Abstract
Age-related cognitive decline is a significant public health concern. Recently, non-pharmacological methods, such as physical activity and mental training practices, have emerged as promising low-cost methods to slow the progression of age-related memory decline. In this study, we investigated if Tai Chi Chuan (TCC) and Baduanjin modulated the fractional amplitude of low-frequency fluctuations (fALFF) in different frequency bands (low-frequency: 0.01–0.08 Hz; slow-5: 0.01–0.027 Hz; slow-4: 0.027–0.073 Hz) and improved memory function. Older adults were recruited for the randomized study. Participants in the TCC and Baduanjin groups received 12 weeks of training (1 h/day for 5 days/week). Participants in the control group received basic health education. Each subject participated in memory tests and fMRI scans at the beginning and end of the experiment. We found that compared to the control group: (1) TCC and Baduanjin groups demonstrated significant improvements in memory function; (2) TCC increased fALFF in the dorsolateral prefrontal cortex (DLPFC) in the slow-5 and low-frequency bands; and (3) Baduanjin increased fALFF in the medial PFC in the slow-5 and low-frequency bands. This increase was positively associated with memory function improvement in the slow-5 and low-frequency bands across the TCC and Baduanjin groups. Our results suggest that TCC and Baduanjin may work through different brain mechanisms to prevent memory decline due to aging.
Keywords: mind-body exercise, memory, aging, fractional amplitude of low-frequency fluctuations (fALFF), resting-state functional magnetic resonance imaging (fMRI), frequency bands
Introduction
Age is the main risk factor for most common neurodegenerative diseases, such as mild cognitive impairment (MCI) and Alzheimer’s disease (AD). Memory dysfunction is the primary cognitive symptom in MCI and AD and has a profound impact on those whom it affects (McKhann et al., 2011). Nevertheless, pharmaceutical treatments for age-related memory decline remain unsatisfactory.
Recently, non-pharmacological methods, such as physical activity and mental training practices, have emerged as promising low-cost methods to slow the progression of age-related memory decline (Hillman et al., 2008; Erickson et al., 2011, 2014; Killgore et al., 2013; Makizako et al., 2013; Voss et al., 2013; Kelly et al., 2014; Tang and Posner, 2014; Tamura et al., 2015). For instance, Ruscheweyh et al. (2011) found that a 6 months intervention of low-intensity physical activity can improve episodic memory performance in healthy elderly individuals, and this improvement is associated with increases in local gray matter volume in the prefrontal and cingulate cortex, and Brain-derived neurotrophic factor (BDNF) levels. Innes et al. (2017) also reported that 12 min/day for 3 months of Kirtan Kriya meditation training can significantly improve memory and cognitive performance. Unlike pharmaceutical treatments, these methods usually lack serious side effects.
Tai Chi Chuan (TCC) and Baduanjin are popular mind-body practices (Wang et al., 2010; Zheng et al., 2014; Tao et al., 2015). Both of these practices combine meditation with slow movements, deep breathing, and relaxation to smooth vital energy (or qi) flow in the body (Wang et al., 2010). However, these practices are also different from each other; TCC involves more complicated body movements and requires moving one’s trunk and all four limbs (Wei et al., 2013), whereas the movement involved in Baduanjin is much simpler and is characterized by eight fixed movements (Xiong et al., 2015). Accumulating evidence has shown that TCC and Baduanjin practice improves cognitive performance and memory function (Wang, 2007; Chang et al., 2010; Lam et al., 2011; Tsai et al., 2013; Fong et al., 2014; Li F. et al., 2014; Wayne et al., 2014; Yin et al., 2014; Zheng et al., 2015). Nevertheless, the mechanisms underlying TCC and Baduanjin are still poorly understood.
In recent years, spontaneous fluctuations in brain activity during rest have drawn the attention of neuroimaging researchers. Investigators believe that these slow-frequency fluctuations may provide information about the intrinsic functional organization of the brain (Fox and Raichle, 2007). Furthermore, studies suggest that the human brain is a complex system that can generate a multitude of oscillatory waves, with different oscillatory classes carrying different dimensions of brain integration. The coupling of different bands of oscillators can provide enhanced combinatorial opportunities for storing complex temporal patterns to accomplish specific functions (Knyazev, 2007).
The low frequency fluctuations (LFF) between 0.01 Hz and 0.08 Hz are of particular relevance to resting state fMRI (rs-fMRI; Biswal et al., 1995). This low frequency range has been further divided into several distinct bands (Buzsáki and Draguhn, 2004), such as slow-4 (0.027–0.073 Hz) and slow-5 (0.01–0.027 Hz), which may indicate the modulation of cortical excitability and neuronal synchronization (Hoptman et al., 2010; Zuo et al., 2010). Recently, investigators have analyzed resting-state fMRI data filtered at the slow-4 and slow-5 bands separately to investigate AD (Liu et al., 2014), MCI (Han et al., 2012; Zhao et al., 2015), social anxiety disorder (SAD; Zhang et al., 2015), Parkinson’s Disease (PD; Esposito et al., 2013), and schizophrenia (Hoptman et al., 2010). Studies have found characteristic differences between these specific bands, further endorsing the value of distinguishing the slow-4 and slow-5 bands.
There are many methods that can be used to investigate the brain’s resting state spontaneous fluctuations. One such method is to characterize the regional spontaneous neuronal activity using the fractional amplitude of low frequency fluctuations (fALFF; Zang et al., 2007; Zou et al., 2008). As a normalized index of amplitude of low frequency fluctuations (ALFF), fALFF is defined as the total power within the low-frequency range divided by the total power in the entire detectable frequency range (Zuo et al., 2010). This method significantly suppresses non-specific signal components in resting state MRI and increases sensitivity to regional spontaneous brain activity (Zuo et al., 2010).
Alterations in fALFF have been found in several diseases. For instance, Sui et al. (2015) reported that in schizophrenic patients, increased cognitive performance was associated with higher fALFF in the striatum and decreased cognitive performance was associated with higher fALFF in the dorsolateral prefrontal cortex (DLPFC). McGill et al. (2014) reported decreased fALFF in the (PFC) and thalamus in patients with idiopathic generalized epilepsy. Additionally, Han et al. (2011) found that MCI is associated with decreased ALFF/fALFF values in the PCC/PCu, mPFC, hippocampus/PHG and prefrontal regions and increased ALFF/fALFF values in the occipital and temporal regions.
In this study, we investigated changes in spontaneous brain activity using fALFF in older adults following 3-months of TCC or Baduanjin practice. We hypothesized that 3-months of TCC and Baduanjin practice would improve memory function and modulate spontaneous brain activity in the brain regions associated with memory. In addition, we hypothesized that the modulatory effects of TCC and Baduanjin might vary in different low frequency bands.
Materials and Methods
In this study, we applied a data driven method to investigate fALFF changes before and after TCC and Baduanjin as compared to a control group. Although, the data has been used previously to investigate the resting state functional connectivity changes of the hippocampus (Tao et al., 2016) and DLPFC (Tao et al., 2017a) and brain structure changes (Tao et al., 2017b) following TCC and Baduanjin practice, we have never reported the results published in this manuscript. Please also see these published studies for more details on the experimental procedure.
