Abstract
Cancer stem cells (CSCs) are rare but accounted for tumor initiation, progression, metastasis, relapse and therapeutic resistance. Ubiquitination and deubiquitination of stemness-related proteins are essential for CSC maintenance and differentiation, even leading to execute various stem cell fate choices. Deubiquitinating enzymes (DUBs), specifically disassembling ubiquitin chains, are important to maintain the balance between ubiquitination and deubiquitination. In this review, we have focused on the DUBs regulation of stem cell fate determination. For example, we discuss deubiquitinase inhibition may lead stem cell transcription factors and CSCs-related protein degradation. Also, CSCs microenvironment is regulated by DUBs activity. Our review provides a new insight into DUBs activity by emphasizing their cellular role in regulating stem cell fate and illustrates the opportunities for the application of DUBs inhibitors in the CSC-targeted therapy.
Keywords: Cancer stem cells, Deubiquitinating enzymes, Cancer therapies, CSCs
Background
The existence of cancer stem cells (CSCs) are considered to play a pivotal role in tumor recurrence, resistance and progression [1, 2]. There are three main aspects to effect CSCs maintenance and differentiation, including transcription factor network, CSC-related proteins and microenvironment [3, 4]. Conventional cancer therapy can’t kill cancer stem cells, which will cause cancer relapse and drug resistance under certain conditions (Fig. 1).
Fig. 1.
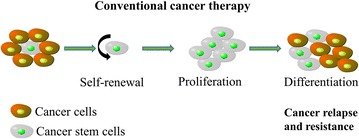
CSCs cause cancer relapse and resistance after conventional cancer therapy. The conventional therapy targeting the tumor bulk without targeting the CSCs leads to tumor recurrence
Ubiquitination is a post-translational modification process that participates in the covalent conjugation of small, highly conserved 76 amino acid protein ubiquitin with the lysine residues of the substrate protein through the cascade of enzyme reactions, including E1-activating enzymes, E2-conjugating enzymes, and E3 ligases, resulting in protein final degradation, relocalization or activity change. On the contrary, DUB-mediated deubiquitination removes the ubiquitin labels to protect substrate proteins from above-mentioned changes caused by ubiquitination. It has been reported that the ubiquitination and deubiquitination of the key proteins in stem cells may determine the fate of cells (Fig. 2). Recently, DUBs have been demonstrated as promising targets for cancer therapy [5–7], their functions in cancer cell stemness remains elusive. For example, USP54 is overexpressed in colorectal cancer stem cells and promotes intestinal tumorigenesis [8]. USP28 confers stem-cell-like traits to breast cancer cells [9].
Fig. 2.
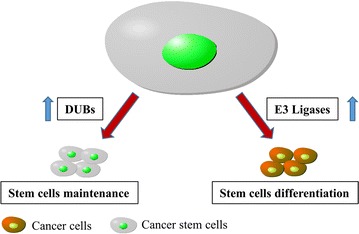
Regulating CSCs differentiation and pluripotency by ubiquitination and deubiquitination. Ubiquitination of core stem cell transcription factors or related key proteins by E3 ligases may drive CSCs differentiation, but deubiquitination of those proteins by DUBs mediates stem pluripotency
Finding deubiquitinates of transcription factors and key protein can provide better understand of the activation mechanism on CSCs, and further deubiquitination inhibitors can be used to eliminate CSCs in cancer radical treatment.
DUBs and CSC-associated transcription factors
Embryonic stem cells (ESCs) self-renewal and differentiation are known to be regulated by a network of transcription factors including Oct3/4, Sox2, c-Myc, Klf4 and Nanog [10, 11]. Cancer stem cells share significant similarity with normal stem cells in biological characteristics such as quiescence, self-renewal and differentiation [12, 13].
Sox2
Sox2 also regulates the differentiation and stemness in cancer stem cells [14]. USP22 is located directly on the Sox2 promoter and negatively regulates Sox2 transcription in ESCs [15]. In brain tumor cells, Usp9x was associated with Sox2 and played key roles in the growth of tumor cells, but the relationship between them was not clear [16]. Sox2 also regulated DUBs activity by binding to the promoter region at the transcriptional level, such as USP7, USP25, USP37, and USP44 [17].
c-Myc
c-Myc is a classical CSC-related marker, which can be stabilized by many DUBs. USP37 directly deubiquitinates and stabilizes c-Myc in lung cancer [18]. USP22 positively regulates c-Myc stability and tumorigenic activity in mammalian and breast cancer cells [19]. In a subset of human breast and lung cancers, USP36 interacts with and deubiquitinates c-Myc [20]. USP28 is required for c-Myc stability in human tumor cells, which binds to c-Myc through an interaction with FBW7alpha, an F-box protein that is part of an SCF-type ubiquitin ligase [21].
