP treatment for the first year, but from one to three years fracture risk was similar, and after three years BP patients were significantly more likely to suffer a typical hip fracture than were the untreated controls. Given that BP treatment reduced fracture risk in the first year, while between one and three years the fracture incidence was equivalent, it is reasonable to assume that the higher incidence of fractures in the group treated with BP for more than three years was not due to the confounding indication that they were more severely affected by osteoporosis; otherwise, fracture risk in BP patients would have been higher than in the controls in the first year. Treatment with BP for more than three years is associated with a higher fracture risk in this large study, which fits with our observations of the material properties of BP-treated bone and the continued accumulation of microcracks reducing the strength of bone.
A recent systematic review of BP therapy, which included the Fracture Intervention Trial (FIT),32,33 reported that the evidence for BP treatment reducing the risk of hip fracture was weak.34 Due to the long half-life of BP in bone, there is a pressing clinical need to establish whether BP therapy can contribute towards the accumulation of microcracks, with the mechanical consequence of reducing the compressive strength of bone in the long term. BP may thereby increase the risk of hip fractures in the long term rather than preventing them. The prevalence of AFFs associated with BP use already appears to be increasing: 10.7% per year on average between 1999 and 2010.35 The number of BP prescriptions are expected to increase due to the ageing population; this may result in an actual increase in fractures.36,37
The clinical impact of our findings, and those of the studies referenced here, needs further evaluation. Conventional metrics of bone quality such as DXA or QCT are unable to detect microcracks, so may have little to contribute to this important topic. A clinical metric of the prevalence of microcracks in bone would be highly desirable and of real clinical relevance in patients who have been exposed to BP, as their bone does not behave like normal bone, and should be considered as a different biomaterial, with less strength per unit volume than bone that has not been exposed to BP.
In comparison with untreated hip fracture and healthy controls, bone from BP-treated hip fracture patients was lower in strength. BP bone may have been lower in strength because the bone was more osteoporotic but the BVF and other microarchitectural properties were identical to those of the naïve fracture patients. The data we report here suggest that the low strength is (at least partly) the result of the greater density of accumulated microcracks found in BP-treated bone. The clinical impact of this finding needs further evaluation. Clinical trials have shown that BP reduces fracture risk but there may be a subgroup of patients who, due to over-suppression of remodelling, do not respond to therapy as well as they could regarding bone strength and, therefore, fracture risk. Similarly, patients exposed to BP may be at higher risk of periprosthetic fractures or of early fixation failure owing to a greater density of microcracks. Perhaps some patients require smaller doses of BP or treatment holidays to allow for the repair of microcracks.
Footnotes
Author Contribution: A. Jin: Designing and conducting the study, Collecting and analysing the data, Drafting the manuscript.
J. Cobb: Designing the study, Analysing the data, Drafting the manuscript.
U. Hansen: Designing the study, Analysing the data, Drafting the manuscript.
R. Bhattacharya: Conducting the study, Drafting the manuscript.
C. Reinhard: Conducting the study, Collecting the data, Drafting the manuscript.
N. Vo: Conducting the study, Collecting and analysing the data, Drafting the manuscript.
R. Atwood: Conducting the study, Collecting the data, Drafting the manuscript.
J. Li: Conducting the study, Collecting the data, Drafting the manuscript.
A. Karunaratne: Conducting the study, Collecting the data, Drafting the manuscript.
C. Wiles: Conducting the study, Collecting the data, Drafting the manuscript.
R. Abel: Designing and conducting the study, Collecting and analysing the data, Drafting the manuscript.
Statement: None declared
Supplementary material
Videos showing the microarchitecture and micro-cracking in the bone cores are available alongside this paper online at www.bjr.boneandjoint.org.uk
Funding Statement
Funding has been received from Wellcome Trust and EPSRC related to this article
References
- 1. Kanis JA. WHO Technical Report. University of Sheffield. 2007. https://www.sheffield.ac.uk/FRAX/pdfs/WHO_Technical_Report.pdf (date last accessed 18 September 2017).