Participants
The study was approved by the Medical Ethics Committee of Affiliated Rehabilitation Hospital, Fujian University of Traditional Chinese Medicine and registered in the Chinese Clinical Trial Registry (ChiCTR1, ChiCTR-IPR-15006131). All participants were informed and signed a written consent.
Two cohorts of older adults from one community were recruited independently and randomized into a TCC or control group in one cohort and a Baduanjin or control group in the other cohort. We recruited the two cohorts separately to avoid potential cross practicing between TCC and Baduanjin. The randomized treatment assignments were sealed in opaque envelopes and opened each time when new participants were included. Outcome raters were blind to the group allocation.
Inclusion criteria were: (1) 50–70 years old; (2) right-handed; and (3) no regular physical exercise for at least 1 year (the minimal standard for regular physical exercise was defined as 30 min 3–4 times per week for the past 3 months). Exclusion criteria were: (1) history of stroke; (2) suffered from severe cerebrovascular disease, musculoskeletal system disease, or other contraindications caused by sports injury; (3) a score of Beck depression inventory (BDI-II) ≥ 14 (Beck et al., 1996); and (4) a score on the Mini-Mental State Exam (MMSE) < 24 (Zhang et al., 1990).
Intervention
Two professional instructors with more than 5 years of training experience from Fujian University of Traditional Chinese Medicine were responsible for TCC and Baduanjin exercise training. To guarantee research quality, two staff members monitored the whole training procedure.
Tai Chi Chuan Exercise Group
TCC exercise, which was based on Yang-style 24-form (China National Sports Commission, 1983), was conducted for 60 min per session, 5 days per week for 12 weeks. Each session consisted of a warm-up and review of Tai Chi principles, TCC exercises, breathing technique training, and relaxation.
Baduanjin Exercise Group
The Baduanjin training regimen was in accordance with “Health Qigong—Baduanjin”, published by the General Administration of Sport of China. Each Baduanjin session consisted of a warm-up, eight fixed movements, and ending posture. The frequency of the Baduanjin exercise was the same as the TCC group, i.e. 60 min per session, one session per day, 5 days per week for 12 weeks.
Control Group
Participants in the control group received basic health education at the beginning of the experiment (Hughes et al., 2014). For the next 12 weeks, they were instructed to keep their original physical activity habits. At the end of the experiment (after the second MRI scan), free TCC or Baduanjin training was offered.
Behavioral Measurement
The Wechsler Memory Scale–Chinese Revision (WMS-CR) was used to assess the memory function of each participant. The WMS-CR is designed to assess memory function (Gong and Wang, 1989; Woodard and Axelrod, 1995) and is one of the most frequently used clinical assessments. It consists of ten subtests: information, orientation, mental control, picture, recognition, visual reproduction, associative learning, touch, comprehension memory, and digit span. It also provides an overall memory quotient (MQ). Two licensed WMS-CR raters who were blinded to the randomization distribution administered the WMS-CR.
MRI Acquisition
All MRI scans were acquired on a 3.0T magnetic resonance scanner (General Electric SignaHDxt, Milwaukee, WI, USA) with an 8-channel phased-array head coil. For the rs-fMRI, the scans were acquired with TR = 2100 ms, TE = 30 ms, flip angle = 90°, slice thickness = 3 mm, gap = 0.6 mm, acquisition matrix = 64 × 64, voxel size = 3.125 × 3.125 × 3.6 mm3, 42 axial slices, FOV = 200 × 200 mm, phases per location = 160. The scan lasted for 5 min and 36 s, and participants were required to stay awake with their eyes closed and ears plugged during the rs-fMRI scanning. In addition, magnetization-prepared rapid gradient echo (MPRAGE) T1-weighted images were collected.
Statistical Analysis
Behavioral Analysis
Baseline characteristics were compared by one-way analysis of variance (ANOVA) and Chi square tests using SPSS 18.0 Software (SPSS Inc., Chicago, IL, USA). During the analysis, all control participants from the two cohorts were combined into one group to increase the power. In order to estimate the effects of TCC and Baduanjin, ANCOVA analysis was applied to compare the change of MQ and the subtests across the three groups with age (years), with gender and education (years) included as covariates in the model. Post hoc analysis (Sidak corrected) was applied to explore the between-group differences.
Resting State Data Analysis
The fMRI data preprocessing was performed using Data Processing Assistant for Resting-State fMRI (DPARSF) Software (available at: http://rfmri.org/DPARSF; Chao-Gan and Yu-Feng, 2010) in MATLAB (Mathworks Inc., Natick, MA, USA). The software is based on Statistical Parametric Mapping (SPM8)2 and the Resting-State fMRI Data Analysis Toolkit3 (Song et al., 2011).
The first 10 volumes of functional data for each subject were discarded for signal equilibrium and participants’ adaptation to the imaging noise. The remaining volumes were slice timing corrected, within-subject spatially realigned, co-registered to the respective structural images for each subject, and then segmented. Subjects were excluded if head movement exceeded 3 mm on any axis or if head rotation was greater than 3°. To perform subject-level correction of head motion, the Friston 24-parameter model (6 head motion parameters, 6 head motion parameters one time point before, and the 12 corresponding squared items; Friston et al., 1996; Yan et al., 2013) was used. Images were normalized using structural image unified segmentation and then re-sampled to 3-mm cubic voxels. After smoothing with a 6 mm full-width at half maximum (FWHM) Gaussian kernel, the linear and quadric trends of the time courses were removed. Similar to previous studies (Han et al., 2011), no temporal filtering was implemented during preprocessing so that the entire frequency band could be calculated. In this study, we applied three frequency bands: slow-5 (0.01–0.027 Hz), slow-4 (0.027–0.073 Hz), and the traditionally used low-frequency (0.01–0.08 Hz) bands.
Group analysis was performed with a random effects model using SPM8. To explore the difference between TCC and Baduanjin after longitudinal treatment, we used a full factorial module in SPM8 with two factors for group analysis. The first factor had three levels (TCC, Baduanjin, control group) and the second factor had two levels (pre- and post-treatment). Age, gender and years of education were also included in the analysis as covariates of non-interest. A threshold of a voxel-wise p < 0.001 uncorrected and cluster-level p < 0.05 family-wise error corrected based on the random Gaussian field theory base (Lindquist et al., 2009) was applied.
Results
102 older adults between 50–70 years old were screened for this study. Of the 90 participants who were qualified for the study and finished baseline scans, 62 participants completed all study procedures (21 in the TCC group, 16 in the Baduanjin group, and 25 in the control group). Four participants in the TCC group dropped out (1 due to relocation, 1 due to unwillingness to get the second MRI scan, and 2 due to scheduling conflicts). Nine participants in the Baduanjin group dropped out (8 due to scheduling conflicts and 1 due to unwillingness to participate in the MRI scan). Fifteen participants in the control group dropped out (11 due to scheduling conflicts and 4 due to inability to participate in post-treatment MRI scans). One subject in the Baduanjin group was excluded from fALFF analysis due to excessive head movement (exceeded 3.0 mm).