Nanog and ID proteins
Recent studies demonstrated that USP21 maintained the stemness of mouse embryonic stem cells via stabilization of Nanog by removing K48-linked ubiquitin chains [22]. Inhibitor of DNA binding (ID) proteins are transcriptional regulators that control the timing of cell fate determination and differentiation in stem and progenitor cells during normal development and adult life [23]. The small molecule inhibitor of USP1 promotes ID1 degradation and has cytotoxicity to leukemic cells [24]. USP1 deubiquitinated and stabilized ID1, ID2, and ID3 proteins to preserve a mesenchymal stem cell program in osteosarcoma [25].
Some pluripotent factors such as Oct3/4, Klf4 and Lin28 have not been found their DUBs, but all of them are affected by the 26S proteasome, suggesting a potential role of DUB for their stabilization in CSCs.
DUBs and CSC-related proteins
Some CSC-related proteins also control the fate of CSC, such as SIRT1, P53, PTEN, LSD1, PRC and so on. SIRT1, a NAD+-dependent histone deacetylase, influences stem cell aging by controlling mitochondrial biogenesis and turnover which may be required for self-renewal [26, 27].
SIRT1
SIRT1 inhibition represents a potential approach to target leukemia stem cells [28, 29]. USP22 interacts with and stabilizes SIRT1 by removing polyubiquitin chains conjugated onto SIRT1 in mouse embryonic development [30].
P53
P53, tumor suppresser, demonstrates a role for p53 deficiency in enhancing the formation of tumors arising from stem cells (embryonal carcinoma cells) [31, 32]. It is reported that USP10 deubiquitinates p53, reversing Mdm2-induced p53 nuclear export and degradation [33]. Ataxin-3, the machado–joseph disease deubiquitinase, interacts with p53 and functions as a novel p53 DUB [34]. USP7 deubiquitinates both p53 and MDM2, one of the ubiquitin ligases that ubiquitylates p53, thereby stabilizing both proteins [35, 36]. OTUD1, OTUD5 and USP11 directly deubiquitinating p53 and functional proteins were required for p53 stabilization [37–39].
PTEN
PTEN loss leads to the development of cancer stem cells, with the capacity of self-renewal and multi-lineage differentiation [40–43]. ATXN3 acts primarily by repressing PTEN transcription, without altering PTEN protein stability [44]. However, USP18 overexpression could stabilize PTEN protein, and USP18 repression decreases mainly cytoplasmic PTEN [45]. PTEN subcellular compartmentalization can be regulated by USP7 [46, 47].
PRC
The dysfunction of polycomb repressive complex (PRC) is closely related to cancer stemness [48, 49]. PRC1 represses transcription is only in part dependent on its ubiquitination activity, and Fbxl10 is reported to recruit PRC1 to CpG islands and regulate H2A ubiquitylation [50, 51]. Polycomb gene silencing may require H2A ubiquitination by PRC1 and H2A deubiquitination by Polycomb repressive deubiquitinase (PR-DUB). In some cancer types, PRC1 can be deubiquitinated by USP7, USP11 and USP26 [52, 53]. PRC2-mediated histone methylation plays an important role in aberrant cancer gene silencing and is a potential target for cancer therapy. The PRC2 proteins EZH2 is frequently overexpressed in mesothelioma with BAP1 mutation [54]. The deubiquitination enzymes of PRC need to be further explored in the future.
LSD
Lysine-specific demethylase 1 (LSD1), the first identified histone demethylase, maintains cell stemness during cancer progression [55, 56]. USP7 and USP28 inhibited LSD1 ubiquitination and stabilized LSD1 protein level [9, 57].
Taken together, CSC-related proteins degradation or activity inhibition by targeting DUBs is effective for eliminating cancer stem cells.
DUBs and CSC microenvironment
The microenvironment of CSC has also been reported to play essential roles in maintenance of cancer stemness. Tumor specific microenvironments comprise stromal cells, immune cells, networks of cytokines and growth factors, hypoxic regions, and the extracellular matrix (ECM). We summarize the role of CSC microenvironment from two aspects: hypoxia and inflammation [58–60].
Hypoxia
Hypoxia is considered to be a major feature of the tumor microenvironment and is a potential contributor to the CSC phenotype. Hypoxia-inducible factor (HIF) transcription factors (HIF-1α and HIF-2α) are key mediators in cancer hypoxia response and help maintain multiple CSC population [61, 62]. In the presence of oxygen, VHL tumor suppressor protein interacts with HIF proteins and this interaction results in the ubiquitination and degradation of HIF proteins, maintaining low levels of these transcription factors [63]. However, HIF proteins stabilization can be regulated by DUBs, such as USP8, USP19 and USP28 [64–66]. In addition, USP52 is a key component of P-bodies required to prevent HIF1α mRNA degradation [67].
Inflammation
The inflammatory cytokines modify the cancer microenvironment, CSCs secretion factors attract the necessary cells into their areas, enabling them better survive and escape chemotherapy [68]. Transforming growth factor β (TGFβ) has the ability to regulate immune cell populations in inhibiting and promoting tumor formation and progression active [69]. Cancer cells exposed to IL-6 are malignant, such as enhanced invasive ability and drug resistance [70, 71]. IL-8 promotes angiogenic activity through the activation of VEGFR2 [78]. USP21 binds to the promoter region of IL-8 and mediates transcriptional initiation in stem-cell like property of human renal cell carcinoma [79]. Also, IL-6 and G-CSF levels have been elevated in lung CSCs [80]. Most inflammatory cytokines are produced by many kinds of signal pathways and the deubiquitination of key proteins in the pathway can block inflammatory cytokines release. For example, TRAF6, a key regulator in toll-like receptor pathway and NF-κB pathway, can be regulated by USP4 and A20 [81, 82].