- 2. Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 2006;17:1726-1733. [DOI] [PubMed] [Google Scholar]
- 3. Blake GM, Herd RJ, Fogelman I. A longitudinal study of supine lateral DXA of the lumbar spine: a comparison with posteroanterior spine, hip and total-body DXA. Osteoporos Int 1996;6:462-470. [DOI] [PubMed] [Google Scholar]
- 4. Boutroy S, Bouxsein ML, Munoz F, Delmas PD. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab 2005;90:6508-6515. [DOI] [PubMed] [Google Scholar]
- 5. Kanis JA, Borgstrom F, De Laet C, et al. Assessment of fracture risk. Osteoporos Int 2005;16:581-589. [DOI] [PubMed] [Google Scholar]
- 6. No authors listed. National Institute for Health and Care Excellence. Alendronate, etidronate, risedronate, raloxifene and strontium ranelate for the primary prevention of osteoporotic fragility fractures in postmenopausal women (amended). 2011. https://www.nice.org.uk/guidance/ta160 (date last accessed 18 September 2017).
- 7. No authors listed. The Food and Drug Administration. Background document for meeting of advisory committee for reproductive health drugs and drug safety and risk management advisory committee. 2011. https://wayback.archive-it.org/7993/20170722221841/https://www.fda.gov/advisorycommittees/calendar/ucm262477.htm (date last accessed 18 September 2017).
- 8. Papapoulos SE, Schimmer RC. Changes in bone remodelling and antifracture efficacy of intermittent bisphosphonate therapy: implications from clinical studies with ibandronate. Ann Rheum Dis 2007;66:853-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burr DB, Liu Z, Allen MR. Duration-dependent effects of clinically relevant oral alendronate doses on cortical bone toughness in beagle dogs. Bone 2015;71:58-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mashiba T, Turner CH, Hirano T, et al. Effects of suppressed bone turnover by bisphosphonates on microdamage accumulation and biomechanical properties in clinically relevant skeletal sites in beagles. Bone 2001;28:524-531. [DOI] [PubMed] [Google Scholar]
- 11. Schilcher J, Koeppen V, Aspenberg P, Michaëlsson K. Risk of atypical femoral fracture during and after bisphosphonate use. N Engl J Med 2014;371:974-976. [DOI] [PubMed] [Google Scholar]
- 12. Ruggiero SL. Bisphosphonate-related osteonecrosis of the jaw (BRONJ): initial discovery and subsequent development. J Oral Maxillofac Surg 2009;67(Suppl):13-18. [DOI] [PubMed] [Google Scholar]
- 13. Allen MR, Burr DB. Mineralization, microdamage, and matrix: How bisphosphonates influence material properties of bone. BoneKEy-Osteovision 2007;4:49-60. [Google Scholar]
- 14. Stepan JJ, Burr DB, Pavo I, et al. Low bone mineral density is associated with bone microdamage accumulation in postmenopausal women with osteoporosis. Bone 2007;41:378-385. [DOI] [PubMed] [Google Scholar]
- 15. Chapurlat RD, Arlot M, Burt-Pichat B, et al. Microcrack frequency and bone remodeling in postmenopausal osteoporotic women on long-term bisphosphonates: a bone biopsy study. J Bone Miner Res 2007;22:1502-1509. [DOI] [PubMed] [Google Scholar]
- 16. Mashiba T, Hirano T, Turner CH, et al. Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. J Bone Miner Res 2000;15:613-620. [DOI] [PubMed] [Google Scholar]
- 17. Komatsubara S, Mori S, Mashiba T, et al. Long-term treatment of incadronate disodium accumulates microdamage but improves the trabecular bone microarchitecture in dog vertebra. J Bone Miner Res 2003;18:512-520. [DOI] [PubMed] [Google Scholar]
- 18. Allen MR, Iwata K, Phipps R, Burr DB. Alterations in canine vertebral bone turnover, microdamage accumulation, and biomechanical properties following 1-year treatment with clinical treatment doses of risedronate or alendronate. Bone 2006;39:872-879. [DOI] [PubMed] [Google Scholar]
- 19. Doube M, Kłosowski MM, Arganda-Carreras I, et al. BoneJ: Free and extensible bone image analysis in ImageJ. Bone 2010;47:1076-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Basham M, Filik J, Wharmby MT, et al. Data Analysis WorkbeNch (DAWN). J Synchrotron Radiat 2015;22:853-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Titarenko V, Bradley R, Martin C, Withers PJ, Titarenko S. Regularization methods for inverse problems in x-ray tomography. Proceedings from SPIE 7804, Developments in X-Ray Tomography VII. 2010. https://www.spiedigitallibrary.org/conference-proceedings-of-spie/7804/1/Regularization-methods-for-inverse-problems-in-x-ray-tomography/10.1117/12.860260.short?SSO=1 (date last accessed 18 September 2017).