Behavioral Results
Group characteristics are shown in Table 1. Age, gender, handedness, average years of education, MMSE score, and BDI score did not significantly differ among the three groups (P > 0.05). Average attendance rates were 95% in the TCC group (ranging from 88% to 100%) and 97% in the Baduanjin group (ranging from 92% to 100%).
Table 1.
Demographics of study participants and clinical outcome measurements.
| Between-group difference | ||||||
|---|---|---|---|---|---|---|
| Characteristics | Control (n = 25) Mean (SD) | Tai Chi Chuan (n = 21) Mean (SD) | Baduanjin (n = 15) Mean (SD) | Tai Chi Chuan vs. Control P value | Baduanjin vs. Control p value | Tai Chi Chuan vs. Baduanjin p value |
| Age† | 59.76 (4.83) | 62.38 (4.55) | 62.33 (3.88) | 0.055 | 0.087 | 0.975 |
| Gender (female/male)‡ | 19/6 | 13/8 | 9/6 | 0.473 | ||
| Handedness (right/left) | 25/0 | 21/0 | 15/0 | - | - | - |
| Average years of education† | 8.52 (3.65) | 9.61 (3.02) | 9.13 (2.69) | 0.255 | 0.563 | 0.658 |
| MQ_Pre treatment† | 99.08 (14.59) | 105.81 (10.24) | 99.20 (9.30) | 0.065 | 0.976 | 0.112 |
| MQ_Post treatment††† | 97.76 (13.92) | 123.57 (11.42) | 124.86 (11.21) | <0.001 | <0.001 | 0.907 |
†p values were calculated with one-way analysis of variance, ‡p values were calculated with the chi-square test, †††p values were calculated with mixed-model regression.
MQ scores before and after exercise are presented in Table 1. No significant differences were found among the three groups at baseline. ANCOVA analysis of change between the baseline and post-treatment MQ scores showed a significant difference among the three groups (F = 25.45, p < 0.001). Post hoc Sidak correction analysis showed that compared with the control group, MQ scores significantly increased in the TCC and Baduanjin groups (Baduanjin: p < 0.001, TCC: p < 0.001). There were no significant differences between the TCC and Baduanjin groups (p = 0.233). The comparisons of the subscores of WMS-CR showed TCC significantly increased visual reproduction subscores compared to controls. Baduanjin produced greater improvement in mental control, recognition, visual reproduction, touch and comprehension memory subscores compared to controls after bonferroni correction (p < 0.0063). Baduanjin also produced greater improvements in touch subscores compared to TCC after bonferroni correction (p < 0.0063). Please also see our previous publications on subscore changes across different treatment (Tao et al., 2017b).
Resting-State fMRI Data Analysis Results
fALFF in Low-Frequency Band (0.01–0.08 Hz)
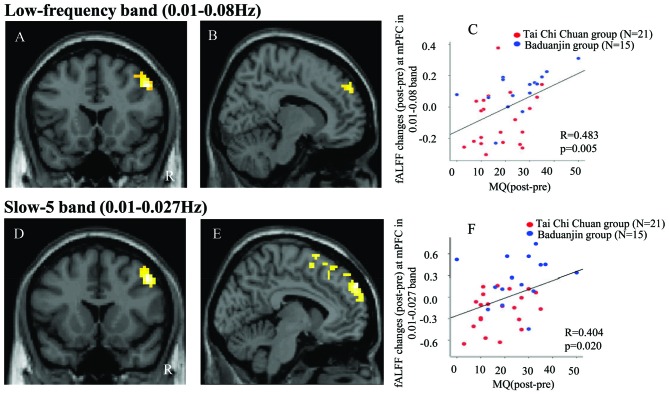
Pre- and post-treatment comparison of fALFF in the low-frequency band (0.01–0.08 Hz) among the three groups showed that after 12 weeks, fALFF was significantly increased in the right DLPFC in the TCC group compared to the control group (Table 2, Figure 1A). In the Baduanjin group, there was a significant increase in fALFF in the bilateral medial prefrontal cortex (mPFC) compared to the control group (Table 2, Figure 1B). No significant difference was observed between the TCC and Baduanjin groups at the threshold we set.
Table 2.
Comparisons of fractional amplitude of low-frequency fluctuations (fALFF) at different bands between groups.
| MNI coordinates | |||||||
|---|---|---|---|---|---|---|---|
| Contrast | Brain regions | Cluster size | Peak z-value | Cluster effect size | X | Y | Z |
| Low-frequency band | |||||||
| TaiChiChuan > control | R DLPFC | 60 | 5.45 | 3.02 | 51 | 18 | 39 |
| Baduanjin > control | L mPFC | 33 | 4.64 | 1.68 | −12 | 12 | 66 |
| R mPFC | 34 | 4.23 | 1.59 | 12 | 54 | 36 | |
| Control > Tai Chi Chuan | No brain region above the threshold | ||||||
| Control > Baduanjin | No brain region above the threshold | ||||||
| Tai Chi Chuan > Baduanjin | No brain region above the threshold | ||||||
| Baduanjin > Tai Chi Chuan | No brain region above the threshold | ||||||
| Slow-5 band | |||||||
| Tai Chi Chuan > control | R DLPFC | 65 | 5.1 | 2.17 | 48 | 15 | 42 |
| Baduanjin > control | R mPFC | 167 | 4.51 | 1.67 | 9 | 57 | 36 |
| L mPFC | 4.41 | −6 | 60 | 39 | |||
| Control > Tai Chi Chuan | No brain region above the threshold | ||||||
| Control > Baduanjin | No brain region above the threshold | ||||||
| Tai Chi Chuan > Baduanjin | No brain region above the threshold | ||||||
| Baduanjin > Tai Chi Chuan | No brain region above the threshold | ||||||
L, left; R, right; DLPFC, dorsolateral prefrontal cortex; mPFC, medial prefrontal cortex.
Figure 1.
(A) Increased fractional amplitude of low-frequency fluctuations (fALFF) in the 0.01–0.08 Hz band in the Tai Chi Chuan (TCC) group compared with the control group. (B) Increased fALFF in the 0.01–0.08 Hz band in the Baduanjin group compared with the control group. (D) Increased fALFF in the 0.01–0.027 Hz band in the TCC group compared with the control group. (E) Increased fALFF in the 0.01–0.027 Hz band in the Baduanjin group compared with the control group. (C,F) Scatter plots showing the association between the prefrontal cortex fALFF value of the significant cluster and improvements in memory across the TCC and Baduanjin groups, corrected for age, gender, years of education (C: r = 0.483, p = 0.005; F: r = 0.404, p = 0.02). R: right.
To explore the difference between Tai Chi vs. Baduanjin intervention, we also applied a relatively less conservative threshold of voxel-wise p < 0.005 uncorrected with 10 continuous voxels. We found that compared to the Baduanjin group, there was a significant increase in fALFF in the periaqueductal gray, bilateral DLPFC, and temporoparietal junction in the TCC group. Compared with the TCC group, the Baduanjin group was associated with a significant fALFF increase in the left mPFC and left precuneus.