Conclusions
CSCs are difficult to eliminate by conventional treatment, mainly due to disorders of signal transduction and epigenetics. The control of ubiquitination and deubiquitination of CSC-related proteins determine the difference in CSCs and the maintenance of pluripotency. DUBs can protect the stemness of the CSC, thereby maintaining its activity and further forming a vicious circle. Therefore, DUBs are very important in the CSC specific treatment. We summarized the effect of deubiquitinating enzymes in the regulation of target proteins in Table 1. The successful inhibition of CSC maintenance and radiation resistance by USP1 specific inhibitor (pimozide) has been provided the basis for further clinical trials [83]. It means that DUB inhibitors may boost more advantages in CSC-specific therapy than other anti-cancer drugs such as proteasome inhibitors. For example, b-AP15, a selective DUB inhibitor, can overcome bortezomib resistance in multiple myeloma [84]. More relevant basic research should be carried out to determine the DUBs related to the CSCs and to identify the mechanisms between them. Currently commercialized DUB inhibitors are summarized in Table 2, showing significant pharmacological effects on cancer cells or cancer stem cells. In general, strategies involving the use of DUB inhibitors to target combination therapy of cancer stem cells and differentiated cancer cells can provide better outcomes for radical cancer treatment.
Table 1.
The effect of deubiquitinating enzymes in the regulation of target proteins
| Proteins | Deubiquitinating enzymes | Effect | References |
|---|---|---|---|
| Sox2 | USP22 | Transcription | [15] |
| USP9X | Unclear | [16] | |
| c-myc | USP37 | Protein stabilization | [18] |
| USP22 | [19] | ||
| USP36 | [20] | ||
| USP28 | [21] | ||
| Nanog | USP21 | Protein stabilization | [22] |
| ID proteins | USP1 | Protein stabilization | [24, 25] |
| SIRT1 | USP22 | Protein stabilization | [30] |
| p53 | USP10 | Protein stabilization | [33] |
| Ataxin-3 | [34] | ||
| USP7 | [35, 36] | ||
| OTUD1 | [37] | ||
| OTUD5 | [38] | ||
| USP11 | [39] | ||
| PTEN | ATXN3 | Transcription | [44] |
| USP18 | Protein stabilization | [45] | |
| USP7 | Location | [46, 47] | |
| PRC1 | USP7 | Protein stabilization | [52] |
| USP11 | [53] | ||
| USP26 | [77] | ||
| PRC2 | BAP1 | Unclear | [54] |
| LSD1 | USP7 | Protein stabilization | [57] |
| USP28 | [9] | ||
| HIF-1α | USP8 | Protein stabilization | [66] |
| USP19 | [65] | ||
| USP28 | [64] | ||
| USP52 | mRNA degradation | [67] | |
| IL-8 | USP21 | Transcription | [79] |
| TRAF6 | USP4 | Activity | [81] |
| A20 | [82] |
Table 2.
DUB inhibitors for preclinical application in CSC-targeted therapy
| Inhibitors | Targeted DUBs | CSC type | References |
|---|---|---|---|
| Pimozide | USP1 | Osteosarcoma, glioblastoma | [25, 83] |
| ML323 | USP1 | ||
| P5091 | USP7, USP47 | Neural, glioblastoma, multiple myeloma | [57, 85–87] |
| P22077 | USP7, USP47 | ||
| WP1130 | USP9x, USP5, UCHL1, USP14, UCH37 | Liver, breast cancer | [72, 73] |
| IU1 | USP14 | Gastric, multiple myeloma | [74, 75] |
| b-AP15 | USP14, UCHL5 | ||
| VLX1570 | USP14 | ||
| LDN-57444 | UCHL1, UCHL3 | Prostate | [76] |
| TCID | UCHL3, UCHL5 | Multiple myeloma | [84] |
Authors’ contributions
HL collected materials and wrote the review. HZS collected materials. YLW modified and corrected the review. All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Not applicable.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
National Natural Science Foundation of China (81570118; 81700475).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- CSCs
cancer stem cells
- DUBs
deubiquitinating enzymes
- ESCs
embryonic stem cells
- ID
inhibitor of DNA binding
- PRC
polycomb repressive complex
- LSD1
lysine-specific demethylase 1
- ECM
extracellular matrix
- HIF
hypoxia-inducible factor
- TGFβ
transforming growth factor β
Contributor Information
Hu Lei, Email: hulei@shsmu.edu.cn.
Yingli Wu, Email: wuyingli@shsmu.edu.cn.