- 22. Ma S, Boughton O, Karunaratne A, et al. Synchrotron imaging assessment of bone quality. Clin Rev Bone Miner Metab 2016;14:150-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chappard C, Basillais A, Benhamou L, et al. Comparison of synchrotron radiation and conventional x-ray microcomputed tomography for assessing trabecular bone microarchitecture of human femoral heads. Med Phys 2006;33:3568-3577. [DOI] [PubMed] [Google Scholar]
- 24. Betz O, Wegst U, Weide D, et al. Imaging applications of synchrotron X-ray phase-contrast microtomography in biological morphology and biomaterials science. I. General aspects of the technique and its advantages in the analysis of millimetre-sized arthropod structure. J Microsc 2007;227:51-71. [DOI] [PubMed] [Google Scholar]
- 25. Raum K, Leguerney I, Chandelier F, et al. Site-matched assessment of structural and tissue properties of cortical bone using scanning acoustic microscopy and synchrotron radiation μCT. Phys Med Biol 2006;51:733-746. [DOI] [PubMed] [Google Scholar]
- 26. Nuzzo S, Lafage-Proust MH, Martin-Badosa E, et al. Synchrotron radiation microtomography allows the analysis of three-dimensional microarchitecture and degree of mineralization of human iliac crest biopsy specimens: effects of etidronate treatment. J Bone Miner Res 2002;17:1372-1382. [DOI] [PubMed] [Google Scholar]
- 27. Ettinger B, Burr DB, Ritchie RO. Proposed pathogenesis for atypical femoral fractures: lessons from materials research. Bone 2013;55:495–500. [DOI] [PubMed] [Google Scholar]
- 28. Greenwood C, Clement JG, Dicken AJ, et al. The micro-architecture of human cancellous bone from fracture neck of femur patients in relation to the structural integrity and fracture toughness of the tissue. Bone Rep 2015;3:67-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen H, Kubo KY. Bone three-dimensional microstructural features of the common osteoporotic fracture sites. World J Orthop 2014;5:486-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Black DM, Cummings SR, Genant HK, et al. Axial and appendicular bone density predict fractures in older women. J Bone Miner Res 1992;7:633-638. [DOI] [PubMed] [Google Scholar]
- 31. Erviti J, Alonso Á, Gorricho J, López A. Oral bisphosphonates may not decrease hip fracture risk in elderly Spanish women: a nested case–control study. BMJ Open 2013;3:e002084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet 1996;348:1535-1541. [DOI] [PubMed] [Google Scholar]
- 33. Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA 1998;280:2077-2082. [DOI] [PubMed] [Google Scholar]
- 34. Järvinen TL, Michaëlsson K, Jokihaara J, et al. Overdiagnosis of bone fragility in the quest to prevent hip fracture. BMJ 2015;350:h2088. [DOI] [PubMed] [Google Scholar]
- 35. Meier RP, Perneger TV, Stern R, Rizzoli R, Peter RE. Increasing occurrence of atypical femoral fractures associated with bisphosphonate use. Arch Intern Med 2012;172:930-936. [DOI] [PubMed] [Google Scholar]
- 36. Schneider EL, Guralnik JM. The aging of America. Impact on health care costs. JAMA 1990;263:2335-2340. [PubMed] [Google Scholar]
- 37. National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation; 2010. [Google Scholar]