To explore the association between the fALFF changes observed above and behavioral outcomes, we also extracted the average fALFF values of the significant clusters (DLPFC and mPFC) and performed a multiple regression analysis including age, gender, and education as covariates. Results showed a significant association between the fALFF changes at mPFC and corresponding MQ (r = 0.48, p = 0.005 significant after Bonferroni correction (0.025 (0.05/2); Figure 1C), as well as a marginal association between the fALFF changes at DLPFC and corresponding MQ changes (p = 0.048, not significant after Bonferroni correction across the TCC and Baduanjin groups).
fALFF in Slow-5 Band
Pre- and post-treatment comparison of fALFF in the slow-5 band among the three groups is shown in Table 2 and Figure 1. After 12 weeks, participants in the TCC group showed significant increases in the right DLPFC compared with participants in the control group (Figure 1D). Participants in the Baduanjin group showed significant increases in the bilateral mPFC compared with participants in the control group (Figure 1E). No significant difference was found between the TCC and Baduanjin groups at the threshold we set.
To further explore the difference between Tai Chi vs. Baduanjin comparisons, we applied a relatively less conservative threshold of voxel-wise p < 0.005 uncorrected with 10 continuous voxels. We found that compared to the Baduanjin group, there was a significant fALFF increase in the right lateral prefrontal cortex and periaqueductal gray/pon, and a significant fALFF decrease in the left DLPFC in the TCC group.
To explore the association between the fALFF changes observed above and behavioral outcomes, we also extracted the average fALFF values of the significant clusters and performed multiple regression analysis respectively including age, gender and education as covariates across the participants in TCC and Baduanjin group. Results showed a significant association between the mPFC fALFF changes and corresponding MQ changes (r = 0.40, p = 0.02, significant after after Bonferroni correction (0.025 (0.05/2; Figure 1F). There was no significant association between DLPFC fALFF changes and corresponding MQ changes (p = 0.078).
fALFF in Slow-4 Band
No significant differences among the three groups (two exercise groups and one control group) were observed. When we applied a relatively less conservative threshold of voxel-wise p < 0.001 uncorrected with 10 continuous voxels, we also did not find any significant results between the three groups.
Discussion
In this study, we investigated the effects of 12 weeks of TCC and Baduanjin exercise on fALFF changes and clinical outcome measures in older adults. We found that: (1) MQ significantly increased in both TCC and Baduanjin groups compared with the control group; (2) TCC increased fALFF in the right DLPFC in the slow-5 band and the 0.01–0.08 Hz band; and (3) Baduanjin increased fALFF in the bilateral mPFC in the slow-5 band and the 0.01–0.08 Hz band following exercise. fALFF changes at the mPFC in the slow-5 and 0.01–0.08 Hz bands showed a significant positive association with corresponding MQ changes.
Both TCC and Baduanjin are mind-body exercises consisting of meditation, breathing, and gentle movements. From the viewpoint of physical exercise, both TCC and Baduanjin are safe aerobic activities (Li R. et al., 2014; Wayne et al., 2014). Aerobic exercise has been shown to improve memory function (Flöel et al., 2010; Erickson et al., 2011; Li L. et al., 2014; Seo et al., 2014). In addition to the physical component, TCC and Baduanjin also include sustained attention, focus, and multi-tasking. Thus, the mind-body exercise component may also have positive effects on cognitive function. Our finding of a significant improvement in general memory function after 3 months of TCC and Baduanjin practice is consistent with previous studies (Chang et al., 2010; Miller and Taylor-Piliae, 2014; Zheng et al., 2015) showing positive cognitive benefits of TCC in older adults. Our study demonstrates the power of TCC and Baduanjin practice in helping older adults improve memory.
We found that compared to controls, participants in the TCC group had increased fALFF in the right DLPFC, while the participants in the Baduanjin group had increased fALFF in the bilateral mPFC in the slow-5 band and the 0.01–0.08 Hz band. Although TCC and Baduanjin are associated with different patterns compared to controls, we did not find significant differences between the TCC and Baduanjin groups at this threshold we set. However, at a less conservative threshold of voxel-wise p < 0.005 uncorrected with 10 continuous voxels, there was a significant fALFF increase in the bilateral DLPFC, and decrease in the left mPFC and left precuneus in the TCC group compared to Baduanjin group in the 0.01–0.08 band. We also found that compared to the Baduanjin group, there was a significant fALFF increase in right lateral prefrontal cortex compared to the Baduanjin group in the slow-5 band. These significant difference activity pattern between TCC and Baduanjin is consistent with the findings when comparing: (1) Tai Chi vs. Control; and (2) Baduanjin vs. Control. Furthermore, we also found that TCC and Baduanjin modulate the subtests of WMS-CR differently, Baduanjin can significantly increase the touch subscore compared to TCC, which further suggests that different mechanism may underlying Tai Chi Quan and Baduanjin. We speculate this difference may due to different exercise characteristics associated with the two mind-body interactions. TCC involves more complicated body movements and requires moving the trunk and all four limbs, whereas the movement involved in Baduanjin is much simpler.
In this study, we found that TCC exercise increased fALFF in the right DLPFC in the slow-5 band and the 0.01–0.08 Hz band compared to the control group. Previous studies have demonstrated that the DLPFC, a task positive region, is a key area in the cognitive control network (CCN; Miller and Cohen, 2001; Cieslik et al., 2013). The CCN is important in top-down modulation of attention–memory interactions (Corbetta and Shulman, 2002; Cole and Schneider, 2007; Chiu and Yantis, 2009; Spreng et al., 2010; Kong et al., 2013; Hwang et al., 2015; Rosen et al., 2016). Recent studies have shown that non-invasive brain stimulation techniques such as repetitive transcranial magnetic stimulation and transcranial direct current stimulation of the DLPFC enhanced memory-guided responses in a visuospatial working memory task (Balconi and Ferrari, 2012; Brunoni and Vanderhasselt, 2014; Giglia et al., 2014). These findings further confirm the DLPFC’s role in memory function.
Previous studies (Baron Short et al., 2010) found significantly increased DLPFC activation during meditation in comparison to a control task. In a recent study, investigators found that compared with control participants, TCC experts show greater functional homogeneity in the right post-central gyrus and lower functional homogeneity in the right DLPFC and the left anterior cingulate cortex. The gain in functional integration was significantly correlated with cognitive performance in TCC experts (Wei et al., 2014). In another study, investigators found that multimodal intervention including TCC exercise enhanced the ALFF in the right middle frontal gyrus/DLPFC in older adults (Yin et al., 2014). In a more recent study, we found that TCC practice significantly modulates the rsFC between the CCN and the superior frontal gyrus and ACC, and that Baduanjin modulates the rsFC between the CCN and the putamen and insula (Tao et al., 2017a). Our results are partially consistent with these findings.
We also found fALFF increases in the slow-5 band and the 0.01–0.08 Hz band in the Baduanjin group at the mPFC. This change was significantly associated with memory function changes. The mPFC is associated with the highest baseline metabolic activity at rest (Gusnard et al., 2001) and is a key region in the default mode network (DMN; Li L. et al., 2014). The mPFC identified in the present study overlaps with the findings observed in previous studies on the impact of physical activity and meditation on cognitive functions (Flöel et al., 2010; Hasenkamp and Barsalou, 2012; Tang and Posner, 2014; Tamura et al., 2015).