References
- 1.Krause M, Dubrovska A, Linge A, Baumann M. Cancer stem cells: radioresistance, prediction of radiotherapy outcome and specific targets for combined treatments. Adv Drug Deliv Rev. 2017;109:63–73. doi: 10.1016/j.addr.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem cells–perspectives on current status and future directions: AACR workshop on cancer stem cells. Cancer Res. 2006;66(19):9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 3.Qiu GZ, Sun W, Jin MZ, Lin J, Lu PG, Jin WL. The bad seed gardener: deubiquitinases in the cancer stem–cell signaling network and therapeutic resistance. Pharmacol Ther. 2017;172:127–138. doi: 10.1016/j.pharmthera.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Suresh B, Lee J, Kim KS, Ramakrishna S. The importance of ubiquitination and deubiquitination in cellular reprogramming. Stem Cells Int. 2016;2016:6705927. doi: 10.1155/2016/6705927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fraile JM, Manchado E, Lujambio A, Quesada V, Campos-Iglesias D, Webb TR, Lowe SW, Lopez-Otin C, Freije JM. USP39 deubiquitinase is essential for KRAS oncogene-driven cancer. J Biol Chem. 2017;292(10):4164–4175. doi: 10.1074/jbc.M116.762757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao C, Chen X, Zang D, Lan X, Liao S, Yang C, Zhang P, Wu J, Li X, Liu N, et al. A novel nickel complex works as a proteasomal deubiquitinase inhibitor for cancer therapy. Oncogene. 2016;35(45):5916–5927. doi: 10.1038/onc.2016.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suresh B, Lee J, Kim H, Ramakrishna S. Regulation of pluripotency and differentiation by deubiquitinating enzymes. Cell Death Differ. 2016;23(8):1257–1264. doi: 10.1038/cdd.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fraile JM, Campos-Iglesias D, Rodriguez F, Espanol Y, Freije JM. The deubiquitinase USP54 is overexpressed in colorectal cancer stem cells and promotes intestinal tumorigenesis. Oncotarget. 2016;7(46):74427–74434. doi: 10.18632/oncotarget.12769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Y, Wang Y, Yang XH, Kang T, Zhao Y, Wang C, Evers BM, Zhou BP. The deubiquitinase USP28 stabilizes LSD1 and confers stem-cell-like traits to breast cancer cells. Cell Rep. 2013;5(1):224–236. doi: 10.1016/j.celrep.2013.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai N, Li M, Qu J, Liu GH, Izpisua Belmonte JC. Post-translational modulation of pluripotency. J Mol Cell Biol. 2012;4(4):262–265. doi: 10.1093/jmcb/mjs031. [DOI] [PubMed] [Google Scholar]
- 11.Ramakrishna S, Kim KS, Baek KH. Posttranslational modifications of defined embryonic reprogramming transcription factors. Cell Reprogr. 2014;16(2):108–120. doi: 10.1089/cell.2013.0077. [DOI] [PubMed] [Google Scholar]
- 12.Zhao J. Cancer stem cells and chemoresistance: the smartest survives the raid. Pharmacol Ther. 2016;160:145–158. doi: 10.1016/j.pharmthera.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bharti R, Dey G, Mandal M. Cancer development, chemoresistance, epithelial to mesenchymal transition and stem cells: a snapshot of IL-6 mediated involvement. Cancer Lett. 2016;375(1):51–61. doi: 10.1016/j.canlet.2016.02.048. [DOI] [PubMed] [Google Scholar]
- 14.Liu K, Lin B, Zhao M, Yang X, Chen M, Gao A, Liu F, Que J, Lan X. The multiple roles for Sox2 in stem cell maintenance and tumorigenesis. Cell Sign. 2013;25(5):1264–1271. doi: 10.1016/j.cellsig.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sussman RT, Stanek TJ, Esteso P, Gearhart JD, Knudsen KE, McMahon SB. The epigenetic modifier ubiquitin-specific protease 22 (USP22) regulates embryonic stem cell differentiation via transcriptional repression of sex-determining region Y-box 2 (SOX2) J Biol Chem. 2013;288(33):24234–24246. doi: 10.1074/jbc.M113.469783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cox JL, Wilder PJ, Gilmore JM, Wuebben EL, Washburn MP, Rizzino A. The SOX2-interactome in brain cancer cells identifies the requirement of MSI2 and USP9X for the growth of brain tumor cells. PLoS ONE. 2013;8(5):e62857. doi: 10.1371/journal.pone.0062857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122(6):947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan J, Deng Q, Jiang C, Wang X, Niu T, Li H, Chen T, Jin J, Pan W, Cai X, et al. USP37 directly deubiquitinates and stabilizes c-Myc in lung cancer. Oncogene. 2015;34(30):3957–3967. doi: 10.1038/onc.2014.327. [DOI] [PubMed] [Google Scholar]