The mPFC is known to undergo both structural and functional changes with aging (Gutchess et al., 2007; Hurtz et al., 2014; van de Vijver et al., 2014). Research suggests that the mPFC’s function is related to different aspects of social cognitive processing (Amodio and Frith, 2006), which involves action monitoring (Barch et al., 2001), self-knowledge (Macrae et al., 2004), person perception, mentalization (Grèzes et al., 2004), and outcome monitoring (Camille et al., 2004). Studies also found the mPFC is involved in the encoding and retrieval of memory (van Kesteren et al., 2010, 2012; Brod et al., 2013). In a previous study based on the same data, we found that TCC and Baduanjin (at a less conservative threshold) can increase the rsFC between the hippocampus and mPFC (Tao et al., 2016). Taken together, our result suggests that Baduanjin may improve memory function through the mPFC and associated brain networks, such as the hippocampus.
In this study, we found that the slow-5 frequency band and the low-frequency band (0.01–0.08 Hz) showed fALFF changes after TCC and Baiduanjin practice, while no significant differences were observed in the slow-4 band. This suggests that the memory-relevant changes induced by 3 months of TCC and Baduanjin practice are specifically reflected difference low-frequency band. In a previous study, Han et al. (2011) investigated changes of ALFF and fALFF in patients with MCI between the slow-4 and slow-5 bands. They found significant differences in fALFF between MCI patients and controls only in the slow-5 band. The pattern of intrinsic functional connectivity is sensitive to specific frequency bands. Chao-Gan and Yu-Feng (2010) found that the low-frequency range (0.01–0.08 Hz) may better reveal the DMN. Zuo et al. (2010) also shown that Slow-5 (0.01–0.027 Hz) amplitudes at low frequency were more dominant within ventromedial prefrontal cortices. Consistent with these results, our findings also predominantly locate at the frontal area in Slow-5 band and low-frequency range. Further studies are needed to confirm and expand our findings.
The current study has several limitations. First, the sample size is relatively small. Second, both TCC and Baduanjin are considered mind-body exercises. Therefore, our design could not disentangle the effect of physical activity vs. mental exercise, and was unable to identify the crucial components of the exercise affecting memory improvement and brain functional fluctuation changes. Further research is needed to compare the effects of exercise, meditation, TCC and Baduanjin directly. Third, we did not record the participants’ original physical activity habits, and only gave them an introduction to keep their original physical activity habits throughout the duration of the experiment. Further study should record the participants original physical activity habits for more accurate documentation of their activity intensity.
Conclusion
In this study, we found that 12 weeks of intensive group TCC/Baduanjin practice can significantly modulate fALFF in different frequency bands and improve memory function. TCC increased fALFF in the slow-5 band and the 0.01–0.08 Hz band in the DLPFC, and Baduanjin increased fALFF in the slow-5 band and the 0.01–0.08 Hz band in the mPFC. fALFF changes in the mPFC in the slow-5 band and 0.01–0.08 Hz band were also correlated with general memory improvement. Our results imply that TCC and Baduanjin may work through different brain mechanisms due to differences in the characteristics and complexity of their respective regimens, but both exercises may hold the potential to prevent age-related memory decline.
Ethics Statement
The Medical Ethics Committee in the Affiliated Rehabilitation Hospital of Fujian University of Traditional Chinese Medicine approved all study procedures. The experiment was performed in accordance with approved guidelines. All participants signed a written consent. This study was registered on the Chinese Clinical Trial Registry (ChiCTR-IPR-15006131).
Author Contributions
LC: experimental design; JK: analysis and manuscript preparation; JT: experimental design, data analysis and manuscript preparation; GZ: data analysis; JL and XX: data collection and data analysis; WL, JW, ML, ZW and KH: data collection; XC and NE: manuscript preparation. All authors contributed to manuscript draft and have read and approved the final manuscript.
Conflict of Interest Statement
JK has a disclosure to report; holding equity in the startup company MNT, but declares no conflict of interest. The other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank two professional instructors from the Fujian University of Traditional Chinese Medicine Hongmei Yi and Tingjin Duan, for their training in Tai Chi Chuan and Baduanjin exercise. We thank Sharon Sun, Courtney Lang and Joel Park for editing the English.
Glossary
Abbreviations
- AD
Alzheimer’s disease
- ALFF
amplitude of low frequency fluctuations
- aMCI
amnestic mild cognitive impairment
- BDI
Beck depression inventory
- CCN
cognitive control network
- CSF
cerebrospinal fluid
- DLPFC
dorsolateral prefrontal cortex
- DPARSF
Data Processing Assistant for Resting-State fMRI
- fALFF
fractional amplitude of low-frequency fluctuations
- FWHM
full-width at half maximum
- LFF
low frequency fluctuations
- MCI
mild cognitive impairment
- MDD
major depressive disorder
- MMSE
Mini-Mental State Exam
- mPFC
medial prefrontal cortex
- MPRAGE
magnetization-prepared rapid gradient echo
- PD
Parkinson’s Disease
- SAD
social anxiety disorder
- TCC
Tai Chi Chuan
- WMS-CR
Wechsler Memory Scale–Chinese Revision.
Funding. This study is supported by the Special Scientific Research Fund of Public Welfare Profession of China (Grant No. 201307004), Ministry of Science and Technology and Ministry of Finance of the People’s Republic of China. It is also supported by the National Rehabilitation Research Center of Traditional Chinese Medicine, Fujian rehabilitation industrial institution and Fujian Rehabilitation Tech Co-innovation Center (Grant No. X2012007-Collaboration). JK is supported by R01AT006364, R01AT008563, R61AT009310, R21AT008707 and P01 AT006663 from NIH/NCCIH.
References
- Amodio D. M., Frith C. D. (2006). Meeting of minds: the medial frontal cortex and social cognition. Nat. Rev. Neurosci. 7, 268–277. 10.1038/nrn1884 [DOI] [PubMed] [Google Scholar]
- Balconi M., Ferrari C. (2012). rTMS stimulation on left DLPFC increases the correct recognition of memories for emotional target and distractor words. Cogn. Affect. Behav. Neurosci. 12, 589–598. 10.3758/s13415-012-0090-1 [DOI] [PubMed] [Google Scholar]
- Barch D. M., Braver T. S., Akbudak E., Conturo T., Ollinger J., Snyder A. (2001). Anterior cingulate cortex and response conflict: effects of response modality and processing domain. Cereb. Cortex 11, 837–848. 10.1093/cercor/11.9.837 [DOI] [PubMed] [Google Scholar]
- Baron Short E., Kose S., Mu Q., Borckardt J., Newberg A., George M. S., et al. (2010). Regional brain activation during meditation shows time and practice effects: an exploratory FMRI study. Evid. Based Complement. Alternat. Med. 7, 121–127. 10.1093/ecam/nem163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A. T., Steer R. A., Brown G. K. (1996). Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Biswal B., Yetkin F. Z., Haughton V. M., Hyde J. S. (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 34, 537–541. 10.1002/mrm.1910340409 [DOI] [PubMed] [Google Scholar]
- Brod G., Werkle-Bergner M., Shing Y. L. (2013). The influence of prior knowledge on memory: a developmental cognitive neuroscience perspective. Front. Behav. Neurosci. 7:139. 10.3389/fnbeh.2013.00139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunoni A. R., Vanderhasselt M. A. (2014). Working memory improvement with non-invasive brain stimulation of the dorsolateral prefrontal cortex: a systematic review and meta-analysis. Brain Cogn. 86, 1–9. 10.1016/j.bandc.2014.01.008 [DOI] [PubMed] [Google Scholar]
- Buzsáki G., Draguhn A. (2004). Neuronal oscillations in cortical networks. Science 304, 1926–1929. 10.1126/science.1099745 [DOI] [PubMed] [Google Scholar]
- Camille N., Coricelli G., Sallet J., Pradat-Diehl P., Duhamel J. R., Sirigu A. (2004). The involvement of the orbitofrontal cortex in the experience of regret. Science 304, 1167–1170. 10.1126/science.1094550 [DOI] [PubMed] [Google Scholar]
- Chang Y.-K., Nien Y.-H., Tsai C.-L., Etnier J. L. (2010). Physical activity and cognition in older adults: the potential of Tai Chi Chuan. J. Aging Phys. Act. 18, 451–472. 10.1123/japa.18.4.451 [DOI] [PubMed] [Google Scholar]
- Chao-Gan Y., Yu-Feng Z. (2010). DPARSF: a MATLAB toolbox for ‘Pipeline’ data analysis of resting-state fMRI. Front. Syst. Neurosci. 4:13. 10.3389/fnsys.2010.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- China National Sports Commission (1983). Simplified Taijiquan. Beijing: People’s Sports Press. [Google Scholar]
- Chiu Y. C., Yantis S. (2009). A domain-independent source of cognitive control for task sets: shifting spatial attention and switching categorization rules. J. Neurosci. 29, 3930–3938. 10.1523/jneurosci.5737-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslik E. C., Zilles K., Caspers S., Roski C., Kellermann T. S., Jakobs O., et al. (2013). Is there “One” DLPFC in cognitive action control? Evidence for heterogeneity from co-activation-based parcellation. Cereb. Cortex 23, 2677–2689. 10.1093/cercor/bhs256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M. W., Schneider W. (2007). The cognitive control network: integrated cortical regions with dissociable functions. Neuroimage 37, 343–360. 10.1016/j.neuroimage.2007.03.071 [DOI] [PubMed] [Google Scholar]
- Corbetta M., Shulman G. L. (2002). Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 3, 201–215. 10.1038/nrn755 [DOI] [PubMed] [Google Scholar]
- Erickson K. I., Leckie R. L., Weinstein A. M. (2014). Physical activity, fitness, and gray matter volume. Neurobiol. Aging 35, S20–S28. 10.1016/j.neurobiolaging.2014.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K. I., Voss M. W., Prakash R. S., Basak C., Szabo A., Chaddock L., et al. (2011). Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. U S A 108, 3017–3022. 10.1073/pnas.1015950108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito F., Tessitore A., Giordano A., De Micco R., Paccone A., Conforti R., et al. (2013). Rhythm-specific modulation of the sensorimotor network in drug-naive patients with Parkinson’s disease by levodopa. Brain 136, 710–725. 10.1093/brain/awt007 [DOI] [PubMed] [Google Scholar]
- Flöel A., Ruscheweyh R., Krüger K., Willemer C., Winter B., Völker K., et al. (2010). Physical activity and memory functions: are neurotrophins and cerebral gray matter volume the missing link? Neuroimage 49, 2756–2763. 10.1016/j.neuroimage.2009.10.043 [DOI] [PubMed] [Google Scholar]
- Fong D.-Y., Chi L.-K., Li F., Chang Y.-K. (2014). The benefits of endurance exercise and Tai Chi Chuan for the task-switching aspect of executive function in older adults: an ERP study. Front. Aging Neurosci. 6:295. 10.3389/fnagi.2014.00295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M. D., Raichle M. E. (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 8, 700–711. 10.1038/nrn2201 [DOI] [PubMed] [Google Scholar]
- Friston K. J., Williams S., Howard R., Frackowiak R. S., Turner R. (1996). Movement-related effects in fMRI time-series. Magn. Reson. Med. 35, 346–355. 10.1002/mrm.1910350312 [DOI] [PubMed] [Google Scholar]
- Giglia G., Brighina F., Rizzo S., Puma A., Indovino S., Maccora S., et al. (2014). Anodal transcranial direct current stimulation of the right dorsolateral prefrontal cortex enhances memory-guided responses in a visuospatial working memory task. Funct. Neurol. 29, 189–193. 10.11138/FNeur/2014.29.3.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y., Wang D. J. (1989). Handbook of Wechsler Memory Scale-Revised (WMS-RC), Chinese Version. Changsha: Bulletin of Hunan Medical College. [Google Scholar]
- Grèzes J., Frith C., Passingham R. E. (2004). Brain mechanisms for inferring deceit in the actions of others. J. Neurosci. 24, 5500–5505. 10.1523/jneurosci.0219-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard D. A., Akbudak E., Shulman G. L., Raichle M. E. (2001). Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc. Natl. Acad. Sci. U S A 98, 4259–4264. 10.1073/pnas.071043098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutchess A. H., Kensinger E. A., Schacter D. L. (2007). Aging, self-referencing, and medial prefrontal cortex. Soc. Neurosci. 2, 117–133. 10.1080/17470910701399029 [DOI] [PubMed] [Google Scholar]
- Han Y., Lui S., Kuang W., Lang Q., Zou L., Jia J. (2012). Anatomical and functional deficits in patients with amnestic mild cognitive impairment. PLoS One 7:e28664. 10.1371/journal.pone.0028664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y., Wang J., Zhao Z., Min B., Lu J., Li K., et al. (2011). Frequency-dependent changes in the amplitude of low-frequency fluctuations in amnestic mild cognitive impairment: a resting-state fMRI study. Neuroimage 55, 287–295. 10.1016/j.neuroimage.2010.11.059 [DOI] [PubMed] [Google Scholar]
- Hasenkamp W., Barsalou L. W. (2012). Effects of meditation experience on functional connectivity of distributed brain networks. Front. Hum. Neurosci. 6:38. 10.3389/fnhum.2012.00038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman C. H., Erickson K. I., Kramer A. F. (2008). Be smart, exercise your heart: exercise effects on brain and cognition. Nat. Rev. Neurosci. 9, 58–65. 10.1038/nrn2298 [DOI] [PubMed] [Google Scholar]