- 19.Kim D, Hong A, Park HI, Shin WH, Yoo L, Jeon SJ, Chung KC. Deubiquitinating enzyme USP22 positively regulates c-Myc stability and tumorigenic activity in mammalian and breast cancer cells. J Cell Physiol. 2017;232(12):3664–3676. doi: 10.1002/jcp.25841. [DOI] [PubMed] [Google Scholar]
- 20.Sun XX, He X, Yin L, Komada M, Sears RC, Dai MS. The nucleolar ubiquitin-specific protease USP36 deubiquitinates and stabilizes c-Myc. Proc Natl Acad Sci USA. 2015;112(12):3734–3739. doi: 10.1073/pnas.1411713112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Popov N, Wanzel M, Madiredjo M, Zhang D, Beijersbergen R, Bernards R, Moll R, Elledge SJ, Eilers M. The ubiquitin-specific protease USP28 is required for MYC stability. Nat Cell Biol. 2007;9(7):765–774. doi: 10.1038/ncb1601. [DOI] [PubMed] [Google Scholar]
- 22.Jin J, Liu J, Chen C, Liu Z, Jiang C, Chu H, Pan W, Wang X, Zhang L, Li B, et al. The deubiquitinase USP21 maintains the stemness of mouse embryonic stem cells via stabilization of Nanog. Nat Commun. 2016;7:13594. doi: 10.1038/ncomms13594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lasorella A, Benezra R, Iavarone A. The ID proteins: master regulators of cancer stem cells and tumour aggressiveness. Nat Rev Cancer. 2014;14(2):77–91. doi: 10.1038/nrc3638. [DOI] [PubMed] [Google Scholar]
- 24.Mistry H, Hsieh G, Buhrlage SJ, Huang M, Park E, Cuny GD, Galinsky I, Stone RM, Gray NS, D’Andrea AD, et al. Small-molecule inhibitors of USP1 target ID1 degradation in leukemic cells. Mol Cancer Ther. 2013;12(12):2651–2662. doi: 10.1158/1535-7163.MCT-13-0103-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams SA, Maecker HL, French DM, Liu J, Gregg A, Silverstein LB, Cao TC, Carano RA, Dixit VM. USP1 deubiquitinates ID proteins to preserve a mesenchymal stem cell program in osteosarcoma. Cell. 2011;146(6):918–930. doi: 10.1016/j.cell.2011.07.040. [DOI] [PubMed] [Google Scholar]
- 26.Mantel C, Broxmeyer HE. Sirtuin 1, stem cells, aging, and stem cell aging. Curr Opin Hematol. 2008;15(4):326–331. doi: 10.1097/MOH.0b013e3283043819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou L, Chen X, Liu T, Zhu C, Si M, Jargstorf J, Li M, Pan G, Gong Y, Luo ZP, et al. SIRT1-dependent anti-senescence effects of cell-deposited matrix on human umbilical cord mesenchymal stem cells. J Tissue Eng Regen Med. 2017 doi: 10.1002/term.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li L, Osdal T, Ho Y, Chun S, McDonald T, Agarwal P, Lin A, Chu S, Qi J, Li L, et al. SIRT1 activation by a c-MYC oncogenic network promotes the maintenance and drug resistance of human FLT3-ITD acute myeloid leukemia stem cells. Cell Stem Cell. 2014;15(4):431–446. doi: 10.1016/j.stem.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin Y, Cao Q, Chen C, Du X, Jin B, Pan J. Tenovin-6-mediated inhibition of SIRT1/2 induces apoptosis in acute lymphoblastic leukemia (ALL) cells and eliminates ALL stem/progenitor cells. BMC Cancer. 2015;15:226. doi: 10.1186/s12885-015-1282-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin Z, Yang H, Kong Q, Li J, Lee SM, Gao B, Dong H, Wei J, Song J, Zhang DD, et al. USP22 antagonizes p53 transcriptional activation by deubiquitinating Sirt1 to suppress cell apoptosis and is required for mouse embryonic development. Mol Cell. 2012;46(4):484–494. doi: 10.1016/j.molcel.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 31.Puzio-Kuter AM, Levine AJ. Stem cell biology meets p53. Nat Biotechnol. 2009;27(10):914–915. doi: 10.1038/nbt1009-914. [DOI] [PubMed] [Google Scholar]
- 32.Aloni-Grinstein R, Shetzer Y, Kaufman T, Rotter V. p53: the barrier to cancer stem cell formation. FEBS Lett. 2014;588(16):2580–2589. doi: 10.1016/j.febslet.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 33.Yuan J, Luo K, Zhang L, Cheville JC, Lou Z. USP10 regulates p53 localization and stability by deubiquitinating p53. Cell. 2010;140(3):384–396. doi: 10.1016/j.cell.2009.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu H, Li X, Ning G, Zhu S, Ma X, Liu X, Liu C, Huang M, Schmitt I, Wullner U, et al. The Machado-Joseph disease deubiquitinase Ataxin-3 regulates the stability and apoptotic function of p53. PLoS Biol. 2016;14(11):e2000733. doi: 10.1371/journal.pbio.2000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brooks CL, Li M, Hu M, Shi Y, Gu W. The p53–Mdm2–HAUSP complex is involved in p53 stabilization by HAUSP. Oncogene. 2007;26(51):7262–7266. doi: 10.1038/sj.onc.1210531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brooks CL, Gu W. p53 regulation by ubiquitin. FEBS Lett. 2011;585(18):2803–2809. doi: 10.1016/j.febslet.