- Hoptman M. J., Zuo X. N., Butler P. D., Javitt D. C., D’Angelo D., Mauro C. J., et al. (2010). Amplitude of low-frequency oscillations in schizophrenia: a resting state fMRI study. Schizophr. Res. 117, 13–20. 10.1016/j.schres.2009.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes T. F., Flatt J. D., Fu B., Butters M. A., Chang C. C., Ganguli M. (2014). Interactive video gaming compared with health education in older adults with mild cognitive impairment: a feasibility study. Int. J. Geriatr. Psychiatry 29, 890–898. 10.1002/gps.4075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtz S., Woo E., Kebets V., Green A. E., Zoumalan C., Wang B., et al. (2014). Age effects on cortical thickness in cognitively normal elderly individuals. Dement. Geriatr. Cogn. Dis. Extra 4, 221–227. 10.1159/000362872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J. W., Egorova N., Yang X. Q., Zhang W. Y., Chen J., Yang X. Y., et al. (2015). Subthreshold depression is associated with impaired resting-state functional connectivity of the cognitive control network. Transl. Psychiatry 5:e683. 10.1038/tp.2015.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innes K. E., Selfe T. K., Khalsa D. S., Kandati S. (2017). Meditation and music improve memory and cognitive function in adults with subjective cognitive decline: a pilot randomized controlled trial. J. Alzheimers Dis. 56, 899–916. 10.3233/JAD-160867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M. E., Loughrey D., Lawlor B. A., Robertson I. H., Walsh C., Brennan S. (2014). The impact of exercise on the cognitive functioning of healthy older adults: a systematic review and meta-analysis. Ageing Res. Rev. 16, 12–31. 10.1016/j.arr.2014.05.002 [DOI] [PubMed] [Google Scholar]
- Killgore W. D., Olson E. A., Weber M. (2013). Physical exercise habits correlate with gray matter volume of the hippocampus in healthy adult humans. Sci. Rep. 3:3457. 10.1038/srep03457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knyazev G. G. (2007). Motivation, emotion, and their inhibitory control mirrored in brain oscillations. Neurosci. Biobehav. Rev. 31, 377–395. 10.1016/j.neubiorev.2006.10.004 [DOI] [PubMed] [Google Scholar]
- Kong J., Jensen K., Loiotile R., Cheetham A., Wey H. Y., Tan Y., et al. (2013). Functional connectivity of the frontoparietal network predicts cognitive modulation of pain. Pain 154, 459–467. 10.1016/j.pain.2012.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam L. C., Chau R. C., Wong B. M., Fung A. W., Lui V. W., Tam C. C., et al. (2011). Interim follow-up of a randomized controlled trial comparing chinese style mind body (Tai Chi) and stretching exercises on cognitive function in subjects at risk of progressive cognitive decline. Int. J. Geriatr. Psychiatry 26, 733–740. 10.1002/gps.2602 [DOI] [PubMed] [Google Scholar]
- Li F., Harmer P., Liu Y., Chou L. S. (2014). Tai Ji Quan and global cognitive function in older adults with cognitive impairment: a pilot study. Arch. Gerontol. Geriatr. 58, 434–439. 10.1016/j.archger.2013.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Jin L., Hong P., He Z.-H., Huang C.-Y., Zhao J.-X., et al. (2014). The effect of baduanjin on promoting the physical fitness and health of adults. Evid. Based Complement. Alternat. Med. 2014:784059. 10.1155/2014/784059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Men W. W., Chang Y. K., Fan M. X., Ji L., Wei G. X. (2014). Acute aerobic exercise increases cortical activity during working memory: a functional MRI study in female college students. PLoS One 9:e99222. 10.1371/journal.pone.0099222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist M. A., Meng Loh J., Atlas L. Y., Wager T. D. (2009). Modeling the hemodynamic response function in fMRI: efficiency, bias and mis-modeling. Neuroimage 45, S187–S198. 10.1016/j.neuroimage.2008.10.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Wang S., Zhang X., Wang Z., Tian X., He Y. (2014). Abnormal amplitude of low-frequency fluctuations of intrinsic brain activity in Alzheimer’s disease. J. Alzheimers Dis. 40, 387–397. 10.3233/JAD-131322 [DOI] [PubMed] [Google Scholar]
- Macrae C. N., Moran J. M., Heatherton T. F., Banfield J. F., Kelley W. M. (2004). Medial prefrontal activity predicts memory for self. Cereb. Cortex 14, 647–654. 10.1093/cercor/bhh025 [DOI] [PubMed] [Google Scholar]
- Makizako H., Shimada H., Doi T., Park H., Yoshida D., Suzuki T. (2013). Six-minute walking distance correlated with memory and brain volume in older adults with mild cognitive impairment: a voxel-based morphometry study. Dement. Geriatr. Cogn. Dis. Extra 3, 223–232. 10.1159/000354189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill M. L., Devinsky O., Wang X., Quinn B. T., Pardoe H., Carlson C., et al. (2014). Functional neuroimaging abnormalities in idiopathic generalized epilepsy. Neuroimage Clin. 6, 455–462. 10.1016/j.nicl.2014.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G. M., Knopman D. S., Chertkow H., Hyman B. T., Jack C. R., Jr., Kawas C. H., et al. (2011). The diagnosis of dementia due to Alzheimer’s disease: recommendations from the national institute on aging- Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 263–269. 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. K., Cohen J. D. (2001). An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 24, 167–202. 10.1146/annurev.neuro.24.1.167 [DOI] [PubMed] [Google Scholar]
- Miller S. M., Taylor-Piliae R. E. (2014). Effects of Tai Chi on cognitive function in community-dwelling older adults: a review. Geriatr. Nurs. 35, 9–19. 10.1016/j.gerinurse.2013.10.013 [DOI] [PubMed] [Google Scholar]
- Rosen M. L., Stern C. E., Michalka S. W., Devaney K. J., Somers D. C. (2016). Cognitive control network contributions to memory-guided visual attention. Cereb. Cortex 26, 2059–2073. 10.1093/cercor/bhv028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscheweyh R., Willemer C., Krüger K., Duning T., Warnecke T., Sommer J., et al. (2011). Physical activity and memory functions: an interventional study. Neurobiol. Aging 32, 1304–1319. 10.1016/j.neurobiolaging.2009.08.001 [DOI] [PubMed] [Google Scholar]
- Seo T. B., Kim T. W., Shin M. S., Ji E. S., Cho H. S., Lee J. M., et al. (2014). Aerobic exercise alleviates ischemia-induced memory impairment by enhancing cell proliferation and suppressing neuronal apoptosis in hippocampus. Int. Neurourol. J. 18, 187–197. 10.5213/inj.2014.18.4.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X. W., Dong Z. Y., Long X. Y., Li S. F., Zuo X. N., Zhu C. Z., et al. (2011). REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One 6:e25031. 10.1371/journal.pone.0025031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng R. N., Stevens W. D., Chamberlain J. P., Gilmore A. W., Schacter D. L. (2010). Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage 53, 303–317. 10.1016/j.neuroimage.2010.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui J., Pearlson G. D., Du Y., Yu Q., Jones T. R., Chen J., et al. (2015). In search of multimodal neuroimaging biomarkers of cognitive deficits in schizophrenia. Biol. Psychiatry 78, 794–804. 10.1016/j.biopsych.2015.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura M., Nemoto K., Kawaguchi A., Kato M., Arai T., Kakuma T., et al. (2015). Long-term mild-intensity exercise regimen preserves prefrontal cortical volume against aging. Int. J. Geriatr. Psychiatry 30, 686–694. 10.1002/gps.4205 [DOI] [PubMed] [Google Scholar]