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piao S, Pei HZ, Huang B, Baek SH. Ovarian tumor domain-containing protein 1 deubiquitinates and stabilizes p53. Cell Sign. 2017;33:22–29. doi: 10.1016/j.cellsig.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 38.Luo J, Lu Z, Lu X, Chen L, Cao J, Zhang S, Ling Y, Zhou X. OTUD5 regulates p53 stability by deubiquitinating p53. PLoS ONE. 2013;8(10):e77682. doi: 10.1371/journal.pone.0077682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ke JY, Dai CJ, Wu WL, Gao JH, Xia AJ, Liu GP, Lv KS, Wu CL. USP11 regulates p53 stability by deubiquitinating p53. J Zhejiang Univ Sci B. 2014;15(12):1032–1038. doi: 10.1631/jzus.B1400180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schubbert S, Jiao J, Ruscetti M, Nakashima J, Wu S, Lei H, Xu Q, Yi W, Zhu H, Wu H. Methods for PTEN in stem cells and cancer stem cells. Methods Mol Biol. 2016;1388:233–285. doi: 10.1007/978-1-4939-3299-3_15. [DOI] [PubMed] [Google Scholar]
- 41.Duan S, Yuan G, Liu X, Ren R, Li J, Zhang W, Wu J, Xu X, Fu L, Li Y, et al. PTEN deficiency reprogrammes human neural stem cells towards a glioblastoma stem cell-like phenotype. Nat Commun. 2015;6:10068. doi: 10.1038/ncomms10068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liao J, Marumoto T, Yamaguchi S, Okano S, Takeda N, Sakamoto C, Kawano H, Nii T, Miyamato S, Nagai Y, et al. Inhibition of PTEN tumor suppressor promotes the generation of induced pluripotent stem cells. Mol Ther. 2013;21(6):1242–1250. doi: 10.1038/mt.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hill R, Wu H. PTEN, stem cells, and cancer stem cells. J Biol Chem. 2009;284(18):11755–11759. doi: 10.1074/jbc.R800071200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sacco JJ, Yau TY, Darling S, Patel V, Liu H, Urbe S, Clague MJ, Coulson JM. The deubiquitylase Ataxin-3 restricts PTEN transcription in lung cancer cells. Oncogene. 2014;33(33):4265–4272. doi: 10.1038/onc.2013.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mustachio LM, Kawakami M, Lu Y, Rodriguez-Canales J, Mino B, Behrens C, Wistuba I, Bota-Rabassedas N, Yu J, Lee JJ, et al. The ISG15-specific protease USP18 regulates stability of PTEN. Oncotarget. 2017;8(1):3–14. doi: 10.18632/oncotarget.13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song MS, Salmena L, Carracedo A, Egia A, Lo-Coco F, Teruya-Feldstein J, Pandolfi PP. The deubiquitinylation and localization of PTEN are regulated by a HAUSP-PML network. Nature. 2008;455(7214):813–817. doi: 10.1038/nature07290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morotti A, Panuzzo C, Crivellaro S, Pergolizzi B, Familiari U, Berger AH, Saglio G, Pandolfi PP. BCR-ABL disrupts PTEN nuclear-cytoplasmic shuttling through phosphorylation-dependent activation of HAUSP. Leukemia. 2014;28(6):1326–1333. doi: 10.1038/leu.2013.370. [DOI] [PubMed] [Google Scholar]
- 48.Gao X, Jin W. The emerging role of tumor-suppressive microRNA-218 in targeting glioblastoma stemness. Cancer Lett. 2014;353(1):25–31. doi: 10.1016/j.canlet.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 49.Suva ML, Riggi N, Janiszewska M, Radovanovic I, Provero P, Stehle JC, Baumer K, Le Bitoux MA, Marino D, Cironi L, et al. EZH2 is essential for glioblastoma cancer stem cell maintenance. Cancer Res. 2009;69(24):9211–9218. doi: 10.1158/0008-5472.CAN-09-1622. [DOI] [PubMed] [Google Scholar]
- 50.Laugesen A, Hojfeldt JW, Helin K. Role of the polycomb repressive complex 2 (PRC2) in transcriptional regulation and cancer. Cold Spring Harb Perspect Med. 2016;6(9):a026575. doi: 10.1101/cshperspect.a026575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu X, Johansen JV, Helin K. Fbxl10/Kdm2b recruits polycomb repressive complex 1 to CpG islands and regulates H2A ubiquitylation. Mol Cell. 2013;49(6):1134–1146. doi: 10.1016/j.molcel.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 52.Maertens GN, El Messaoudi-Aubert S, Elderkin S, Hiom K, Peters G. Ubiquitin-specific proteases 7 and 11 modulate Polycomb regulation of the INK4a tumour suppressor. EMBO J. 2010;29(15):2553–2565. doi: 10.1038/emboj.