- Tang Y.-Y., Posner M. I. (2014). Training brain networks and states. Trends. Cogn. Sci. 18, 345–350. 10.1016/j.tics.2014.04.002 [DOI] [PubMed] [Google Scholar]
- Tao J., Chen X., Egorova N., Liu J., Xue X., Wang Q., et al. (2017a). Tai Chi Chuan and baduanjin practice modulates functional connectivity of the cognitive control network in older adults. Sci. Rep. 7:41581. 10.1038/srep41581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J., Liu J., Liu W., Huang J., Xue X., Chen X., et al. (2017b). Tai Chi Chuan and baduanjin increase grey matter volume in older adults: a brain imaging study. J. Alzheimers Dis. 60, 389–400. 10.3233/JAD-170477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J., Liu J., Egorova N., Chen X., Sun S., Xue X., et al. (2016). Increased hippocampus–medial prefrontal cortex resting state functional connectivity and memory function after Tai Chi Chuan practice in elder adults. Front. Aging Neurosci. 8:25. 10.3389/fnagi.2016.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J., Rao T., Lin L., Liu W., Wu Z., Zheng G., et al. (2015). Evaluation of Tai Chi yunshou exercises on community-based stroke patients with balance dysfunction: a study protocol of a cluster randomized controlled trial. BMC Complement. Altern. Med. 15:31. 10.1186/s12906-015-0555-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai P. F., Chang J. Y., Beck C., Kuo Y. F., Keefe F. J. (2013). A pilot cluster-randomized trial of a 20-week Tai Chi program in elders with cognitive impairment and osteoarthritic knee: effects on pain and other health outcomes. J. Pain Symptom Manage. 45, 660–669. 10.1016/j.jpainsymman.2012.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Vijver I., Cohen M. X., Ridderinkhof K. R. (2014). Aging affects medial but not anterior frontal learning-related theta oscillations. Neurobiol. Aging 35, 692–704. 10.1016/j.neurobiolaging.2013.09.006 [DOI] [PubMed] [Google Scholar]
- van Kesteren M. T., Rijpkema M., Ruiter D. J., Fernández G. (2010). Retrieval of associative information congruent with prior knowledge is related to increased medial prefrontal activity and connectivity. J. Neurosci. 30, 15888–15894. 10.1523/JNEUROSCI.2674-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kesteren M. T., Ruiter D. J., Fernández G., Henson R. N. (2012). How schema and novelty augment memory formation. Trends Neurosci. 35, 211–219. 10.1016/j.tins.2012.02.001 [DOI] [PubMed] [Google Scholar]
- Voss M. W., Heo S., Prakash R. S., Erickson K. I., Alves H., Chaddock L., et al. (2013). The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: results of a one- year exercise intervention. Hum. Brain Mapp. 34, 2972–2985. 10.1002/hbm.22119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. T. (2007). Effect of baduanjin on physiological age of intelligence for old people. J. Clin. Rehabil. Tissue Eng. Res. 11, 7910–7913. [Google Scholar]
- Wang C., Schmid C. H., Rones R., Kalish R., Yinh J., Goldenberg D. L., et al. (2010). A randomized trial of Tai Chi for fibromyalgia. N. Engl. J. Med. 363, 743–754. 10.1056/NEJMoa0912611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne P. M., Walsh J. N., Taylor-Piliae R. E., Wells R. E., Papp K. V., Donovan N. J., et al. (2014). Effect of Tai Chi on cognitive performance in older adults: systematic review and meta-analysis. J. Am. Geriatr. Soc. 62, 25–39. 10.1111/jgs.12611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei G. X., Dong H. M., Yang Z., Luo J., Zuo X. N. (2014). Tai Chi Chuan optimizes the functional organization of the intrinsic human brain architecture in older adults. Front. Aging Neurosci. 6:74. 10.3389/fnagi.2014.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei G.-X., Xu T., Fan F.-M., Dong H.-M., Jiang L.-L., Li H.-J., et al. (2013). Can taichi reshape the brain? a brain morphometry study. PLoS One 8:e61038. 10.1371/journal.pone.0061038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodard J. L., Axelrod B. N. (1995). Parsimonious prediction of wechsler memory scale—revised memory indices. Psychol. Assess. 7, 445–449. 10.1037//1040-3590.7.4.445 [DOI] [PubMed] [Google Scholar]
- Xiong X., Wang P., Li S., Zhang Y., Li X. (2015). Effect of baduanjin exercise for hypertension: a systematic review and meta-analysis of randomized controlled trials. Maturitas 80, 370–378. 10.1016/j.maturitas.2015.01.002 [DOI] [PubMed] [Google Scholar]
- Yan C. G., Cheung B., Kelly C., Colcombe S., Craddock R. C., Di Martino A., et al. (2013). A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage 76, 183–201. 10.1016/j.neuroimage.2013.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin S., Zhu X., Li R., Niu Y., Wang B., Zheng Z., et al. (2014). Intervention-induced enhancement in intrinsic brain activity in healthy older adults. Sci. Rep. 4:7309. 10.1038/srep07309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang Y. F., He Y., Zhu C. Z., Cao Q. J., Sui M. Q., Liang M., et al. (2007). Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 29, 83–91. 10.1016/j.braindev.2006.07.002 [DOI] [PubMed] [Google Scholar]
- Zhang M. Y., Katzman R., Salmon D., Jin H., Cai G. J., Wang Z. Y., et al. (1990). The prevalence of dementia and Alzheimer’s disease in shanghai, china: impact of age, gender, and education. Ann. Neurol. 27, 428–437. 10.1002/ana.410270412 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zhu C., Chen H., Duan X., Lu F., Li M., et al. (2015). Frequency-dependent alterations in the amplitude of low-frequency fluctuations in social anxiety disorder. J. Affect. Disord. 174, 329–335. 10.1016/j.jad.2014.12.001 [DOI] [PubMed] [Google Scholar]
- Zhao Z.-L., Fan F.-M., Lu J., Li H.-J., Jia L.-F., Han Y., et al. (2015). Changes of gray matter volume and amplitude of low-frequency oscillations in amnestic MCI: an integrative multi-modal MRI study. Acta Radiol. 56, 614–621. 10.1177/0284185114533329 [DOI] [PubMed] [Google Scholar]
- Zheng G., Chen B., Fang Q., Yi H., Lin Q., Chen L., et al. (2014). Primary prevention for risk factors of ischemic stroke with baduanjin exercise intervention in the community elder population: study protocol for a randomized controlled trial. Trials 15:113. 10.1186/1745-6215-15-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G., Liu F., Li S., Huang M., Tao J., Chen L. (2015). Tai Chi and the protection of cognitive ability. Am. J. Prev. Med. 49, 89–97. 10.1016/j.amepre.2015.01.002 [DOI] [PubMed] [Google Scholar]
- Zou Q. H., Zhu C. Z., Yang Y., Zuo X. N., Long X. Y., Cao Q. J., et al. (2008). An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J. Neurosci. Methods 172, 137–141. 10.1016/j.jneumeth.2008.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X. N., Di Martino A., Kelly C., Shehzad Z. E., Gee D. G., Klein D. F., et al. (2010). The oscillating brain: complex and reliable. Neuroimage 49, 1432–1445. 10.1016/j.neuroimage.2009.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]