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lecona E, Narendra V, Reinberg D. USP7 cooperates with SCML2 to regulate the activity of PRC1. Mol Cell Biol. 2015;35(7):1157–1168. doi: 10.1128/MCB.01197-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kemp CD, Rao M, Xi S, Inchauste S, Mani H, Fetsch P, Filie A, Zhang M, Hong JA, Walker RL, et al. Polycomb repressor complex-2 is a novel target for mesothelioma therapy. Clin Cancer Res. 2012;18(1):77–90. doi: 10.1158/1078-0432.CCR-11-0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amente S, Lania L, Majello B. The histone LSD1 demethylase in stemness and cancer transcription programs. Biochim Biophys Acta. 2013;1829(10):981–986. doi: 10.1016/j.bbagrm.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 56.Hino S, Kohrogi K, Nakao M. Histone demethylase LSD1 controls the phenotypic plasticity of cancer cells. Cancer Sci. 2016;107(9):1187–1192. doi: 10.1111/cas.13004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yi L, Cui Y, Xu Q, Jiang Y. Stabilization of LSD1 by deubiquitinating enzyme USP7 promotes glioblastoma cell tumorigenesis and metastasis through suppression of the p53 signaling pathway. Oncol Rep. 2016;36(5):2935–2945. doi: 10.3892/or.2016.5099. [DOI] [PubMed] [Google Scholar]
- 58.Carnero A, Lleonart M. The hypoxic microenvironment: a determinant of cancer stem cell evolution. BioEssays. 2016;38(Suppl 1):S65–S74. doi: 10.1002/bies.201670911. [DOI] [PubMed] [Google Scholar]
- 59.Kise K, Kinugasa-Katayama Y, Takakura N. Tumor microenvironment for cancer stem cells. Adv Drug Deliv Rev. 2016;99(Pt B):197–205. doi: 10.1016/j.addr.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 60.Lau EY, Ho NP, Lee TK. Cancer stem cells and their microenvironment: biology and therapeutic implications. Stem Cells Int. 2017;2017:3714190. doi: 10.1155/2017/3714190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van den Beucken T, Koch E, Chu K, Rupaimoole R, Prickaerts P, Adriaens M, Voncken JW, Harris AL, Buffa FM, Haider S, et al. Hypoxia promotes stem cell phenotypes and poor prognosis through epigenetic regulation of DICER. Nat Commun. 2014;5:5203. doi: 10.1038/ncomms6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao M, Zhang Y, Zhang H, Wang S, Zhang M, Chen X, Wang H, Zeng G, Chen X, Liu G, et al. Hypoxia-induced cell stemness leads to drug resistance and poor prognosis in lung adenocarcinoma. Lung Cancer. 2015;87(2):98–106. doi: 10.1016/j.lungcan.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 63.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399(6733):271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 64.Flugel D, Gorlach A, Kietzmann T. GSK-3beta regulates cell growth, migration, and angiogenesis via Fbw7 and USP28-dependent degradation of HIF-1alpha. Blood. 2012;119(5):1292–1301. doi: 10.1182/blood-2011-08-375014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Altun M, Zhao B, Velasco K, Liu H, Hassink G, Paschke J, Pereira T, Lindsten K. Ubiquitin-specific protease 19 (USP19) regulates hypoxia-inducible factor 1alpha (HIF-1alpha) during hypoxia. J Biol Chem. 2012;287(3):1962–1969. doi: 10.1074/jbc.M111.305615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Troilo A, Alexander I, Muehl S, Jaramillo D, Knobeloch KP, Krek W. HIF1alpha deubiquitination by USP8 is essential for ciliogenesis in normoxia. EMBO Rep. 2014;15(1):77–85. doi: 10.1002/embr.201337688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bett JS, Ibrahim AF, Garg AK, Kelly V, Pedrioli P, Rocha S, Hay RT. The P-body component USP52/PAN2 is a novel regulator of HIF1A mRNA stability. Biochem J. 2013;451(2):185–194. doi: 10.1042/BJ20130026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shigdar S, Li Y, Bhattacharya S, O’Connor M, Pu C, Lin J, Wang T, Xiang D, Kong L, Wei MQ, et al. Inflammation and cancer stem cells. Cancer Lett. 2014;345(2):271–278. doi: 10.1016/j.canlet.2013.07.031. [DOI] [PubMed] [Google Scholar]
- 69.Bierie B, Moses HL. Transforming growth factor beta (TGF-beta) and inflammation in cancer. Cytokine Growth Factor Rev. 2010;21(1):49–59. doi: 10.1016/j.cytogfr.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dethlefsen C, Hojfeldt G, Hojman P. The role of intratumoral and systemic IL-6 in breast cancer. Breast Cancer Res Treat. 2013;138(3):657–664. doi: 10.1007/s10549-013-2488-z. [DOI] [PubMed] [Google Scholar]
- 71.Hollier BG, Evans K, Mani SA. The epithelial-to-mesenchymal transition and cancer stem cells: a coalition against cancer therapies. J Mammary Gland Biol Neoplasia. 2009;14(1):29–43. doi: 10.1007/s10911-009-9110-3. [DOI] [PubMed] [Google Scholar]
- 72.Shen G, Lin Y, Yang X, Zhang J, Xu Z, Jia H. MicroRNA-26b inhibits epithelial-mesenchymal transition in hepatocellular carcinoma by targeting USP9X. BMC Cancer. 2014;14:393. doi: 10.1186/1471-2407-14-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fu P, Du F, Liu Y, Yao M, Zhang S, Zheng X, Zheng S. WP1130 increases cisplatin sensitivity through inhibition of usp9x in estrogen receptor-negative breast cancer cells. Am J Transl Res. 2017;9(4):1783–1791. [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu Y, Zhang Y, Sui Z, Zhang Y, Liu M, Tang H. USP14 de-ubiquitinates vimentin and miR-320a modulates USP14 and vimentin to contribute to malignancy in gastric cancer cells. Oncotarget. 2016;8(30):48725–48736. doi: 10.18632/oncotarget.10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang X, Mazurkiewicz M, Hillert EK, Olofsson MH, Pierrou S, Hillertz P, Gullbo J, Selvaraju K, Paulus A, Akhtar S, et al. Corrigendum: the proteasome deubiquitinase inhibitor VLX1570 shows selectivity for ubiquitin-specific protease-14 and induces apoptosis of multiple myeloma cells. Sci Rep. 2016;6:30667. doi: 10.1038/srep30667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Song HM, Lee JE, Kim JH. Ubiquitin C-terminal hydrolase-L3 regulates EMT process and cancer metastasis in prostate cell lines. Biochem Biophys Res Commun. 2014;452(3):722–727. doi: 10.1016/j.bbrc.2014.08.144. [DOI] [PubMed] [Google Scholar]
- 77.Ning B, Zhao W, Qian C, Liu P, Li Q, Li W, Wang RF. USP26 functions as a negative regulator of cellular reprogramming by stabilising PRC1 complex components. Nat Commun. 2017;8(1):349. doi: 10.1038/s41467-017-00301-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martin D, Galisteo R, Gutkind JS. CXCL8/IL8 stimulates vascular endothelial growth factor (VEGF) expression and the autocrine activation of VEGFR2 in endothelial cells by activating NFkappaB through the CBM (Carma3/Bcl10/Malt1) complex. J Biol Chem. 2009;284(10):6038–42. doi: 10.1074/jbc.C800207200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peng L, Hu Y, Chen D, Jiao S, Sun S. Ubiquitin specific peptidase 21 regulates interleukin-8 expression, stem-cell like property of human renal cell carcinoma. Oncotarget. 2016;7(27):42007–16. doi: 10.18632/oncotarget.9751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Levina V, Marrangoni AM, DeMarco R, Gorelik E, Lokshin AE. Drug-selected human lung cancer stem cells: cytokine network, tumorigenic and metastatic properties. PLoS ONE. 2008;3(8):e3077. doi: 10.1371/journal.pone.0003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xiao N, Li H, Luo J, Wang R, Chen H, Chen J, Wang P. Ubiquitin-specific protease 4 (USP4) targets TRAF2 and TRAF6 for deubiquitination and inhibits TNFalpha-induced cancer cell migration. Biochem J. 2012;441(3):979–86. doi: 10.1042/BJ20111358. [DOI] [PubMed] [Google Scholar]
- 82.Sun SC. Deubiquitylation and regulation of the immune response. Nat Rev Immunol. 2008;8(7):501–11. doi: 10.1038/nri2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee JK, Chang N, Yoon Y, Yang H, Cho H, Kim E, Shin Y, Kang W, Oh YT, Mun GI, et al. USP1 targeting impedes GBM growth by inhibiting stem cell maintenance and radioresistance. Neuro-oncology. 2016;18(1):37–47. doi: 10.1093/neuonc/nov091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tian Z, D’Arcy P, Wang X, Ray A, Tai YT, Hu Y, Carrasco RD, Richardson P, Linder S, Chauhan D, et al. A novel small molecule inhibitor of deubiquitylating enzyme USP14 and UCHL5 induces apoptosis in multiple myeloma and overcomes bortezomib resistance. Blood. 2014;123(5):706–16. doi: 10.1182/blood-2013-05-500033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang Z, Wu Q, Guryanova OA, Cheng L, Shou W, Rich JN, Bao S. Deubiquitylase HAUSP stabilizes REST and promotes maintenance of neural progenitor cells. Nat Cell Biol. 2011;13(2):142–52. doi: 10.1038/ncb2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chauhan D, Tian Z, Nicholson B, Kumar KG, Zhou B, Carrasco R, McDermott JL, Leach CA, Fulcinniti M, Kodrasov MP, et al. A small molecule inhibitor of ubiquitin-specific protease-7 induces apoptosis in multiple myeloma cells and overcomes bortezomib resistance. Cancer Cell. 2012;22(3):345–58. doi: 10.1016/j.ccr.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fan YH, Cheng J, Vasudevan SA, Dou J, Zhang H, Patel RH, Ma IT, Rojas Y, Zhao Y, Yu Y, et al. USP7 inhibitor P22077 inhibits neuroblastoma growth via inducing p53-mediated apoptosis. Cell Death Dis. 2013;4:e867. doi: 10.1038/cddis.2013.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


